Phenotyping, Genotyping, and Selections within Italian Local Landraces of Romanesco Globe Artichoke
Abstract
:1. Introduction
2. Materials and Methods
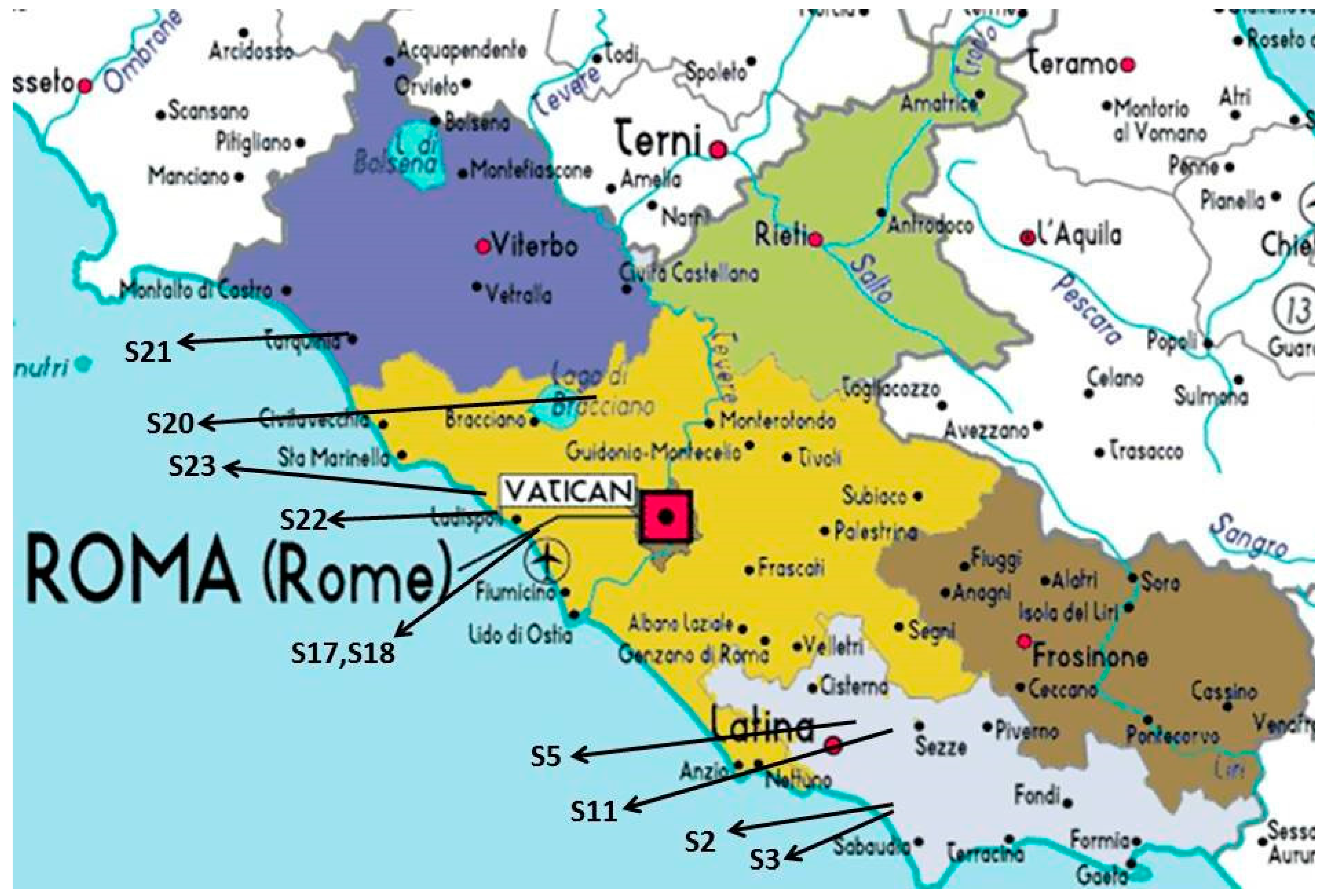
2.1. Experimental Field and Plant Material
2.2. Morphological Analysis
2.3. DNA Extraction and Molecular Marker Analysis
2.4. Biochemical Analysis
2.4.1. Solvents and Reagents
2.4.2. Polyphenol Extraction and HPLC Analysis
2.5. Statistical Analysis
3. Results
3.1. Morphological Characterization
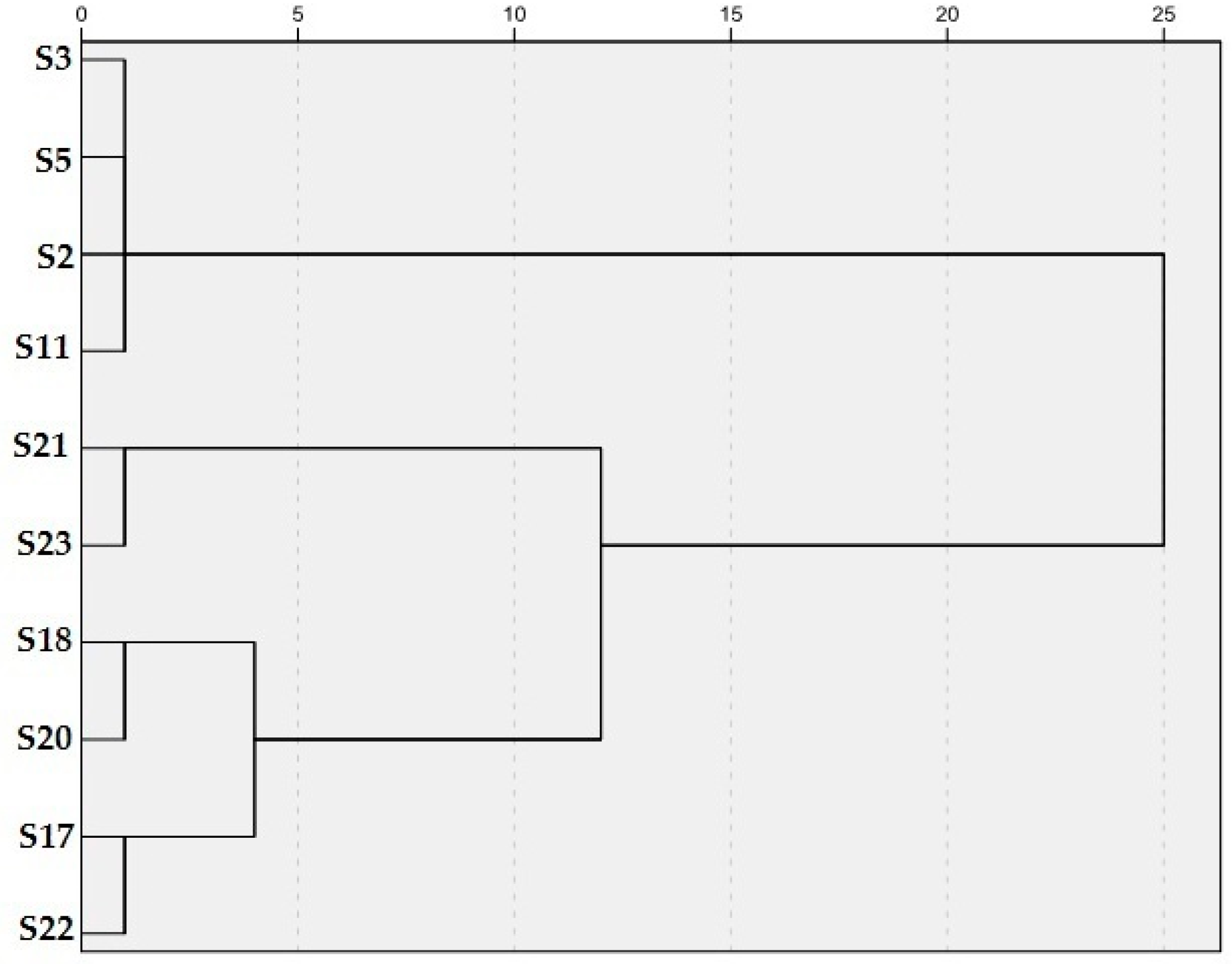
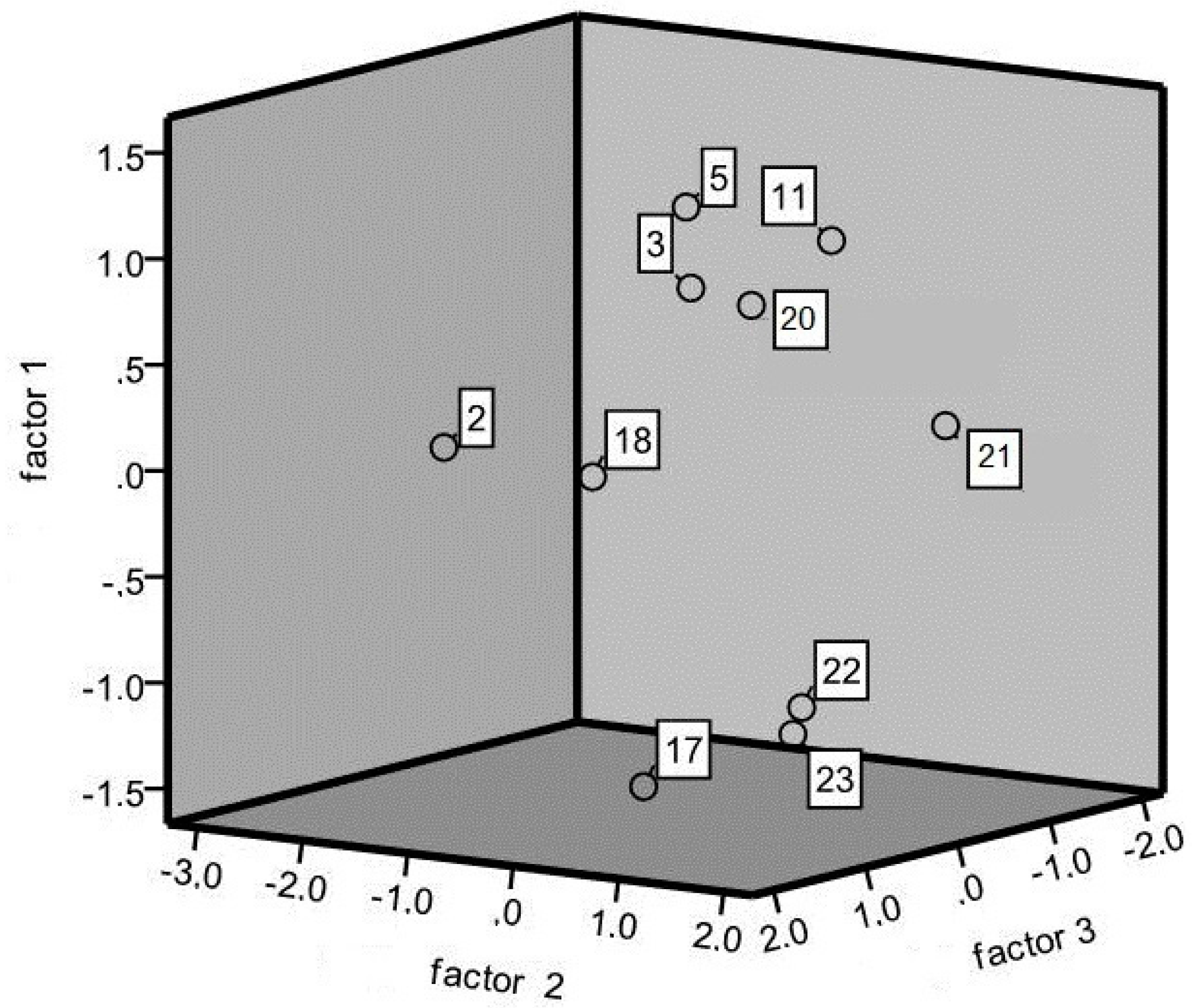
3.2. DNA Marker Characterization
3.3. Biochemical Characterization
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bianco, V.V. Carciofo (Cynara scolymus). In Orticoltura; Orticoltura Bianco, V.V., Rimpini, F., Eds.; Patron Editore: Bologna, Italy, 1990; pp. 209–251. [Google Scholar]
- Mauro, R.; Portis, E.; Acquadro, A.; Lombardo, S.; Mauromicale, G.; Lanteri, S. Genetic diversity of globe artichoke landraces from Sicilian small-holdings: Implications for evolution and domestication of the species. Conserv. Genet. 2009, 10, 431–440. [Google Scholar] [CrossRef]
- Ierna, A.; Mauro, R.P.; Mauromicale, G. Biomass, grain and Energy yield in Cynara cardunculus L. as affected by fertilization, genotype and harvest time. Biomass Bioenergy 2012, 36, 404–410. [Google Scholar] [CrossRef]
- Ledda, L.; Deligios, P.A.; Farci, R.; Sulas, L. Biomass supply for energetic purposes from some Cardueae species grown in Mediterranean farming systems. Ind. Crops Prod. 2013, 47, 218–226. [Google Scholar] [CrossRef]
- Lombardo, S.; Pandino, G.; Ierna, A.; Mauromicale, G. Variation of polyphenols in a germplasm collection of globe artichoke. Food Res. Int. 2012, 46, 544–553. [Google Scholar] [CrossRef]
- Ciancolini, A.; Alignan, M.; Pagnotta, M.A.; Miquel, J.; Vilarem, G.; Crinò, P. Morphological characterization, biomass and pharmaceutical compounds in Italian globe artichoke genotypes. Ind. Crops Prod. 2013, 49, 326–333. [Google Scholar] [CrossRef]
- FAOSTAT Data 2013. Available online: http://www.fao.org/faostat/en/#data (accessed on 30 October 2016).
- ISTAT (Italian National Institute of Statistics) 2009. Available online: http://dati.istat.it/Index.aspx?DataSetCode=DCSP_COLTIVAZ&Lang=en (accessed on 25 October 2016).
- Pignone, D.; Sonnante, G. Wild artichokes of south Italy: Did the story begin here? Genet. Resour. Crop Evol. 2004, 51, 577–580. [Google Scholar] [CrossRef]
- Porceddu, E.; Dellacecca, V.; Blanco, V.V. Classificazione numerica di cultivar di carciofo. In Proceedings of the II International Congress of Artichoke, Bari, Italy, 17–21 October 1976; pp. 1105–1119.
- Mauromicale, G.; Ierna, A. Characteristics of heads of seed-grown globe artichoke [Cynara cardunculus L. var. scolymus (L.) Fiori] as affected by harvest period, sowing date and gibberellic acid. Agronomie 2000, 20, 197–204. [Google Scholar]
- Acquadro, A.; Papanice, M.A.; Lanteri, S.; Bottalico, G.; Portis, E.; Campanale, A.; Finetti-Sialer, M.M.; Mascia, T.; Sumerano, P.; Gallitelli, D. Production and fingerprinting of virus-free clones in a reflowering globe artichoke. Plant Cell Tissue Organ Cult. 2010, 100, 329–337. [Google Scholar] [CrossRef]
- Portis, E.; Mauromicale, G.; Barchi, L.; Mauro, R.; Lanteri, S. Population structure and genetic variation in autochthonous globe artichoke germplasm from Sicily Island. Plant Sci. 2005, 168, 1591–1598. [Google Scholar] [CrossRef]
- Bonasia, A.; Conversa, G.; Lazzizera, C.; Gambacorta, G.; Elia, A. Morphological and qualitative characterisation of globe artichoke head from new seed-propagated cultivars. J. Sci. Food Agric. 2010, 90, 2689–2693. [Google Scholar] [CrossRef] [PubMed]
- Soressi, G. Available variability usable for breeding of globe artichoke [Cynara cardunculus L. var. scolymus L.]. Inf Agrar. 2003, 22, 47. [Google Scholar]
- Ancora, G.; Crinò, P.; Tavazza, R.; Pagnotta, M.A.; Temperini, O.; Campanelli, R.; Saccardo, F. The first three clones selected from the traditional artichoke Romanesco populations and proposed for the release of new varieties. Acta Horticult. 2012, 942, 125–132. [Google Scholar] [CrossRef]
- De Vos, N.E. Artichoke production in California. HortTechnology 1992, 2, 438–444. [Google Scholar]
- Crinò, P.; Tavazza, R.; Rey Muñoz, N.A.; Trionfetti Nisini, P.; Saccardo, F.; Ancora, G.; Pagnotta, M.A. Recovery, morphological and molecular characterisation of globe artichoke ‘Romanesco’ landraces. Genet. Resour. Crop Evol. 2008, 55, 823–833. [Google Scholar] [CrossRef]
- Cappelletti, R.; Mezzetti, B.; Balducci, F.; Diamanti, J.; Capocasa, F. Morphological, nutraceutical and chemical characterization of globe artichoke (Cynara cardunculus L. var. scolymus (L.) Fiori) landraces typically cultivated in Marche area. Acta Hortic. 2013, 983, 39–46. [Google Scholar] [CrossRef]
- Mauro, R.; Portis, E.; Lanteri, S.; Mauromicale, G. Genotypic and bio-agronomical characterization of an early Sicilian landrace of globe artichoke. Euphytica 2012, 186, 357–366. [Google Scholar] [CrossRef]
- Mauro, R.; Portis, E.; Lanteri, S.; Mauromicale, G. Bio-agronomical and molecular characterization of selected clones in the early Sicilian globe artichoke landrace ‘violetto di Sicilia’. Acta Horticult. 2013, 983, 209–214. [Google Scholar] [CrossRef]
- Ciancolini, A.; Rey, N.A.; Pagnotta, M.A.; Crinò, P. Characterization of Italian spring globe artichoke germplasm: morphological and molecular profiles. Euphytica 2012, 186, 433–443. [Google Scholar] [CrossRef]
- Pagnotta, M.A.; Rey, N.A.; Ciancolini, A.; Crinò, P. Are UPOV Descriptors a Useful and Feasible Tool? Acta Hort. 2013, 983, 145–149. [Google Scholar] [CrossRef]
- Vos, P.; Hogers, R.; Bleeker, M.; Reijand, M.; Van de Lee, T.; Hornes, M.; Fritjers, A.; Pot, J.; Paleman, J.; Kuiper, M.; et al. AFLP: A new technique for DNA fingerprinting. Nucleic Acids Res. 1995, 23, 4407–4414. [Google Scholar] [CrossRef] [PubMed]
- Acquadro, A.; Portis, E.; Lee, D.; Donini, P.; Lanteri, S. Development and characterization of microsatellite markers in Cynara cardunculus L. Genome 2005, 48, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Acquadro, A.; Portis, E.; Lanteri, S. Isolation of microsatellite loci in artichoke (Cynara cardunculus L. var. scolymus). Mol. Ecol. Notes 2003, 3, 37–39. [Google Scholar] [CrossRef]
- Acquadro, A.; Portis, E.; Albertini, E.; Lanteri, S. M-AFLP-based protocol for microsatellite loci isolation in Cynara cardunculus L. (Asteraceae). Mol. Ecol. Notes 2005, 5, 272–274. [Google Scholar] [CrossRef]
- Sonnante, G.; Carluccio, A.V.; De Paolis, A.; Pignone, D. Identification of artichoke SSR markers: Molecular variation and patterns of diversity in genetically cohesive taxa and wild allies. Genet. Resour. Crop Evol. 2008, 55, 1029–1046. [Google Scholar] [CrossRef]
- Pagnotta, M.A.; Fernández, J.A.; Sonnante, G.; Egea Gilabert, C. Genetic diversity and accession structure in European Cynara cardunculus collections. 2017. (in press) [Google Scholar]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research-an update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Muse, S.V. PowerMarker: An integrated analysis environment for genetic marker analysis. Bioinformatics 2005, 21, 2128–2129. [Google Scholar] [CrossRef] [PubMed]
- Nei, M. Genetic distance between populations. Am. Nat. 1972, 106, 183–192. [Google Scholar] [CrossRef]
- Mantel, N.A. The detection of disease clustering and a generalized regression approach. Cancer Res. 1967, 27, 209–220. [Google Scholar] [PubMed]
- Lahoz, I.; Fernández, J.A.; Migliaro, D.; Macua, J.I.; Egea-Gilabert, C. Using molecular markers, nutritional traits and field performance data to characterize cultivated cardoon germplasm resources. Sci. Hortic. 2011, 127, 188–197. [Google Scholar] [CrossRef]
- Lanteri, S.; Acquadro, A.; Saba, E.; Portis, E. Molecular fingerprinting and evaluation of genetic distances among selected clones of globe artichoke (Cynara cardunculus L. var. scolymus L.). J. Horticult. Sci. Biotechnol. 2004, 79, 863–870. [Google Scholar] [CrossRef]
- Scaglione, D.; Reyes-Chin Who, S.; Acquadro, A.; Froenicke, L.; Portis, E.; Beitel, C.; Tirone, M.; Mauro, R.; Lo Monaco, A.; Mauromicale, G.; et al. The genome sequence of the outbreeding globe artichoke constructed de novo incorporating a phase-aware low-pass sequestrategy of a F1 progeny. Sci. Rep. 2016, 6, 25323. [Google Scholar] [CrossRef] [PubMed]
- Lo Bianco, C.L.; Fernández, J.A.; Migliaro, D.; Crinò, P.; Egea-Gilabert, C. Identification of F1 hybrids of artichoke by ISSR markers and morphological analysis. Mol. Breed. 2011, 27, 157–170. [Google Scholar] [CrossRef]
- Pandino, G.; Lombardo, S.; Mauro, R.P.; Mauromicale, G. Variation in polyphenol profile and head morphology among clones of globe artichoke selected from a landrace. Sci. Horticult. 2012, 138, 259–265. [Google Scholar] [CrossRef]
- Pandino, G.; Lombardo, S.; Mauromicale, G. Globe artichoke leaves and floral stems as a source of bioactive compounds. Ind. Crops Prod. 2013, 44, 44–49. [Google Scholar] [CrossRef]
- Lombardo, S.; Pandino, G.; Mauromicale, G.; Knödler, M.; Carle, R.; Schieber, A. Influence of genotype, harvest time and plant part on polyphenolic composition of globe artichoke [Cynara cardunculus L. var. scolymus (L.) Fiori]. Food Chem. 2010, 119, 1175–1181. [Google Scholar] [CrossRef]
- Pandino, G.; Lombardo, S.; Mauromicale, G.; Williamson, G. Profile of poyphenols and phenolic acids in bracts and receptacles of globe artichoke (Cynara cardunculus var. scolymus), germplasm. J. Food Compos. Anal. 2011, 24, 148–153. [Google Scholar] [CrossRef]
- Rodriguez, T.S.; Giménez, D.G.; De La Puerta Vázquez, R. Choleretic activity and biliary elimination of lipids and bile acids induced by an artichoke leaf extract in rats. Phytomedicine 2002, 9, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Coinu, R.; Carta, S.; Urgeghe, P.P.; Mulinacci, N.; Pinelli, P.; Franconi, F.; Romani, A. Dose-effect study on the antioxidant properties of leaves and outer bracts of extracts obtained from Violetto di Toscana artichoke. Food Chem. 2007, 101, 524–531. [Google Scholar] [CrossRef]
- Rondanelli, M.; Giacosa, A.; Opizzi, A.; Faliva, M.A.; Sala, P.; Perna, S.; Bombardelli, E. Beneficial effects of artichoke leaf extract supplementation on increasing HDL-cholesterol in subjects with primary mild hypercholesterolaemia: A double-blind, randomized, placebo-controlled trial. Int. J. Food Sci. Nutr. 2013, 64, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Fantini, N.; Colombo, G.; Giori, A.; Riva, A.; Morazzoni, P.; Bombardelli, E.; Carai, M.A. Evidence of glycemia-lowering effect by a Cynara scolymus L. extract in normal and obese rats. Phytother. Res. 2011, 25, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Lattanzio, V.; Cardinali, A.; Di Venere, D.; Linsalata, V.; Palmieri, S. Browning phenomena in stored artichoke (Cynara scolymus L.) heads: Enzymic or chemical reactions? Food Chem. 1994, 50, 1–7. [Google Scholar] [CrossRef]

| No. | Descriptor | No. | Descriptor |
|---|---|---|---|
| 1. | Plant: height (including central flower-head) | 25. | Central flower-head: shape of tip |
| 2. | Plant: number of lateral shoots on main stem | 26. | First flower-head on lateral shoot: length |
| 3. | Main stem: height (excluding central flower-head) | 27. | First flower-head on lateral shoot: diameter |
| 4. | Main stem: distance between central flower-head and youngest well developed leaf | 28. | First flower-head on lateral shoot: size |
| 5. | Main stem: diameter (at about 10 cm below central flower-head) | 29. | First flower-head on lateral shoot: shape |
| 6. | Leaf: attitude | 30. | Outer bract: length of base |
| 7. | Leaf: long spines | 31. | Outer bract: width of base |
| 8. | Leaf: length | 32. | Outer bract: thickness at base |
| 9. | Leaf: incisions | 33. | Outer bract: main shape |
| 10. | Leaf: number of lobes, | 34. | Outer bract: shape of apex |
| 11. | Lobe: shape of tip (excluding terminal lobe) | 35. | Outer bract: depth of emargination |
| 12. | Lobe: number of secondary lobes | 36. | Outer bract: colour (external side) |
| 13. | Lobe: shape of tip of secondary lobes | 37. | Outer bract: hue of secondary colour |
| 14. | Leaf blade: shape in cross section | 38. | Outer bract: size of spine |
| 15. | Leaf blade: intensity of green colour | 39. | Outer bract: mucron |
| 16. | Leaf blade: hue of green colour | 40. | Central flower-head: anthocyanin coloration of inner bracts |
| 17. | Leaf blade: intensity of grey hue | 41. | Central flower-head: density of inner bracts |
| 18. | Leaf: hairiness on upper side | 42. | Receptacle: diameter |
| 19. | Leaf blade: blistering | 43. | Receptacle: thickness |
| 20. | Petiole: anthocyanin coloration at base | 44. | Receptacle: shape in longitudinal |
| 21. | Central flower-head: length | 45. | Main head weight |
| 22. | Central flower-head: diameter | 46. | Total number of heads |
| 23. | Central flower-head: size | 47. | Main Head date of maturity |
| 24. | Central flower-head: shape in longitudinal section |
| Molecular Markers | Ta | NPB | He | Ho | PIC | |
|---|---|---|---|---|---|---|
| AFLP | EcoACC/MseCTA | 5 | 0.37 | |||
| EcoACG/MseCTT | 3 | 0.45 | ||||
| EcoAGC/MseCTT | 4 | 0.34 | ||||
| MseAC/PstCA | 81 | 0.31 | ||||
| MseAC/PstCG | 68 | 0.27 | ||||
| MseGC/PstCA | 95 | 0.28 | ||||
| MseGC/PstCG | 67 | 0.28 | ||||
| ISSR | 810 (GA)8T | 43 | 4 | 0.24 | ||
| 827 (AG)8G | 52 | 1 | 0.41 | |||
| 841 (GA)8YC | 45 | 30 | 0.20 | |||
| 857 (AC)8YG | 54 | 35 | 0.22 | |||
| SSR | CMAL11 | 54 | 2 | 0.32 | 0.40 | 0.268 |
| CMAL117 | 60 | 2 | 0.48 | 0.60 | 0.365 | |
| CMAL24 | 60 | 4 | 0.66 | 0.90 | 0.592 | |
| CMAL06 | 60 | 2 | 0.50 | 0.80 | 0.375 | |
| CMAL108 | 60 | 2 | 0.10 | 0.10 | 0.091 | |
| CDAT01 | 54 | 3 | 0.56 | 1.00 | 0.442 | |
| CLIB02 | 59 | 2 | 0.50 | 0.90 | 0.373 | |
| CLIB12 | 55 | 2 | 0.50 | 0.90 | 0.373 | |
| CMAFLP18 | 55 | 2 | 0.50 | 0.90 | 0.373 | |
| CsPal03 | 52 | 3 | 0.55 | 1.00 | 0.442 | |
| CsPal02 | 60 | 2 | 0.10 | 0.10 | 0.091 | |
| CsEST03 | 62 | 2 | 0.38 | 0.50 | 0.305 | |
| CsCiCaCa05 | 55 | 2 | 0.10 | 0.10 | 0.091 | |
| Clones | PH | DHL | MSD | HeadL | HeadD | ShapeIndex | ShapeTip | ColBract | RecD | RecThick | RecShape | HeadW | TimeHead | HeadN | TotHeadW |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S2 | 68.6 d,e | 23.4 b,c | 2.80 a,b | 7.72 b,c | 9.93 a,b | 0.78 e | 3.54 a | 3.00 b,c | 4.88 a | 0.69 b,c | 1.96 c,d | 358.4 a,b | 191.5 a | 8.08 d | 1291.7 e |
| S3 | 71.4 c,d | 29.6 a,b | 2.66 b,c | 8.34 a,b | 9.38 b,c | 0.89 a,c | 2.54 b,d | 3.08 b,c | 4.20 a,b | 0.57 c | 2.29 a,c | 326.8 a,b | 186.5 a | 10.67 a,d | 1342.1 e |
| S5 | 85.1 a,b | 27.5 b,c | 2.54 c | 8.52 a,b | 9.47 a,c | 0.90 a,b | 2.46 c,d | 2.67 c,d | 4.62 a,b | 0.61 c | 2.61 a | 340.3 a,b | 188.6 a | 9.5 b,d | 1323.8 e |
| S11 | 79.8 b,c | 29.5 a,b | 2.75 a,c | 8.48 a,b | 9.51 a,c | 0.89 a,c | 1.67 e | 3.67 a,b | 4.38 a,b | 0.64 c | 2.54 a,b | 354.6 a,b | 188.7 a | 10.88 a,d | 1399.0 d,e |
| S17 | 54.1 f | 25.2 b,c | 2.93 a | 7.28 c | 9.13 b,c | 0.80 b,e | 3.38 a | 2.25 d | 4.68 a,b | 0.90 a,b | 1.13 e | 336.9 a,b | 148.7 e | 10.58 a,d | 1820.8 a,d |
| S18 | 86.4 a,b | 35.0 a | 2.94 a | 7.86 a,c | 9.33 b,c | 0.84 a,d | 2.96 a,d | 3.50 a,b | 4.85 a | 0.64 c | 1.58 d,e | 355.2 a,b | 183.0 a,b | 9.21 c,d | 1687.1 b,e |
| S20 | 90.7 a | 34.6 a | 2.61 b,c | 8.21 a,c | 8.79 c | 0.93 a | 3.00 a,c | 3.21 b,c | 4.01 b | 0.60 c | 2.13 b,c | 314.2 b | 183.5 a,b | 11.38 a,c | 1647.6 c,e |
| S21 | 72.0 c,d | 29.4 a,b | 2.64 b,c | 8.76 a | 9.91 a,b | 0.88 a,c | 2.33 d | 3.92 a | 4.31 a,b | 0.78 a,c | 1.96 c,d | 378.4 a | 175.1 b,c | 12.50 a,b | 2174.0 a |
| S22 | 59.9 e,f | 23.1 b,c | 2.97 a | 8.09 a,c | 10.32 a | 0.78 e | 3.08 a,c | 2.58 c,d | 4.89 a | 0.94 a | 1.58 d,e | 359.1 a,b | 166.1 c,d | 13.54 a | 1908.1 a,c |
| S23 | 54.2 f | 21.3 c | 2.85 a,b | 7.65 b,c | 9.61 a,c | 0.79 c,e | 3.17 a,b | 2.54 c,d | 4.80 a | 0.99 a | 1.58 d,e | 329.9 a,b | 161.8 d | 13.42 a | 2107.0 a,b |
| Std.Er. | 1.62 | 1.44 | 0.39 | 0.20 | 0.18 | 0.02 | 0.14 | 0.13 | 0.14 | 0.04 | 0.09 | 13.68 | 2.082 | 0.596 | 89.719 |
| Y | *** | *** | *** | ** | ** | *** | ns | *** | ns | *** | *** | *** | *** | *** | *** |
| CxY | *** | *** | ns | ns | *** | ns | ns | *** | ns | *** | ** | ns | *** | * | ns |
| Clones | N. Private Alleles | Private Bands |
|---|---|---|
| S11 | 4 | MgcPca/10, MgcPca/202, 841/302, 857/168 |
| S17 | 7 | MacPca/107, MgcPca/131, MgcPca/159, MgcPca/228, MgcPca/267, MgcPcg/9, 857/277 |
| S18 | 6 | MacPcg/4, MacPca/12, MacPca/54, MacPca/150, MacPcg/24, MacPcg/63, MacPcg/86, MgcPca/30, MgcPca/39, MgcPca/43, MgcPca/60, MgcPca/180, MgcPcg/3, MgcPcg/22, MgcPcg/35, MgcPcg/151 |
| S2 | 9 | 810/4, 857/172, MacPca/24, MacPcg/95, MacPcg/228, MgcPca/5, MgcPca/73, MgcPcg/15, MgcPcg/94 |
| S22 | 20 | MacPca/50, MacPca/32, MacPca/167, MacPca/194, MacPca/215, MacPca/236, MacPca/238, MacPcg/16, MacPcg/48, MacPcg/137, MgcPca/4, MgcPca/80, MgcPca/87, MgcPca/107, MgcPca/201, MgcPca/252, MgcPcg/8, MgcPcg/105, MgcPcg/122, MgcPcg/126 |
| S23 | 8 | MacPca/275, MacPcg/115, MgcPca/68, MgcPca/268, MgcPcg/31, MgcPcg/82, 857/41, 857/191 |
| S3 | 15 | MacPcg/23, MgcPcg/26, MacPcg/234, MgcPca/78, MgcPcg/17, MgcPcg/20, MgcPcg/48, EaccMcta/91, 810/5, 841/32, 841/34, 841/220, 841/223, 857/74, 857/76 |
| S5 | 14 | MacPca/70, MacPca/133, MacPca/134, MgcPca/117, MacPca/222, MgcPcg/154, MgcPcg/167, 841/51, 841/53, 841/131, 857/62, 857/193, 857/262,857/275 |
| S21 | 20 | 841/76, 841/194, 841/195, 841/330, 857/315, 857/321, 857/323, MacPcg/26, MacPcg/37, MacPcg/179, MgcPca/27, MgcPca/32, MgcPca/41, MgcPca/75, MgcPca/133, MgcPca/193, MgcPca/240, MgcPcg/40, MgcPcg/138, MgcPcg/191 |
| S20 | 37 | 857/2, MacPca/5, MacPca/15, MacPca/35, MacPca/48, MacPca/73, MacPca/74, MacPca/158, MacPca/193, MacPca/278, MacPcg/9, MacPcg/18, MacPcg/25, MacPcg/34, MacPcg/38, MacPcg/44, MacPcg/45, MacPcg/46, MacPcg/92, MacPcg/145, MgcPca/17, MgcPca/49, MgcPca/53, MgcPca/97, MgcPca/141, MgcPca/169, MgcPca/207, MgcPca/210, MgcPcg/135, MgcPcg/153, MgcPcg/162, MgcPcg/168, MgcPcg/178, MgcPcg/182, MgcPcg/19, MgcPcg/36, MgcPcg/71 |
| Clones | 1,5-O-Dicaffeoylquinic Acid | Chlorogenic Acid | Cynaroside | Total Caffeolyquinic Acids | Total Measured Polyphenols |
|---|---|---|---|---|---|
| S20 | 4.17 d | 3.62 d | 0.46 b | 7.79 e | 8.25 e |
| S21 | 5.99 c | 6.52 a,b | 0.34 c,d | 12.51 b,c | 12.85 b,c |
| S2 | 6.44 b,c | 7.00 a | 0.40 b,c | 13.44 a,b | 13.84 a,b |
| S3 | 7.05 a,b | 6.74 a | 0.31 d | 13.79 a,b | 14.01 a,b |
| S5 | 7.38 a | 7.30 a | 0.59 a | 14.68 a | 15.27 a |
| S11 | 5.96 c | 5.93 b | 0.43 b | 11.89 c | 12.32 c |
| S17 | 3.49 e | 6.63 a,b | 0.42 b,c | 10.12 d | 10.54 d |
| S18 | 6.60 b,c | 7.32 a | 0.28 d | 13.92 a,b | 14.20 a,b |
| S22 | 4.93 e | 6.69 a,b | 0.41 b,c | 11.62 c | 12.03 c |
| S23 | 3.53 e | 4.42 c | 0.55 a | 7.95 e | 8.50 e |
| Std. Error | 0.265 | 0.266 | 0.030 | 0.527 | 0.535 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crinò, P.; Pagnotta, M.A. Phenotyping, Genotyping, and Selections within Italian Local Landraces of Romanesco Globe Artichoke. Diversity 2017, 9, 14. https://doi.org/10.3390/d9010014
Crinò P, Pagnotta MA. Phenotyping, Genotyping, and Selections within Italian Local Landraces of Romanesco Globe Artichoke. Diversity. 2017; 9(1):14. https://doi.org/10.3390/d9010014
Chicago/Turabian StyleCrinò, Paola, and Mario Augusto Pagnotta. 2017. "Phenotyping, Genotyping, and Selections within Italian Local Landraces of Romanesco Globe Artichoke" Diversity 9, no. 1: 14. https://doi.org/10.3390/d9010014
APA StyleCrinò, P., & Pagnotta, M. A. (2017). Phenotyping, Genotyping, and Selections within Italian Local Landraces of Romanesco Globe Artichoke. Diversity, 9(1), 14. https://doi.org/10.3390/d9010014







