A Room-Temperature Operation Formaldehyde Sensing Material Printed Using Blends of Reduced Graphene Oxide and Poly(methyl methacrylate)
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Device Fabrication
2.3. Analysis
2.4. PMMA/RGO Sensing Film Preparation
2.5. Characteristics and Measurements
3. Results and Discussion
3.1. SEM and FTIR Analysis
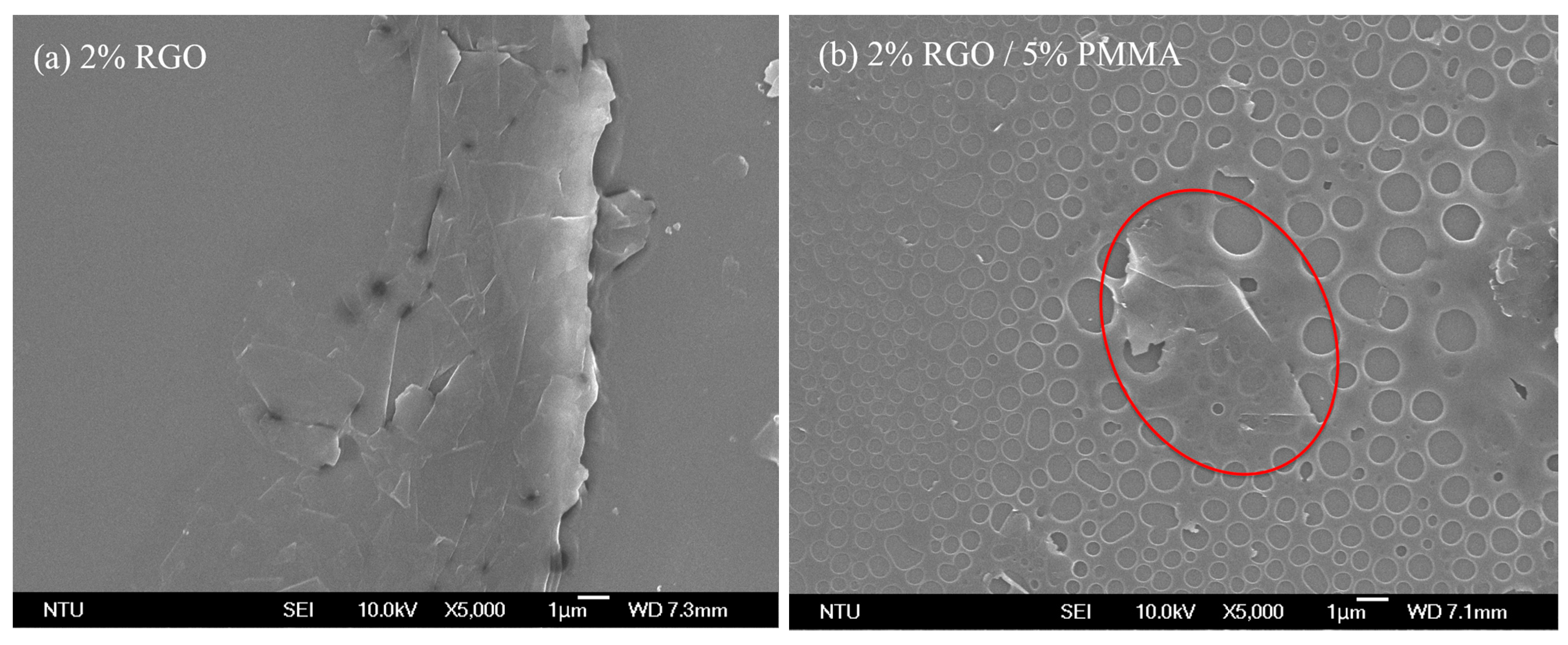

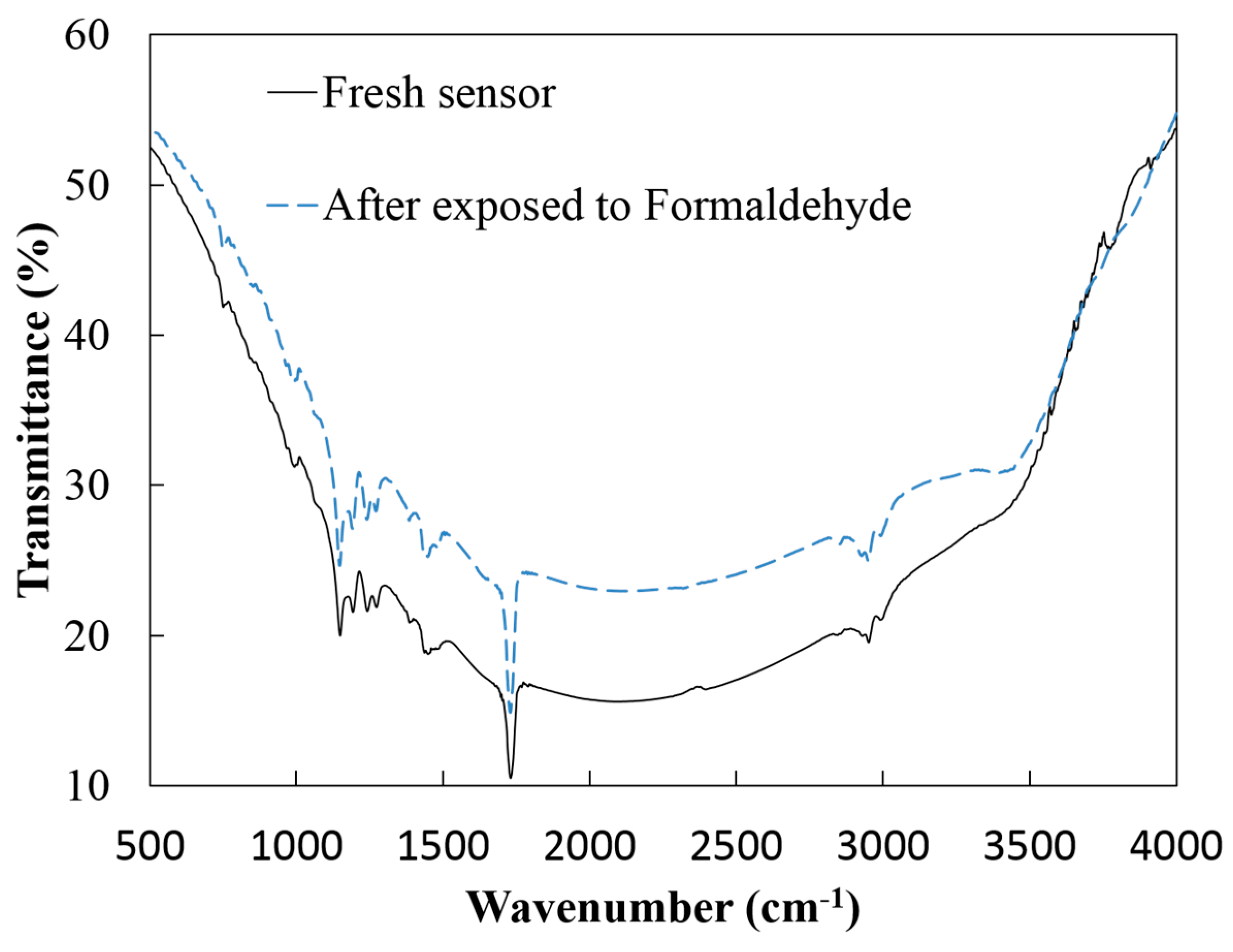
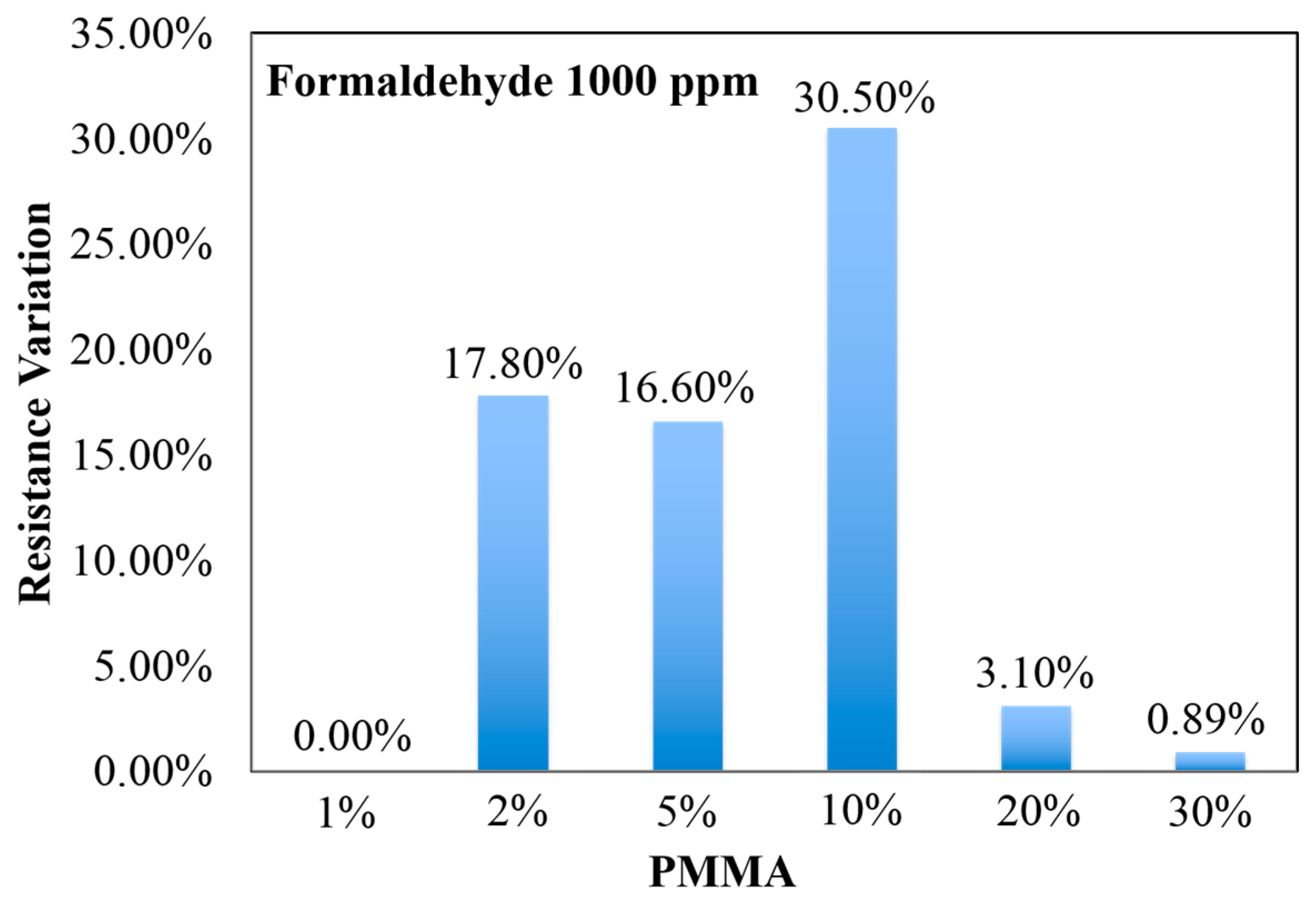
3.2. Sensor Characterization for Formaldehyde Detection
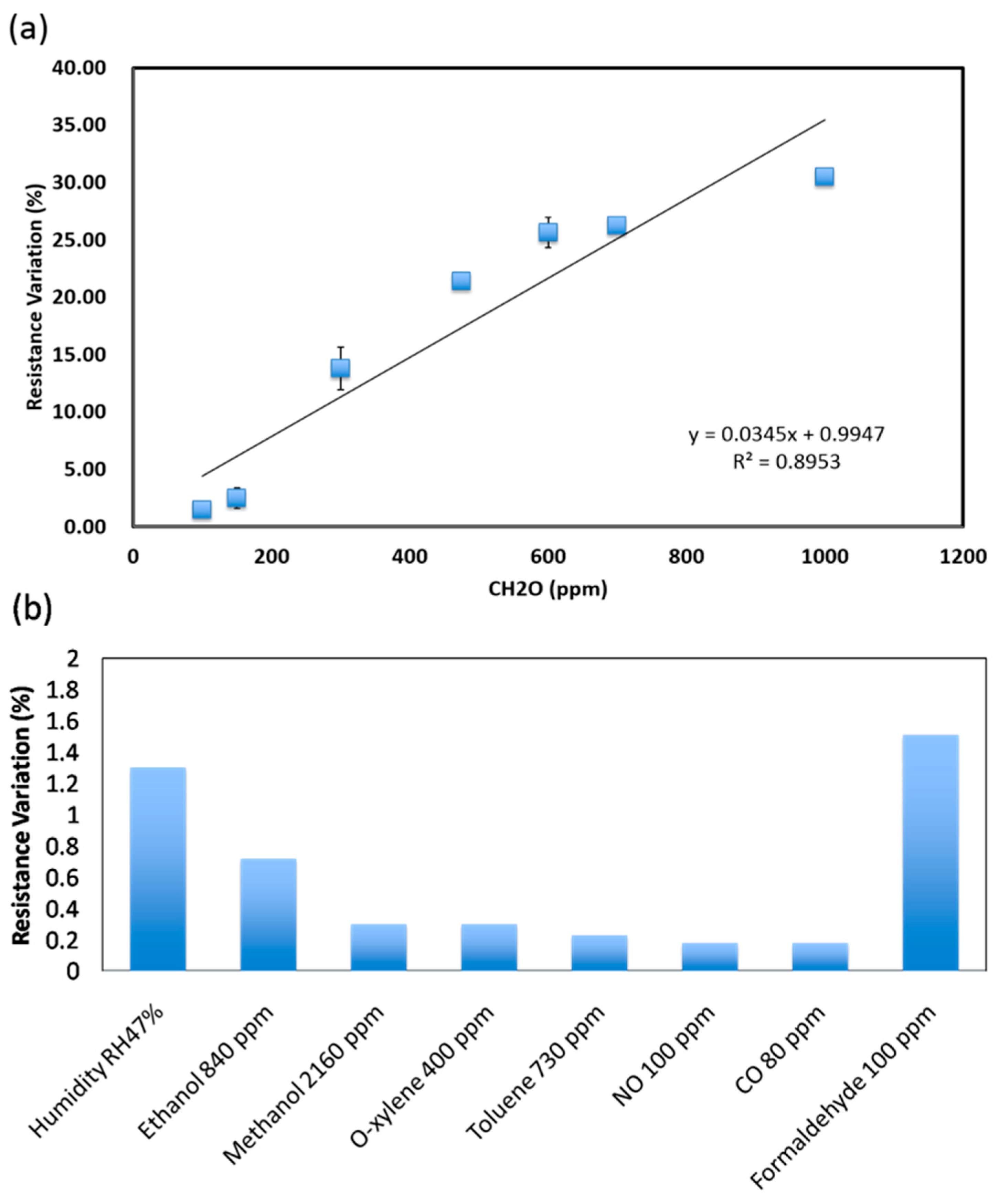
| Year [Reference] | Sensing Material(s) | Working Temperature | Sensitivity | Sensing Range |
|---|---|---|---|---|
| 2011 [22] | ZnO | 400 °C | 0.564 ppm−1 (Ra/Rg) | 1–1000 ppm |
| 2011 [24] | Cd activated Sn-ZnO | 200 °C | 10 ppm−1 (Ra/Rg) | 1–205 ppm |
| 2014 [35] | Polyaniline | 25 °C | 0.21% ppm−1 (△R/R0) | 0.4–400 ppm |
| 2015 [23] | In2O3/ZnO | 300 °C | 0.3 ppm−1 (Ra/Rg) | 100–2000 ppm |
| 2015 [19] | Pd-SnO2 | 160 °C | 0.188 ppm−1 (Ra/Rg) | 5–1000 ppm |
| This Work | RGO/PMMA | 25 °C | 0.043% ppm−1 (△R/R0) 0.007 ppm−1 (Rg/R0) | 10–1000 ppm |
3.3. Raman Spectroscopy
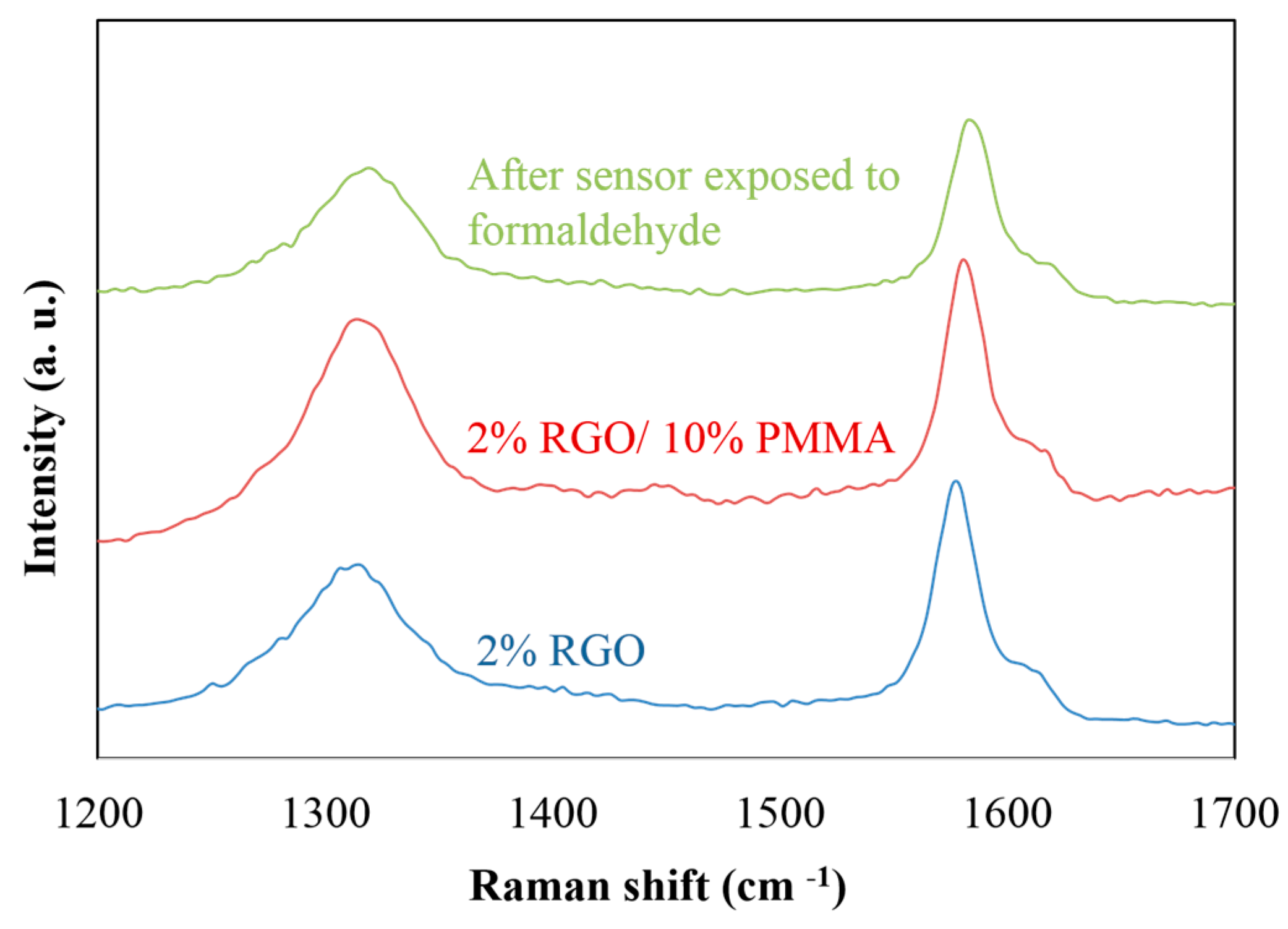
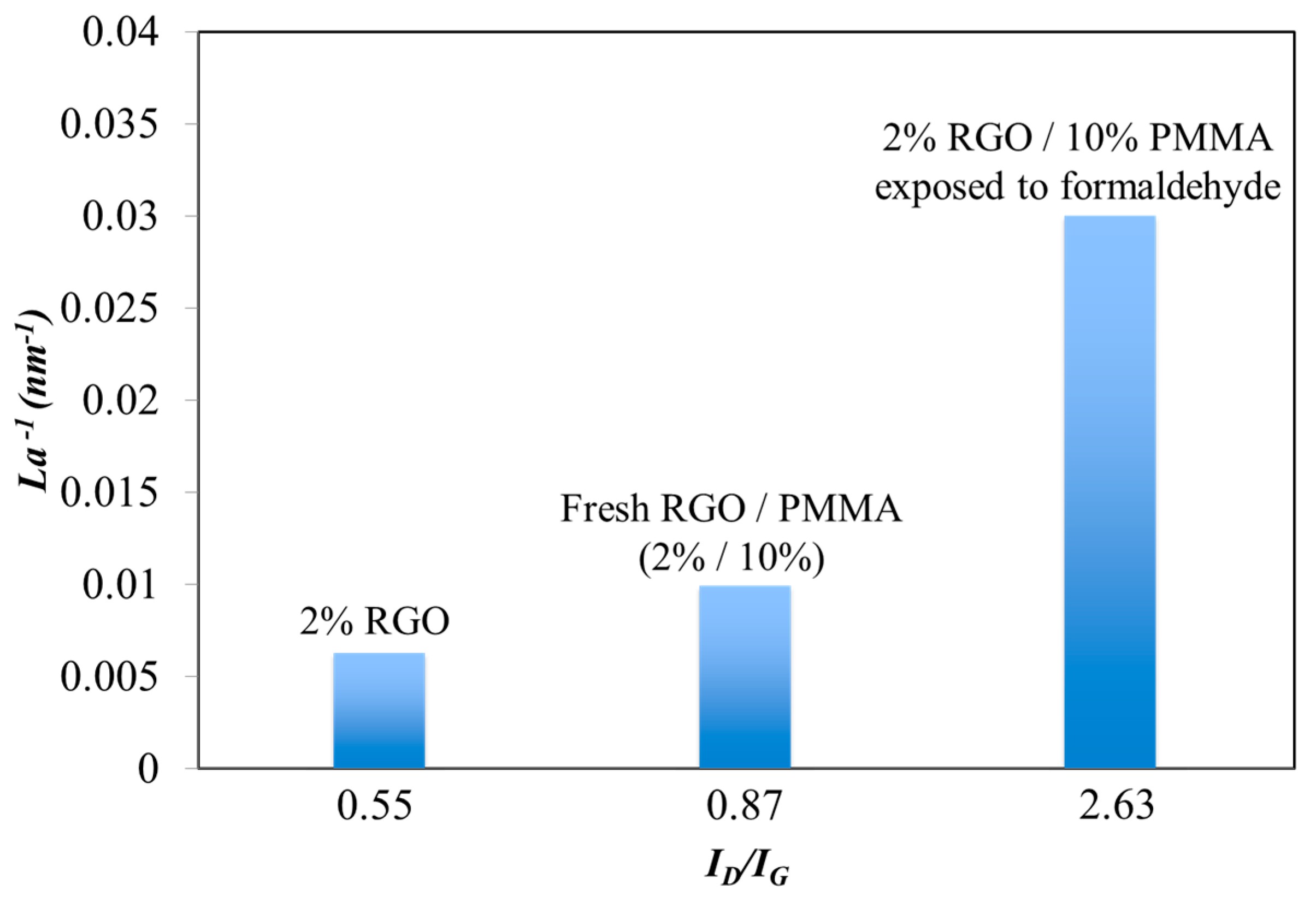
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chang, C.T.; Lin, K.L. Assessment of the strategies for reducing VOCs emission from polyurea-formaldehyde resin synthetic fiber leather industry in Taiwan. Resour. Conserv. Recycl. 2006, 46, 321–334. [Google Scholar] [CrossRef]
- Knake, R.; Jacquinot, P.; Hauser, P.C. Amperometric detection of gaseous formaldehyde in the ppb range. Electroanalysis 2001, 13, 631–634. [Google Scholar] [CrossRef]
- Salthammer, T.; Mentese, S.; Marutzky, R. Formaldehyde in the indoor environment. Chem. Rev. 2010, 110, 2536–2572. [Google Scholar] [CrossRef] [PubMed]
- Chung, F.C.; Zhu, Z.; Luo, P.Y.; Wu, R.J.; Li, W. Au@ZnO core–shell structure for gaseous formaldehyde sensing at room temperature. Sens. Actuators B: Chem. 2014, 199, 314–319. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, P.; Qi, J.Q.; Yao, P.J. Silicon-based micro-gas sensors for detecting formaldehyde. Sens. Actuators B Chem. 2009, B136, 399–404. [Google Scholar] [CrossRef]
- Xia, Y.X. Toxicity of Chemicals; Shanghai Science and Technology press: Shanghai, China, 1991. [Google Scholar]
- Cogliano, V.J.; Grosse, Y.; Bean, R.A.; Straif, K.; Secretan, M.B.; El Ghissassi, F. Summary of IARC monographs on formaldehydes, 2-butoxyethanol and 1-tert-butoxy-2-propanol. Environ. Health Perspect. 2005, 113, 1205–1208. [Google Scholar] [CrossRef] [PubMed]
- IARC. Formaldehyde, 2-butoxyethanol and 1-tert-butoxy-2-propanol in Evaluating Carcinogenic. In IARC Monographs on the Evaluation of Carcinogenic Risks to Human; IARC: Lyon, France, 2006; Volume 88, pp. 37–325. [Google Scholar]
- Rumchev, K.B.; Spickett, J.T.; Bulsara, M.K.; Philips, M.R.; Stick, S.M. Domestic exposure to formaldehyde significantly increases the risk of asthma in young children. Eur. Respir. J. 2002, 20, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Beane Freeman, L.E.; Blair, A.; Lubin, J.H.; Stewart, P.A.; Hayes, R.B.; Hoover, R.N.; Hauptmann, M. Mortality from lymphohematopoietic malignancies among workers in formaldehyde industries: The National Cancer Institute cohort. J. Natl. Cancer Inst. 2009, 101, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Kerns, W.D.; Pavkov, K.L.; Donofrio, D.J.; Gralla, E.J.; Swenberg, J.A. Carcinogenicity of formaldehyde in rats and mice after long-term inhalation exposure. Cancer Res. 1983, 43, 4382–4392. [Google Scholar] [PubMed]
- Blair, A.; Stewart, P.; O’Berg, M.; Gaffey, W.; Walrath, J.; Ward, J.; Bales, R.; Kaplan, S.; Cubit, D. Mortality among industrial workers exposed to formaldehyde. J. Natl. Cancer Inst. 1986, 76, 1071–1084. [Google Scholar] [PubMed]
- Liu, J.; Wang, W.; Li, S.; Liu, M.; He, S. Advances in SAW gas sensors based on the condensate-adsorption effect. Sensors 2011, 11, 11871–11884. [Google Scholar] [CrossRef] [PubMed]
- Mine, Y.; Melander, N.; Richter, D.; Lancaster, D.G.; Petrov, K.P.; Curl, R.F. Detection of formaldehyde using mid-infrared difference-frequency generation. Appl. Phys. B 1997, 65, 771–774. [Google Scholar] [CrossRef]
- Chung, P.R.; Tzeng, C.T.; Ke, M.T.; Lee, C.Y. Formaldehyde Gas Sensors: A Review. Sensors 2013, 13, 4468–4484. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wei, W.; Li, Y.; Li, F.; Zhou, J.; Sun, D.; Chen, Y.; Ruan, S. Preparation of Pd nanoparticle-decorated hollow SnO2 nanofibers and their enhanced formaldehyde sensing properties. J. Alloy. Compd. 2015, 651, 690–698. [Google Scholar] [CrossRef]
- Lv, P.; Tang, Z.A.; Yu, J.; Zhang, F.T.; Wei, G.F.; Huang, Z.X.; Hu, Y. Study on a micro-gas sensor with SnO2–NiO sensitive film for indoor formaldehyde detection. Sens. Actuators B Chem. 2008, 132, 54–80. [Google Scholar] [CrossRef]
- Daza, L.; Dassy, S.; Delmon, B. Chemical sensors based on SnO2 and WO3 for the detection of formaldehyde: Cooperative effects. Sens. Actuators B Chem. 1993, 10, 99–105. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, J.; Lu, H.; Gong, L.; Li, L.; Zheng, J.; Li, H.; Zhu, Z. High sensitive and selective formaldehyde sensors based on nanoparticle-assembled ZnO micro-octahedrons synthesized by homogeneous precipitation method. Sens. Actuators B Chem. 2011, 160, 364–370. [Google Scholar] [CrossRef]
- Dong, C.; Liu, X.; Han, B.; Deng, S.; Xiao, X.; Wang, Y. Nonaqueous synthesis of Ag-functionalized In2O3/ZnO nanocomposites for highly sensitive formaldehyde sensor. Sens. Actuators B Chem. 2015. [Google Scholar] [CrossRef]
- Han, N.; Wu, X.; Zhang, D.; Shen, G.; Liu, H.; Chen, Y. CdO activated Sn-doped ZnO for highly sensitive, selective and stable formaldehyde sensor. Sens. Actuators B Chem. 2011, 152, 324–329. [Google Scholar] [CrossRef]
- Chen, T.; Zhou, Z.; Wang, Y. Effects of calcining temperature on the phase structure and the formaldehyde gas sensing properties of CdO-mixed In2O3. Sens. Actuators B Chem. 2008, 135, 219–223. [Google Scholar] [CrossRef]
- Castro-Hurtado, I.; Mandayo, G.G.; Castaño, E. Conductometric Formaldehyde Gas Sensors. A Review: From Conventional Films to Nanostructured Materials. Thin Solid Films 2013, 548, 665–676. [Google Scholar] [CrossRef]
- Lee, C.-H.; Chuang, W.-Y.; Cowan, M.A.; Wu, W.-J.; Lin, C.-T. A low-power integrated humidity CMOS sensor by printing-on-chip technology. Sensors 2014, 14, 9247–9255. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.Y.; Lee, D.S.; Kyw Choi, H.; Lee, D.H.; Kim, J.-E.; Lee, J.Y.; Lee, W.J.; Kim, S.O.; Choi, S.-Y. Flexible room-temperature NO2 gas sensors based on carbon nanotubes/reduced graphene hybrid films. Appl. Phys. Lett. 2010, 96, 213105–213113. [Google Scholar] [CrossRef]
- Basua, S.; Bhattacharyya, P. Recent developments on graphene and graphene oxide based solid state gas sensors. Sens. Actuators B Chem. 2012, 173, 1–21. [Google Scholar] [CrossRef]
- Hill, E.W.; Vijayaragahvan, A.; Novoselov, K. Graphene sensors. IEEE Sens. J. 2011, 11, 3161–3170. [Google Scholar] [CrossRef]
- Chen, C.; Xu, K.; Ji, X.; Miao, L.; Jiang, J. Enhanced adsorption of acidic gases (CO2, NO2 and SO2) on light metal decorated grapheme oxide. Phys. Chem. Chem. Phys. 2014, 16, 11031–11036. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Jiang, Y.; Xie, T.; Tai, H.; Xie, G. A novel sensing mechanism for resistive gas sensors based on layered reduced graphene oxide thin films at room temperature. Sens. Actuators B Chem. 2014, 203, 135–142. [Google Scholar] [CrossRef]
- Yoon, H.J.; Jun, D.H.; Yang, J.H.; Zhou, Z.; Yang, S.S.; Cheng, M.M.C. Carbon dioxide gas sensor using a graphene sheet. Sens. Actuators B Chem. 2011, 157, 310–313. [Google Scholar] [CrossRef]
- Dua, V.; Surwade, S.P.; Ammu, S.; Agnihotra, S.R.; Jain, S.; Roberts, K.E.; Park, S.; Ruoff, R.S.; Manohar, S.K. All organic vapor sensor using inkjet-printed reduced graphene oxide. Angew. Chem. Int. Ed. 2010, 49, 2154–2157. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, T.; Soltani, L.H. Graphene/poly (methyl methacrylate) chemiresistor sensor for formaldehyde odor sensing. J. Hazard. Mater. 2013, 248–249, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Antwi-Boampong, S.; BelBruno, J.J. Detection of formaldehyde vapor using conductive polymer films. Sens. Actuators B Chem. 2013, 182, 300–306. [Google Scholar] [CrossRef]
- Tripathi, S.N.; Saini, P.; Gupta, D.; Choudhary, V. Electrical and mechanical properties of PMMA/reduced graphene oxide nanocomposites prepared via in situ polymerization. J. Mater. Sci. 2013, 48, 6223–6232. [Google Scholar] [CrossRef]
- Srinives, S.; Sarkar, T.; Mulchandani, A. Primary amine-functionalized polyaniline nanothin film sensor for detecting formaldehyde. Sens. Actuators B Chem. 2014, 194, 255–259. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Raman spectroscopy of amorphous, nanostructured, diamond-like carbon, and nanodiamond. Phil. Trans. R. Soc. Lond. 2004, A362, 2477–2512. [Google Scholar] [CrossRef] [PubMed]
- Cancado, L.G.; Jorio, A.; Martins Ferreira, E.H.; Stavale, F.; Achete, C.A.; Capaz, R.B.; Moutinho, M.V.O.; Lombardo, A.; Kulmala, T.S.; Ferrari, A.C. Quantifying Defects in Graphene via Raman Spectroscopy at Different Excitation Energies. Nano Lett. 2011, 11, 3190–3196. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuang, W.-Y.; Yang, S.-Y.; Wu, W.-J.; Lin, C.-T. A Room-Temperature Operation Formaldehyde Sensing Material Printed Using Blends of Reduced Graphene Oxide and Poly(methyl methacrylate). Sensors 2015, 15, 28842-28853. https://doi.org/10.3390/s151128842
Chuang W-Y, Yang S-Y, Wu W-J, Lin C-T. A Room-Temperature Operation Formaldehyde Sensing Material Printed Using Blends of Reduced Graphene Oxide and Poly(methyl methacrylate). Sensors. 2015; 15(11):28842-28853. https://doi.org/10.3390/s151128842
Chicago/Turabian StyleChuang, Wen-Yu, Sung-Yuan Yang, Wen-Jong Wu, and Chih-Ting Lin. 2015. "A Room-Temperature Operation Formaldehyde Sensing Material Printed Using Blends of Reduced Graphene Oxide and Poly(methyl methacrylate)" Sensors 15, no. 11: 28842-28853. https://doi.org/10.3390/s151128842
APA StyleChuang, W.-Y., Yang, S.-Y., Wu, W.-J., & Lin, C.-T. (2015). A Room-Temperature Operation Formaldehyde Sensing Material Printed Using Blends of Reduced Graphene Oxide and Poly(methyl methacrylate). Sensors, 15(11), 28842-28853. https://doi.org/10.3390/s151128842






