A Programmable High-Voltage Compliance Neural Stimulator for Deep Brain Stimulation in Vivo
Abstract
:1. Introduction

2. Materials and Methods
2.1. Schematic of in Vivo Deep Brain Stimulation (DBS)

2.2. Design of Stimulator with High-Voltage Compliance
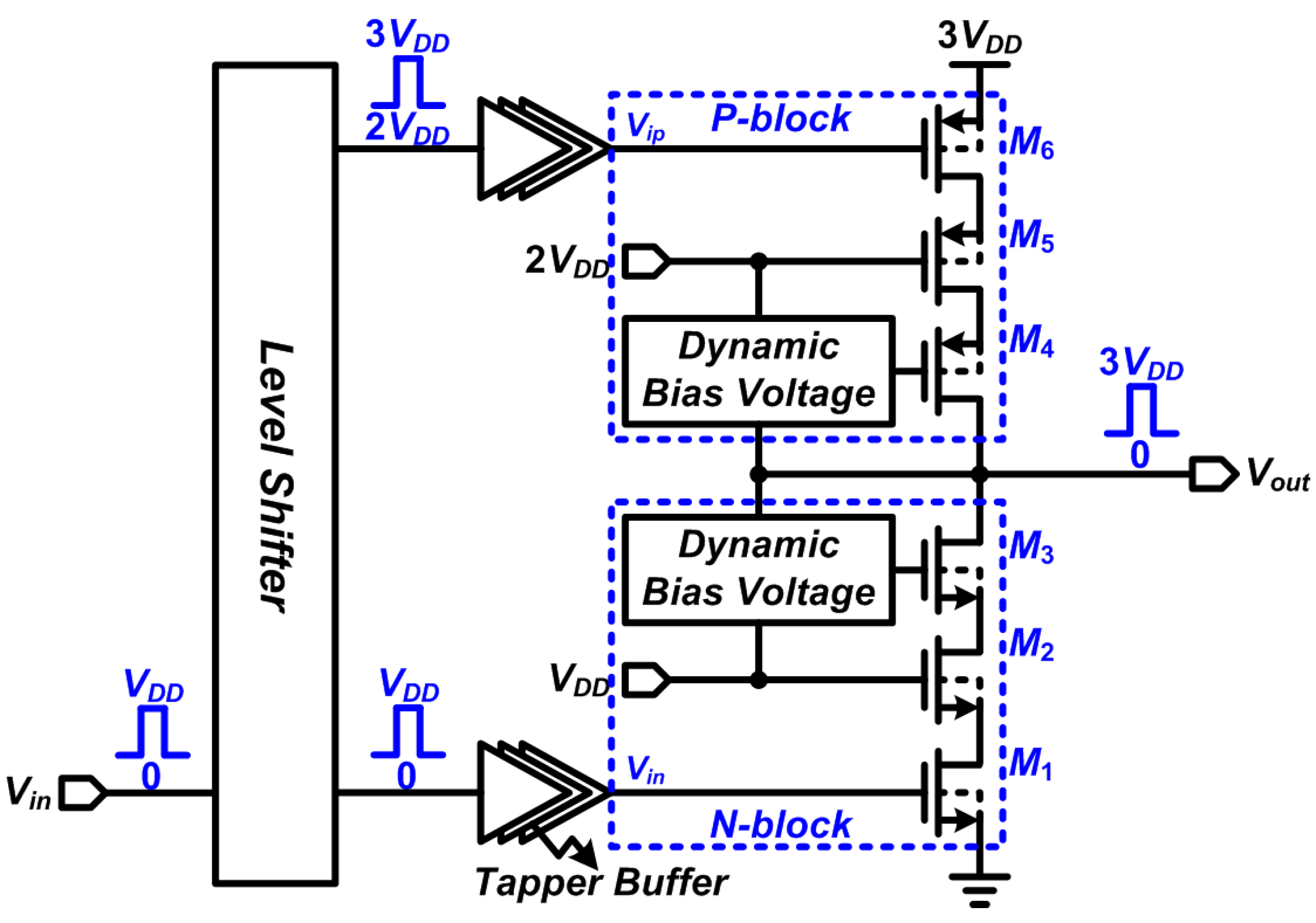
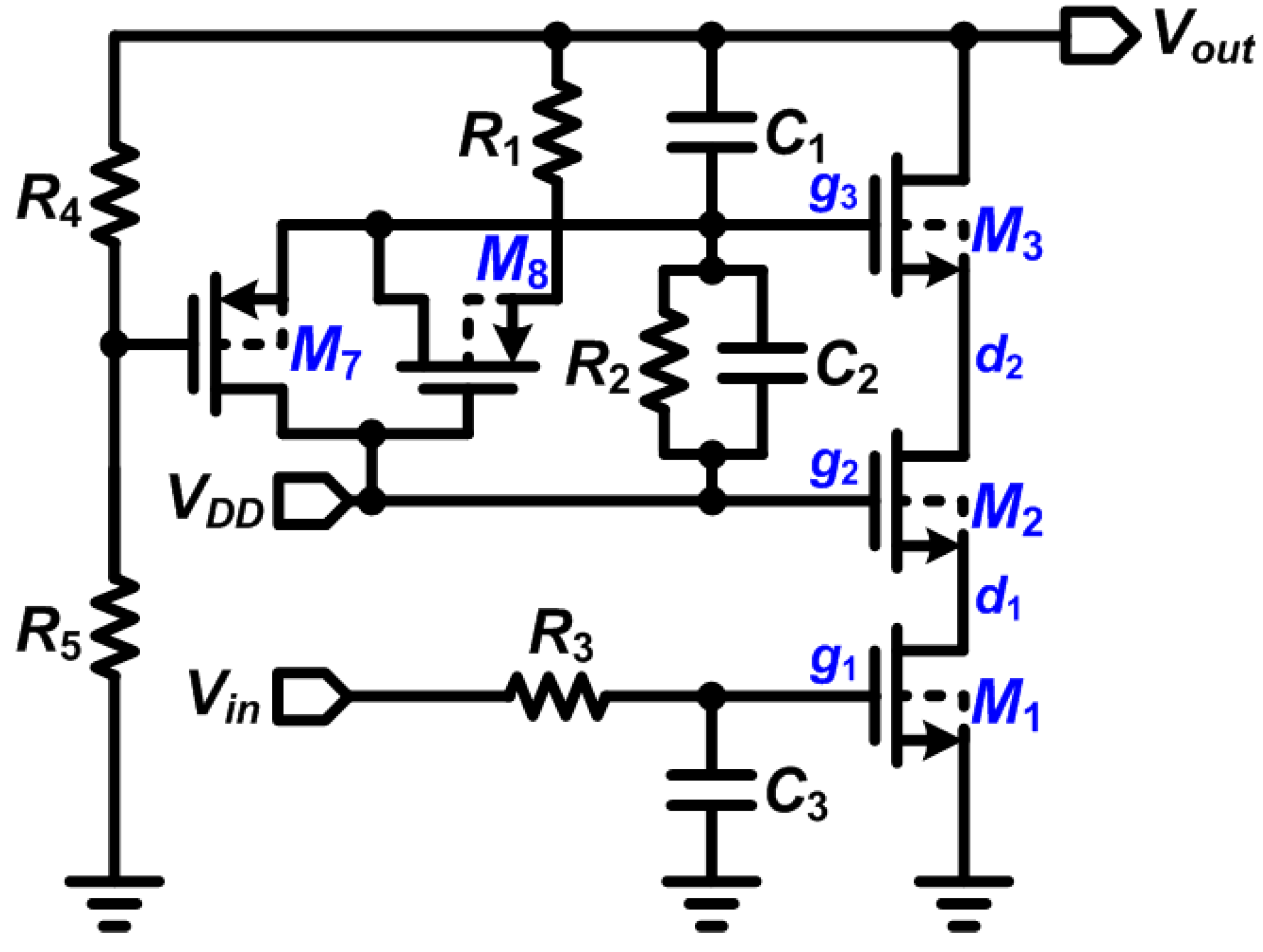
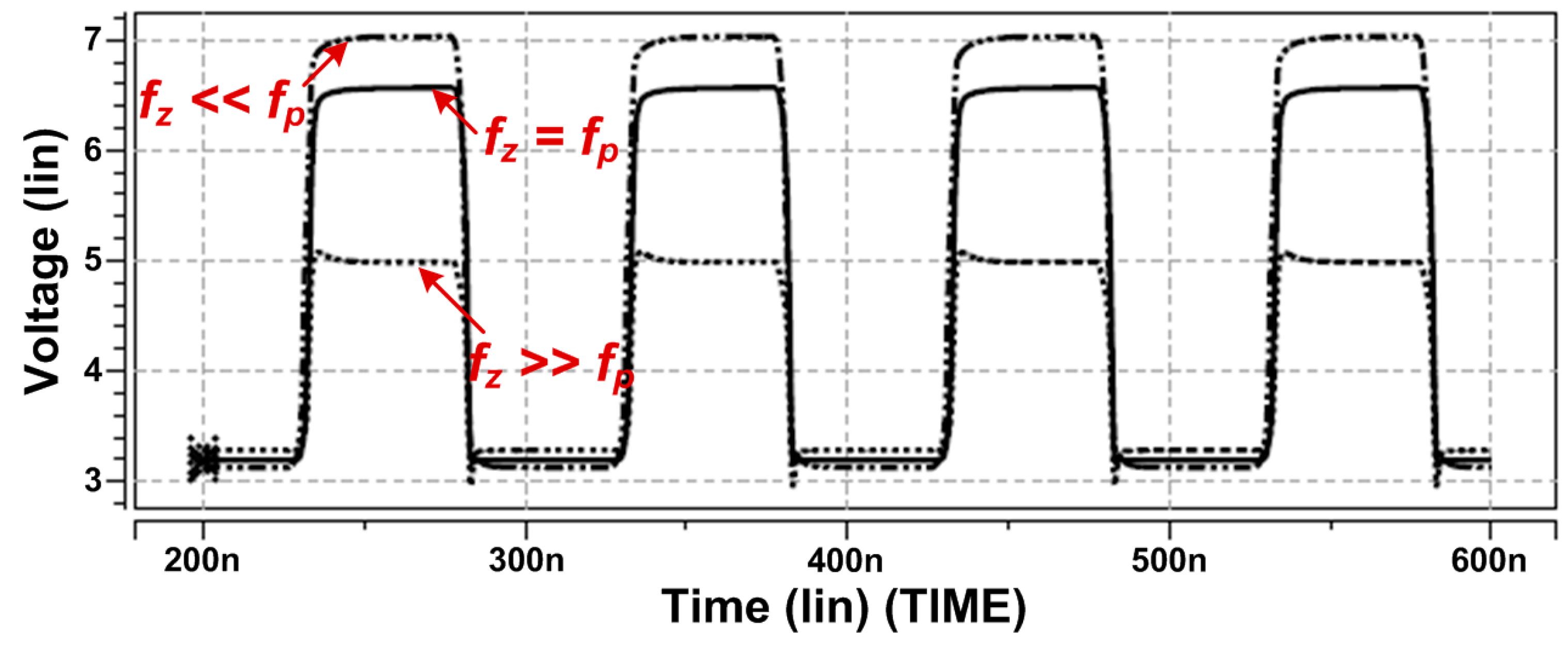
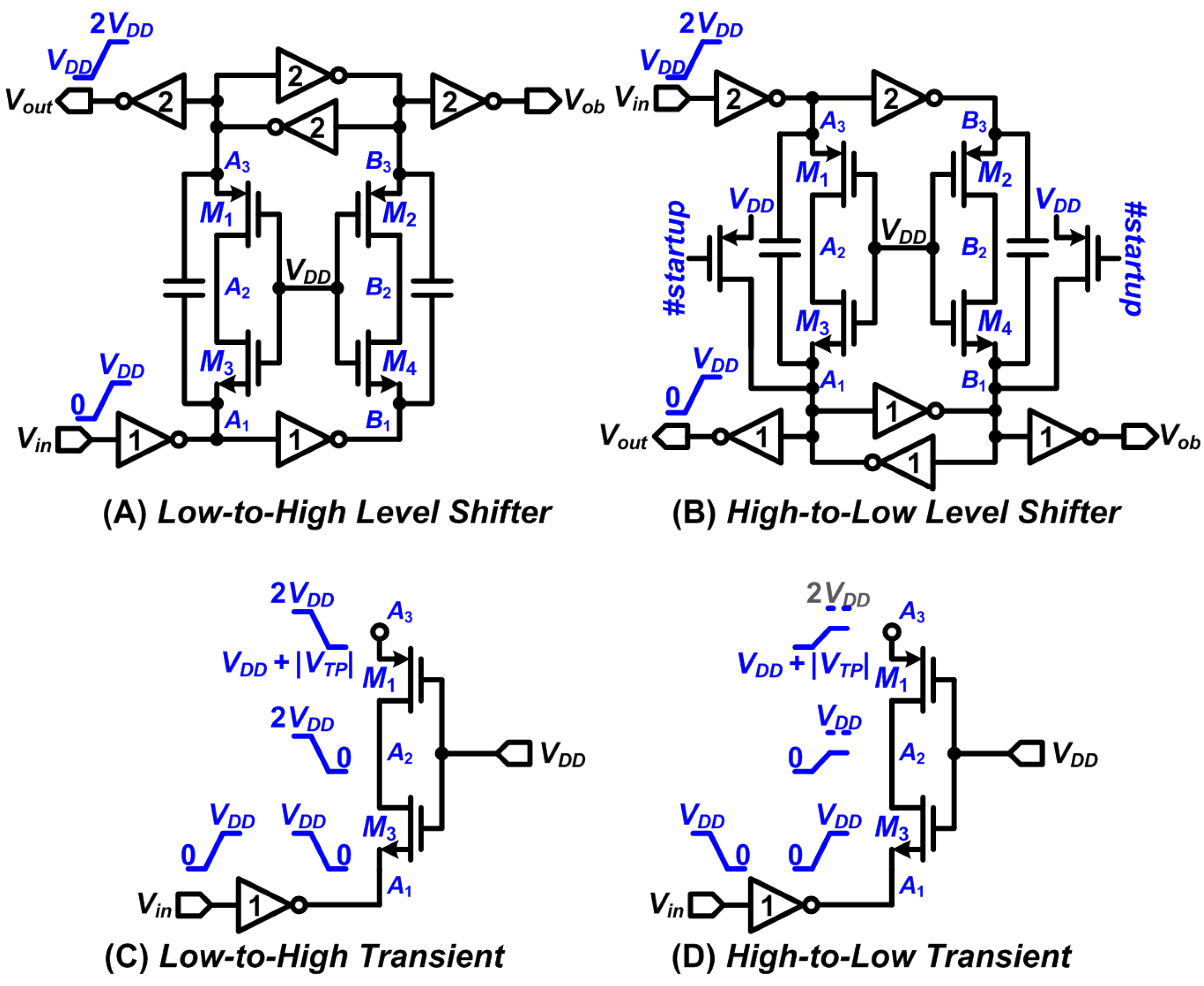
2.3. The DBS Platform
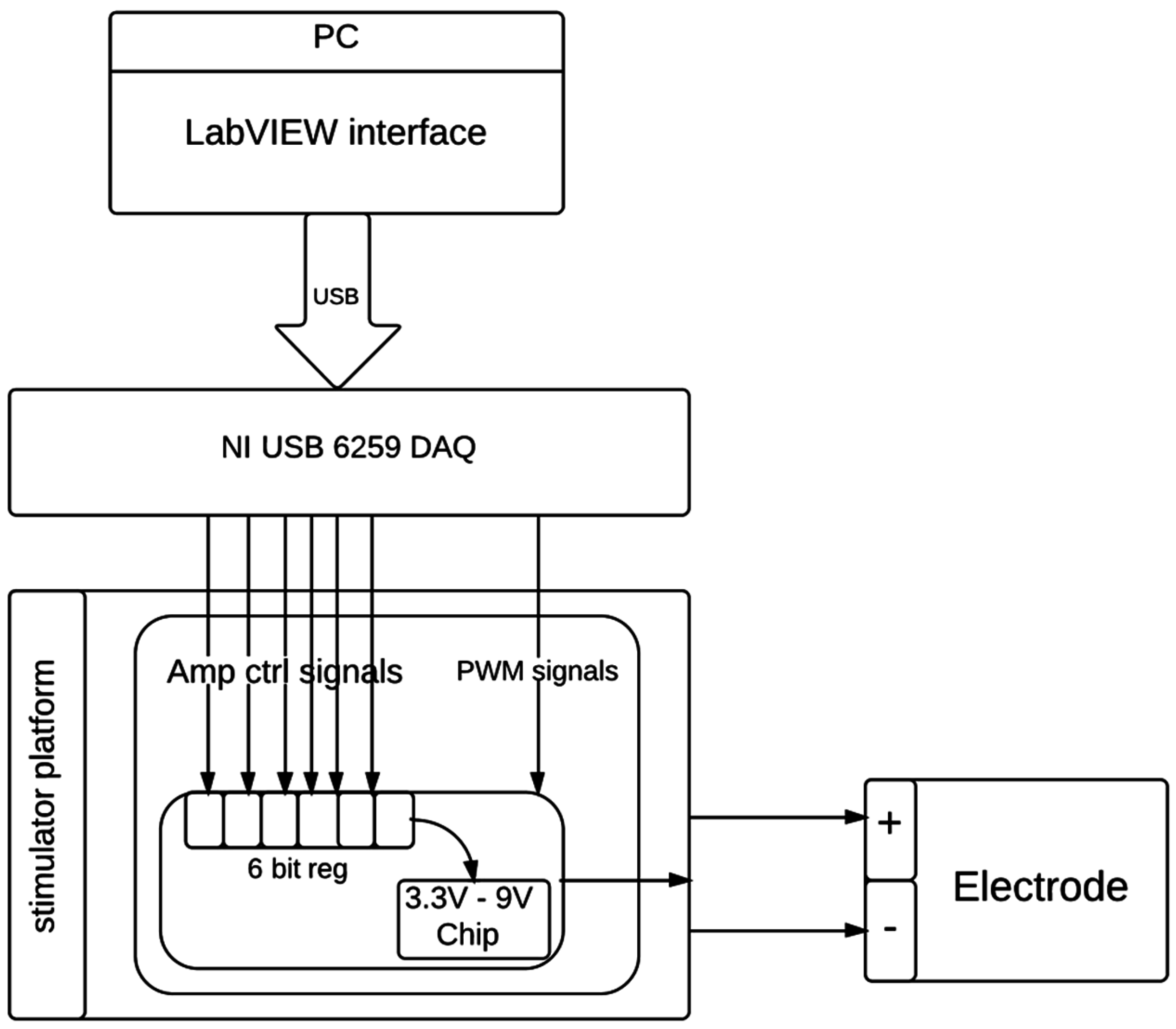
2.4. In Vivo Thalamic Stimuli and in Vivo Impedance Measurement
2.5. Electrophysiological Data Analysis
3. Results and Discussion
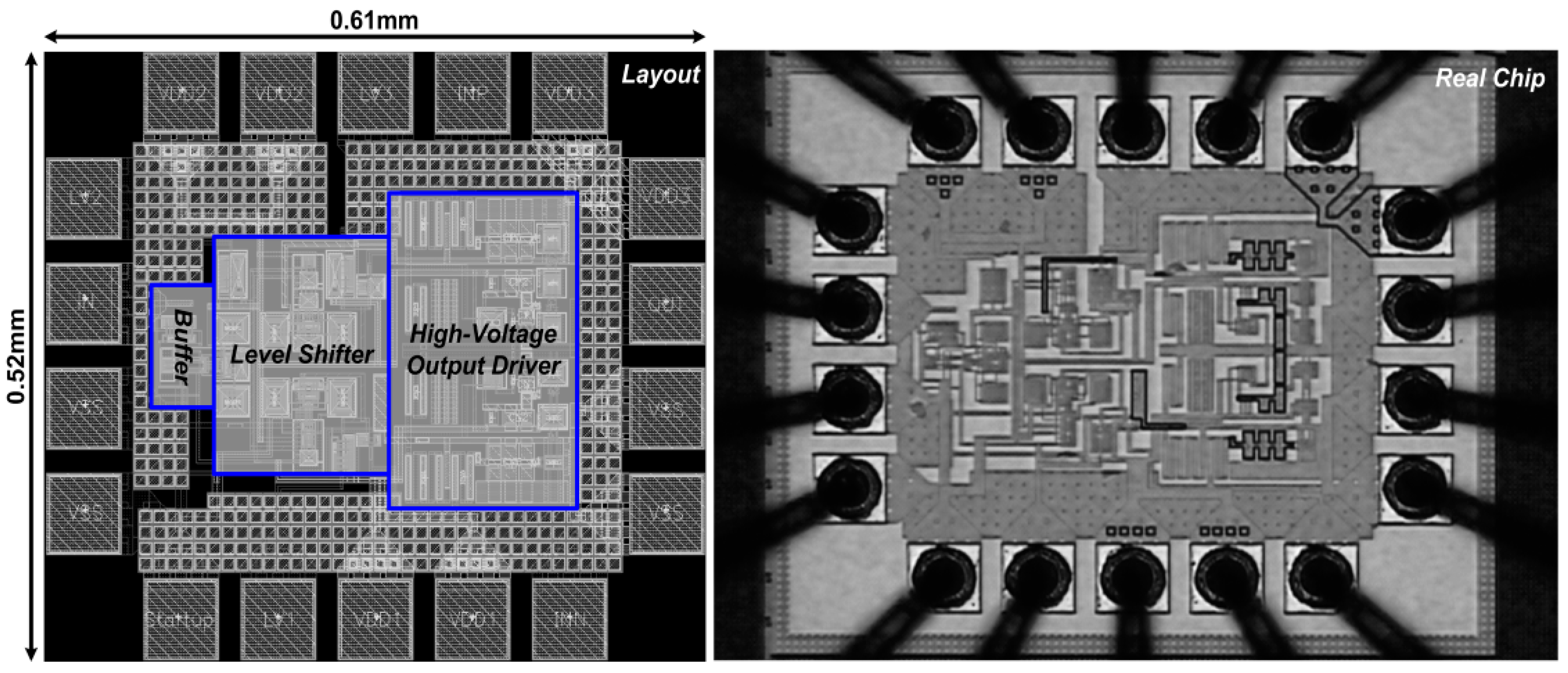
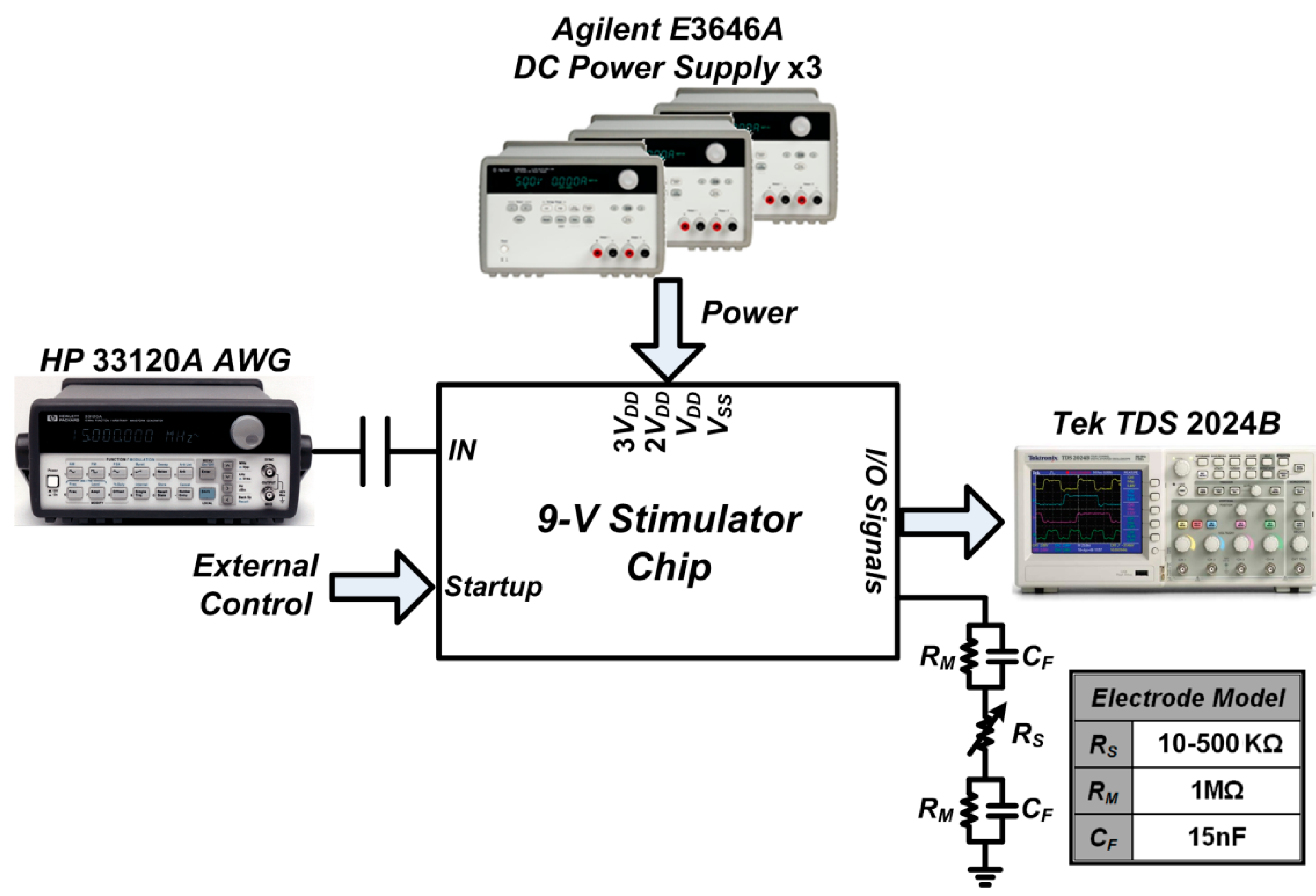
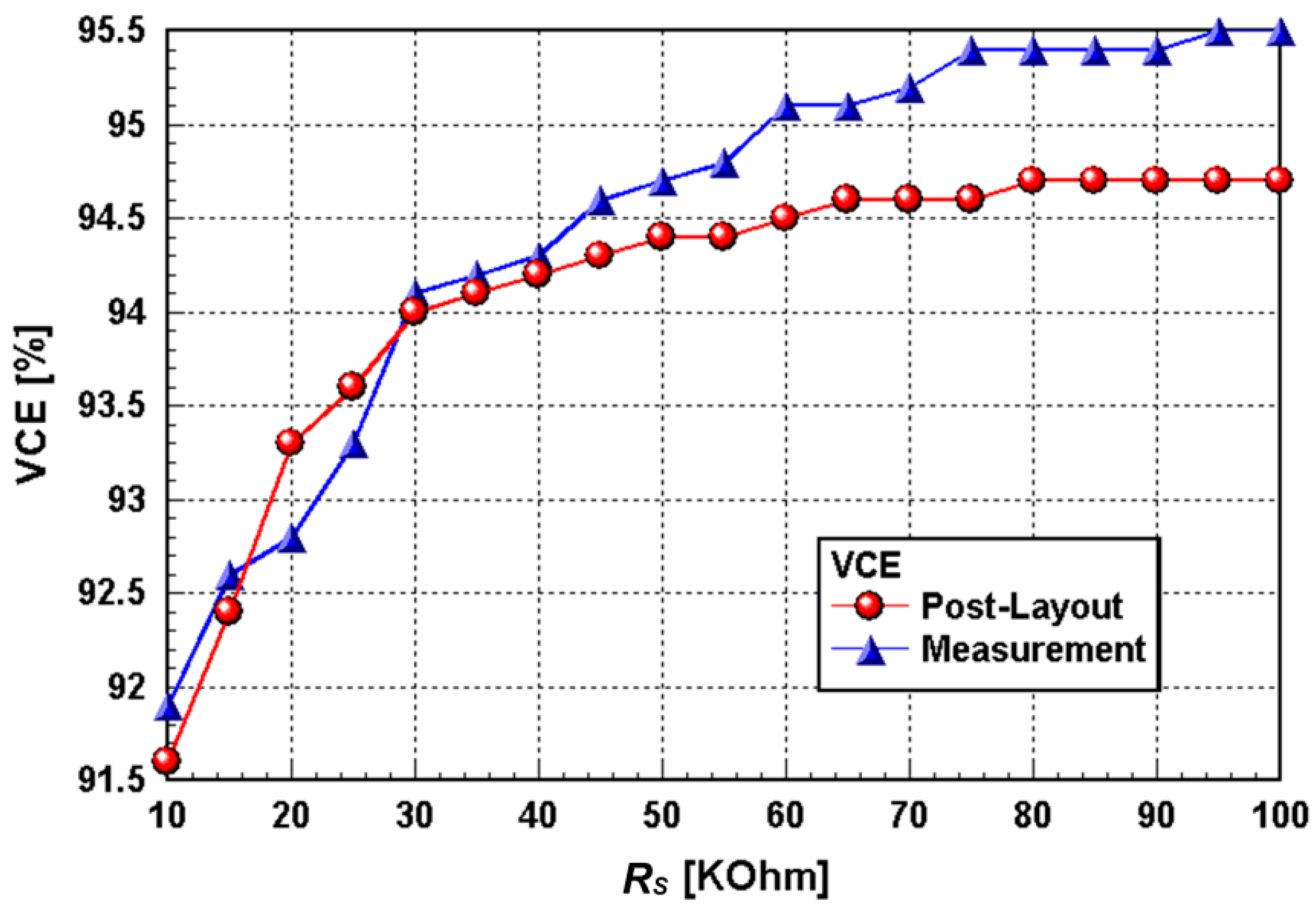
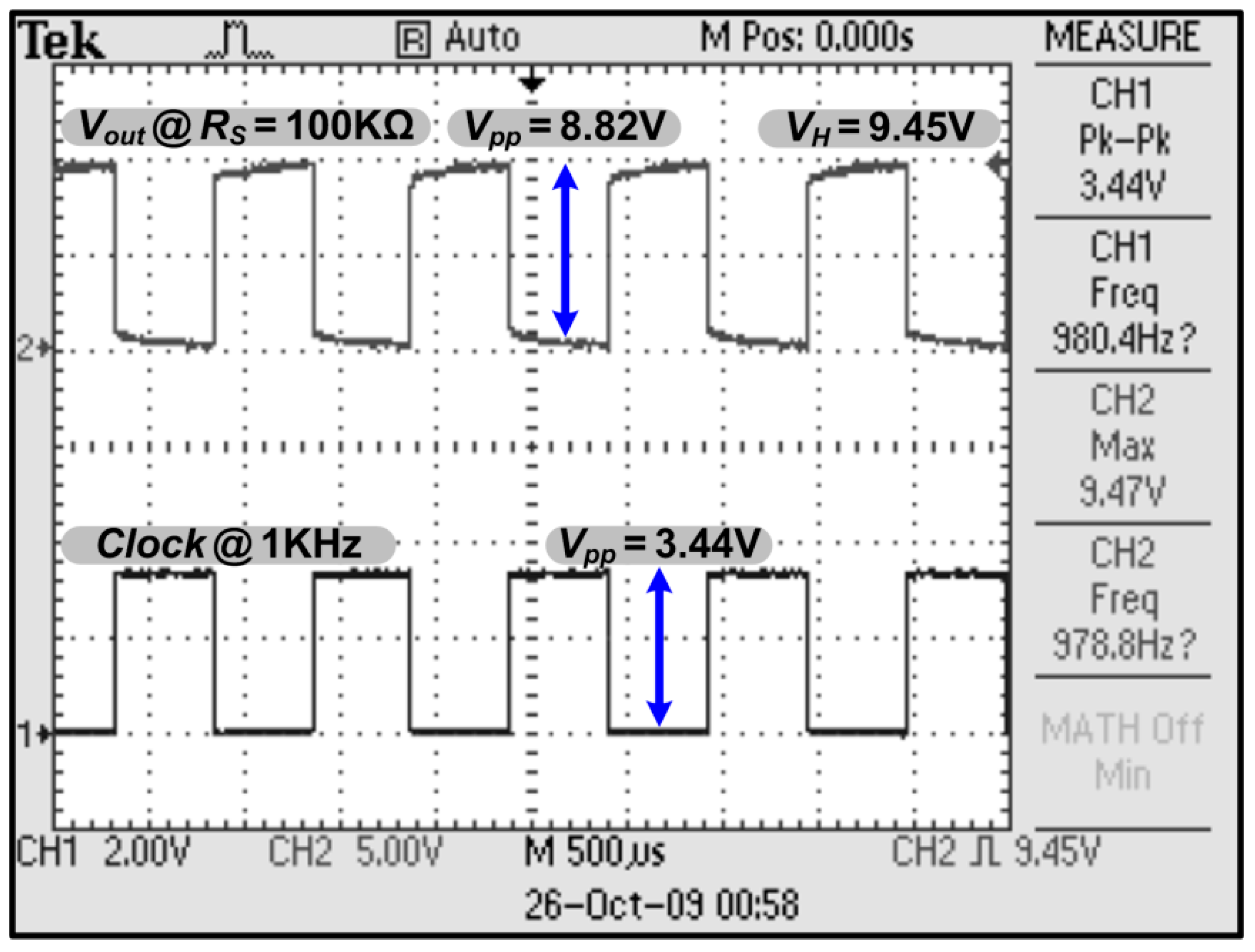
3.1. Chip Testing and Characterization
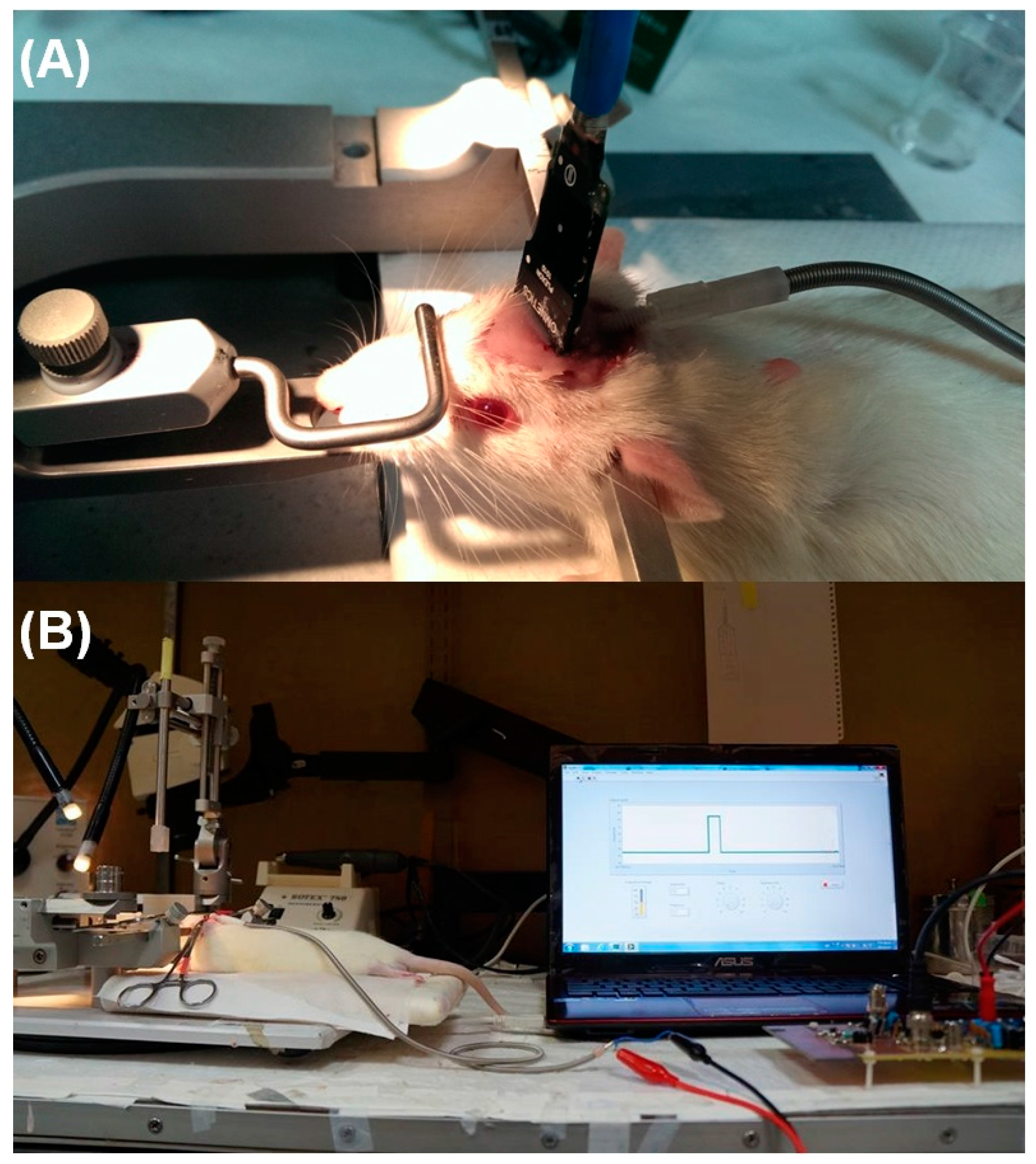
3.2. In Vivo Stimuli in Rat Thalamus Using the Prototype DBS System
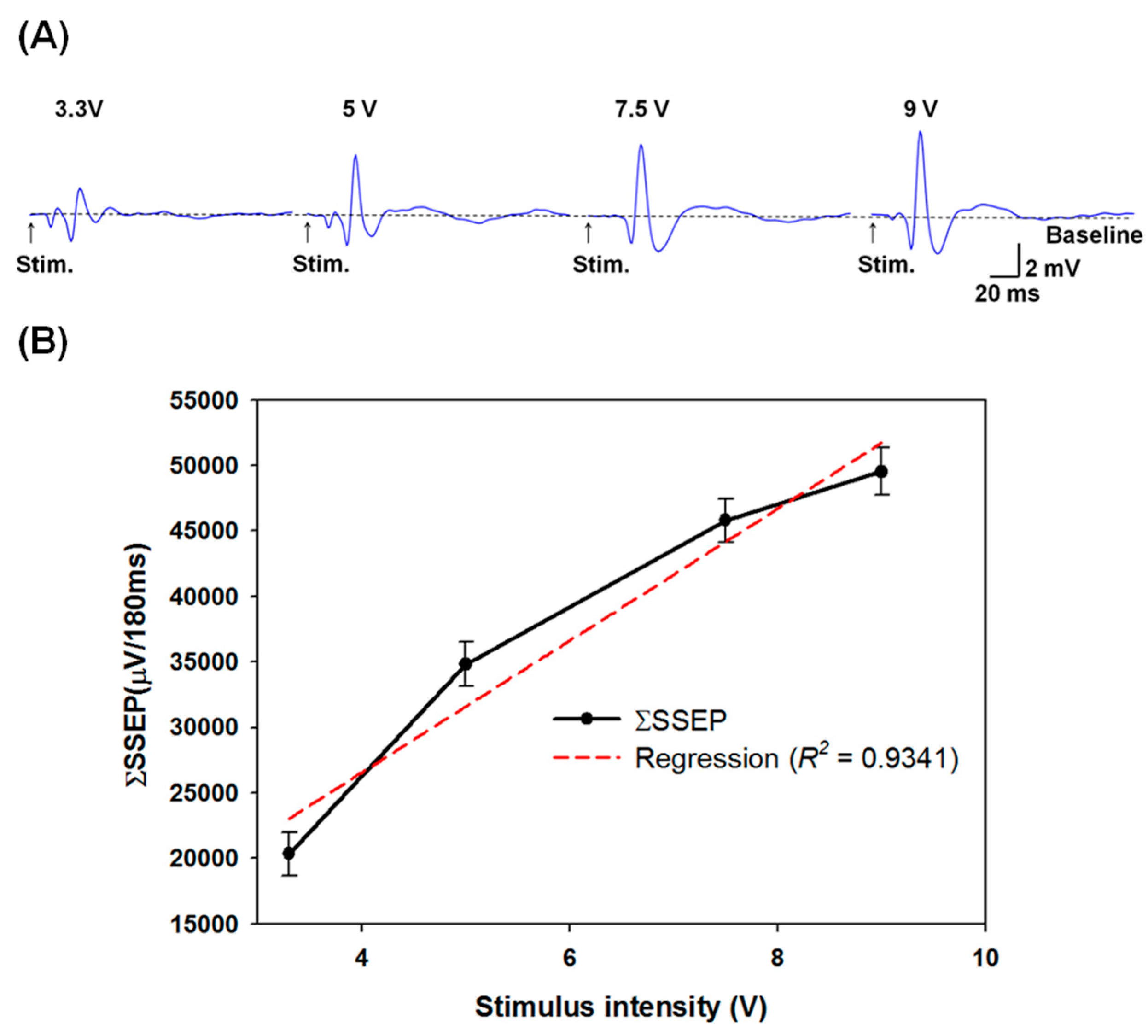
3.3. Grading Thalamic Stimuli Induced Somatosensory Evoked Potentials
3.4. In Vivo Electrochemical Characterization for Electrode-Electrolyte Interface and the Evaluation of its Effect on the Stimulator
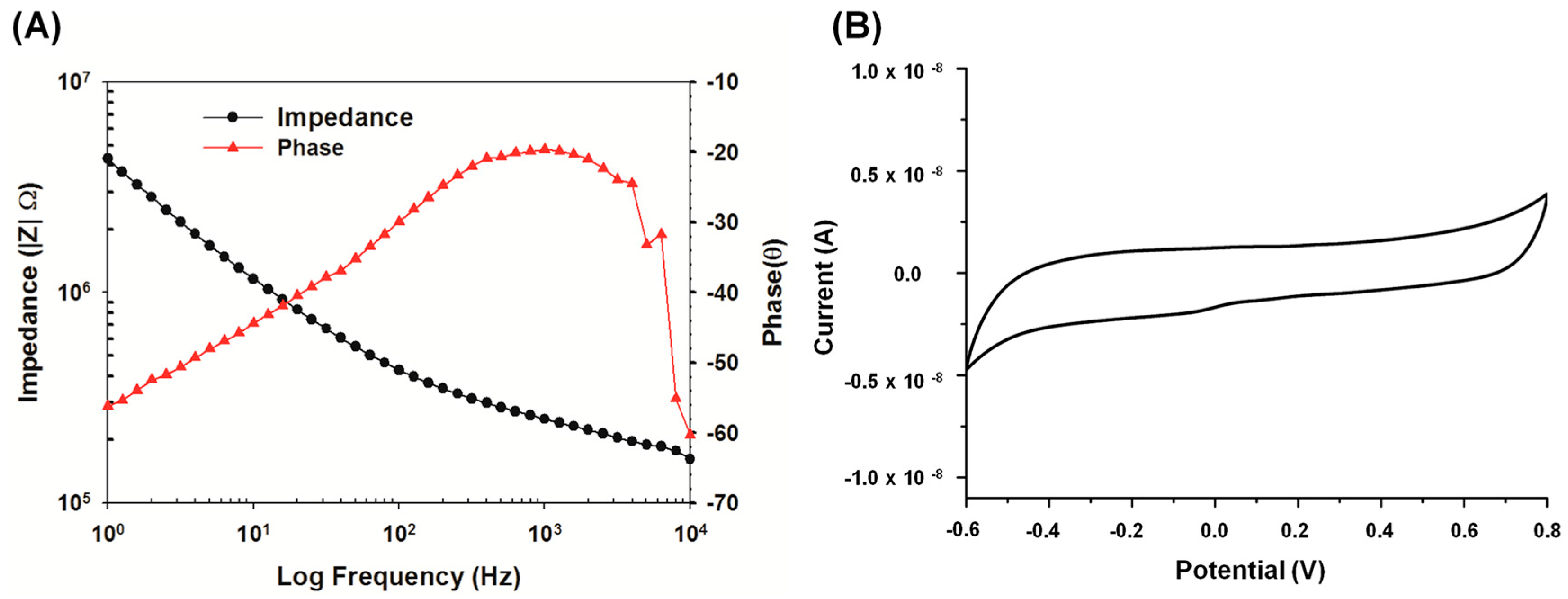
3.5. Advantages and Discussions of the Proposed System
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Incerti, P.V.; Ching, T.Y.; Cowan, R. A systematic review of electric-acoustic stimulation: Device fitting ranges, outcomes, and clinical fitting practices. Trends Amplif. 2013, 17, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Niparko, J.K.; Wilson, B.W. History of cochlear implants. In Cochlear Implants: Principles and Practices; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2000; pp. 103–108. [Google Scholar]
- North, R.B. Neural interface devices: Spinal cord stimulation technology. IEEE Proc. 2008, 96, 1108–1119. [Google Scholar] [CrossRef]
- Velez, F.G.; Isobe, J.; Zealear, D.; Judy, J.W.; Edgerton, V.R.; Patnode, S.; Lee, H.; Hahn, B.T. Toward an implantable functional electrical stimulation device to correct strabismus. J. AAPOS 2009, 13, 229–35 e1. [Google Scholar] [CrossRef] [PubMed]
- Morishita, T.; Fayad, S.M.; Higuchi, M.A.; Nestor, K.A.; Foote, K.D. Deep brain stimulation for treatment-resistant depression: Systematic review of clinical outcomes. Neurotherapeutics 2014, 11, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Overbeek, J.M.; de Koning, P.; Luigjes, J.; van den Munckhof, P.; Schuurman, P.R.; Denys, D. Deep brain stimulation for psychiatric disorders. Ned. Tijdschr. Geneeskd. 2013, 157, A7015. [Google Scholar] [PubMed]
- Williams, N.R.; Okun, M.S. Deep brain stimulation (DBS) at the interface of neurology and psychiatry. J. Clin. Investig. 2013, 123, 4546–4556. [Google Scholar] [CrossRef] [PubMed]
- Schlaepfer, T.E.; Bewernick, B.H. Neuromodulation for treatment resistant depression: State of the art and recommendations for clinical and scientific conduct. Brain Topogr. 2014, 27, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Dommel, N.; Wong, Y.T.; Preston, P.J.; Lehmann, T.; Lovell, N.H.; Suaning, G.J. The design and testing of an epi-retinal vision prosthesis neurostimulator capable of concurrent parallel stimulation. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2006, 1, 4700–4709. [Google Scholar] [PubMed]
- Ortmanns, M.; Unger, N.; Rocke, A.; Gehrke, M.; Tietdke, H.J. A 0.1 mm/sup 2/, digitally programmable nerve stimulation pad cell with high-voltage capability for a retinal implant. In Proceedings of the IEEE International ISSCC Conference, San Francisco, CA, USA, 6–9 February 2006; pp. 89–98.
- Ethier, S.; Sawan, M.; Aboulhamid, E.M.; el-Gamal, M. A ±9 v fully integrated CMOS electrode driver for high-impedance microstimulation. In Proceedingss of the 52nd IEEE International Midwest Symposium on Circuits and Systems, Cancun, Mexico, 2–5 August 2009; pp. 192–195.
- Nadeau, P.; Sawan, M. A flexible high voltage biphasic current-controlled stimulator. In Proceedingss of the IEEE BIOCAS Conference, London, UK, 29 November–1 December 2006; pp. 206–209.
- Noorsal, E.; Sooksood, K.; Hongcheng, X.; Hornig, R.; Becker, J.; Ortmanns, M. A neural stimulator frontend with high-voltage compliance and programmable pulse shape for epiretinal implants. IEEE J. Solid State Circuits 2012, 47, 244–256. [Google Scholar] [CrossRef]
- Ghovanloo, M. Switched-capacitor based implantable low-power wireless microstimulating systems. In Proceedingss of the IEEE ISCAS Conference, Island of Kos, Greece, 21–24 May 2006; p. 4.
- Yousif, N.; Purswani, N.; Bayford, R.; Nandi, D.; Bain, P.; Liu, X. Evaluating the impact of the deep brain stimulation induced electric field on subthalamic neurons: A computational modelling study. J. Neurosci. Meth. 2010, 188, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.A.; Robinson, B.K.; Rubinstein, J.T.; Abbas, P.J.; Runge-Samuelson, C.L. Auditory nerve responses to monophasic and biphasic electric stimuli. Hear. Res. 2001, 151, 79–94. [Google Scholar] [CrossRef]
- Hao, J.; Junmin, Z.; Di, L.; Chao, K.K.; Shyshenq, L.; Shahnasser, H.; Fechter, R.; Hirose, S.; Harrison, M.; Roy, S. A low-frequency versatile wireless power transfer technology for biomedical implants. IEEE Trans. Biomed. Circuits Syst. 2013, 7, 526–535. [Google Scholar]
- Tierney, T.S.; Abd-El-Barr, M.M.; Stanford, A.D.; Foote, K.D.; Okun, M.S. Deep brain stimulation and ablation for obsessive compulsive disorder: Evolution of contemporary indications, targets and techniques. Int. J. Neurosci. 2014, 124, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Henn, F.A. Circuits, cells, and synapses: Toward a new target for deep brain stimulation in depression. Neuropsychopharmacology 2012, 37, 307–308. [Google Scholar] [CrossRef] [PubMed]
- Van Laere, K.; Nuttin, B.; Gabriels, L.; Dupont, P.; Rasmussen, S.; Greenberg, B.D.; Cosyns, P. Metabolic imaging of anterior capsular stimulation in refractory obsessive-compulsive disorder: A key role for the subgenual anterior cingulate and ventral striatum. J. Nucl. Med. 2006, 47, 740–747. [Google Scholar] [PubMed]
- Kuhn, J.; Grundler, T.O.; Lenartz, D.; Sturm, V.; Klosterkotter, J.; Huff, W. Deep brain stimulation for psychiatric disorders. Dtsch. Arztebl. Int. 2010, 107, 105–113. [Google Scholar] [PubMed]
- Hemm, S.; Vayssiere, N.; Mennessier, G.; Cif, L.; Zanca, M.; Ravel, P.; Frerebeau, P.; Coubes, P. Evolution of brain impedance in dystonic patients treated by GPI electrical stimulation. Neuromodulation 2004, 7, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Sillay, K.A.; Rutecki, P.; Cicora, K.; Worrell, G.; Drazkowski, J.; Shih, J.J.; Sharan, A.D.; Morrell, M.J.; Williams, J.; Wingeier, B. Long-term measurement of impedance in chronically implanted depth and subdural electrodes during responsive neurostimulation in humans. Brain Stimul. 2013, 6, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Waln, O.; Jimenez-Shahed, J. Constant voltage versus constant current deep brain stimulation: Is impedance fluctuation sufficient to favor constant current stimulation? [abstract]. Mov. Disord. 2014, 29, 1237. [Google Scholar]
- Nowak, L.G.; Bullier, J. Axons, but not cell bodies, are activated by electrical stimulation in cortical gray matter. I. Evidence from chronaxie measurements. Exp. Brain Res. 1998, 118, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Nowak, L.G.; Bullier, J. Axons, but not cell bodies, are activated by electrical stimulation in cortical gray matter. II. Evidence from selective inactivation of cell bodies and axon initial segments. Exp. Brain Res. 1998, 118, 489–500. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, C.C.; Savasta, M.; Kerkerian-Le Goff, L.; Vitek, J.L. Uncovering the mechanism(s) of action of deep brain stimulation: Activation, inhibition, or both. Clin. Neurophysiol. 2004, 115, 1239–1248. [Google Scholar] [CrossRef] [PubMed]
- Serneels, B.; Piessens, T.; Steyaert, M.; Dehaene, W. A high-voltage output driver in a 2.5-v 0.25-μm CMOS technology. IEEE J. Solid State Circuits 2005, 40, 576–583. [Google Scholar] [CrossRef]
- Merrill, D.R.; Bikson, M.; Jefferys, J.G.R. Electrical stimulation of excitable tissue: Design of efficacious and safe protocols. J. Neurosci. Meth. 2005, 141, 171–198. [Google Scholar] [CrossRef] [PubMed]
- Simmons, A.D.; Montgomery, E.B.; Grill, W.M. Excitation Characteristics of Paresthesias Produced by Deep Brain Stimulation. In Proceedings of the IFESS Conference, Cleveland, OH, USA, 14–16 October 2001; p. 71.
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates: Compact, 6th ed.; Academic Press: New York, NY, USA, 2008. [Google Scholar]
- Chang, Y.J.; Liao, L.D.; Lin, C.T.; Lai, H.Y.; Chen, J.L.; Yang, Y.T.; Ting, Y.C.; Huang, Y.P.; Wu, R.; Thakor, N.V.; et al. A low-cost multi-electrode array system for the simultaneous acquisition of electrophysiological signal and cellular morphology. J. Neurosci. Neuroeng. 2012, 1, 131–142. [Google Scholar] [CrossRef]
- Chang, C.W.; Chiou, J.C. Development of a three dimensional neural sensing device by a stacking method. Sensors 2010, 10, 4238–4252. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Lai, H.Y.; Lin, S.H.; Cho, C.W.; Chao, W.H.; Liao, C.H.; Tsang, S.; Chen, Y.F.; Lin, S.Y. Design and fabrication of a polyimide-based microelectrode array: Application in neural recording and repeatable electrolytic lesion in rat brain. J. Neurosci. Meth. 2009, 182, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Butson, C.R.; Maks, C.B.; McIntyre, C.C. Sources and effects of electrode impedance during deep brain stimulation. Clin. Neurophysiol. 2006, 117, 447–544. [Google Scholar] [CrossRef] [PubMed]
- Sawan, M.; Mounaim, F.; Lesbros, G. Wireless monitoring of electrode-tissues interfaces for long term characterization. Analog Integr. Circuits Signal Process. 2008, 55, 103–114. [Google Scholar] [CrossRef]
- Fakhar, K.; Hastings, E.; Butson, C.R.; Foote, K.D.; Zeilman, P.; Okun, M.S. Management of deep brain stimulator battery failure: Battery estimators, charge density, and importance of clinical symptoms. PLoS ONE 2013, 8, e58665. [Google Scholar] [CrossRef] [PubMed]
- Kern, D.S.; Kumar, R. Deep brain stimslation. Neurologist 2007, 13, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Boex, C.; Seeck, M.; Vulliemoz, S.; Rossetti, A.O.; Staedler, C.; Spinelli, L.; Pegna, A.J.; Pralong, E.; Villemure, J.G.; Foletti, G.; et al. Chronic deep brain stimulation in mesial temporal lobe epilepsy. Seizure 2011, 20, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Cleary, D.R.; Raslan, A.M.; Rubin, J.E.; Bahgat, D.; Viswanathan, A.; Heinricher, M.M.; Burchiel, K.J. Deep brain stimulation entrains local neuronal firing in human globus pallidus internus. J. Neurophysiol. 2013, 109, 978–987. [Google Scholar] [CrossRef] [PubMed]
- Butson, C.R.; McIntyre, C.C. Differences among implanted pulse generator waveforms cause variations in the neural response to deep brain stimulation. Clin. Neurophysiol. 2007, 118, 1889–1894. [Google Scholar] [CrossRef] [PubMed]
- Whitmer, D.; de Solages, C.; Hill, B.; Yu, H.; Henderson, J.M.; Bronte-Stewart, H. High frequency deep brain stimulation attenuates subthalamic and cortical rhythms in Parkinson’s disease. Front. Hum. Neurosci. 2012, 6, 155. [Google Scholar] [CrossRef] [PubMed]
- Cogan, S.F. Neural Stimulation and Recording Electrodes. Annu. Rev. Biomed. Eng. 2008, 10, 275–309. [Google Scholar] [CrossRef] [PubMed]
- Negi, S.; Bhandari, R.; Rieth, L.; Solzbacher, F. In vitro comparison of sputtered iridium oxide and platinum-coated neural implantable microelectrode arrays. Biomed. Mater. 2010, 5, 015007. [Google Scholar] [CrossRef] [PubMed]
- Hoogerwerf, A.C.; Wise, K.D. A three-dimensional microelectrode array for chronic neural recording. IEEE Trans. Bio-Med. Eng. 1994, 41, 1136–1146. [Google Scholar] [CrossRef] [PubMed]
- Irazoqui-Pastor, P.; Mody, I.; Judy, J.W. In-vivo EEG recording using a wireless implantable neural transceiver. In Proceedings of the IEEE EMBS Conference, Capri Island, Italy, 20–22 March 2003; pp. 622–625.
- Olsson, R.H.; Wise, K.D. A three-dimensional neural recording microsystem with implantable data compression circuitry. IEEE J. Solid-State Circuits 2005, 40, 2796–2804. [Google Scholar] [CrossRef]
- Harrison, R.R.; Watkins, P.T.; Kier, R.J.; Lovejoy, R.O.; Black, D.J.; Greger, B.; Solzbacher, F. A low-power integrated circuit for a wireless 100-electrode neural recording system. IEEE J. Solid State Circuits 2007, 42, 123–133. [Google Scholar] [CrossRef]
- Clarke, C.T.; Xu, X.; Rieger, R.; Taylor, J.; Donaldson, N. An implanted system for multi-site nerve cuff-based ENG recording using velocity selectivity. Analog Integr. Circuits Signal Process. 2009, 58, 91–104. [Google Scholar] [CrossRef]
- Lungu, C.; Malone, P.; Wu, T.; Ghosh, P.; McElroy, B.; Zaghloul, K.; Patterson, T.; Hallett, M.; Levine, Z. Temporal macrodynamics and microdynamics of the postoperative impedance at the tissue-electrode interface in deep brain stimulation patients. J. Neurol. Neurosurg. Psychiatry 2014, 85, 816–819. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.Y.; Liao, L.D.; Lin, C.T.; Hsu, J.H.; He, X.; Chen, Y.Y.; Chang, J.Y.; Chen, H.F.; Tsang, S.; Shih, Y.Y. Design, simulation and experimental validation of a novel flexible neural probe for deep brain stimulation and multichannel recording. J. Neural Eng. 2012, 9, 036001. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.Y.; Yash, T.V.; Rogers, B.; Duong, T.Q. FMRI of deep brain stimulation at the rat ventral posteromedial thalamus. Brain Stimul. 2014, 7, 190–193. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, C.-S.A.; Lai, H.-Y.; Huang, S.-H.; Lo, Y.-C.; Lee, N.; Chen, P.-Y.; Tu, P.-H.; Yang, C.-Y.; Lin, J.C.-C.; Chen, Y.-Y. A Programmable High-Voltage Compliance Neural Stimulator for Deep Brain Stimulation in Vivo. Sensors 2015, 15, 12700-12719. https://doi.org/10.3390/s150612700
Gong C-SA, Lai H-Y, Huang S-H, Lo Y-C, Lee N, Chen P-Y, Tu P-H, Yang C-Y, Lin JC-C, Chen Y-Y. A Programmable High-Voltage Compliance Neural Stimulator for Deep Brain Stimulation in Vivo. Sensors. 2015; 15(6):12700-12719. https://doi.org/10.3390/s150612700
Chicago/Turabian StyleGong, Cihun-Siyong Alex, Hsin-Yi Lai, Sy-Han Huang, Yu-Chun Lo, Nicole Lee, Pin-Yuan Chen, Po-Hsun Tu, Chia-Yen Yang, James Chang-Chieh Lin, and You-Yin Chen. 2015. "A Programmable High-Voltage Compliance Neural Stimulator for Deep Brain Stimulation in Vivo" Sensors 15, no. 6: 12700-12719. https://doi.org/10.3390/s150612700
APA StyleGong, C.-S. A., Lai, H.-Y., Huang, S.-H., Lo, Y.-C., Lee, N., Chen, P.-Y., Tu, P.-H., Yang, C.-Y., Lin, J. C.-C., & Chen, Y.-Y. (2015). A Programmable High-Voltage Compliance Neural Stimulator for Deep Brain Stimulation in Vivo. Sensors, 15(6), 12700-12719. https://doi.org/10.3390/s150612700







