Molecular Assisted Identification Reveals Hidden Red Algae Diversity from the Burica Peninsula, Pacific Panama
Abstract
1. Introduction
“…our surroundings seemed ideal for successful work with the marine flora—except for the one unhappy fact, which gradually became apparent, that a marine flora, in the ordinary sense, was in that region almost non-existent.”—Marshall Avery Howe [1]
2. Materials and Methods
3. Results
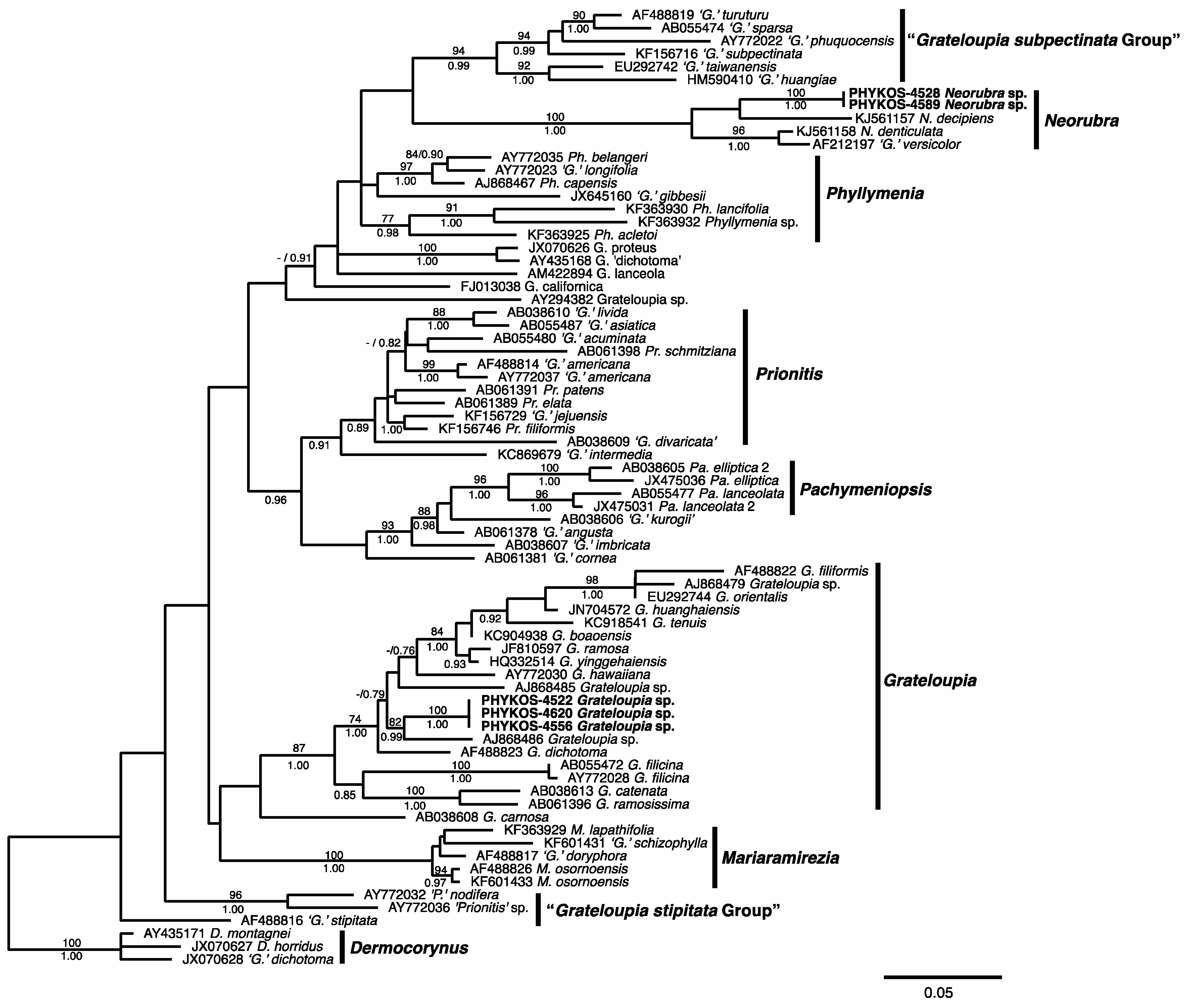
3.1. Overall “Barcoding” Cluster Analyses
3.2. Species Treatments
3.2.1. Tricleocarpa cylindrica (J. Ellis and Solander) Huisman and Borowitska
3.2.2. Hommersandiophycus borowitzkae (Huisman) S.-M. Lin and Huisman
3.2.3. Izziella sp.
3.2.4. Liagora ceranoides J.V. Lamouroux
3.2.5. Neoizziella asiatica Showe M. Lin, S.-Y. Yang and Huisman
3.2.6. Gelidium sclerophyllum W.R. Taylor
3.2.7. Gelidium sp.
3.2.8. Millerella sp.
3.2.9. Asparagopsis sp.
3.2.10. Plocamium sp.
3.2.11. Hypnea flava Nauer, Cassano and M.C. Oliveira
3.2.12. Hypnea pannosa J. Agardh
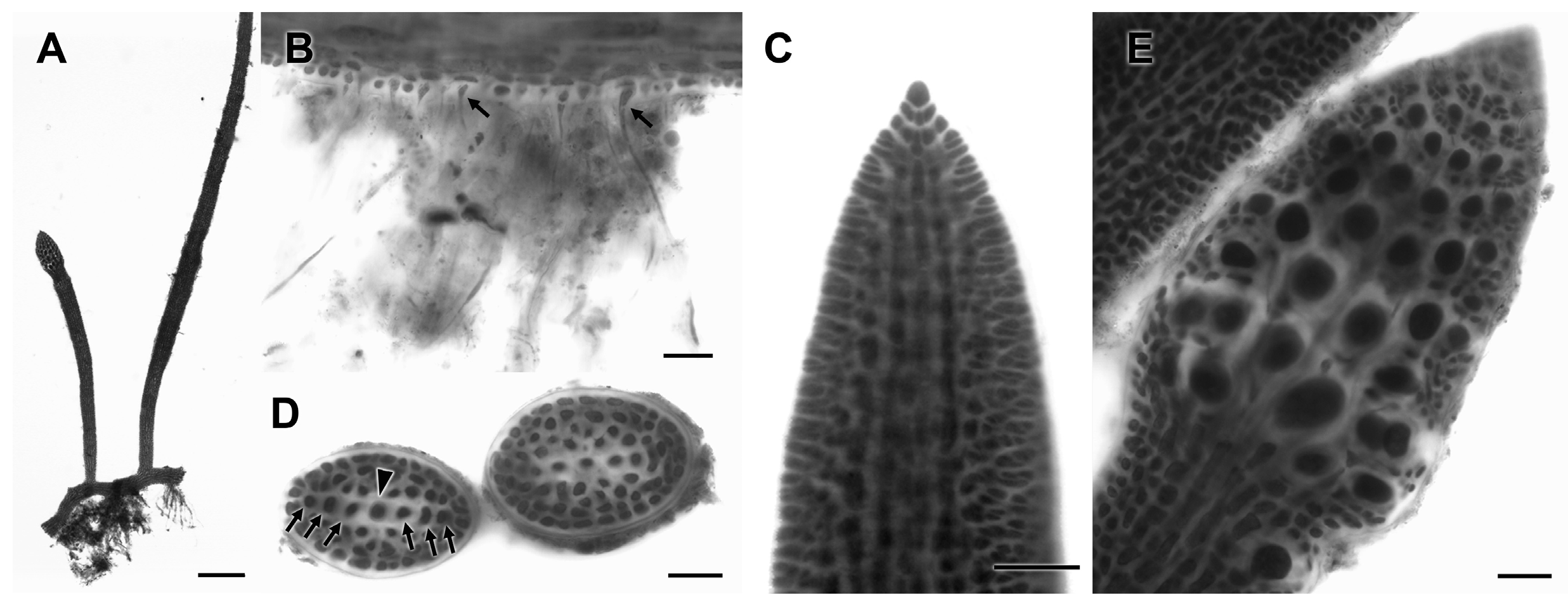
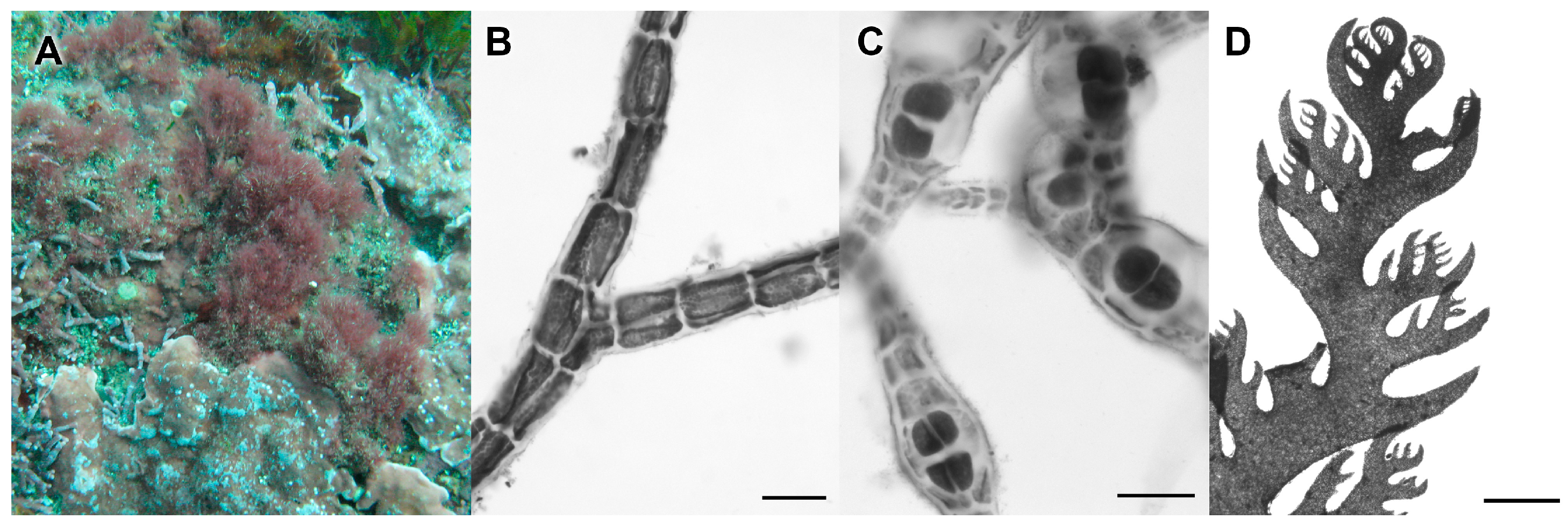
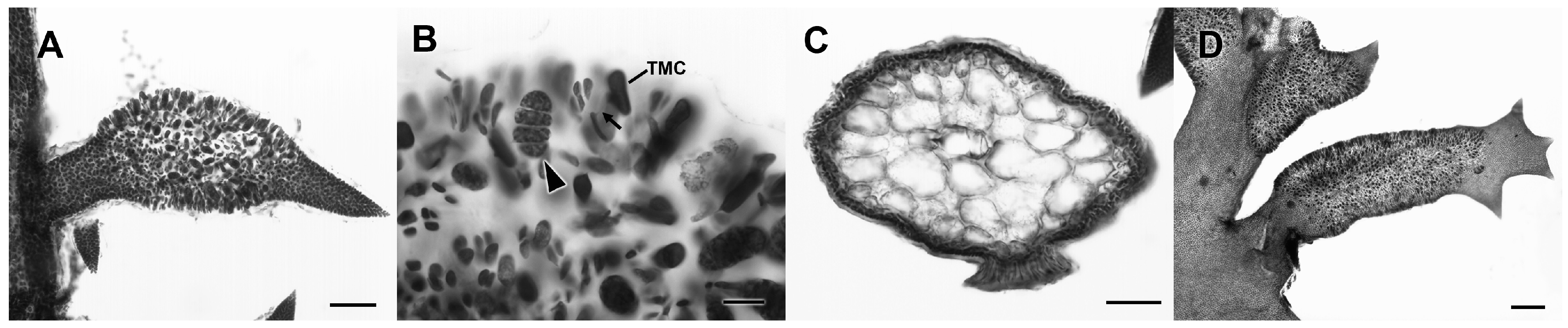
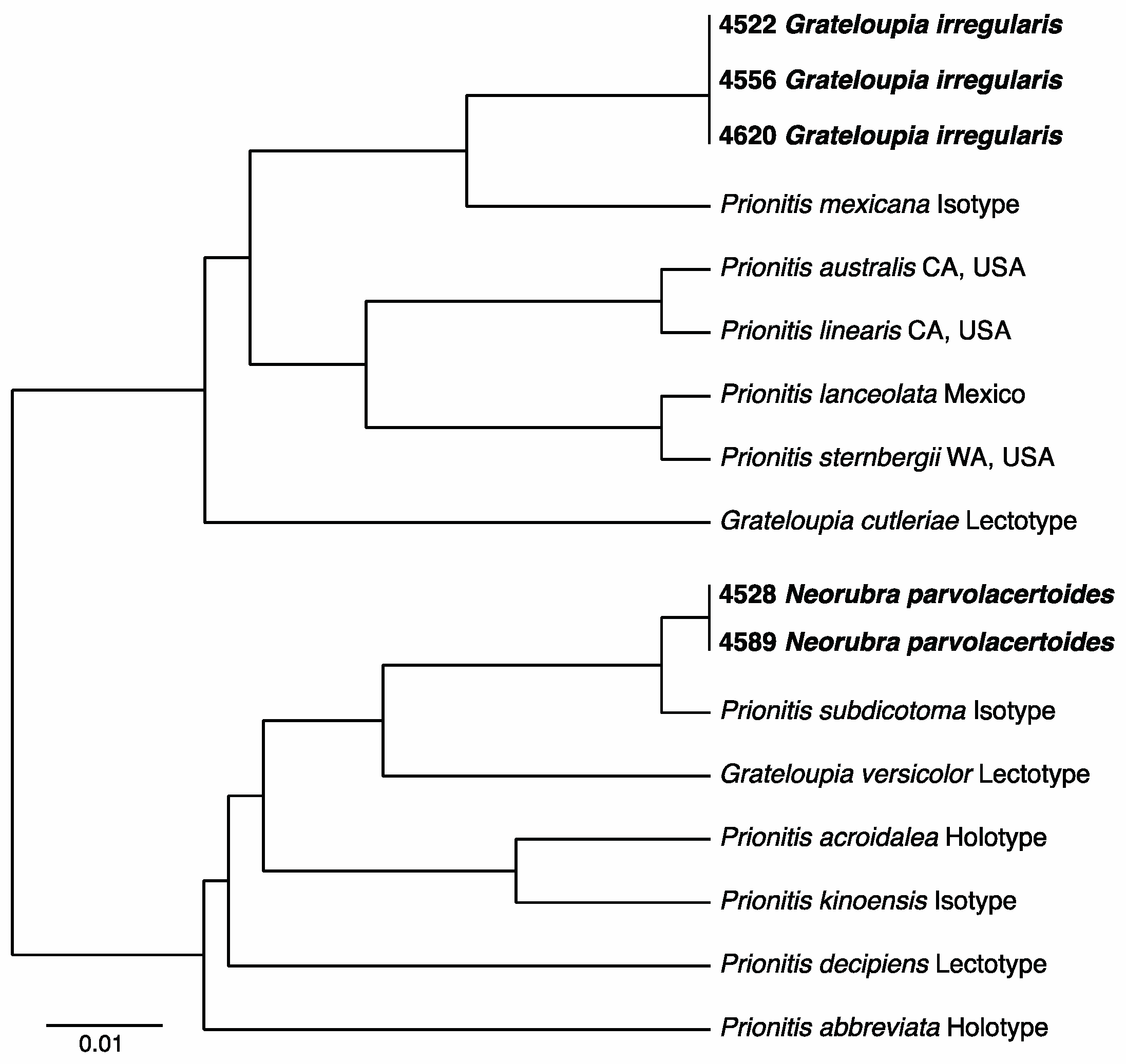
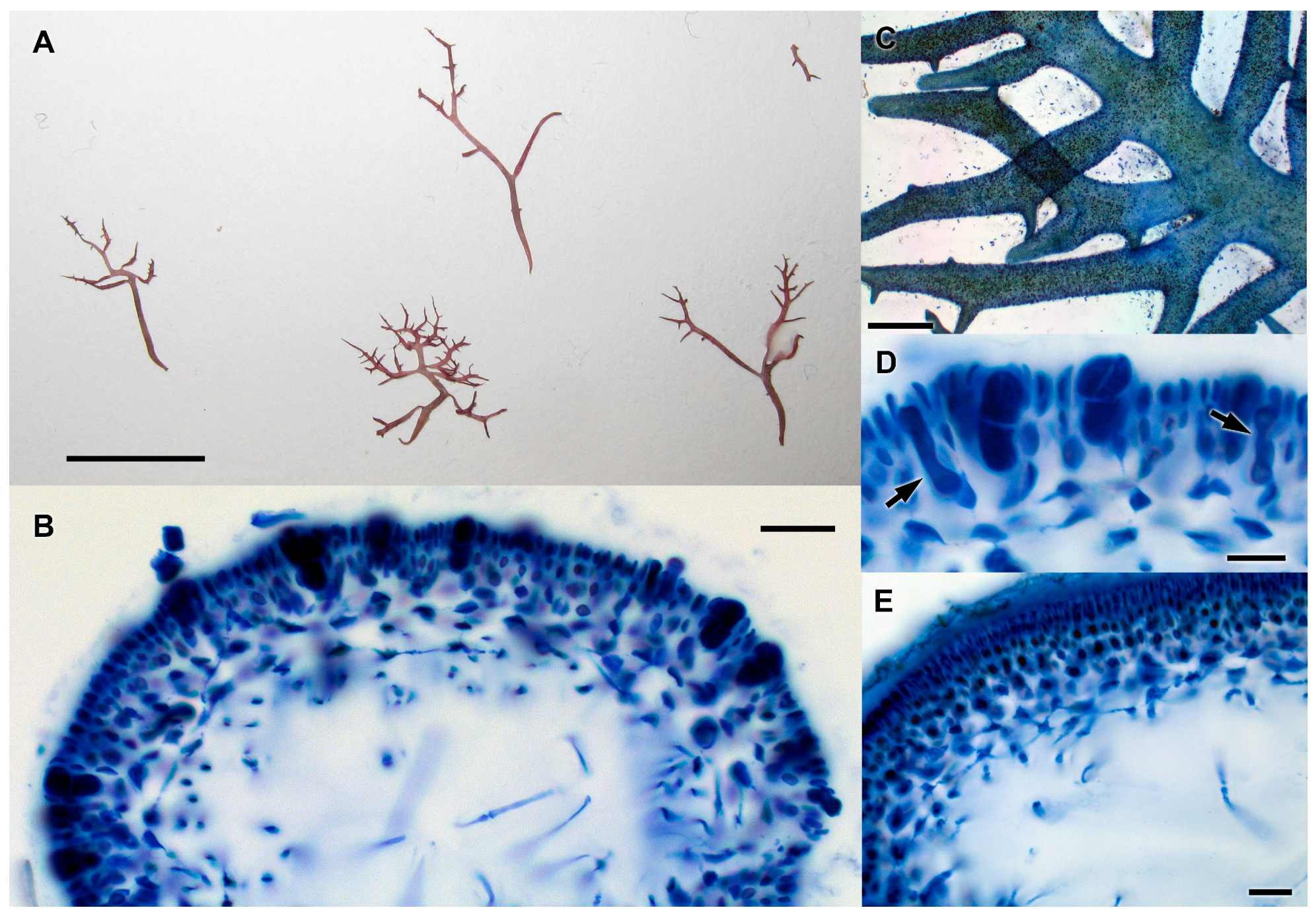
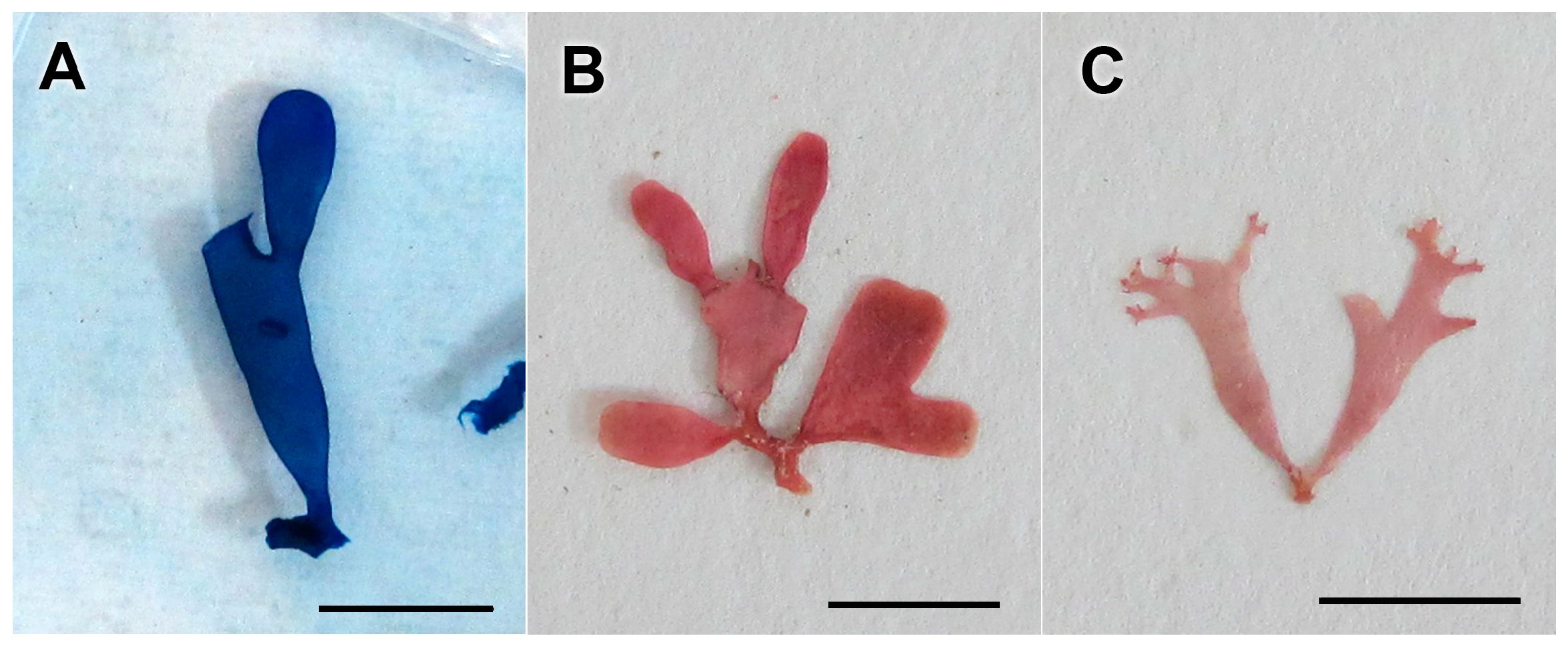
3.2.13. Neorubra parvolacertoides Freshwater and P.W. Gabrielson sp. nov.
3.2.14. Grateloupia irregularis P.W. Gabrielson and Freshwater sp. nov
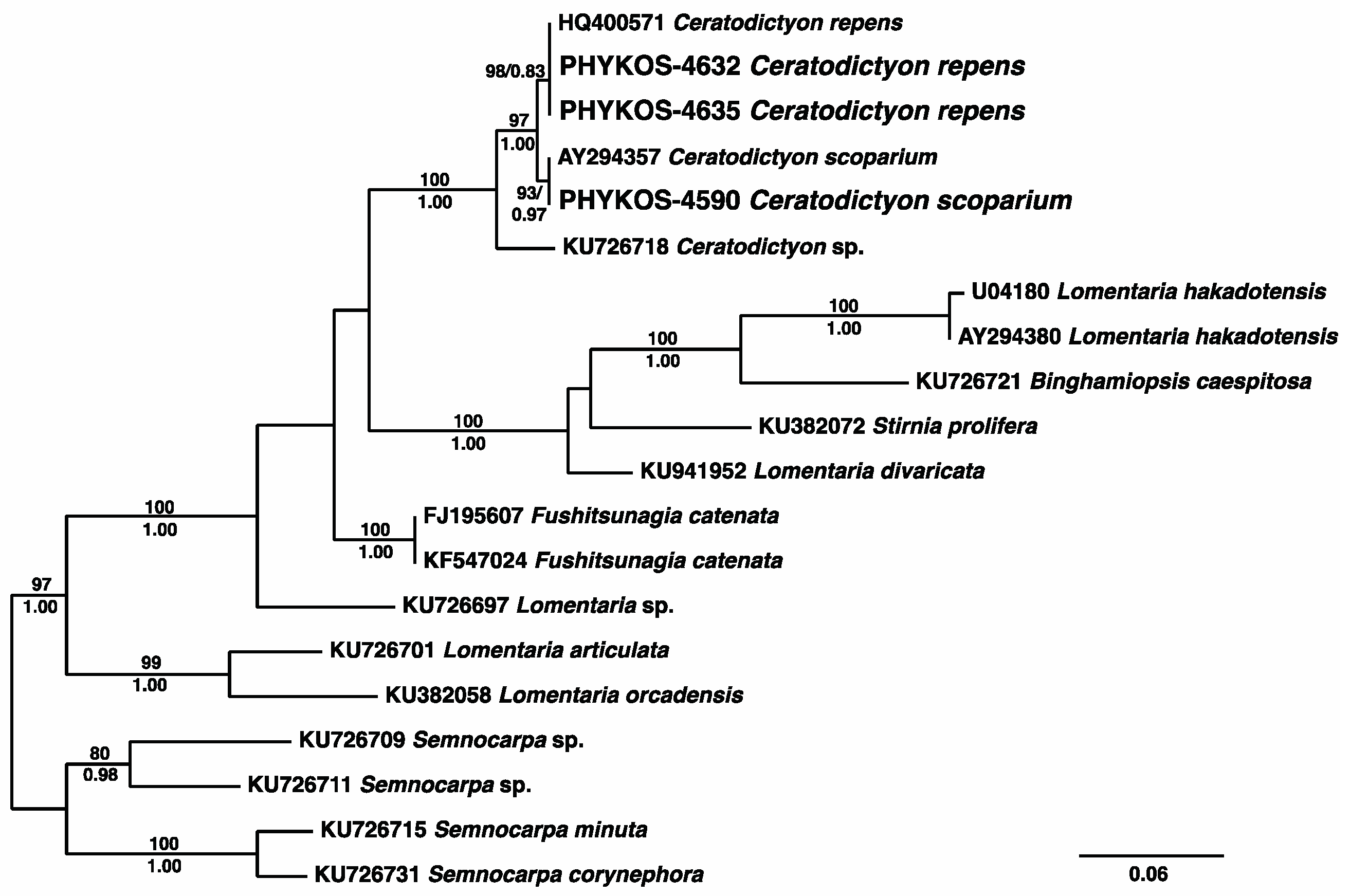
3.2.15. Ceratodictyon repens (Kützing) R.E. Norris
3.2.16. Ceratodictyon scoparium (Montagne and Millardet) R.E. Norris
3.2.17. Gracilaria Species
3.2.18. Aglaothamnion sp.
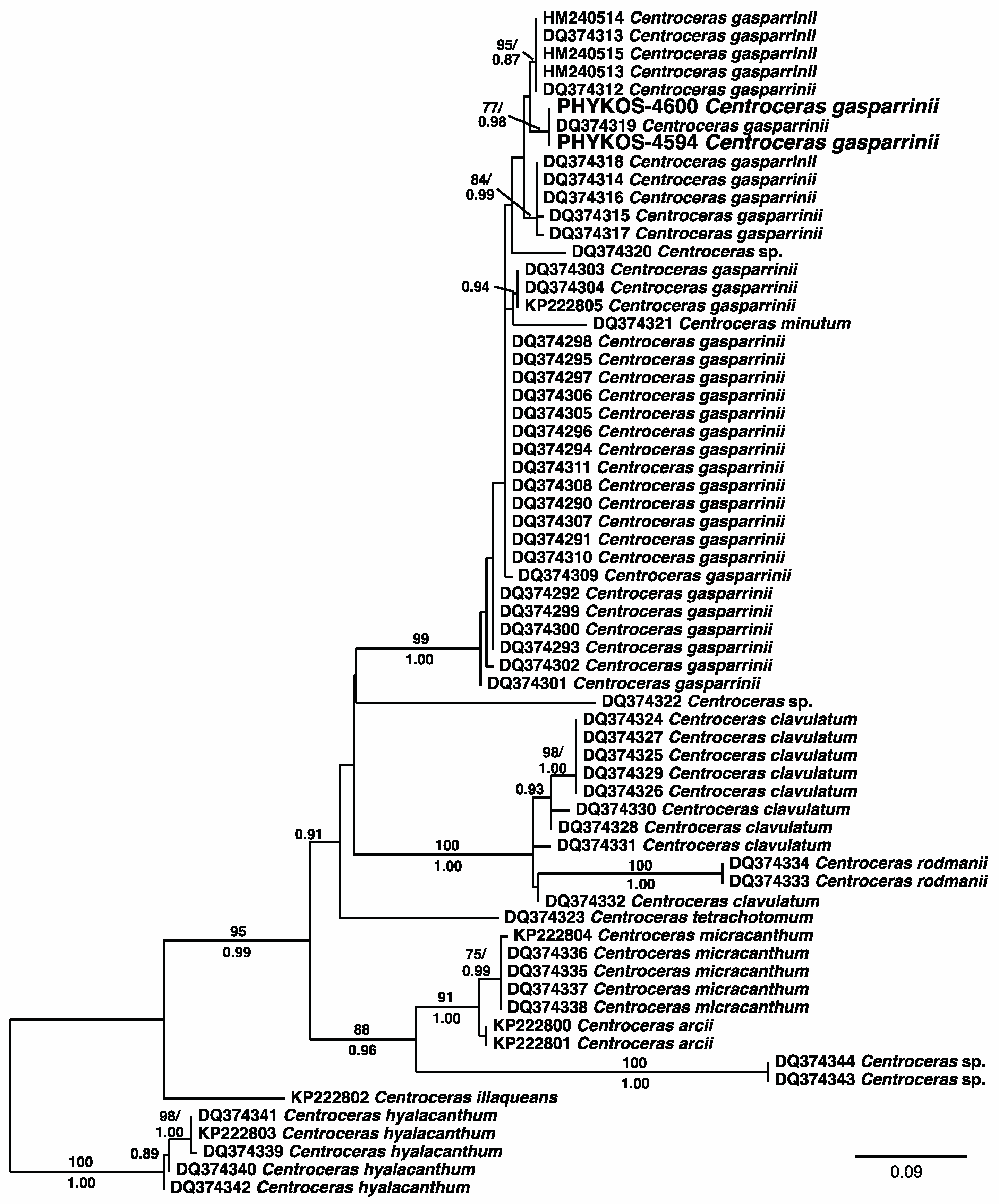
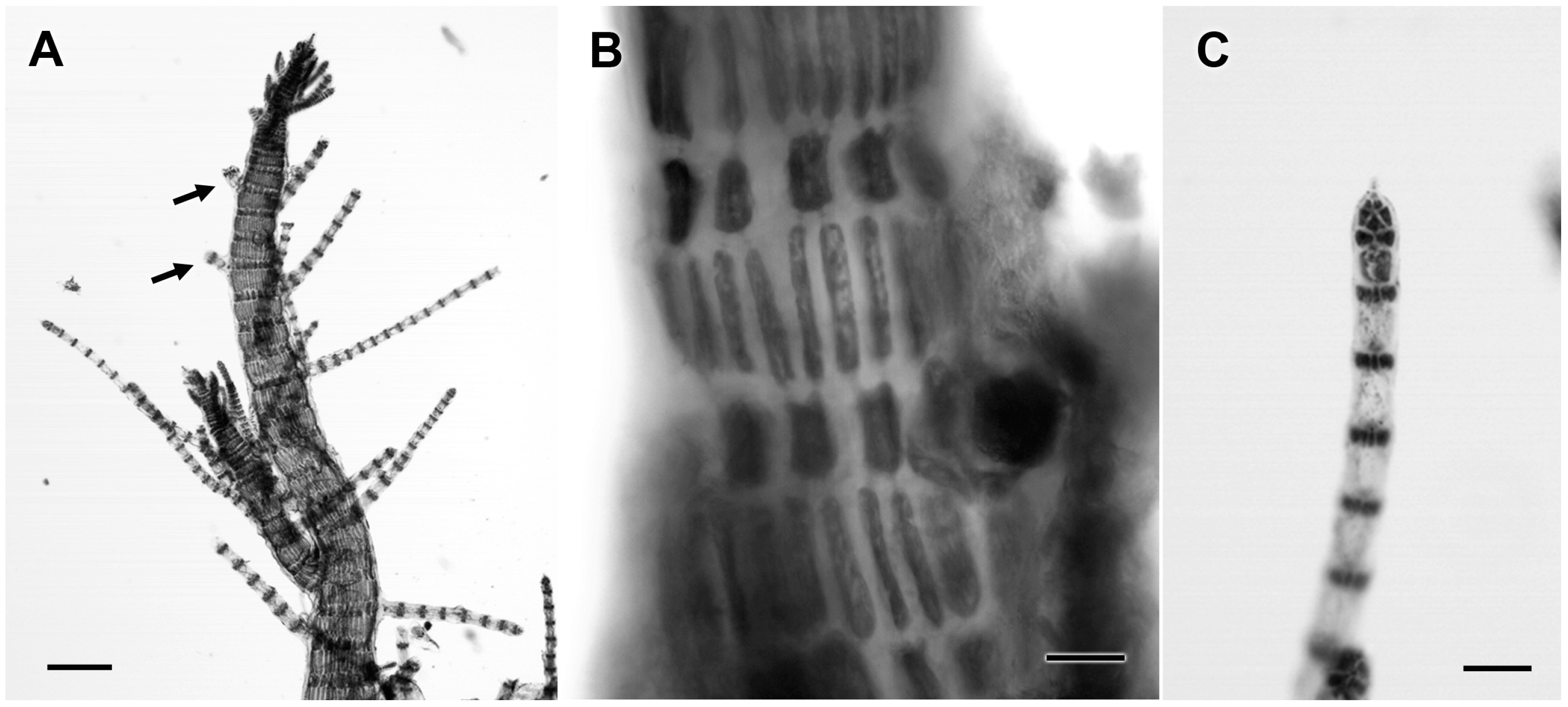
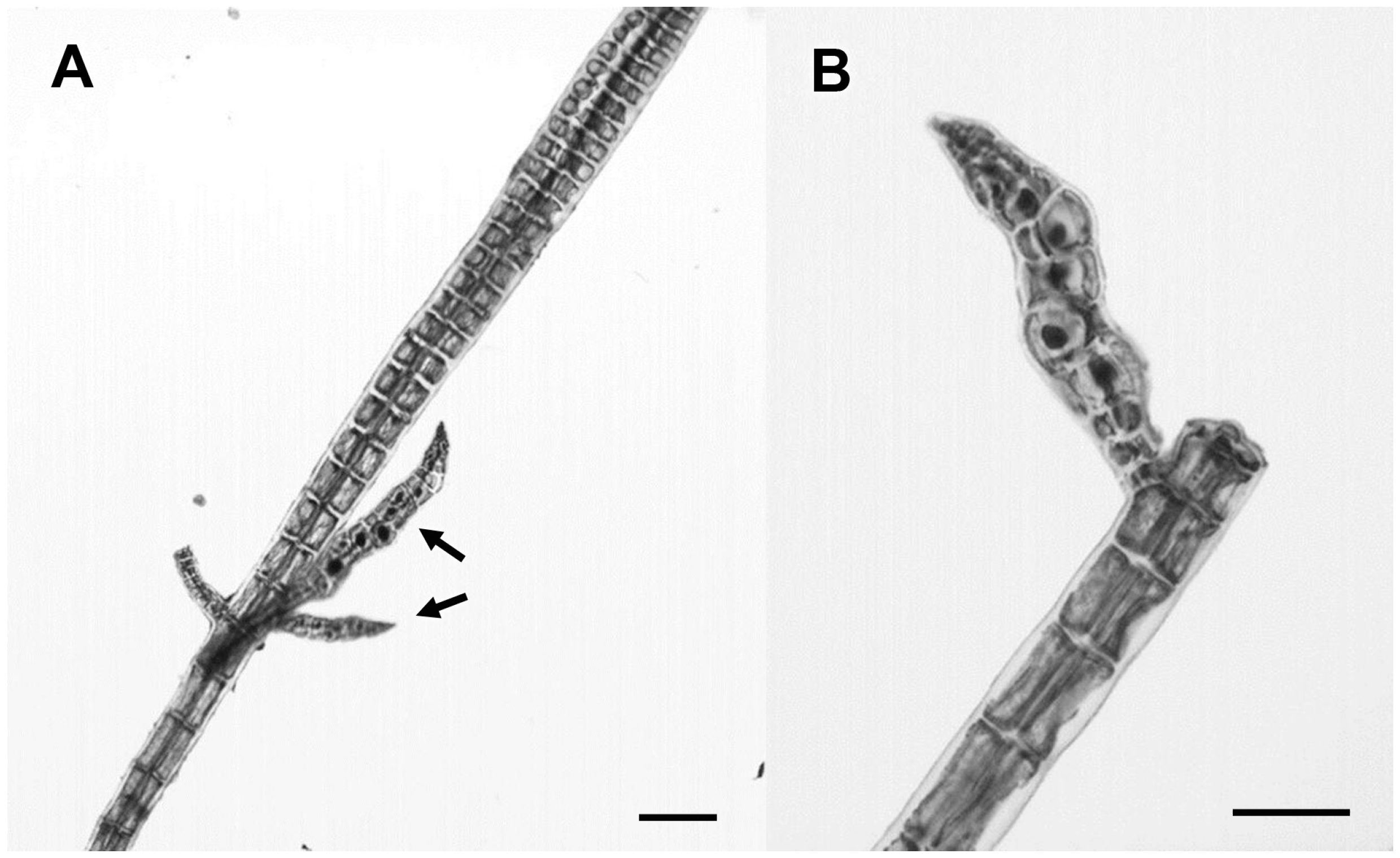
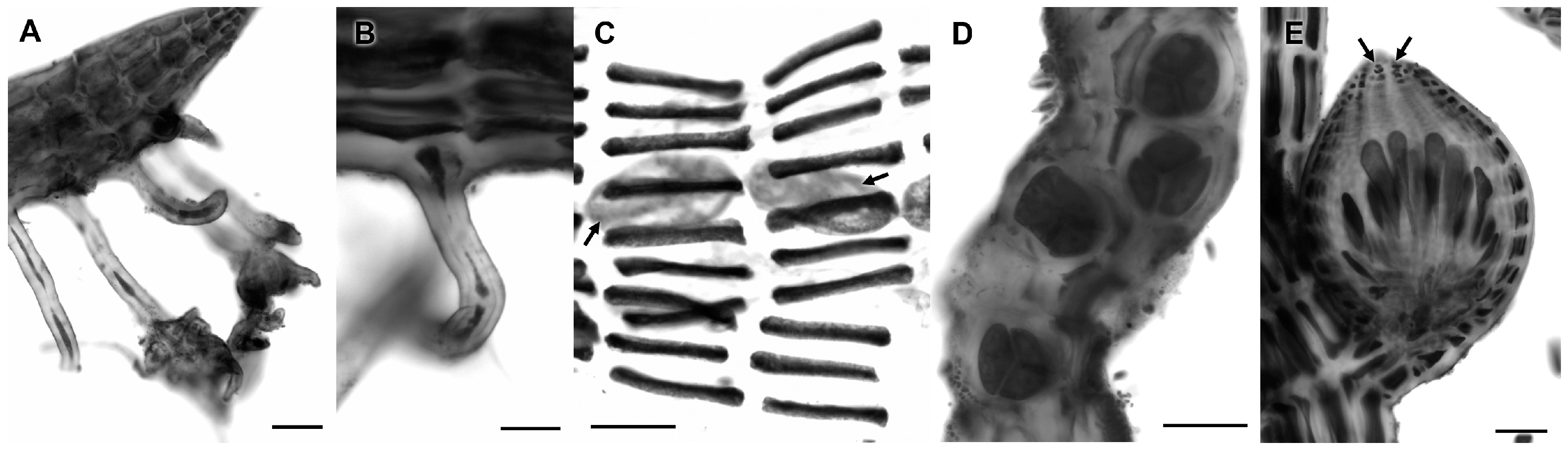
3.2.19. Centroceras gasparrinii (Meneghini) Kützing
3.2.20. Spyridia sp.
3.2.21. Melanothamnus sp.
3.2.22. ‘Polysiphonia’ binneyi Harvey
3.2.23. ‘Polysiphonia’ sp.
3.2.24. Wilsonosiphonia howei (Hollenberg) D. Bustamante, Won and T.O. Cho
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Howe, M.A. Report on a botanical visit to the Isthmus of Panama. J. N. Y. Bot. Gard. 1910, 11, 30–44. [Google Scholar]
- Taylor, W.R. Pacific Marine Algae of the Allan Hancock Expeditions to the Galapagos Islands; The University of Southern California Press: Los Angeles, CA, USA, 1945; p. 528. [Google Scholar]
- Wysor, B. An annotated list of marine Chlorophyta from the Pacific coast of the republic of Panama with a comparison to Caribbean Panama species. Nova Hedwigi. 2004, 78, 209–241. [Google Scholar] [CrossRef]
- Littler, M.M.; Littler, D.S. Coralline algal rhodoliths form extensive benthic communities in the Gulf of Chiriqui, Pacific Panama. Coral Reefs 2008, 27, 553. [Google Scholar] [CrossRef]
- Fernández-García, C.; Riosmena-Rodríguez, R.; Wysor, B.; Tejada, O.L.; Cortés, J. Checklist of the Pacific marine macroalgae of Central America. Bot. Mar. 2011, 54, 53–73. [Google Scholar] [CrossRef]
- Dawson, E.Y. Marine algae from the Pacific Costa Rican gulfs. Contrib. Sci. 1957, 15, 1–28. [Google Scholar]
- Morell, K.D.; Fisher, D.M.; Gardner, T.W.; Femina, P.L.; Davidson, D.; Teletzke, A. Quaternary outer fore-arc deformation and uplift inboard of the Panama Triple Junction, Burica Peninsula. J. Geophys. Res. 2011, 116, B05402. [Google Scholar] [CrossRef]
- Buchs, D.M.; Baumgartner, P.O.; Baumgartner-Mora, C.; Bandini, A.N.; Jackett, S.-J.; Diserens, M.-O.; Stucki, J. Late Cretaceous to Miocene seamount accretion and mélange formation in the Osa and Burica Peninsulas (Southern Costa Rica): Episodic growth of a convergent margin. In The Origin and Evolution of the Caribbean Plate; Lorente, J.K., Pindell, M.A., Eds.; Geological Society: London, UK, 2007; Volume 28, pp. 411–456. [Google Scholar]
- Mamoozadeh, N.R.; Freshwater, D.W. Taxonomic notes on Caribbean Neosiphonia and Polysiphonia (Ceramiales, Flordeophyceae): Five species from Florida, USA and Mexico. Bot. Mar. 2011, 54, 269–292. [Google Scholar] [CrossRef]
- Hebert, P.D.N.; Cywinska, A.; Ball, S.L.; de Waard, J.R. Biological identifications through DNA barcodes. Philos. Trans. R. Soc. B 2003, 270, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Saunders, G.W. Applying DNA barcoding to red macroalgae: A preliminary appraisal holds promise for future applications. Phil. Trans. R. Soc. B 2005, 360, 1879–1888. [Google Scholar] [CrossRef] [PubMed]
- Robba, L.; Russell, S.J.; Barker, G.L.; Brodie, J. Assessing the use of the mitochondrial cox1 marker for use in DNA barcoding of red algae (Rhodophyta). Am. J. Bot. 2006, 93, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Le Gall, L.; Saunders, G.W. DNA barcoding is a powerful tool to uncover algal diversity: A case study of the Phyllophoraceae (Gigartinales, Rhodophyta) in the Canadian flora. J. Phycol. 2010, 46, 374–389. [Google Scholar] [CrossRef]
- Milstein, D.; Saunders, G.W. DNA barcoding of Canadian Ahnfeltiales (Rhodophyta) reveals a new species—Ahnfeltia borealis sp. nov. Phycologia 2012, 51, 247–259. [Google Scholar] [CrossRef]
- Lyra, G.D.M.; Gurgel, C.F.D.; Costa, E.D.S.; de Jesus, P.B.; Oliveira, M.C.; Oliveira, E.C.; Davis, C.C.; Nunes, J.M.D.C. Delimitating cryptic species in the Gracilaria domingensis complex (Gracilariaceae, Rhodophyta) using molecular and morphological data. J. Phycol. 2016, 52, 997–1017. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, A.R.; Presting, G.G. Universal primers amplify a 23S rDNA plastid marker in eukaryotic algae and cyanobacteria. J. Phycol. 2007, 43, 605–608. [Google Scholar] [CrossRef]
- Sherwood, A.R.; Sauvage, T.; Kurihara, A.; Conklin, K.T.; Presting, G.G. A comparative analysis of COI, LSU and UPA marker data for the Hawaiian florideophyte Rhodophyta: Implications for DNA barcoding of red algae. Cryptogam. Algologie 2010, 31, 451–465. [Google Scholar]
- Iha, C.; Milstein, D.; Guimarães, S.M.P.B.; Freshwater, D.W.; Oliveira, M.C. DNA barcoding reveals high diversity in the Gelidiales of the Brazilian southeast coast. Bot. Mar. 2015, 58, 295–305. [Google Scholar] [CrossRef]
- Chase, M.W.; Hills, H.H. Silica gel: An ideal material for field preservation of leaf samples for DNA studies. Taxon 1991, 40, 215–220. [Google Scholar] [CrossRef]
- Hughey, J.R.; Silva, P.C.; Hommersand, M.H. Solving taxonomic and nomenclatural problems in Pacific Gigartinaceae (Rhodophyta) using DNA from type material. J. Phycol. 2001, 37, 1091–1109. [Google Scholar] [CrossRef]
- Freshwater, D.W.; Braly, S.K.; Stuercke, B.; Hamner, R.M.; York, R.A. Phylogenetic analyses of North Carolina Rhodymeniales. I. The genus Asteromenia. J. N. C. Acad. Sci. 2005, 121, 49–55. [Google Scholar]
- Saunders, G.W. A DNA barcode examination of the red algal family Dumontiaceae in Canadian waters reveals substantial cryptic species diversity. 1. The foliose Disea-Neodilsea complex and Weeksia. Botany 2008, 86, 773–789. [Google Scholar] [CrossRef]
- Saunders, G.W.; McDevit, D.C. Methods for DNA barcoding photosynthetic protists emphasizing the macroalgae and diatoms. Methods Mol. Biol. 2012, 858, 207–222. [Google Scholar] [PubMed]
- Freshwater, D.W.; Rueness, J. Phylogenetic relationships of some European Gelidium (Gelidiales, Rhodophyta) species, based on rbcL nucleotide sequence analysis. Phycologia 1994, 33, 187–194. [Google Scholar] [CrossRef]
- Stuercke, B.; Freshwater, D.W. Consistency of morphological characters used to delimit Polysiphonia sensu lato species (Ceramiales, Florideophyceae): Analyses of North Carolina, USA specimens. Phycologia 2008, 47, 541–559. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 2006, 22, 2688–2690. [Google Scholar] [CrossRef] [PubMed]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef] [PubMed]
- Won, B.Y.; Cho, T.O.; Fredericq, S. Morphological and molecular characterization of species of the genus Centroceras (Ceramiaceae, Ceramiales), including two new species. J. Phycol. 2009, 45, 227–250. [Google Scholar] [CrossRef] [PubMed]
- Grusz, A.L.; Freshwater, D.W. Studies of Costa Rican Gelidiales (Florideophyceae). II. Two Pacific taxa including Gelidium microglossum, n. sp. Pac. Sci. 2014, 68, 97–110. [Google Scholar] [CrossRef]
- Boo, G.H.; Hughey, J.R.; Miller, K.A.; Boo, S.M. Mitogenomes from type specimens, a genotyping tool for morphologically simple species: Ten genomes of agar-producing red algae. Sci. Rep. 2016, 6, 35337. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-L.; Liu, S.-L.; Lin, S.-M. Systematics of the calcified genera of the Galaxauraceae (Nemaliales, Rhodophyta) with an emphasis on Taiwan species. J. Phycol. 2005, 41, 685–703. [Google Scholar] [CrossRef]
- Wiriyadamrikul, J.; Geraldino, P.J.L.; Huisman, J.M.; Lewmanomont, K.; Boo, S.M. Molecular diversity of the calcified red algal genus Tricleocarpa (Galaxauraceae, Nemaliales) with the description of T. jejuensis and T. natalensis. Phycologia 2013, 52, 338–351. [Google Scholar] [CrossRef]
- Huisman, J.M.; Borowitzka, M.A. A revision of the Australian species of Galaxaura (Rhodophyta, Galaxauraceae), with a description of Tricleocarpa gen. nov. Phycologia 1990, 29, 150–172. [Google Scholar] [CrossRef]
- Le Gall, L.; Saunders, G.W. Establishment of a DNA-barcode library for the Nemaliales (Rhodophyta) from Canada and France uncovers overlooked diversity in the species Nemalion helminthoides (Velley) Batters. Cryptogam. Algologie 2010, 31, 403–421. [Google Scholar]
- Machín-Sánchez, M.; Rousseau, F.; Le Gall, L.; Cassano, V.; Neto, A.I.; Sentíes, A.; Fuji, M.T.; Gil-Rodríguez, M.C. Species diversity of the genus Osmundea (Ceramiales, Rhodophyta) in the Macaronesian region. J. Phycol. 2016, 52, 664–681. [Google Scholar] [CrossRef] [PubMed]
- Taylor, W.R. Marine Algae of the Eastern Tropical and Subtropical Coasts of the Americas; The University of Michigan Press: Ann Arbor, MI, USA, 1960; p. 870. [Google Scholar]
- Costa, J.; Lin, S.-M.; Macaya, E.; Fernández-Garcia, C.; Verbruggen, H. Chloroplast genomes as a tool to resolve red algal phylogenies: A case study in the Nemaliales. BMC Evol. Biol. 2016, 16, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Huisman, J.M. The type and Australian species of the red algal genera Liagora and Ganonema (Liagoraceae, Nemaliales). Aust. Syst. Bot. 2002, 15, 773–838. [Google Scholar] [CrossRef]
- Lin, S.-M.; Huisman, J.M.; Ballantine, D.L. Revisiting the systematics of Ganonema (Liagoraceae, Rhodophyta) with emphasis on species from the northwest Pacific Ocean. Phycologia 2014, 53, 37–51. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. World-Wide Electronic Publication, National University of Ireland, Galway, 2017. Available online: www.algaebase.org (accessed on 30 January 2017).
- Lin, S.-M.; Yang, S.-Y.; Huisman, J.M. Systematic revision of the genera Liagora and Izziella (Liagoraceae, Rhodophyta) from Taiwan based on molecular analyses an carposporophyte development, with the description of two new species. J. Phycol. 2011, 47, 352–365. [Google Scholar] [CrossRef] [PubMed]
- Abbott, I.A. Marine Red Algae of the Hawaiian Islands; Bishop Museum Press: Honolulu, HI, USA, 1999; p. 477. [Google Scholar]
- Lin, S.-M.; Huisman, J.M.; Payri, C. Characterization of Liagora ceranoides (Liagoraceae, Rhodopyta) on the basis of rbcL sequence analyses and carposporophyte development, including Yoshizakia indopacifica gen. et sp. nov. from the Indo-Pacific region. Phycologia 2013, 52, 161–170. [Google Scholar] [CrossRef]
- Lin, S.-M.; Yang, S.-Y.; Huisman, J.M. Systematics of Liagora with diffuse gonimoblasts based on rbcL sequences and carposporophyte development, including the description of the genera Neoizziella and Macrocarpus (Liagoraceae, Rhodophyta). Eur. J. Phycol. 2011, 46, 249–262. [Google Scholar] [CrossRef]
- Boo, G.H.; Kim, K.M.; Nelson, W.A.; Riosmena-Rodríguez, R.; Yoon, K.J.; Boo, S.M. Taxonomy and distribution of selected species of the agarophyte genus Gelidium (Gelidiales, Rhodophyta). J. Appl. Phycol. 2014, 26, 1243–1251. [Google Scholar] [CrossRef]
- Thomas, D.T.; Freshwater, D.W. Studies of Costa Rican Gelidiales (Rhodophyta): Four Caribbean taxa including Pterocladiella beachii sp. nov. Phycologia 2001, 40, 340–350. [Google Scholar] [CrossRef]
- Littler, D.S.; Littler, M.M. Marine Plants of Pacific Panama. 2009. Available online: http://biogeodb.stri.si.edu/pacificalgae/list (accessed on 30 January 2017).
- Kim, K.M.; Boo, S.M. Phylogenetic relationships and distribution of Gelidium crinale and G. pusillum (Gelidiales, Rhodophyta) using cox1 and rbcL sequences. Algae 2012, 27, 83–94. [Google Scholar] [CrossRef][Green Version]
- Santelices, B. Parviphycus, a new genus in the Gelidiellaceae (Gelidiales, Rhodophyta). Cryptogam. Algologie 2004, 25, 313–326. [Google Scholar]
- Boo, G.H.; Nguyen, T.V.; Kim, J.Y.; Le Gall, L.; Rico, J.M.; Bottalico, A.; Boo, S.M. A revised classification of the Gelidiellaceae (Rhodophyta) with descriptions of three new genera: Huismaniella, Millerella and Perronella. Taxon 2016, 65, 965–979. [Google Scholar] [CrossRef]
- Dawson, E.Y. The marine algae of the Gulf of California. Allan Hancock Pac. Exped. 1944, 3, 189–454. [Google Scholar]
- Dawson, E.Y. Marine red algae of Pacific Mexico. Part I. Bangiales to Corallinaceae subf. Corallionoidea. Allan Hancock Pac. Exped. 1953, 17, 1–239. [Google Scholar]
- Dawson, E.Y. Plantas Marinas de la zona de las mareas de El Salvador. Pac. Nat. 1961, 2, 389–461. [Google Scholar]
- Dijoux, L.; Viard, F.; Payri, C. The more we search, the more we find: Discovery of a new lineage and new species complex in the genus Asparagopsis. PLoS ONE 2014, 9, e103826. [Google Scholar] [CrossRef] [PubMed]
- Andreakis, N.; Procaccini, G.; Maggs, C.; Kooistra, W.H.C.F. Phylogeography of the invasive seaweed Asparagopsis (Bonnemaisoniales, Rhodophyta) reveals cryptic diversity. Mol. Ecol. 2007, 16, 2285–2299. [Google Scholar] [CrossRef] [PubMed]
- Zanolla, M.; Carmona, R.; De La Rosa, J.; Salvador, N.; Sherwood, A.R.; Andreakis, N.; Altamirano, M. Morphological differentiation of cryptic lineages within the invasive genus Asparagopsis (Bonnemaisoniales, Rhodophyta). Phycologia 2014, 53, 233–242. [Google Scholar] [CrossRef]
- Abbott, I.A.; Hollenberg, G.J. Marine Algae of California; Stanford University Press: Stanford, CA, USA, 1976; p. 827. [Google Scholar]
- Farlow, W.G. On some algae new to the United States. Proc. Am. Acad. Arts Sci. 1877, 4, 235–245. [Google Scholar] [CrossRef]
- Nauer, F.; Guimarães, N.R.; Cassano, V.; Yokoya, N.S.; Oliveira, M.C. Hypnea species (Gigartinales, Rhodophyta) from the southeastern coast of Brazil based on molecular studies complemented with morphological analyses, including descriptions of Hypnea edeniana sp. nov. and H. flava sp. nov. Eur. J. Phycol. 2014, 49, 550–575. [Google Scholar] [CrossRef]
- Dawson, E.Y. Marine red algae of Pacific Mexico, part 4. Gigartinales. Pac. Nat. 1961, 2, 191–343. [Google Scholar]
- Norris, J.N. Marine Algae of the Northern Gulf of California II: Rhodophyta; Smithsonian Institution Scholarly Press: Washington, DC, USA, 2014; Volume 96, pp. 1–555. [Google Scholar]
- Payri, C.; De Ramon N’Yeurt, A.; Orempuller, J. Algae of French Polynesia; Au Vent Des Iles: Tahiti, France, 2000; p. 320. [Google Scholar]
- De Ramon N’Yeurt, A. Marine algae from the Suva Lagoon and reef, Fiji. Aust. Syst. Bot. 2001, 14, 689–869. [Google Scholar]
- Littler, D.S.; Littler, M.M. South Pacific Reef Plants, A Diver’s Guide to the Plant Life of South Pacific Coral Reefs; Offshore Graphics, Inc.: Washington, DC, USA, 2003; p. 331. [Google Scholar]
- Gargiulo, G.M.; Morabito, M.; Manghisi, A. A re-assessment of reproductive anatomy and postfertilization development in the systematics of Grateloupia (Halymeniales, Rhodophyta). Cryptogam. Algologie 2013, 34, 3–35. [Google Scholar] [CrossRef]
- Calderon, M.S.; Boo, G.H.; Boo, S.M. Neorubra decipiens gen. & comb. nov. and Phyllymenia lancifolia comb. nov. (Halymeniales, Rhodophyta) from South America. Phycologia 2014, 53, 409–422. [Google Scholar]
- Calderon, M.S.; Boo, G.H.; Boo, S.M. Morphology and phylogeny of Ramirezia osornoensis gen. & sp. nov. and Phyllymenia acletoi sp. nov. (Halymeniales, Rhodophyta) from South America. Phycologia 2014, 53, 23–36. [Google Scholar]
- Calderon, M.S.; Boo, G.H.; Boo, S.M. Corrigendum of Morphology and phylogeny of Ramirezia osornoensis gen. & sp. nov. and Phyllymenia acletoi sp. nov. (Halymeniales, Rhodophyta) from South America. Phycologia 2016, 55, 610. [Google Scholar]
- Saunders, G.W.; Strachan, I.M.; Kraft, G.T. The families of the order Rhodymeniales (Rhodophyta): A molecular-systematic investigation with a description of Faucheaceae fam. nov. Phycologia 1999, 38, 23–40. [Google Scholar] [CrossRef]
- Zanardini, G. Phyceae papuanae novae vel minus cognitae a Cl. O. Beccari in itinere ad Novam Guineam annis 1872-75 collectae. Nuovo Giorn. Bot. Ital. 1878, 10, 34–40. [Google Scholar]
- Norris, R.E. The systematic position of Gelidiopsis and Ceratodictyon (Gigartinales, Rhodophyceae), genera new to South Africa. S. Afr. J. Bot. 1987, 53, 239–246. [Google Scholar] [CrossRef]
- Le Gall, L.; Dalen, J.L.; Saunders, G.W. Phylogenetic analyses of the red algal order Rhodymeniales supports recognition of the Hymenocldiaceae fam. nov., Fryeellaceae fam. nov., and Neogastroclonium gen. nov. J. Phycol. 2008, 44, 1556–1571. [Google Scholar] [CrossRef] [PubMed]
- Filloramo, G.V.; Saunders, G.W. Application of multigene phylogenetics and site-stripping to resolve intraordinal relationships in the Rhodymeniales (Rhodophyta). J. Phycol. 2016, 52, 339–355. [Google Scholar] [CrossRef] [PubMed]
- De Clerck, O.; Tronchin, E.M.; Schils, T. Red Algae, Rhodophyceae. In Guide to the Seaweeds of Kwazulu-Natal; De Clerk, O., Bolton, J.J., Anderson, R.J., Coppejans, E., Eds.; Scripta Botanica Belgica 33, National Botanic Garden of Belgium: Meise, Belgium, 2005; pp. 132–267. [Google Scholar]
- Tejada, O.L. Listado de macroalgas en el litoral de El Salvador, basado en registros entre 1961 al 2001. In Diagnóstico de la Diversidad Biológica de El Salvador; Flores, V.O., Handal, A., Eds.; Red Mesoamericana de Recursos Bióticos: Mexico City, Mexico, 2003; pp. 1–171. [Google Scholar]
- Hommersand, M.H.; Freshwater, D.W.; Lopez-Bautista, J.M.; Fredericq, S. Proposal of the Euptiloteae Hommersand et Fredericq trib. nov. and transfer of some southern hemisphere Ptiloteae to the Callithamnieae (Ceramiaceae, Rhodophyta). J. Phycol. 2005, 42, 203–225. [Google Scholar] [CrossRef]
- Rodríguez-Prieto, C.; Freshwater, D.W.; Hommersand, M.H. Vegetative and reproductive development of Mediterranean Gulsonia nodulosa (Ceramiales, Rhodophyta) and its genetic affinities. Phycologia 2013, 52, 357–367. [Google Scholar] [CrossRef]
- McIvor, L.; Maggs, C.A.; Stanhope, M.J. RbcL sequences indicate a single evolutionary origin of multinucleate cells in the red algal tribe Callithamnieae. Mol. Phylogenet. Evol. 2002, 23, 433–446. [Google Scholar] [CrossRef]
- Aponte, N.E.; Ballantine, D.L.; Norris, J.N. Aglaothamnion halliae comb. nov. and A. collinsii sp. nov. (Ceramiales, Rhodophyta): Resolution of nomenclatural and taxonomic confusion. J. Phycol. 1997, 33, 81–87. [Google Scholar] [CrossRef]
- Dawson, E.Y. An annotated list of marine algae from Eniwetok Atoll, Marshall Islands. Pac. Sci. 1957, 11, 92–132. [Google Scholar]
- Børgesen, F. The marine algae of the Danish West Indies. Part 3. Rhodophyceae (3). Dansk Bot. Arkiv. 1917, 3, 145–240. [Google Scholar]
- Won, B.Y.; Fredericq, S.; Cho, T.O. Two new species of Centroceras (Ceramiales, Rhodophyta) from KwaZulu-Natal, South Africa. Eur. J. Phycol. 2010, 45, 240–246. [Google Scholar] [CrossRef]
- Schneider, C.W.; Cianciola, E.N.; Popolizio, T.R.; Spagnuolo, D.S.; Lane, C.E. A molecular-assisted alpha taxonomic study of the genus Centroceras (Ceramiaceae, Rhodophyta) in Bermuda reveals two novel species. Algae 2015, 30, 15–33. [Google Scholar] [CrossRef]
- Dawson, E.Y. New records of marine algae from Pacific Mexico and Central America. Pac. Nat. 1960, 1, 31–52. [Google Scholar]
- Zuccarello, G.C.; Sandercock, B.; West, J.A. Diversity within red algal species: Variations in world-wide samples of Spyridia filamentosa (Ceramiaceae) and Murrayella periclados (Rhodomelaceae) using DNA markers and breeding studies. Eur. J. Phycol. 2002, 37, 403–417. [Google Scholar] [CrossRef]
- Zuccarello, G.C.; Purd’homme van Reine, W.F.; Stegenga, H. Recognition of Spyridia griffithsiana comb. nov. (Ceramiales, Rhodophyta): A taxon previously misidentified as Spyridia filamentosa from Europe. Bot. Mar. 2004, 47, 481–489. [Google Scholar] [CrossRef]
- Conklin, K.Y.; Sherwood, A.R. Molecular and morphological variation of the red alga Spyridia filamentosa (Ceramiales, Rhodophyta) in the Hawaiian Archipelago. Phycologia 2012, 51, 347–357. [Google Scholar] [CrossRef]
- Kapraun, D.F. An Illustrated Guide to the Benthic Marine Algae of Coastal North Carolina I. Rhodophyta; The University of North Carolina Press: Chapel Hill, NC, USA, 1980; p. 206. [Google Scholar]
- Schneider, C.W.; Searles, R.B. Seaweeds of the Southeastern United States, Cape Hatteras to Cape Canaveral; Duke University Press: Durham, NC, USA, 1991; p. 553. [Google Scholar]
- Wynne, M.J.; Banaimoon, S.A. The occurrence of Jolyna laminarioides (Phaeophyta) in the Arabian Sea and the Indian Ocean, and a new report of Melanothamnus somalensis (Rhodophyta). Bot. Mar. 1990, 33, 213–218. [Google Scholar] [CrossRef]
- Shameel, M. Melanothamnus afaqhusainii, a new red alga from the coast of Karachi. Pak. J. Bot. 1999, 31, 211–214. [Google Scholar]
- Díaz-Tapia, P.; McIvor, L.; Freshwater, D.W.; Verbruggen, H.; Wynne, M.J.; Maggs, C.A. The genera Melanothamnus Bornet & Falkenberg and Vertebrata S.F. Gray constitute well-defined clades of the red algal tribe Polysiphonieae (Rhodomelaceae, Ceramiales). Eur. J. Phycol. 2017, 52, 1–30. [Google Scholar]
- Mamoozadeh, N.R.; Freshwater, D.W. Polysiphonia sensu lato (Ceramiales, Florideophyceae) species of Caribbean Panama including Polysiphonia lobophoralis sp. nov. and Polysiphonia nuda sp. nov. Bot. Mar. 2012, 55, 317–347. [Google Scholar] [CrossRef]
- McIvor, L.; Maggs, C.A.; Provan, J.; Stanhope, M.J. rbcL sequences reveal multiple cryptic introductions of the Japanese red alga Polysiphonia harveyi. Mol. Ecol. 2001, 10, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Yang, E.C. Taxonomic note of Polysiphonia pacifica (Ceramiales, Rhodophyta) complex with focus on Pacific isolates. Algae 2005, 20, 15–23. [Google Scholar] [CrossRef]
- Bustamante, D.E.; Won, B.Y.; Cho, T.O. Polysiphonia ulleungensis sp. nov. (Rhodomelaceae, Rhodophyta): A new diminutive species from Korea belonging to Polysiphonia sensu stricto. Algae 2014, 29, 111–120. [Google Scholar] [CrossRef]
- Freshwater, D.W.; Tudor, K.; O’Shaughnessy, K.; Wysor, B. DNA barcoding in the red algal order Gelidiales: Comparison of COI with rbcL and verification of the “barcoding gap”. Cryptogam. Algologie 2010, 31, 435–449. [Google Scholar]
- Tan, J.; Lim, P.-E.; Phang, S.-M.; Hong, D.D.; Sunarpi, H.; Hurtado, A.Q. Assessment of four molecular markers as potential DNA barcodes for red algae Kappaphycus Doty and Eucheuma J. Agardh (Solieriaceae, Rhodophyta). PLoS ONE 2012, 7, e52905. [Google Scholar] [CrossRef] [PubMed]
- Savoie, A.M.; Saunders, G.W. Evidence for the introduction of the Asian red alga Neosiphonia japonica and its introgression with Neosiphonia harveyi (Ceramiales, Rhodophyta) in the Northwest Atlantic. Mol. Ecol. 2015, 24, 5927–5937. [Google Scholar] [CrossRef] [PubMed]
- Mamoozadeh, N.R. Morphological and Molecular Analyses of Polysiphonia sensu lato in Southern Central America and the Caribbean. Master’s Thesis, University of North Carolina Wilmington, Wilmington, NC, USA, 2010. [Google Scholar]
- Bustamante, D.E.; Won, B.Y.; Miller, K.A.; Cho, T.O. Wilsonosiphonia gen. nov. (Rhodomelaceae, Rhodophyta) based on molecular and morpho-anatomical characters. J. Phycol. 2017, 53. [Google Scholar] [CrossRef] [PubMed]
- Kapraun, D.F.; Lemus, A.J.; Bula Meyer, G. Genus Polysiphonia (Rhodophyta, Ceramiales) in the tropical western Atlantic. I. Colombia and Venezuela. Bull. Mar. Sci. 1983, 33, 881–898. [Google Scholar]
- Skelton, P.A.; South, G.R. The benthic marine algae of the Samoan Archipelago, South Pacific, with emphasis on the Apia District. Beih. Nova Hedwig. 2007, 132, 1–350. [Google Scholar]
- Hollenberg, G.J. An account of the species of the red alga Polysiphonia of the central and western tropical Pacific Ocean. II. Polysiphonia. Pac. Sci. 1968, 22, 198–207. [Google Scholar]
- Dawes, C.J.; Mathieson, A.C. The seaweeds of Florida; University Press of Florida: Gainesville, FL, USA, 2008; p. 591. [Google Scholar]
- Savoie, A.M.; Saunders, G.W. A molecular phylogenetic and DNA barcode assessment of the tribe Pterosiphonieae (Ceramiales, Rhodophyta) emphasizing the Northeast Pacific. Botany 2016, 94, 917–939. [Google Scholar] [CrossRef]
- Lemoine, P. Les Corallinacees de l’Archipel des Galapagos et du Golfo de Panama. Arch. Mus. Hist. Nat. 1929, 6, 47–88. [Google Scholar]
- Knowlton, N.; Weigt, L.A. New dates and new rates for divergence across the Isthmus of Panama. Proc. Biol. Sci. 1998, 265, 2257–2263. [Google Scholar] [CrossRef]
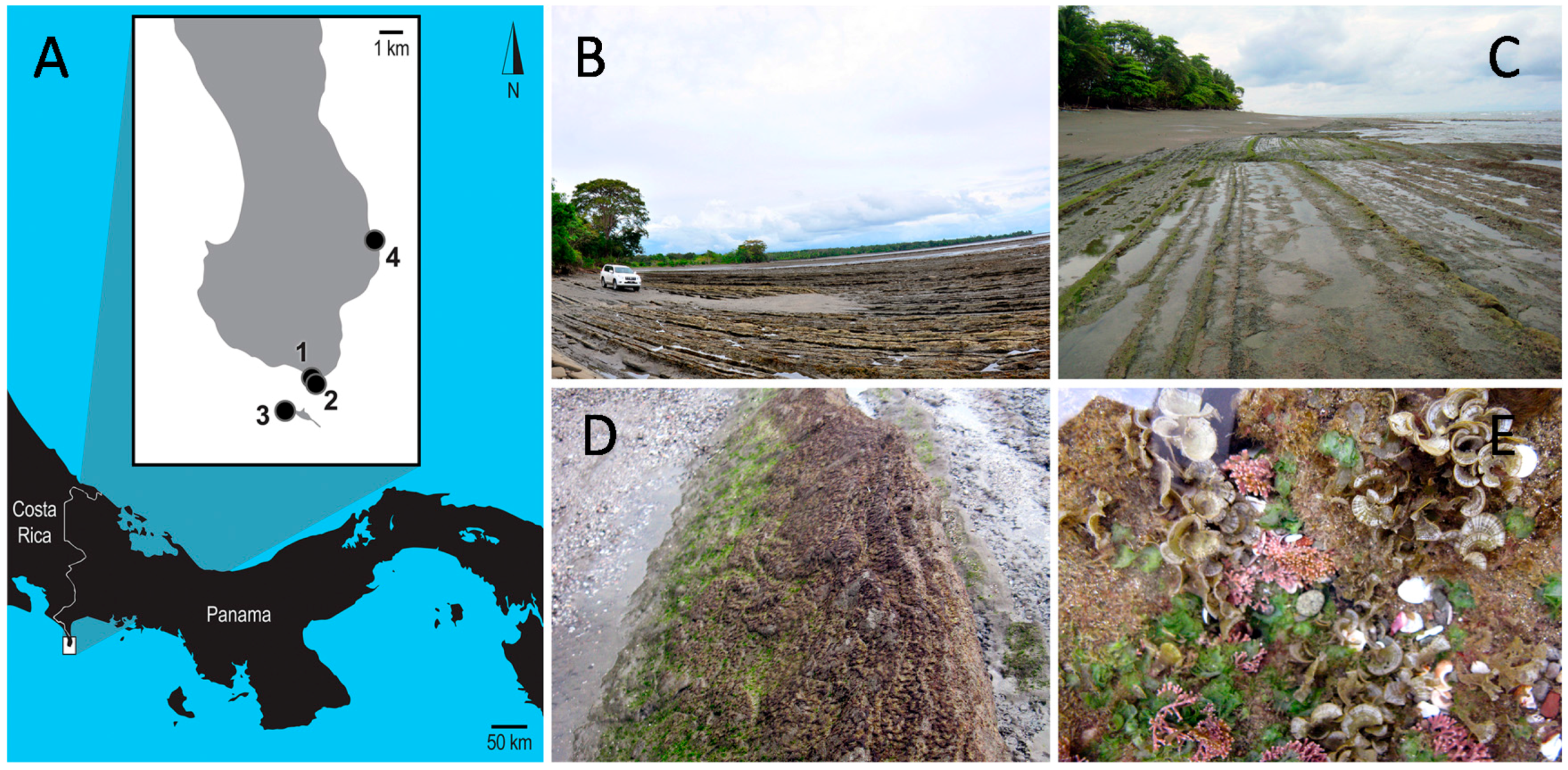

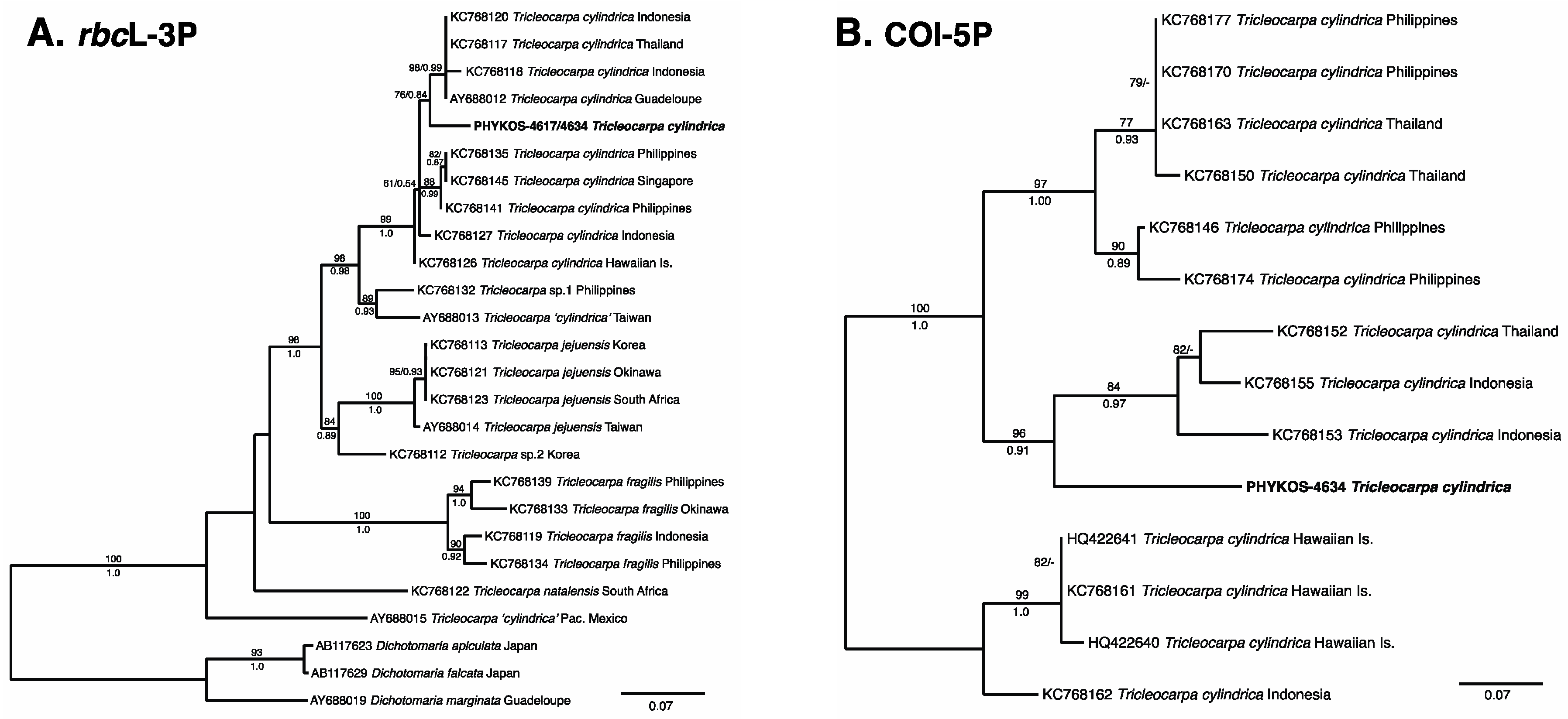

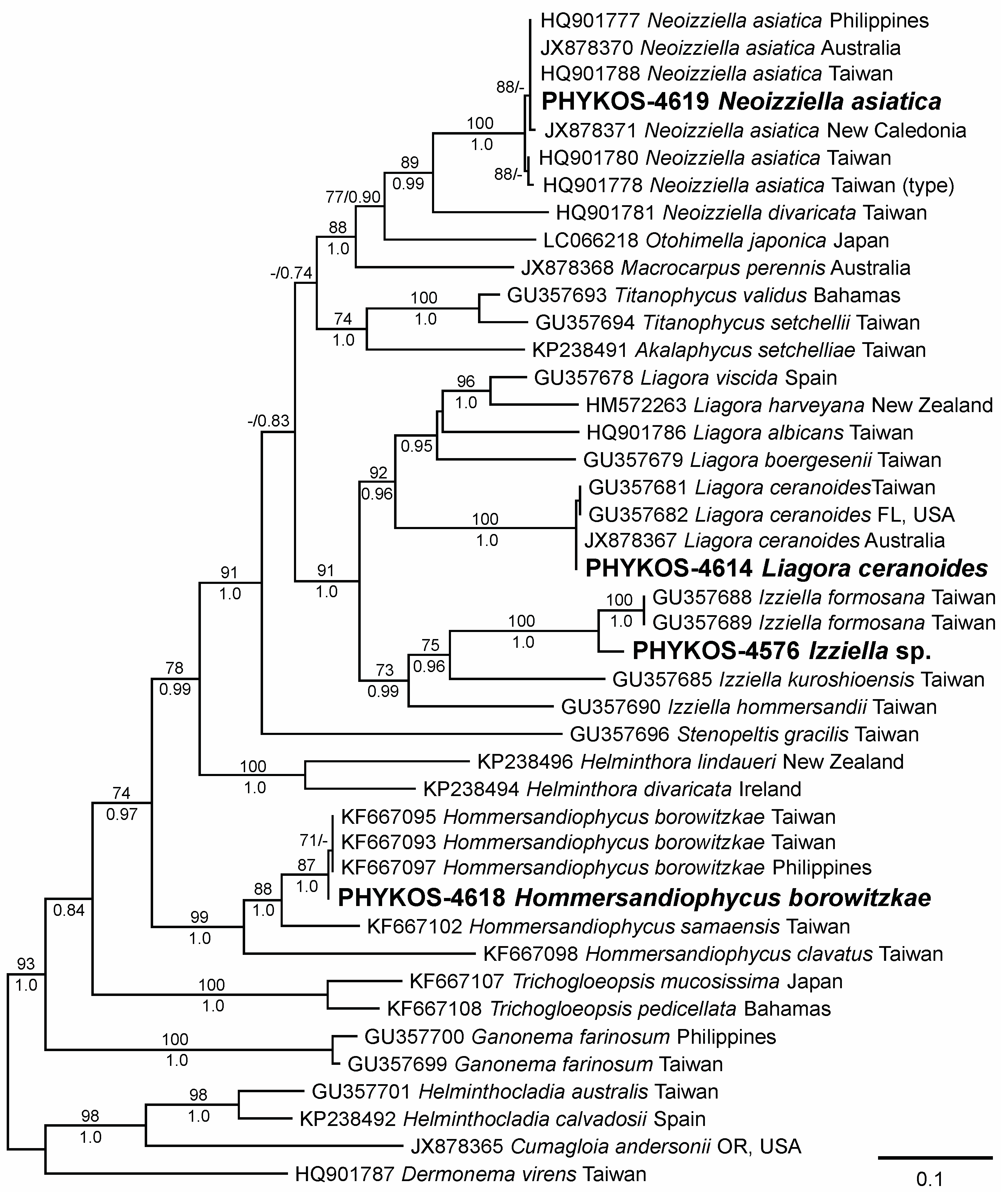


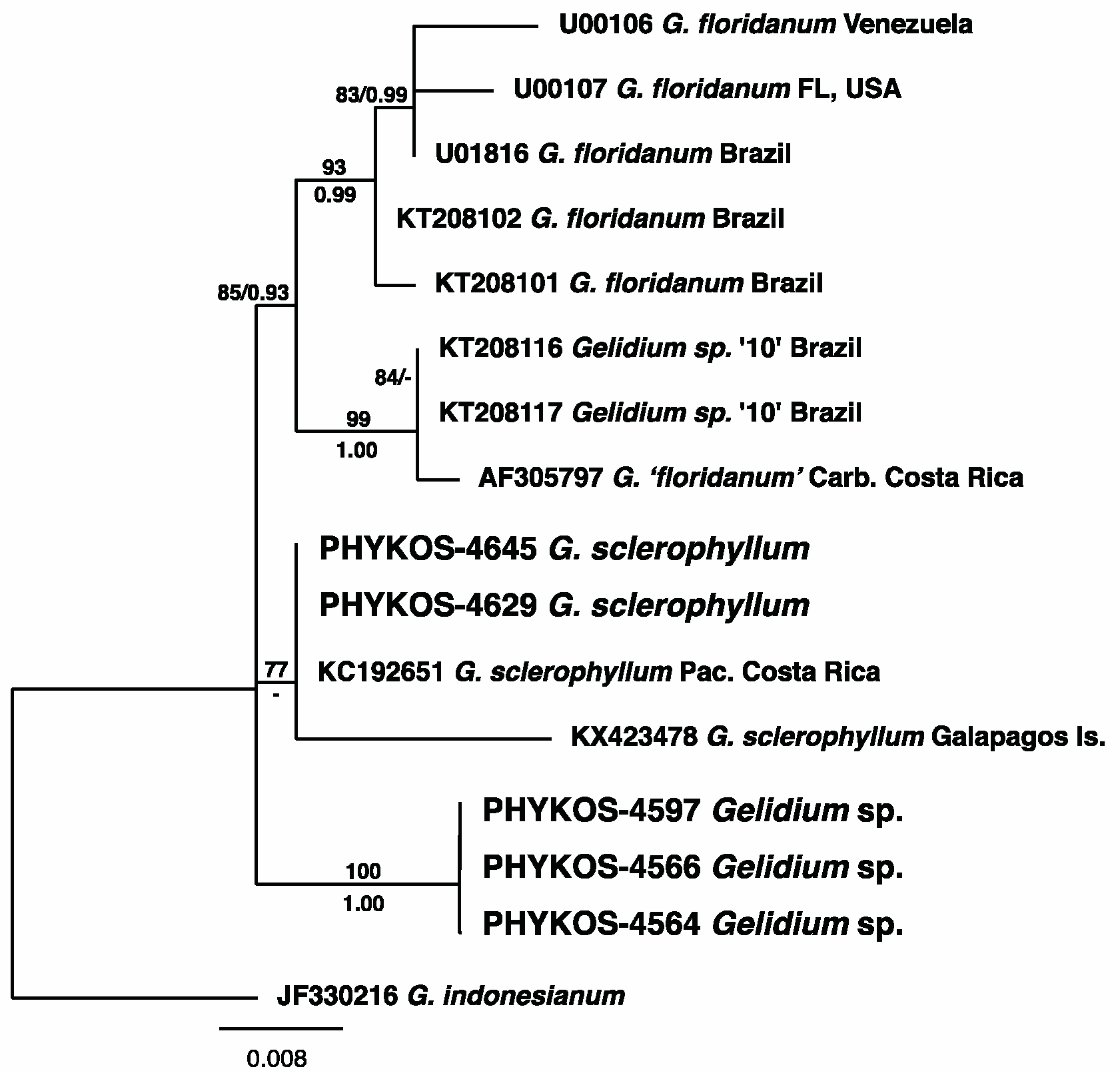











| Locus | Primer | Sequence | Citation |
|---|---|---|---|
| COI-5P | GHalF | TCAACAAATCATAAAGATATYGG | [22] |
| GazR1 | ACTTCTGGATGTCCAAAAAAYCA | [11] | |
| GWSFn | TCAACAAAYCAYAAAGATATYGG | [13] | |
| GWSRx | ACTTCTGGRTGICCRAARAAYCA | [23] | |
| rbcL-3P | F753 | GGAAGATATGTATGAAAGAGC | [24] |
| RrbcSstart | GTTCCTTGTGTTAATCTCAC | Modified from [24] | |
| 5P-rbcL 1 | F57 | GTAATTCCATATGCTAAAATGGG | [24] |
| R893 | GAATAAGTTGARTTWCCIGCAC | [25] | |
| UPA | p23SrV-f1 | GGACAGAAAGACCCTATGAA | [16] |
| p23SrV-r1 | TCAGCCTGTTATCCCTAGAG | [16] |
| PHYKOS | rbcL-3P | COI-5P | UPA | |
|---|---|---|---|---|
| MAI ID | Coll. No. | BLAST | BLAST | BLAST |
| 1 Tricleocarpa cylindrica (J. Ellis and Solander) Huisman and Borowitzka | 4617 | Tricleocarpa cylindrica (98%) | - | Dichotomaria marginata and Galaxaura rugosa (97%) |
| 4634 | „ | Tricleocarpa cylindrica (96%) | „ | |
| 1,2 Hommersandiophycus borowitzkae (Huisman) S.-M. Lin and Huisman | 4618 | Hommersandiophycus borowitzkae (99%) | Ganonema yoshizakii (91%) | Ganonema yoshizaki and Hommersandiophycus borowitzkae (99%) |
| 1 Izziella sp. | 4576 | Izziella formosana (98%) | Izziella orientalis (95%) | Izziella formosana (100%) |
| 4599 | - | „ | „ | |
| 1 Liagora ceranoides J.V. Lamouroux | 4614 | Liagora ceranoides (100%) | Liagora sp. (96%) | Liagora ceranoides (99%) |
| 1 Neoizziella asiatica S.-M. Lin, S.-Y. Yang and Huisman | 4619 | Neoizziella asiatica (100%) | Neoizziella divaricata (99%) | Neoizziella asiatica (100%) |
| 1 Gelidium sclerophyllum W.R. Taylor | 4514 | - | Gelidium sclerophyllum (98%) | Gelidium sclerophyllum (100%) |
| 4629 | Gelidium sclerophyllum (100%) | - | „ | |
| 4645 | „ | „ | „ | |
| 1 Gelidium sp. | 4564 | Gelidium floridanum and G. sclerophyllum (99%) | Gelidium floridanum and Gelidium sp. (94%) | Gelidium sclerophyllum (99%) |
| 4566 | „ | „ | „ | |
| 4597 | „ | „ | „ | |
| Millerella sp. | 4570 | Millerella myriocladus, M. tinerfensis and M. felicinii (93%) | Milerella myriocladus (86%) | Millerella sp. (97%) |
| Asparagopsis sp. | 4636 | Asparagopsis taxiformis (97%) | Asparagopsis taxiformis (100%) | Asparagopsis taxiformis (100%) |
| 4646 | - | - | „ | |
| 1 Plocamium sp. | 4643 | Plocamium pacificum [as P. cartilagineum] (99%) | Plocamium pacificum (95%) | Plocamium cartilagineum and P. telfairiae (99%) |
| 1,2 Hypnea flava Nauer, Cassano and M.C. Oliveira | 4574 | Hypnea flava (99%) | Hypnea ’spinella’ (96%) | Hypnea ’spinella’ (99%) |
| Hypnea pannosa J. Agardh | 4631 | Hypnea pannosa (100%) | Hypnea panosa (99%) | Hypnea nudifica + 4 others (99%) |
| 1,2 Neorubra parvolacertoides sp. nov. | 4528 | Neorubra decipiens (96%) | Grateloupia angusta, G. taiwanensis (91%) | Grateloupia phuquocensis + 6 others (98%) |
| 4589 | „ | „ | „ | |
| 1,2 Grateloupia irregularis sp. nov. | 4522 | Grateloupia dichotoma and G. filicina (97%) | Prionitis filiformis (92%) | Grateloupia phuquocensis (99%) |
| 4556 | „ | „ | „ | |
| 4620 | „ | „ | „ | |
| 1 Ceratodictyon repens (Kützing) R.E. Norris | 4627 | - | Ceratodictyon scoparia (94%) | Ceratodictyon scoparia (99%) |
| 4632 | Ceratodictyon repens (100%) | „ | „ | |
| 4635 | „ | „ | „ | |
| 1,2 Ceratodictyon scoparium (Montagne and Millardet) R.E. Norris | 4590 | Ceratodictyon sp. ’Calerita’ (100%) | Ceratodictyon scoparium (99%) | Ceratodictyon scoparium (100%) |
| 1 Gracilaria sp.1 | 4640 | Gracilaria tikvahiae, G. cuneifolia and G. isabellana (97%) | Gracilaria incurvata (94%) | Gracilaria parvispora + 6 others (99%) |
| 1 Gracilaria sp.2 | 4519 | - | Gracilaria galetensis (92%) | Gracilaria galetensis (98%) |
| 4541 | Gracilaria galetensis (97%) | „ | „ | |
| 4630 | „ | „ | „ | |
| 1 Gracilaria sp.3 | 4548 | Gracilaria damaecornis, G. isabellana, G. chouae and G. parvispora (98%) | Gracilaria isabellana, G. caudata (94%) | Gracilaria parvispora + 7 others (99%) |
| 1,2 Aglaothamnion sp. | 4553 | Aglaothamnion halliae and A. hookeri (96%) | Callithamnion tetragonum and Aglaothamnion sp. (91%) | Callithamnion corymbosum and Aglaothamnion spp. (96%) |
| Centroceras gasparrinii (Meneghini) Kützing | 4594 | C. gasparrini (100%) | Centroceras clavulatum (94%) | Centroceras sp. (99%) |
| 4600 | „ | „ | „ | |
| 1,2 Spyridia sp. | 4584 | - | Spyridia filamentosa (95%) | - |
| 1 Melanothamnus sp. | 4568 | Melanothamnus pseudovillum (98%) | Melanothamnus pseudovillum (97%) | Melanothamnus spp. and ’Polysiphonia’ spp. (98%) |
| 1,2 ’Polysiphonia’ binneyi Harvey | 4534 | ’Polysiphonia’ binneyi (99%) | ’Polysiphonia’ echinata (93%) | ’Polysiphonia’ sp. (97%) |
| 1,2 ’Polysiphonia’ sp. | 4515 | ’Polysiphonia’ sp. (99%) | ’Polysiphonia’ sp. (99%) | Herposiphonia sp., Symphyocladia latiuscula (97%) |
| 4525 | „ | „ | „ | |
| 4526 | „ | „ | „ | |
| 4552 | „ | - | „ | |
| Wilsonosiphonia howei (Hollenberg) D. Bustamante, Won and T.O. Cho | 4657 | Wilsonosiphonia howei (99%) | - | Wilsonosiphonia howei (99%) |
| 4663 | „ | Wilsonosiphonia howei (97%) | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freshwater, D.W.; Idol, J.N.; Parham, S.L.; Fernández-García, C.; León, N.; Gabrielson, P.W.; Wysor, B. Molecular Assisted Identification Reveals Hidden Red Algae Diversity from the Burica Peninsula, Pacific Panama. Diversity 2017, 9, 19. https://doi.org/10.3390/d9020019
Freshwater DW, Idol JN, Parham SL, Fernández-García C, León N, Gabrielson PW, Wysor B. Molecular Assisted Identification Reveals Hidden Red Algae Diversity from the Burica Peninsula, Pacific Panama. Diversity. 2017; 9(2):19. https://doi.org/10.3390/d9020019
Chicago/Turabian StyleFreshwater, David Wilson, Jennifer N. Idol, Seth L. Parham, Cindy Fernández-García, Noemi León, Paul W. Gabrielson, and Brian Wysor. 2017. "Molecular Assisted Identification Reveals Hidden Red Algae Diversity from the Burica Peninsula, Pacific Panama" Diversity 9, no. 2: 19. https://doi.org/10.3390/d9020019
APA StyleFreshwater, D. W., Idol, J. N., Parham, S. L., Fernández-García, C., León, N., Gabrielson, P. W., & Wysor, B. (2017). Molecular Assisted Identification Reveals Hidden Red Algae Diversity from the Burica Peninsula, Pacific Panama. Diversity, 9(2), 19. https://doi.org/10.3390/d9020019








