Electrochemical Metal Ion Sensors. Exploiting Amino Acids and Peptides as Recognition Elements
Abstract
:Introduction
Complexes of Metal Ions with Peptides
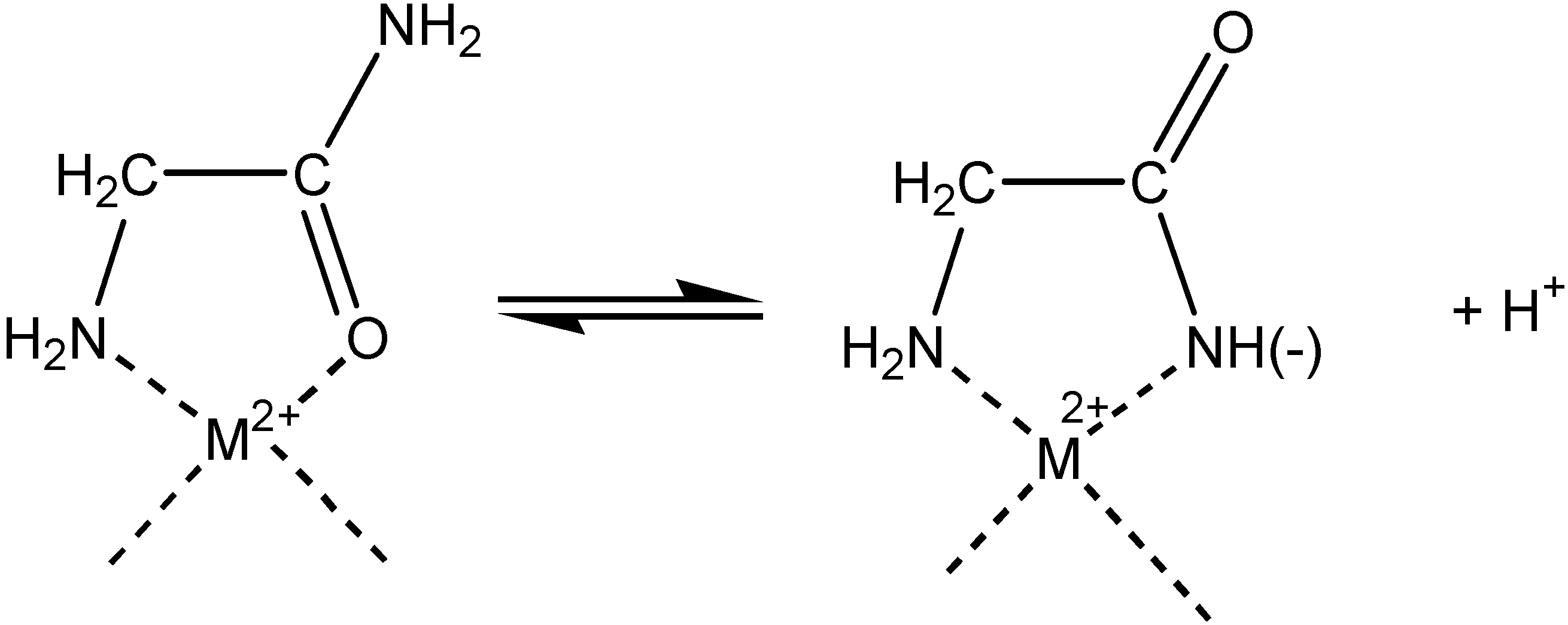
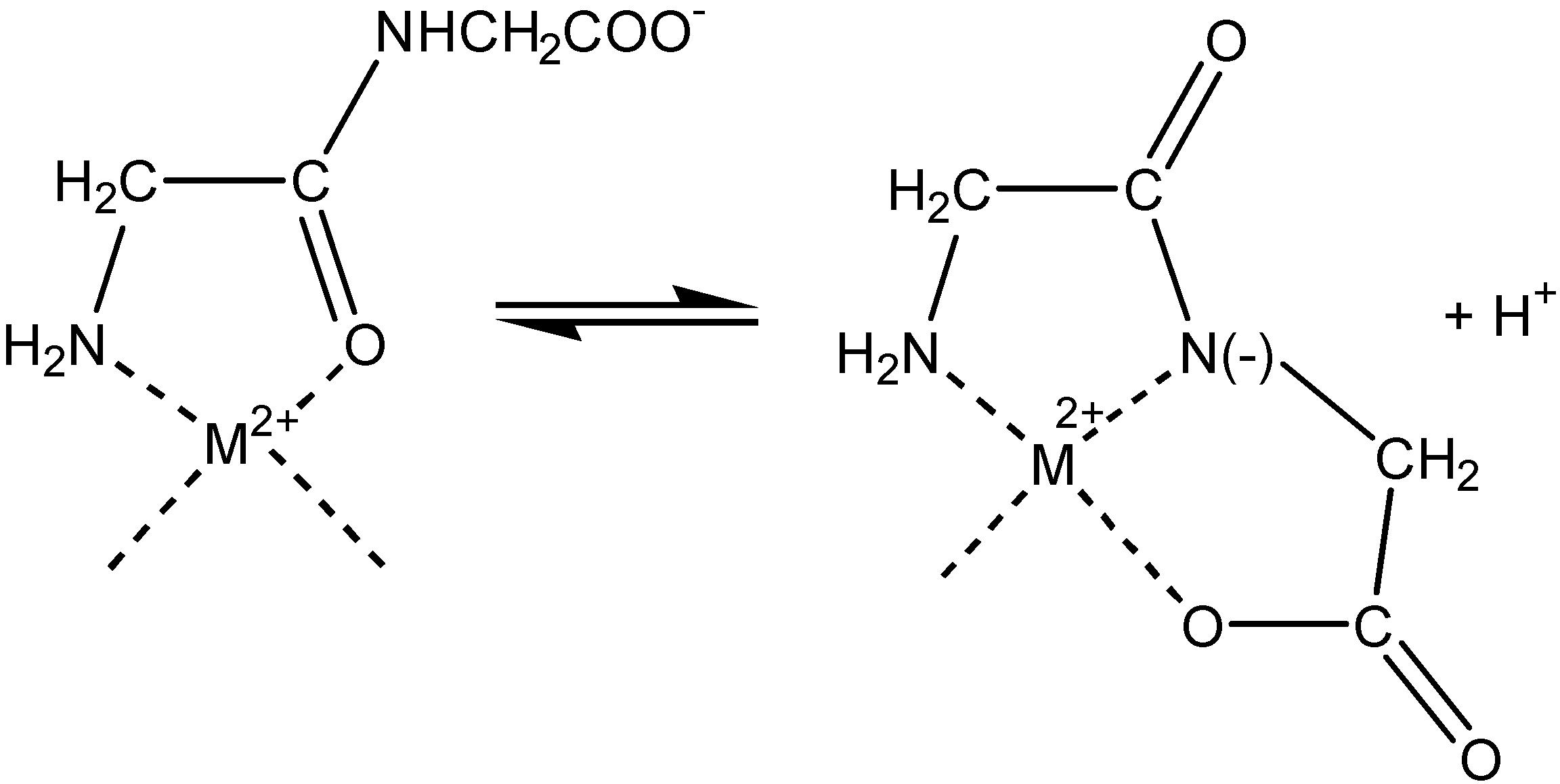
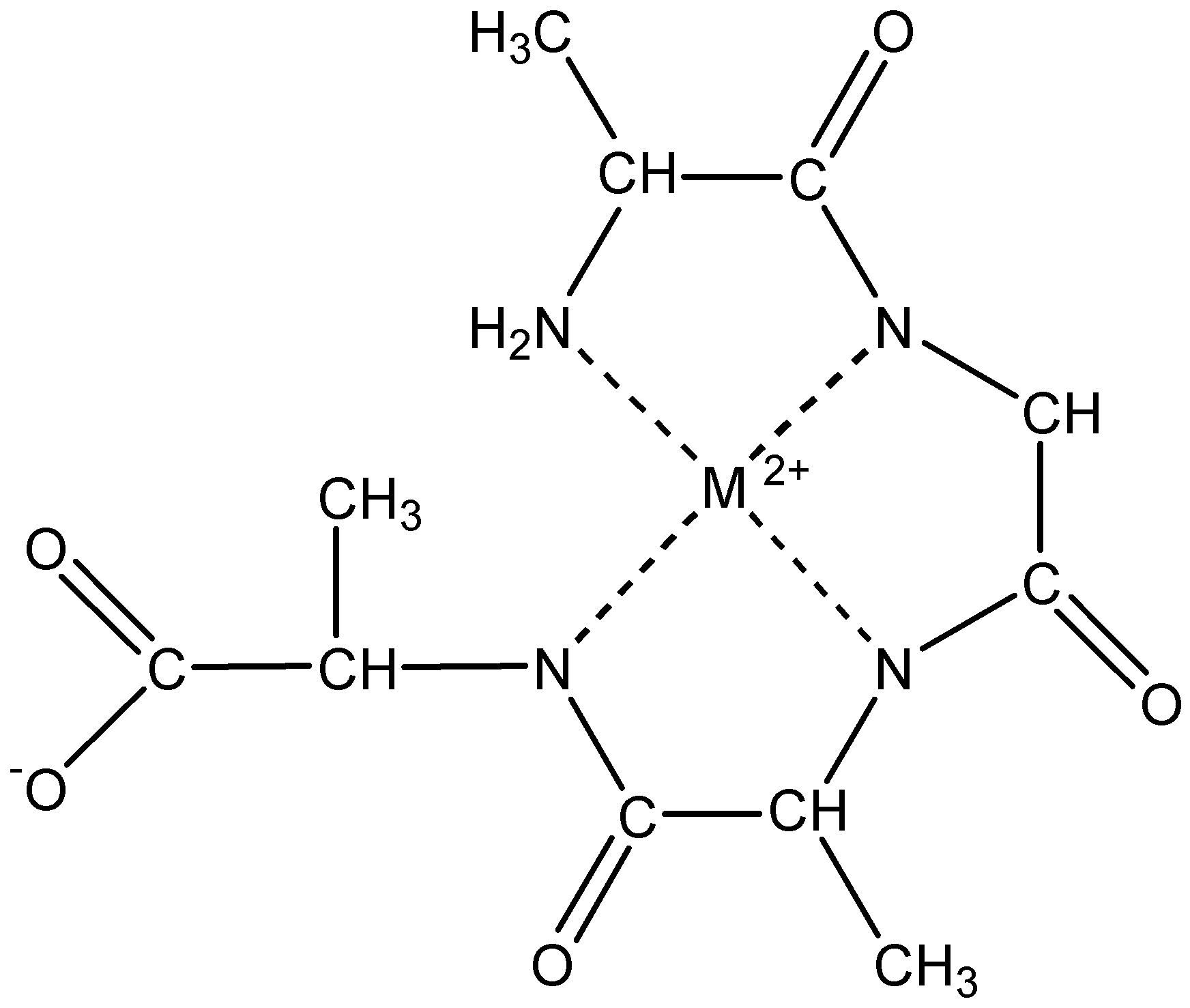
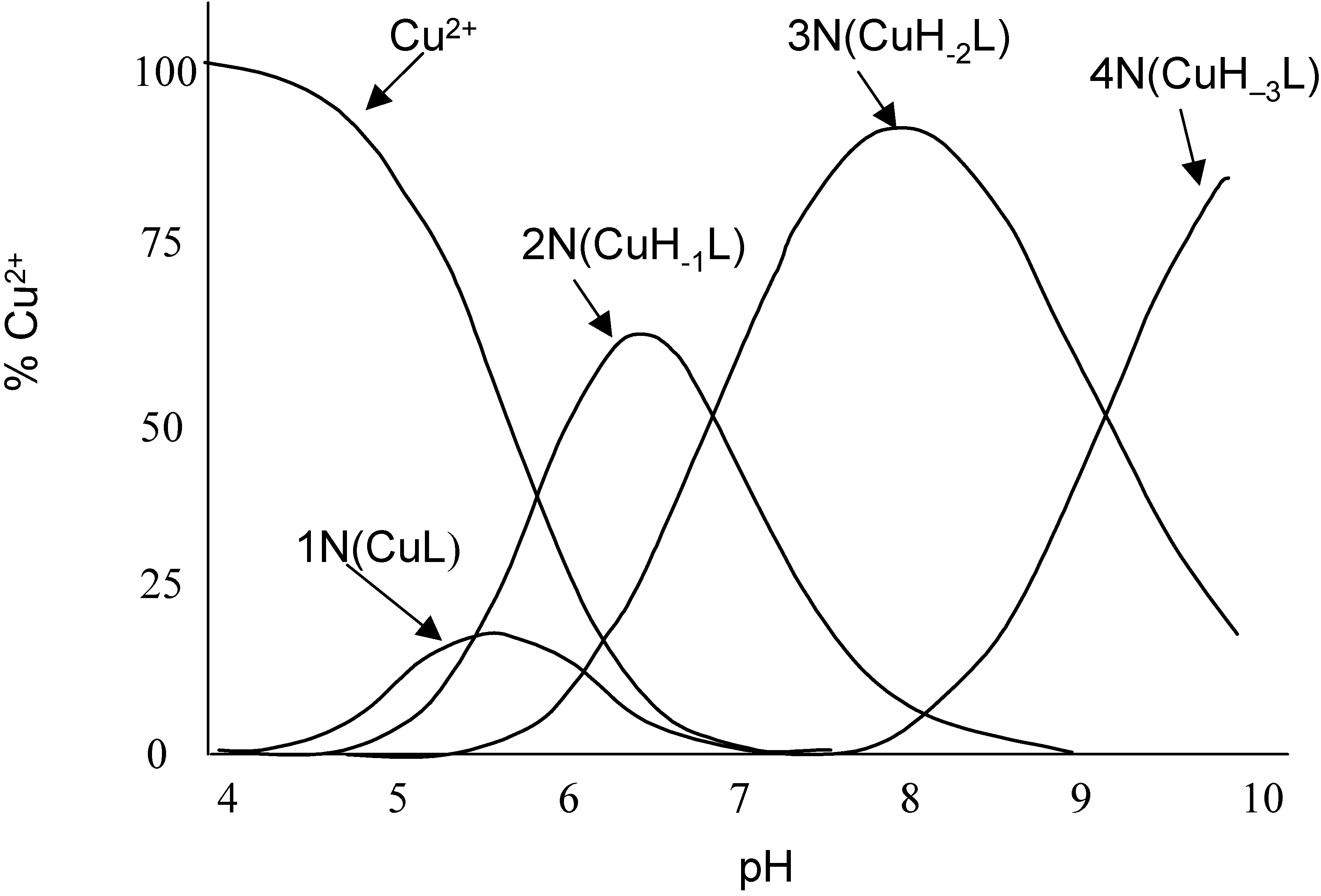

Electrochemistry of peptides and amino acids in the presence of complexing metal ions
Peptides on surfaces
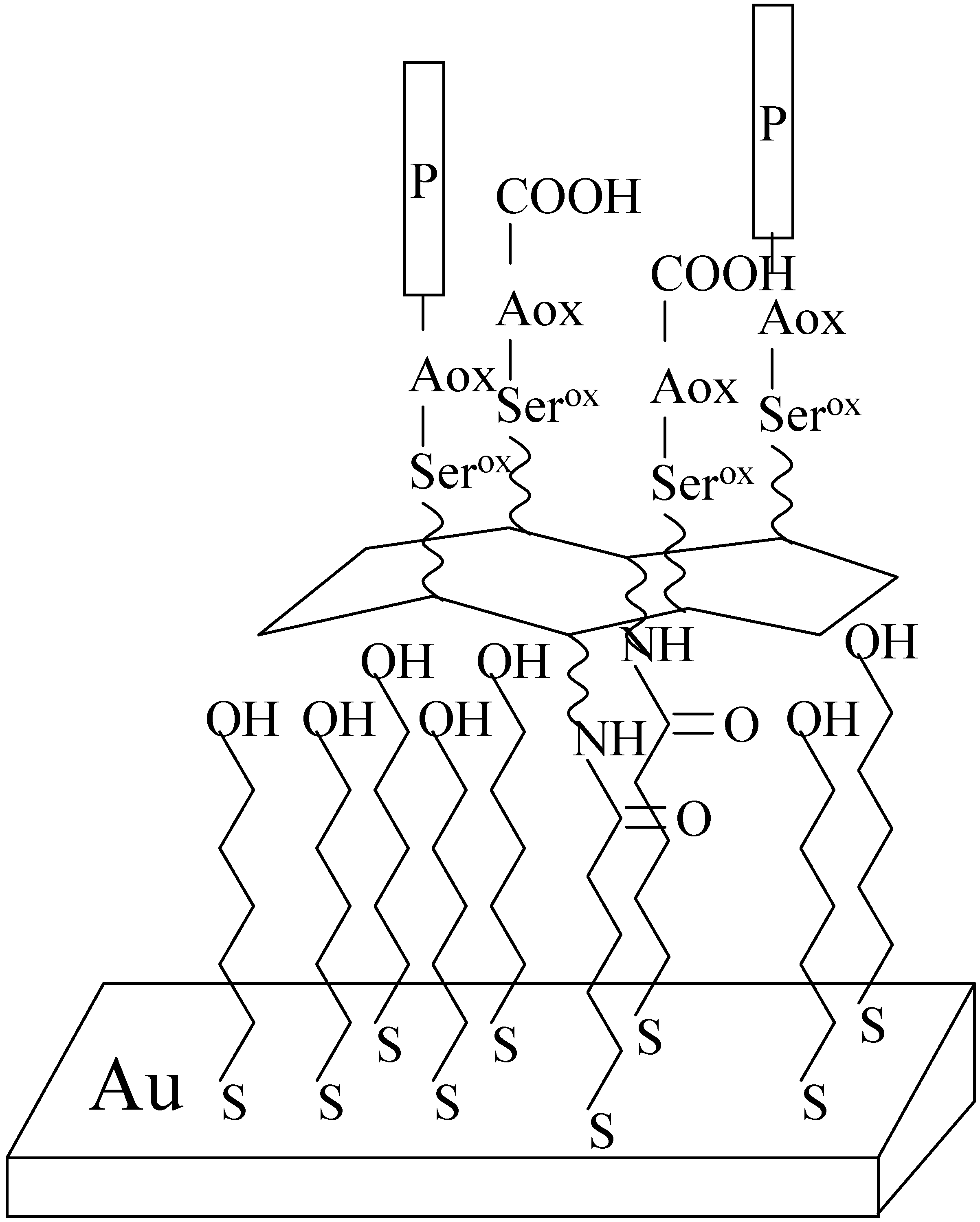
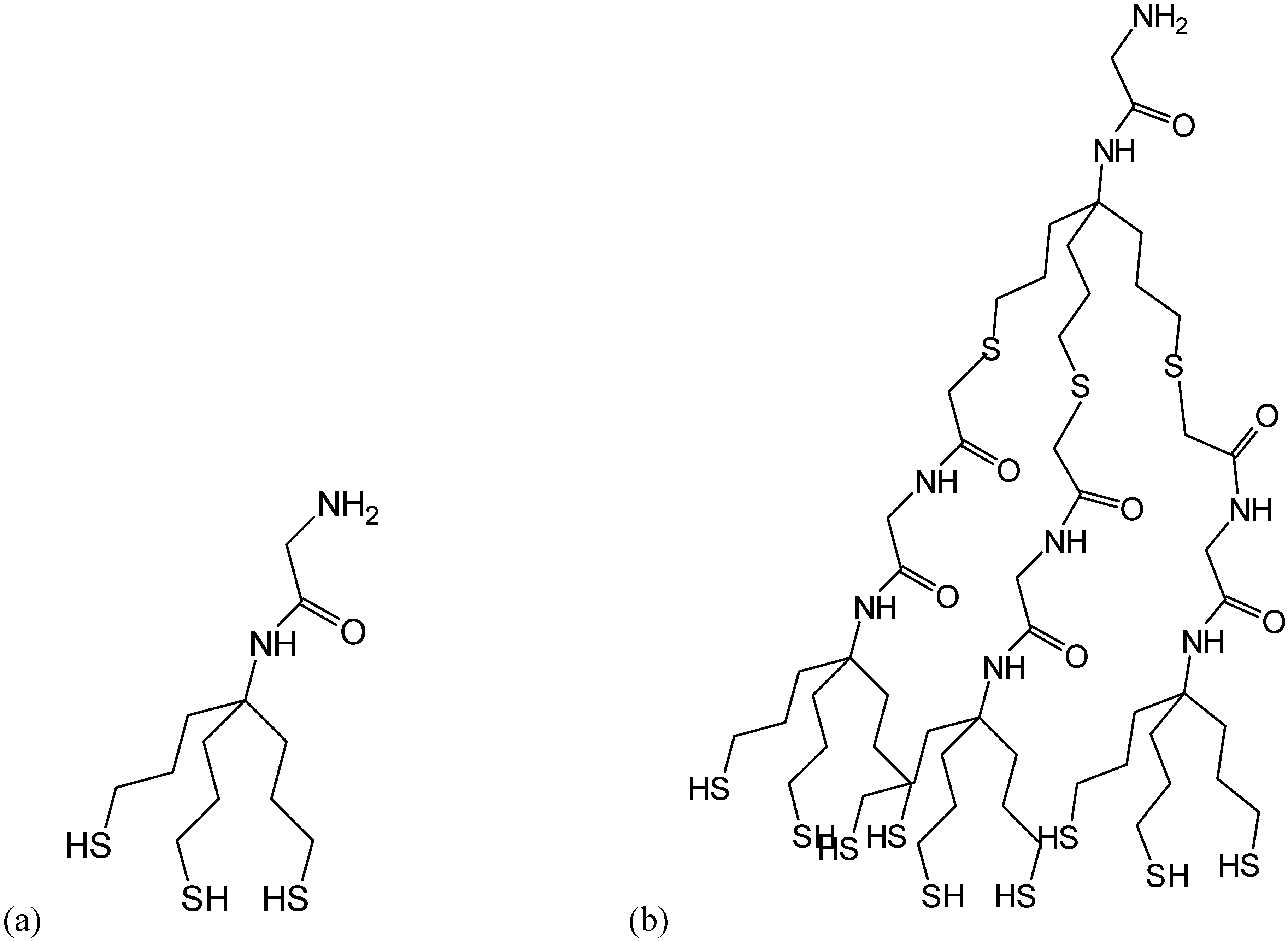
Methods for investigation peptides on surfaces
Peptides on surfaces as metal sensors


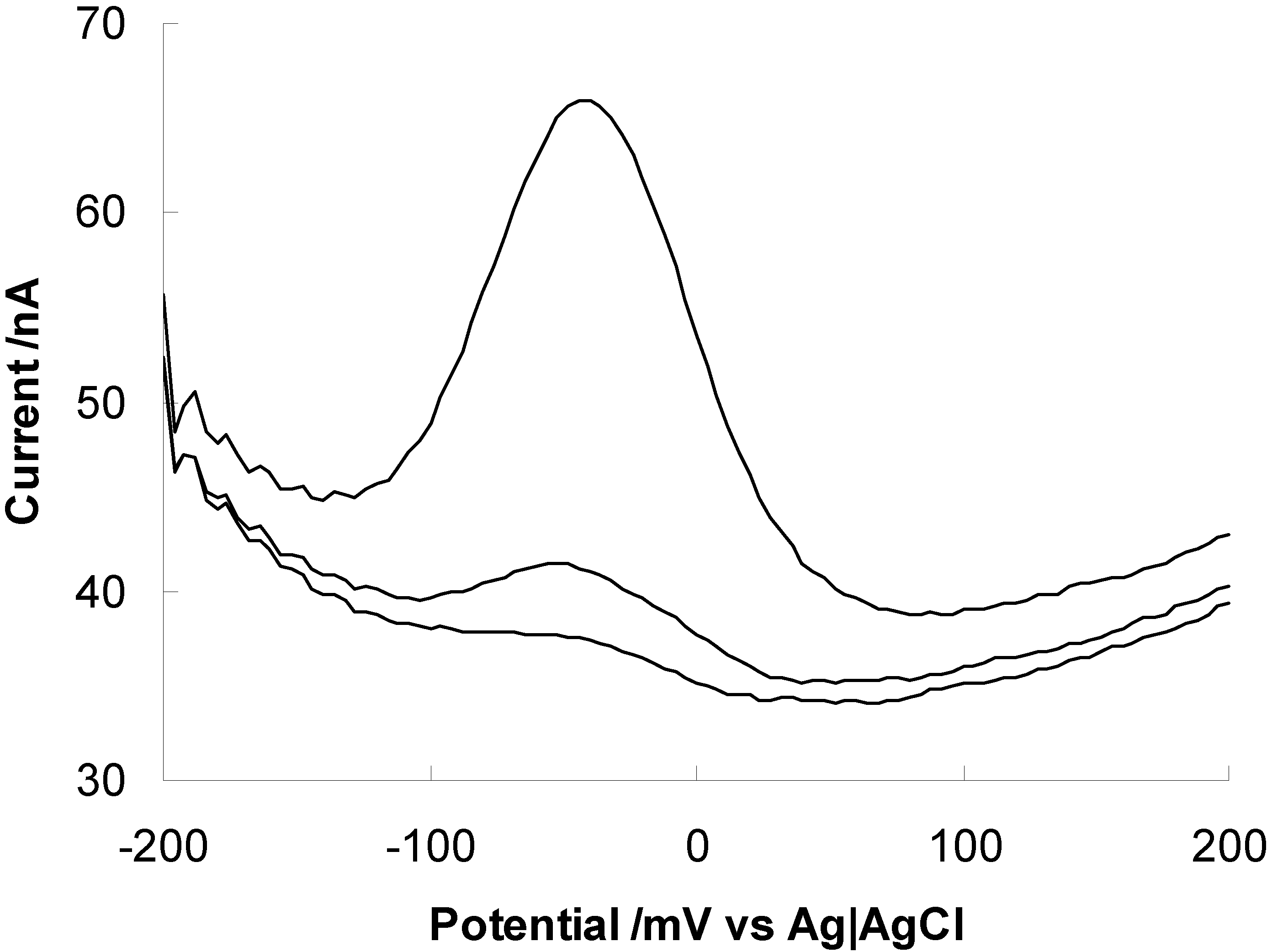
Future Prospects
References
- Sigel, H.; Martin, R.B. Coordinating Properties of the Amide Bond. Stability and Structure of Metal Ion Complexes of Peptides and Related Ligands. Chem. Rev. 1982, 82, 385–426. [Google Scholar] [CrossRef]
- Kozlowski, H.; Bal, W.; Dyba, M.; Kowalik-Jankowska, T. Specific structure-stability relations in metallopeptides. Coord. Chem. Rev. 1999, 184, 319–346. [Google Scholar] [CrossRef]
- Mackay, K.M.; Mackay, R.A. Introduction to Modern Inorganic Chemistry, 3rd ed.; International Textbook Company: London, 1981. [Google Scholar]
- Krezel, A.; Bal, W. Coordination chemistry of glutathione. Acta Biochim. Pol. 1999, 46, 567–580. [Google Scholar] [PubMed]
- Mendieta, J.; Diaz-Cruz, M.S.; Tauler, R.; Esteban, M. Application of multivariate curve resolution to voltammetric data. II. Study of metal-binding properties of the peptides. Anal. Biochem. 1996, 240, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Cruz, M.S.; Diaz-Cruz, J.M.; Mendieta, J.; Tauler, R.; Esteban, M. Soft- and hard-modeling approaches for the determination of stability constants of metal-peptide systems by voltammetry. Anal. Biochem. 2000, 279, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Harlyk, C.; Nieto, O.; Bordin, G.; Rodriguez, A.R. Electrochemical study of a metallothionein related peptide in the presence of zinc using cyclic voltammetry. J. Electroanal. Chem. 1998, 451, 267–272. [Google Scholar] [CrossRef]
- Harlyk, C.; Bordin, G.; Nieto, O.; Rodriguez, A.R. Electrochemical behavior of a metallothionein related peptide in the presence of cadmium using cyclic voltammetry. J. Electroanal. Chem. 1998, 446, 139–150. [Google Scholar] [CrossRef]
- Mendieta, J.; Diaz-Cruz, M.S.; Monjonell, A.; Tauler, R.; Esteban, M. Complexation of cadmium by the C-terminal hexapeptide Lys-Cys-Thr-Cys-Cys-Ala from mouse metallothionein: study by differential pulse polarography and circular dichroism spectroscopy with multivariate curve resolution analysis. Anal. Chim. Acta 1999, 390, 15–25. [Google Scholar] [CrossRef]
- Diaz-Cruz, M.S.; Esteban, M.; Rodriguez, A.R. Square wave voltammetry data analysis by multivariate curve resolution: application to the mixed-metal system Cd-Zn-{Lys-Cys-Thr-Cys-Cys-Ala}. Anal. Chim. Acta 2001, 428, 285–299. [Google Scholar] [CrossRef]
- Hibbert, D.B.; Weitzner, K.; Carter, P. Voltammetry of platinum in artificial perilymph solution. J. Electrochem. Soc. 2001, 148, E1–E7. [Google Scholar] [CrossRef]
- Saurina, J.; Hernandez-Cassou, S.; Fabregas, E.; Alegret, S. Cyclic voltammetric simultaneous determination of oxidizable amino acids using multivariate calibration methods. Anal. Chim. Acta 2000, 405, 153–160. [Google Scholar] [CrossRef]
- Heyrovsky, M.; Vavricka, S. Adsorption effects of electroactive species in dc polarography demonstrated in the case of the anodic waves of cysteine. J. Electroanal. Chem. 1997, 423, 125–130. [Google Scholar] [CrossRef]
- Heyrovsky, M.; Vavricka, S. Electrochemical reactivity of homocysteine at mercury electrodes as compared with cysteine. Bioelectrochem. Bioenerg. 1999, 48, 43–51. [Google Scholar] [CrossRef]
- Bilewicz, R. The reduction of copper(II) complexes of histidine and histidyl peptides at mercury electrodes. J. Electroanal. Chem. 1989, 267, 231–41. [Google Scholar] [CrossRef]
- Banica, F.G.; Fogg, A.G.; Moreira, J.C. Catalytic cathodic stripping voltammetry at a hanging mercury drop electrode of glutathione in the presence of nickel ion. Analyst 1994, 119, 2343–9. [Google Scholar] [CrossRef]
- Kolthoff, I.M.; Stricks, W.; Kapoor, R.C. Equilibrium constants of exchange reactions of cystine with glutathione and with thioglycolic acid both in the oxidized and reduced state. J. Am. Chem. Soc. 1955, 77, 4733–4739. [Google Scholar] [CrossRef]
- Witwicki, J. Polarographic studies of blood using mercury drop electrodes. I. Polarographic study of individual components of blood. Postepy Biochem. 1955, 1, 63–83. [Google Scholar]
- Banica, F.G.; Moreira, J.C.; Fogg, A.G. Application of catalytic stripping voltammetry for the determination of organic sulfur compounds at a hanging mercury drop electrode. Behavior of cysteine, cystine and N-acetylcysteine in the presence of nickel ion. Analyst (Cambridge, U. K.) 1994, 119, 309–318. [Google Scholar] [CrossRef]
- Banica, F.G.; Fogg, A.G.; Moreira, J.C. Catalytic cathodic stripping voltammetry of oxidized glutathione at a hanging mercury drop electrode in the presence of nickel ion. Talanta 1995, 42, 227–234. [Google Scholar] [CrossRef]
- Forsman, U. Cathodic stripping voltammetry of the peptide felypressin in trace amounts. Anal. Chim. Acta 1984, 156, 43–49. [Google Scholar] [CrossRef]
- Scarano, G.; Morelli, E. Polarographic behavior of metal phytochelatin complexes. Electroanalysis 1998, 10, 39–43. [Google Scholar] [CrossRef]
- Ertas, F.N.; Fogg, A.G.; Moreira, J.C.; Barek, J. Differential pulse cathodic stripping voltammetry of the copper complexes of glycyl-L-histidyl-glycine at a hanging mercury drop electrode. Talanta 1993, 40, 1481–1488. [Google Scholar] [CrossRef]
- Moreira, J.C.; Fogg, A.G. Determination of nanomolar levels of histidine by differential-pulse adsorptive-cathodic stripping voltammetry of its copper(II) complex. Analyst 1990, 115, 41–43. [Google Scholar] [CrossRef]
- Humble, A.V.; Gadd, G.M.; Codd, G.A. Binding of copper and zinc to three cyanobacterial microcystins quantified by differential pulse polarography. Water Res. 1997, 31, 1679–1686. [Google Scholar] [CrossRef]
- Yan, F.; Ozsoz, M.; Sadik, O.A. Electrochemical and conformational studies of microcystin-LR. Anal. Chim. Acta 2000, 409, 247–255. [Google Scholar] [CrossRef]
- Tanaka, S.; Yoshida, H. Enhancement Effect of Cysteine on Anodic Stripping Voltammetry of Copper. J. Electroanal. Chem. 1982, 137, 261–270. [Google Scholar] [CrossRef]
- Bai, Y.; Ruan, X.Y.; Mo, J.Y.; Xie, Y.Q. Potentiometric stripping analysis of copper using cysteine modified mercury film electrode. Anal. Chim. Acta 1998, 373, 39–46. [Google Scholar] [CrossRef]
- Moreira, J.C.; Zhao, R.; Fogg, A.G. Modification of electrodes with adsorbed polyamino acids. Part 1. Cathodic stripping voltammetric determination of copper(II) at a hanging mercury drop electrode using adsorptive accumulation on an adsorbed layer of poly-L-histidine. Analyst 1990, 115, 1561–1564. [Google Scholar] [CrossRef]
- Pirzad, R.; Moreira, J.C.; Rangel, A.O.S.S.; Alonso, R.M.; Edmonds, T.E.; Fogg, A.G. Differential-pulse cathodic stripping voltammetric determination of sodium nitroprusside at a hanging mercury drop electrode aided by copper(II) and poly-L-lysine modification. Analyst 1994, 119, 963–968. [Google Scholar] [CrossRef]
- Moreira, J.C.; Fogg, A.G. Modification of electrodes with adsorbed polyamino acids. Part 2. Adsorptive stripping voltammetric determination of hexacyanoferrate(III) at a hanging mercury drop electrode in the presence of an adsorbed layer of copper-modified poly-L-lysine. Analyst 1990, 115, 1565–1568. [Google Scholar] [CrossRef]
- Walkup, G.K.; Imperiali, B. Fluorescent chemosensors for divalent zinc based on zinc finger domains. Enhanced oxidative stability, metal binding affinity, and structural and functional characterization. J. Am. Chem. Soc. 1997, 119, 3443–3450. [Google Scholar] [CrossRef]
- Torrado, A.; Walkup, G.K.; Imperiali, B. Exploiting polypeptide motifs for the design of selective Cu(II) ion chemosensors. J. Am. Chem. Soc. 1998, 120, 609–610. [Google Scholar] [CrossRef]
- Miura, Y.; Kimura, S.; Imanishi, Y.; Umemura, J. Oriented helical peptide layer on the carboxylate-terminated alkanethiol immobilized on a gold surface. Langmuir 1999, 15, 1155–1160. [Google Scholar] [CrossRef]
- Scheibler, L.; Dumy, P.; Stamou, D.; Duschl, C.; Vogel, H.; Mutter, M. Functionalization of gold surfaces via topological templates. Tetrahedron 1998, 54, 3725–3734. [Google Scholar] [CrossRef]
- Scheibler, L.; Dumy, P.; Boncheva, M.; Leufgen, K.; Mathieu, H.-J.; Mutter, M.; Vogel, H. Functional molecular thin films: topological templates for the chemoselective ligation of antigenic peptides to self-assembled monolayers. Angew. Chem., Int. Ed. 1999, 38, 696–699. [Google Scholar] [CrossRef]
- Whitesell, J.K.; Chang, H.K. Directionally aligned helical peptides on surfaces. Science (Washington, D. C., 1883-) 1993, 261, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Strong, A.E.; Moore, B.D. Self-assembling chiral monolayers of helical peptides bound to gold via side-chain thioethers. Chem. Commun. 1998, 473, 473–474. [Google Scholar] [CrossRef]
- Strong, A.E.; Moore, B.D. Self-assembling monolayers of helical oligopeptides on gold with applications in molecular electronics. J. Mater. Chem. 1999, 9, 1097–1105. [Google Scholar] [CrossRef]
- Cheng, Y.F.; Corn, R.M. Ultrathin polypeptide multilayer films for the fabrication of model liquid/liquid electrochemical interfaces. J. Phys. Chem. B 1999, 103, 8726–8731. [Google Scholar] [CrossRef]
- Cheng, Y.F.; Murtomaki, L.; Corn, R.M. Electrochemical characterization of the ultrathin polypeptide film/1,2-dichloroethane liquid vertical bar liquid interface. J. Electroanal. Chem. 2000, 483, 88–94. [Google Scholar] [CrossRef]
- Frey, B.L.; Corn, R.M. Covalent Attachment and Derivatization of Poly(L-lysine) Monolayers on Gold Surfaces as Characterized by Polarisation-Modulation FT-IR Spectroscopy. Anal. Chem. 1996, 68, 3187–3193. [Google Scholar] [CrossRef]
- Autry, H.A.; Holcombe, J.A. Cadmium, Copper and Zinc-Complexes of Poly-L-Cysteine. Analyst 1995, 120, 2643–2647. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, E.; Miller, T.C.; Gonzalez-Redondo, J.R.; Holcombe, J.A. Characterization of immobilized poly-L-aspartate as a metal chelator. Environ. Sci. Technol. 1999, 33, 1664–1670. [Google Scholar] [CrossRef]
- Howard, M.; Jurbergs, H.A.; Holcombe, J.A. Effects of oxidation of immobilized poly(L-cysteine) on trace metal chelation and preconcentration. Anal. Chem. 1998, 70, 1604–1609. [Google Scholar] [CrossRef]
- Jurbergs, H.A.; Holcombe, J.A. Characterization of immobilized poly(L-cysteine) for cadmium chelation and preconcentration. Anal. Chem. 1997, 69, 1893–1898. [Google Scholar] [CrossRef]
- Miller, T.C.; Holcombe, J.A. An ion-exchange microcolumn employing gold minigrids as supports for the on line immobilization of poly(L-aspartate). Anal. Chem. 1999, 71, 2667–2671. [Google Scholar] [CrossRef]
- Gooding, J.J.; Hibbert, D.B. The Application of Alkanethiol Self-Assembled Monolayers to Enzyme Electrodes. TrAC 1999, 18, 525–533. [Google Scholar] [CrossRef]
- Takehara, K.; Aihara, M.; Ueda, N. An Ion-Gate Response of a Glutathione Monolayer Assembly Highly Sensitive to Lanthanide Ions. Electroanalysis 1994, 6, 1083–1086. [Google Scholar] [CrossRef]
- Ju, H.X.; Leech, D. Electrochemical study of a metallothionein modified gold disk electrode and its action on Hg2+ cations. J. Electroanal. Chem. 2000, 484, 150–156. [Google Scholar] [CrossRef]
- Arrigan, D.W.M.; Le Bihan, L. A study of L-cysteine adsorption on gold via electrochemical desorption and copper(II) ion complexation. Analyst 1999, 124, 1645–1649. [Google Scholar] [CrossRef]
- Liu, A.-C.; Chen, D.-c.; Lin, C.-C.; Chou, H.-H.; Chen, C.-h. Application of Cysteine Monolayers for Electrochemical Determination of Sub-ppb Copper(II). Anal. Chem. 1999, 71, 1549–1552. [Google Scholar] [CrossRef]
- Yang, W.; Gooding, J.J.; Hibbert, D.B. Characterisation of Gold Electrodes Modified with Self Assembled Monolayers of L-Cysteine for the Adsorptive Stripping Analysis of Copper. J. Electroanal. Chem. 2001. submitted. [Google Scholar] [CrossRef]
- Gooding, J.J.; Praig, V.; Hall, E.A.H. Platinum Mediated Enzyme Electrodes Immobilised on Gold using Self-Assembled Monolayers. Anal. Chem. 1998, 70, 2396–2402. [Google Scholar] [CrossRef] [PubMed]
- Gaus, K. Ph. D., Cambridge University, 1999.
- Imperiali, B.; Walkup, G.K. U.S.; (California Institute of Technology, USA); US 6083758; 2000; p. 19. [Google Scholar]
- Sample Availability: Not available.
© 2001 by MDPI (http://www.mdpi.net). Reproduction is permitted for noncommercial purposes.
Share and Cite
Gooding, J.J.; Hibbert, D.B.; Yang, W. Electrochemical Metal Ion Sensors. Exploiting Amino Acids and Peptides as Recognition Elements. Sensors 2001, 1, 75-90. https://doi.org/10.3390/s10300075
Gooding JJ, Hibbert DB, Yang W. Electrochemical Metal Ion Sensors. Exploiting Amino Acids and Peptides as Recognition Elements. Sensors. 2001; 1(3):75-90. https://doi.org/10.3390/s10300075
Chicago/Turabian StyleGooding, J. Justin, D. Brynn Hibbert, and Wenrong Yang. 2001. "Electrochemical Metal Ion Sensors. Exploiting Amino Acids and Peptides as Recognition Elements" Sensors 1, no. 3: 75-90. https://doi.org/10.3390/s10300075
APA StyleGooding, J. J., Hibbert, D. B., & Yang, W. (2001). Electrochemical Metal Ion Sensors. Exploiting Amino Acids and Peptides as Recognition Elements. Sensors, 1(3), 75-90. https://doi.org/10.3390/s10300075




