Manipulation and Immobilization of a Single Fluorescence Nanosensor for Selective Injection into Cells
Abstract
:1. Introduction
2. Materials and Methods
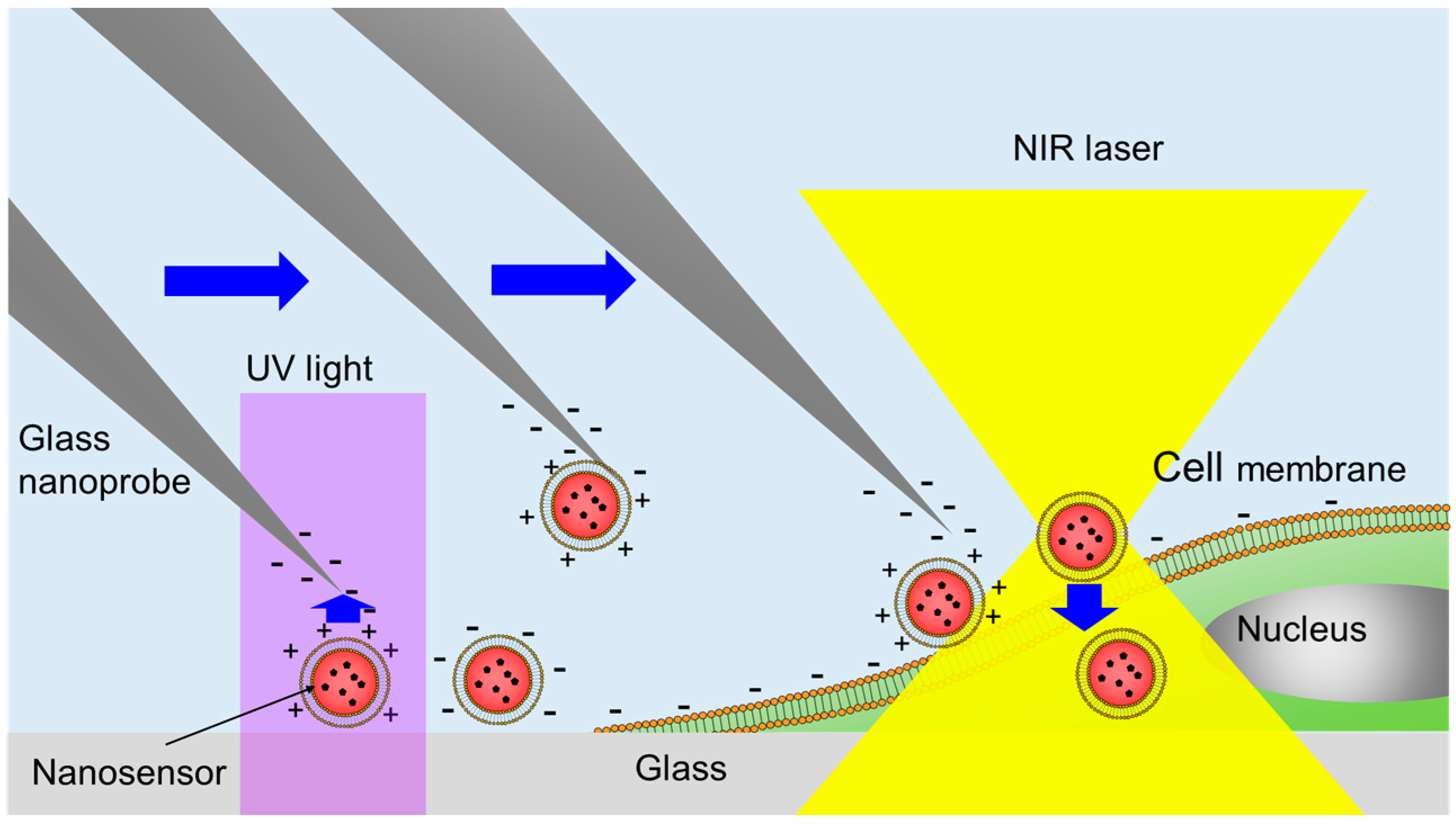
2.1. Principle of Manipulation and Cell Injection of the Nanosensor Using Optical Control of the Zeta Potential
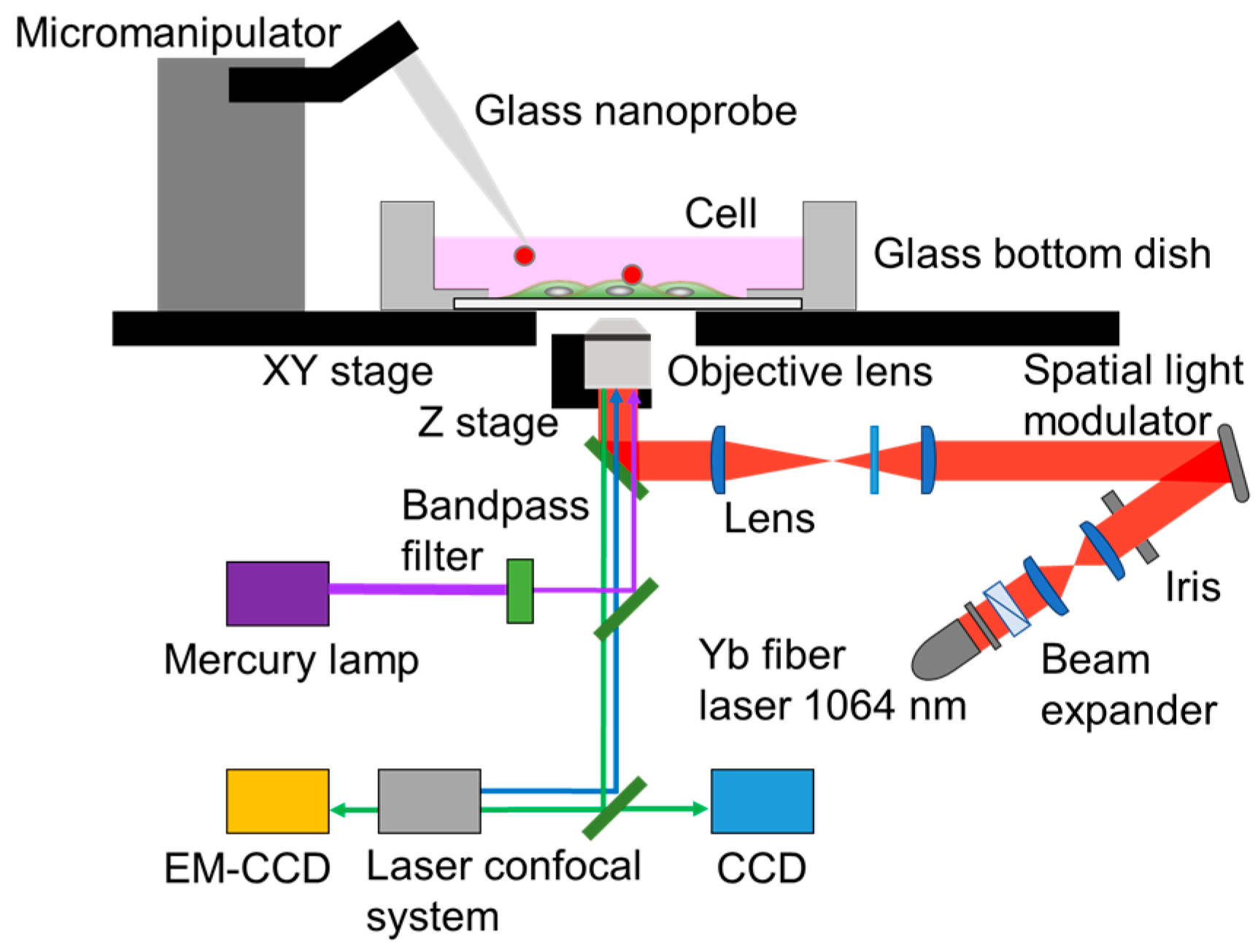
2.2. Experimental System Setup
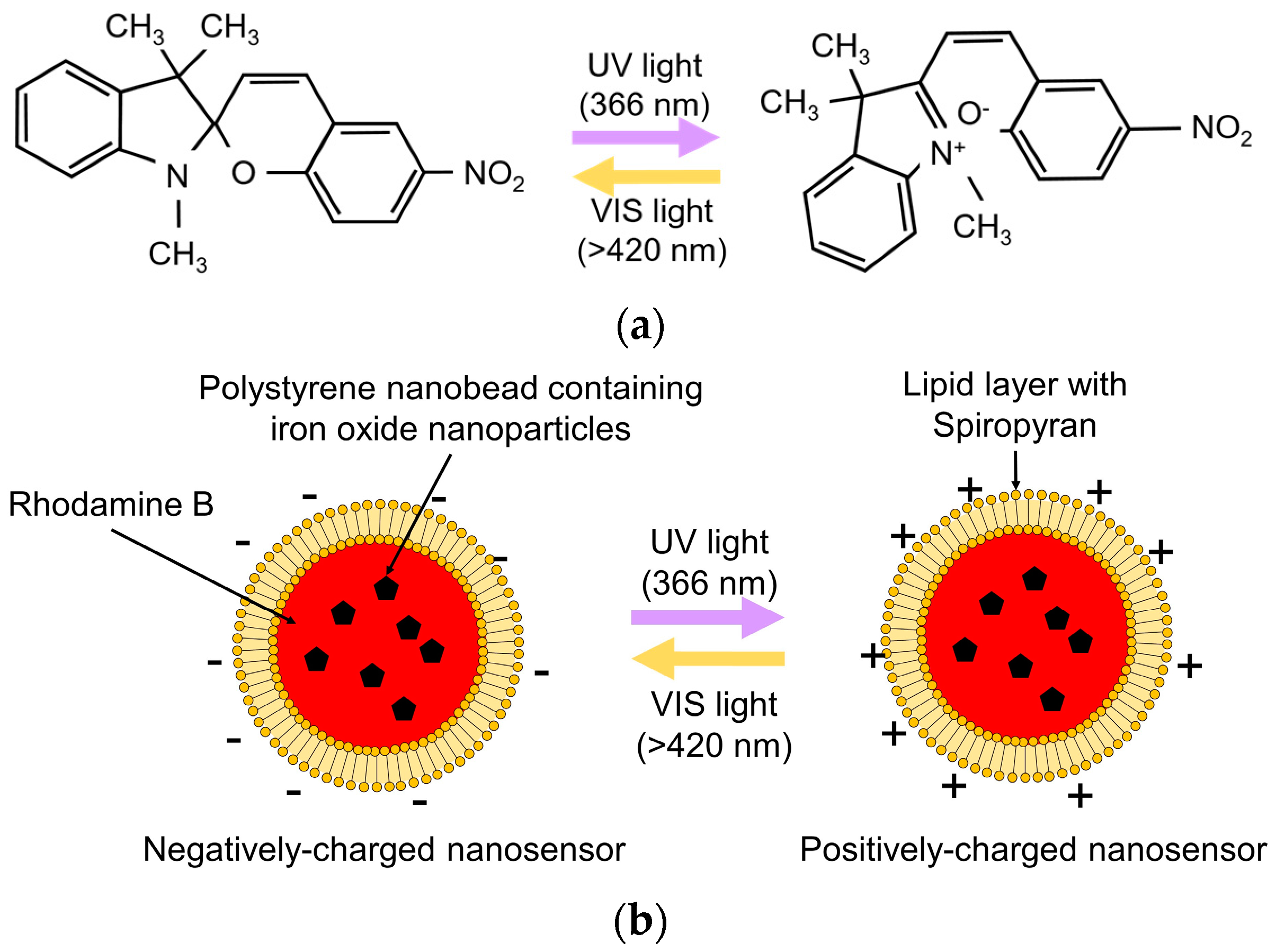
2.3. Optical Control of Zeta Potential Using Photochromic Material
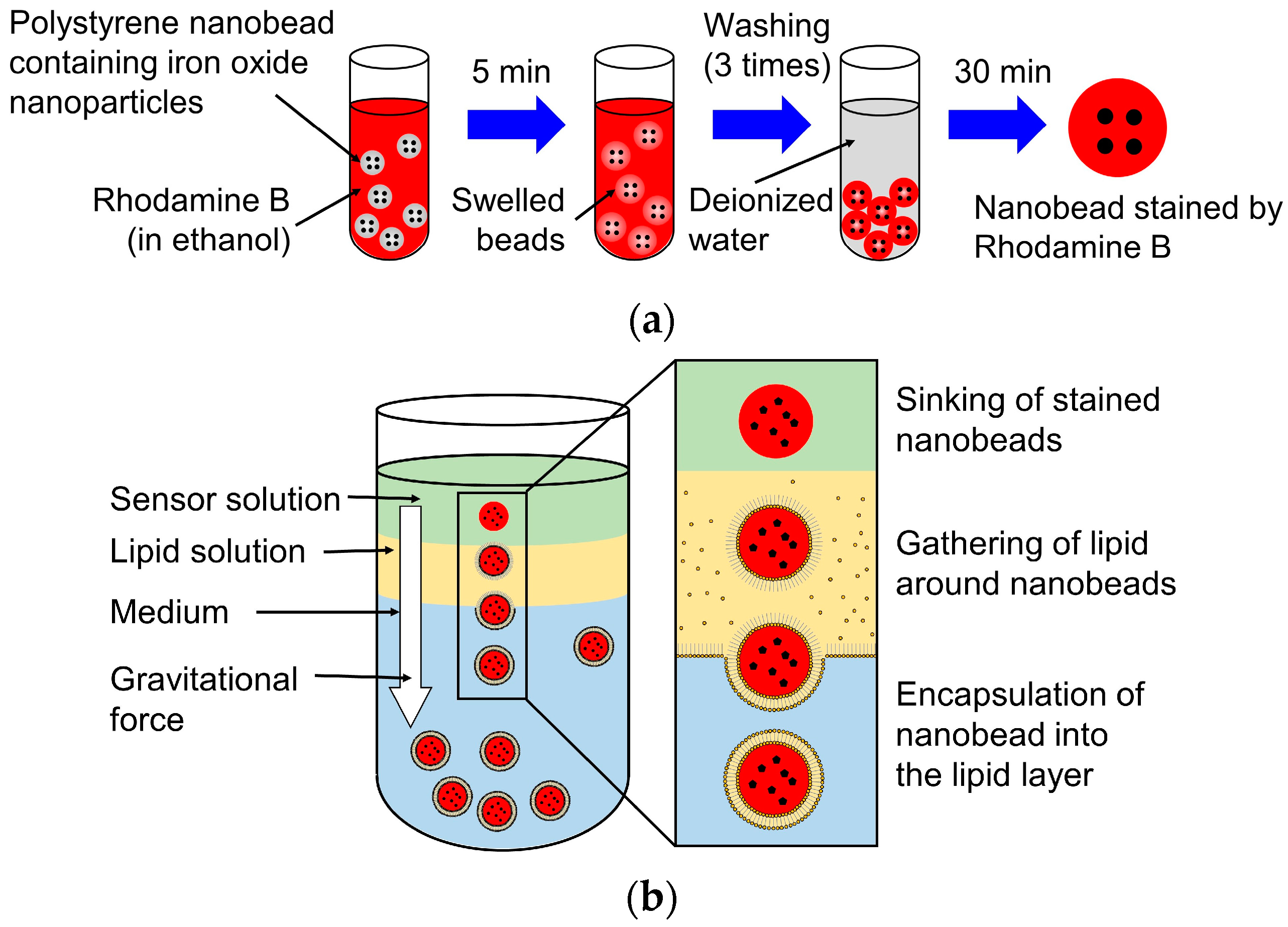
2.4. Fabrication of the Nanosensor with a Photochromic Lipid Layer
2.5. Fabrication of the Glass Nanoprobe
2.6. Cell Culture
3. Results and Discussion
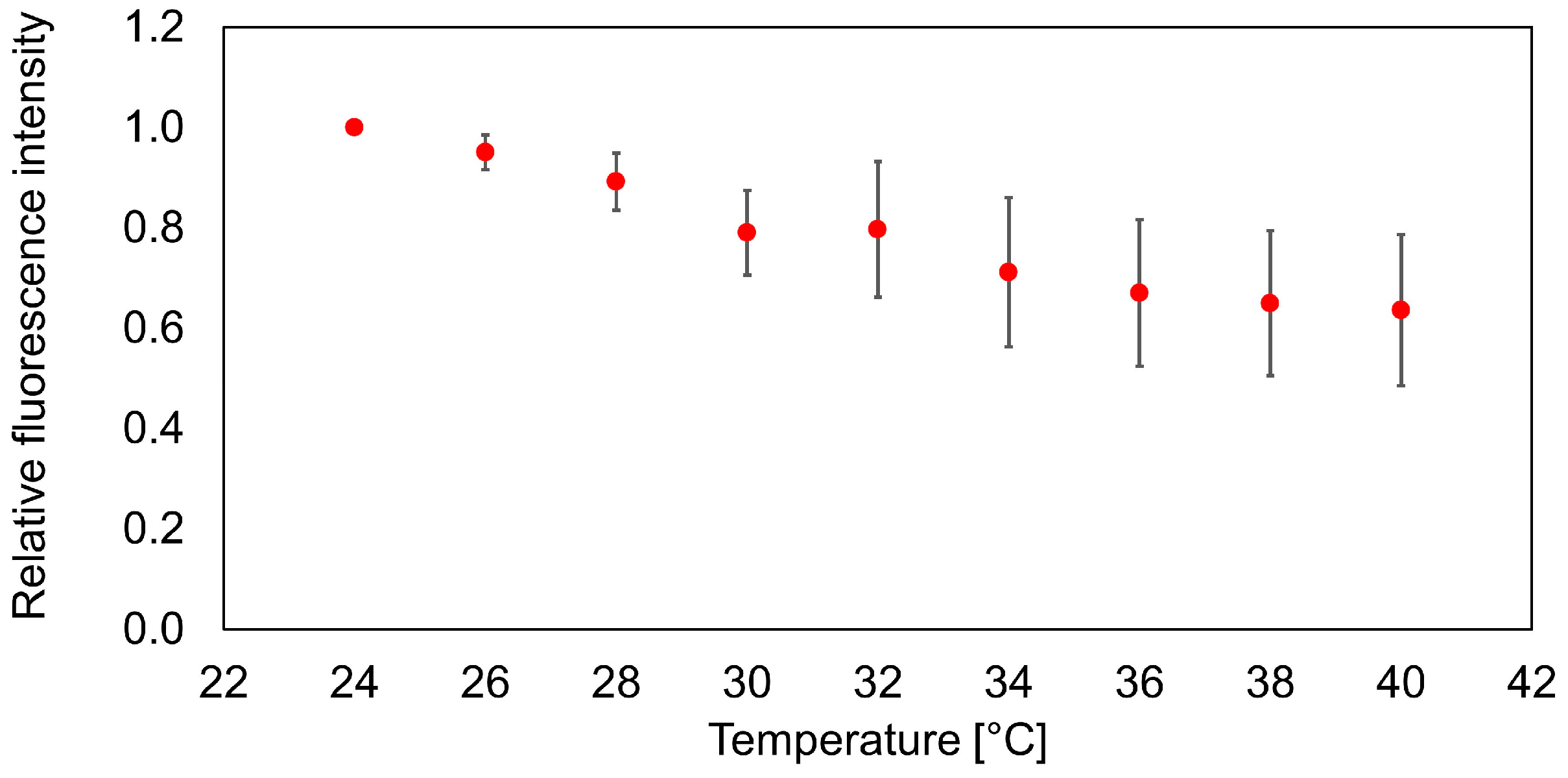
3.1. Temperature Calibration of the Nanosensor
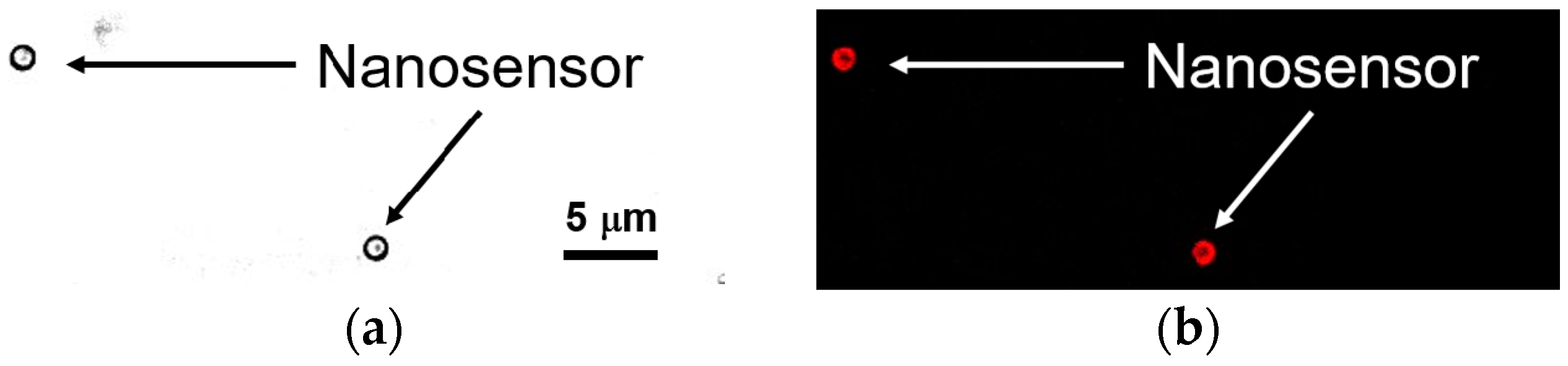
3.2. Manipulation and Immobilization of the Nanosensor Using Zeta Potential Control
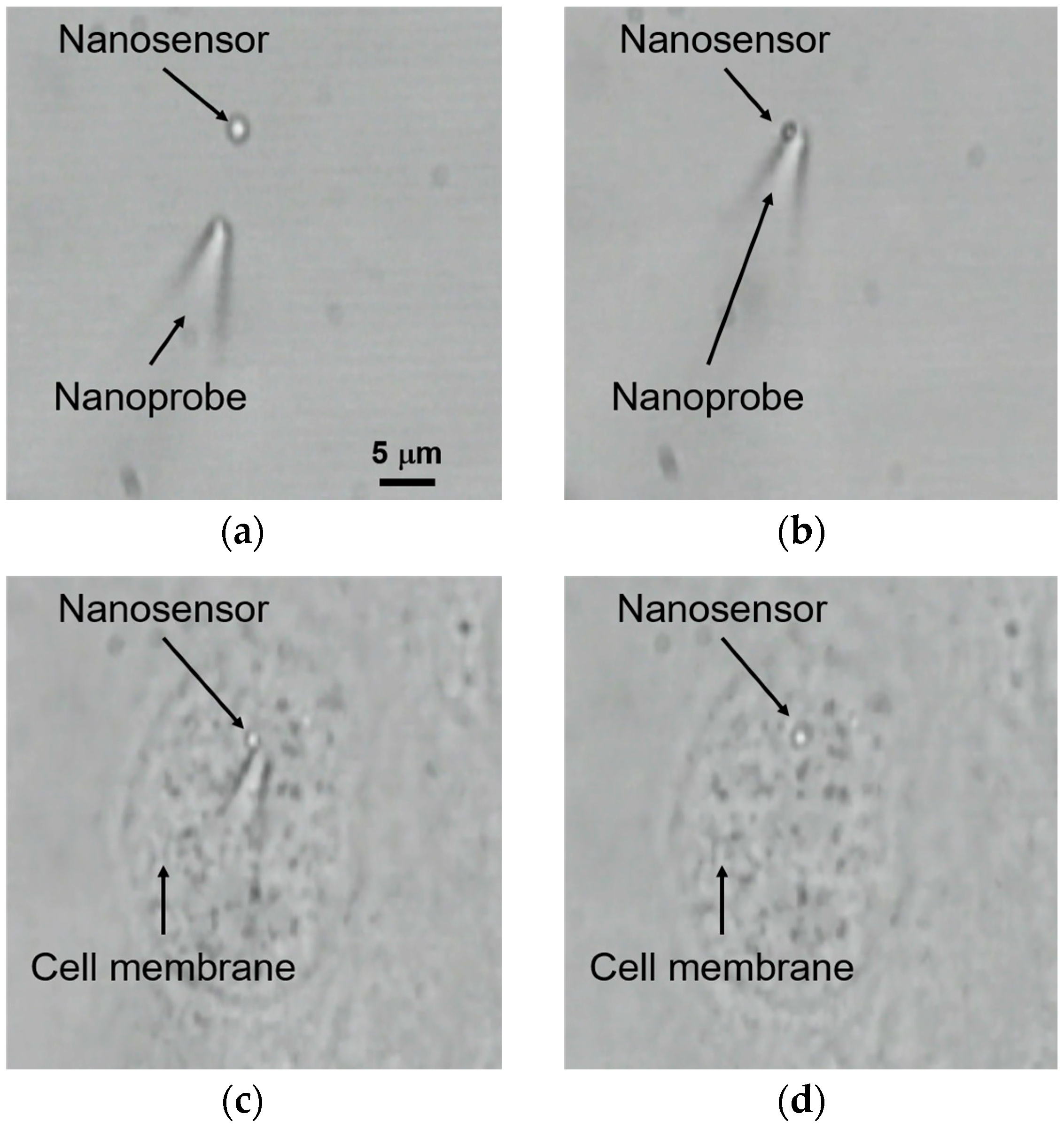
3.3. Injection of the Nanosensor by Local Laser Heating
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Torchilin, V.P. Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat. Rev. Drug Discov. 2014, 13, 813–827. [Google Scholar] [CrossRef] [PubMed]
- Ciampor, F.; Thompson, C.A.; Grambas, S.; Hay, A.J. Regulation of pH by the M2 protein of influenza A viruses. Virus Res. 1992, 22, 247–258. [Google Scholar] [CrossRef]
- Okabe, K.; Inada, N.; Gota, C.; Harada, Y.; Funatsu, T.; Uchiyama, S. Intracellular temperature mapping with a fluorescent polymeric thermometer and fluorescence lifetime imaging microscopy. Nat. Commun. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Himms-Hagen, J. Cellular thermogenesis. Annu. Rev. Physiol. 1976, 38, 315–351. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.L.; Bostick, D.A.; Garry, R.F. Sindbis virus infection decreases intracellular pH: Alkaline medium inhibits processing of sindbis virus polyproteins. Virology 1988, 166, 1–9. [Google Scholar] [CrossRef]
- Donner, J.S.; Thompson, S.A.; Kreuzer, M.P.; Baffou, G.; Quidant, R. Mapping intracellular temperature using green fluorescent protein. Nano Lett. 2012, 12, 2107–2111. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.M.; Yang, H.; Lin, L. Quantum dot nano thermometers reveal heterogeneous local thermogenesis in living cells. ACS Nano 2011, 5, 5067–5071. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Maruyama, H.; Masuda, T.; Honda, A.; Arai, F. Multi-fluorescent micro-sensor for accurate measurement of pH and temperature variations in micro-environments. Sens. Actuators B Chem. 2014, 203, 54–62. [Google Scholar] [CrossRef]
- LaVan, D.A.; McGuire, T.; Langer, R. Small-scale systems for in vivo drug delivery. Nat. Biotechnol. 2003, 21, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Zhang, J.; Lee, C.S.; Chen, X. Micro- and nanotechnologies for intracellular delivery. Small 2014, 10, 4487–4504. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Kis, A.; Zettl, A.; Bertozzi, C.R. A cell nanoinjector based on carbon nanotubes. Proc. Natl. Acad. Sci. USA 2007, 104, 8218–8222. [Google Scholar] [CrossRef] [PubMed]
- Truschel, S.T.; Wang, E.; Ruiz, W.G.; Leung, S.-M.; Rojas, R.; Lavelle, J.; Zeidal, M.; Stoffer, D.; Apodaca, G. Stretch-regulated exocytosis/endocytosis in bladder umbrella cells. Mol. Biol. Cell 2002, 13, 830–846. [Google Scholar] [CrossRef] [PubMed]
- Delgado, D.; Del Pozo-Rodríguez, A.; Solinís, M.Á.; Rodríguez-Gascón, A. Understanding the mechanism of protamine in solid lipid nanoparticle-based lipofection: The importance of the entry pathway. Eur. J. Pharm. Biopharm. 2011, 79, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Yavari, F.; Minary-Jolandan, M.; Giraldo-Vela, J.P.; Safi, A.; McNaughton, R.L.; Parpoil, V.; Espinosa, H.D. Nanofountain probe electroporation of single cells. Nano Lett. 2013, 13, 2448–2457. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lohmuller, T.; Feldmann, J.; Lohmüller, T.; Feldmann, J. Optical injection of gold nanoparticles into living cells. Nano Lett. 2015, 15, 770–775. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Maruyama, H.; Masuda, T.; Arai, F. Vibration-assisted optical injection of a single fluorescent sensor into a target cell. Sens. Actuators B Chem. 2015, 220, 40–49. [Google Scholar] [CrossRef]
- Zhong, J.; Liu, H.; Maruyama, H.; Masuda, T.; Arai, F. Continuous-wave laser-assisted injection of single magnetic nanobeads into living cells. Sens. Actuators B Chem. 2016, 230, 298–305. [Google Scholar] [CrossRef]
- Zhong, J.; Liu, H.; Maruyama, H.; Arai, F. Optical heating of metallic nanoparticles for fast injection of nanoscale sensor into living cells. In Proceedings of the IEEE International Conference on Nanotechnology, Rome, Italy, 27–30 July 2015; pp. 721–723.
- Arai, F.; Ng, C.; Maruyama, H.; Ichikawa, A.; El-Shimy, H.; Fukuda, T. On chip single-cell separation and immobilization using optical tweezers and thermosensitive hydrogel. Lab Chip 2005, 5, 1399–1403. [Google Scholar] [CrossRef] [PubMed]
- De Vlaminck, I.; Dekker, C. Recent advances in magnetic tweezers. Annu. Rev. Biophys. 2012, 41, 453–472. [Google Scholar] [CrossRef] [PubMed]
- De Vries, A.H.B.; Krenn, B.E.; van Driel, R.; Kanger, J.S. Micro magnetic tweezers for nanomanipulation inside live cells. Biophys. J. 2005, 88, 2137–2144. [Google Scholar] [CrossRef] [PubMed]
- Blonder, R.; Katz, E.; Willner, I.; Wray, V.; Bückmann, A.F. Application of a nitrospiropyran-FAD-reconstituted glucose oxidase and charged electron mediators as optobioelectronic assemblies for the amperometric transduction of recorded optical signals: Control of the “on”-“off” direction of the photoswitch. J. Am. Chem. Soc. 1997, 119, 11747–11757. [Google Scholar] [CrossRef]
- Vasiliadou, I.A.; Chrysikopoulos, C.V. Cotransport of Pseudomonas putida and kaolinite particles through water-saturated columns packed with glass beads. Water Resour. Res. 2011, 47, 1–14. [Google Scholar] [CrossRef]
- Paulmichl, M.; Friedrich, F.; Wöll, E.; Weiss, H.; Lang, F. Effects of serotonin on electrical properties of Madin-Darby canine kidney cells. Pflügers Arch. Eur. J. Physiol. 1988, 411, 394–400. [Google Scholar] [CrossRef]
- Onda, K.; Arai, F. Multi-beam bilateral teleoperation of holographic optical tweezers. Opt. Express 2012, 20, 3633–3641. [Google Scholar] [CrossRef] [PubMed]
- Edahiro, J.I.; Sumaru, K.; Tada, Y.; Ohi, K.; Takagi, T.; Kameda, M.; Shinbo, T.; Kanamori, T.; Yoshimi, Y. In situ control of cell adhesion using photoresponsive culture surface. Biomacromolecules 2005, 6, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Yamada, A.; Yamanaka, T.; Hamada, T.; Hase, M.; Yoshikawa, K.; Baigl, D. Spontaneous transfer of phospholipid-coated oil-in-oil and water-in-oil micro-droplets through an oil/water interface. Langmuir 2006, 22, 9824–9828. [Google Scholar] [CrossRef] [PubMed]
- Hashim, H.; Lei, W.; Maruyama, H.; Masuda, T.; Arai, F. Optical control of adhesion property of magnetic nanosensor using photochromism for effective manipulation and cell injection. In Proceedings of the 16th International Conference on Nanotechnology, Sendai, Japan, 22–25 August 2016.
- Liu, H.; Maruyama, H.; Masuda, T.; Honda, A.; Arai, F. The Influence of Virus Infection on the Extracellular pH of the Host Cell Detected on Cell Membrane. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]

| Solution | Without UV | With UV |
|---|---|---|
| PBS | 10% | 75% |
| DMEM + FBS | 5% | 43% |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashim, H.; Maruyama, H.; Masuda, T.; Arai, F. Manipulation and Immobilization of a Single Fluorescence Nanosensor for Selective Injection into Cells. Sensors 2016, 16, 2041. https://doi.org/10.3390/s16122041
Hashim H, Maruyama H, Masuda T, Arai F. Manipulation and Immobilization of a Single Fluorescence Nanosensor for Selective Injection into Cells. Sensors. 2016; 16(12):2041. https://doi.org/10.3390/s16122041
Chicago/Turabian StyleHashim, Hairulazwan, Hisataka Maruyama, Taisuke Masuda, and Fumihito Arai. 2016. "Manipulation and Immobilization of a Single Fluorescence Nanosensor for Selective Injection into Cells" Sensors 16, no. 12: 2041. https://doi.org/10.3390/s16122041
APA StyleHashim, H., Maruyama, H., Masuda, T., & Arai, F. (2016). Manipulation and Immobilization of a Single Fluorescence Nanosensor for Selective Injection into Cells. Sensors, 16(12), 2041. https://doi.org/10.3390/s16122041







