1. Introduction
The choice of a host plant is crucial for fitness in phytophagous insects. In particular, females are generally selected to choose the oviposition sites that will maximize the performance (survival, fecundity) of their offspring (Optimal Oviposition Theory) [
1,
2]. Discrimination of suitable oviposition sites is especially critical in species where the larvae have low mobility, or are limited to a restricted amount of resources, as in species for which oviposition and larval development occur within a fruit. The choice of oviposition sites is guided by forces acting on the ovipositing female and/or her offspring, such as plant nutritional value and defenses [
3,
4]. Plant traits that may influence this choice include external factors that act at a distance (e.g., plant size, volatiles) or by contact (e.g., tissue thickness), and internal factors (e.g., primary and secondary metabolites), often in an integrated way [
5]. These cues can provide the insect with information for host plant recognition, and information about plant quality, defenses, and the presence of competitors or enemies. Oviposition choice of phytophagous insects can thus follow the optimal foraging theories initially developed for parasitoids [
6]. However, oviposition choice of phytophagous insects has been much less studied than oviposition choice of parasitoids.
In plant insect interactions, plant resistance to herbivory and insect host choice may greatly impact fitness of both partners. Natural selection can lead to changes in plant traits that confer resistance against herbivores [
7,
8] and to changes in the population of herbivores, for instance, in morphology, physiology, or behaviour (e.g., [
9]). Further, the reciprocal selection pressure exerted by the two partners can lead to coevolution [
10], especially when their relationship is very specific and each other’s influence is strong [
11]. In addition, the geographical structure of herbivore and host plant populations can lead to local coadaptation of the two partners, when local genotypes of one species are selected to fit local genotypes of the other (e.g., [
12,
13]).
Invasive plant species offer good systems to study the relationships between plants and phytophagous insects and the processes linked to host plant choice and oviposition choice, since they have both native populations that live in sympatry with their specific herbivores and populations introduced into environments devoid of these herbivores [
14,
15]. Using such an invasive host species and populations coming from both native and introduced ranges offers great opportunity to study processes and traits linked to host choice by its herbivores, because plants coming from populations with contrasting evolutionary histories are expected to differ in their sensitivity to local phytophagous insects [
16,
17], and thus, in traits associated with host choice.
We studied oviposition choice of the seed-eating weevil
Exapion ulicis L. Forster (Cucurlionidae: Apionidae) on its host plant, the invasive species
Ulex europaeus (Fabaceae: Genistae).
Ulex europaeus (the common gorse) is native to Western Europe, where all its populations are naturally infested by
E.
ulicis. It was introduced into many parts of the world during the 19th Century (e.g., in South America, western North America, Reunion Island, New Zealand, Hawaii), initially without its natural enemies. In some countries,
E.
ulicis was later introduced as a biological control agent, like in New Zealand in 1931 [
18]. This system is particularly interesting because the relationship between
U.
europaeus and
E.
ulicis is very specific and very strong [
19]. Indeed, the weevil undergoes its whole life-cycle on gorse, and gorse may suffer considerable damage from the weevils, which may infest up to 90% of the mature pods [
19,
20]. However, pod infestation rates are extremely variable in natural and experimental populations, and can vary from 20–98%, even among neighbouring individuals [
19,
20,
21]. This proportion has been shown to depend on many factors, including plant traits such as plant architecture, flowering phenology, chemical compounds and flower and pod densities [
21,
22]. This suggests that
E.
ulicis females use all these cues to choose the plant on which they will lay their eggs. The next step,
i.
e., the choice of pods in which to oviposit, has not been explored previously.
To investigate how Exapion ulicis weevils choose pods to oviposit in, we performed choice experiments in the lab. We also tested weevil feeding preference on flowers, to compare oviposition preference and feeding preference. The gorse studied came from two native regions where the weevil is present (Brittany and Scotland), one invaded region where the weevil is absent (Reunion), and one invaded region where the weevil was initially absent, but later introduced for biological control (New Zealand). By using gorses from regions with various histories of coevolution between the plant and the weevil, we expected to detect variations in oviposition and feeding preferences. Our objective was to explore the oviposition choice of E. ulicis and to answer the following questions: (i) Do female E. ulicis weevils express a preference between gorse pods from different regions? (ii) If so, is their oviposition choice based on external cues (i.e., detected before the female begin to dig a hole on the pod wall) or on internal cues (i.e., detected once the hole has been achieved)? (iii) If so, is their oviposition choice based on external cues (i.e., detected before the female begins to bore a hole in the pod wall) or on internal cues (i.e., detected once the hole has been made)? (iii) Are the regions chosen for oviposition the same as those chosen for feeding?
2. Materials and Methods
2.1. Study Species
Gorse,
Ulex europaeus (Fabaceae: Genistae), is a perennial spiny shrub. It can live up to 30 years, and adult height usually varies from one to four meters. The hermaphrodite flowers are pollinated by large insects such as honeybees or bumblebees [
23]. Pods start to form immediately after flowers have been fertilized and pod maturation takes at least two months. When initiated, young pods are green and soft, and about 4 mm long. Mature pods are lignified, brown, and about 13 mm long. In Europe, pods are attacked by several seed predators, of which the weevil
Exapion ulicis is the most common [
19,
24]. In its invasive range,
U.
europaeus was introduced around the 1800s, without its natural enemies. In New Zealand,
E.
ulicis was introduced in 1931 for biological control and is now widespread [
18,
25]. In Reunion, there is no biological control program, and gorse plants still have no known natural enemies.
Exapion ulicis is naturally present in Europe and is specific to
U.
europaeus on which it spends its whole life cycle [
19]. It is present in all European gorse populations. In Brittany, the oviposition season lasts about six weeks in spring [
19]. Gorse pod are susceptible for oviposition when they are 10–35 days old,
i.
e., when they are longer than 8 mm but still green and soft [
23,
26]. Larvae develop within the maturing pods, feeding on the developing seeds, and adults emerge from the ripe pods at dehiscence, about two months later. Adults then feed on the vegetative parts and on flowers of
U.
europaeus. The laying period lasts about six weeks in early spring, and a female can lay up to several dozen eggs [
19].
To focus on pod and flower cues and avoid the confounding effects of whole-plant traits such as pod and flower density or plant size [
22], we worked on fresh isolated pods and flowers that were collected just before the experiments in the common garden described by Hornoy
et al. [
27]. In this garden, located in Rennes (Brittany, France), gorse plants from seeds collected in Brittany and Scotland (native range), and Reunion and New Zealand (invaded range) have been grown in a randomized pattern since October 2006. For each of these four regions, three populations are represented, with 10 plants grown per population. During the choice experiments done in 2009 (see below), the adult weevils used were regularly collected on gorse branches of a natural population located 700 m away from the experimental garden. The weevils collected were sorted by sex and reared in the laboratory for, at most, four days before use in boxes containing water and fresh gorse shoots. Rearing and experiments were performed in dedicated rooms, at ambient temperature and humidity.
2.2. Oviposition Choice Experiments
Preliminary experiments were performed in early spring 2009, by exposing different numbers of females to different numbers of pods to observe female oviposition behaviour and determine optimal experimental conditions. Indeed, female egg-laying rates vary with year, and had to be calibrated. Females did not lay eggs on pods collected more than 24 h before the experiment. According to these preliminary experiments, we decided to do the choice experiments by exposing each morning a set of four females to six pods collected the same morning. In this way, we obtained eggs on about half of the pods after 24 h of exposure.
For each region, the three gorse populations of the common garden were sampled for pods on three gorse plants per population. Gorse plants used for each experiment were all in a similar phenological stage. Pods offered to E. ulicis females were chosen for their developmental state (i.e., susceptible to oviposition) and checked for their infestation status. A pod was considered as uninfested when observation under a dissecting microscope revealed no holes or scars. Infested pods were discarded from the experiment. The pool of pods of each population was made up of an equal number of uninfested pods collected on each of the three selected individuals. For each choice experiment, a random pod from each of the three populations of each region was offered to weevils in Petri dishes.
Female weevils used for these oviposition experiments were reared in the presence of males for at least two days. They were fed with pod-free shoots coming from the natural population in which the weevils were collected. Choice experiments were performed in Petri dishes.
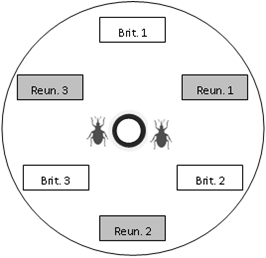
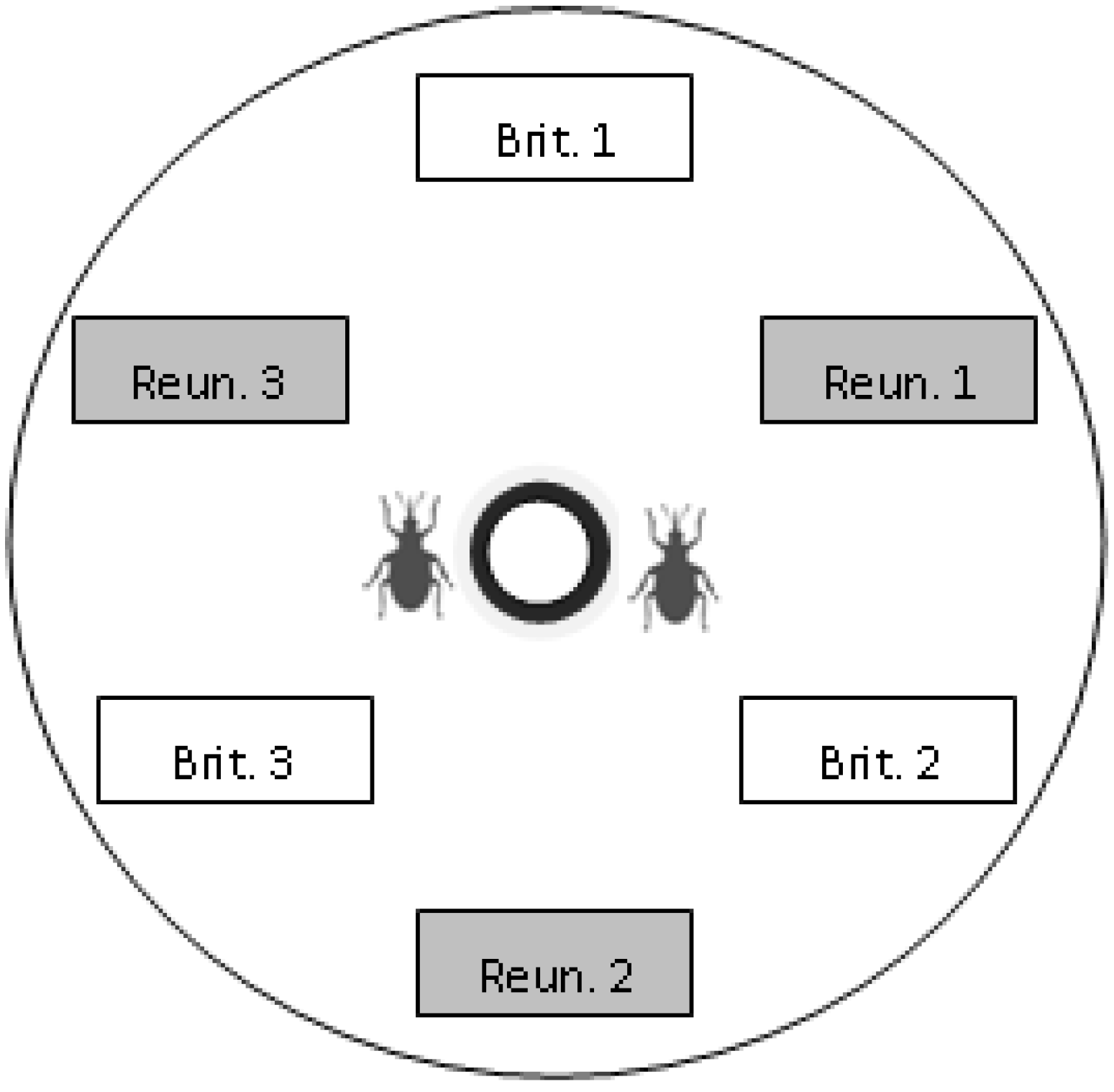
Within each Petri dish we put six uninfested pods collected in the morning (three pods from each of the two regions tested), four female weevils, two male weevils (to prevent male limitation), two flowers collected (one from each of the two regions tested) for feeding and oviposition stimulation, and a water supply (cotton wool soaked in water and placed in a small cup), as described in
Figure 1.
Each set of females was tested during two days. To ensure oviposition activity, pods and flowers were replaced by fresh ones every 24 h. After pod removal, pod length was measured with a caliper, and pods were opened to estimate the number of seeds, the number of holes in the pod wall, the number of egg clusters, and the total number of eggs.
The duration of the experiment, and thus, the number of comparisons that could be performed was limited by the duration of the weevil oviposition season (about six weeks). During that period, we first performed preliminary experiments, and during the remaining weeks we were able to make only three pairwise comparisons with sufficient replication (18–24 sets of females tested for each comparison), due to technical constraints. Two comparisons were made between native and invaded regions: Brittany vs. Reunion and Scotland vs. New Zealand. One was made between two regions of the native range: Brittany vs. Scotland.
Figure 1.
Scheme of the choice device. Pods of each region and population were placed alternately, at an equal distance from the centre of the dish. In this example, the “Brit. 1” box represents a pod from population 1 of Brittany. At the beginning of an experiment, weevils were placed at the centre of the dish, near the water supply (bold black circle).
Figure 1.
Scheme of the choice device. Pods of each region and population were placed alternately, at an equal distance from the centre of the dish. In this example, the “Brit. 1” box represents a pod from population 1 of Brittany. At the beginning of an experiment, weevils were placed at the centre of the dish, near the water supply (bold black circle).
Since these experiments were performed at different periods of the reproductive season, the weevil oviposition activity and gorse pod characteristics may have differed between experiments. Analyses were thus performed separately within each choice experiment without pooling the whole dataset.
2.3. Weevil Mass at Emergence and Pod Traits in the Common Garden
Weevil eggs obtained in the lab during choice experiments desiccated quickly and did not develop into larvae. Thus, to link weevil offspring performance to pod traits, we made measurements on the plants grown in the common garden. These were made on 103–106 plants (8–10 plants per population, 21–28 plants per region, see Hornoy
et al. [
27] for details). For each gorse individual in the garden, 30 ripe pods were randomly chosen and opened. The number of seeds per pod was estimated from at least 10 uninfested pods. Seed mass was estimated on 10 seeds per plant, collected from at least three different pods. Pod length was estimated from three pods per plant. Seed mass per pod was then estimated by multiplying mean seed number per pod by mean seed mass per plant. When pods were infested by weevils, we counted the number of adult weevils, and estimated their fresh weight.
2.4. Feeding Choice Experiments
To compare feeding on gorses from different regions, we performed the same pairwise choice experiments as for the oviposition experiments in spring 2010. Selection of gorse individuals, flower collection and arrangement of the dishes was done as for pods in the oviposition choice experiments (see above and
Figure 1). We collected flowers that were recently opened, unpollinated (
i.
e., with keel not opened), and barely, if at all, eaten (showing no or very few feeding holes through the petals). The number of holes present in the petals before the experiment was noted.
We tested male and female weevils separately to detect any sex effect on the feeding choice. Male and female weevils used for this test were reared separately and fed with flowering shoots from the two regions tested.
Each experiment consisted of a single weevil introduced to the six offered flowers from the two regions for 24 h, after which the flowers were removed. Flower length was then measured with a caliper, and flowers were inspected under a dissecting microscope to count the number of holes in the petals. Weevil consumption was estimated as the difference between the number of holes present before and after the experiment. Each pairwise choice experiment consisted of 20 such replicates (10 with males, and 10 with females).
2.5. Statistical Analyses
The effect of the region of origin was tested for the number of holes per pod, the number of eggs per hole, and the number of eggs per pod to explore oviposition preference, and for the number of holes per flower to explore feeding preference. Two types of test were performed:
- (1)
Because the oviposition behaviour resulted in non-normal data, we first analyzed them with nonparametric statistics. We used a Chi-squared test, because it does not make any assumption about data distribution. For this test, all data of a given experiment were pooled for each of the two regions compared, and the significance of the difference was tested with a chi-squared with one d.f.
- (2)
For the number of eggs per pod and the number of holes per flower, we confirmed the results of the Chi-squared test with an exact test of proportions, the null hypothesis being an equal result for both regions studied in a given experiment (see Appendix).
Other comparisons of means were performed using Student’s
t-test for pod length, seed content, and flower length or one-way ANOVA for cluster size. Correlations between pod characteristics and other variables were tested using Spearman’s rank order correlation coefficients. All these analyses were performed with R [
28].
3. Results
3.1. Oviposition Choice Experiments
3.1.1. Oviposition Behaviour
In the lab, the oviposition behaviour of E. ulicis females consists of sequential steps: (i) the female walks, exploring, and eventually climbs onto a pod; (ii) she explores the pod and (iii) may start to bore a hole with her rostrum through the pod wall. Then (iv), either she turns around and inserts her ovipositor through the hole to lay eggs in the pod’s seed chamber, or leaves the pod without ovipositing; (v) eventually, after ovipositing, she walks all over the pod rubbing the back end of her abdomen on it, probably to mark it. The whole sequence can last several hours.
Under the experimental conditions used for the choice experiments, most hole-borings did not result in egg-laying, and the mean number of clusters laid per hole was 0.28. When females laid eggs, they laid between 1 and 13 eggs per cluster, with a mean cluster size of 4.56 ± 2.60. Cluster size did not differ between the three choice experiments (one-way ANOVA, F2,455 = 0.45, p = 0.64). On average, 3.62 eggs were laid per female per day. After the experiment, a single pod exhibited between 0 and 18 holes, and contained between 0 and 25 eggs.
3.1.2. Oviposition Choice
The number of eggs per pod was significantly different between the two regions tested in all three choice experiments (
Table 1). The results of the exact test of proportions for the number of eggs per pod gave similar significance levels as the Chi-square tests (
p<0.001 for the three choice experiments).
Table 1.
Results of the X2 tests for oviposition choice by Exapion ulicis on Ulex europaeus pods.
Table 1.
Results of the X2 tests for oviposition choice by Exapion ulicis on Ulex europaeus pods.
| | | Brittany vs. Reunion | Scotland vs. New Zealand | Brittany vs. Scotland |
|---|
| number of pods * | 276 | 288 | 176 |
| number of holes * | 757 | 698 | 790 |
| number of eggs * | 650 | 613 | 639 |
| | | X² | P | X² | P | X² | P |
| holes per pod | 0.00 | NS | 0.01 | NS | 42.86 | p < 0.001 |
| eggs per hole | 0.64 | p < 0.01 | 10.77 | p < 0.01 | 0.10 | NS |
| eggs per pod | 9.85 | p < 0.01 | 11.24 | p < 0.001 | 31.11 | p < 0.001 |
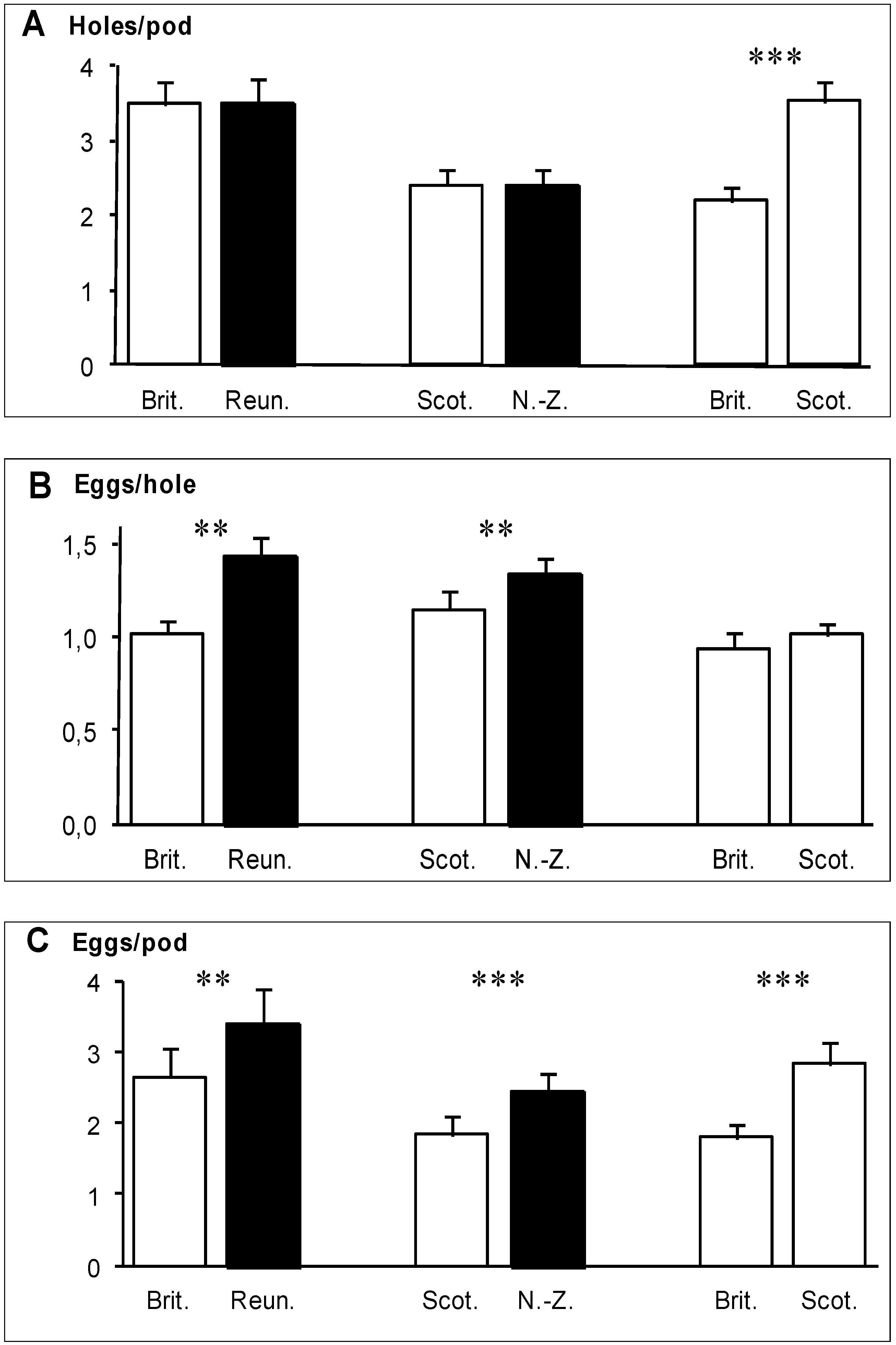
In the two experiments involving a native and an invaded region (Brittany
vs. Reunion, Scotland
vs. New Zealand), the total number of holes bored in pods did not differ significantly between regions (
Figure 2A). However, the number of eggs laid per hole was significantly greater in pods from the invaded regions (
Figure 2B), so that pods from the invaded region received significantly more eggs than pods from the native region (
Figure 2C). In the Reunion
vs. Brittany experiment, this difference arose from the number of egg clusters per hole (0.24 for Brittany
vs. 0.32 for Reunion), while the number of eggs per cluster was very similar (4.68 for Brittany
vs. 4.66 for Reunion). In the Scotland
vs. New Zealand experiment, this difference arose also mainly from the number of egg clusters per hole (0.28 for Scotland
vs. 0.32 for New Zealand) rather than from the number of eggs per cluster (4.27 for Scotland
vs. 4.53 for New Zealand). In the Brittany
vs. Scotland experiment, the number of holes bored in the pods was significantly higher in pods of Scottish gorses (
Figure 2A) but the number of eggs per hole did not differ between pods from the two regions (
Figure 2B). As a consequence, pods of Scottish gorses received significantly more eggs in total than pods of Brittany gorses (
Figure 2C).
Figure 2.
(
A) Mean number of holes bored by the weevils
E.
ulicis through the pod walls of
U.
europaeus; (
B) mean number of eggs laid per hole; (
C) mean number of eggs per pod (
C). White bars: native regions, black bars: invaded regions. Means are shown with 1 SE. Significant differences (
Table 1) are indicated by stars: *
p<0.05, **
p<0.01, ***
p<0.001.
Figure 2.
(
A) Mean number of holes bored by the weevils
E.
ulicis through the pod walls of
U.
europaeus; (
B) mean number of eggs laid per hole; (
C) mean number of eggs per pod (
C). White bars: native regions, black bars: invaded regions. Means are shown with 1 SE. Significant differences (
Table 1) are indicated by stars: *
p<0.05, **
p<0.01, ***
p<0.001.
3.1.3. Characteristics of the Pods Used for the Experiment
Pod length differed significantly between regions in all three choice experiments: pods from Reunion were larger than pods from Brittany, pods from New Zealand were larger than pods from Scotland, and pods from Scotland were larger than pods from Brittany (
Table 2). The mean number of seeds per pod differed significantly between regions only in the Brittany
vs. Scotland experiment, where pods of Scottish gorses had significantly more seeds than pods of Brittany gorses (
Table 2).
Table 2.
Characteristics of Ulex europaeus pods and flowers used in each choice experiment, and results of Student’s t-test for pairwise comparisons of means.
Table 2.
Characteristics of Ulex europaeus pods and flowers used in each choice experiment, and results of Student’s t-test for pairwise comparisons of means.
| | Brittany vs. Reunion | Scotland vs. New Zealand | Brittany vs. Scotland |
|---|
| | mean ± sd | t | mean ± sd | t | mean ± sd | t |
|---|
| | Brittany | Reunion | | Scotland | N. Zealand | | Brittany | Scotland | |
|---|
| Pod length (mm) | 12.59 ± 1.32 | 13.09 ± 1.40 | −2.71 ** | 13.46 ± 1.23 | 13.86 ± 1.55 | −2.45 * | 12.33 ± 1.35 | 13.27 ± 1.19 | −6.19 *** |
| Seeds per pod | 3.81 ± 1.55 | 3.84 ± 1.21 | −0.15 | 3.96 ± 1.68 | 4.08 ± 1.56 | −0.62 | 3.01 ± 1.48 | 3.46 ± 1.52 | −2.44 * |
| Flower length (mm) | 18.70 ± 1.55 | 18.96 ± 1.35 | −0.97 | 19.52 ± 1.25 | 19.35 ± 1.88 | 0.61 | 18.37 ± 1.57 | 19.01 ± 1.51 | 2.26 * |
We found positive and significant correlations between pod length and the number of eggs laid per pod (
Table 3). These correlations still held when considering only pods from one region, except for Scotland in the Brittany-Scotland choice experiment (not shown). The number of eggs laid significantly correlated with the number of seeds per pod only in the Brittany
vs. Scotland experiment. In all cases, these effects are very weak (R
2 < 0.07).
Table 3.
Spearman’s rank order correlation coefficients between egg laying (or consumption) and Ulex europaeus’ pod (or flower) characteristics.
Table 3.
Spearman’s rank order correlation coefficients between egg laying (or consumption) and Ulex europaeus’ pod (or flower) characteristics.
| | Brit. vs. Reun. | Scot. vs. N. Z. | Brit. vs. Scot. |
|---|
| eggs per pod × pod length | +0.25 *** | +0.18 ** | +0.12 * |
| eggs per pod × seed per pod | +0.07 | +0.06 | +0.15 * |
| holes per flower × flower length | +0.03 | +0.06 | +0.32 *** |
3.2. Weevil Mass at Emergence and Pod Traits in the Common Garden
In the common garden, pod length was correlated with the number of seeds they contained (R = 0.28, N = 103, P = 0.004), with seed mass (R = 0.35, N = 105, P = 0.0002), and with total seed mass in a pod (seed number × seed mass; R = 0.42, N = 102, P<0.0001). Mass of adult weevils emerging from the pods in summer was positively correlated with seed number (males: R = 0.34, N = 94, P = 0.0009; females: R = 0.25, N = 93, P = 0.01), with seed mass only for males (males: R = 0.24, N = 93, P = 0.02; females: R = 0.15, N = 92, P = 0.15), and with total seed mass in a pod (males: R = 0.43, N = 92, P < 0.0001; females: R = 0.31, N = 91, P = 0.003).
3.3. Feeding Choice Experiments
After 24 h, a single flower exhibited between 0 and 36 new feeding holes. Flower consumption depended on both sex and regions, with females generally boring more holes than males. However, the interaction between sex and regions was not significant, indicating that both sexes made the same feeding choices (
Table 4). The results of the exact test of proportions gave similar significance levels as Chi-square tests (
p < 0.05 for the Brittany
vs. Reunion experiment,
P < 0.001 for the two other experiments).
Table 4.
Results of the X² tests for difference in feeding choice by Exapion ulicis on Ulex europaeus flowers.
Table 4.
Results of the X² tests for difference in feeding choice by Exapion ulicis on Ulex europaeus flowers.
| | | Brittany vs Reunion | Scotland vs New Zealand | Brittany vs Scotland |
|---|
| number of flowers * | 120 | 120 | 120 |
| number of holes * | 646 | 775 | 575 |
| holes per flower | X² | P | X² | P | X² | P |
| sex | 1.23 | NS | 64.82 | P<0.001 | 54.82 | P<0.001 |
| region | 14.06 | P < 0.05 | 54.23 | P<0.001 | 7.14 | P<0.01 |
| sex × region | 0.939 | NS | 0.903 | NS | 0.132 | NS |
Flowers from Brittany were significantly more consumed in both experiments they were involved in (
Figure 3). In the Scotland
vs. New Zealand choice experiment, flowers from New Zealand were more consumed than flowers from Scotland (
Figure 3).
Figure 3.
Consumption of U. europaeus flowers by the weevils E. ulicis. White bars: native regions, black bars: invaded regions. Significant differences after chi-squared test are indicated by stars: * P < 0.05, ** P < 0.01, *** P < 0.001. N = 60 flowers per region in each of the three experiments.
Figure 3.
Consumption of U. europaeus flowers by the weevils E. ulicis. White bars: native regions, black bars: invaded regions. Significant differences after chi-squared test are indicated by stars: * P < 0.05, ** P < 0.01, *** P < 0.001. N = 60 flowers per region in each of the three experiments.
Weevil feeding choice was independent of the number of holes present on the flowers before the experiment: the Spearman correlation between the number of holes made during the experiment (flower consumption) and the number of holes present before the experiment was non-significant in all three two-choice experiments (Brittany vs. Reunion: N = 120, RS = 0.03, P = 0.78; Scotland vs. New Zealand: N = 120, RS = 0.17, P = 0.06; Brittany vs. Scotland: N = 120, RS = 0.07, P = 0.45).
Flower length only differed between regions in the Brittany
vs. Scotland experiment, where flowers of Scottish gorses were significantly longer than flowers of gorses from Brittany (
Table 2). Consumption was correlated with flower length only in this same experiment, with longer flowers being more consumed (
Table 3), although this effect was weak (R
2 = 0.10).
4. Discussion
In the three pairwise comparisons, females of E. ulicis exhibited a clear preference, both for oviposition and feeding. However, the expression of their choice varied depending on cues detected at different times of the oviposition process, and the preferred region was not the same for oviposition and feeding.
4.1. Oviposition Choice
The number of eggs laid per pod depended on three decision steps. First, females will bore a hole or not, a decision that can only be taken from external cues. Second, females will use the hole to lay eggs or not. Indeed, most hole-borings did not result in egg-laying. Since hole-boring takes several hours and is probably costly in energy, the decision to lay eggs or not once a hole is dug is presumably taken from cues that could not be detected before, i.e., from internal cues. The third step is the number of eggs laid per hole. This last decision may depend both on internal and external cues.
Depending on the choice offered, the female’s oviposition preference resulted from different processes. In the Brittany vs. Reunion and Scotland vs. New Zealand choice experiments, females laid more eggs in pods from the two invaded regions, Reunion and New Zealand. However, the number of holes bored through the pod wall was similar, and the difference in egg number per hole resulted mainly from the proportions of holes with or without egg clusters. The female’s choice to lay eggs or not was thus taken after hole boring, i.e., from internal cues. On the contrary, in the Brittany vs. Scotland choice experiment, females’ oviposition preference for pods from Scotland was expressed as the number of holes per pod, which differed between the two regions while the number of eggs per hole was similar. The preference thus appeared to result from external cues detected by the females before hole digging. The capacity of E. ulicis females to make a choice at two different stages—before and after hole digging—clearly shows that they are able to use both external and internal pod cues when foraging for oviposition sites.
The assessment of fruit quality before oviposition was previously suggested for other weevil species (e.g., [
29,
30,
31,
32]). In
E.
ulicis, our results suggest that females tend to lay more eggs in the bigger pods and/or in pods with the most seeds. The adaptive value of such a preference was tested in the common garden by testing the correlation between the weight of emerging weevils (a trait generally related to insect fitness [
33,
34]) and pod size and content. We found that the weight of weevil emerging from a pod was positively correlated with both pod size and the number of seeds per pod. Ovipositing more in pods that are bigger and/or contains more seeds thus seems to be adaptive. However, the correlations we observed between pod length, seed number and eggs per pod were too weak to explain the differences observed between regions, so that other external and internal traits are likely to be involved (e.g., texture, volatile and nonvolatile chemicals [
5,
35]). Among the chemicals that could be involved, lupanine—a toxic alkaloid—is a good candidate, since it has been shown to reduce pod infestation rate by weevils in the common garden [
22,
36].
4.2. Role of the Origin of Plants in Oviposition and Feeding Preference
In the two experiments where female weevils were given the choice between pods from native and invaded regions (Brittany
vs. Reunion and Scotland
vs. New Zealand), they laid more eggs in pods from the invaded regions. This preference for pods from invaded regions compared to native ones may result from the loss of resistance cues in gorse from invaded regions, which is in agreement with theoretical expectations from the Enemy Release Hypothesis and the Evolution of Increased Competitive Ability Hypothesis [
16,
17]. Although, more regions should be used and more comparisons should be performed to fully explore the role of the region’s status (native or invasive) in the results obtained. Still, our results are in agreement with experimental observations in other species. Indeed, while relatively few studies have compared fruits’ defense/susceptibility among plant populations living with or without enemies, most found a decrease in defense and/or higher susceptibility in the absence of enemies [
37,
38,
39,
40].
In the experiments where female weevils were given the choice between pods from two native regions (Brittany and Scotland), gorses from both regions had been living in sympatry with
E.
ulicis weevils. Although processes such as genetic drift or indirect selection cannot be excluded, the differences observed likely result from local adaptation between gorses and weevils. Indeed, according to the theory of host-parasite coevolution [
11], local adaptation is expected to evolve in this system because: (i) the interaction between
U.
europaeus and
E.
ulicis is very specific [
19,
24]; and (ii) both species exert strong selective pressures on each other [
19,
20]. In that case, the preference of weevil females collected in Brittany for pods from Scotland suggests that the plants may defend more efficiently against local herbivores than against alien ones [
15]. Further exploration of this hypothesis would need reciprocal experiments to be done in Scotland.
4.3. Feeding Preference
The pattern observed for feeding preference was similar for both sexes, and differed from the pattern of oviposition preference. In particular, when Brittany was involved in a pairwise comparison, the weevils preferred to feed on flowers from Brittany, while they preferred to lay in pods from the other regions. This feeding preference could have been induced by the weevils’ past diets [
5,
41]. Indeed, the weevils used were collected on gorses from Brittany where they fed since their emergence. Testing this hypothesis further was not possible in the absence of weevils from different regions and reciprocal experiments.
In the Scotland
vs. New Zealand comparison, an interference with a local preference or past diet cannot occur, because the weevils used originated from neither of these two regions. In this experiment, the feeding preference resembled the oviposition preference: weevils of both sexes preferred to feed on flowers from the invaded region, as expected following Enemy Release [
16,
17].
Flower size may be involved in weevils’ choices, since weevils had a tendency to prefer bigger flowers. Yet, the effect of flower size was only observed in the Brittany/Scotland comparison, in which it was rather low and could not explain the direction of the observed preference (weevils fed more on flowers of gorses from Brittany, although they were on average smaller than flowers of Scottish gorses). Other attractants such as visual cues (e.g., flower spectral quality), feeding stimulants (e.g., sugars), and/or defensive compounds may have played a significant role in the choices observed.
5. Conclusions
By comparing
Exapion ulicis oviposition choice on gorses with different histories of coevolution with the weevil, we were able to find quantitative and qualitative differences in pod traits involved in the weevils’ choices. While further experiments would be necessary to precisely identify all of the cues involved, our results show that
E.
ulicis females use both internal and external cues in their oviposition choice, and that response to at least some of these cues is adaptive. However, the preferences observed here on isolated pods were different from those observed on isolated flowers, and did not explain the whole-plant infestation rate in the common garden [
27]. Together with previous studies, these results reveal that the foraging behaviour of
E.
ulicis weevils requires the capacity to respond to a wide variety of cues: whole-plant traits [
21], flower traits, internal and external pod cues (this study), and cues indicating the presence of conspecifics [
26] or the presence of the competitor
Cydia succedana [
19]. It would be interesting to apply more widely the theoretical framework developed for parasitoid wasp oviposition [
6], to study the evolution of the interaction between phytophagous insects—especially those whose larvae develop at the expense of seeds—and their host plant. The context of enemy release frequently encountered in invasive plant species offers a good opportunity for further exploration in that direction.


 .
.  is therefore:
is therefore:


 a Student-Snedecor distribution (as insured by a sum of more than five binomial random variables). A preference for a region was declared, with risk α, when this confidence interval did not include A5. The accuracy of formula (A6) was verified by simulation, using R random generators.
a Student-Snedecor distribution (as insured by a sum of more than five binomial random variables). A preference for a region was declared, with risk α, when this confidence interval did not include A5. The accuracy of formula (A6) was verified by simulation, using R random generators.