Drastic Population Size Change in Two Populations of the Golden-Striped Salamander over a Forty-Year Period—Are Eucalypt Plantations to Blame?
Abstract
:1. Introduction

2. Materials and Methods
2.1. Study Areas and Fieldwork
2.2. Pattern of Annual Migration
2.3. Natural History of Chioglossa lusitanica and Unknowns
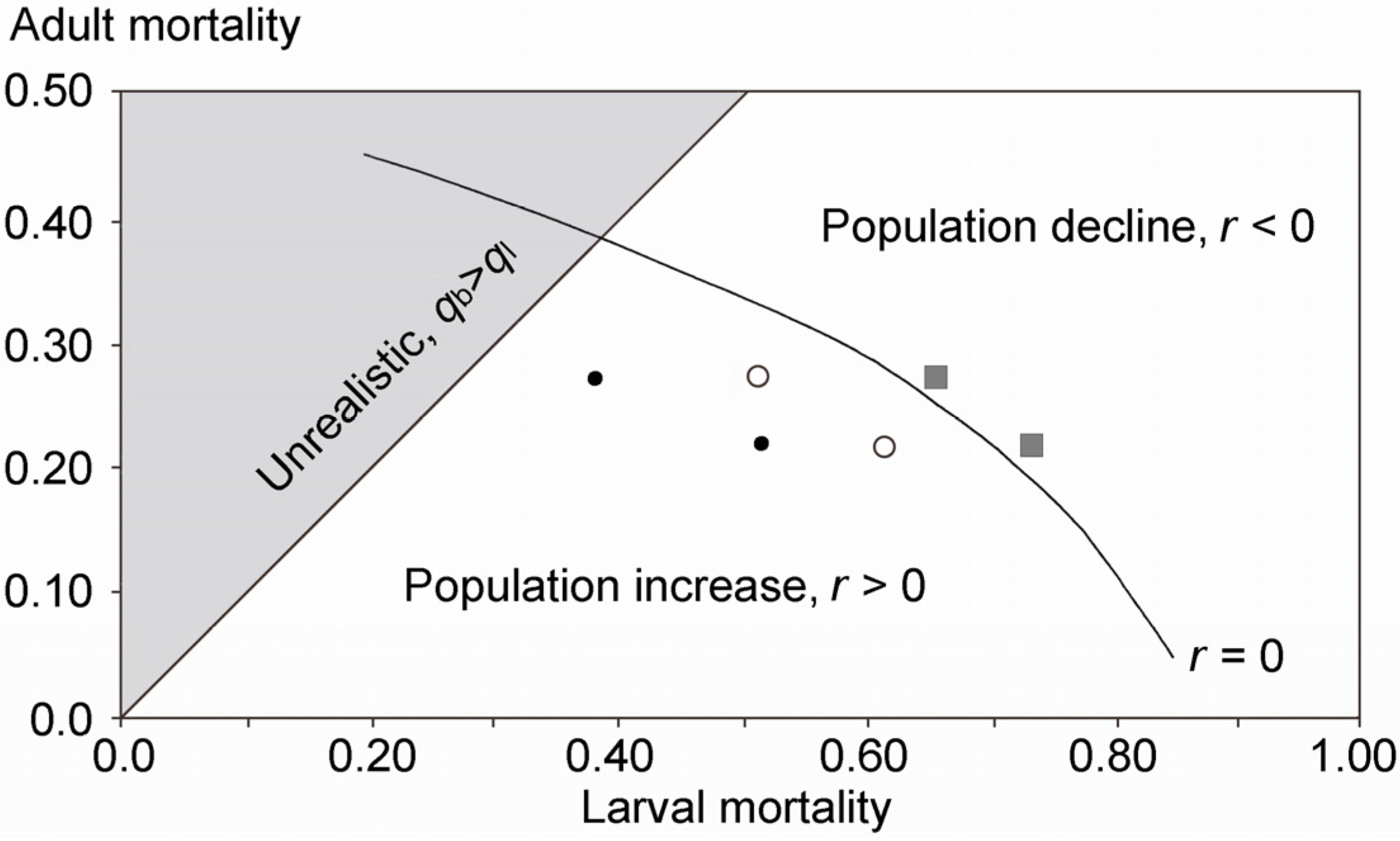
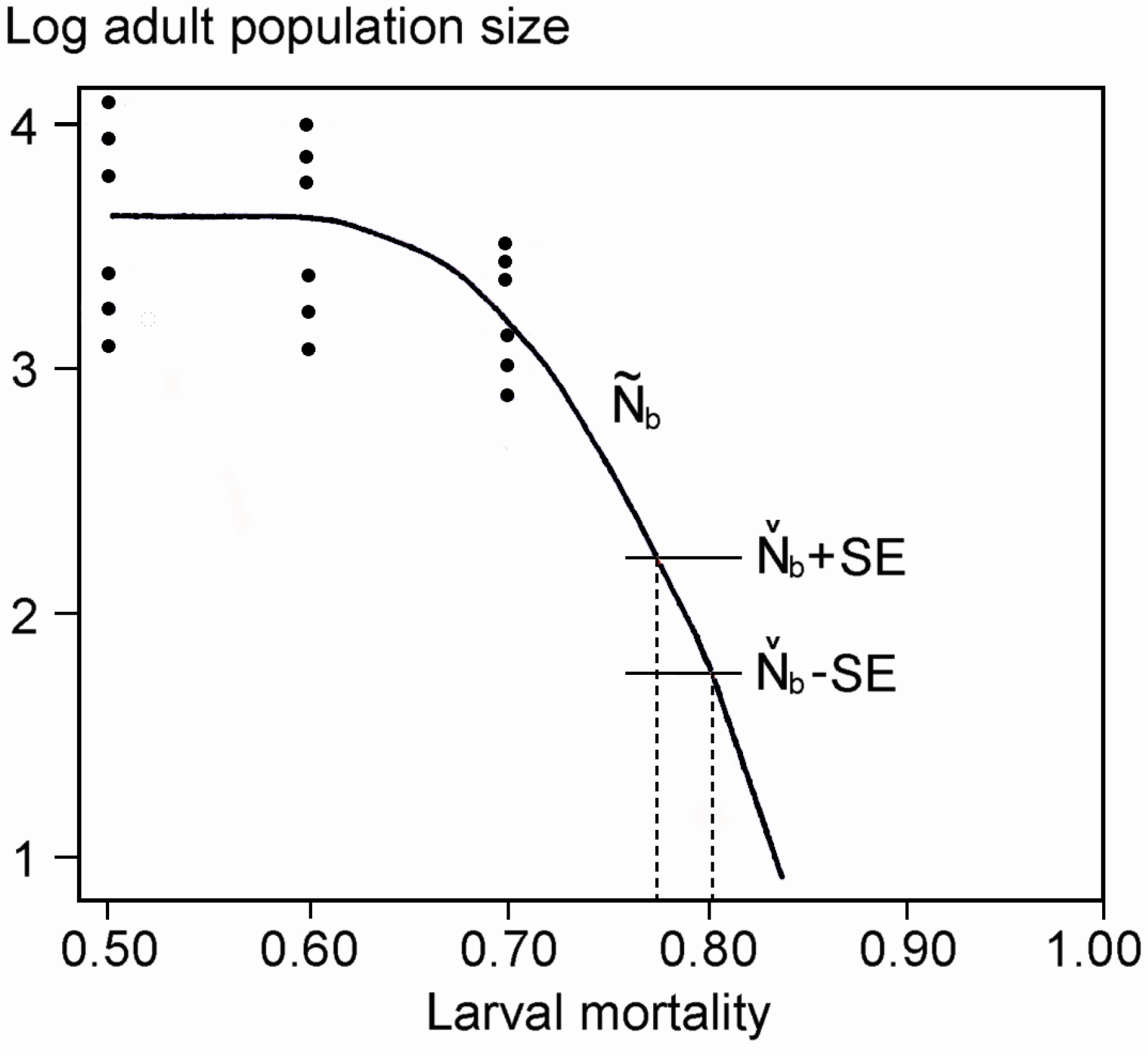
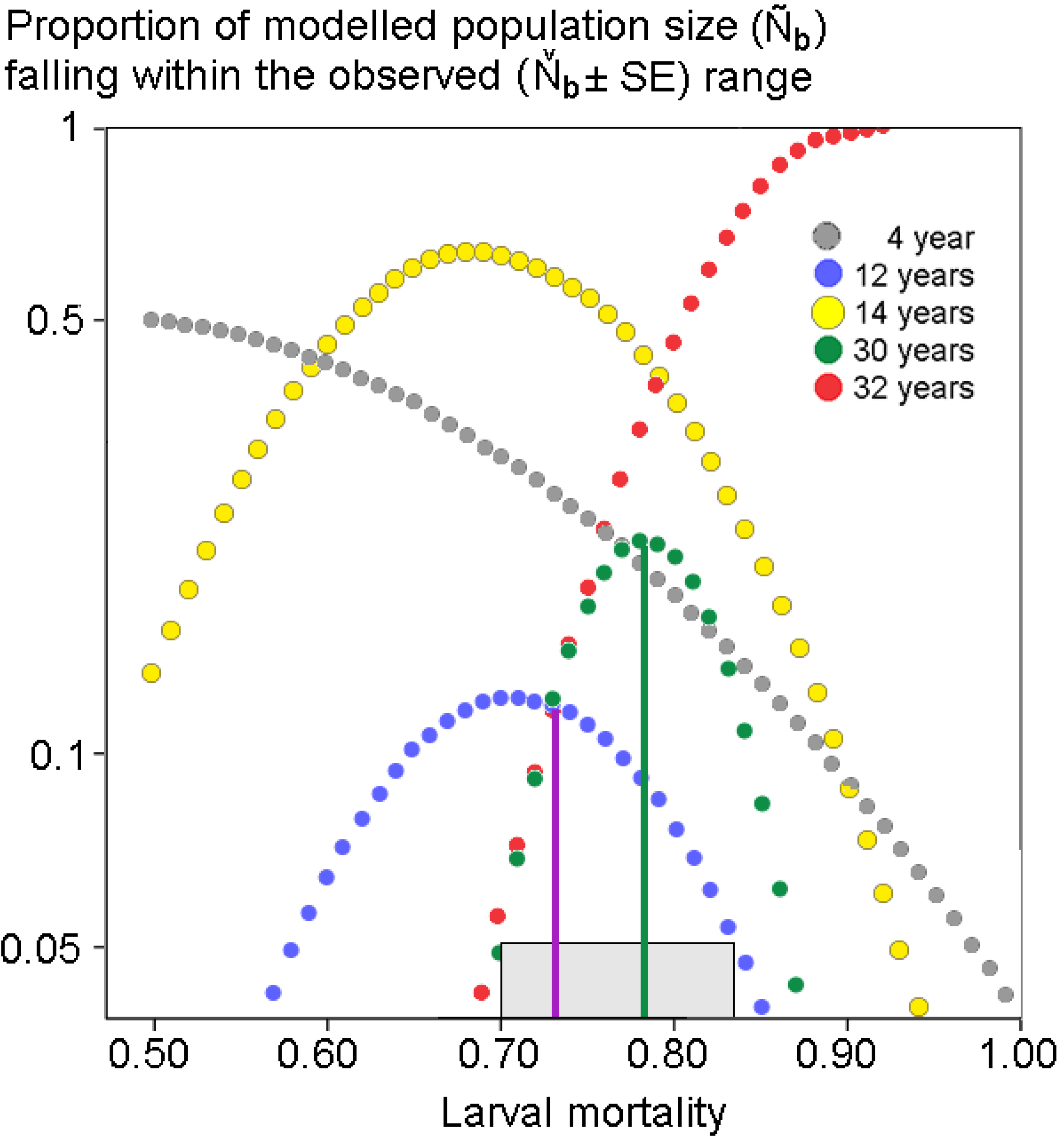
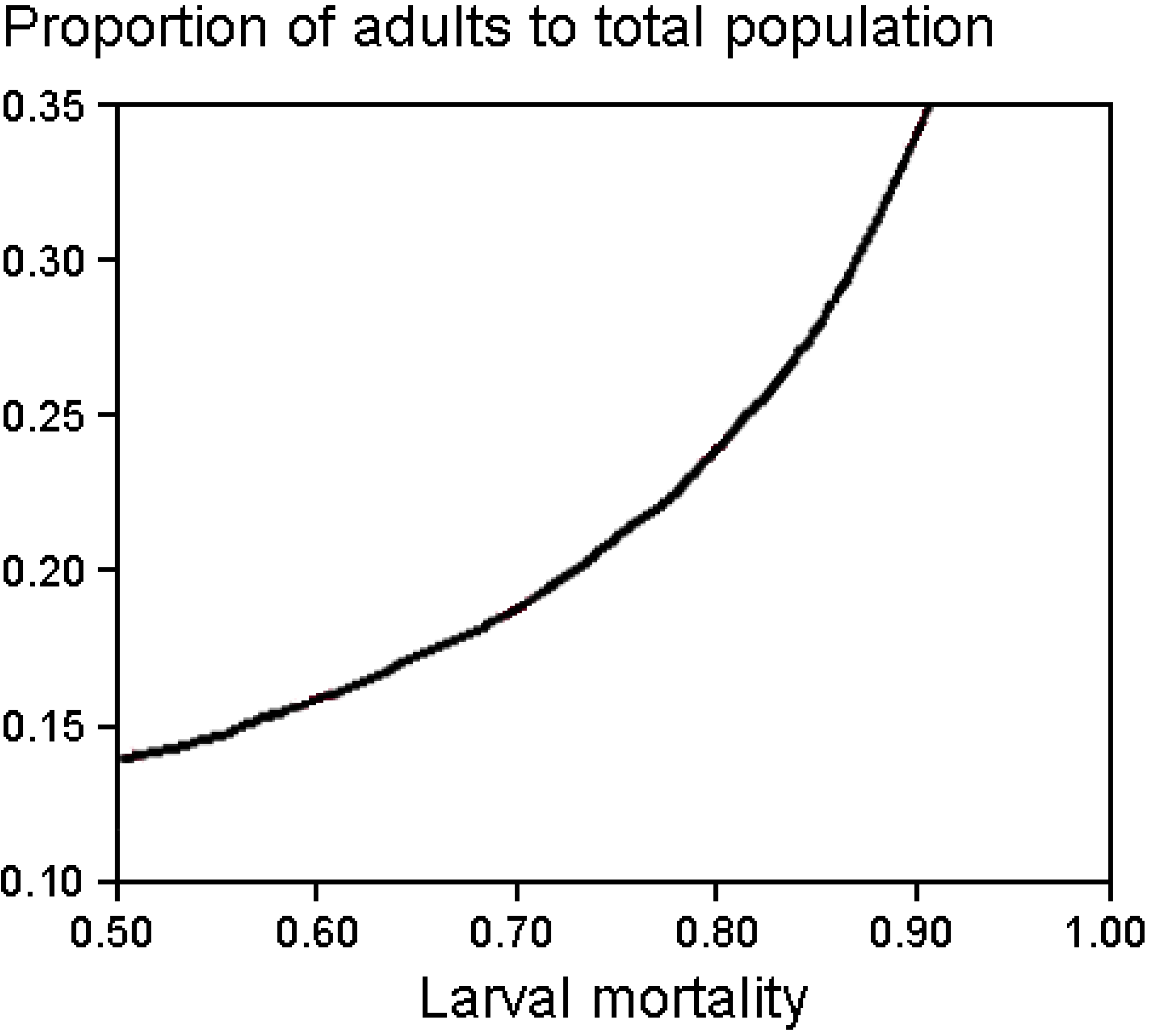
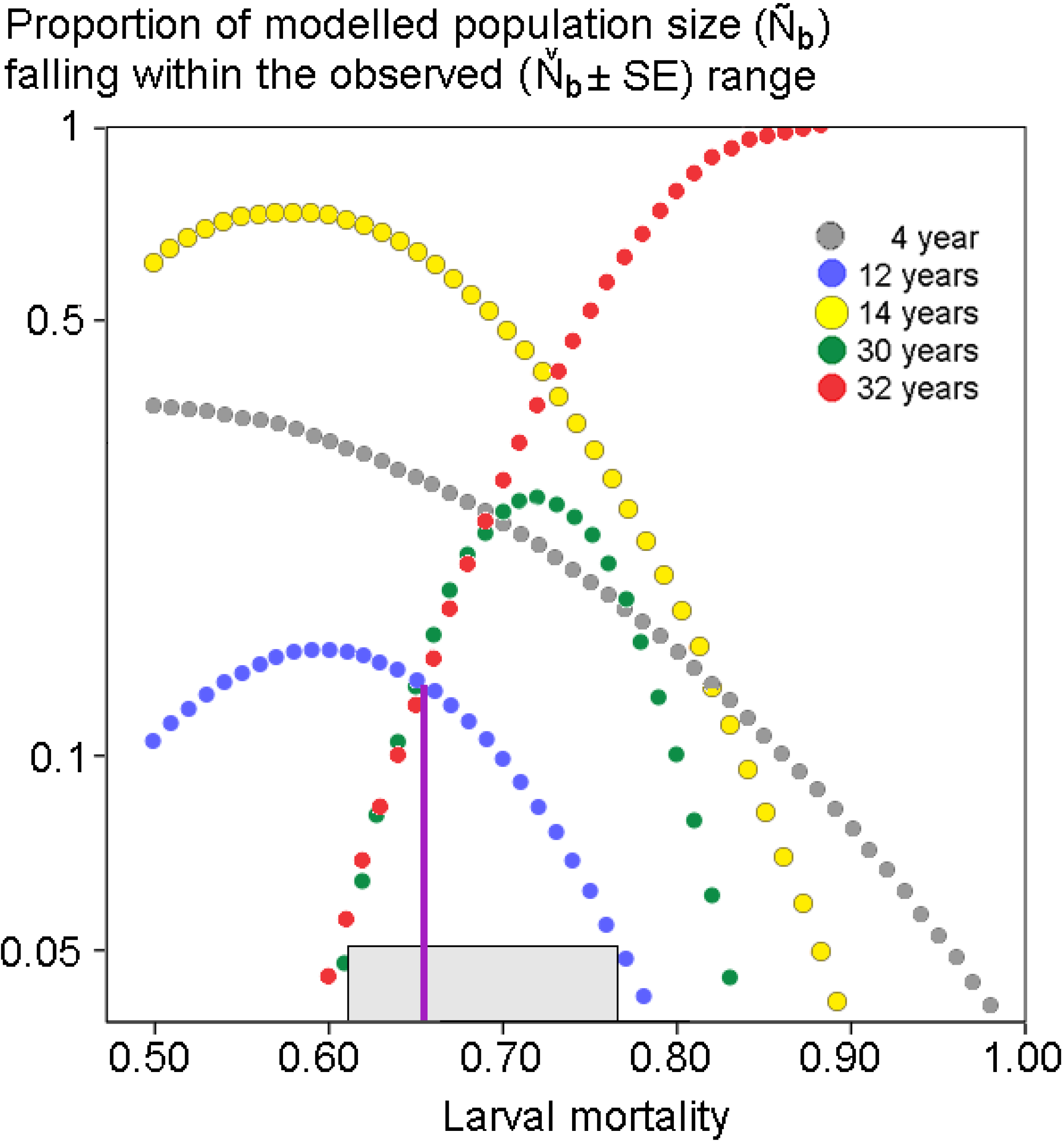
2.4. Population Modeling
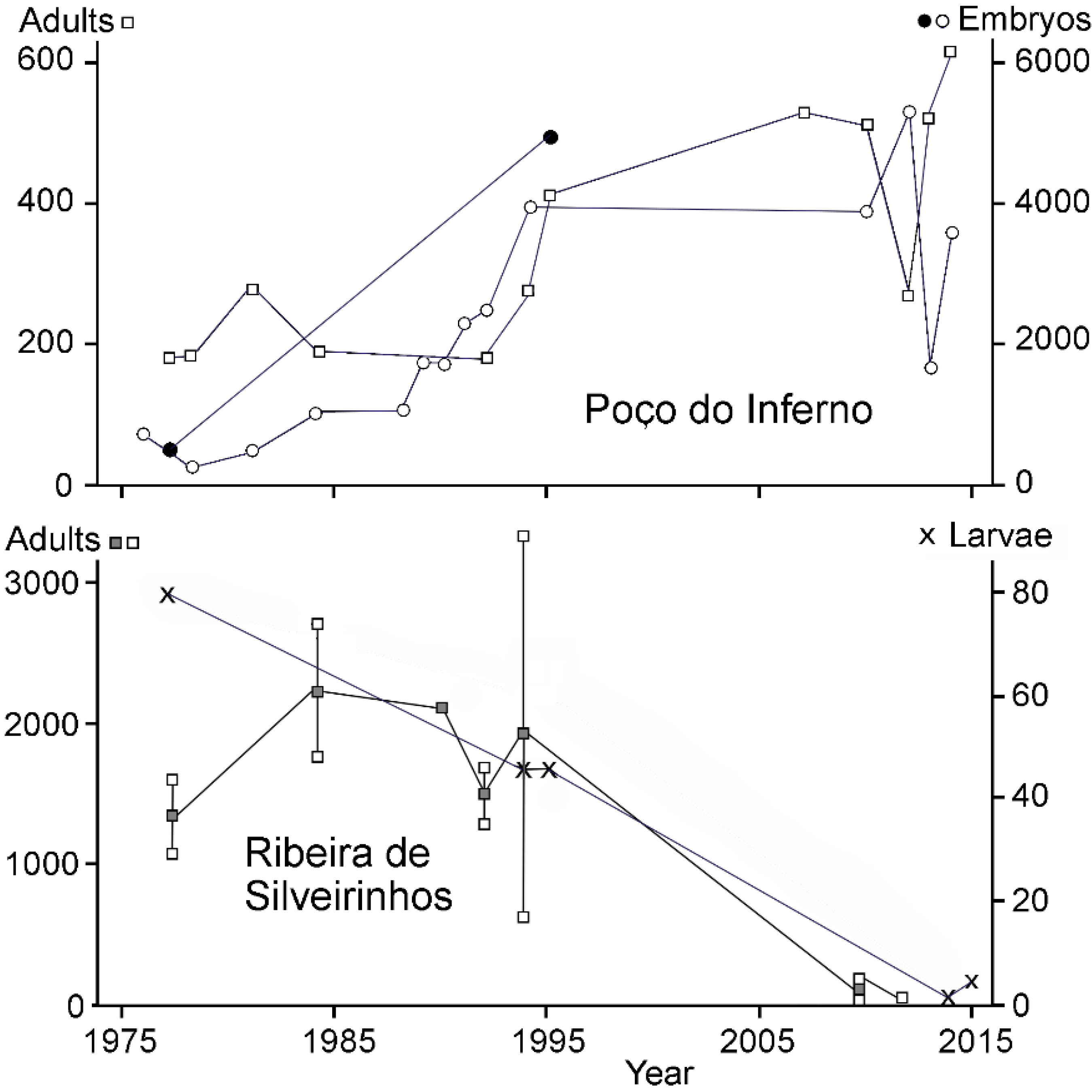
3. Results
4. Discussion
4.1. Population Development at Ribeira de Silveirinhos
4.2. Potential Reasons for the Decline
4.3. Population Development at Poço do Inferno
4.4. Are Eucalypt Plantations to Blame? Suggestions for Future Investigations
Acknowledgments
Conflicts of Interest
Appendix 1
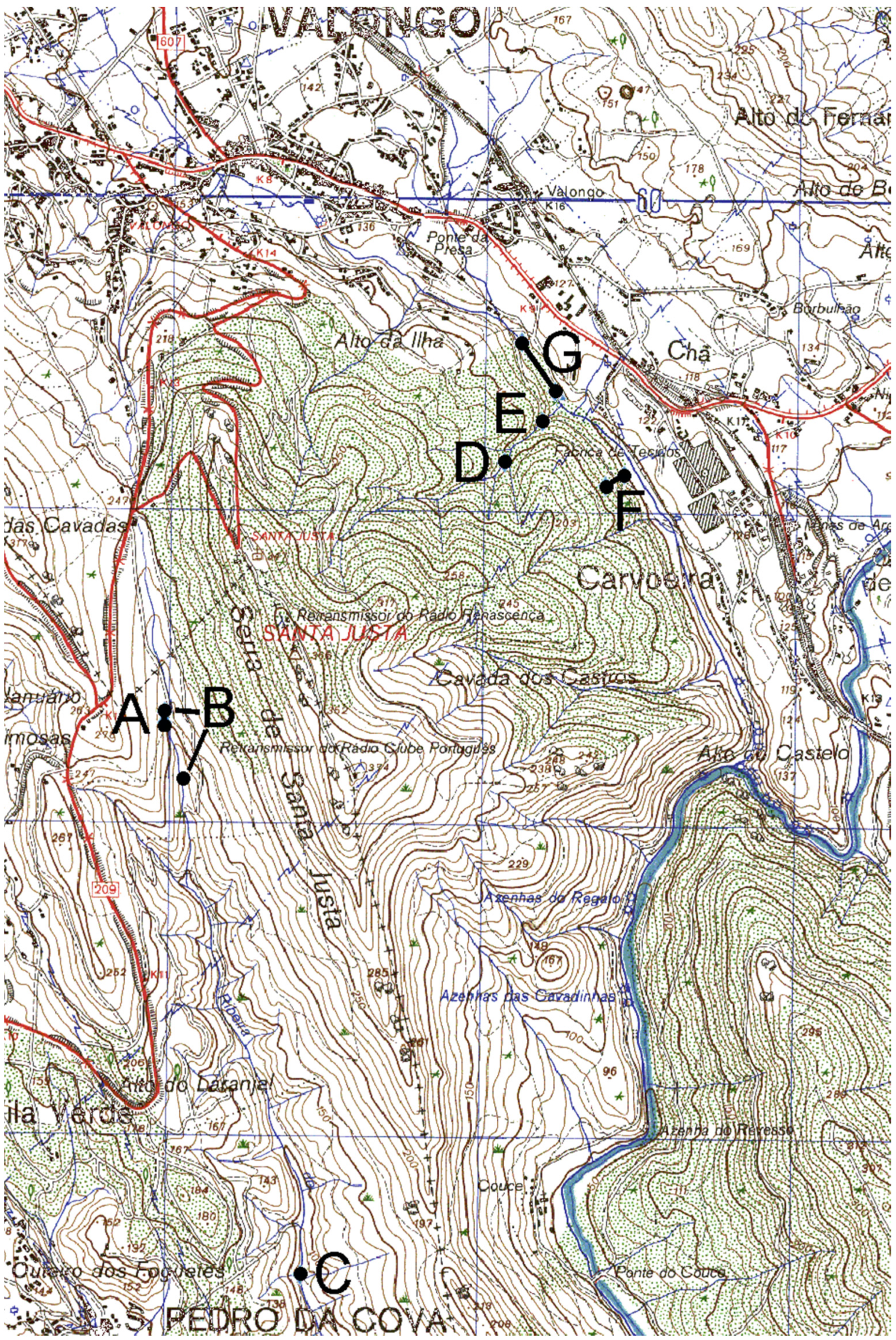
Appendix 2
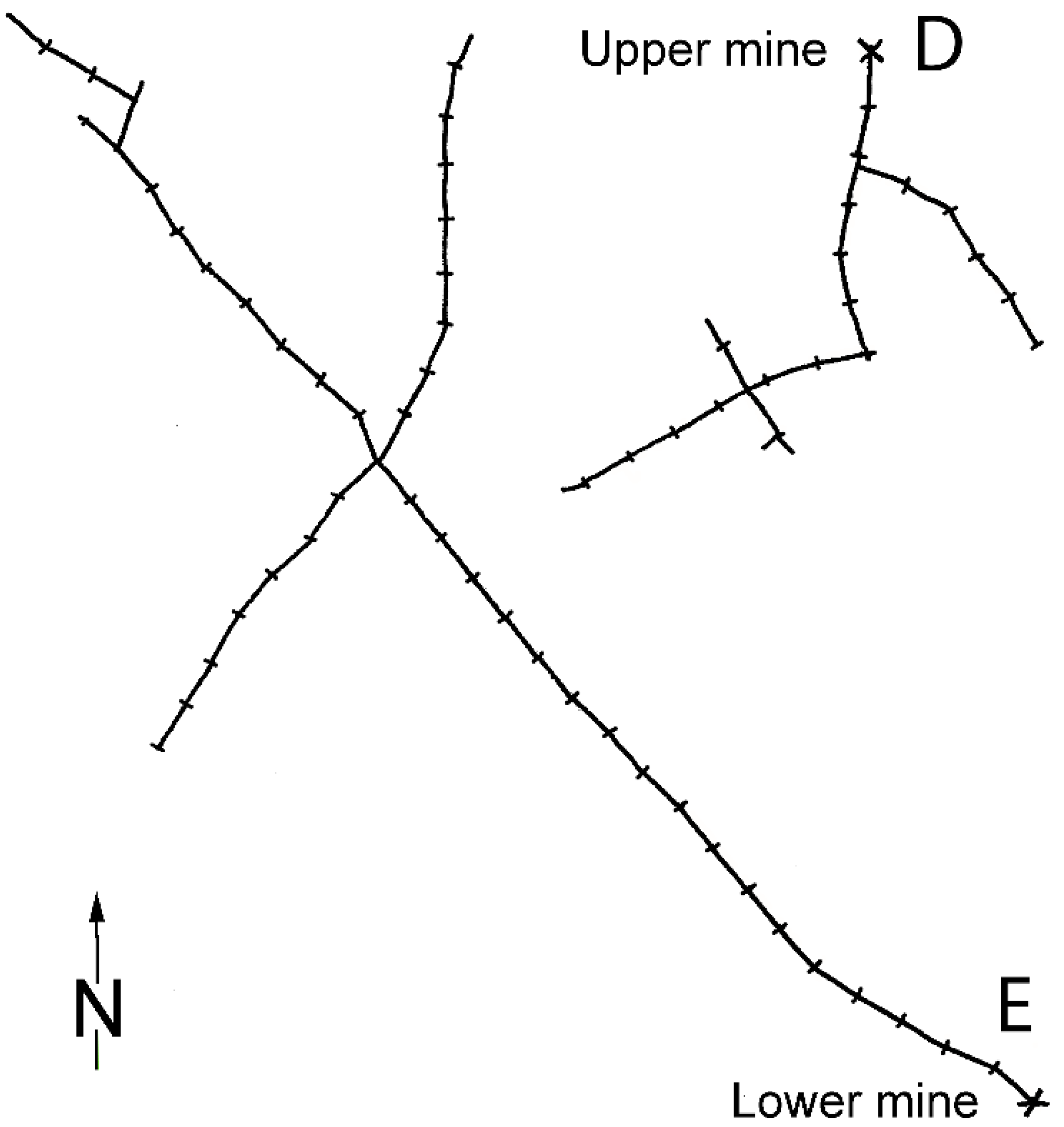
Appendix 3. Parameter Settings for the Population Demographic Modelling with Vortex [31].
A3.1. Hypothetical Population of Chioglossa lusitanica
A3.1.1. Scenario Settings
A3.1.2. Reproductive System
Polygynous
Reproductive Rates
Mortality Rates
A3.2. Ribeira de Silveirinhos Population of Chioglossa lusitanica
A3.3. Poço de Inferno Population of Chioglossa lusitanica
Appendix 4. Numerical Data on the Abundance of Chioglossa lusitanica in Two Study Areas of the Serra de Santa Justa, Portugal over the 1976–2015 Period
| Year | Adult Population Size ± Standard Error | Method | Source |
|---|---|---|---|
| 1977 | 1324 ± 265 | Weighted mean | [8,15] |
| 1984 | 2234 ± 488 | Weighted mean | [3] |
| 1990 | 2117 | Nil-recapture | [4] |
| 1992 | 1477 ± 207 | Weighted mean | [68] |
| 1994 | 1974 ± 1352 | Weighted mean | [32] |
| 2010 | 113 ± 56 | Weighted mean | |
| 2012 | 10–50 | Interpretation | [34] |
| Year | Number of Larvae Observed in the Silveirinhos, Maximum (Date) * | Number of Visits | Source | |
|---|---|---|---|---|
| First Year Cohort | Second Year Cohort | |||
| 1977 | 81 (3/7) | 8 (20/4) | 5 | [8,15] |
| 1994 | 46 (December) | 40 (December) | 1 | [32] |
| 1995 | 46 (June) | 18 (March) | 4 | [32] |
| 2014 | 1 (26/12) | 2 (26/12) | 1 | |
| 2015 | 5 (15–16/4) | 3 (15–16/4) | 1 | |
| Year | Largest Number of Adults Observed (Date) | Number of Visits | Source | |
| Upper Mine | Lower Mine | |||
| 1977 | 78 (19/9) | 100 (20/9) | 22, 16 | [8,15] |
| 1978 | 63 (13/10) | 116 (22/10) | 4, 3 | [8,15] |
| 1981 | 100 (13/10) | 175 (13/10) | 6, 6 | |
| 1984 | 56 (5/10) | 134 (28/8) | 6, 4 | [3] |
| 1992 | 37 (25/9) | 141 (25/9) | 1, 1 | [68] |
| 1994 | 117 (29/10) | 153 (9/10) | 6, 10 | [51,66] |
| 1995 | 209 (4/11) | 199 (9/9) | 25, 25 | [51,66] |
| 2007 | 311 (18/10) | 216 (17/10) | 1, 1 | |
| 2010 | 284 (3/11) | 227 (28/10) | 3, 2 | |
| 2012 | 146 (October) | 121 (October) | 1, 1 | [34] |
| 2013 | 264 (11/11) | 253 (13/11) | 2, 2 | |
| 2014 | 279 (30/9) | 336 (30/9) | 1, 1 | |
| Year | Newly Deposited Eggs Totaled over Multiple Visits | Number of Visits | Source | |
| Upper Mine | Lower Mine | |||
| 1977 | 334 | 208 | 22, 16 | [15] |
| 1995 | 2975 | 2050 | 25, 25 | [51] |
| Year | Maximum Number of Eggs and Embryos Observed | Number of Visits | Source | |
| Upper Mine | Lower Mine | |||
| 1976 ** | 487 | 169 | 1, 1 | [15] |
| 1978 | 130 | 78 | 4, 3 | [8,15] |
| 1981 | 219 | 233 | 6, 6 | |
| 1984 | 97 | 922 | 6, 4 | [3] |
| 1988 | 56 | 987 | Not reported | [4] |
| 1989 | 506 | 1200 | Not reported | [4] |
| 1990 | 150 | 1562 | Not reported | [69] in [4] |
| 1991 | 71 | 1778 | 2, 2 | |
| 1991 | 213 | 2043 | Not reported | [4] |
| 1992 | 46 | 2420 | 5, 5 | [68] |
| 1994 | 2536 | 1394 | Not reported | [32] |
| 2010 | 2503 | 1369 | 3, 2 | |
| 2012 | 2017 | 3283 | 3, 3 | [34] |
| 2013 | 970 | 720 | 2, 2 | |
| 2014 | 1520 | 2039 | 1, 1 | |
Appendix 5
References
- Turnbill, J.W. Eucalypt plantations. New For. 1999, 17, 37–52. [Google Scholar] [CrossRef]
- Reboredo, F.; Pais, J. Evolution of forest cover in Portugal: A review of the 12th–20th centuries. J. For. Res. 2014, 25, 249–256. [Google Scholar] [CrossRef]
- Veenstra, G. Heeft de aanplant van Eucalyptus gevolgen voor de goudstreepsalamander, Chioglossa lusitanica ? Lacerta 1986, 44, 106–113. (In Dutch) [Google Scholar]
- Vences, M. Habitat choice of the salamander Chioglossa lusitanica: The effects of eucalypt plantations. Amphibia-Reptilia 1993, 14, 201–212. [Google Scholar] [CrossRef]
- Ferreira, A.J.D.; Coelho, C.O.A.; Walsh, R.P.D.; Shakesby, R.A.; Ceballos, A.; Doerr, S.H. Hydrological implications of soil water-repellency in Eucalyptus globulus forests, north-central Portugal. J. Hydrol. 2000, 231, 165–177. [Google Scholar] [CrossRef]
- Arntzen, J.W.; Beukema, W.; Galis, F.; Ivanović, A. Vertebral number is highly evolvable in salamanders and newts (family Salamandridae) and variably associated with climatic parameters. Contrib. Zool. 2015, 84, 85–113. [Google Scholar]
- Arntzen, J.W. Speedy salamanders: Sedentariness and migration of Chioglossa lusitanica. Rev. Esp. Herpetol. 1994, 8, 81–86. [Google Scholar]
- Arntzen, J.W. Ecological observations on Chioglossa lusitanica (Caudata, Salamandridae). Amphibia-Reptilia 1981, 1, 187–203. [Google Scholar] [CrossRef]
- Pinto Oliveira, C.I. Susceptibility to Forest Fire in the Municipality of Valongo. Implications for area Planning. Master’s Thesis, Department of Geography, Faculty of Arts, University of Porto, Porto, Portugal, 2006. [Google Scholar]
- Gonçalves, L. A reprodução de Chioglossa lusitanica Bocage. Algumas notas. Naturalia 1962, 8, 1–3. (In Portuguese) [Google Scholar]
- Arntzen, J.W. Temporal and spatial distribution of the Golden-striped salamander (Chioglossa lusitanica) along two mountain brooks in northern Portugal. Herpetol. J. 1995, 5, 213–216. [Google Scholar]
- Begon, M. Investigating Animal Abundance; Edward Arnold: London, UK, 1979. [Google Scholar]
- Bocage, B. Note sur un nouveau batracien du Portugal, Chioglossa lusitanica, et sur une grenouille nouvelle de l’Afrique occidentale. Rev. Mag. Zool. Pure Appl. 1864, 16, 248–254. (In French) [Google Scholar]
- Goux, L. Contribution a l’étude écologique, biologique et biogéographique de Chioglossa lusitanica Barb. (Urodela, Salamandridae). Bull. Soc. Zool. Fr. 1957, 82, 361–377. (In French) [Google Scholar]
- Arntzen, J.W. De Levenswijze van de Goudstreepsalamander, Chioglossa lusitanica Bocage, 1864. Master’s Thesis, Institute of Taxonomic Zoology, University of Amsterdam, Amsterdam, The Netherlands, 1979. [Google Scholar]
- Arntzen, J.W. Chioglossa lusitanica Bocage 1864—Der Goldstreifen-salamander. In Handbuch der Reptilien und Amphibien Europas; Grossenbacher, K., Thiesmeier, B., Eds.; Akademische Verlagsgesellschaft: Wiesbaden, Germany, 1999; pp. 301–321. (In German) [Google Scholar]
- Vences, M. Untersuchungen zur Ökologie, Ethologie end geografischen Variation von Chioglossa lusitanica BOCAGE, 1864. Salamandra 1990, 26, 267–297. (In German) [Google Scholar]
- Sequeira, F.; Gonçalves, H.; Faria, M.M.; Meneses, V.; Arntzen, J.W. Habitat-structural and meteorological parameters influencing the activity and local distribution of the Golden-striped salamander, Chioglossa lusitanica. Herpetol. J. 2001, 11, 85–90. [Google Scholar]
- Lima, V.; Arntzen, J.W.; Ferrand, N.M. Age structure and growth pattern in two populations of the golden-striped salamander Chioglossa lusitanica (Caudata, Salamandridae). Amphibia-Reptilia 2000, 22, 55–68. [Google Scholar] [CrossRef]
- Sequeira, F.; Ferrand, N.; Crespo, E.G. Reproductive cycle of the golden-striped salamander Chioglossa lusitanica (Caudata, Salamandridae) in NW Portugal. Amphibia-Reptilia 2003, 24, 1–12. [Google Scholar] [CrossRef]
- Pleguezuelos, J.M.; Márquez, R.; Lizana, M. Atlas y Libro Rojo de los Anfibios y Reptiles de España; Ministerio de Medio Ambiante: Madrid, Spain, 2002. (In Spanish) [Google Scholar]
- Arntzen, J.W.; Teixeira, J. History and new developments in the mapping and modelling of the distribution of the golden-striped salamander, Chioglossa lusitanica. Z. Feldherpetol. 2006, 10, 113–126. [Google Scholar]
- Loureiro, A.; de Almeida, N.F.; Carretero, M.A.; Paulo, O.S. Atlas dos Anfíbios e Répteis de Portugal; Instituto da Conservação da Natureza e da Biodiversidade: Lisboa, Portugal, 2008. (In Portuguese) [Google Scholar]
- Arntzen, J.W. Allometry and autotomy of the tail in the Golden-striped salamander, Chioglossa lusitanica. Amphibia-Reptilia 1994, 15, 267–274. [Google Scholar] [CrossRef]
- Alexandrino, J.; Arntzen, J.W. Morphological variation in two genetically distinct groups of the golden-striped salamander, Chioglossa lusitanica (Amphibia: Urodela). Contrib. Zool. 2005, 74, 213–222. [Google Scholar]
- Arntzen, J.W.; Groenenberg, D.S.J.; Alexandrino, J.; Ferrand, N.; Sequeira, F. Geographical variation in the golden-striped salamander, Chioglossa lusitanica Bocage, 1864 and the description of a newly recognized subspecies. J. Nat. Hist. 2007, 41, 925–936. [Google Scholar] [CrossRef]
- Alexandrino, J.; Froufe, E.; Arntzen, J.W.; Ferrand, N. Genetic subdivision, glacial refugia and postglacial recolonization in the golden-striped salamander, Chioglossa lusitanica (Amphibia: Urodela). Mol. Ecol. 2000, 9, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Alexandrino, J.; Arntzen, J.W.; Ferrand, N. Nested clade analysis and the genetic evidence for population expansion in the phylogeography of the golden-striped salamander, Chioglossa lusitanica (Amphibia: Urodela). Heredity 2002, 88, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Arntzen, J.W.; Alexandrino, J. Ecological modelling of genetically differentiated forms of the Iberian endemic Golden-striped salamander, Chioglossa lusitanica. Herpetol. J. 2004, 14, 137–141. [Google Scholar]
- Sequeira, F.; Alexandrino, J.; Rocha, R.; Arntzen, J.W.; Ferrand, N. Genetic exchange across a hybrid zone within the Iberian endemic golden-striped salamander, Chioglossa lusitanica. Mol. Ecol. 2005, 14, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Lacy, R.C.; Pollak, J.P. Vortex: A Stochastic Simulation of the Extinction Process. Version 10.0; Chicago Zoological Society: Brookfield, IL, USA, 2014. [Google Scholar]
- Lima, V. Estudo Comparativo de Alguns Aspectos da Biologia de Chioglossa lusitanica em Duas Populaçŏes do Noroeste de Portugal. Master’s Thesis, Faculty of Sciences, University of Porto, Porto, Portugal, 1995. [Google Scholar]
- Science Plus Group. PRISM, v. 6; Science Plus Group: Groningen, The Netherlands.
- Silva Santos, J. Estudo Sobre o Tamanho de Três Populações de Salamandra Lusitânica (Chioglossa lusitanica) nas Serras de Valongo. Master’s Thesis, Faculty of Sciences, University of Porto, Porto, Portugal, 2013. [Google Scholar]
- Smith, K.F.; Acevedo-Whitehouse, K.; Pedersen, A.B. The role of infectious diseases in biological conservation. Anim. Conserv. 2009, 12, 1–12. [Google Scholar] [CrossRef]
- Pryor, L.D. The Biology of Eucalypts; Edward Arnold: London, UK, 1976. [Google Scholar]
- Thiesmeier, B. Trophische Beziehungen und Habitatpräferenzen sympatrisch lebender Salamandra salamandra und Chioglossa lusitanica-Larven. In Abhandlungen und Berichte für Naturkunde; Museum für Naturkunde Magdeburg: Magdeburg, Germany, 1994; Volume 17, pp. 119–126. (In German) [Google Scholar]
- Hall, N.; Johnston, R.D.; Chippendale, G.M. Forest Trees of Australia; Australian Government Publishing Service: Canberra, Australia, 1970. [Google Scholar]
- Sellers, C.H. Eucalyptus, Its History, Growth and Utilization; A.J. Johnston Co.: Sacramento, CA, USA, 1910. [Google Scholar]
- Calder, I.R.; Rosier, P.T.W.; Prasanna, K.T.; Parameswarappa, S. Eucalyptus water use greater than rainfall input—A possible explanation from southern India. Hydrol. Earth Syst. Sci. 1997, 1, 249–256. [Google Scholar] [CrossRef]
- Arntzen, J.W.; (Naturalis Biodiversity Center, Leiden, The Netherlands). Unpublished data. 2014, 2015.
- Bosch, J.; Martínez-Solano, I.; García-París, M. Evidence of a chytrid fungus infection involved in the decline of the common midwife toad (Alytes obstetricans) in protected areas of Central Spain. Biol. Conserv. 2001, 97, 331–337. [Google Scholar] [CrossRef]
- Bosch, J.; Martínez-Solano, I. Chytrid fungus infection related to unusual mortalities of Salamandra salamandra and Bufo bufo in the Peñalara Natural Park (Central Spain). Oryx 2006, 40, 84–89. [Google Scholar] [CrossRef]
- Price, S.J.; Garner, T.W.J.; Nichols, R.A.; Balloux, F.; Ayres, C.; de Alba, A.M.; Bosch, J. Collapse of amphibian communities due to an introduced ranavirus. Curr. Biol. 2014, 24, 2586–2591. [Google Scholar] [CrossRef] [PubMed]
- Froufe, E.; Arntzen, J.W.; Loureiro, A. Dead Newts in Peneda-Gerês. Available online https://www.amphibians.org/wp-content/uploads/2012/05/Froglog31.pdf (accessed on 21 July 2015).
- Soares, C.; de Matos, A.A.; Arntzen, J.W.; Carretero, M.; Loureiro, A. Amphibian Mortality in a National Park in the North of Portugal. Available online: http://issuu.com/amphibiansdotorg/docs/froglog56/1 (accessed on 21 July 2015).
- Prowse, T.A.A.; Johnson, C.N.; Lacy, R.C.; Bradshaw, C.J.A.; Pollak, J.P.; Watts, M.J.; Brook, B.W. No need for disease: Testing extinction hypotheses for the thylacine using multi-species metamodels. J. Anim. Ecol. 2013, 82, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Arntzen, J.W.; (Naturalis Biodiversity Center, Leiden, The Netherlands). Unpublished data. 1981.
- Arntzen, J.W.; (Naturalis Biodiversity Center, Leiden, The Netherlands). Unpublished data. 1977, 2010.
- Arntzen, J.W.; (Naturalis Biodiversity Center, Leiden, The Netherlands); Gerats, A.G.M.; (University of Amsterdam, Amsterdam, The Netherlands). Unpublished data. 1977.
- Faria, M.M.; (R. Cooperativa 7 Bicas 130 3° E, Senhora da Hora, Portugal). Personal communication, 2015.
- Poore, M.E.D.; Fries, C. The Ecological Effects of Eucalyptus; Food and Agriculture Organization of the United Nations: Rome, Italy, 1985. [Google Scholar]
- Florence, R.G. Cultural problems of Eucalyptus as exotics. Commonw. For. Rev. 1986, 65, 141–163. [Google Scholar]
- Del Moral, R.; Muller, C.H. Fog drip: A mechanism of toxin transport from Eucalyptus globulus. Bull. Torrey Bo. Club 1969, 96, 467–475. [Google Scholar] [CrossRef]
- De la Hera, I.; Arizaga, J.; Galarza, A. Exotic tree plantations and avian conservation in northern Iberia: A view from a nest-box monitoring study. Anim. Biodivers. Conserv. 2013, 36, 153–163. [Google Scholar]
- Proença, V.M.; Pereira, H.M.; Guilherme, J.; Vicente, L. Plant and bird diversity in natural forests and in native and exotic plantations in NW Portugal. Acta Oecol. 2010, 36, 219–226. [Google Scholar] [CrossRef]
- Zahn, A.; Rainho, A.; Rodrigues, I.; Palmeirim, J.M. Low macro-arthropod abundance in exotic Eucalyptus plantations in the Mediterranean. Appl. Ecol. Environ. Res. 2009, 7, 297–301. [Google Scholar] [CrossRef]
- Abelho, M.; Graça, M.A.S. Effects of eucalyptus afforestation on leaf litter dynamics and macroinvertebrate community structure of streams in Central Portugal. Hydrobiologia 1996, 324, 195–204. [Google Scholar] [CrossRef]
- Graça, M.A.S.; Pozo, J.; Canhoto, C.; Elosegi, A. Effects of Eucalyptus plantations on detritus, decomposers, and detritivores in streams. Sci. World 2002, 2, 1173–1185. [Google Scholar] [CrossRef] [PubMed]
- Larrañaga, A.; Basaguren, A.; Pozo, J. Impacts of Eucalyptus globulus plantations on physiology and population densities of invertebrates inhabiting Iberian Atlantic streams. Int. Rev. Hydrobiol. 2009, 94, 497–511. [Google Scholar] [CrossRef]
- Greenberg, D.A.; Green, D.M. Effects of an invasive plant on population dynamics in toads. Conserv. Biol. 2013, 27, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Sequeira, F.; Teixeira, J.; Alexandrino, J.; Lima, V.; Ferrand, N. Distribución de Chioglossa lusitanica en Portugal. Bol. Aso. Herpetol. Esp. 1996, 7, 7–8. (In Portuguese) [Google Scholar]
- IGC—Instituto Geográfico e Cadastral. Carta Corográfica de Portugal na Escala 1/50,000; IGC: Lisboa, Portugal, 1977. (In Portuguese) [Google Scholar]
- Francillon-Vieillot, H.; Arntzen, J.W.; Géraudie, J. Age, growth and longevity of sympatric Triturus cristatus, T. marmoratus and their hybrids (Amphibia, Urodela). A skeletochronological comparison. J. Herpetol. 1990, 24, 13–22. [Google Scholar] [CrossRef]
- Caughley, G. Analysis of Vertebrate Populations; John Wiley & Sons: London, UK, 1977. [Google Scholar]
- Faria, M.M.; Gonçalves, H.; Meneses, C.; Sequeira, F. Reproductive habits of the golden-striped salamander, Chioglossa lusitanica in three mine galleries in Valongo (North of Portugal). In Proceedings of the IV Congresso Luso-Espanhol de Herpetologia, Porto, Portugal, 5–8 December 1996; p. 82.
- Ortiz-Santaliestra, M.E.; Fernández-Benéitez, M.J.; Lizana, M.; Marco, A. Influence of a combination of agricultural chemicals on embryos of the endangered Gold-striped salamander (Chioglossa lusitanica). Arch. Environ. Contam. Toxicol. 2011, 60, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Belcher, J.; Cornish, C. Personal communication, 1992.
- Arntzen, J.W.; (Naturalis Biodiversity Center, Leiden, The Netherlands); Vences, M.; (Technical University of Braunschweig, Braunschweig, Germany). Unpublished data, 1990.
© 2015 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arntzen, J.W. Drastic Population Size Change in Two Populations of the Golden-Striped Salamander over a Forty-Year Period—Are Eucalypt Plantations to Blame? Diversity 2015, 7, 270-294. https://doi.org/10.3390/d7030270
Arntzen JW. Drastic Population Size Change in Two Populations of the Golden-Striped Salamander over a Forty-Year Period—Are Eucalypt Plantations to Blame? Diversity. 2015; 7(3):270-294. https://doi.org/10.3390/d7030270
Chicago/Turabian StyleArntzen, Jan W. 2015. "Drastic Population Size Change in Two Populations of the Golden-Striped Salamander over a Forty-Year Period—Are Eucalypt Plantations to Blame?" Diversity 7, no. 3: 270-294. https://doi.org/10.3390/d7030270
APA StyleArntzen, J. W. (2015). Drastic Population Size Change in Two Populations of the Golden-Striped Salamander over a Forty-Year Period—Are Eucalypt Plantations to Blame? Diversity, 7(3), 270-294. https://doi.org/10.3390/d7030270






