Determination of Zinc, Cadmium and Lead Bioavailability in Contaminated Soils at the Single-Cell Level by a Combination of Whole-Cell Biosensors and Flow Cytometry
Abstract
:1. Introduction
2. Experimental Section
2.1. Strain
2.2. Soil Samples
2.3. Cell Culture
2.4. Propidium Iodide Staining
2.5. Recovery of Biosensors from Soils by Nycodenz®
2.6. Flow Cytometry Analysis
2.7. Metal Analysis by Atomic Absorption Spectroscopy
3. Results and Discussion
3.1. Development of Fluorescence-Based Biosensors for MTE Detection in Liquid Media
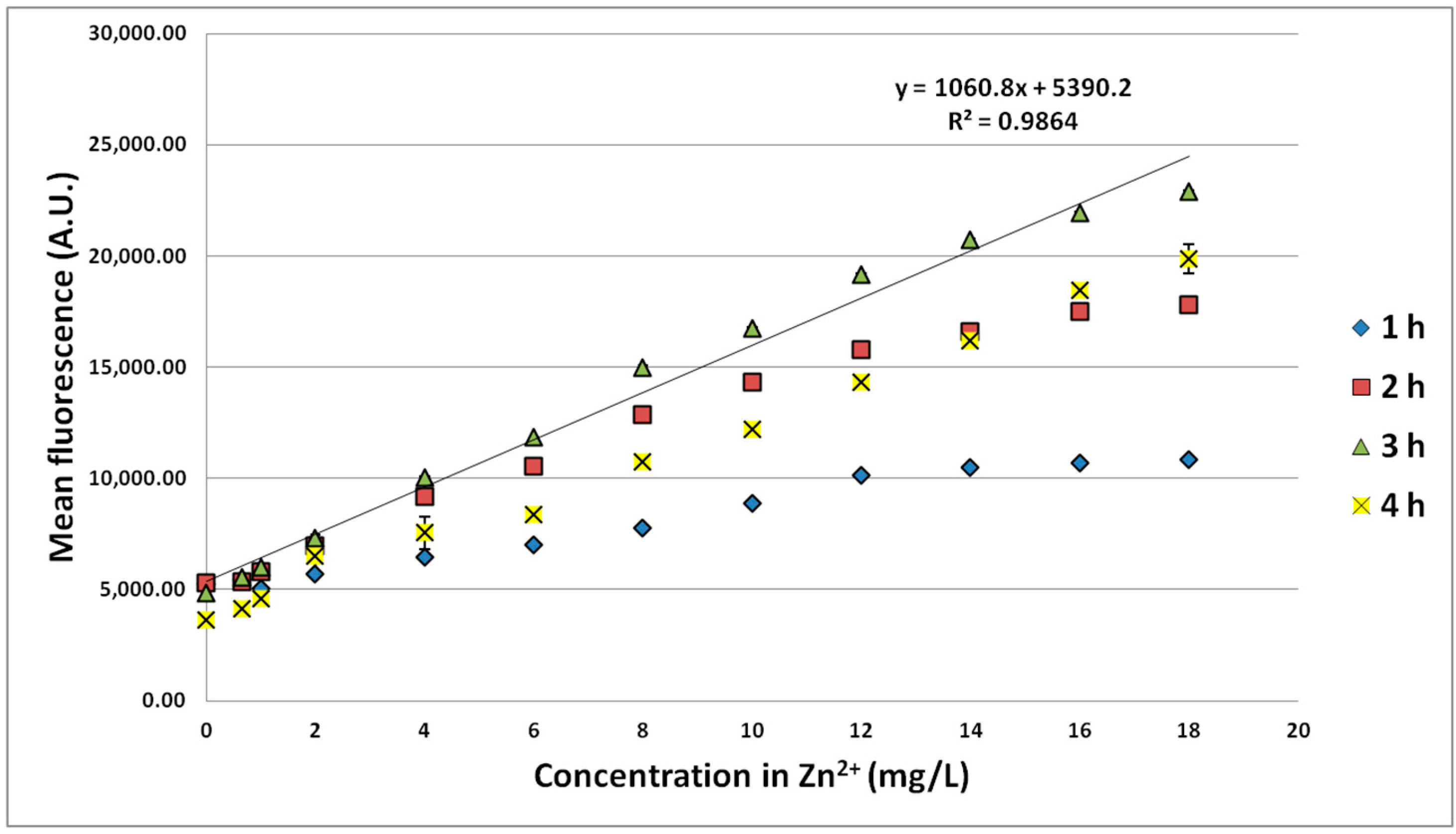
3.1.1. Effect of Zn Concentration and Incubation Time
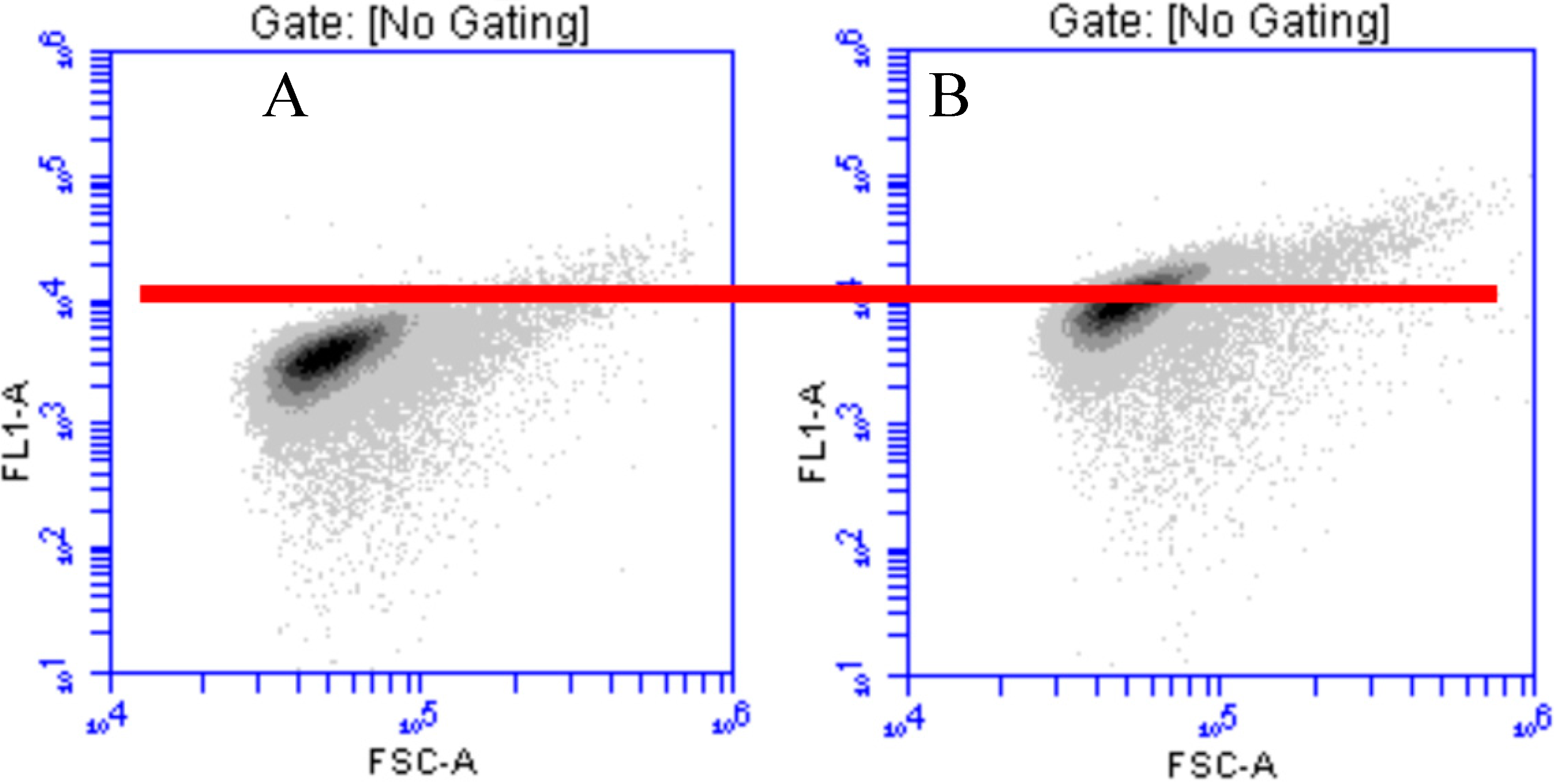
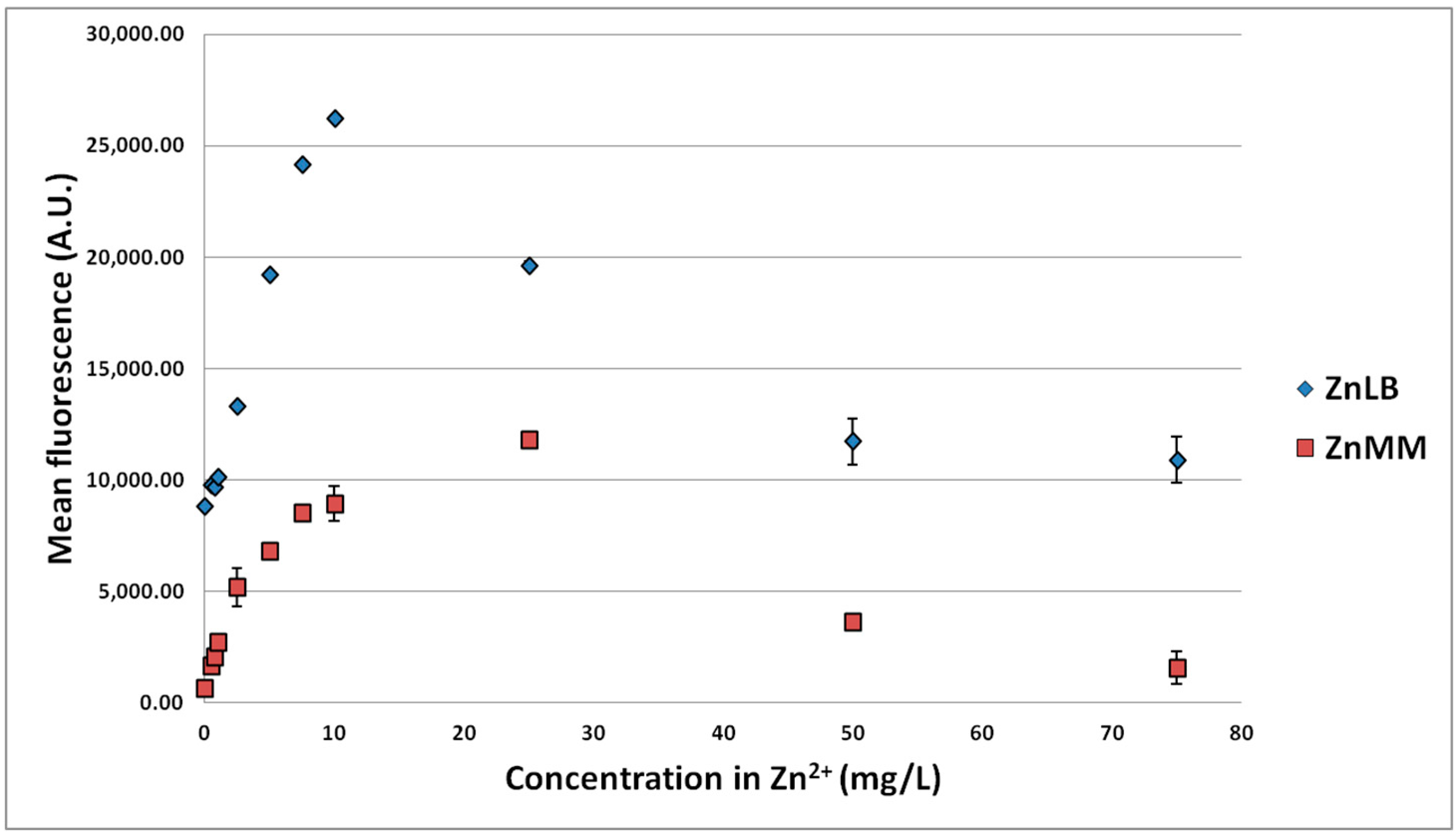
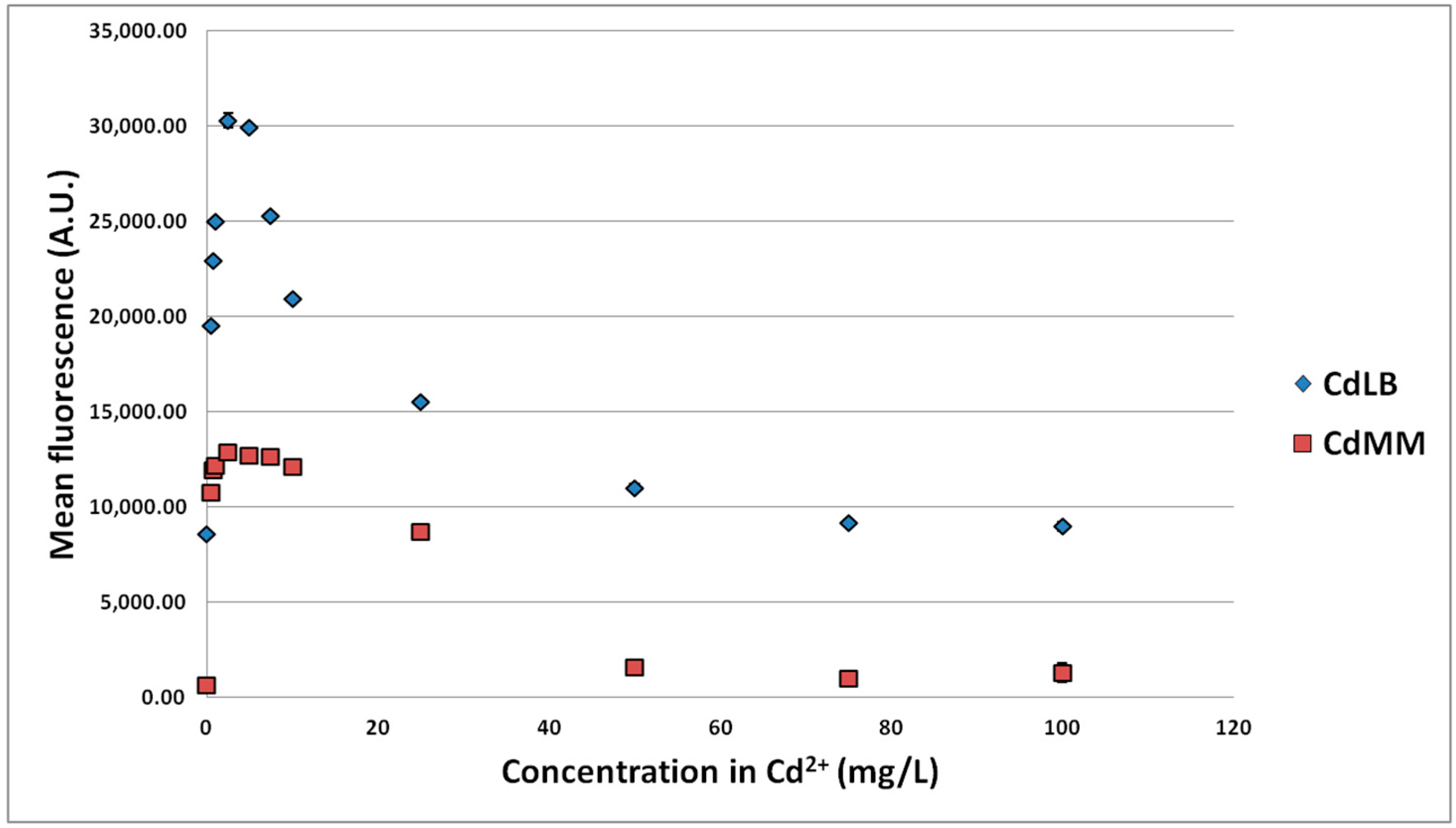
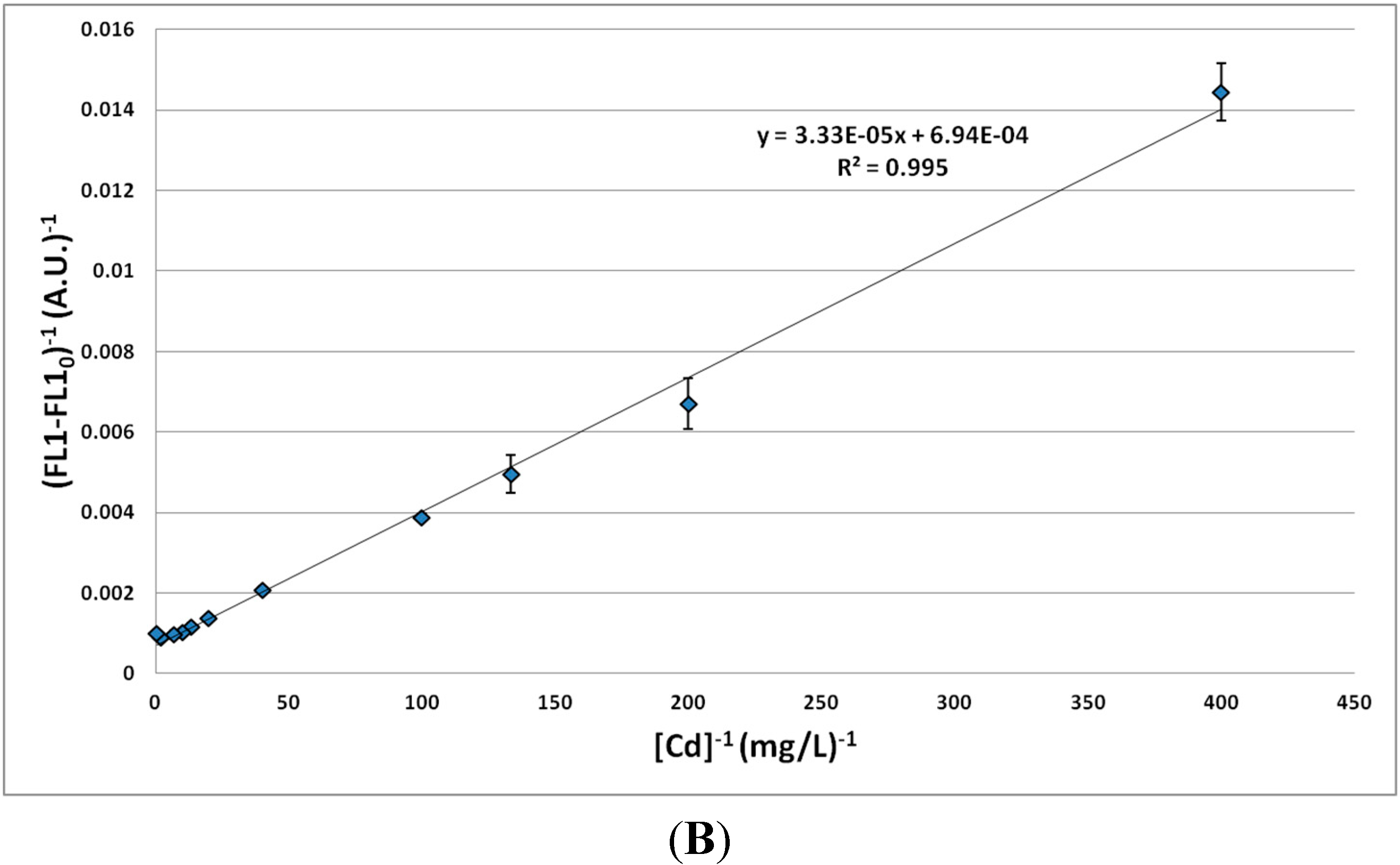
3.1.2. Effect of MTE Concentrations
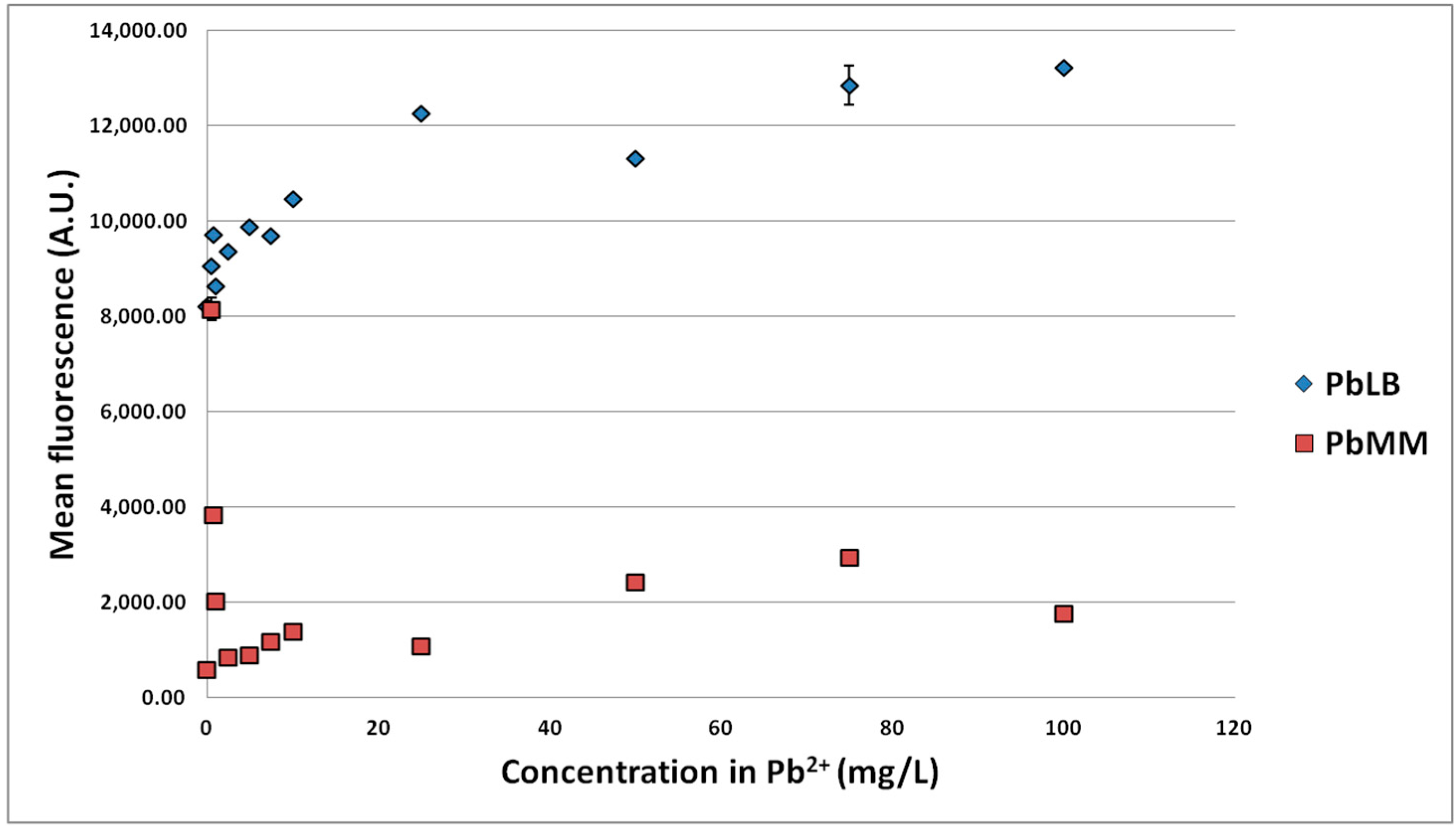
3.1.3. Impact of Zn2+/Cd2+ Ratio on Biosensor Response

3.2. Soil Analysis
3.2.1. Soil Characterization by Atomic Absorption Spectroscopy
| Soil ID | Soil pH | Extraction Method | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Aqua Regia Total MTEs (mg/kg) | EDTA + Ammonium Acetate Solution, pH 4.65 Available MTEs (mg/kg) | CaCl2 Solution Soluble MTEs (mg/kg) | ||||||||
| Cd | Pb | Zn | Cd | Pb | Zn | Cd | Pb | Zn | ||
| S1 | 6.9 | 5 | 131 | 410 | 3 | 73 | 69 | ND * | ND * | 0.547 |
| S2 | 6.8 | 4 | 117 | 318 | 3 | 69 | 91 | ND * | ND * | 0.274 |
| S3 | 6.9 | 2 | 59 | 200 | 1 | 30 | 51 | ND * | ND * | 0.434 |
3.2.2. Soil Characterization by Flow Cytometry and MTEs Bioavailability Assessment
| Soil ID | Soil Concentration (mg/mL) | Bioavailable Cd2+ in Soils (mg/kg) | |||
|---|---|---|---|---|---|
| Soil Sieved at 2 mm | Soil Ground at 0.2 mm | ||||
| Average | Amplitude | Average | Amplitude | ||
| S1 | 25 | 0.26 | 0.14–0.38 | 0.31 | 0.30–0.39 |
| 50 | 0.22 | 0.11–0.36 | 0.20 | 0.17–0.50 | |
| 100 | 0.20 | 0.06–0.41 | 0.15 | 0.13–0.50 | |
| S2 | 25 | 0.33 | 0.16–0.50 | 0.34 | 0.32–0.36 |
| 50 | 0.24 | 0.14–0.41 | 0.22 | 0.16–0.27 | |
| 100 | 0.14 | 0.10–0.23 | 0.14 | 0.13–0.24 | |
| S3 | 25 | 0.14 | 0.06–0.22 | 0.22 | 0.21–0.22 |
| 50 | 0.10 | 0.04–0.13 | 0.13 | 0.10–0.13 | |
| 100 | 0.08 | 0.03–0.10 | 0.08 | 0.07–0.10 | |
3.2.3 Relevance of Biosensors for Soil Characterization
| Method | Abbr. | Limits of Detection and Dynamic Range | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 ppq | 1 ppt | 1 ppb | 1 ppm | 103 ppm | ||||||||||
| X-ray fluorescence spectrometry | XRF | |||||||||||||
| Inductively coupled plasma-mass spectrometry | ICP-MS | |||||||||||||
| Inductively coupled plasma-atomic emission spectroscopy | ICP-AES | |||||||||||||
| Graphite furnace-atomic absorption spectroscopy | GFAAS | |||||||||||||
| Atomic absorption spectroscopy | AAS | |||||||||||||
| E. coli pPZntAgfpl | ||||||||||||||
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Baize, D. Eléments traces dans les sols. Courrier de l’Environnement de l’INRA 2009, 57, 63–72. (In French) [Google Scholar]
- Alloway, B.J. Introduction. In Heavy Metals in Soils: Trace Metals and Metalloids in Soils and Their Bioavailability, 3rd ed.; Alloway, B.J., Ed.; Springer: Berlin, Germany, 2013; Volume 22, pp. 3–9. [Google Scholar]
- Hooda, P.S. Introduction. In Trace Elements in Soils; Hooda, P.S., Ed.; Blackwell Publishing Ltd.: Chippenham, UK, 2010; pp. 3–8. [Google Scholar]
- Al-Garni, S.M.; Ghanem, K.M.; Bahobail, A.S. Biosorption characteristics of aspergillus fumigatus in removal of cadmium from an aqueous solution. Afr. J. Biotechnol. 2009, 8, 4163–4172. [Google Scholar]
- Wuana, R.A.; Okieimen, F.E. Heavy metals in contaminated soils: A review of sources chemistry, risks and best available strategies for remediation. ISRN 2011, 2011, 1–20. [Google Scholar]
- Smolders, E.; Mertens, J. Cadmium. In Heavy Metals in Soils: Trace Metals and Metalloids in Soils and Their Bioavailability; Alloway, B., Ed.; Springer: Berlin, Germany, 2013; Volume 22, pp. 283–311. [Google Scholar]
- Steinnes, E. Lead. In Heavy Metals in Soils: Trace Metals and Metalloids in Soils and Their Bioavailability, 3rd ed.; Alloway, B.J., Ed.; Springer: Berlin, Germany, 2013; Volume 22, pp. 395–409. [Google Scholar]
- Su, C.; Jiang, L.; Zhang, W. A review on heavy metal contamination in the soil worldwide: Situation, impact and remediation techniques. Environ. Skept. Crit. 2014, 3, 24–38. [Google Scholar]
- Mertens, J.; Smolders, E. Zinc. In Heavy Metals in Soils: Trace Metals and Metalloids in Soils and Their Bioavailability, 3rd ed.; Alloway, B.J., Ed.; Springer: Berlin, Germanry, 2013; Volume 22, pp. 465–493. [Google Scholar]
- Davidson, C. Methods for the determination of heavy metals and metalloids in soils. In Heavy Metals in Soils: Trace Metals and Metalloids in Soils and Their Bioavailability; Alloway, B.J., Ed.; Springer: Berlin, Germany, 2013; Volume 22, pp. 97–140. [Google Scholar]
- Tack, F.M.G. Trace elements: General soil chemistry, principles and processes. In Trace Elements in Soils; Hooda, P.S., Ed.; Blackwell Publishing Ltd.: Chippenham, UK, 2010; pp. 9–37. [Google Scholar]
- Su, L.; Jia, W.; Hou, C.; Lei, Y. Microbial biosensors: A review. Biosens. Bioelectron. 2011, 26, 1788–1799. [Google Scholar] [CrossRef] [PubMed]
- Karube, I.; Matsunaga, T.; Mitsuda, S.; Suzuki, S. Microbial electrode bod sensors. Biotechnol. Bioeng. 1977, 19, 1535–1547. [Google Scholar] [CrossRef] [PubMed]
- Greene, J.C.; Miller, W.E.; Debacon, M.K.; Long, M.A.; Bartels, C.L. A comparison of three microbial assay procedures for measuring toxicity of chemical residues. Arch. Environ. Con. Tox. 1985, 14, 659–667. [Google Scholar] [CrossRef]
- Korpela, M.; Karp, M. Stable-light producing Escherichia coli. Biotechnol. Lett. 1988, 10, 383–388. [Google Scholar] [CrossRef]
- King, J.M.H.; DiGrazia, P.M.; Applegate, B.; Burlage, R.S.; Sanseverino, J.; Dunbar, P.; Larimer, F.; Sayler, G.S. Rapid, sensitive bioluminescent reporter technology for naphtalene exposure and biodegradation. Science 1990, 249, 778–781. [Google Scholar] [CrossRef] [PubMed]
- Burlage, R.S.; Palumbo, A.V.; Heitzer, A.; Sayler, G.S. Bioluminescent reporter bacteria dectect contaminants in soil samples. Appl. Biochem. Biotech. 1994, 45–46, 731–741. [Google Scholar] [CrossRef]
- Geiselhart, L.; Osgook, M.; Holmes, D.S. Construction and evaluation of a self-luminescent biosensor. Ann. N. Y. Acad. Sci. 1991, 646, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Guzzo, A.; Diorio, C.; Dubow, M.S. Transcription of the escherichia coli flic gene is regulated by metal ions. Appl. Environ. Microb. 1991, 57, 2255–2259. [Google Scholar]
- Yoon, K.P.; Silver, S. A second gene in the staphylococcus aureus cada cadmium resistance determinant of plasmid pl258. J. Bacteriol. 1991, 173, 7636–7642. [Google Scholar] [PubMed]
- Yoon, K.P.; Silver, S. Regulation of the cadA cadmium resistance determinant of staphylococcus aureus plasmid pl258. J. Bacteriol. 1991, 173, 7643–7649. [Google Scholar] [PubMed]
- Selifonova, O.; Burlage, R.S.; Barkay, T. Bioluminescent snsors for detection of bioavailable Hg(ii) in the environment. Appl. Environ. Microb. 1993, 59, 3083–3090. [Google Scholar]
- Erbe, J.L.; Adams, A.C.; Taylor, K.B.; Hall, L.M. Cyanobacteria carrying ansmt-lux transcriptional fusion as biosensors for the detection of heavy metal cations. J. Ind. Microb. 1996, 17, 80–83. [Google Scholar] [CrossRef]
- Huckle, J.W.; Morby, A.P.; Turner, J.S.; Robinson, N.J. Isolation of a prokaryotic metallothionein locus and analysis of transcriptional control by trace metal ions. Mol. Microbiol. 1993, 7, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Paton, G.I.; Palmer, G.; Kindness, A.; Campbell, C.D.; Glover, L.A.; Killham, K. Use of luminescence-marked bacteria to assess copper bioavailability in malt whisky distillery effluent. Chemosphere 1995, 31, 3217–3224. [Google Scholar] [CrossRef]
- Corbisier, P.; Thiry, E.; Diels, L. Bacterial biosensors for the toxicity assesment of solid wastes. Environ. Toxic. Water 1996, 11, 171–177. [Google Scholar] [CrossRef]
- Corbisier, P.; van der Lelie, D.; Boremans, B.; Provoost, A.; de Lorenzo, V.; Brown, N.L.; Lloyd, J.R.; Hobman, J.L.; Csöregi, E.; Johansson, G.; et al. Whole cell- and protein-based biosensors for the detection of bioavailable heavy metals in environmental samples. Anal. Chim. Acta 1999, 387, 235–244. [Google Scholar] [CrossRef]
- Van der Lelie, D.; Schwuchow, T.; Schwidetzky, U.; Wuertz, S.; Baeyens, W.; Mergeay, M.; Nies, D.H. Two-component regulatory system involved in transcriptional control of heavy-metal homoeostasis in alcaligenes eutrophus. Mol. Microbiol. 1997, 23, 493–503. [Google Scholar]
- Tsien, R. The green fluorescent protein. Annu. Rev. Biochem. 1998, 67, 509–544. [Google Scholar] [CrossRef] [PubMed]
- Blattner, F.R. The complet genome sequence of Escherichia coli K-12. Science 1997, 277, 1453–1462. [Google Scholar] [CrossRef] [PubMed]
- Bansal, V.; Bharde, A.; Ramanathan, R.; Bhargava, S.K. Inorganic materials using “unusual” microorganisms. Adv. Colloid Interface Sci. 2012, 179–182, 150–168. [Google Scholar] [CrossRef] [PubMed]
- Beard, S.J.; Hashim, R.; Membrillo-Hernandez, J.; Hughes, M.N.; Poole, R.K. Zinc(ii) tolerance in Escherichia coli K-12: Evidence that the ZntA gene (o732) encodes a cation transport atpase. Mol. Microbiol. 1997, 25, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Rensing, C.; Mitra, B.; Rosen, B.P. The ZntA gene of Escherichia coli encodes a Zn(ii)-translocating p-type atpase. Proc. Natl. Acad. Sci. USA 1997, 94, 14326–14331. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.J.; Liu, J.; Stemmler, A.J.; Mitra, B. Conservative and nonconservative mutations of the transmembrane CPC motif in ZntA: Effect on metal selectivity and activity. Biochemistry 2007, 46, 3692–3703. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Rensing, C.; Rosen, B.P.; Mitra, B. The ATP hydrolytic activity of purified ZntA, a Pb(ii)/Cd(ii)/Zn(ii)-translocating ATPase from Escherichia coli. J. Biol. Chem. 2000, 275, 3873–3878. [Google Scholar] [CrossRef] [PubMed]
- Rensing, C.; Sun, Y.; Mitra, B.; Rosen, B.P. Pb(ii)-translocating p-type ATPases. J. Biol. Chem. 1998, 273, 32614–32617. [Google Scholar] [CrossRef] [PubMed]
- Brocklehurst, K.R.; Hobman, J.L.; Lawley, B.; Blank, L.; Marshall, S.J.; Brown, N.L.; Morby, A.P. ZntR is a Zn(ii)-responsive MerR-like transcriptional regulator of ZntA in Escherichia coli. Mol. Microbiol. 1999, 31, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Liénard, A.; Colinet, G.; Dere, C. Spatial distribution of metallic trace elements in soils contaminated by atmospheric fallouts: Case study: Sclaigneaux (Belgium). In Proceedings of 4th International Congress EUROSOIL 2012 Soil Science for the Benefit for the Mankind and Environment, Bari, Italy, 2–7 July 2012.
- Riether, K.B. Développement D’un Biocapteur Bactérien Pour la Détection de Métaux Lourds. Ph.D. Thesis, Université de Metz, Metz, France, 2001. [Google Scholar]
- Burmolle, M.; Hansen, L.H.; Oregaard, G.; Sorensen, S.J. Presence of n-acyl homoserine lactones in soil detected by a whole-cell biosensor and flow cytometry. Microb. Ecol. 2003, 45, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Axis-Shield PoC AS. Available online: http://www.axis-shield-density-gradient-media.com/Catalog%202013.pdf (accessed on 16 April 2015).
- Poté, J.; Bravo, A.G.; Mavingui, P.; Ariztegui, D.; Wildi, W. Evaluation of quantitative recovery of bacterial cells and DNA from different lake sediments by nycodenz density gradient centrifugation. Ecol. Indic. 2010, 10, 234–240. [Google Scholar] [CrossRef]
- Liénard, A.; Brostaux, Y.; Colinet, G. Soil contamination near a former Zn-Pb ore-treatment plant: Evaluation of deterministic factors and spatial structures at the landscape scale. J. Geochem. Explor. 2014, 147, 107–116. [Google Scholar] [CrossRef]
- Labanowski, J.; Monna, F.; Bermond, A.; Cambier, P.; Fernandez, C.; Lamy, I.; van Oort, F. Kinetic extractions to assess mobilization of Zn, Pb, Cu, and Cd in a metal-contaminated soil: Edta vs. Citrate. Environ. Pollut. 2008, 152, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Binet, M.R.; Poole, R.K. Pb(ii) and Zn(ii) ions regulate expression of the metal-transporting p-type ATPase ZntA in Escherichia coli. FEBS Lett. 2000, 473, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Gireesh-Babu, P.; Chaudhari, A. Development of a broad-spectrum fluorescent heavy metal bacterial biosensor. Mol. Biol. Rep. 2012, 39, 11225–11229. [Google Scholar] [CrossRef] [PubMed]
- Vallee, B.; Ulmer, D. Biochemical effects of mercury, cadmium, and lead. Annu. Rev. Biochem. 1972, 41, 91–128. [Google Scholar] [CrossRef] [PubMed]
- Davidovich, R.; Stavila, V.; Whitmire, K. Stereochemistry of lead(ii) complexes containing sulfur and selenium donoratom ligands. Coordin. Chem. Rev. 2010, 254, 2193–2226. [Google Scholar] [CrossRef]
- Lee, L.J.; Barrett, J.A.; Poole, R.K. Genome-wide transcriptional response of chemostat-cultured escherichia coli to zinc. J. Bacteriol. 2005, 187, 1124–1134. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, M.; Marks, A. Mark’s Basic Medical Biochemistry; Lippincott Williams and Wilikins: Philadelphia, PA, USA, 2012. [Google Scholar]
- Chaney, R. Cadmium and zinc. In Trace Elements in Soils; Hooda, P.S., Ed.; Blackwell Publishing Ltd.: Chippenham, UK, 2010; pp. 409–439. [Google Scholar]
- Arrêté du Gouvernement Wallon Relatif à la Gestion des Sols. Available online: http://environnement.wallonie.be/legis/solsoussol/sol004.htm (accessed on 16 April 2015).
- Nies, D.H. Microbial heavy-metal resistance. Appl. Microbiol. Biot. 1999, 51, 730–750. [Google Scholar] [CrossRef]
- Pham, V.D.; Ravikumar, S.; Lee, S.H.; Hong, S.H.; Yoo, I.K. Modification of response behavior of zinc sensing hydhg two-component system using a self-activation loop and genomic integration. Bioproc. Biosyst. Eng. 2013, 36, 1185–1190. [Google Scholar] [CrossRef]
- Ivask, A.; Virta, M.; Kahru, A. Construction and use of specific luminescent recombinant bacterial sensors for the assessment of bioavailable fraction of cadmium, zinc, mercury and chromium in the soil. Soil Biol. Biochem. 2002, 34, 1439–1447. [Google Scholar] [CrossRef]
- Bondarenko, O.; Rõlova, T.; Kahru, A.; Ivask, A. Bioavailability of Cd, Zn and Hg in soil to nine recombinant luminescent metal sensor bacteria. Sensors 2008, 8, 6899–6923. [Google Scholar] [CrossRef]
- Ivask, A.; Rolova, T.; Kahru, A. A suite of recombinant luminescent bacterial strains for the quantification of bioavailable heavy metals and toxicity testing. BMC Biotechnol. 2009, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- De Gennaro, G.; Daresta, B.E.; Ielpo, P.; Placentino, M. Analytical Methods for Determination of Metals in Environmental Sampes. Available online: http://www.regione.abruzzo.it/rivernet/docs/attivita/metodologie/lezioni/analytical-methods-for-determination-of-metals-in-environmental-samples.pdf (accessed on 16 April 2015).
- Tyler, G.; Jobin Yvon, S.A.S. ICP-OES, ICP-MS and AAS Techniques Compared; Horiba Group: Longjumeau, France, 2011; p. 11. [Google Scholar]
- Alloway, B.J. Heavy Metals in Soils; Springer: Berlin, Germany, 2013; p. 615. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hurdebise, Q.; Tarayre, C.; Fischer, C.; Colinet, G.; Hiligsmann, S.; Delvigne, F. Determination of Zinc, Cadmium and Lead Bioavailability in Contaminated Soils at the Single-Cell Level by a Combination of Whole-Cell Biosensors and Flow Cytometry. Sensors 2015, 15, 8981-8999. https://doi.org/10.3390/s150408981
Hurdebise Q, Tarayre C, Fischer C, Colinet G, Hiligsmann S, Delvigne F. Determination of Zinc, Cadmium and Lead Bioavailability in Contaminated Soils at the Single-Cell Level by a Combination of Whole-Cell Biosensors and Flow Cytometry. Sensors. 2015; 15(4):8981-8999. https://doi.org/10.3390/s150408981
Chicago/Turabian StyleHurdebise, Quentin, Cédric Tarayre, Christophe Fischer, Gilles Colinet, Serge Hiligsmann, and Frank Delvigne. 2015. "Determination of Zinc, Cadmium and Lead Bioavailability in Contaminated Soils at the Single-Cell Level by a Combination of Whole-Cell Biosensors and Flow Cytometry" Sensors 15, no. 4: 8981-8999. https://doi.org/10.3390/s150408981
APA StyleHurdebise, Q., Tarayre, C., Fischer, C., Colinet, G., Hiligsmann, S., & Delvigne, F. (2015). Determination of Zinc, Cadmium and Lead Bioavailability in Contaminated Soils at the Single-Cell Level by a Combination of Whole-Cell Biosensors and Flow Cytometry. Sensors, 15(4), 8981-8999. https://doi.org/10.3390/s150408981






