Stand-Off Biodetection with Free-Space Coupled Asymmetric Microsphere Cavities
Abstract
:1. Introduction
2. Directional Emission
2.1. The Ray Model for ARCs
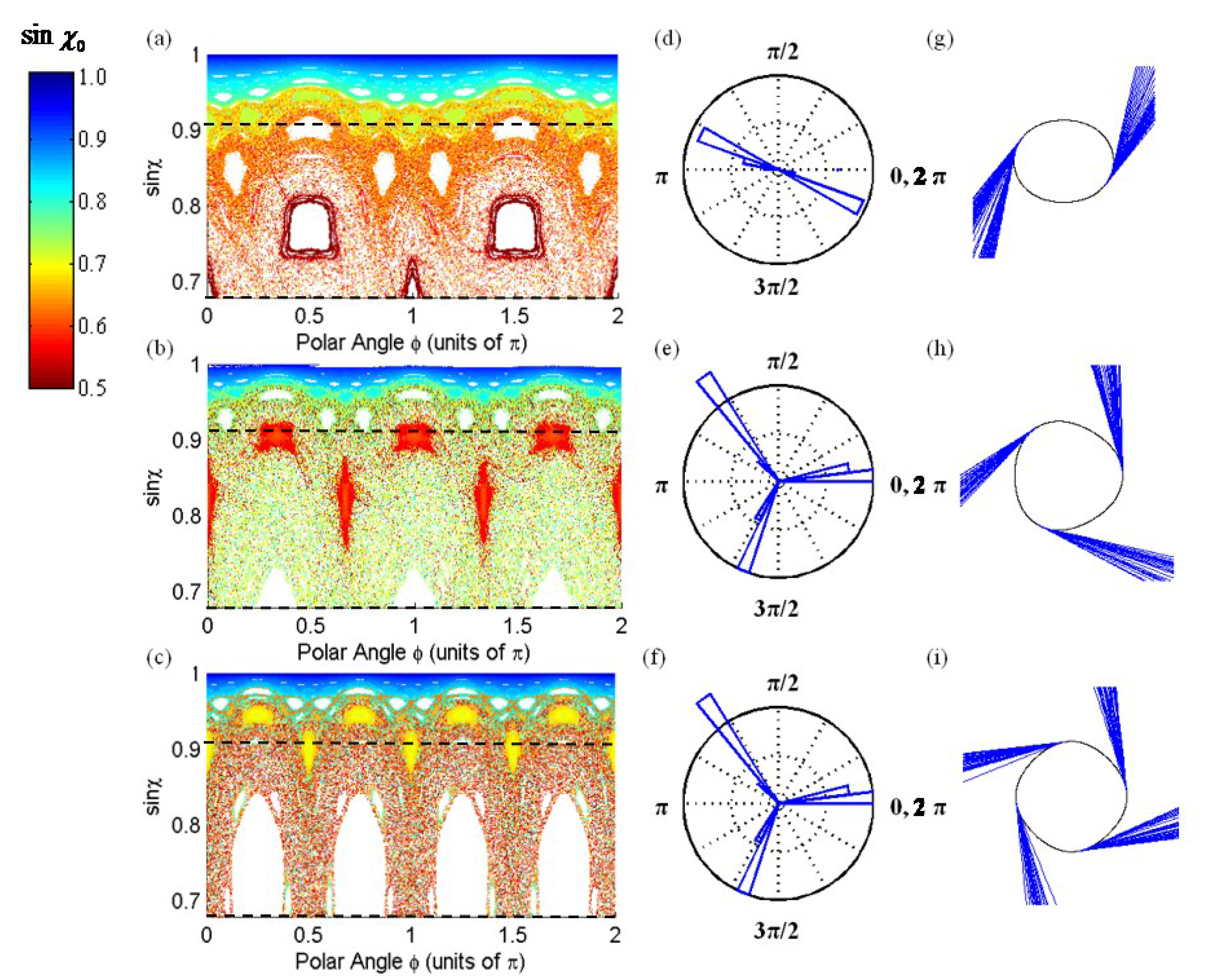

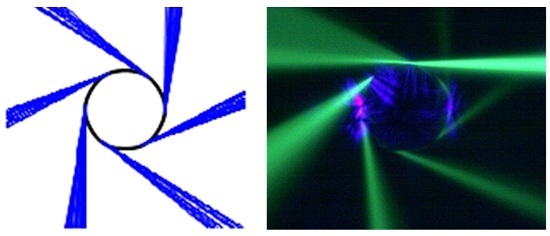
2.2. Visualizing Directional Emission Patterns from ARCs
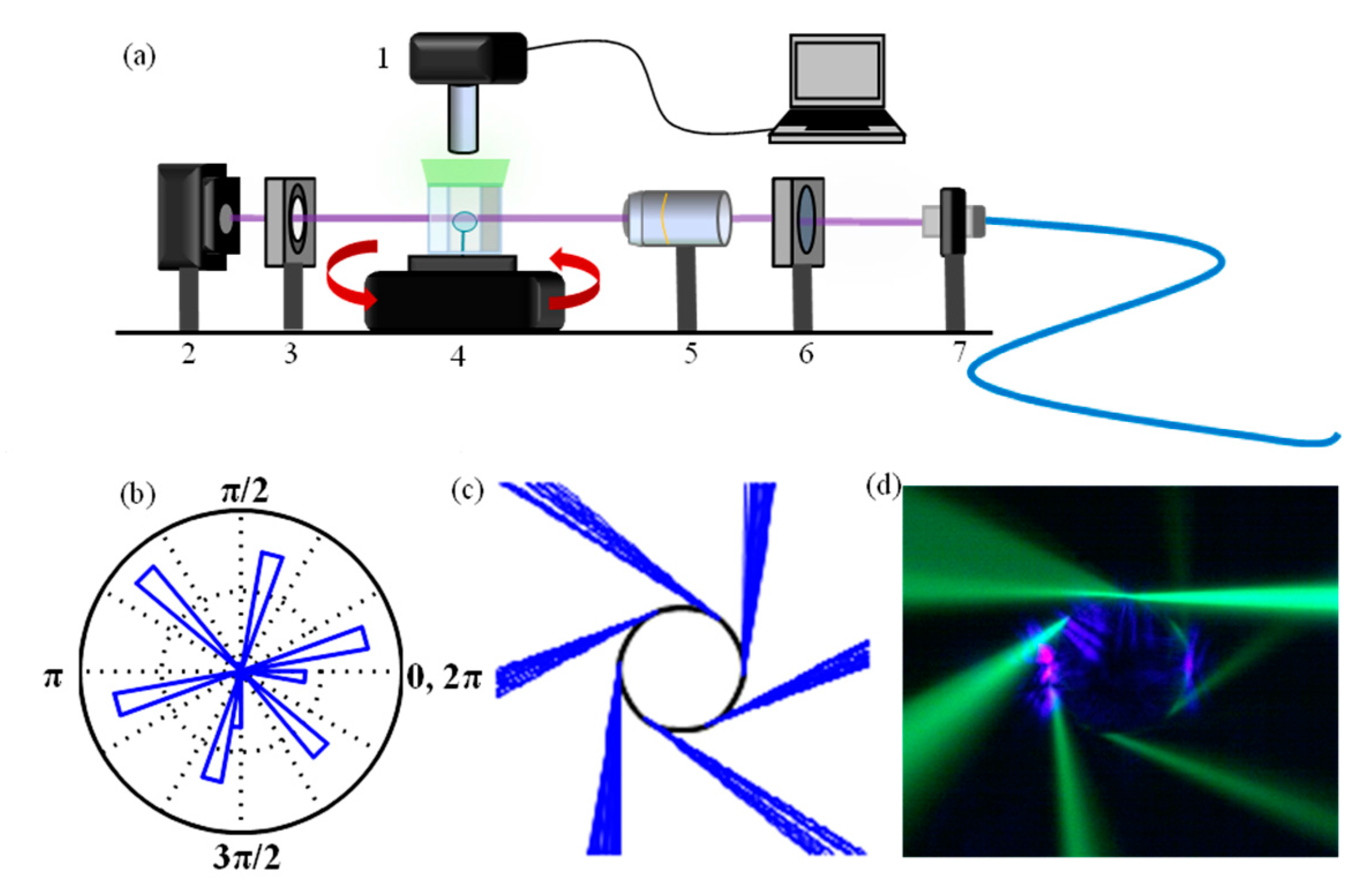
3. Measuring Modes in the Far-Field

4. Biosensing with Free-Space Coupled ARC
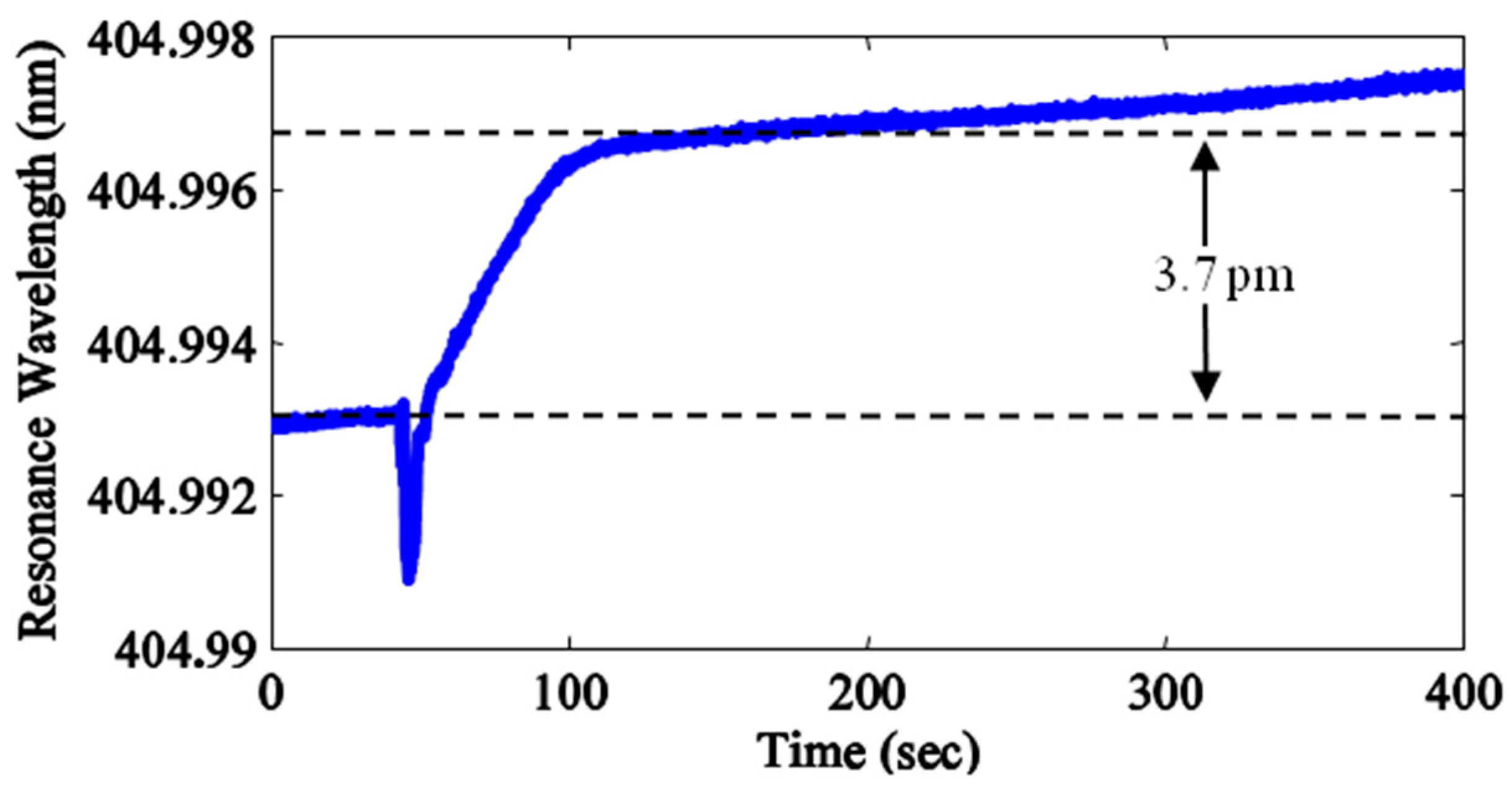
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, B.-B.; Clements, W.R.; Yu, X.-C.; Shi, K.; Gong, Q.; Xiao, Y.-F. Single nanoparticle detection using split-mode microcavity Raman lasers. Proc. Natl. Acad. Sci. USA 2014, 111, 14657–14662. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, Ş.K.; Zhu, J.; Yang, X.; Peng, B.; Yilmaz, H.; He, L.; Monifi, F.; Huang, S.H.; Long, G.L.; Yang, L. Highly sensitive detection of nanoparticles with a self-referenced and self-heterodyned whispering-gallery Raman microlaser. Proc. Natl. Acad. Sci. USA 2014, 111, E3836–E3844. [Google Scholar] [CrossRef] [PubMed]
- Baaske, M.D.; Foreman, M.R.; Vollmer, F. Single-molecule nucleic acid interactions monitored on a label-free microcavity biosensor platform. Nat. Nanotechnol. 2014, 9, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, F.; Arnold, S.; Keng, D. Single virus detection from the reactive shift of a whispering-gallery mode. Proc. Natl. Acad. Sci. USA 2008, 105, 20701–20704. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Ozdemir, S.K.; Xiao, Y.-F.; Li, L.; He, L.; Chen, D.-R.; Yang, L. On-chip single nanoparticle detection and sizing by mode splitting in an ultrahigh-Q microresonator. Nat. Photonics 2010, 4, 46–49. [Google Scholar] [CrossRef]
- Shao, L.; Jiang, X.-F.; Yu, X.-C.; Li, B.-B.; Clements, W.R.; Vollmer, F.; Wang, W.; Xiao, Y.-F.; Gong, Q. Detection of Single Nanoparticles and Lentiviruses Using Microcavity Resonance Broadening. Adv. Mater. 2013, 25, 5616–5620. [Google Scholar] [CrossRef] [PubMed]
- Gorodetsky, M.L.; Savchenkov, A.A.; Ilchenko, V.S. Ultimate Q of optical microsphere resonators. Opt. Lett. 1996, 21, 453–455. [Google Scholar] [CrossRef] [PubMed]
- Knight, J.C.; Cheung, G.; Jacques, F.; Birks, T. A. Phase-matched excitation of whispering-gallery-mode resonances by a fiber taper. Opt. Lett. 1997, 22, 1129–1131. [Google Scholar] [CrossRef] [PubMed]
- Collot, L.; Lefèvre-Seguin, V.; Brune, M.; Raimond, J.M.; HarocheL, S. Very High-Q Whispering-Gallery Mode Resonances Observed on Fused Silica Microspheres. Europhys. Lett. 2007, 23, 327. [Google Scholar] [CrossRef]
- Spillane, S.M.; Kippenberg, T.J.; Painter, O.J.; Vahala, K.J. Ideality in a Fiber-Taper-Coupled Microresonator System for Application to Cavity Quantum Electrodynamics. Phys. Rev. Lett. 2003, 91, 043902. [Google Scholar] [CrossRef] [PubMed]
- Gorodetsky, M.L.; Ilchenko, V.S. Optical microsphere resonators: Optimal coupling to high-Q whispering-gallery modes. J. Opt. Soc. Am. B 1999, 16, 147–154. [Google Scholar] [CrossRef]
- Ilchenko, V.S.; Yao, X.S.; Maleki, L. Pigtailing the high-Q microsphere cavity: A simple fiber coupler for optical whispering-gallery modes. Opt. Lett. 1999, 24, 723–725. [Google Scholar] [CrossRef] [PubMed]
- Baaske, M.; Vollmer, F. Optical Resonator Biosensors: Molecular Diagnostic and Nanoparticle Detection on an Integrated Platform. Chem. Phys. Chem. 2012, 13, 427–436. [Google Scholar] [PubMed]
- Wilson, K.A.; Finch, C.A.; Anderson, P.; Vollmer, F.; Hickman, J.J. Whispering gallery mode biosensor quantification of fibronectin adsorption kinetics onto alkylsilane monolayers and interpretation of resultant cellular response. Biomaterials 2012, 33, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Swaim, J.D.; Knittel, J.; Bowen, W.P. Detection of nanoparticles with a frequency locked whispering gallery mode microresonator. Appl. Phys. Lett. 2013, 102, 183106. [Google Scholar] [CrossRef]
- Zhu, J.; Ozdemir, S.K.; Yang, L. Optical Detection of Single Nanoparticles with a Subwavelength Fiber-Taper. IEEE Photonics Technol. Lett. 2011, 23, 1346–1348. [Google Scholar] [CrossRef]
- Park, Y.-S.; Cook, A.K.; Wang, H. Cavity QED with Diamond Nanocrystals and Silica Microspheres. Nano Lett. 2006, 6, 2075–2079. [Google Scholar] [CrossRef] [PubMed]
- Conti, G.N.; Berneschi, S.; Brenci, M.; Cosi, F.; Pelli, S.; Soria, S.; Righini, G.C. WGM microresonators: Light coupling issues and integration perspectives. In Proceedings of 2011 13th International Conference on Transparent Optical Networks (ICTON), Stockholm, Sweden, 26–30 June 2011; pp. 1–4.
- Zou, C.-L.; Shu, F.-J.; Sun, F.-W.; Gong, Z.-J.; Han, Z.-F.; Guo, G.-C. Theory of free space coupling to high-Q whispering gallery modes. Opt. Express 2013, 21, 9982–9995. [Google Scholar] [CrossRef] [PubMed]
- Podolskiy, V.A.; Narimanov, E.E. Chaos-assisted tunneling in dielectric microcavities. Opt. Lett. 2005, 30, 474–476. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.-F.; Jiang, X.-F.; Cui, Y.-L.; Shao, L.; Xiao, Y.-F. Dynamical tunneling-assisted coupling of high-Q deformed microcavities using a free-space beam. Phys. Rev. A 2013, 88, 023810. [Google Scholar] [CrossRef]
- Cao, H.; Wiersig, J. Dielectric microcavities: Model systems for wave chaos and non-Hermitian physics. Rev. Mod. Phys. 2015, 87, 61–111. [Google Scholar] [CrossRef]
- Xiao, Y.-F.; Dong, C.-H.; Han, Z.-F.; Guo, G.-C.; Park, Y.-S. Directional escape from a high-Q deformed microsphere induced by short CO2 laser pulses. Opt. Lett. 2007, 32, 644–646. [Google Scholar] [CrossRef] [PubMed]
- Nöckel, J.U.; Stone, A.D.; Chen, G.; Grossman, H.L.; Chang, R.K. Directional emission from asymmetric resonant cavities. Opt. Lett. 1996, 21, 1609–1611. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Wang, Q.J.; Diehl, L.; Hentschel, M.; Wiersig, J.; Yu, N.; Pflügl, C.; Capasso, F.; Belkin, M.A.; Edamura, T.; et al. Directional emission and universal far-field behavior from semiconductor lasers with limaçon-shaped microcavity. Appl. Phys. Lett. 2009, 94, 251101. [Google Scholar]
- Wang, Q.J.; Yan, C.; Yu, N.; Unterhinninghofen, J.; Wiersig, J.; Pflügl, C.; Diehl, L.; Edamura, T.; Yamanishi, M.; Kan, H.; et al. Whispering-gallery mode resonators for highly unidirectional laser action. Proc. Natl. Acad. Sci. USA 2010, 107, 22407–22412. [Google Scholar] [CrossRef]
- Aveline, D.C.; Baumgartel, L.M.; Lin, G.; Yu, N. Whispering gallery mode resonators augmented with engraved diffraction gratings. Opt. Lett. 2013, 38, 284–286. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Özdemir, Ş.K.; Yilmaz, H.; Peng, B.; Dong, M.; Tomes, M.; Carmon, T.; Yang, L. Interfacing whispering-gallery microresonators and free space light with cavity enhanced Rayleigh scattering. Sci. Rep. 2014, 4, 6396. [Google Scholar]
- Dong, C.; Xiao, Y.; Yang, Y.; Han, Z.; Guo, G.; Yang, L. Directly mapping whispering gallery modes in a microsphere through modal coupling and directional emission. Chin. Opt. Lett. 2008, 6, 300–302. [Google Scholar] [CrossRef]
- Park, Y.-S.; Wang, H. Radiation pressure driven mechanical oscillation in deformed silica microspheres via free-space evanescent excitation. Opt. Express 2007, 15, 16471–16477. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.-F.; Xiao, Y.-F.; Zou, C.-L.; He, L.; Dong, C.-H.; Li, B.-B.; Li, Y.; Sun, F.-W.; Yang, L.; Gong, Q. Highly Unidirectional Emission and Ultralow-Threshold Lasing from On-Chip Ultrahigh-Q Microcavities. Adv. Mater. 2012, 24, OP260–OP264. [Google Scholar] [CrossRef] [PubMed]
- Nöckel, J.U.; Stone, A.D. Ray and wave chaos in asymmetric resonant optical cavities. Nature 1997, 385, 45–47. [Google Scholar] [CrossRef]
- Lee, S.-Y. Optical Mode Properties of 2-D Deformed Microcavities. In Advances in Optical and Photonic Devices; Young, K., Ed.; INTECH Open Access Publisher: Rijeka, Croatia, 2010. [Google Scholar]
- Zou, C.-L.; Sun, F.-W.; Dong, C.-H.; Shu, F.-J.; Wu, X.-W.; Cui, J.-M.; Yang, Y.; Han, Z.-F.; Guo, G.-C. High-Q and Unidirectional Emission Whispering Gallery Modes: Principles and Design. IEEE J. Sel. Top. Quantum Electron. 2013, 19, 1–6. [Google Scholar]
- Lacey, S.; Wang, H. Directional emission from whispering-gallery modes in deformed fused-silica microspheres. Opt. Lett. 2001, 26, 1943–1945. [Google Scholar] [CrossRef]
- Schwefel, H.G.L.; Rex, N.B.; Tureci, H.E.; Chang, R.K.; Stone, A.D.; Ben-Messaoud, T.; Zyss, J. Dramatic shape sensitivity of directional emission patterns from similarly deformed cylindrical polymer lasers. J. Opt. Soc. Am. B 2004, 21, 923–934. [Google Scholar] [CrossRef]
- Shinohara, S.; Harayama, T.; Fukushima, T.; Hentschel, M.; Sasaki, T.; Narimanov, E.E. Chaos-Assisted Directional Light Emission from Microcavity Lasers. Phys. Rev. Lett. 2010, 104, 163902. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.-F.; Dong, C.-H.; Zou, C.-L.; Han, Z.-F.; Yang, L.; Guo, G.-C. Low-threshold microlaser in a high-Q asymmetrical microcavity. Opt. Lett. 2009, 34, 509–511. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.-M.; Dong, C.-H.; Zou, C.-L.; Sun, F.-W.; Xiao, Y.-F.; Han, Z.-F.; Guo, G.-C. Controlling deformation in a high quality factor silica microsphere toward single directional emission. Appl. Opt. 2013, 52, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Lacey, S.; Wang, H.; Foster, D.H.; Nöckel, J.U. Directional Tunneling Escape from Nearly Spherical Optical Resonators. Phys. Rev. Lett. 2003, 91, 033902. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.-F.; Jiang, X.-F.; Yang, Q.-F.; Wang, L.; Shi, K.; Li, Y.; Gong, Q. Chaos-induced transparency in an ultrahigh-Q optical microcavity. Laser Photonics Rev. 2013, 7, L51–L54. [Google Scholar] [CrossRef]
- Vollmer, F.; Braun, D.; Libchaber, A.; Khoshsima, M.; Teraoka, I.; Arnold, S. Protein detection by optical shift of a resonant microcavity. Appl. Phys. Lett. 2002, 80, 4057–4059. [Google Scholar] [CrossRef]
- Foreman, M.R.; Jin, W.-L.; Vollmer, F. Optimizing detection limits in whispering gallery mode biosensing. Opt. Express 2014, 22, 5491–5511. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.; Lee, S.-Y.; Song, E.Y.; Chun, H.; Lee, B. Plasmonic Nanostructures for Nano-Scale Bio-Sensing. Sensors 2011, 11, 10907–10929. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.E.; Yao, J.; Maria, J.; Gray, S.K.; Rogers, J.A.; Nuzzo, R.G. Multispectral Thin Film Biosensing and Quantitative Imaging Using 3D Plasmonic Crystals. Anal. Chem. 2009, 81, 5980–5989. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ballard, Z.; Baaske, M.D.; Vollmer, F. Stand-Off Biodetection with Free-Space Coupled Asymmetric Microsphere Cavities. Sensors 2015, 15, 8968-8980. https://doi.org/10.3390/s150408968
Ballard Z, Baaske MD, Vollmer F. Stand-Off Biodetection with Free-Space Coupled Asymmetric Microsphere Cavities. Sensors. 2015; 15(4):8968-8980. https://doi.org/10.3390/s150408968
Chicago/Turabian StyleBallard, Zachary, Martin D. Baaske, and Frank Vollmer. 2015. "Stand-Off Biodetection with Free-Space Coupled Asymmetric Microsphere Cavities" Sensors 15, no. 4: 8968-8980. https://doi.org/10.3390/s150408968
APA StyleBallard, Z., Baaske, M. D., & Vollmer, F. (2015). Stand-Off Biodetection with Free-Space Coupled Asymmetric Microsphere Cavities. Sensors, 15(4), 8968-8980. https://doi.org/10.3390/s150408968