A Review on Microfluidic Paper-Based Analytical Devices for Glucose Detection
Abstract
:1. Introduction
2. Colorimetric Detection of Glucose
2.1. Fabrication Process of Colorimetric Glucose μPADs
2.2. Alternative Color Indicators for Glucose μPADs
2.3. 3D-μPADs
3. Electrochemical Detection of Glucose
3.1. Advanced Fabrications of Electrochemical Glucose μPADs
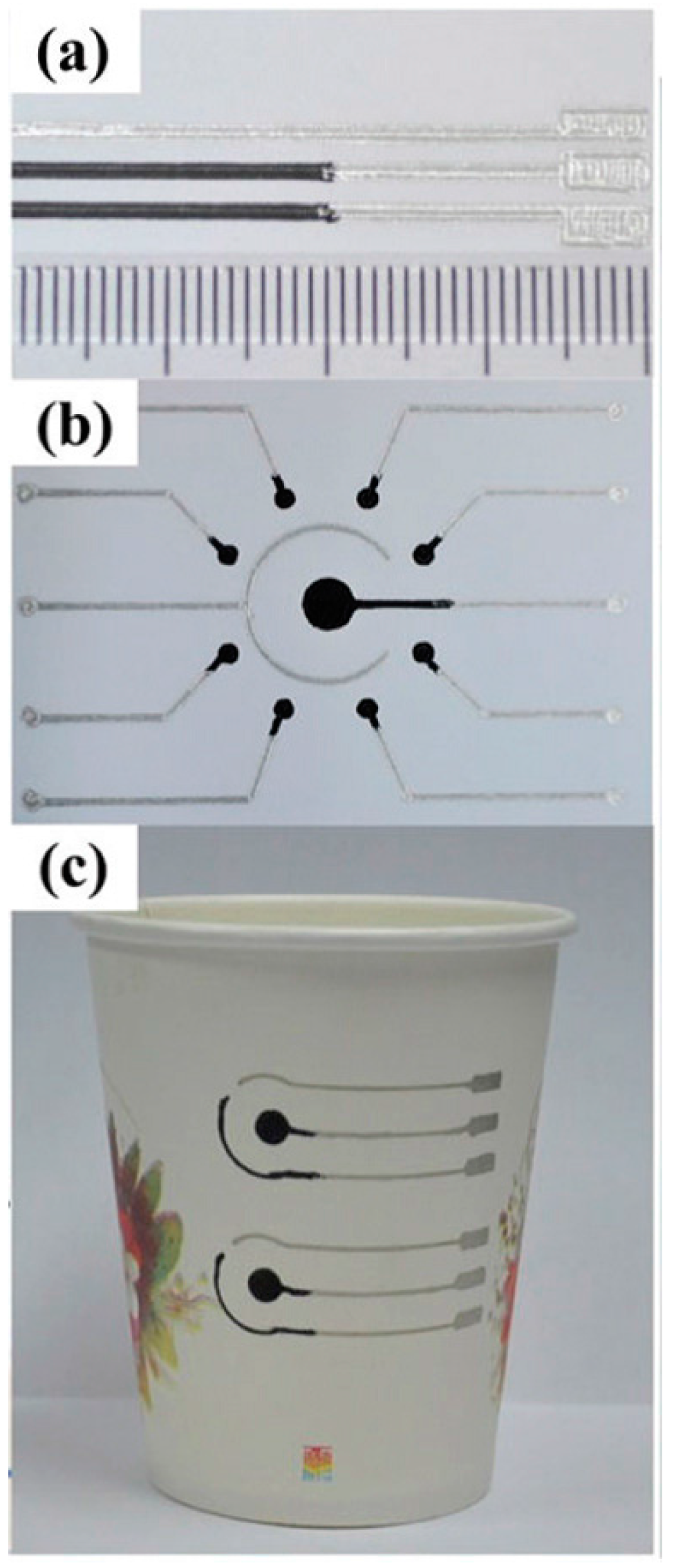
3.2. Electrochemical Glucose μPADs with Printed Electrodes
4. Other Glucose Detection Platforms
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Kaefer, M.; Piva, S.J.; De Carvalho, J.A.M.; Da Silva, D.B.; Becker, A.M.; Coelho, A.C.; Duarte, M.; Moresco, R.N. Association between ischemia modified albumin, inflammation and hyperglycemia in type 2 diabetes mellitus. Clin. Biochem. 2010, 43, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Si, P.; Kannan, P.; Guo, L.H.; Son, H.S.; Kim, D.H. Highly stable and sensitive glucose biosensor based on covalently assembled high density Au nanostructures. Biosens. Bioelectron. 2011, 26, 3845–3851. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Bu, R.F.; Sun, Z.L.; Lu, Q.S.; Jin, H.; Wang, Y.; Wang, S.H.; Li, L.; Xie, Z.L.; Yang, B.Q. Comparable efficacy of self-monitoring of quantitative urine glucose with self-monitoring of blood glucose on glycaemic control in non-insulin-treated type 2 diabetes. Diabetes Res. Clin. Pract. 2011, 93, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, J.; Wang, F.B.; Xiang, X.; Luo, M.; Ji, X.H.; He, Z.K. Determination of glucose and uric acid with bienzyme colorimetry on microfluidic paper-based analysis devices. Biosens. Bioelectron. 2012, 35, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Vaurs, C.; Brun, J.-F.; Bertrand, M.; Burcelin, R.; du Rieu, M.C.; Anduze, Y.; Hanaire, H.; Ritz, P. Post-prandial hypoglycemia results from a non-glucose-dependent inappropriate insulin secretion in Roux-en-Y gastric bypassed patients. Metab. Clin. Exp. 2016, 65, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Amer Diabet, A. Diagnosis and classification of diabetes mellitus American diabetes association. Diabetes Care 2011, 34, S62–S69. [Google Scholar]
- Maeda, E.; Kataoka, M.; Hino, M.; Kajimoto, K.; Kaji, N.; Tokeshi, M.; Kido, J.I.; Shinohara, Y.; Baba, Y. Determination of human blood glucose levels using microchip electrophoresis. Electrophoresis 2007, 28, 2927–2933. [Google Scholar] [CrossRef] [PubMed]
- Riddle, M.C. Oral pharmacologic management of type 2 diabetes. Am. Fam. Physician 1999, 60, 2613–2620. [Google Scholar] [PubMed]
- Allemann, S.; Houriet, C.; Diem, P.; Stettler, C. Self-monitoring of blood glucose in non-insulin treated patients with type 2 diabetes: A systematic review and meta-analysis. Curr. Med. Res. Opin. 2009, 25, 2903–2913. [Google Scholar] [CrossRef] [PubMed]
- Clar, C.; Barnard, K.; Cummins, E.; Royle, P.; Waugh, N. Aberdeen Health Technology Assessment Group. Self-monitoring of blood glucose in type 2 diabetes: Systematic review. Health Technol. Assess. 2010, 14, 1–140. [Google Scholar] [CrossRef] [PubMed]
- Hu, J. The evolution of commercialized glucose sensors in China. Biosens. Bioelectron. 2009, 24, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
- Lockwood, C.; Xue, Y.F.; McArthur, A. Self-monitoring of blood glucose in type 2 diabetes mellitus: A systematic review of economic evidence. J. Adv. Nurs. 2010, 66, 1931–1936. [Google Scholar]
- Martinez, A.W.; Phillips, S.T.; Whitesides, G.M.; Carrilho, E. Diagnostics for the developing world: Microfluidic paper-based analytical devices. Anal. Chem. 2010, 82, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.W.; Phillips, S.T.; Butte, M.J.; Whitesides, G.M. Patterned paper as a platform for inexpensive, low-volume, portable bioassays. Angew. Chem. Int. Edit. 2007, 46, 1318–1320. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.A.; van den Berg, A. Lab on paper. Lab Chip 2008, 8, 1988–1991. [Google Scholar] [PubMed]
- Carvalhal, R.F.; Carrilho, E.; Kubota, L.T. The potential and application of microfluidic paper-based separation devices. Bioanalysis 2010, 2, 1663–1665. [Google Scholar] [CrossRef] [PubMed]
- Coltro, W.K.T.; de Jesus, D.P.; da Silva, J.A.F.; do Lago, C.L.; Carrilho, E. Toner and paper-based fabrication techniques for microfluidic applications. Electrophoresis 2010, 31, 2487–2498. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D. Papermaking: The History and Technique of an Ancient Craft; Courier Corporation: North Chelmsford, MA, USA, 1978. [Google Scholar]
- Yager, P.; Edwards, T.; Fu, E.; Helton, K.; Nelson, K.; Tam, M.R.; Weigl, B.H. Microfluidic diagnostic technologies for global public health. Nature 2006, 442, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Parolo, C.; Merkoci, A. Paper-based nanobiosensors for diagnostics. Chem. Soc. Rev. 2013, 42, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Tokel, O.; Inci, F.; Demirci, U. Advances in plasmonic technologies for point of care applications. Chem. Rev. 2014, 114, 5728–5752. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.S.; Xianyu, Y.L.; Jiang, X.Y. Point-of-care biochemical assays using gold nanoparticle-implemented microfluidics. Chem. Soc. Rev. 2014, 43, 6239–6253. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, K.; Ferguson, B.S.; Eisenstein, M.; Plaxco, K.W.; Soh, H.T. Integrated electrochemical microsystems for genetic detection of pathogens at the point of care. Acc. Chem. Res. 2015, 48, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Yetisen, A.K.; Akram, M.S.; Lowe, C.R. Paper-based microfluidic point-of-care diagnostic devices. Lab Chip 2013, 13, 2210–2251. [Google Scholar] [CrossRef] [PubMed]
- Aragay, G.; Pons, J.; Merkoci, A. Recent trends in macro-, micro-, and nanomaterial-based tools and strategies for heavy-metal detection. Chem. Rev. 2011, 111, 3433–3458. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Ma, J.Z.; Zhang, Q.L.; Li, N.N.; Yang, J.C.; Raju, P.A.; Peng, M.L.; Luo, Y.L.; Hui, W.L.; Chen, C.; et al. Polyelectrolyte-coated gold magnetic nanoparticles for immunoassay development: Toward point of care diagnostics for syphilis screening. Anal. Chem. 2013, 85, 6688–6695. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.L.; Lam, A.W.Y.; Chan, W.C.W. Automating quantum dot barcode assays using microfluidics and magnetism for the development of a point-of-care device. ACS Appl. Mater. Interfaces 2013, 5, 2853–2860. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, L.; Sun, J.S.; Liu, Y.L.; Ma, X.J.; Cui, S.J.; Ma, L.Y.; Xi, J.J.; Jiang, X.Y. Point-of-care multiplexed assays of nucleic acids using microcapillary-based loop-mediated isothermal amplification. Anal. Chem. 2014, 86, 7057–7062. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Xin, X.L.; Fan, C.Y.; Li, X.; Tian, X.L.; Xu, K.F.; Zheng, Z. A paper-based skin patch for the diagnostic screening of cystic fibrosis. Chem. Commun. 2015, 51, 6365–6368. [Google Scholar] [CrossRef] [PubMed]
- Chapman, R.; Lin, Y.; Burnapp, M.; Bentham, A.; Hillier, D.; Zabron, A.; Khan, S.; Tyreman, M.; Stevens, M.M. Multivalent nanoparticle networks enable point-of-care detection of human phospholipase-A2 in serum. ACS Nano 2015, 9, 2565–2573. [Google Scholar] [CrossRef] [PubMed]
- Aid, T.; Kaljurand, M.; Vaher, M. Colorimetric determination of total phenolic contents in ionic liquid extracts by paper microzones and digital camera. Anal. Methods 2015, 7, 3193–3199. [Google Scholar] [CrossRef]
- Tram, K.; Kanda, P.; Salena, B.J.; Huan, S.Y.; Li, Y.F. Translating bacterial detection by dnazymes into a litmus test. Angew. Chem. Int. Edit. 2014, 53, 12799–12802. [Google Scholar] [CrossRef] [PubMed]
- Sanjay, S.T.; Fu, G.L.; Dou, M.W.; Xu, F.; Liu, R.T.; Qi, H.; Li, X.J. Biomarker detection for disease diagnosis using cost-effective microfluidic platforms. Analyst 2015, 140, 7062–7081. [Google Scholar] [CrossRef] [PubMed]
- Sameenoi, Y.; Panymeesamer, P.; Supalakorn, N.; Koehler, K.; Chailapakul, O.; Henry, C.S.; Volckens, J. Microfluidic paper-based analytical device for aerosol oxidative activity. Environ. Sci. Technol. 2013, 47, 932–940. [Google Scholar] [CrossRef] [PubMed]
- Cate, D.M.; Dungchai, W.; Cunningham, J.C.; Volckens, J.; Henry, C.S. Simple, distance-based measurement for paper analytical devices. Lab Chip 2013, 13, 2397–2404. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.Q.; Liu, S.L.; Zhao, W.; Zhang, W.P.; Yu, X.; Li, Y.; Li, A.J.; Pang, D.W.; Zhang, Z.L. A simple point-of-care microfluidic immunomagnetic fluorescence assay for pathogens. Anal. Chem. 2013, 85, 2645–2651. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhang, W.P.; Zhang, Z.L.; He, R.L.; Lin, Y.; Xie, M.; Wang, H.Z.; Pang, D.W. Robust and highly sensitive fluorescence approach for point-of-care virus detection based on immunomagnetic separation. Anal. Chem. 2012, 84, 2358–2365. [Google Scholar] [CrossRef] [PubMed]
- Dou, M.W.; Sanjay, S.T.; Benhabib, M.; Xu, F.; Li, X.J. Low-cost bioanalysis on paper-based and its hybrid microfluidic platforms. Talanta 2015, 145, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.W.; Yan, X.H.; Feng, X.J.; Wang, J.; Du, W.; Wang, Y.C.; Chen, P.; Xiong, L.; Liu, B.F. Agarose-based microfluidic device for point-of-care concentration and detection of pathogen. Anal. Chem. 2014, 86, 10653–10659. [Google Scholar] [CrossRef] [PubMed]
- Taudte, R.V.; Beavis, A.; Wilson-Wilde, L.; Roux, C.; Doble, P.; Blanes, L. A portable explosive detector based on fluorescence quenching of pyrene deposited on coloured wax-printed μPADs. Lab Chip 2013, 13, 4164–4172. [Google Scholar] [CrossRef] [PubMed]
- He, Q.H.; Ma, C.C.; Hu, X.Q.; Chen, H.W. Method for fabrication of paper-based microfluidic devices by alkylsilane self-assembling and UV/O3-patterning. Anal. Chem. 2013, 85, 1327–1331. [Google Scholar] [CrossRef] [PubMed]
- Klasner, S.A.; Price, A.K.; Hoeman, K.W.; Wilson, R.S.; Bell, K.J.; Culbertson, C.T. Paper-based microfluidic devices for analysis of clinically relevant analytes present in urine and saliva. Anal. Bioanal. Chem. 2010, 397, 1821–1829. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Shi, Z.Z. Microfluidic paper-based analytical devices fabricated by low-cost photolithography and embossing of Parafilm®. Lab Chip 2015, 15, 1642–1645. [Google Scholar] [CrossRef] [PubMed]
- Park, T.S.; Baynes, C.; Cho, S.I.; Yoon, J.Y. Paper microfluidics for red wine tasting. RSC Adv. 2014, 4, 24356–24362. [Google Scholar] [CrossRef]
- Martinez, A.W.; Phillips, S.T.; Wiley, B.J.; Gupta, M.; Whitesides, G.M. Flash: A rapid method for prototyping paper-based microfluidic devices. Lab Chip 2008, 8, 2146–2150. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.W.; Phillips, S.T.; Whitesides, G.M. Three-dimensional microfluidic devices fabricated in layered paper and tape. Proc. Natl. Acad. Sci. USA 2008, 105, 19606–19611. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Shi, W.; Jiang, L.; Qin, J.; Lin, B. Rapid prototyping of paper-based microfluidics with wax for low-cost, portable bioassay. Electrophoresis 2009, 30, 1497–1500. [Google Scholar] [CrossRef] [PubMed]
- Carrilho, E.; Martinez, A.W.; Whitesides, G.M. Understanding wax printing: A simple micropatterning process for paper-based microfluidics. Anal. Chem. 2009, 81, 7091–7095. [Google Scholar] [CrossRef] [PubMed]
- Renault, C.; Koehne, J.; Ricco, A.J.; Crooks, R.M. Three-dimensional wax patterning of paper fluidic devices. Langmuir 2014, 30, 7030–7036. [Google Scholar] [CrossRef] [PubMed]
- Chaiyo, S.; Siangproh, W.; Apilux, A.; Chailapakul, O. Highly selective and sensitive paper-based colorimetric sensor using thiosulfate catalytic etching of silver nanoplates for trace determination of copper ions. Anal. Chim. Acta 2015, 866, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Dungchai, W.; Chailapakul, O.; Henry, C.S. A low-cost, simple, and rapid fabrication method for paper-based microfluidics using wax screen-printing. Analyst 2011, 136, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Nie, Z.H.; Nijhuis, C.A.; Gong, J.L.; Chen, X.; Kumachev, A.; Martinez, A.W.; Narovlyansky, M.; Whitesides, G.M. Electrochemical sensing in paper-based microfluidic devices. Lab Chip 2010, 10, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tian, J.F.; Nguyen, T.; Shen, W. Paper-based microfluidic devices by plasma treatment. Anal. Chem. 2008, 80, 9131–9134. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tian, J.F.; Shen, W. Quantitative biomarker assay with microfluidic paper-based analytical devices. Anal. Bioanal. Chem. 2010, 396, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Määttänen, A.; Fors, D.; Wang, S.; Valtakari, D.; Ihalainen, P.; Peltonen, J. Paper-based planar reaction arrays for printed diagnostics. Sens. Actuators B Chem. 2011, 160, 1404–1412. [Google Scholar] [CrossRef]
- Sameenoi, Y.; Nongkai, P.N.; Nouanthavong, S.; Henry, C.S.; Nacapricha, D. One-step polymer screen-printing for microfluidic paper-based analytical device (μPAD) fabrication. Analyst 2014, 139, 6580–6588. [Google Scholar] [CrossRef] [PubMed]
- Olkkonen, J.; Lehtinen, K.; Erho, T. Flexographically printed fluidic structures in paper. Anal. Chem. 2010, 82, 10246–10250. [Google Scholar] [CrossRef] [PubMed]
- Chitnis, G.; Ding, Z.W.; Chang, C.L.; Savran, C.A.; Ziaie, B. Laser-treated hydrophobic paper: An inexpensive microfluidic platform. Lab Chip 2011, 11, 1161–1165. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.F.; Wang, Y.; Wu, Y.Y.; Xu, C.X.; Zhong, M.H.; Lai, H.Y.; Huang, J.S. Fabrication of a microfluidic paper-based analytical device by silanization of filter cellulose using a paper mask for glucose assay. Analyst 2014, 139, 4593–4598. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.A.; Hou, L.Y.; Zhang, W.Y.; Zhu, L. Preparation of paper micro-fluidic devices used in bio-assay based on drop-on-demand wax droplet generation. Anal. Methods 2014, 6, 878–885. [Google Scholar] [CrossRef]
- Zhou, M.; Yang, M.H.; Zhou, F.M. Paper based colorimetric biosensing platform utilizing cross-linked siloxane as probe. Biosens. Bioelectron. 2014, 55, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Garcia, P.T.; Cardoso, T.M.G.; Garcia, C.D.; Carrilho, E.; Coltro, W.K.T. A handheld stamping process to fabricate microfluidic paper-based analytical devices with chemically modified surface for clinical assays. RSC Adv. 2014, 4, 37637–37644. [Google Scholar] [CrossRef]
- Wei, X.F.; Tian, T.; Jia, S.S.; Zhu, Z.; Ma, Y.L.; Sun, J.J.; Lin, Z.Y.; Yang, C.J. Microfluidic distance readout sweet hydrogel integrated paper based analytical device (μDiSH-PAD) for visual quantitative point-of-care testing. Anal. Chem. 2016, 88, 2345–2352. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.B.; Liu, H.; Gu, Z.Z. Bottom-up fabrication of paper-based microchips by blade coating of cellulose microfibers on a patterned surface. Langmuir 2014, 30, 15041–15046. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Nam, Y.G.; Lee, S.K.; Kim, C.S.; Koo, Y.M.; Chang, W.J.; Gunasekaran, S. Paper-fluidic electrochemical biosensing platform with enzyme paper and enzymeless electrodes. Sens. Actuators B Chem. 2014, 203, 44–53. [Google Scholar] [CrossRef]
- Li, W.B.; Qian, D.P.; Wang, Q.H.; Li, Y.B.; Bao, N.; Gu, H.Y.; Yu, C.M. Fully-drawn origami paper analytical device for electrochemical detection of glucose. Sens. Actuators B Chem. 2016, 231, 230–238. [Google Scholar] [CrossRef]
- Maattanen, A.; Vanamo, U.; Ihalainen, P.; Pulkkinen, P.; Tenhu, H.; Bobacka, J.; Peltonen, J. A low-cost paper-based inkjet-printed platform for electrochemical analyses. Sens. Actuators B Chem. 2013, 177, 153–162. [Google Scholar] [CrossRef]
- Zhao, C.; Thuo, M.M.; Liu, X.Y. A microfluidic paper-based electrochemical biosensor array for multiplexed detection of metabolic biomarkers. Sci. Technol. Adv. Mater. 2013, 14, 54402–54408. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.H.; Wang, S.M.; Ge, L.; Ge, S.G. A novel chemiluminescence paper microfluidic biosensor based on enzymatic reaction for uric acid determination. Biosens. Bioelectron. 2011, 26, 3284–3289. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.H.; Ge, L.; Huang, J.D.; Wang, S.M.; Ge, S.G. Microfluidic paper-based chemiluminescence biosensor for simultaneous determination of glucose and uric acid. Lab Chip 2011, 11, 1286–1291. [Google Scholar] [CrossRef] [PubMed]
- Delaney, J.L.; Hogan, C.F.; Tian, J.F.; Shen, W. Electrogenerated chemiluminescence detection in paper-based microfluidic sensors. Anal. Chem. 2011, 83, 1300–1306. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Luo, Y.; Shi, B.; Liu, X.M.; Gao, Z.G.; Du, Y.G.; Zhao, W.J.; Lin, B.C. Chemiluminescence diminishment on a paper-based analytical device: High throughput determination of beta-agonists in swine hair. Anal. Methods 2014, 6, 9684–9690. [Google Scholar] [CrossRef]
- Ge, L.; Wang, S.M.; Song, X.R.; Ge, S.G.; Yu, J.H. 3D origami-based multifunction-integrated immunodevice: Low-cost and multiplexed sandwich chemiluminescence immunoassay on microfluidic paper-based analytical device. Lab Chip 2012, 12, 3150–3158. [Google Scholar] [CrossRef] [PubMed]
- Thom, N.K.; Lewis, G.G.; Yeung, K.; Phillips, S.T. Quantitative fluorescence assays using a self-powered paper-based microfluidic device and a camera-equipped cellular phone. RSC Adv. 2014, 4, 1334–1340. [Google Scholar] [CrossRef] [PubMed]
- Jang, G.; Kim, J.; Kim, D.; Lee, T.S. Synthesis of triphenylamine-containing conjugated polyelectrolyte and fabrication of fluorescence color-changeable, paper-based sensor strips for biothiol detection. Polym. Chem. 2015, 6, 714–720. [Google Scholar] [CrossRef]
- Dou, M.W.; Dominguez, D.C.; Li, X.J.; Sanchez, J.; Scott, G. A versatile pdms/paper hybrid microfluidic platform for sensitive infectious disease diagnosis. Anal. Chem. 2014, 86, 7978–7986. [Google Scholar] [CrossRef] [PubMed]
- Thom, N.K.; Lewis, G.G.; DiTucci, M.J.; Phillips, S.T. Two general designs for fluidic batteries in paper-based microfluidic devices that provide predictable and tunable sources of power for on-chip assays. RSC Adv. 2013, 3, 6888–6895. [Google Scholar] [CrossRef]
- Ho, J.; Tan, M.K.; Go, D.B.; Yeo, L.Y.; Friend, J.R.; Chang, H.C. Paper-based microfluidic surface acoustic wave sample delivery and ionization source for rapid and sensitive ambient mass spectrometry. Anal. Chem. 2011, 83, 3260–3266. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Wang, H.; Manicke, N.E.; Lin, J.M.; Cooks, R.G.; Ouyang, Z. Development, characterization, and application of paper spray ionization. Anal. Chem. 2010, 82, 2463–2471. [Google Scholar] [CrossRef] [PubMed]
- Li, B.W.; Zhang, W.; Chen, L.X.; Lin, B.C. A fast and low-cost spray method for prototyping and depositing surface-enhanced Raman scattering arrays on microfluidic paper based device. Electrophoresis 2013, 34, 2162–2168. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.W.; White, I.M. Inkjet printed surface enhanced Raman spectroscopy array on cellulose paper. Anal. Chem. 2010, 82, 9626–9630. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, J.A.; McKee, A.E.; Keasling, J.D. High-throughput metabolic engineering: Advances in small-molecule screening and selection. Annu. Rev. Biochem. 2010, 79, 563–590. [Google Scholar]
- Jiang, Y.; Zhao, H.; Lin, Y.Q.; Zhu, N.N.; Ma, Y.R.; Mao, L.Q. Colorimetric detection of glucose in rat brain using gold nanoparticles. Angew. Chem. Int. Edit. 2010, 49, 4800–4804. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Amsden, J.J.; Boriskina, S.V.; Gopinath, A.; Mitropolous, A.; Kaplan, D.L.; Omenetto, F.G.; Dal Negro, L. Spatial and spectral detection of protein monolayers with deterministic aperiodic arrays of metal nanoparticles. Proc. Natl. Acad. Sci. USA 2010, 107, 12086–12090. [Google Scholar] [CrossRef] [PubMed]
- Namera, A.; Nakamoto, A.; Saito, T.; Nagao, M. Colorimetric detection and chromatographic analyses of designer drugs in biological materials: A comprehensive review. Forensic Toxicol. 2011, 29, 1–24. [Google Scholar] [CrossRef]
- Li, Z.A.; Yang, J.Q.; Zhu, L.; Tang, W.C. Fabrication of paper micro-devices with wax jetting. RSC Adv. 2016, 6, 17921–17928. [Google Scholar] [CrossRef]
- Li, B.W.; Fu, L.W.; Zhang, W.; Feng, W.W.; Chen, L.X. Portable paper-based device for quantitative colorimetric assays relying on light reflectance principle. Electrophoresis 2014, 35, 1152–1159. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.; Maeki, M.; Mohamadi, R.M.; Ishida, A.; Tani, H.; Tokeshi, M. An instrument-free, screen-printed paper microfluidic device that enables bio and chemical sensing. Analyst 2015, 140, 6493–6499. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.L.; Zha, Y. Fabrication of paper-based microfluidic device using printed circuit technology. AIP Adv. 2012, 2, 6. [Google Scholar] [CrossRef]
- Sun, J.Y.; Cheng, C.M.; Liao, Y.C. Screen printed paper-based diagnostic devices with polymeric inks. Anal. Sci. 2015, 31, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.X.; Forouzan, O.; Brown, T.P.; Shevkoplyas, S.S. Integrated separation of blood plasma from whole blood for microfluidic paper-based analytical devices. Lab Chip 2012, 12, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Oyola-Reynoso, S.; Heim, A.P.; Halbertsma-Black, J.; Zhao, C.; Tevis, I.D.; Cinar, S.; Cademartiri, R.; Liu, X.Y.; Bloch, J.F.; Thuo, M.M. Draw your assay: Fabrication of low-cost paper-based diagnostic and multi-well test zones by drawing on a paper. Talanta 2015, 144, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Sones, C.L.; Katis, I.N.; He, P.J.W.; Mills, B.; Namiq, M.F.; Shardlow, P.; Ibsen, M.; Eason, R.W. Laser-induced photo-polymerisation for creation of paper-based fluidic devices. Lab Chip 2014, 14, 4567–4574. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.J.; Feng, D.Q.; Chen, M.; Chen, Z.D.; Zhu, R.; Fang, H.L.; Wang, W. Bienzyme colorimetric detection of glucose with self-calibration based on tree-shaped paper strip. Sens. Actuators B Chem. 2014, 190, 414–418. [Google Scholar] [CrossRef]
- Cha, R.T.; Wang, D.; He, Z.B.; Ni, Y.H. Development of cellulose paper testing strips for quick measurement of glucose using chromogen agent. Carbohydr. Polym. 2012, 88, 1414–1419. [Google Scholar] [CrossRef]
- Gabriel, E.F.M.; Garcia, P.T.; Cardoso, T.M.G.; Lopes, F.M.; Martins, F.T.; Coltro, W.K.T. Highly sensitive colorimetric detection of glucose and uric acid in biological fluids using chitosan-modified paper microfluidic devices. Analyst 2016, 141, 4749–4756. [Google Scholar] [CrossRef] [PubMed]
- Soni, A.; Jha, S.K. A paper strip based non-invasive glucose biosensor for salivary analysis. Biosens. Bioelectron. 2015, 67, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Palazzo, G.; Facchini, L.; Mallardi, A. Colorimetric detection of sugars based on gold nanoparticle formation. Sens. Actuators B Chem. 2012, 161, 366–371. [Google Scholar] [CrossRef]
- Figueredo, F.; Garcia, P.T.; Corton, E.; Coltro, W.K.T. Enhanced analytical performance of paper microfluidic devices by using Fe3O4 nanoparticles, mwcnt, and graphene oxide. ACS Appl. Mater. Interfaces 2016, 8, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Evans, E.; Gabriel, E.F.M.; Benavidez, T.E.; Coltro, W.K.T.; Garcia, C.D. Modification of microfluidic paper-based devices with silica nanoparticles. Analyst 2014, 139, 5560–5567. [Google Scholar] [CrossRef] [PubMed]
- Yasui, K.; Kimizuka, N. Enzymatic synthesis of gold nanoparticles wrapped by glucose oxidase. Chem. Lett. 2005, 34, 416–417. [Google Scholar] [CrossRef]
- Caseli, L.; dos Santos, D.S.; Aroca, R.F.; Oliveira, O.N. Controlled fabrication of gold nanoparticles biomediated by glucose oxidase immobilized on chitosan layer-by-layer films. Mater. Sci. Eng. 2009, 29, 1687–1690. [Google Scholar] [CrossRef]
- Song, Y.J.; Qu, K.G.; Zhao, C.; Ren, J.S.; Qu, X.G. Graphene oxide: Intrinsic peroxidase catalytic activity and its application to glucose detection. Adv. Mater. 2010, 22, 2206–2210. [Google Scholar] [CrossRef] [PubMed]
- Ornatska, M.; Sharpe, E.; Andreescu, D.; Andreescu, S. Paper bioassay based on ceria nanoparticles as colorimetric probes. Anal. Chem. 2011, 83, 4273–4280. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Chen, C.G.; Zhu, C.Z.; Dong, S.J.; Lu, H.M. Multiplexed bioactive paper based on GO@SiO2@CeO2 nanosheets for a low-cost diagnostics platform. Biosens. Bioelectron. 2014, 52, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, H.T.; Noxon, I.C.; Chaplan, C.A.; Carlton, S.J.; Liu, C.H.; Ganaja, K.A.; Martinez, N.W.; Immoos, C.E.; Costanzo, P.J.; Martinez, A.W. Reagent pencils: A new technique for solvent-free deposition of reagents onto paper-based microfluidic devices. Lab Chip 2015, 15, 2213–2220. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, X.Y. Fabrication of three-dimensional microfluidic channels in a single layer of cellulose paper. Microfluid. Nanofluid. 2014, 16, 819–827. [Google Scholar] [CrossRef]
- Chun, H.J.; Park, Y.M.; Han, Y.D.; Jang, Y.H.; Yoon, H.C. Paper-based glucose biosensing system utilizing a smartphone as a signal reader. BioChip J. 2014, 8, 218–226. [Google Scholar] [CrossRef]
- Im, S.H.; Kim, K.R.; Park, Y.M.; Yoon, J.H.; Hong, J.W.; Yoon, H.C. An animal cell culture monitoring system using a smartphone-mountable paper-based analytical device. Sens. Actuators B Chem. 2016, 229, 166–173. [Google Scholar] [CrossRef]
- Costa, M.N.; Veigas, B.; Jacob, J.M.; Santos, D.S.; Gomes, J.; Baptista, P.V.; Martins, R.; Inacio, J.; Fortunato, E. A low cost, safe, disposable, rapid and self-sustainable paper-based platform for diagnostic testing: Lab-on-paper. Nanotechnology 2014, 25, 94006–94017. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.G.; DiTucci, M.J.; Baker, M.S.; Phillips, S.T. High throughput method for prototyping three-dimensional, paper-based microfluidic devices. Lab Chip 2012, 12, 2630–2633. [Google Scholar] [CrossRef] [PubMed]
- Li, C.G.; Joung, H.A.; Noh, H.; Song, M.B.; Kim, M.G.; Jung, H. One-touch-activated blood multidiagnostic system using a minimally invasive hollow microneedle integrated with a paper-based sensor. Lab Chip 2015, 15, 3286–3292. [Google Scholar] [CrossRef] [PubMed]
- Schilling, K.M.; Jauregui, D.; Martinez, A.W. Paper and toner three-dimensional fluidic devices: Programming fluid flow to improve point-of-care diagnostics. Lab Chip 2013, 13, 628–631. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Kim, S.K.; Lee, G.J.; Park, H.K. Paper-based 3D microfluidic device for multiple bioassays. Sens. Actuators B Chem. 2015, 219, 245–250. [Google Scholar] [CrossRef]
- Sechi, D.; Greer, B.; Johnson, J.; Hashemi, N. Three-dimensional paper-based microfluidic device for assays of protein and glucose in urine. Anal. Chem. 2013, 85, 10733–10737. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.G.; Lee, S.H.; Choi, C.H.; Kim, J.; Lee, C.S. Toward instrument-free digital measurements: A three-dimensional microfluidic device fabricated in a single sheet of paper by double-sided printing and lamination. Lab Chip 2015, 15, 1188–1194. [Google Scholar] [CrossRef] [PubMed]
- Sekar, N.C.; Shaegh, S.A.M.; Ng, S.H.; Ge, L.; Tan, S.N. A paper-based amperometric glucose biosensor developed with Prussian blue-modified screen-printed electrodes. Sens. Actuators B Chem. 2014, 204, 414–420. [Google Scholar] [CrossRef]
- Lawrence, C.S.K.; Tan, S.N.; Floresca, C.Z. A “green” cellulose paper based glucose amperometric biosensor. Sens. Actuators B Chem. 2014, 193, 536–541. [Google Scholar] [CrossRef]
- Noiphung, J.; Songjaroen, T.; Dungchai, W.; Henry, C.S.; Chailapakul, O.; Laiwattanapaisal, W. Electrochemical detection of glucose from whole blood using paper-based microfluidic devices. Anal. Chim. Acta 2013, 788, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.A.; Cardoso, T.M.G.; Cardoso, R.M.; Duarte, L.C.; Munoz, R.A.A.; Richter, E.M.; Coltro, W.K.T. Paper-based enzymatic reactors for batch injection analysis of glucose on 3D printed cell coupled with amperometric detection. Sens. Actuators B Chem. 2016, 226, 196–203. [Google Scholar] [CrossRef]
- Miki, M.; Iwahara, S.; Uno, S. A hybrid system of carbon ink electrodes and chromatography paper on a cmos chip and its application to enzymatic glucose sensor. Jpn. J. Appl. Phys. 2014, 53, 04EL06. [Google Scholar] [CrossRef]
- Rungsawang, T.; Punrat, E.; Adkins, J.; Henry, C.; Chailapakul, O. Development of electrochemical paper-based glucose sensor using cellulose-4-aminophenylboronic acid-modified screen-printed carbon electrode. Electroanalysis 2016, 28, 462–468. [Google Scholar] [CrossRef]
- Li, Z.D.; Li, F.; Hu, J.; Wee, W.H.; Han, Y.L.; Pingguan-Murphy, B.; Lu, T.J.; Xu, F. Direct writing electrodes using a ball pen for paper-based point-of-care testing. Analyst 2015, 140, 5526–5535. [Google Scholar] [CrossRef] [PubMed]
- Labroo, P.; Cui, Y. Graphene nano-ink biosensor arrays on a microfluidic paper for multiplexed detection of metabolites. Anal. Chim. Acta 2014, 813, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Santhiago, M.; Kubota, L.T. A new approach for paper-based analytical devices with electrochemical detection based on graphite pencil electrodes. Sens. Actuators B Chem. 2013, 177, 224–230. [Google Scholar] [CrossRef]
- Chen, Y.A.; Tsai, F.J.; Zeng, Y.T.; Wang, J.C.; Hong, C.P.; Huang, P.H.; Chuang, H.L.; Lin, S.Y.; Chan, C.T.; Ko, Y.C.; et al. Fast and effective turn-on paper-based phosphorescence biosensor for detection of glucose in serum. J. Chin. Chem. Soc. 2016, 63, 424–431. [Google Scholar] [CrossRef]
- Durán, G.M.; Benavidez, T.E.; Ríos, A.; García, C.D. Quantum dot-modified paper-based assay for glucose screening. Microchim. Acta 2016, 183, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Davaji, B.; Lee, C.H. A paper-based calorimetric microfluidics platform for bio-chemical sensing. Biosens. Bioelectron. 2014, 59, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Colletes, T.C.; Garcia, P.T.; Campanha, R.B.; Abdelnur, P.V.; Romao, W.; Coltro, W.K.T.; Vaz, B.G. A new insert sample approach to paper spray mass spectrometry: A paper substrate with paraffin barriers. Analyst 2016, 141, 1707–1713. [Google Scholar] [CrossRef] [PubMed]
- Torul, H.; Ciftci, H.; Cetin, D.; Suludere, Z.; Boyaci, I.H.; Tamer, U. Paper membrane-based sers platform for the determination of glucose in blood samples. Anal. Bioanal. Chem. 2015, 407, 8243–8251. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.I.; Chang, B.Y. Development of the smartphone-based colorimetry for multi-analyte sensing arrays. Lab Chip 2014, 14, 1725–1732. [Google Scholar] [CrossRef] [PubMed]
- De Souza, F.R.; Alves, G.L.; Coltro, W.K.T. Capillary-driven toner-based microfluidic devices for clinical diagnostics with colorimetric detection. Anal. Chem. 2012, 84, 9002–9007. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, K.A.; Silva, P.; de Souza, F.R.; Martins, F.T.; Coltro, W.K.T. Kinetic study of glucose oxidase on microfluidic tolener-based analytical devices for clinical diagnostics with image-based detection. Anal. Methods 2014, 6, 4995–5000. [Google Scholar] [CrossRef]
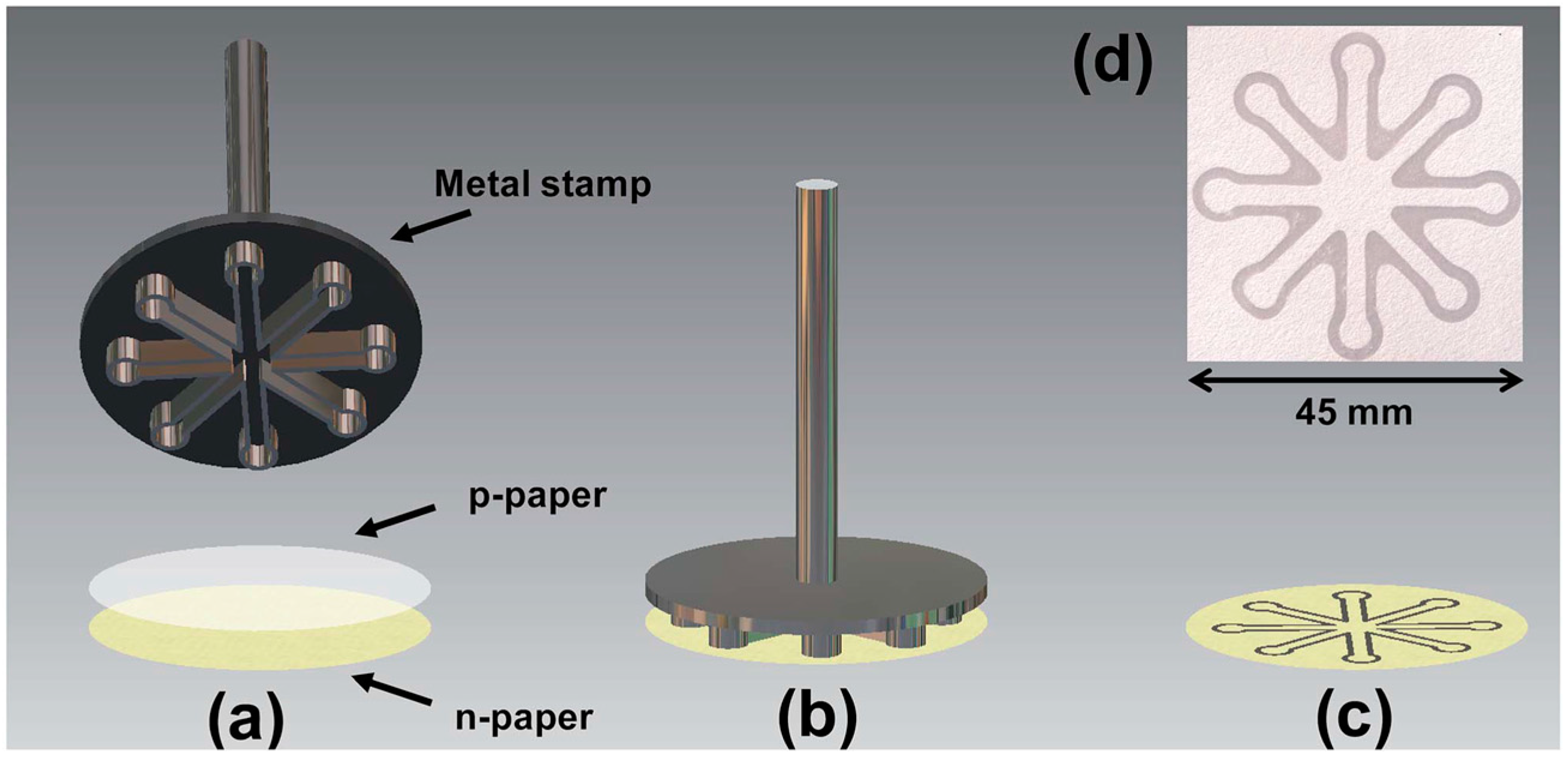
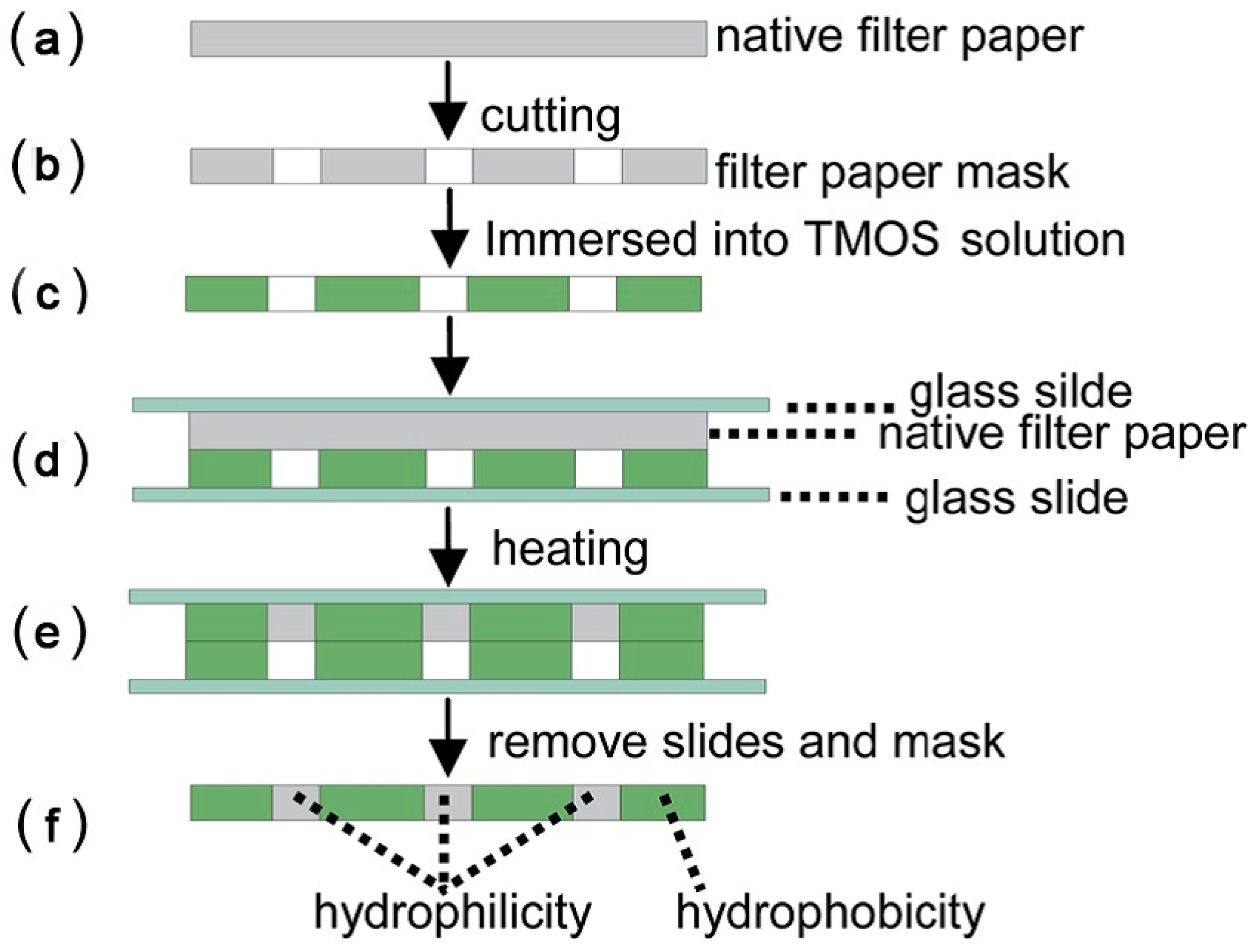
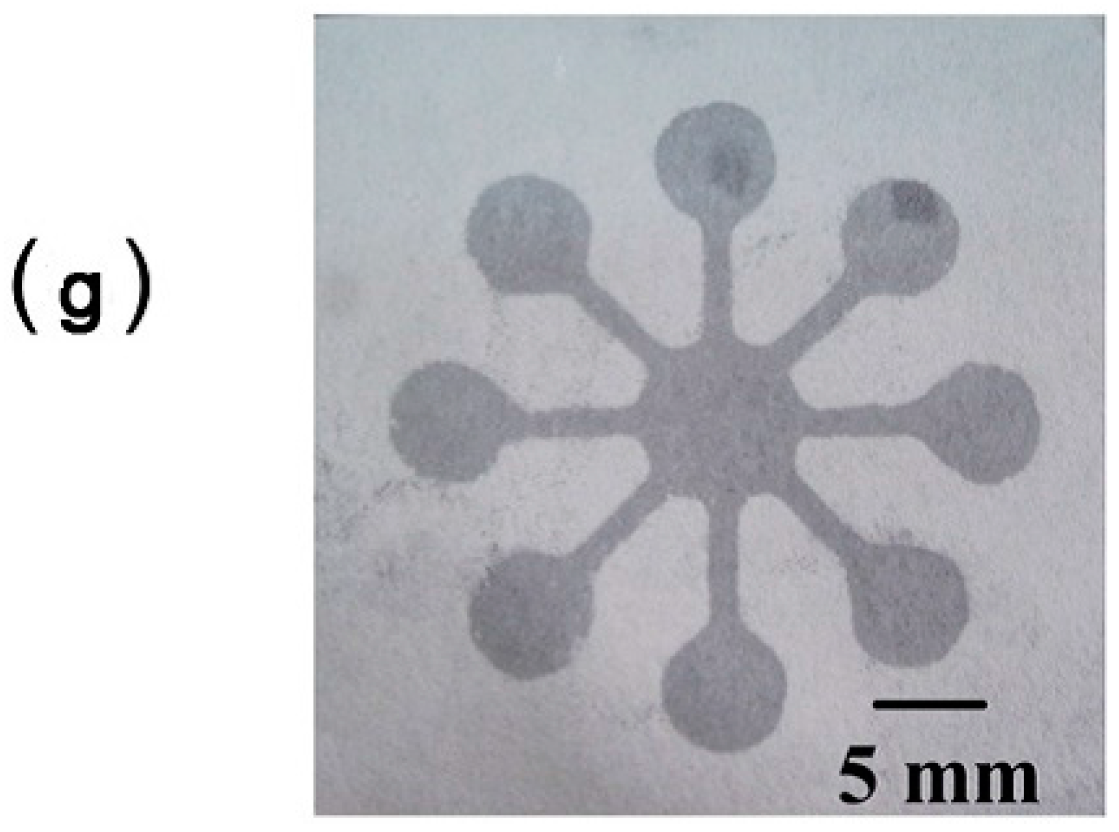
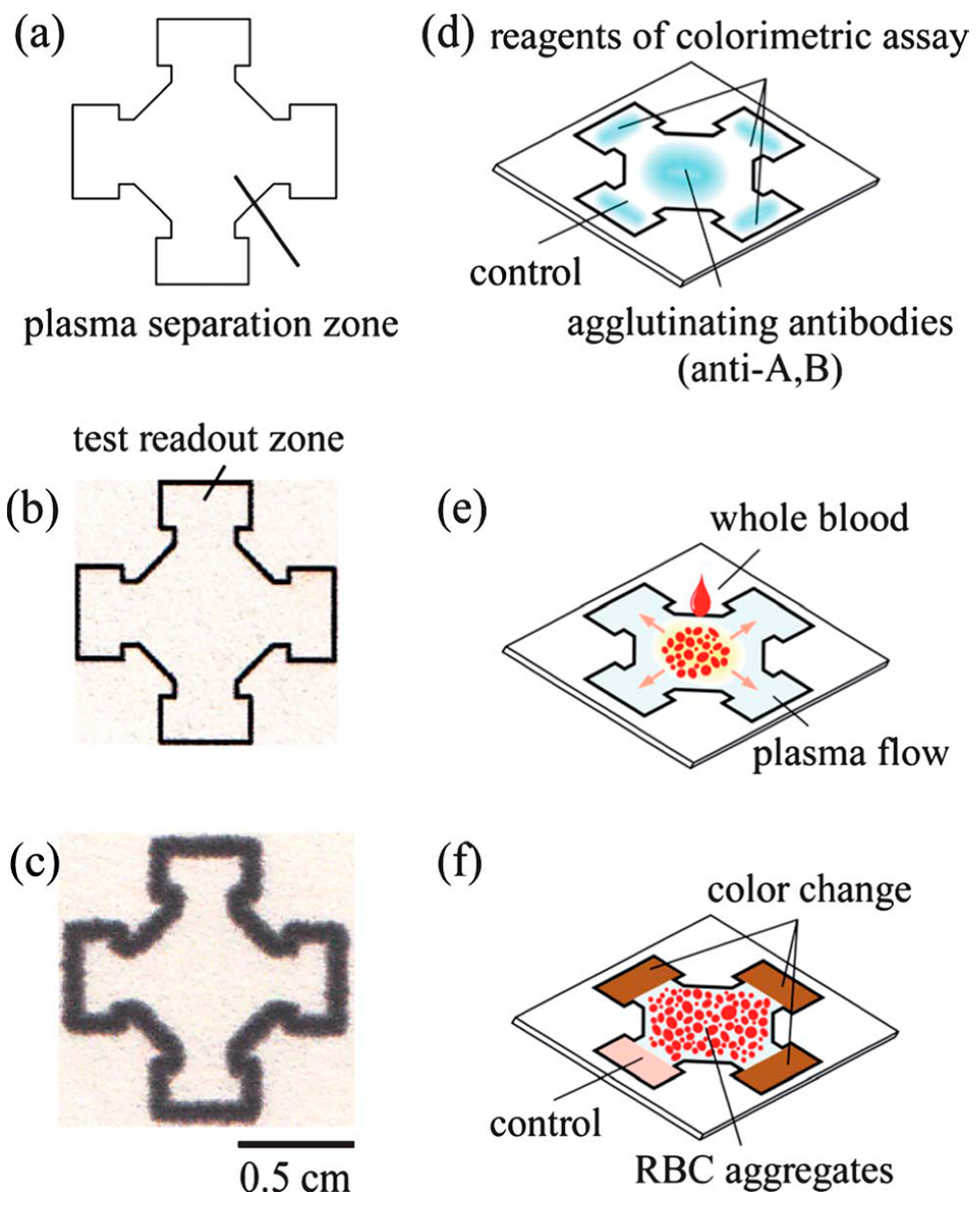
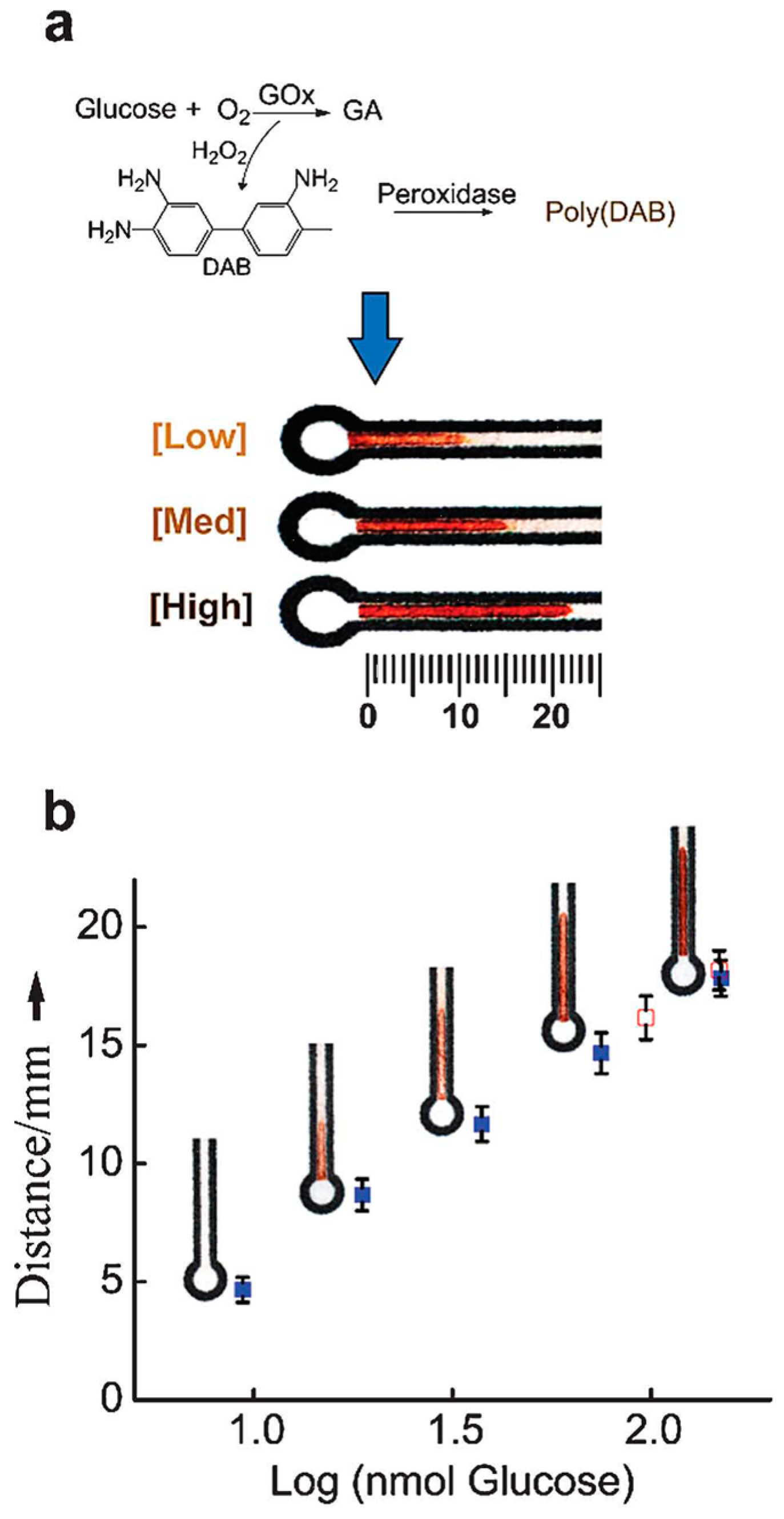
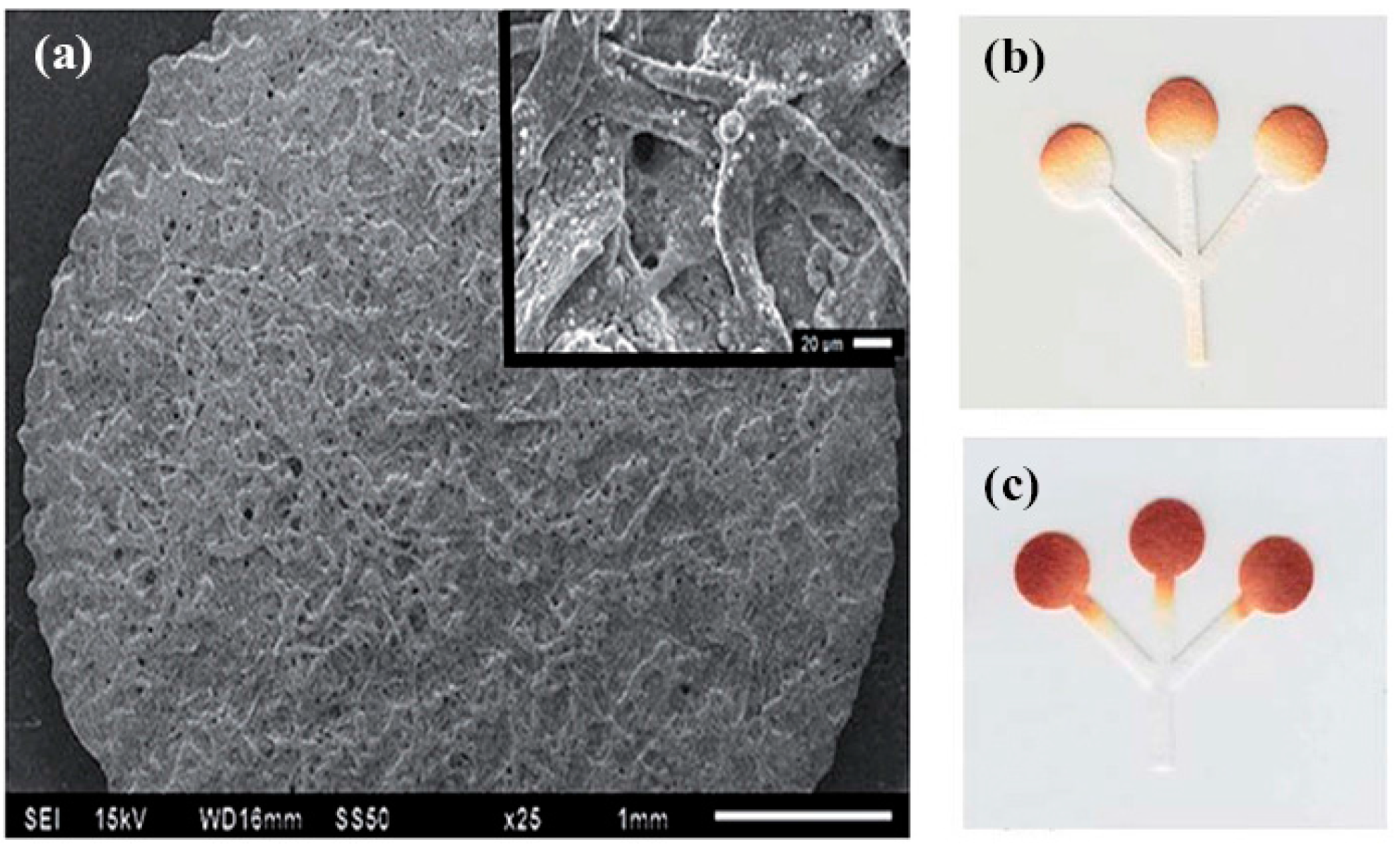
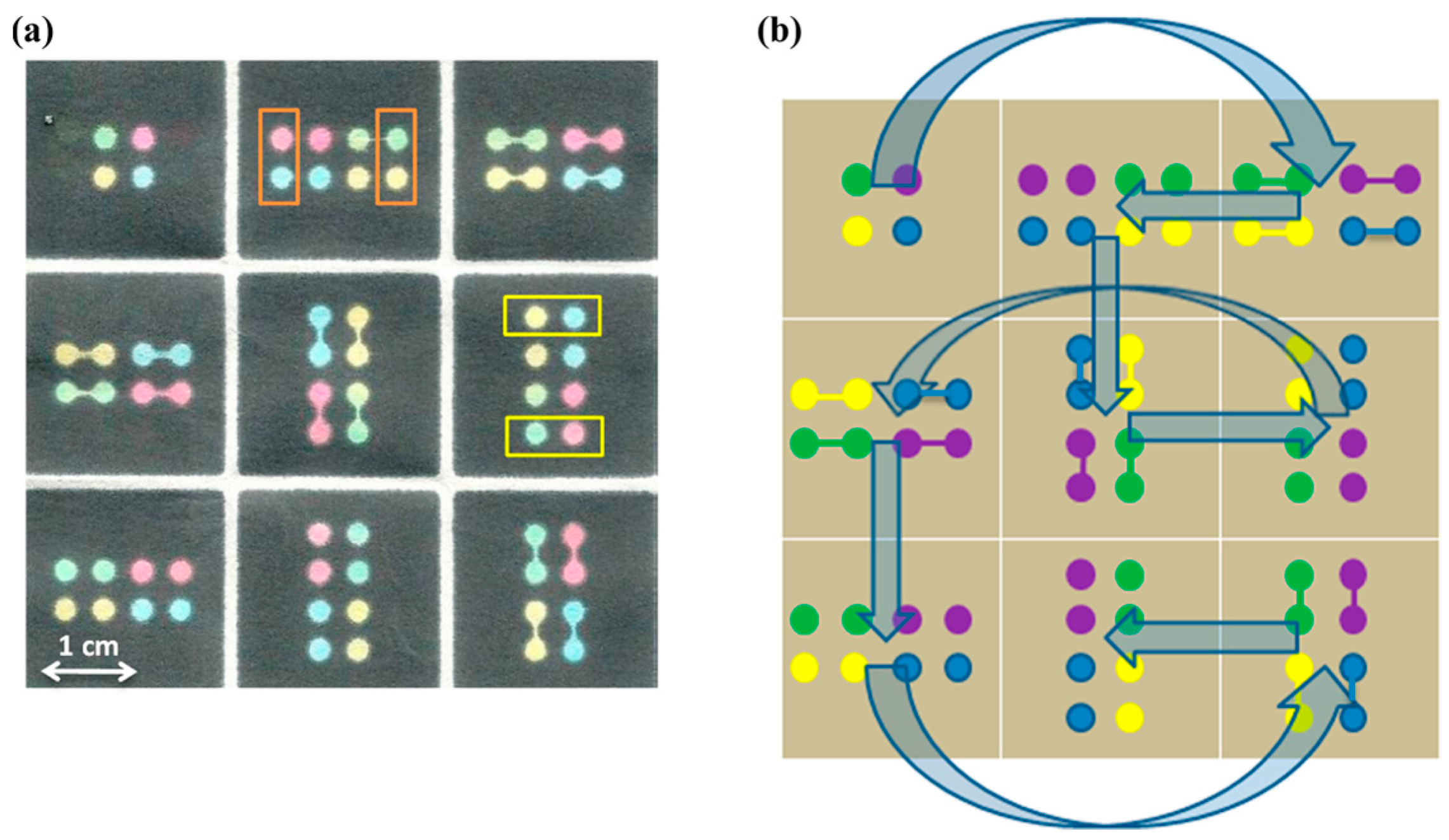
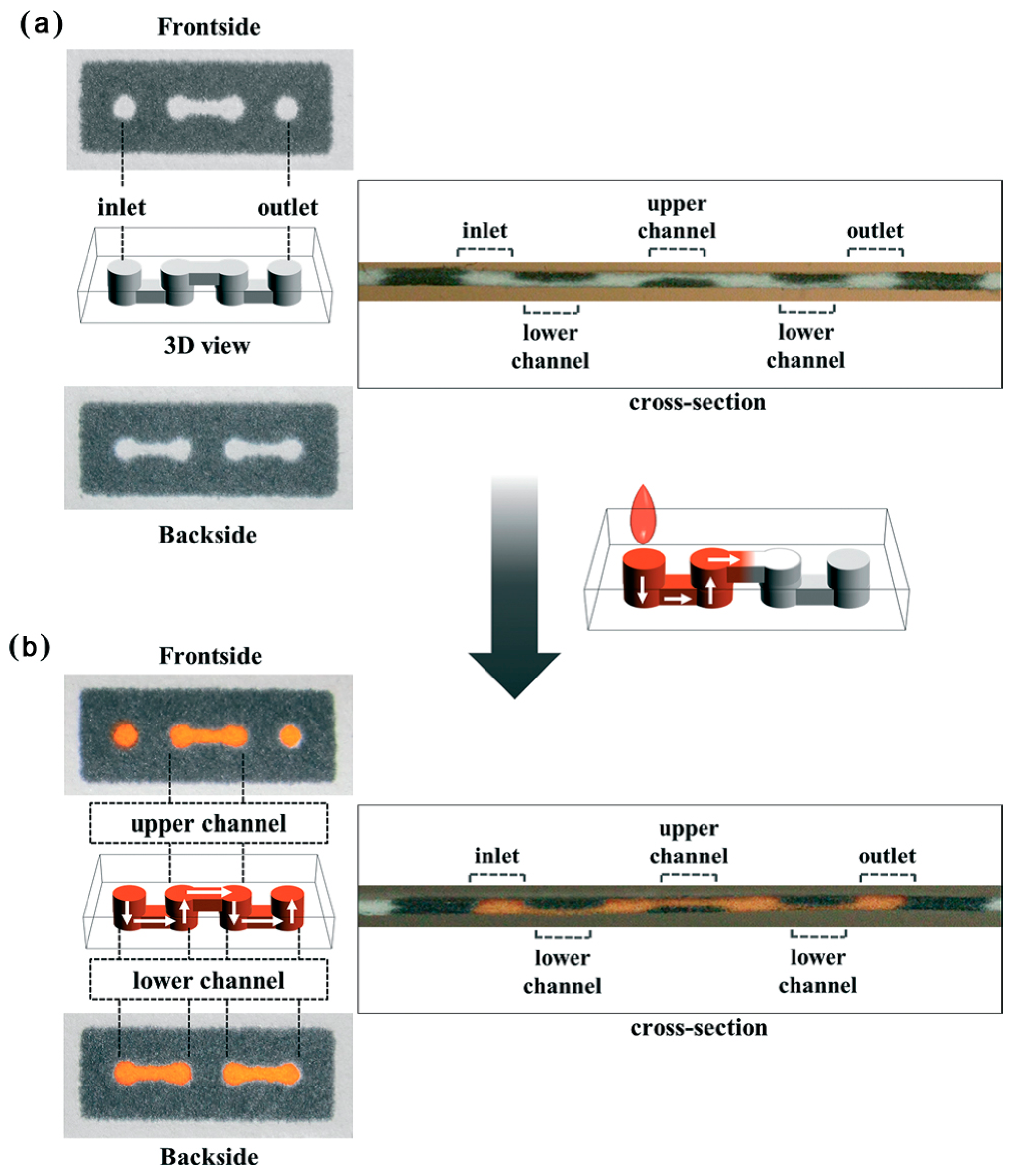
| Reference | Indicator | Barrier | Limits of Detection |
|---|---|---|---|
| Garcia et al. (2014) [62] | KI | Paraffin | 0.1 mM |
| Cai et al. (2014) [59] | KI | TMOS | Not mentioned |
| Li et al. (2016) [86] | KI | Wax | Not mentioned |
| Mohammadi et al. (2015) [88] | KI | PDMS | 5 mM |
| Oyola-Reynoso et al. (2015) [92] | KI | Trichloro silane | 5.5 mM |
| Chen et al. (2012) [4] | 4-AAP/TOPS | Paper pieces | 0.21 mM |
| Zhu et al. (2014) [94] | 4-AAP/TBHBA | Paper pieces | 0.3 mM |
| Zhou et al. (2014) [61] | APTMS/GA | Paper pieces | 0.25 mM |
| Soni et al. (2015) [97] | Methyl red | Paper pieces | 1.23 mM |
| Cate et al. (2013) [35] | DAB | Paper pieces | 1.11 mM |
| Figueredo et al. (2016) [99] | TMB | Paper pieces | 0.043, 0.062 and 0.018 mM (with different nanomaterials) |
| Palazzo et al. (2012) [98] | AuNPs | Paper pieces | 0.1 mM |
| Deng et al. (2014) [105] | ABTS | Paper pieces | 9 nM |
| Im et al. (2016) [109] | 4-AAP/MAOS | Wax | 0.3 mM |
| Gabriel et al. (2016) [96] | 4-AAP/DHBS | Paraffin | 0.023 mM |
| Reference | Mediator | Electrode | Limits of Detection |
|---|---|---|---|
| Lawrence et al. (2014) [118] | Fc-COOH | Commercial SPE | 0.18 mM |
| Sekar et al. (2014) [117] | PB | Commercial SPE | 0.01 mM |
| Yang et al. (2014) [65] | PtNPs | Commercial SPE | 0.0093 mM |
| Miki et al. (2014) [121] | K3[Fe(CN)6] | CMOS | 1 mM |
| Rungsawang et al. (2016) [122] | 4-APBA | Wax-printing electrode | 0.86 mM |
| Li et al. (2015) [123] | K3[Fe(CN)6] | Writing electrode | 2 mM |
| Li et al. (2016) [66] | FcA | Graphite drawing electrode | 0.05 mM |
| Santhiago et al. (2013) [125] | 4-APBA | Graphite dual-electrode | 0.38 μM |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Su, W.; Ding, X. A Review on Microfluidic Paper-Based Analytical Devices for Glucose Detection. Sensors 2016, 16, 2086. https://doi.org/10.3390/s16122086
Liu S, Su W, Ding X. A Review on Microfluidic Paper-Based Analytical Devices for Glucose Detection. Sensors. 2016; 16(12):2086. https://doi.org/10.3390/s16122086
Chicago/Turabian StyleLiu, Shuopeng, Wenqiong Su, and Xianting Ding. 2016. "A Review on Microfluidic Paper-Based Analytical Devices for Glucose Detection" Sensors 16, no. 12: 2086. https://doi.org/10.3390/s16122086
APA StyleLiu, S., Su, W., & Ding, X. (2016). A Review on Microfluidic Paper-Based Analytical Devices for Glucose Detection. Sensors, 16(12), 2086. https://doi.org/10.3390/s16122086







