Uniformly Porous Nanocrystalline CaMgFe1.33Ti3O12 Ceramic Derived Electro-Ceramic Nanocomposite for Impedance Type Humidity Sensor
Abstract
:1. Introduction
2. Experimental Details
2.1. Preparation of Sensing Material
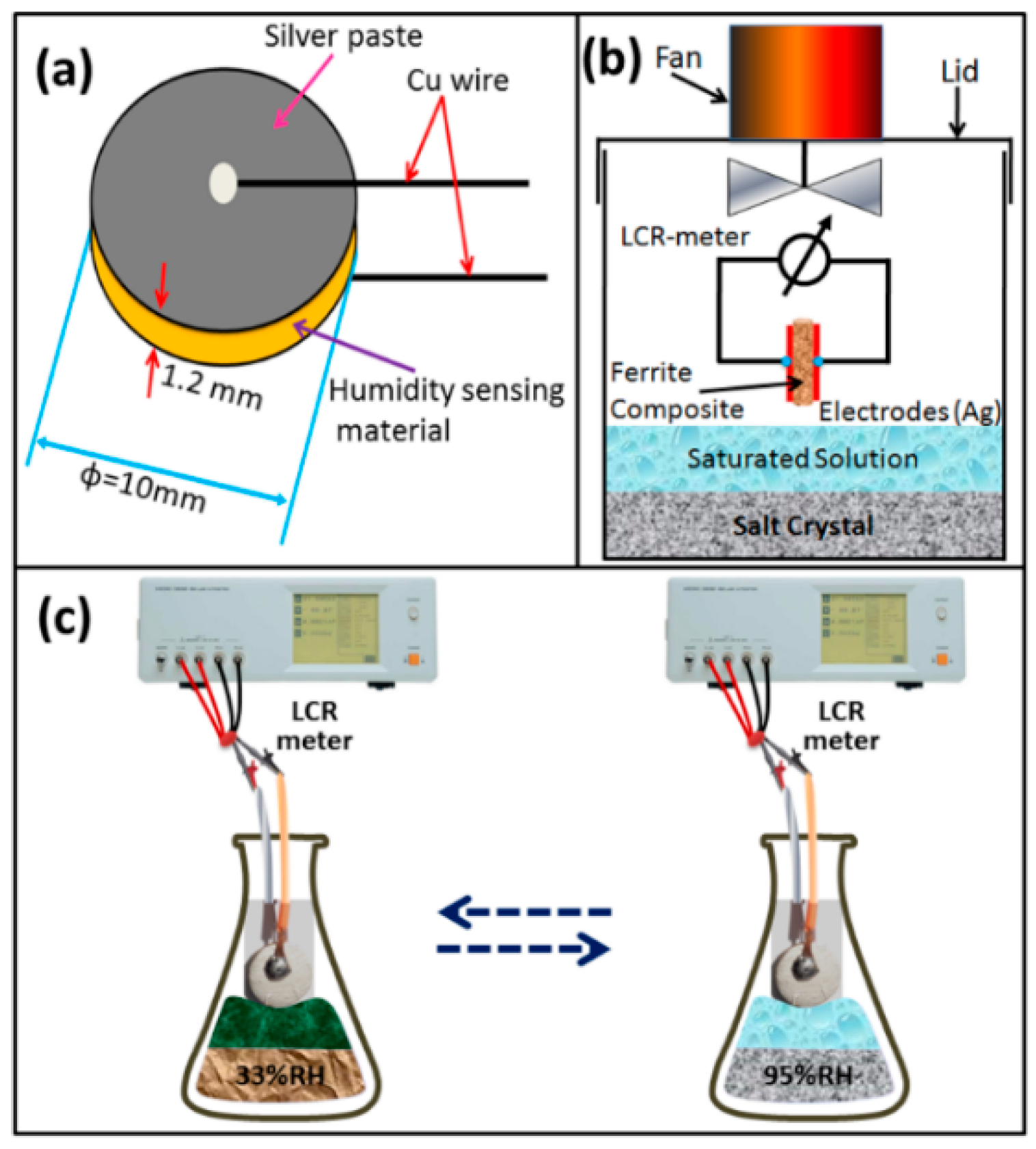
2.2. Humidity Sensor Fabrication and Measuring Setup
3. Results and Discussion
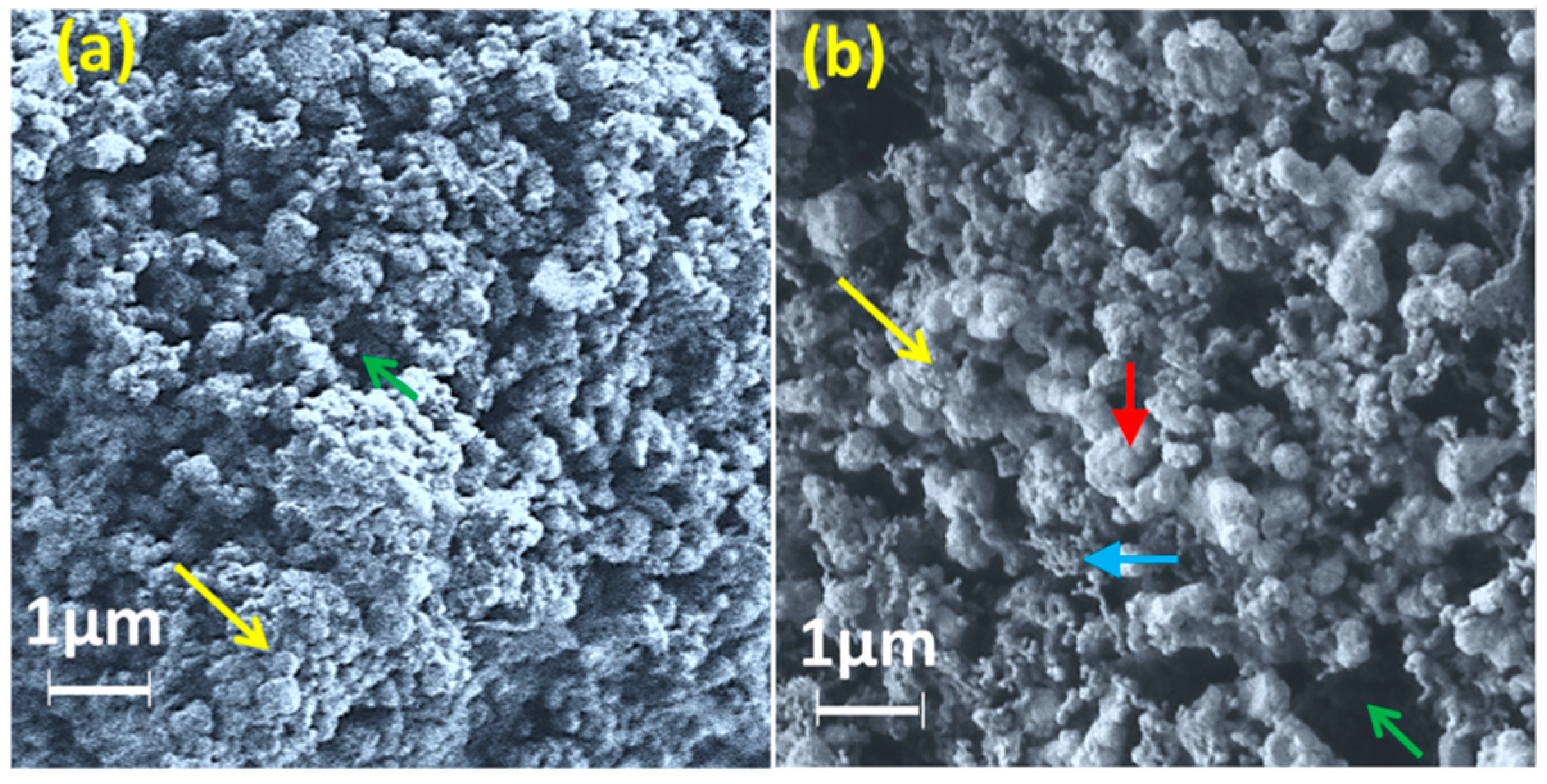
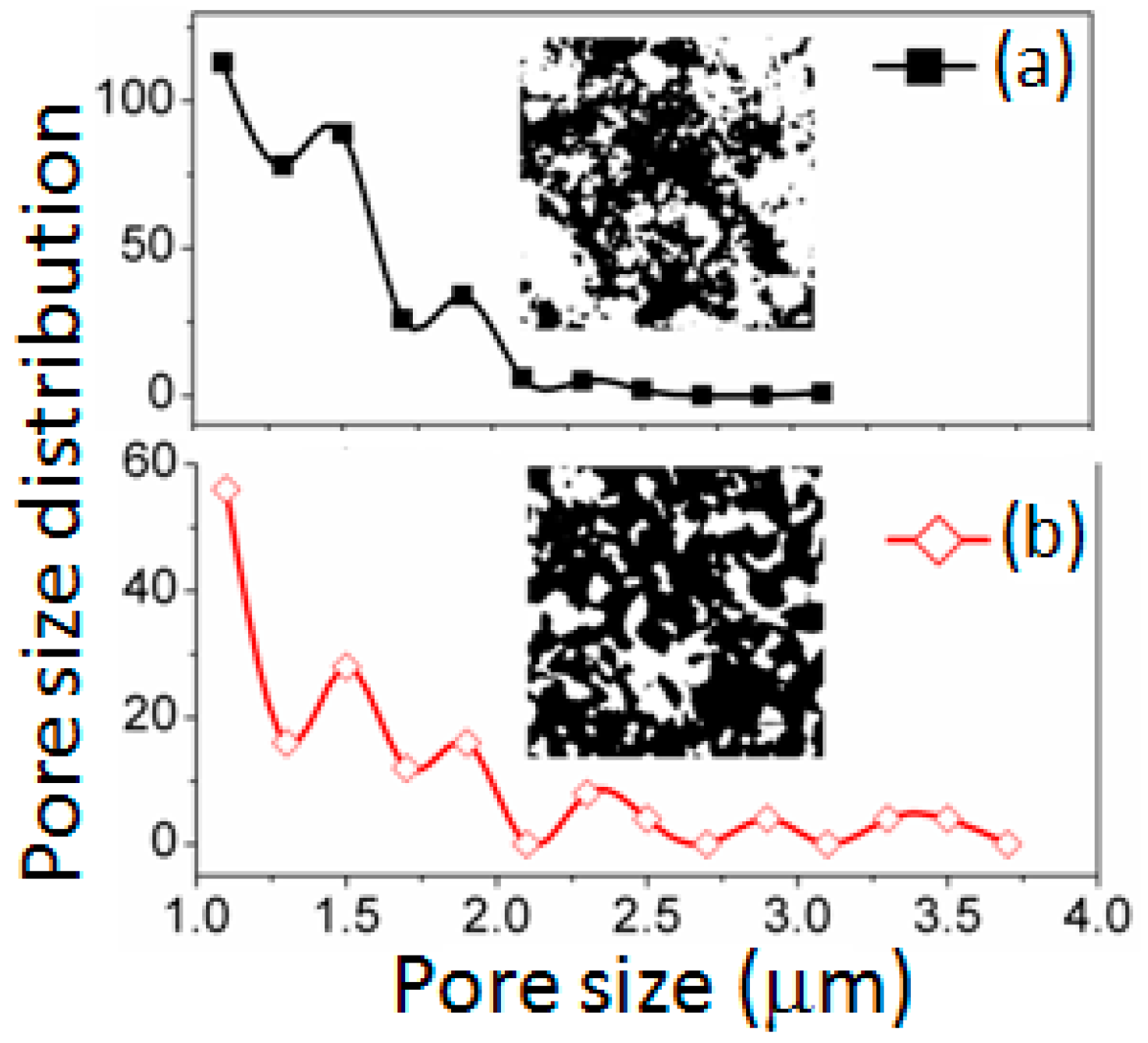
3.1. Structural and Morphological Analysis
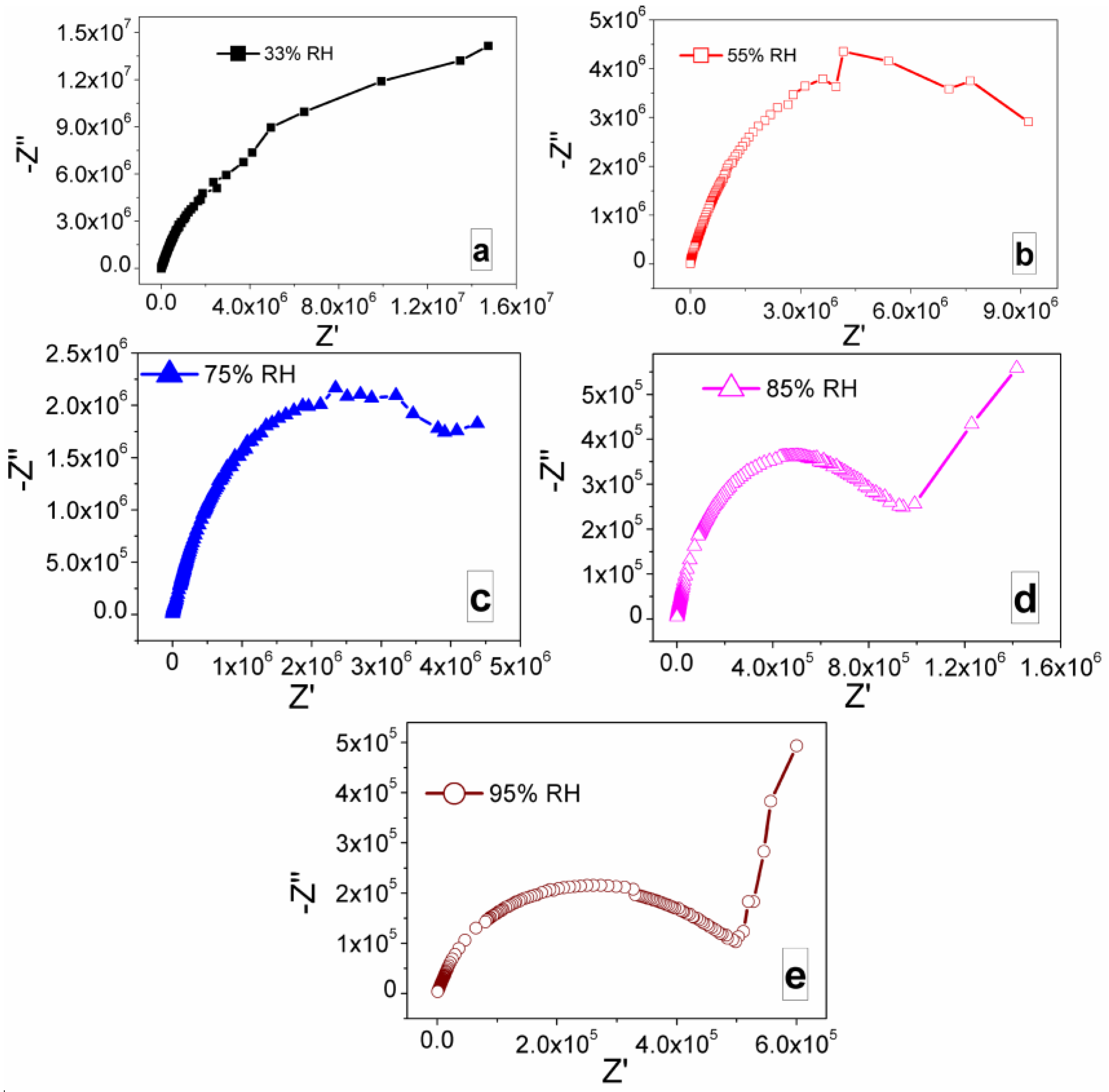
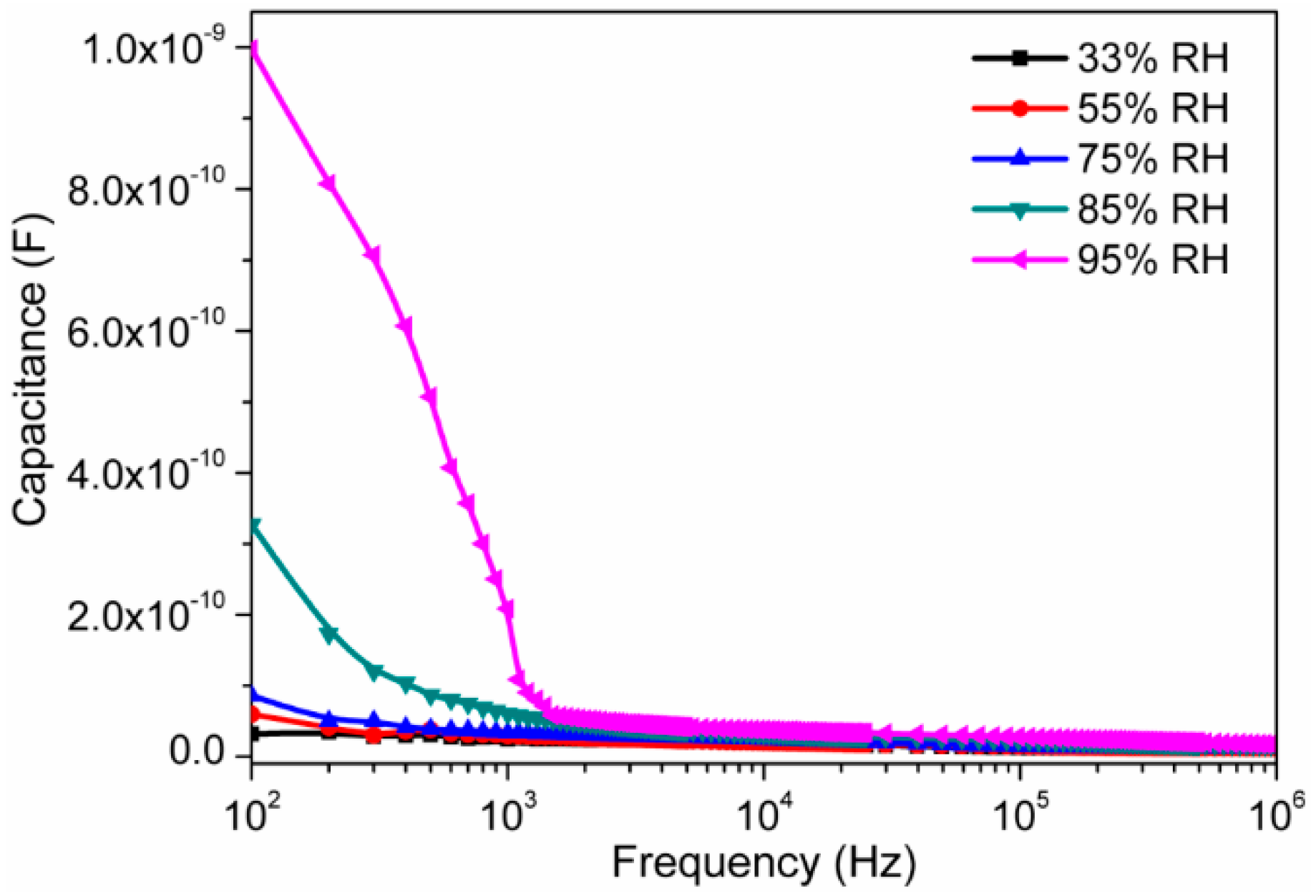
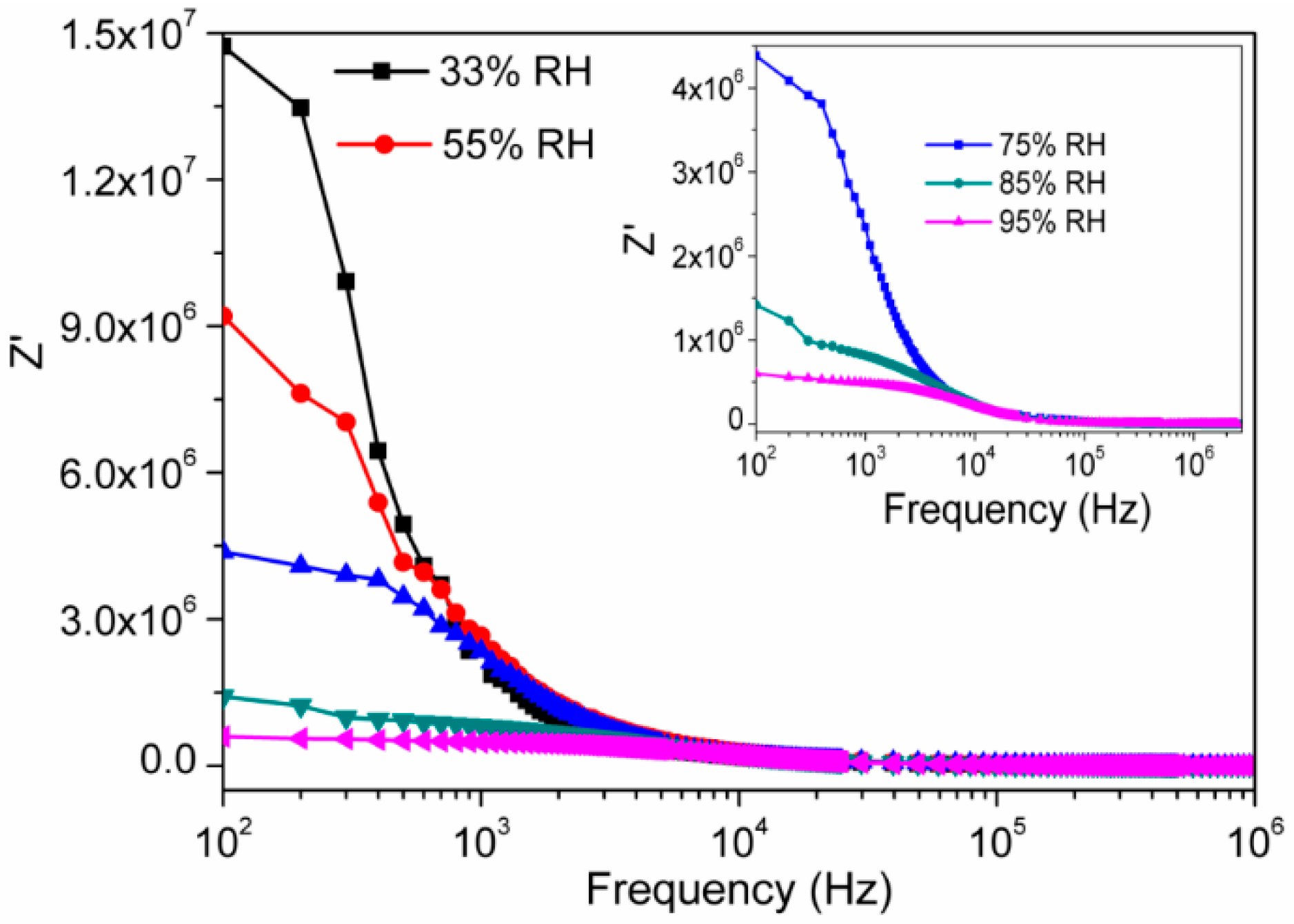
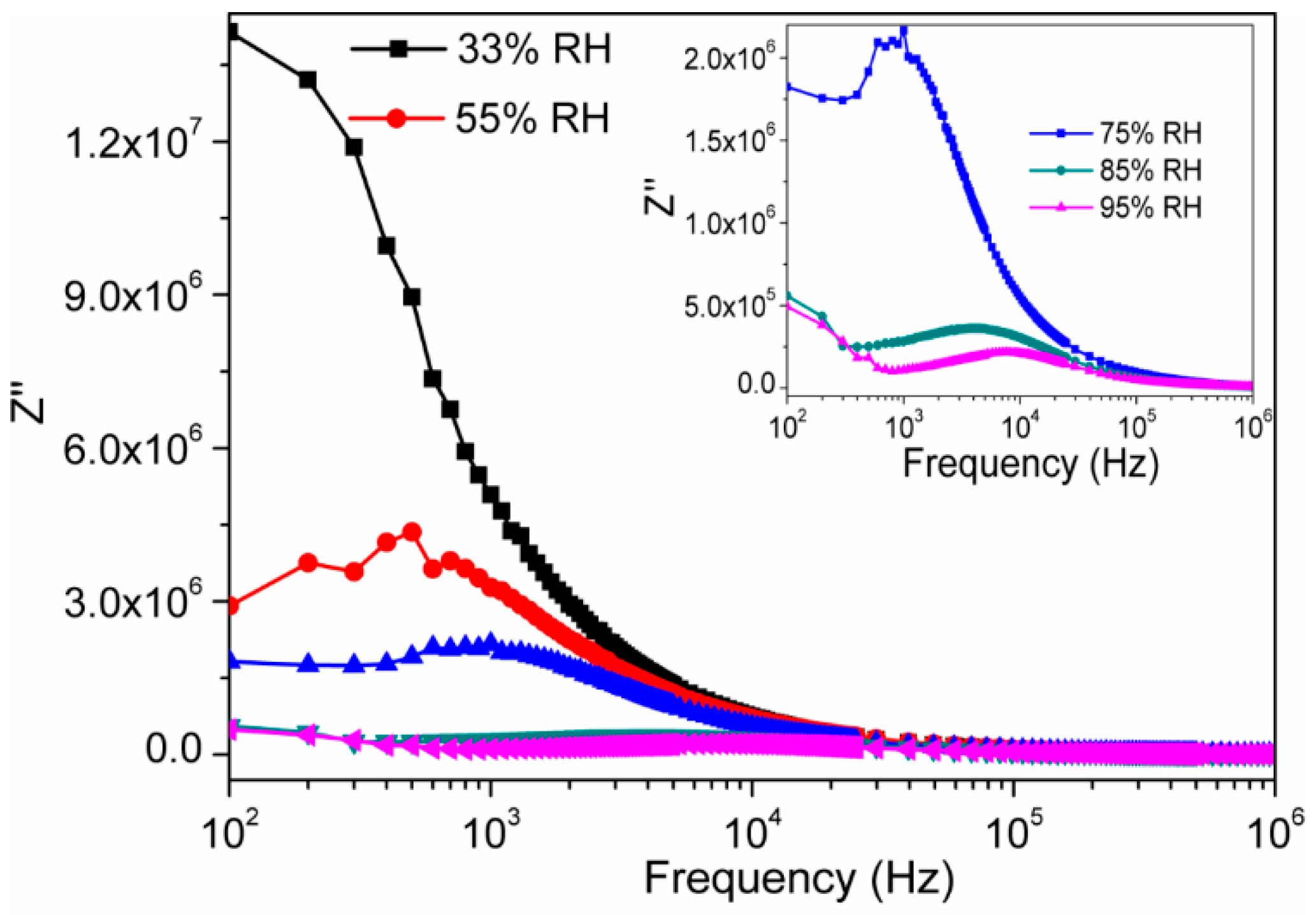
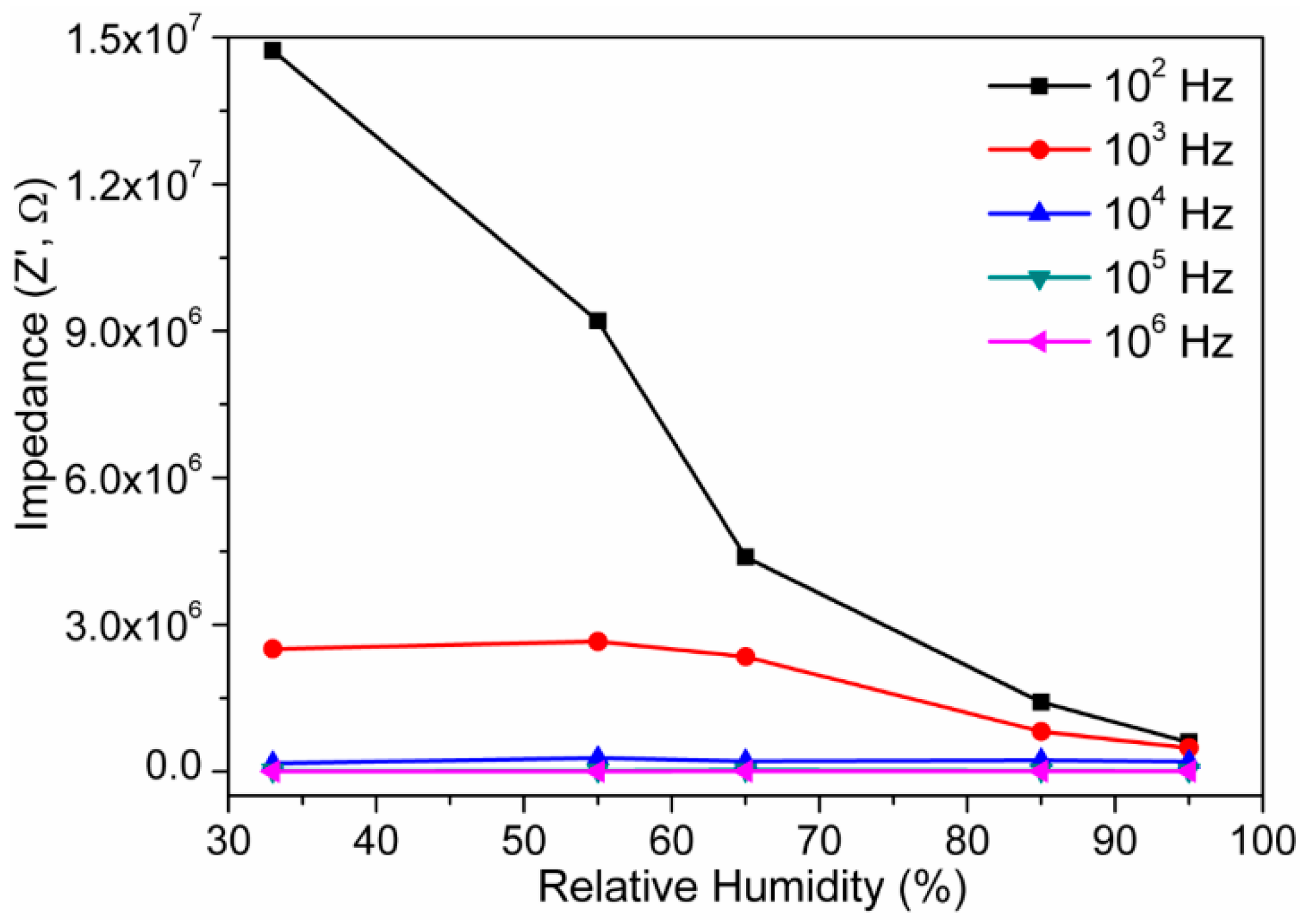
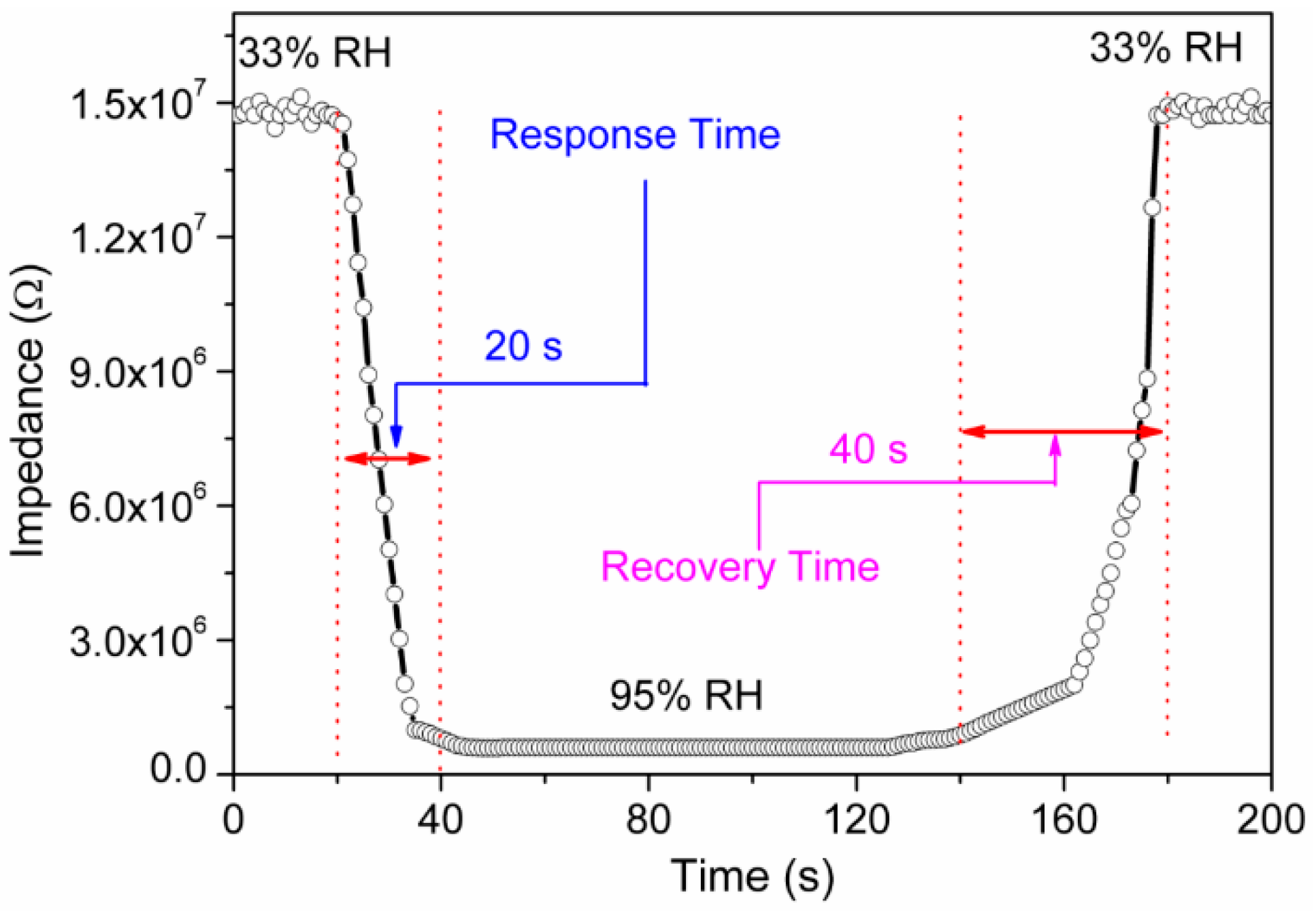
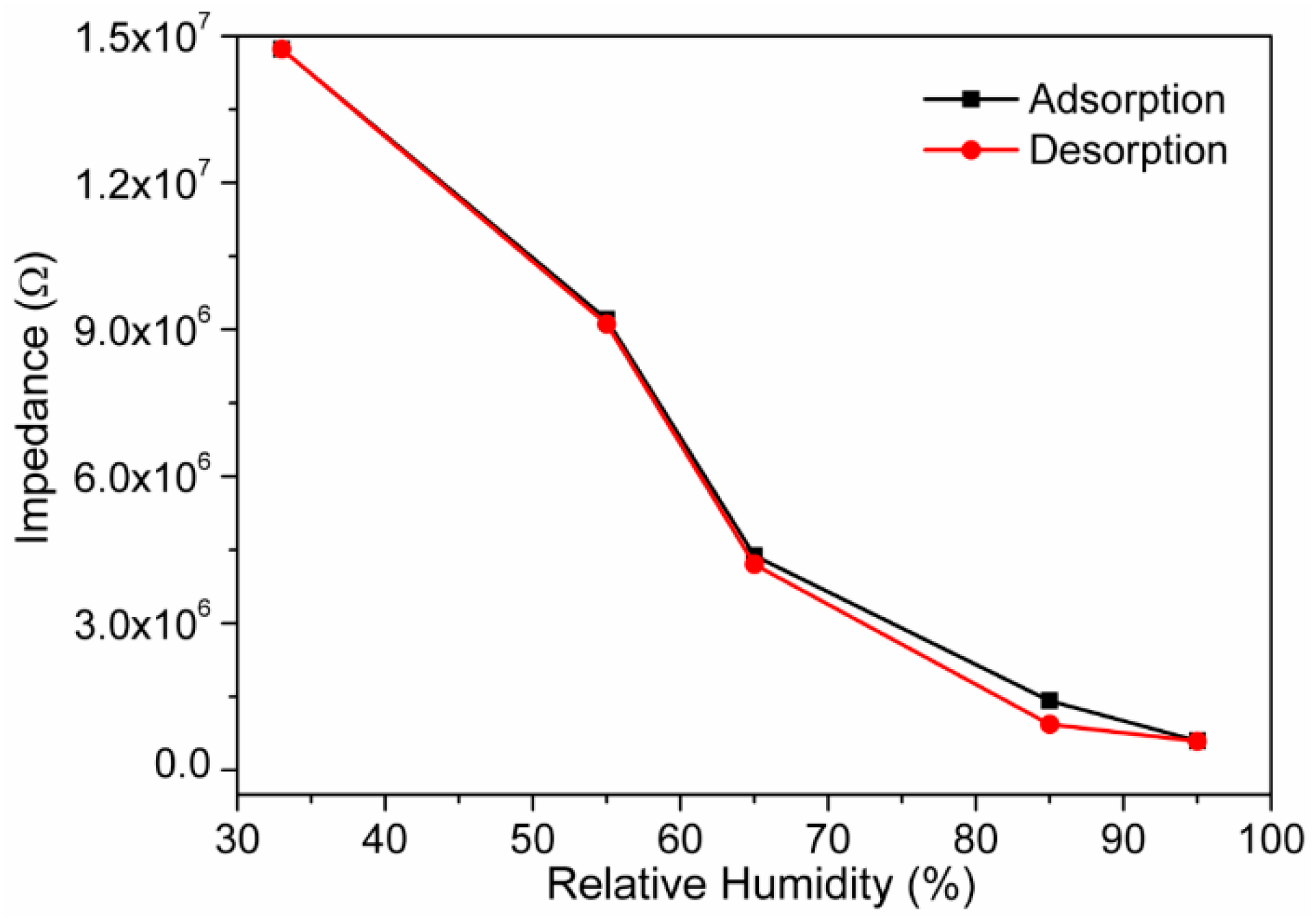
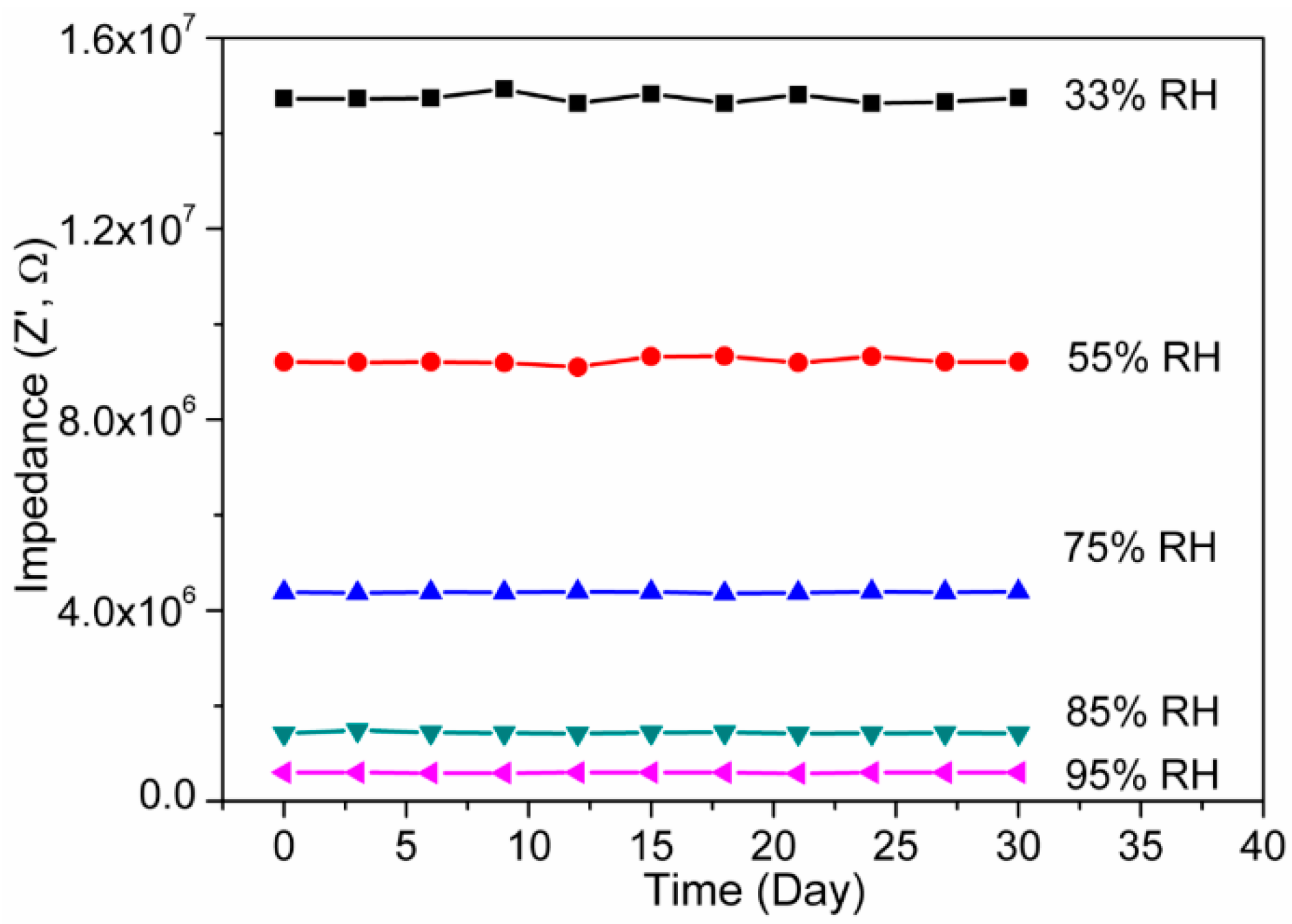
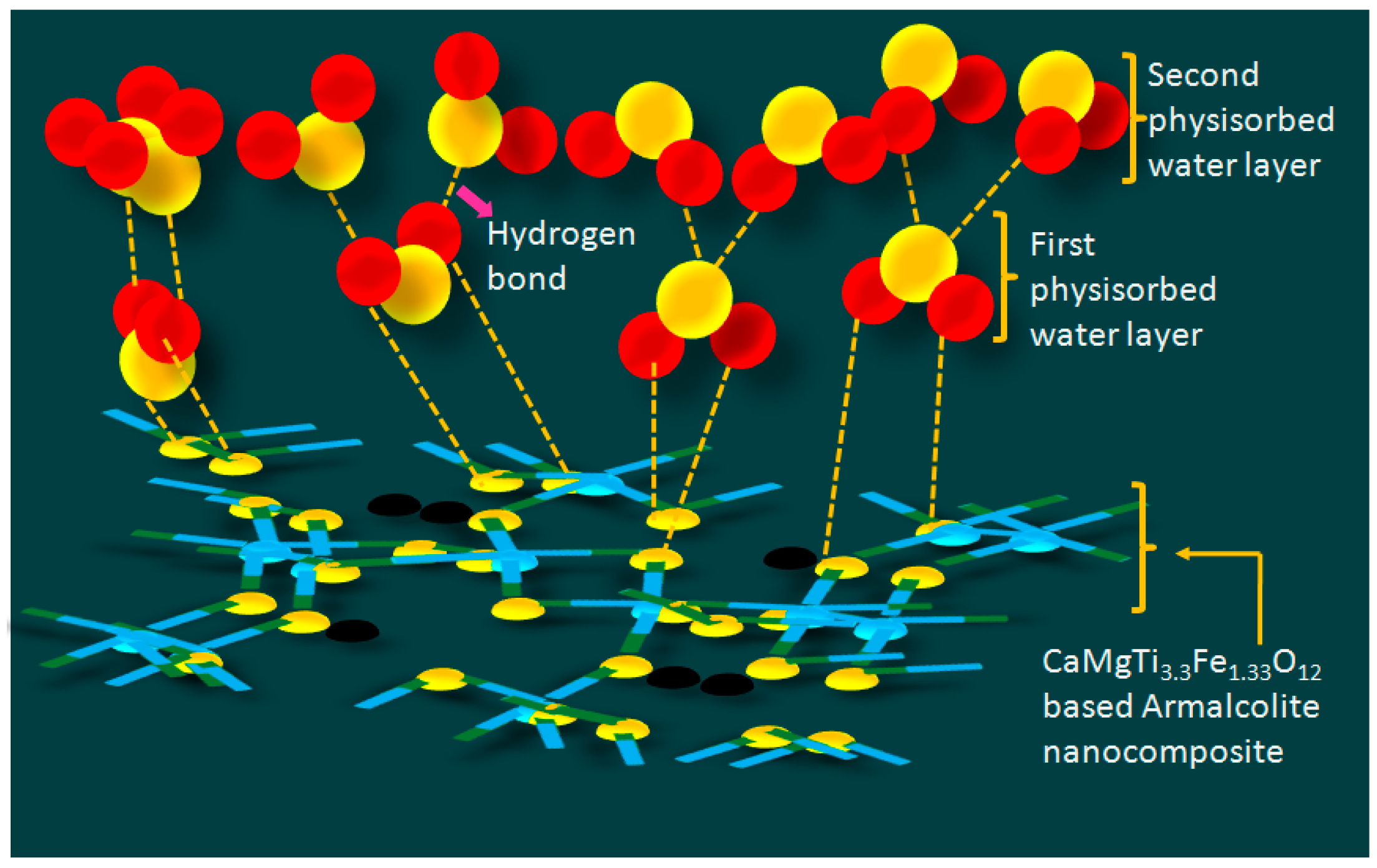
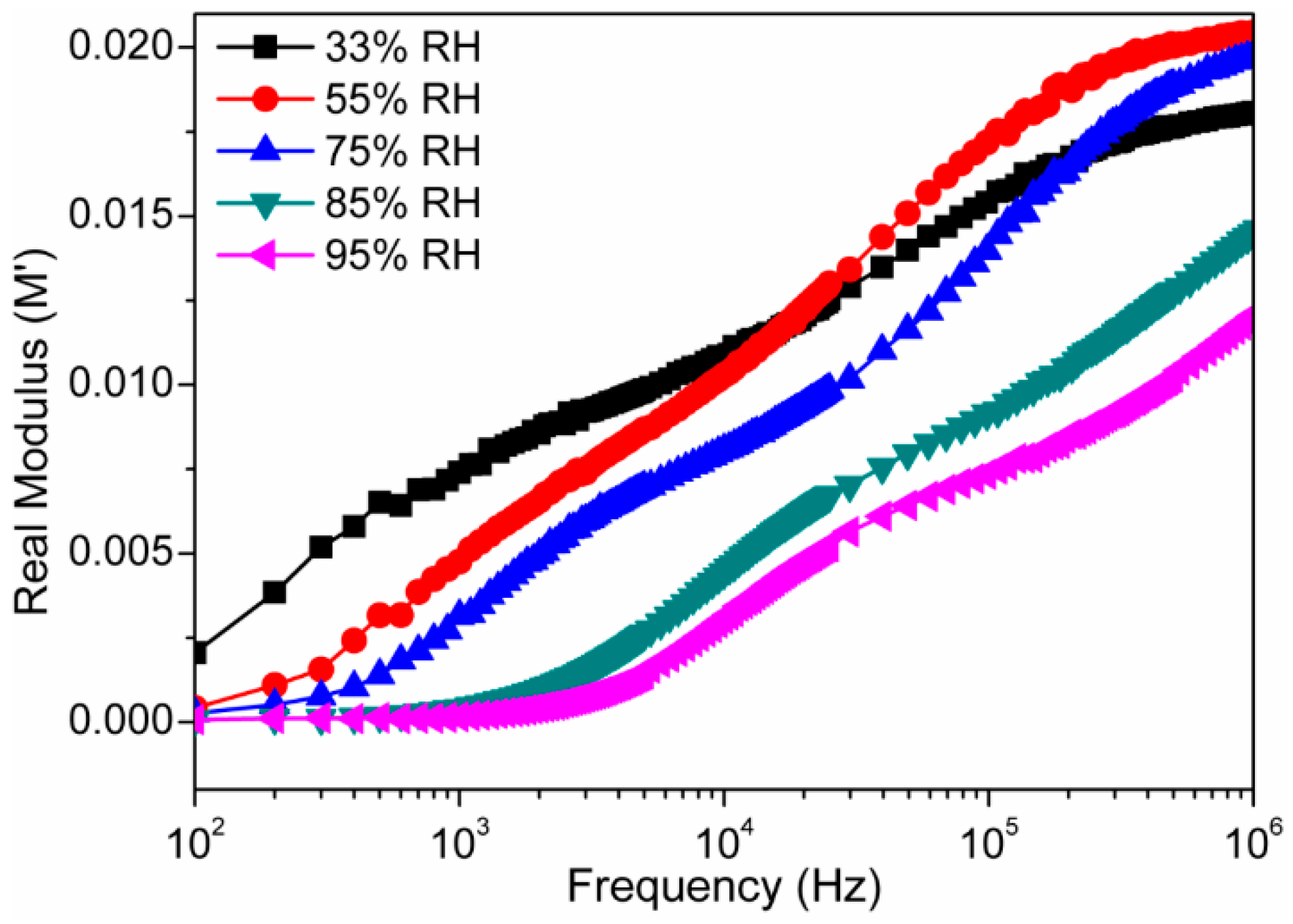
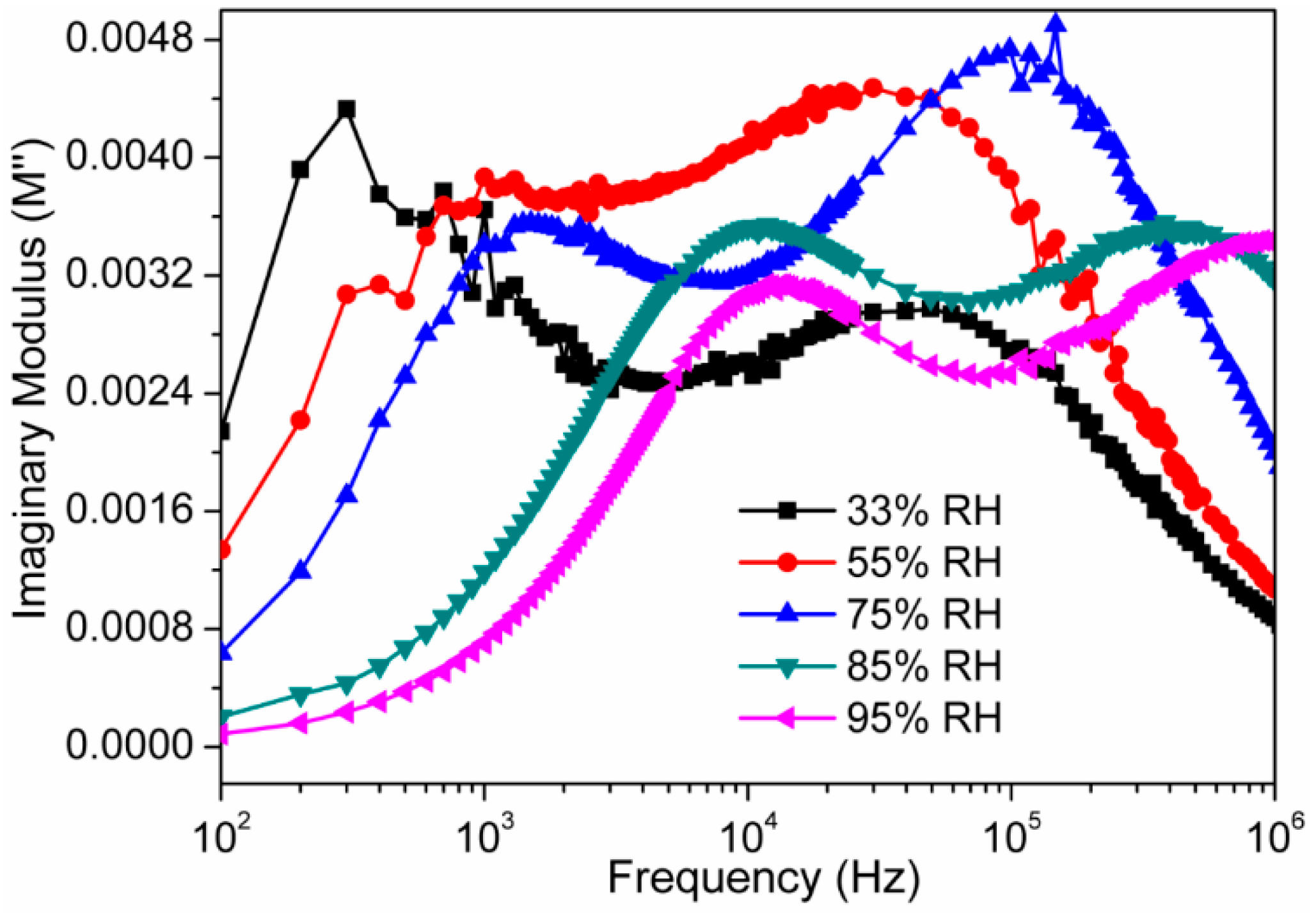
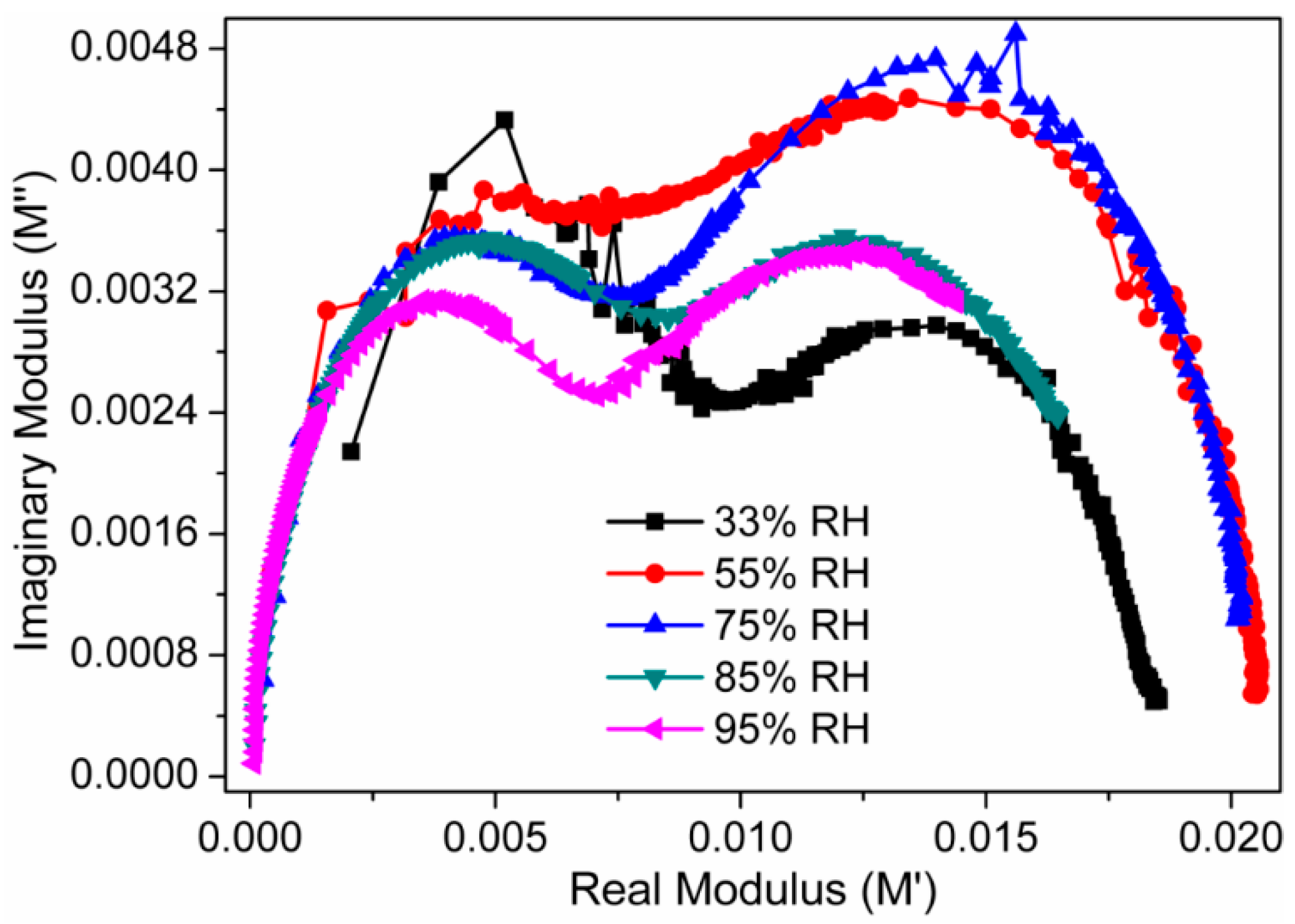
3.2. Humidity Sensing Measurements
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, Z.; Lu, C. Humidity sensors: A review of materials and mechanisms. Sens. Lett. 2005, 3, 274–295. [Google Scholar] [CrossRef]
- Comini, E.; Sberveglieri, G. Metal oxide nanowires as chemical sensors. Mater. Today 2010, 13, 36–44. [Google Scholar] [CrossRef]
- Kong, J.; Franklin, N.R.; Zhou, C.; Chapline, M.G.; Peng, S.; Cho, K.; Dai, H. Nanotube molecular wires as chemical sensors. Science 2000, 287, 622–625. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, H.; Hsueh, T.; Chang, S.; Hung, F.; Weng, W.; Hsu, C.; Dai, B. Si nanowire-based humidity sensors prepared on glass substrate. IEEE Sens. J. 2011, 11, 3036–3041. [Google Scholar] [CrossRef]
- Huang, J.; Virji, S.; Weiller, B.H.; Kaner, R.B. Polyaniline nanofibers: Facile synthesis and chemical sensors. J. Am. Chem. Soc. 2003, 125, 314–315. [Google Scholar] [CrossRef] [PubMed]
- Traversa, E. Ceramic sensors for humidity detection: The state-of-the-art and future developments. Sens. Actuators B Chem. 1995, 23, 135–156. [Google Scholar] [CrossRef]
- Patissier, B. Humidity sensors for automotive, appliances and consumer applications. Sens. Actuators B Chem. 1999, 59, 231–234. [Google Scholar] [CrossRef]
- Tripathy, A.; Pramanik, S.; Cho, J.; Santhosh, J.; Abu Osman, N.A. Role of morphological structure, doping, and coating of different materials in the sensing characteristics of humidity sensors. Sensors 2014, 14, 16343–16422. [Google Scholar] [CrossRef] [PubMed]
- Segev-Bar, M.; Haick, H. Flexible sensors based on nanoparticles. ACS Nano 2013, 7, 8366–8378. [Google Scholar] [CrossRef] [PubMed]
- Matko, V.; Donlagic, D. Sensor for high-air-humidity measurement. IEEE Trans. Instrum. Meas. 1996, 45, 561–563. [Google Scholar] [CrossRef]
- Matko, V. Next generation at-cut quartz crystal sensing devices. Sensors 2011, 11, 4474–4482. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.L.; Schuhmann, W.; Scheller, F.W.; Schubert, F.; Gopel, W.; Hesse, J.; Zemel, J.N. Chemical and biological sensors, part II. In Sensors: A Comprehensive Survey; Göpel, W., Hesse, J., Zemel, J.N., Eds.; Wiley-VCH: Weinheim, Germany, 1992; Volume 3, pp. 717–817. [Google Scholar]
- Venditti, I.; Fratoddi, I.; Bearzotti, A. Self-assembled copolymeric nanoparticles as chemically interactive materials for humidity sensors. Nanotechnology 2010, 21, 355502. [Google Scholar] [CrossRef] [PubMed]
- Fratoddi, I.; Bearzotti, A.; Venditti, I.; Cametti, C.; Russo, M. Role of nanostructured polymers on the improvement of electrical response-based relative humidity sensors. Sens. Actuators B Chem. 2016, 225, 96–108. [Google Scholar] [CrossRef]
- Yagi, H. Humidity Sensors Using Ceramic Materials. In Proceedings of the Symposium on Chemical Sensors II; Butler, M., Ricco, A., Yamazoe, N., Eds.; The Electrochemical Society: Pennington, NJ, USA, 1993; Volume 93-7, pp. 498–509. [Google Scholar]
- Yamazoe, N.; Shimizu, Y. Humidity sensors: Principles and applications. Sens. Actuators 1986, 10, 379–398. [Google Scholar] [CrossRef]
- Fagan, J.G.; Amarakoon, R. Reliability and reproducibility of ceramic sensors. III: Humidity sensors. Am. Ceram. Soc. Bull. 1993, 72, 119–130. [Google Scholar]
- Shimizu, Y.; Arai, H.; Seiyama, T. Theoretical studies on the impedance-humidity characteristics of ceramic humidity sensors. Sens. Actuators 1985, 7, 11–22. [Google Scholar] [CrossRef]
- Hong-Tao, S.; Ming-Tang, W.; Ping, L.; Xi, Y. Porosity control of humidity-sensitive ceramics and theoretical model of humidity-sensitive characteristics. Sens. Actuators 1989, 19, 61–70. [Google Scholar] [CrossRef]
- Pramanik, S.; Pingguan-Murphy, B.; Abu Osman, N.A. Developments of immobilized surface modified piezoelectric crystal biosensors for advanced applications. Int. J. Electrochem. Sci. 2013, 8, 8863–8892. [Google Scholar]
- Shukla, S.K.; Shukla, S.K.; Govender, P.P.; Agorku, E.S. A resistive type humidity sensor based on crystalline tin oxide nanoparticles encapsulated in polyaniline matrix. Microchim. Acta 2016, 183, 573–580. [Google Scholar] [CrossRef]
- Rezlescu, N.; Doroftei, C.; Rezlescu, E.; Popa, P. Structure and humidity sensitive electrical properties of the Sn4+ and/or Mo6+ substituted Mg ferrite. Sens. Actuators B Chem. 2006, 115, 589–595. [Google Scholar] [CrossRef]
- Candeia, R.; Souza, M.; Bernardi, M.I.B.; Maestrelli, S.; Santos, I.; Souza, A.; Longo, E. MgFe2O4 pigment obtained at low temperature. Mater. Res. Bull. 2006, 41, 183–190. [Google Scholar] [CrossRef]
- Menetrey, M.; Markovits, A.; Minot, C. Reactivity of a reduced metal oxide surface: Hydrogen, water and carbon monoxide adsorption on oxygen defective rutile TiO2 (110). Surf. Sci. 2003, 524, 49–62. [Google Scholar] [CrossRef]
- Misra, P.; Shukla, R.; Bali, L.; Gupta, C.; Dubey, G. EPR studies of some thick film ferrates, aluminates and their correlation with humidity sensitivity. Sens. Actuators B Chem. 2003, 94, 210–215. [Google Scholar] [CrossRef]
- Chen, N.-S.; Yang, X.-J.; Liu, E.-S.; Huang, J.-L. Reducing gas-sensing properties of ferrite compounds MFe2O4 (M=Cu, Zn, Cd and Mg). Sens. Actuators B Chem. 2000, 66, 178–180. [Google Scholar] [CrossRef]
- Fu, X.; Wang, C.; Yu, H.; Wang, Y.; Wang, T. Fast humidity sensors based on CeO2 nanowires. Nanotechnology 2007, 18, 145503. [Google Scholar] [CrossRef]
- Ramesh, B.; Ramesh, S.; Kumar, R.V.; Rao, M.L. Ac impedance studies on LiFe5−xMnxO8 ferrites. J. Alloys Compd. 2012, 513, 289–293. [Google Scholar] [CrossRef]
- Hashim, M.; Kumar, S.; Ali, S.; Koo, B.; Chung, H.; Kumar, R. Structural, magnetic and electrical properties of Al3+ substituted Ni–Zn ferrite nanoparticles. J. Alloys Compd. 2012, 511, 107–114. [Google Scholar] [CrossRef]
- Du, J.; Liu, Y.; Yao, G.; Long, X.; Zu, G.; Ma, J. Influence of MnO2 on the sintering behavior and magnetic properties of NiFe2O4 ferrite ceramics. J. Alloys Compd. 2012, 510, 87–91. [Google Scholar] [CrossRef]
- Khan, M.A.; Islam, M.; Ishaque, M.; Rahman, I. Magnetic and dielectric behavior of terbium substituted Mg1−xTbxFe2O4 ferrites. J. Alloys Compd. 2012, 519, 156–160. [Google Scholar] [CrossRef]
- Shinde, T.; Gadkari, A.; Vasambekar, P. Influence of Nd3+ substitution on structural, electrical and magnetic properties of nanocrystalline nickel ferrites. J. Alloys Compd. 2012, 513, 80–85. [Google Scholar] [CrossRef]
- Li, J.; Yu, Z.; Sun, K.; Jiang, X.; Xu, Z.; Lan, Z. Grain growth kinetics and magnetic properties of Ni–Zn ferrite thin films. J. Alloys Compd. 2012, 513, 606–609. [Google Scholar] [CrossRef]
- Birajdar, D.; Mane, D.; More, S.; Kawade, V.; Jadhav, K. Structural and magnetic properties of ZnxCu1.4−xMn0.4Fe1.2O4 ferrites. Mater. Lett. 2005, 59, 2981–2985. [Google Scholar] [CrossRef]
- Suryavanshi, S.; Patil, R.; Patil, S.; Sawant, S. Dc conductivity and dielectric behavior of Ti4+ substituted Mg–Zn ferrites. J. Less Common Met. 1991, 168, 169–174. [Google Scholar] [CrossRef]
- Reddy, M.B.; Reddy, P.V. Electrical conductivity of some mixed Li–Ti ferrites. Phys. Status Solidi A 1990, 120, 575–580. [Google Scholar] [CrossRef]
- Meaz, T.; Attia, S.; El Ata, A.A. Effect of tetravalent titanium ions substitution on the dielectric properties of Co–Zn ferrites. J. Magn. Magn. Mater. 2003, 257, 296–305. [Google Scholar] [CrossRef]
- Prakash, C.; Baijal, J. Dielectric behaviour of tetravalent titanium-substituted Ni–Zn ferrites. J. Less Common Met. 1985, 107, 51–57. [Google Scholar] [CrossRef]
- Suryavanshi, S.; Patil, S.; Jadhav, C.; Sawant, S. Xrd and magnetic studies on Ti-substituted Mg–Zn ferrites. Mater. Res. Bull. 1989, 24, 1201–1206. [Google Scholar] [CrossRef]
- Farea, A.; Kumar, S.; Batoo, K.M.; Yousef, A.; Lee, C.G. Influence of the doping of Ti4+ ions on electrical and magnetic properties of Mn1+xFe2−2xTixO4 ferrite. J. Alloys Compd. 2009, 469, 451–457. [Google Scholar] [CrossRef]
- Tripathy, A.; Pramanik, S.; Manna, A.; Bhuyan, S.; Azrin Shah, N.F.; Radzi, Z.; Abu Osman, N.A. Design and development for capacitive humidity sensor applications of lead-free Ca, Mg, Fe, Ti-oxides-based electro-ceramics with improved sensing properties via physisorption. Sensors 2016, 16, 1135. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, S.; Mohd Hanif, A.S.; Pingguan-Murphy, B.; Abu Osman, N.A. Morphological change of heat treated bovine bone: A comparative study. Materials 2013, 6, 65–75. [Google Scholar] [CrossRef]
- Tripathy, A.; Pramanik, S.; Manna, A.; Azrin Shah, N.F.; Shasmin, H.N.; Radzi, Z.; Abu Osman, N.A. Synthesis and characterizations of novel Ca-Mg-Ti-Fe-oxides based ceramic nanocrystals and flexible film of polydimethylsiloxane composite with improved mechanical and dielectric properties for sensors. Sensors 2016, 16, 292. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, S.; Ataollahi, F.; Pingguan-Murphy, B.; Oshkour, A.A.; Abu Osman, N.A. In vitro study of surface modified poly (ethylene glycol)-impregnated sintered bovine bone scaffolds on human fibroblast cells. Sci. Rep. 2015, 5, 9806. [Google Scholar] [CrossRef] [PubMed]
- Doroftei, C.; Popa, P.; Rezlescu, N. The influence of the heat treatment on the humidity sensitivity of magnesium nanoferrite. J. Optoelectron. Adv. Mater. 2010, 12, 881–884. [Google Scholar]
- Pramanik, S.; Pingguan-Murphy, B.; Cho, J.; Abu Osman, N.A. Design and development of potential tissue engineering scaffolds from structurally different longitudinal parts of a bovine-femur. Sci. Rep. 2014, 4, 5843. [Google Scholar] [CrossRef] [PubMed]
- Ataollahi, F.; Pramanik, S.; Moradi, A.; Dalilottojari, A.; Pingguan-Murphy, B.; Wan Abas, W.A.B.; Abu Osman, N.A. Endothelial cell responses in terms of adhesion, proliferation, and morphology to stiffness of polydimethylsiloxane elastomer substrates. J. Biomed. Mater. Res. Part A 2015, 103, 2203–2213. [Google Scholar] [CrossRef] [PubMed]
- Chuang, S.-H.; Gao, R.-H.; Wang, D.-Y.; Liu, H.-P.; Chen, L.-M.; Chiang, M.Y. Synthesis and characterization of ilmenite-type cobalt titanate powder. J. Chin. Chem. Soc. 2010, 57, 932–937. [Google Scholar] [CrossRef]
- Yazawa, Y.; Yamaguchi, A.; Takeda, H. Lunar minerals and their resource utilization with particular reference to solar power satellites and potential roles for humic substances for lunar agriculture. In Moon; Springer: Berlin, Germany, 2012; pp. 105–138. [Google Scholar]
- Usman, M.; Rasool, K.; Batool, S.; Imran, Z.; Ahmad, M.; Jamil, H.; Rafiq, M.; Hasan, M. Humidity effect on transport properties of titanium dioxide nanoparticles. J. Mater. Sci. Technol. 2014, 30, 748–752. [Google Scholar] [CrossRef]
- Hu, S.; Fu, G. Humidity-sensitive properties based on liquid state LiZnVO4-doped SnO2. Sens. Actuators A Phys. 2010, 163, 481–485. [Google Scholar] [CrossRef]
- Pandey, N.; Tiwari, K. Morphological and relative humidity sensing properties of pure ZnO nanomaterial. Sens. Transducers 2010, 122, 9. [Google Scholar]
- Imran, Z.; Batool, S.; Jamil, H.; Usman, M.; Israr-Qadir, M.; Shah, S.; Jamil-Rana, S.; Rafiq, M.; Hasan, M.; Willander, M. Excellent humidity sensing properties of cadmium titanate nanofibers. Ceram. Int. 2013, 39, 457–462. [Google Scholar] [CrossRef]
- Aoki, H.; Azuma, Y.; Asaka, T.; Higuchi, M.; Asaga, K.; Katayama, K. Improvement of response characteristics of TiO2 humidity sensors by simultaneous addition of Li2O and V2O5. Ceram. Int. 2008, 34, 819–822. [Google Scholar] [CrossRef]
- Yuk, J.; Troczynski, T. Sol–gel batio3 thin film for humidity sensors. Sens. Actuators B Chem. 2003, 94, 290–293. [Google Scholar] [CrossRef]
- Cheng, B.; Ouyang, Z.; Tian, B.; Xiao, Y.; Lei, S. Porous ZnAl2O4 spinel nanorods: High sensitivity humidity sensors. Ceram. Int. 2013, 39, 7379–7386. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, K.; Jiang, D.; Zhu, Z.; Geng, H.; Luo, L. Zinc oxide nanorod and nanowire for humidity sensor. Appl. Surf. Sci. 2005, 242, 212–217. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, R.; Geng, W.; Li, X.; Qi, Q.; He, Y.; Wang, S. Study on humidity sensing properties based on composite materials of Li-doped mesoporous silica A-SBA-15. Sens. Actuators B Chem. 2008, 128, 482–487. [Google Scholar] [CrossRef]
- Doroftei, C.; Popa, P.; Iacomi, F. Study of the influence of nickel ions substitutes in barium stannates used as humidity resistive sensors. Sens. Actuators A Phys. 2012, 173, 24–29. [Google Scholar] [CrossRef]
- Wang, L.; Li, D.; Wang, R.; He, Y.; Qi, Q.; Wang, Y.; Zhang, T. Study on humidity sensing property based on Li-doped mesoporous silica MCM-41. Sens. Actuators B Chem. 2008, 133, 622–627. [Google Scholar] [CrossRef]
- Yuan, Q.; Li, N.; Tu, J.; Li, X.; Wang, R.; Zhang, T.; Shao, C. Preparation and humidity sensitive property of mesoporous ZnO–SiO2 composite. Sens. Actuators B Chem. 2010, 149, 413–419. [Google Scholar] [CrossRef]
- He, Y.; Zhang, T.; Zheng, W.; Wang, R.; Liu, X.; Xia, Y.; Zhao, J. Humidity sensing properties of batio3 nanofiber prepared via electrospinning. Sens. Actuators B Chem. 2010, 146, 98–102. [Google Scholar] [CrossRef]
- Wang, J.; Xu, B.K.; Ruan, S.P.; Wang, S.P. Preparation and electrical properties of humidity sensing films of BaTiO3/polystrene sulfonic sodium. Mater. Chem. Phys. 2003, 78, 746–750. [Google Scholar] [CrossRef]
- Agmon, N. The grotthuss mechanism. Chem. Phys. Lett. 1995, 244, 456–462. [Google Scholar] [CrossRef]
- Nahar, R. Study of the performance degradation of thin film aluminum oxide sensor at high humidity. Sens. Actuators B Chem. 2000, 63, 49–54. [Google Scholar] [CrossRef]
- Gerhardt, R. Impedance and dielectric spectroscopy revisited: Distinguishing localized relaxation from long-range conductivity. J. Phys. Chem. Solids 1994, 55, 1491–1506. [Google Scholar] [CrossRef]
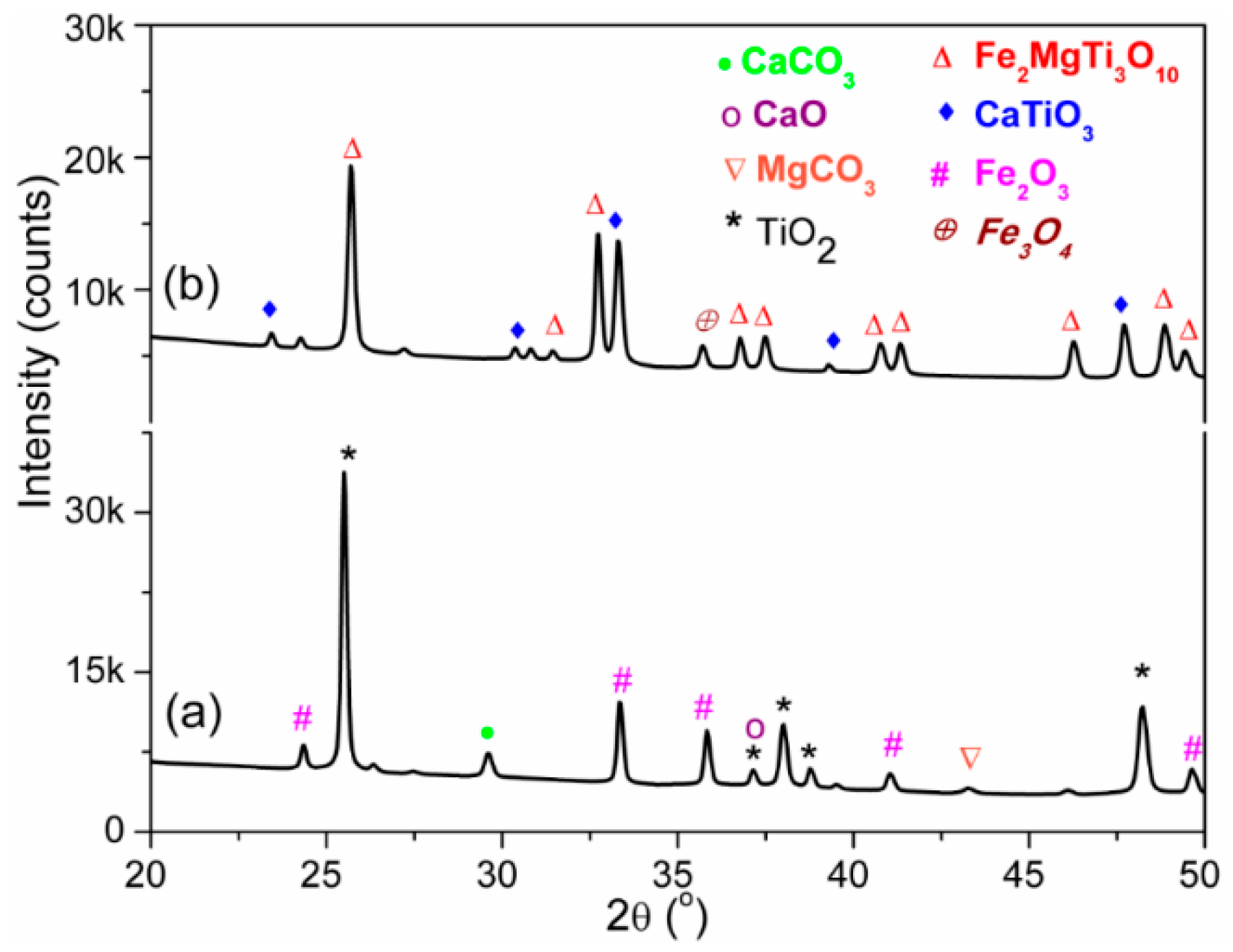
| Crystalline Phase | Area under XRD Peak (s) | Phase Concentration (%) |
|---|---|---|
| Fe2MgTi3O10 | 140,066 | 85.80 |
| CaTiO3 | 19,857 | 12.16 |
| Fe3O4 | 3319 | 2.04 |
| Lattice Parameters | Fe2MgTi3O10 (Calculated) | PDF No. 00-013-0353 (Standard) | CaTiO3 (Calculated) | PDF No. 00-008-0092 (Standard) |
|---|---|---|---|---|
| a (nm) | 0.9658 | 0.9770 | 1.5164 | 1.5250 |
| b (nm) | 1.0046 | 0.9950 | - | - |
| c (nm) | 0.3725 | 0.3730 | - | - |
| Volume (nm3) | 0.3614 | 0.3626 | 3.4869 | 3.5466 |
| Lattice volume strain (%) | −0.3309 | - | −1.6833 | - |
| Crystal system | Orthorhombic | Orthorhombic | Cubic | Cubic |
| Crystallite size (nm) | 20.7 | - | 5.9 | - |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tripathy, A.; Pramanik, S.; Manna, A.; Shasmin, H.N.; Radzi, Z.; Abu Osman, N.A. Uniformly Porous Nanocrystalline CaMgFe1.33Ti3O12 Ceramic Derived Electro-Ceramic Nanocomposite for Impedance Type Humidity Sensor. Sensors 2016, 16, 2029. https://doi.org/10.3390/s16122029
Tripathy A, Pramanik S, Manna A, Shasmin HN, Radzi Z, Abu Osman NA. Uniformly Porous Nanocrystalline CaMgFe1.33Ti3O12 Ceramic Derived Electro-Ceramic Nanocomposite for Impedance Type Humidity Sensor. Sensors. 2016; 16(12):2029. https://doi.org/10.3390/s16122029
Chicago/Turabian StyleTripathy, Ashis, Sumit Pramanik, Ayan Manna, Hanie Nadia Shasmin, Zamri Radzi, and Noor Azuan Abu Osman. 2016. "Uniformly Porous Nanocrystalline CaMgFe1.33Ti3O12 Ceramic Derived Electro-Ceramic Nanocomposite for Impedance Type Humidity Sensor" Sensors 16, no. 12: 2029. https://doi.org/10.3390/s16122029
APA StyleTripathy, A., Pramanik, S., Manna, A., Shasmin, H. N., Radzi, Z., & Abu Osman, N. A. (2016). Uniformly Porous Nanocrystalline CaMgFe1.33Ti3O12 Ceramic Derived Electro-Ceramic Nanocomposite for Impedance Type Humidity Sensor. Sensors, 16(12), 2029. https://doi.org/10.3390/s16122029





