Design Strategies of Fluorescent Biosensors Based on Biological Macromolecular Receptors
Abstract
:1. Introduction
2. Auto-Fluorescent Protein (AFP) Based Biosensors
2.1. Dual AFP-Fused FRET-Based Biosensors
2.2. Single Circularly Permuted (cp) AFP-Based Biosensors
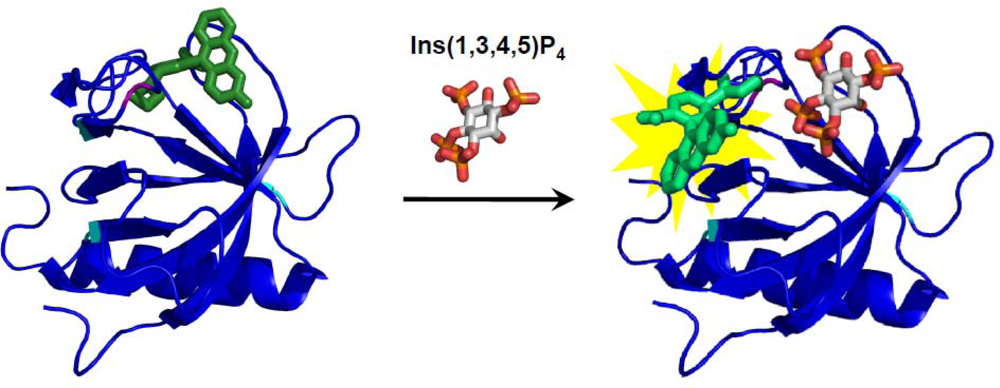
2.3. Split AFP-Based Biosensors
3. Chemically-Modified Protein Based Sensors
3.1. Incorporation of a Thiol Reactive Fluorophore by Engineering of a Mutant Receptor with a Unique Cysteine Residue
3.2. Site-Specific Unnatural Amino Acid Mutagenesis with an Expanded Genetic Code
3.3. Post-Photoaffinity Labeling Modification
4. Signaling Aptamers
4.1. Aptamer Sensors Covalently Modified with Synthetic Fluorophores
4.2. Dual Fluorophore-Labeled Aptamer Sensors
4.3. Modular Strategies for Tailoring Aptamer Sensors
5. Perspective
Acknowledgments
References and Notes
- Giepmans, B.N.; Adams, S.R.; Ellisman, M.H.; Tsien, R.Y. The Fluorescent Toolbox for Assessing Protein Location and Function. Science 2006, 312, 217–224. [Google Scholar]
- Johnsson, N.; Johnsson, K. Chemical Tools for Biomolecular Imaging. ACS Chem. Biol 2007, 2, 31–38. [Google Scholar]
- Rao, J.; Dragulescu-Andrasi, A.; Yao, H. Fluorescence Imaging in vivo: Recent Advances. Curr. Opin. Biotechnol 2007, 18, 17–25. [Google Scholar]
- Johnsson, K. Visualizing Biochemical Activities in Living Cells. Nat. Chem. Biol 2009, 5, 63–65. [Google Scholar]
- Wang, H.; Nakata, E.; Hamachi, I. Recent Progress in Strategies for the Creation of Protein-Based Fluorescent Biosensors. ChemBioChem 2009, 10, 2560–2577. [Google Scholar]
- de Silva, A.P.; Gunaratne, H.Q.; Gunnlaugsson, T.; Huxley, A.J.; McCoy, C.P.; Rademacher, J.T.; Rice, T.E. Signaling Recognition Events with Fluorescent Sensors and Switches. Chem. Rev 1997, 97, 1515–1566. [Google Scholar]
- Johnson, I. Fluorescent Probes for Living Cells. Histochem. J 1998, 30, 123–140. [Google Scholar]
- Terai, T.; Nagano, T. Fluorescent Probes for Bioimaging Applications. Curr. Opin. Chem. Biol 2008, 12, 515–521. [Google Scholar]
- Domaille, D.W.; Que, E.L.; Chang, C.J. Synthetic Fluorescent Sensors for Studying the Cell Biology of Metals. Nat. Chem. Biol 2008, 4, 168–175. [Google Scholar]
- Cao, H.; Heagy, M.D. Fluorescent Chemosensors for Carbohydrates: a Decade's Worth of Bright Spies for Saccharides in Review. J. Fluoresc 2004, 14, 569–584. [Google Scholar]
- Gomes, A.; Fernandes, E.; Lima, J.L. Use of Fluorescence Probes for Detection of Reactive Nitrogen Species: a Review. J. Fluoresc 2006, 16, 119–139. [Google Scholar]
- Soh, N. Recent Advances in Fluorescent Probes for the Detection of Reactive Oxygen Species. Anal. Bioanal. Chem 2006, 386, 532–543. [Google Scholar]
- Nolan, E.M.; Lippard, S.J. Small-Molecule Fluorescent Sensors for Investigating Zinc Metalloneurochemistry. Acc. Chem. Res 2009, 42, 193–203. [Google Scholar]
- Hellinga, H.W.; Marvin, J.S. Protein Engineering and the Development of Generic Biosensors. Trends Biotechnol 1998, 16, 183–189. [Google Scholar]
- Liu, J.; Cao, Z.; Lu, Y. Functional Nucleic Acid Sensors. Chem. Rev 2009, 109, 1948–1998. [Google Scholar]
- Stojanovic, M.N.; Kolpashchikov, D.M. Modular Aptameric Sensors. J. Am. Chem. Soc 2004, 126, 9266–9270. [Google Scholar]
- Hagihara, M.; Fukuda, M.; Hasegawa, T.; Morii, T. A Modular Strategy for Tailoring Fluorescent Biosensors from Ribonucleopeptide Complexes. J. Am. Chem. Soc 2006, 128, 12932–12940. [Google Scholar]
- Shimomura, O.; Johnson, F.H.; Saiga, Y. Extraction, Purification and Properties of Aequorin, a Bioluminescent Protein from the Luminous Hydromedusan, Aequorea. J. Cell Comp. Physiol 1962, 59, 223–239. [Google Scholar]
- Chalfie, M.; Tu, Y.; Euskirchen, G.; Ward, W.W.; Prasher, D.C. Green Fluorescent Protein as a Marker for Gene Expression. Science 1994, 263, 802–805. [Google Scholar]
- Lippincott-Schwartz, J.; Snapp, E.; Kenworthy, A. Studying Protein Dynamics in Living Cells. Nat. Rev. Mol. Cell. Biol 2001, 2, 444–456. [Google Scholar]
- Heim, R.; Prasher, D.C.; Tsien, R.Y. Wavelength Mutations and Posttranslational Autoxidation of Green Fluorescent Protein. Proc. Natl. Acad. Sci. USA 1994, 91, 12501–12504. [Google Scholar]
- Remington, S.J. Fluorescent Proteins: Maturation, Photochemistry and Photophysics. Curr. Opin. Struct. Biol 2006, 16, 714–721. [Google Scholar]
- Zhang, J.; Campbell, R.E.; Ting, A.Y.; Tsien, R.Y. Creating New Fluorescent Probes for Cell Biology. Nat. Rev. Mol. Cell Biol 2002, 3, 906–918. [Google Scholar]
- Kneen, M.; Farinas, J.; Li, Y.; Verkman, A.S. Green Fluorescent Protein as a Noninvasive Intracellular pH Indicator. Biophys. J 1998, 74, 1591–1599. [Google Scholar]
- Llopis, J.; McCaffery, J.M.; Miyawaki, A.; Farquhar, M.G.; Tsien, R.Y. Measurement of Cytosolic, Mitochondrial, and Golgi pH in Single Living Cells with Green Fluorescent Proteins. Proc. Natl. Acad. Sci. USA 1998, 95, 6803–6808. [Google Scholar]
- Miesenbock, G.; De Angelis, D.A.; Rothman, J.E. Visualizing Secretion and Synaptic Transmission with pH-Sensitive Green Fluorescent Proteins. Nature 1998, 394, 192–195. [Google Scholar]
- Wachter, R.M.; Yarbrough, D.; Kallio, K.; Remington, S.J. Crystallographic and Energetic Analysis of Binding of Selected Anions to the Yellow Variants of Green Fluorescent Protein. J. Mol. Biol 2000, 301, 157–171. [Google Scholar]
- Wachter, R.M.; Remington, S.J. Sensitivity of the Yellow Variant of Green Fluorescent Protein to Halides and Nitrate. Curr. Biol 1999, 9, R628–629. [Google Scholar]
- Jayaraman, S.; Haggie, P.; Wachter, R.M.; Remington, S.J.; Verkman, A.S. Mechanism and Cellular Applications of a Green Fluorescent Protein-Based Halide Sensor. J. Biol. Chem 2000, 275, 6047–6050. [Google Scholar]
- Chapleau, R.R.; Blomberg, R.; Ford, P.C.; Sagermann, M. Design of a Highly Specific and Noninvasive Biosensor Suitable for Real-Time in vivo Imaging of Mercury (II) Uptake. Protein Sci 2008, 17, 614–622. [Google Scholar]
- Barondeau, D.P.; Kassmann, C.J.; Tainer, J.A.; Getzoff, E.D. Structural Chemistry of a Green Fluorescent Protein Zn Biosensor. J. Am. Chem. Soc 2002, 124, 3522–3524. [Google Scholar]
- Mank, M.; Griesbeck, O. Genetically Encoded Calcium Indicators. Chem. Rev 2008, 108, 1550–1564. [Google Scholar]
- Garaschuk, O.; Griesbeck, O.; Konnerth, A. Troponin C-Based Biosensors: a New Family of Genetically Encoded Indicators for in vivo Calcium Imaging in the Nervous System. Cell Calcium 2007, 42, 351–361. [Google Scholar]
- Palmer, A.E.; Jin, C.; Reed, J.C.; Tsien, R.Y. Bcl-2-Mediated Alterations in Endoplasmic Reticulum Ca2+ Analyzed with an Improved Genetically Encoded Fluorescent Sensor. Proc. Natl. Acad. Sci. USA 2004, 101, 17404–17409. [Google Scholar]
- Palmer, A.E.; Giacomello, M.; Kortemme, T.; Hires, S.A.; Lev-Ram, V.; Baker, D.; Tsien, R.Y. Ca2+ Indicators Based on Computationally Redesigned Calmodulin-Peptide Pairs. Chem. Biol 2006, 13, 521–530. [Google Scholar]
- Miyawaki, A.; Llopis, J.; Heim, R.; McCaffery, J.M.; Adams, J.A.; Ikura, M.; Tsien, R.Y. Fluorescent Indicators for Ca2+ Based on Green Fluorescent Proteins and Calmodulin. Nature 1997, 388, 882–887. [Google Scholar]
- Mank, M.; Reiff, D.F.; Heim, N.; Friedrich, M.W.; Borst, A.; Griesbeck, O. A FRET-Based Calcium Biosensor with Fast Signal Kinetics and High Fluorescence Change. Biophys. J 2006, 90, 1790–1796. [Google Scholar]
- Nakai, J.; Ohkura, M.; Imoto, K. A High Signal-to-Noise Ca2+ Probe Composed of a Single Green Fluorescent Protein. Nat. Biotechnol 2001, 19, 137–141. [Google Scholar]
- Souslova, E.A.; Belousov, V.V.; Lock, J.G.; Stromblad, S.; Kasparov, S.; Bolshakov, A.P.; Pinelis, V.G.; Labas, Y.A.; Lukyanov, S.; Mayr, L.M.; Chudakov, D.M. Single Fluorescent Protein-Based Ca2+ Sensors with Increased Dynamic Range. BMC Biotechnol 2007, 7, 37. [Google Scholar]
- Baird, G.S.; Zacharias, D.A.; Tsien, R.Y. Circular Permutation and Receptor Insertion within Green Fluorescent Proteins. Proc. Natl. Acad. Sci. USA 1999, 96, 11241–11246. [Google Scholar]
- Romoser, V.A.; Hinkle, P.M.; Persechini, A. Detection in Living Cells of Ca2+-Dependent Changes in the Fluorescence Emission of an Indicator Composed of Two Green Fluorescent Protein Variants Linked by a Calmodulin-Binding Sequence. A New Class of Fluorescent Indicators. J. Biol. Chem 1997, 272, 13270–13274. [Google Scholar]
- Truong, K.; Sawano, A.; Mizuno, H.; Hama, H.; Tong, K.I.; Mal, T.K.; Miyawaki, A.; Ikura, M. FRET-Based in vivo Ca2+ Imaging by a New Calmodulin-GFP Fusion Molecule. Nat. Struct. Biol 2001, 8, 1069–1073. [Google Scholar]
- Nagai, T.; Sawano, A.; Park, E.S.; Miyawaki, A. Circularly Permuted Green Fluorescent Proteins Engineered to Sense Ca2+. Proc Natl. Acad. Sci. USA 2001, 98, 3197–3202. [Google Scholar]
- Imamura, H.; Nhat, K.P.; Togawa, H.; Saito, K.; Iino, R.; Kato-Yamada, Y.; Nagai, T.; Noji, H. Visualization of ATP Levels inside Single Living Cells with Fluorescence Resonance Energy Transfer-Based Genetically Encoded Indicators. Proc. Natl. Acad. Sci. USA 2009, 106, 15651–15656. [Google Scholar]
- DiPilato, L.M.; Cheng, X.; Zhang, J. Fluorescent Indicators of cAMP and Epac Activation Reveal Differential Dynamics of cAMP Signaling within Discrete Subcellular Compartments. Proc. Natl. Acad. Sci. USA 2004, 101, 16513–16518. [Google Scholar]
- Nikolaev, V.O.; Bunemann, M.; Schmitteckert, E.; Lohse, M.J.; Engelhardt, S. Cyclic AMP Imaging in Adult Cardiac Myocytes Reveals Far-Reaching β1-Adrenergic but Locally Confined β2-Adrenergic Receptor-Mediated Signaling. Circ. Res 2006, 99, 1084–1091. [Google Scholar]
- Zaccolo, M.; De Giorgi, F.; Cho, C.Y.; Feng, L.; Knapp, T.; Negulescu, P.A.; Taylor, S.S.; Tsien, R.Y.; Pozzan, T. A Genetically Encoded, Fluorescent Indicator for Cyclic AMP in Living Cells. Nat. Cell Biol 2000, 2, 25–29. [Google Scholar]
- Zaccolo, M.; Pozzan, T. Discrete Microdomains with High Concentration of cAMP in Stimulated Rat Neonatal Cardiac Myocytes. Science 2002, 295, 1711–1715. [Google Scholar]
- Sato, M.; Nakajima, T.; Goto, M.; Umezawa, Y. Cell-Based Indicator to Visualize Picomolar Dynamics of Nitric Oxide Release from Living Cells. Anal. Chem 2006, 78, 8175–8182. [Google Scholar]
- Honda, A.; Adams, S.R.; Sawyer, C.L.; Lev-Ram, V.; Tsien, R.Y.; Dostmann, W.R. Spatiotemporal Dynamics of Guanosine 3′,5′-Cyclic Monophosphate Revealed by a Genetically Encoded, Fluorescent Indicator. Proc. Natl. Acad. Sci. USA 2001, 98, 2437–2442. [Google Scholar]
- Nikolaev, V.O.; Gambaryan, S.; Lohse, M.J. Fluorescent Sensors for Rapid Monitoring of Intracellular cGMP. Nat. Methods 2006, 3, 23–25. [Google Scholar]
- Kaper, T.; Looger, L.L.; Takanaga, H.; Platten, M.; Steinman, L.; Frommer, W.B. Nanosensor Detection of an Immunoregulatory Tryptophan Influx/Kynurenine Efflux Cycle. PLoS Biol 2007, 5, e257. [Google Scholar]
- Okumoto, S.; Looger, L.L.; Micheva, K.D.; Reimer, R.J.; Smith, S.J.; Frommer, W.B. Detection of Glutamate Release from Neurons by Genetically Encoded Surface-Displayed FRET Nanosensors. Proc. Natl. Acad. Sci. USA 2005, 102, 8740–8745. [Google Scholar]
- Tsien, R.Y. Building and Breeding Molecules to Spy on Cells and Tumors. FEBS Lett 2005, 579, 927–932. [Google Scholar]
- Hirose, K.; Kadowaki, S.; Tanabe, M.; Takeshima, H.; Iino, M. Spatiotemporal Dynamics of Inositol 1,4,5-Trisphosphate That Underlies Complex Ca2+ Mobilization Patterns. Science 1999, 284, 1527–1530. [Google Scholar]
- Sakaguchi, R.; Endoh, T.; Yamamoto, S.; Tainaka, K.; Sugimoto, K.; Fujieda, N.; Kiyonaka, S.; Mori, Y.; Morii, T. A Single Circularly Permuted GFP Sensor for Inositol-1,3,4,5-Tetrakisphosphate Based on a Split PH Domain. Bioorg. Med. Chem 2009, 17, 7381–7386. [Google Scholar]
- Sapsford, K.E.; Berti, L.; Medintz, I.L. Materials for Fluorescence Resonance Energy Transfer Analysis: Beyond Traditional Donor-Acceptor Combinations. Angew. Chem. Int. Ed 2006, 45, 4562–4589. [Google Scholar]
- Piston, D.W.; Kremers, G.J. Fluorescent Protein FRET: The Good, the Bad and the Ugly. Trends Biochem. Sci 2007, 32, 407–414. [Google Scholar]
- Ohashi, T.; Galiacy, S.D.; Briscoe, G.; Erickson, H.P. An Experimental Study of GFP-Based FRET, with Application to Intrinsically Unstructured Proteins. Protein Sci 2007, 16, 1429–1438. [Google Scholar]
- Souslova, E.A.; Chudakov, D.M. Genetically Encoded Intracellular Sensors Based on Fluorescent Proteins. Biochemistry (Mosc) 2007, 72, 683–697. [Google Scholar]
- VanEngelenburg, S.B.; Palmer, A.E. Fluorescent Biosensors of Protein Function. Curr. Opin. Chem. Biol 2008, 12, 60–65. [Google Scholar]
- Carlson, H.J.; Campbell, R.E. Genetically Encoded FRET-Based Biosensors for Multiparameter Fluorescence Imaging. Curr. Opin. Biotechnol 2009, 20, 19–27. [Google Scholar]
- Brun, M.A.; Tan, K.T.; Nakata, E.; Hinner, M.J.; Johnsson, K. Semisynthetic Fluorescent Sensor Proteins Based on Self-Labeling Protein Tags. J. Am. Chem. Soc 2009, 131, 5873–5884. [Google Scholar]
- Keppler, A.; Gendreizig, S.; Gronemeyer, T.; Pick, H.; Vogel, H.; Johnsson, K. A General Method for the Covalent Labeling of Fusion Proteins with Small Molecules in vivo. Nat. Biotechnol 2003, 21, 86–89. [Google Scholar]
- Nausch, L.W.; Ledoux, J.; Bonev, A.D.; Nelson, M.T.; Dostmann, W.R. Differential Patterning of cGMP in Vascular Smooth Muscle Cells Revealed by Single GFP-Linked Biosensors. Proc. Natl. Acad. Sci. USA 2008, 105, 365–370. [Google Scholar]
- Belousov, V.V.; Fradkov, A.F.; Lukyanov, K.A.; Staroverov, D.B.; Shakhbazov, K.S.; Terskikh, A.V.; Lukyanov, S. Genetically Encoded Fluorescent Indicator for Intracellular Hydrogen Peroxide. Nat. Methods 2006, 3, 281–286. [Google Scholar]
- Dooley, C.T.; Dore, T.M.; Hanson, G.T.; Jackson, W.C.; Remington, S.J.; Tsien, R.Y. Imaging Dynamic Redox Changes in Mammalian Cells with Green Fluorescent Protein Indicators. J. Biol. Chem 2004, 279, 22284–22293. [Google Scholar]
- Mizuno, T.; Murao, K.; Tanabe, Y.; Oda, M.; Tanaka, T. Metal-Ion-Dependent GFP Emission in vivo by Combining a Circularly Permutated Green Fluorescent Protein with an Engineered Metal-Ion-Binding Coiled-Coil. J. Am. Chem. Soc 2007, 129, 11378–11383. [Google Scholar]
- Ghosh, I.; Hamilton, A.D.; Regan, L. Antiparallel Leucine Zipper-Directed Protein Reassembly: Application to the Green Fluorescent Protein. J. Am. Chem. Soc 2000, 122, 5658–5659. [Google Scholar]
- Stains, C.I.; Porter, J.R.; Ooi, A.T.; Segal, D.J.; Ghosh, I. DNA Sequence-Enabled Reassembly of the Green Fluorescent Protein. J. Am. Chem. Soc 2005, 127, 10782–10783. [Google Scholar]
- Demidov, V.V.; Dokholyan, N.V.; Witte-Hoffmann, C.; Chalasani, P.; Yiu, H.W.; Ding, F.; Yu, Y.; Cantor, C.R.; Broude, N.E. Fast Complementation of Split Fluorescent Protein Triggered by DNA Hybridization. Proc. Natl. Acad. Sci. USA 2006, 103, 2052–2056. [Google Scholar]
- Stains, C.I.; Furman, J.L.; Segal, D.J.; Ghosh, I. Site-Specific Detection of DNA Methylation Utilizing mCpG-SEER. J. Am. Chem. Soc 2006, 128, 9761–9765. [Google Scholar]
- Ozawa, T.; Natori, Y.; Sato, M.; Umezawa, Y. Imaging Dynamics of Endogenous Mitochondrial RNA in Single Living Cells. Nat. Methods 2007, 4, 413–419. [Google Scholar]
- Valencia-Burton, M.; McCullough, R.M.; Cantor, C.R.; Broude, N.E. RNA Visualization in Live Bacterial Cells Using Fluorescent Protein Complementation. Nat. Methods 2007, 4, 421–427. [Google Scholar]
- Marrero, M.B.; Schieffer, B.; Paxton, W.G.; Schieffer, E.; Bernstein, K.E. Electroporation of pp60c-src Antibodies Inhibits the Angiotensin II Activation of Phospholipase C-γ1 in Rat Aortic Smooth Muscle Cells. J. Biol. Chem 1995, 270, 15734–15738. [Google Scholar]
- Fenton, M.; Bone, N.; Sinclair, A.J. The Efficient and Rapid Import of a Peptide into Primary B and T Lymphocytes and a Lymphoblastoid Cell Line. J. Immunol. Methods 1998, 212, 41–48. [Google Scholar]
- Sakaguchi, R.; Tainaka, K.; Shimada, N.; Nakano, S.; Inoue, M.; Kiyonaka, S.; Mori, Y.; Morii, T. An in vivo Fluorescent Sensor Reveals Intracellular Ins(1,3,4,5)P4 Dynamics in Single Cells. Angew. Chem. Int. Ed 2009, 48. in press. [Google Scholar]
- Zelphati, O.; Wang, Y.; Kitada, S.; Reed, J.C.; Felgner, P.L.; Corbeil, J. Intracellular Delivery of Proteins with a New Lipid-Mediated Delivery System. J. Biol. Chem 2001, 276, 35103–35110. [Google Scholar]
- Zheng, X.; Lundberg, M.; Karlsson, A.; Johansson, M. Lipid-Mediated Protein Delivery of Suicide Nucleoside Kinases. Cancer Res 2003, 63, 6909–6913. [Google Scholar]
- Abarzua, P.; LoSardo, J.E.; Gubler, M.L.; Neri, A. Microinjection of Monoclonal Antibody PAb421 into Human SW480 Colorectal Carcinoma Cells Restores the Transcription Activation Function to Mutant p53. Cancer Res 1995, 55, 3490–3494. [Google Scholar]
- Wadia, J.S.; Dowdy, S.F. Transmembrane Delivery of Protein and Peptide Drugs by TAT-Mediated Transduction in the Treatment of Cancer. Adv. Drug. Deliv. Rev 2005, 57, 579–596. [Google Scholar]
- Sugimoto, K.; Nishida, M.; Otsuka, M.; Makino, K.; Ohkubo, K.; Mori, Y.; Morii, T. Novel Real-Time Sensors to Quantitatively Assess in vivo Inositol 1,4,5-Trisphosphate Production in Intact Cells. Chem. Biol 2004, 11, 475–485. [Google Scholar]
- Dwyer, M.A.; Hellinga, H.W. Periplasmic Binding Proteins: A Versatile Superfamily for Protein Engineering. Curr. Opin. Struct. Biol 2004, 14, 495–504. [Google Scholar]
- Marvin, J.S.; Corcoran, E.E.; Hattangadi, N.A.; Zhang, J.V.; Gere, S.A.; Hellinga, H.W. The Rational Design of Allosteric Interactions in a Monomeric Protein and Its Applications to the Construction of Biosensors. Proc. Natl. Acad. Sci. USA 1997, 94, 4366–4371. [Google Scholar]
- Marvin, J.S.; Hellinga, H.W. Engineering Biosensors by Introducing Fluorescent Allosteric Signal Transducers: Construction of a Novel Glucose Sensor. J. Am. Chem. Soc 1998, 120, 7–11. [Google Scholar]
- de Lorimier, R.M.; Smith, J.J.; Dwyer, M.A.; Looger, L.L.; Sali, K.M.; Paavola, C.D.; Rizk, S.S.; Sadigov, S.; Conrad, D.W.; Loew, L.; Hellinga, H.W. Construction of a Fluorescent Biosensor Family. Protein Sci 2002, 11, 2655–2675. [Google Scholar]
- Marvin, J.S.; Hellinga, H.W. Conversion of a Maltose Receptor into a Zinc Biosensor by Computational Design. Proc. Natl. Acad. Sci. USA 2001, 98, 4955–4960. [Google Scholar]
- Dwyer, M.A.; Looger, L.L.; Hellinga, H.W. Computational Design of a Zn2+ Receptor That Controls Bacterial Gene Expression. Proc. Natl. Acad. Sci. USA 2003, 100, 11255–11260. [Google Scholar]
- Looger, L.L.; Dwyer, M.A.; Smith, J.J.; Hellinga, H.W. Computational Design of Receptor and Sensor Proteins with Novel Functions. Nature 2003, 423, 185–190. [Google Scholar]
- Allert, M.; Rizk, S.S.; Looger, L.L.; Hellinga, H.W. Computational Design of Receptors for an Organophosphate Surrogate of the Nerve Agent Soman. Proc. Natl. Acad. Sci. USA 2004, 101, 7907–7912. [Google Scholar]
- Schreier, B.; Stumpp, C.; Wiesner, S.; Hocker, B. Computational Design of Ligand Binding Is Not a Solved Problem. Proc. Natl. Acad. Sci. USA 2009, 106, 18491–18496. [Google Scholar]
- Telmer, P.G.; Shilton, B.H. Structural Studies of an Engineered Zinc Biosensor Reveal an Unanticipated Mode of Zinc Binding. J. Mol. Biol 2005, 354, 829–840. [Google Scholar]
- Brune, M.; Hunter, J.L.; Corrie, J.E.; Webb, M.R. Direct, Real-Time Measurement of Rapid Inorganic Phosphate Release Using a Novel Fluorescent Probe and Its Application to Actomyosin Subfragment 1 ATPase. Biochemistry 1994, 33, 8262–8271. [Google Scholar]
- Gilardi, G.; Zhou, L.Q.; Hibbert, L.; Cass, A.E. Engineering the Maltose Binding Protein for Reagentless Fluorescence Sensing. Anal. Chem 1994, 66, 3840–3847. [Google Scholar]
- Brune, M.; Hunter, J.L.; Howell, S.A.; Martin, S.R.; Hazlett, T.L.; Corrie, J.E.; Webb, M.R. Mechanism of Inorganic Phosphate Interaction with Phosphate Binding Protein from Escherichia coli. Biochemistry 1998, 37, 10370–10380. [Google Scholar]
- Hirshberg, M.; Henrick, K.; Haire, L.L.; Vasisht, N.; Brune, M.; Corrie, J.E.; Webb, M.R. Crystal Structure of Phosphate Binding Protein Labeled with a Coumarin Fluorophore, a Probe for Inorganic Phosphate. Biochemistry 1998, 37, 10381–10385. [Google Scholar]
- Gilbert, S.P.; Webb, M.R.; Brune, M.; Johnson, K.A. Pathway of Processive ATP Hydrolysis by Kinesin. Nature 1995, 373, 671–676. [Google Scholar]
- Morii, T.; Sugimoto, K.; Makino, K.; Otsuka, M.; Imoto, K.; Mori, Y. A New Fluorescent Biosensor for Inositol Trisphosphate. J. Am. Chem. Soc 2002, 124, 1138–1139. [Google Scholar]
- Nishida, M.; Sugimoto, K.; Hara, Y.; Mori, E.; Morii, T.; Kurosaki, T.; Mori, Y. Amplification of Receptor Signaling by Ca2+ Entry-Mediated Translocation and Activation of PLCγ2 in B Lymphocytes. EMBO J 2003, 22, 4677–4688. [Google Scholar]
- Chan, P.H.; Liu, H.B.; Chen, Y.W.; Chan, K.C.; Tsang, C.W.; Leung, Y.C.; Wong, K.Y. Rational Design of a Novel Fluorescent Biosensor for β-Lactam Antibiotics from a Class a β-Lactamase. J. Am. Chem. Soc 2004, 126, 4074–4075. [Google Scholar]
- Chan, P.H.; So, P.K.; Ma, D.L.; Zhao, Y.; Lai, T.S.; Chung, W.H.; Chan, K.C.; Yiu, K.F.; Chan, H.W.; Siu, F.M.; Tsang, C.W.; Leung, Y.C.; Wong, K.Y. Fluorophore-Labeled β-Lactamase as a Biosensor for β-Lactam Antibiotics: A Study of the Biosensing Process. J. Am. Chem. Soc 2008, 130, 6351–6361. [Google Scholar]
- Wang, L.; Schultz, P.G. Expanding the Genetic Code. Angew. Chem. Int. Ed 2005, 44, 34–66. [Google Scholar]
- Xie, J.; Schultz, P.G. A Chemical Toolkit for Proteins—an Expanded Genetic Code. Nat. Rev. Mol. Cell Biol 2006, 7, 775–782. [Google Scholar]
- Hohsaka, T.; Sisido, M. Incorporation of Non-Natural Amino Acids into Proteins. Curr. Opin. Chem. Biol 2002, 6, 809–815. [Google Scholar]
- Anderson, R.D., III; Zhou, J.; Hecht, S.M. Fluorescence Resonance Energy Transfer between Unnatural Amino Acids in a Structurally Modified Dihydrofolate Reductase. J. Am. Chem. Soc 2002, 124, 9674–9675. [Google Scholar]
- Taki, M.; Hohsaka, T.; Murakami, H.; Taira, K.; Sisido, M. Position-Specific Incorporation of a Fluorophore-Quencher Pair into a Single Streptavidin through Orthogonal Four-Base Codon/Anticodon Pairs. J. Am. Chem. Soc 2002, 124, 14586–14590. [Google Scholar]
- Kajihara, D.; Abe, R.; Iijima, I.; Komiyama, C.; Sisido, M.; Hohsaka, T. FRET Analysis of Protein Conformational Change through Position-Specific Incorporation of Fluorescent Amino Acids. Nat. Methods 2006, 3, 923–929. [Google Scholar]
- Nakata, E.; Tsukiji, S.; Hamachi, I. Development of New Methods to Introduce Unnatural Functional Molecules into Native Proteins for Protein Engineering. Bull. Chem. Soc. Jpn 2007, 80, 1268–1279. [Google Scholar]
- Hamachi, I.; Nagase, T.; Shinkai, S. A General Semisynthetic Method for Fluorescent Saccharide-Biosensors Based on a Lectin. J. Am. Chem. Soc 2000, 122, 12065–12066. [Google Scholar]
- Nakata, E.; Nagase, T.; Shinkai, S.; Hamachi, I. Coupling a Natural Receptor Protein with an Artificial Receptor to Afford a Semisynthetic Fluorescent Biosensor. J. Am. Chem. Soc 2004, 126, 490–495. [Google Scholar]
- Koshi, Y.; Nakata, E.; Hamachi, I. Luminescent Saccharide Biosensor by Using Lanthanide-Bound Lectin Labeled with Fluorescein. ChemBioChem 2005, 6, 1349–1352. [Google Scholar]
- Nakata, E.; Koshi, Y.; Koga, E.; Katayama, Y.; Hamachi, I. Double-Modification of Lectin Using Two Distinct Chemistries for Fluorescent Ratiometric Sensing and Imaging Saccharides in Test Tube or in Cell. J. Am. Chem. Soc 2005, 127, 13253–13261. [Google Scholar]
- Nakata, E.; Wang, H.; Hamachi, I. Ratiometric Fluorescent Biosensor for Real-Time and Label-Free Monitoring of Fine Saccharide Metabolic Pathways. ChemBioChem 2008, 9, 25–28. [Google Scholar]
- Tsukiji, S.; Miyagawa, M.; Takaoka, Y.; Tamura, T.; Hamachi, I. Ligand-Directed Tosyl Chemistry for Protein Labeling in vivo. Nat. Chem. Biol 2009, 5, 341–343. [Google Scholar]
- Tsukiji, S.; Wang, H.; Miyagawa, M.; Tamura, T.; Takaoka, Y.; Hamachi, I. Quenched Ligand-Directed Tosylate Reagents for One-Step Construction of Turn-on Fluorescent Biosensors. J. Am. Chem. Soc 2009, 131, 9046–9054. [Google Scholar]
- Ellington, A.D. RNA Selection. Aptamers Achieve the Desired Recognition. Curr. Biol 1994, 4, 427–429. [Google Scholar]
- Ellington, A.D.; Szostak, J.W. In vitro Selection of RNA Molecules That Bind Specific Ligands. Nature 1990, 346, 818–822. [Google Scholar]
- Gold, L.; Polisky, B.; Uhlenbeck, O.; Yarus, M. Diversity of Oligonucleotide Functions. Annu. Rev. Biochem 1995, 64, 763–797. [Google Scholar]
- Osborne, S.E.; Ellington, A.D. Nucleic Acid Selection and the Challenge of Combinatorial Chemistry. Chem. Rev 1997, 97, 349–370. [Google Scholar]
- Wilson, D.S.; Szostak, J.W. In vitro Selection of Functional Nucleic Acids. Annu. Rev. Biochem 1999, 68, 611–647. [Google Scholar]
- Westhof, E.; Patel, D.J. Nucleic Acids. From Self-Assembly to Induced-Fit Recognition. Curr. Opin. Struct. Biol 1997, 7, 305–309. [Google Scholar]
- Mok, W.; Li, Y. Recent Progress in Nucleic Acid Aptamer-Based Biosensors and Bioassays. Sensors 2008, 8, 7050–7084. [Google Scholar]
- Jhaveri, S.D.; Kirby, R.; Conrad, R.; Maglott, E.J.; Bowser, M.; Kennedy, R.T.; Glick, G.; Ellington, A.D. Designed Signaling Aptamers That Transduce Molecular Recognition to Changes in Fluorescence Intensity. J. Am. Chem. Soc 2000, 122, 2469–2473. [Google Scholar]
- Jhaveri, S.; Rajendran, M.; Ellington, A.D. In vitro Selection of Signaling Aptamers. Nat. Biotechnol 2000, 18, 1293–1297. [Google Scholar]
- Yamana, K.; Ohtani, Y.; Nakano, H.; Saito, I. Bis-Pyrene Labeled DNA Aptamer as an Intelligent Fluorescent Biosensor. Bioorg. Med. Chem. Lett 2003, 13, 3429–3431. [Google Scholar]
- Chamberlin, S.I.; Merino, E.J.; Weeks, K.M. Catalysis of Amide Synthesis by RNA Phosphodiester and Hydroxyl Groups. Proc. Natl. Acad. Sci. USA 2002, 99, 14688–14693. [Google Scholar]
- Merino, E.J.; Wilkinson, K.A.; Coughlan, J.L.; Weeks, K.M. RNA Structure Analysis at Single Nucleotide Resolution by Selective 2′-Hydroxyl Acylation and Primer Extension (SHAPE). J. Am. Chem. Soc 2005, 127, 4223–4231. [Google Scholar]
- Kamekawa, N.; Shimomura, Y.; Nakamura, M.; Yamana, K. Pyrene-Modified DNA Aptamer as a Fluorescent Biosensor with High Affinity and Specificity for ATP Sensing. Chem. Lett 2006, 35, 660–661. [Google Scholar]
- Merino, E.J.; Weeks, K.M. Facile Conversion of Aptamers into Sensors Using a 2′-Ribose-Linked Fluorophore. J. Am. Chem. Soc 2005, 127, 12766–12767. [Google Scholar]
- Katilius, E.; Katiliene, Z.; Woodbury, N.W. Signaling Aptamers Created Using Fluorescent Nucleotide Analogues. Anal. Chem 2006, 78, 6484–6489. [Google Scholar]
- Ueyama, H.; Takagi, M.; Takenaka, S. A Novel Potassium Sensing in Aqueous Media with a Synthetic Oligonucleotide Derivative. Fluorescence Resonance Energy Transfer Associated with Guanine Quartet-Potassium Ion Complex Formation. J. Am. Chem. Soc 2002, 124, 14286–14287. [Google Scholar]
- Nagatoishi, S.; Nojima, T.; Galezowska, E.; Juskowiak, B.; Takenaka, S. G Quadruplex-Based FRET Probes with the Thrombin-Binding Aptamer (TBA) Sequence Designed for the Efficient Fluorometric Detection of the Potassium Ion. ChemBioChem 2006, 7, 1730–1737. [Google Scholar]
- Ono, A.; Togashi, H. Highly Selective Oligonucleotide-Based Sensor for Mercury(II) in Aqueous Solutions. Angew. Chem. Int. Ed 2004, 43, 4300–4302. [Google Scholar]
- Stojanovic, M.N.; de Prada, P.; Landry, D.W. Aptamer-Based Folding Fluorescent Sensor for Cocaine. J. Am. Chem. Soc 2001, 123, 4928–4931. [Google Scholar]
- Ozaki, H.; Nishihira, A.; Wakabayashi, M.; Kuwahara, M.; Sawai, H. Biomolecular Sensor Based on Fluorescence-Labeled Aptamer. Bioorg. Med. Chem. Lett 2006, 16, 4381–4384. [Google Scholar]
- Urata, H.; Nomura, K.; Wada, S.; Akagi, M. Fluorescent-Labeled Single-Strand ATP Aptamer DNA: Chemo- and Enantio-Selectivity in Sensing Adenosine. Biochem. Biophys. Res. Commun 2007, 360, 459–463. [Google Scholar]
- Li, J.J.; Fang, X.; Schuster, S.M.; Tan, W. Molecular Beacons: A Novel Approach to Detect Protein - DNA Interactions. Angew. Chem. Int. Ed 2000, 39, 1049–1052. [Google Scholar]
- Hamaguchi, N.; Ellington, A.; Stanton, M. Aptamer Beacons for the Direct Detection of Proteins. Anal. Biochem 2001, 294, 126–131. [Google Scholar]
- Yang, C.J.; Jockusch, S.; Vicens, M.; Turro, N.J.; Tan, W. Light-Switching Excimer Probes for Rapid Protein Monitoring in Complex Biological Fluids. Proc. Natl. Acad. Sci. USA 2005, 102, 17278–17283. [Google Scholar]
- Yamamoto, R.; Baba, T.; Kumar, P.K. Molecular Beacon Aptamer Fluoresces in the Presence of Tat Protein of HIV-1. Genes Cells 2000, 5, 389–396. [Google Scholar]
- Stojanovic, M.N.; de Prada, P.; Landry, D.W. Fluorescent Sensors Based on Aptamer Self-Assembly. J. Am. Chem. Soc 2000, 122, 11547–11548. [Google Scholar]
- Nutiu, R.; Li, Y. Structure-Switching Signaling Aptamers. J. Am. Chem. Soc 2003, 125, 4771–4778. [Google Scholar]
- Nutiu, R.; Li, Y. A DNA-Protein Nanoengine For “On-Demand” Release and Precise Delivery of Molecules. Angew. Chem. Int. Ed 2005, 44, 5464–5467. [Google Scholar]
- Morii, T.; Hagihara, M.; Sato, S.; Makino, K. In vitro Selection of ATP-Binding Receptors Using a Ribonucleopeptide Complex. J. Am. Chem. Soc 2002, 124, 4617–4622. [Google Scholar]
- Fukuda, M.; Hayashi, H.; Hasegawa, T.; Morii, T. Development of a Fluorescent Ribonucleopeptide Sensor for Histamine. Trans. Mat. Res. Soc. Jpn 2009, 34, 525–527. [Google Scholar]
- Hasegawa, T.; Ohkubo, K.; Yoshikawa, S.; Morii, T. A Ribonucleopeptide Receptor Targets Phosphotyrosine. J. Surf. Sci. Nanotech 2005, 3, 33–37. [Google Scholar]
- Hasegawa, T.; Hagihara, M.; Fukuda, M.; Nakano, S.; Fujieda, N.; Morii, T. Context-Dependent Fluorescence Detection of a Phosphorylated Tyrosine Residue by a Ribonucleopeptide. J. Am. Chem. Soc 2008, 130, 8804–8812. [Google Scholar]




© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tainaka, K.; Sakaguchi, R.; Hayashi, H.; Nakano, S.; Liew, F.F.; Morii, T. Design Strategies of Fluorescent Biosensors Based on Biological Macromolecular Receptors. Sensors 2010, 10, 1355-1376. https://doi.org/10.3390/s100201355
Tainaka K, Sakaguchi R, Hayashi H, Nakano S, Liew FF, Morii T. Design Strategies of Fluorescent Biosensors Based on Biological Macromolecular Receptors. Sensors. 2010; 10(2):1355-1376. https://doi.org/10.3390/s100201355
Chicago/Turabian StyleTainaka, Kazuki, Reiko Sakaguchi, Hironori Hayashi, Shun Nakano, Fong Fong Liew, and Takashi Morii. 2010. "Design Strategies of Fluorescent Biosensors Based on Biological Macromolecular Receptors" Sensors 10, no. 2: 1355-1376. https://doi.org/10.3390/s100201355
APA StyleTainaka, K., Sakaguchi, R., Hayashi, H., Nakano, S., Liew, F. F., & Morii, T. (2010). Design Strategies of Fluorescent Biosensors Based on Biological Macromolecular Receptors. Sensors, 10(2), 1355-1376. https://doi.org/10.3390/s100201355



