Chemical Composition of the Essential Oil from Aerial Parts of Javanian Pimpinella pruatjan Molk. and Its Molecular Phylogeny
Abstract
:1. Introduction
2. Experimental Section
2.1. The Plant Material and Isolation of the Oils
2.2. Gas Liquid Chromatography—Mass Spectrometry (GLC-MS)
2.3. DNA Barcoding of P. pruatjan
2.4. Antimicrobial Activity
2.4.1. Microorganisms
2.4.2. Culture Preparation and Cultivation of Microorganism
2.4.3. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Microbicidal Concentration (MMC)
3. Results and Discussion
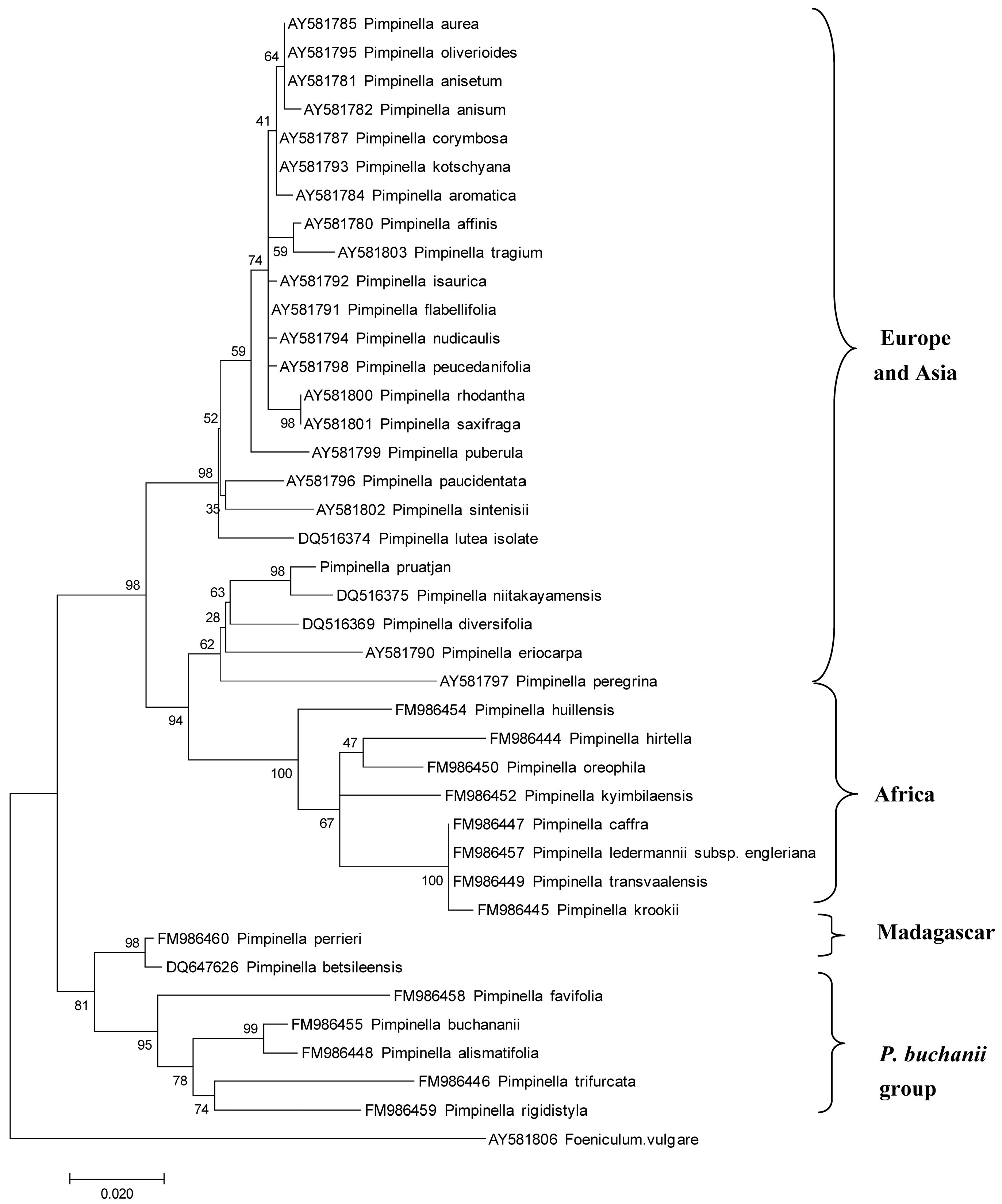
3.1. Relationships between P. pruatjan and Other Pimpinella Species
3.2. Chemical Composition of the Essential Oil
3.3. Antimicrobial Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Velasco-Negueruela, A.; Pérez-Alonso, M.J.; Pérez de Paz, P.L.; Vallejo, C.G.; Palá-Pául, J.; Iñigo, A. Chemical composition of the essential oils from the roots, fruits, leaves and stems of Pimpinella cumbrae Link growing in the Canary Islands (Spain). Flavour Fragr. J. 2002, 17, 468–471. [Google Scholar] [CrossRef]
- Tabanca, N.; Bedir, E.; Ferreira, D.; Slade, D.; Wedge, D.E.; Jacob, M.R.; Khan, S.I.; Bedir, E.; Baser, K.H.; Khan, I.A. Bioactive constituents from Turkish Pimpinella species. Chem. Biodivers. 2005, 2, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Delazar, A.; Biglari, F.; Esnaashari, S.; Nazemiyeh, H.; Talebpour, A.H.; Nahar, L.; Sarker, S.D. GC-MS analysis of the essential oils, and the isolation of phenylpropanoid derivatives from the aerial parts of Pimpinella aurea. Phytochemistry 2006, 67, 2176–2181. [Google Scholar] [CrossRef] [PubMed]
- Lewis, W.H. Medicinal Botany; Wiley-Interscience: New York, NY, USA, 1977. [Google Scholar]
- De Pasquale, A. Therapeutic and pharmacologic properties of the genus pimpinella. In Illicium, Pimpinella, and Foeniculum; Jodral, M.M., Ed.; CRC Press LLC: Boca Raton, FL, USA, 2004; pp. 167–197. [Google Scholar]
- Rahardjo, M.; Sudiarto; Endjo, D.; Wahyuni, S.; Sukarman; Trisilawati, O.; Shusena, A.; Kosasih; Susi, N. Studi Teknologi Perbanyakan, Budidaya dan Konservasi ex Situ Tanaman Obat Langka Purwoceng (Pimpinela pruatjan Molkenb.); Balai Tanaman Obat dan Aromatik: Jawa Barat, Indonesia, 2004; pp. 277–292. [Google Scholar]
- Syahid, S.F.; Rostiana, O.; Miftakhurohmah; Seswita, D.; Rosita; Darwati, I.; Surachman, D.; Aisyah, S. Teknik perbanyakan, produksi metabolit sekunder melalui kultur akar rambut purwoceng secara in vitro dan aklimatisasi di lapang. In Laporan Penyelesaian DIP Bagian Proyek Penelitian Tanaman Rempah dan Obat; Balittro: Bogor, Indonesia, 2004; pp. 128–142. [Google Scholar]
- Darwati, I.; Roostika, I. Status penelitian Purwoceng (Pimpinella alpina Molk.) di Indonesia. Bul. Plasma Nutfah 2006, 12, 1–6. [Google Scholar]
- Embong, M.B.; Hadziyev, D.; Molnar, S. Essential oil from spices grown in Alberta. Anise oil (Pimpinella anisum). Can. J. Plant Sci. 1977, 57, 681–688. [Google Scholar] [CrossRef]
- Bakshu, L.M.; Raju, R.R.V. Essential oil composition and antimicrobial activity of tuberous roots of Pimpinella tirupatiensis Bal. & Subr., an endemic taxon from eastern ghats, India. Flavour Fragr. J. 2002, 17, 413–415. [Google Scholar]
- Velasco-Negueruela, A.; Pérez-Alonso, M.J.; Pérez de Paz, P.L.; Palá-Pául, J.; Sanz, J. Analysis by gas chromatography-mass spectrometry of the essential oil from the aerial parts of Pimpinella junoniae Ceb. & Ort., gathered in La Gomera, Canary Islands, Spain. J. Chromatogr. A 2003, 1011, 241–244. [Google Scholar] [PubMed]
- Bohn, I.; Kubeczka, K.H.; Schultze, W. The essential root oil of Pimpinella major. Planta Med. 1989, 55, 489–490. [Google Scholar] [CrossRef] [PubMed]
- Askari, F.; Sefidkon, F. Volatile components of Pimpinella tragium Vill. from Iran. Iran. J. Pharm. Res. 2005, 2, 117–120. [Google Scholar]
- Velasco-Negueruela, A.; Pérez-Alonso, M.J.; Pérez de Paz, P.L.; Palá-Pául, J.; Sanz, J. Analysis by gas chromatography-mass spectrometry of the essential oils from the aerial parts of Pimpinella anagodendron Bolle and Pimpinella rupicola Svent., two endemic species to the Canary Islands, Spain. J. Chromatogr. A 2005, 1095, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Tabanca, N.; Demirci, B.; Ozek, T.; Kirimer, N.; Baser, K.H.; Bedir, E.; Khan, I.A.; Wedge, D.E. Gas chromatographic-mass spectrometric analysis of essential oils from Pimpinella species gathered from Central and Northern Turkey. J. Chromatogr. A 2006, 1117, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Tabanca, N.; Bedir, E.; Kirimer, N.; Baser, K.H.; Khan, S.I.; Jacob, M.R.; Khan, I.A. Antimicrobial compounds from Pimpinella species growing in Turkey. Planta Med. 2003, 69, 933–938. [Google Scholar] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Khanuja, S.P.S.; Shasany, A.K.; Darokar, M.P.; Kumar, S. Rapid isolation of DNA from dry and fresh samples of plants producing large amounts of secondary metabolites and essential oils. Plant Mol. Biol. Rep. 1999, 17, 1–7. [Google Scholar] [CrossRef]
- Herrmann, F.; Wink, M. Use of rbcL sequences for DNA barcoding and authentication of plant drugs used in Traditional Chinese Medicine. PeerJ PrePr. 2014, 2, e196v1. [Google Scholar] [CrossRef]
- Tamura, K.; Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2015. submitted. [Google Scholar] [CrossRef] [PubMed]
- National Committee for Clinical Laboratory Standards (NCCLS). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; NCCLS: Wayne, PA, USA, 2006. [Google Scholar]
- Shukla, H.S.; Tripathi, S.C. Antifungal substance in the essential oil of anise (Pimpinella anisum L.). Agric. Biol. Chem. 1987, 7, 1991–1993. [Google Scholar] [CrossRef]
- Shukla, H.S.; Dubey, P.; Chaturvedi, R.V. Antiviral properties of essential oils of Foeniculum vulgare and Pimpinella anisum L. Agronomie 1989, 9, 277–279. [Google Scholar] [CrossRef]
- Pourgholami, M.H.; Majzoob, S.; Javadi, M.; Kamalinejad, M.; Fanaee, G.H.; Sayyah, M. The fruit essential oil of Pimpinella anisum exerts anticonvulsant effects in mice. J. Ethnopharmacol. 1999, 66, 211–215. [Google Scholar] [CrossRef]
- Lazarević, J.; Radulović, N.; Palić, R.; Zlatković, B. Chemical analysis of volatile constituents of Berula erecta (Hudson) Coville subsp erecta (Apiaceae) from Serbia. J. Essent. Oil. Res. 2010, 22, 153–156. [Google Scholar] [CrossRef]
- Andriamaharavo, N.R. Retention Data; NIST Mass Spectrometry Data Center: Gaithersburg, MD, USA, 2014. [Google Scholar]
- Nadaf, M.; Nasrabadi, M.; Halimi, M. GC-MS analysis of n-hexane extract from aerial parts of Salvia nemorosa. Middle East J. Sci. Res. 2012, 11, 1127–1130. [Google Scholar]
- Askari, F.; Sefidkon, F.; Mozafarian, V. Essential oil composition of Pimpinella aurea D.C. from Iran. Flavour Fragr. J. 2005, 20, 115–117. [Google Scholar] [CrossRef]
- Stahl, E.; Herting, D. Die Verteilung von Inhaltsstoffen in drei Pimpinella-Arten. Phytochemistry 1976, 15, 997–1001. [Google Scholar] [CrossRef]
- Reichling, J.; Merkel, B.; Hofmeister, P. Studies on the biological activities of rare phenylpropanoids of the genus Pimpinella. J. Nat. Prod. 1991, 54, 1416–1418. [Google Scholar] [CrossRef] [PubMed]
- Wink, M. Modes of action of herbal medicines and plant secondary metabolites. Medicines 2015, 2, 251–286. [Google Scholar] [CrossRef]
- Marcetić, M.; Bozić, D.; Milenković, M.; Lakusić, B.; Kovacević, N. Chemical composition and antimicrobial activity of essential oil of different parts of Seseli rigidum. Nat. Prod. Commun. 2012, 7, 1091−1094. [Google Scholar] [PubMed]
- Herrmann, F.; Hamoud, R.; Sporer, F.; Tahrani, A.; Wink, M. Carlina oxide–a natural polyacetylene from Carlina acaulis (Asteraceae) with potent antitrypanosomal and antimicrobial properties. Planta Med. 2011, 77, 1905−1911. [Google Scholar] [CrossRef] [PubMed]
| No. | Identified Compounds | RI * | RI [15] and Ref. | Abundance (%) * | Identification |
|---|---|---|---|---|---|
| 1 | 2-Hexanol | 800 | 803 [26] | 0.36 | MS |
| 2 | 3-Methylcyclopentanol | 835 | 836 [27] | 0.25 | MS |
| 3 | Heptanal | 891 | 902 | 0.12 | MS, RI |
| 4 | Unidentified I | 899 | 0.66 | ||
| 5 | Nonane (n) | 902 | 900 | 3.70 | MS, RI |
| 6 | Unidentified II | 914 | 0.12 | ||
| 7 | 2,2-Dimethylpentanal | 920 | 826 [28] | 4.53 | MS |
| 8 | Unidentified III | 936 | 0.21 | ||
| 9 | Unidentified IV | 940 | 0.20 | ||
| 10 | Unidentified V | 943 | 1.93 | ||
| 11 | Unidentified VI | 955 | 2.76 | ||
| 12 | Isopropyl tiglate | 968 | 976 | 6.92 | MS, RI |
| 13 | Unidentified VII | 994 | 0.38 | ||
| 14 | Unidentified VIII | 997 | 0.50 | ||
| 15 | Octanal | 1003 | 998 | 0.25 | MS, RI |
| 16 | Unidentified IX | 1005 | 0.24 | ||
| 17 | p-Cymene | 1023 | 1024 | 0.60 | MS, RI |
| 18 | Benzene acetaldehyde | 1042 | 1042 | 0.33 | MS, RI |
| 19 | Unidentified X | 1043 | 0.23 | ||
| 20 | γ-Terpinene | 1059 | 1059 | 0.44 | MS, RI |
| 21 | trans-Linalool oxide | 1072 | 1072 | 0.11 | MS, RI |
| 22 | p-Cymenene | 1089 | 1091 | 0.18 | MS, RI |
| 23 | 2-Nonanone | 1093 | 1090 | 0.10 | MS, RI |
| 24 | Linalool | 1101 | 1096 | 0.20 | MS, RI |
| 25 | Nonanal | 1105 | 1100 | 0.12 | MS, RI |
| 26 | Geijerene | 1141 | 1143 | Tr | MS, RI |
| 27 | trans-Verbenol | 1149 | 1144 | 0.18 | MS, RI |
| 28 | (E)-2-Nonenal | 1160 | 1161 | 0.25 | MS, RI |
| 29 | Borneol | 1165 | 1169 | 0.12 | MS, RI |
| 30 | 2-Methoxy-3-(1-methylpropyl) pyrazine | 1172 | 1172 | Tr | MS, RI |
| 31 | Terpinen-4-ol | 1176 | 1177 | 0.54 | MS, RI |
| 32 | Octanoic acid | 1179 | 1171 | 0.16 | MS, RI |
| 33 | p-Cymen-8-ol | 1184 | 1183 | 0.74 | MS |
| 34 | α-Terpineol | 1190 | 1188 | 0.14 | MS, RI |
| 35 | Unidentified XI | 1195 | 0.11 | ||
| 36 | trans-3-Caren-2-ol | 1223 | 0.31 | MS | |
| 37 | 2-Isopropenyl-5-methyl-1,4-benzenediol | 1232 | 7.20 | MS | |
| 38 | Thymol methyl ether | 1237 | 1235 | 7.80 | MS, RI |
| 39 | Carvacrol methyl ether | 1246 | 1244 | 0.13 | MS, RI |
| 40 | Geraniol | 1256 | 1252 | 0.21 | MS, RI |
| 41 | Bornyl acetate | 1289 | 1288 | 0.34 | MS, RI |
| 42 | 6-Hydroxy-m-anisaldehyde | 1295 | 0.28 | MS | |
| 43 | Thymol | 1298 | 1290 | 0.13 | MS, RI |
| 44 | Carvacrol | 1304 | 1299 | 0.37 | MS, RI |
| 45 | 4-Ethenyl-2-methoxyphenol | 1315 | 1309 | 0.20 | MS, RI |
| 46 | Unidentified XII | 1343 | 0.14 | ||
| 47 | α-Cubebene | 1353 | 1348 | 0.20 | MS, RI |
| 48 | γ-Nonalactone | 1363 | 1361 | 0.14 | MS, RI |
| 49 | 1-(4-Hydroxybenzylidine)acetone | 1367 | 0.12 | MS | |
| 50 | α-Copaene | 1379 | 1376 | 0.56 | MS, RI |
| 51 | 8-epi-Dictamnol | 1382 | 1380 | 0.53 | MS, RI |
| 52 | β-Cubebene | 1387 | 1388 | 0.58 | MS, RI |
| 53 | β-Elemene | 1394 | 1390 | 0.40 | MS, RI |
| 54 | Methyl eugenol | 1405 | 1403 | 0.52 | MS, RI |
| 55 | α-Cedrene | 1418 | 1411 | 0.22 | MS, RI |
| 56 | (E)-Caryophyllene | 1423 | 1419 | 1.12 | MS, RI |
| 57 | 2,5-Dimethoxy-p-cymene | 1427 | 1426 | 4.39 | MS, RI |
| 58 | Dictamnol | 1432 | 1429 | 1.92 | MS, RI |
| 59 | 6,9-Guaiadiene | 1440 | 1444 | 0.16 | MS, RI |
| 60 | α-Himachalene | 1448 | 1451 | Tr | MS, RI |
| 61 | allo-Aromadendrene | 1454 | 1460 | Tr | MS, RI |
| 62 | α-Humulene | 1458 | 1454 | 0.26 | MS, RI |
| 63 | Sesquisabinene | 1461 | 1459 | 0.22 | MS, RI |
| 64 | cis-Cadina-1(6)-4-diene | 1466 | 1463 | 2.86 | MS, RI |
| 65 | Unidentified XIII | 1472 | 0.14 | ||
| 66 | γ-Muurolene | 1482 | 1479 | 0.26 | MS, RI |
| 67 | ar-Curcumene | 1488 | 1480 | 2.96 | MS, RI |
| 68 | (E)-Methyl isoeugenol | 1492 | 1492 | 0.38 | MS, RI |
| 69 | Viridiflorene | 1501 | 1496 | 0.36 | MS, RI |
| 70 | trans-β-Guaiene | 1505 | 1502 | 0.47 | MS, RI |
| 71 | cis-γ-Bisabolene | 1518 | 1515 | 25.28 | MS, RI |
| 72 | δ-Cadinene | 1530 | 1523 | 1.10 | MS, RI |
| 73 | Spathulenol | 1582 | 1578 | 0.13 | MS, RI |
| 74 | Caryophyllene oxide | 1589 | 1583 | 2.80 | MS, RI |
| 75 | Unidentified XIV | 1611 | 0.23 | ||
| 76 | Humulene epoxide II | 1614 | 1608 | 0.96 | MS, RI |
| 77 | Unidentified XV | 1625 | 0.51 | ||
| 78 | 3-Thujopsanone | 1651 | 1654 | 0.14 | MS, RI |
| 79 | α-Cadinol | 1660 | 1654 | 0.54 | MS, RI |
| 80 | Unidentified XVI | 1674 | 0.22 | ||
| 81 | 14-Hydroxy-9-epi-(E)-caryophyllene | 1677 | 1669 | 0.24 | MS, RI |
| 82 | Unidentified XVII | 1682 | 0.12 | ||
| 83 | Amorpha-4,9-dien-2-ol | 1692 | 1700 | 0.46 | MS, RI |
| 84 | Unidentified XVIII | 1714 | 0.10 | ||
| 85 | Unidentified XIX | 1728 | 0.13 | ||
| 86 | Unidentified XX | 1732 | 0.11 | ||
| 87 | trans-Pseudoisoeugenol isobutyrate | 1747 | 1742 | 0.13 | MS, RI |
| 88 | Epoxypseudoisoeugenyl isobutyrate | 1797 | 1793 | 0.18 | MS, RI |
| 89 | trans-Pseudoisoeugenyl-2-metylbutyrate | 1842 | 1842 | 1.38 | MS, RI |
| 90 | Epoxypseudoisoeugenyl 2-methylbutyrate | 1901 | 1895 | 0.14 | MS, RI |
| 91 | Hexadecanoic acid | 1963 | 1960 | 0.35 | MS, RI |
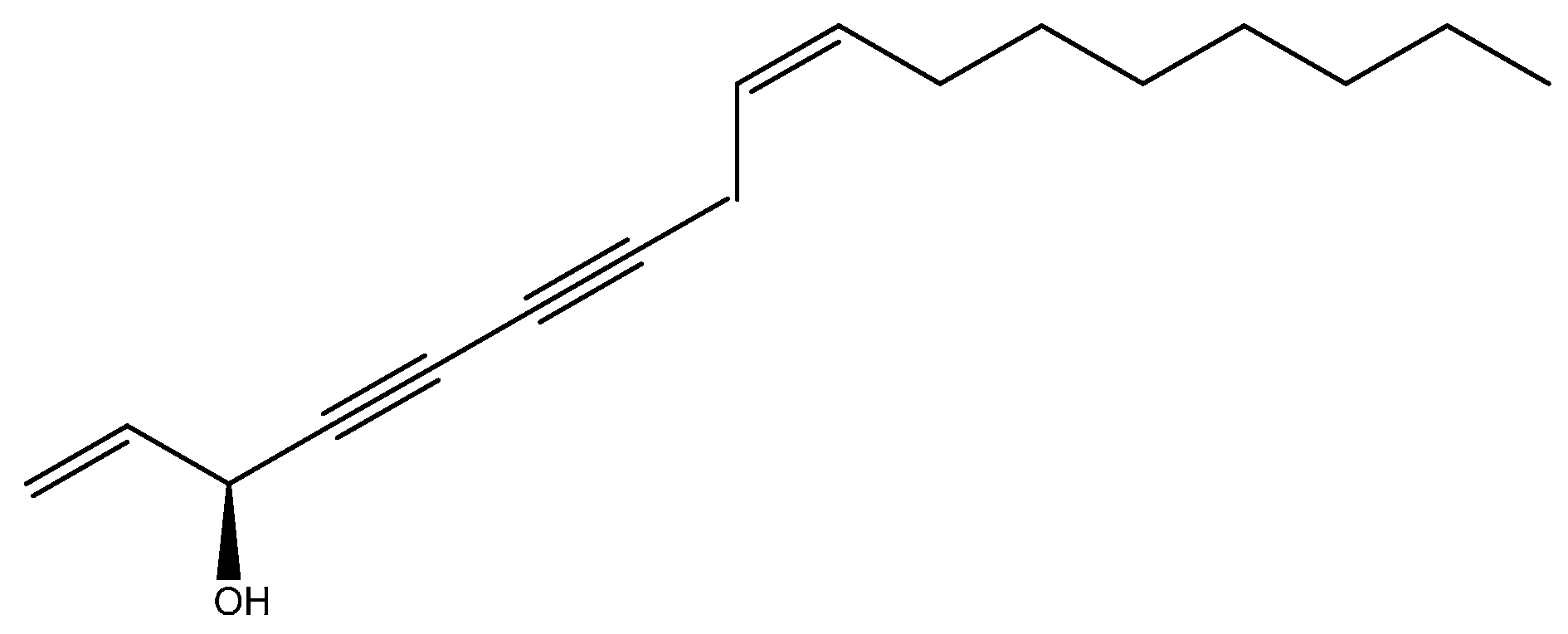
| 92 | cis-Falcarinol | 2038 | 2036 | 0.51 | MS, RI |
| 93 | Nezukol | 2141 | 2133 | 0.18 | MS, RI |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nurcahyanti, A.D.R.; Nasser, I.J.; Sporer, F.; Graf, J.; Bermawie, N.; Reichling, J.; Wink, M. Chemical Composition of the Essential Oil from Aerial Parts of Javanian Pimpinella pruatjan Molk. and Its Molecular Phylogeny. Diversity 2016, 8, 15. https://doi.org/10.3390/d8030015
Nurcahyanti ADR, Nasser IJ, Sporer F, Graf J, Bermawie N, Reichling J, Wink M. Chemical Composition of the Essential Oil from Aerial Parts of Javanian Pimpinella pruatjan Molk. and Its Molecular Phylogeny. Diversity. 2016; 8(3):15. https://doi.org/10.3390/d8030015
Chicago/Turabian StyleNurcahyanti, Agustina D. R., Issam J. Nasser, Frank Sporer, Jürgen Graf, Nurliani Bermawie, Jürgen Reichling, and Michael Wink. 2016. "Chemical Composition of the Essential Oil from Aerial Parts of Javanian Pimpinella pruatjan Molk. and Its Molecular Phylogeny" Diversity 8, no. 3: 15. https://doi.org/10.3390/d8030015
APA StyleNurcahyanti, A. D. R., Nasser, I. J., Sporer, F., Graf, J., Bermawie, N., Reichling, J., & Wink, M. (2016). Chemical Composition of the Essential Oil from Aerial Parts of Javanian Pimpinella pruatjan Molk. and Its Molecular Phylogeny. Diversity, 8(3), 15. https://doi.org/10.3390/d8030015







