Challenges for Managing Fisheries on Diverse Coral Reefs
Abstract
:1. Introduction
2. Resilience
2.1. Climate Change
2.2. Reef Resilience
2.3. Apex Predators

2.4. The Role of Herbivores
2.5. Nutrients
2.6. Phase Shifts and Herbivores
3. Fisheries Stock Assessment and Diversity
3.1. Conventional Stock Assessment

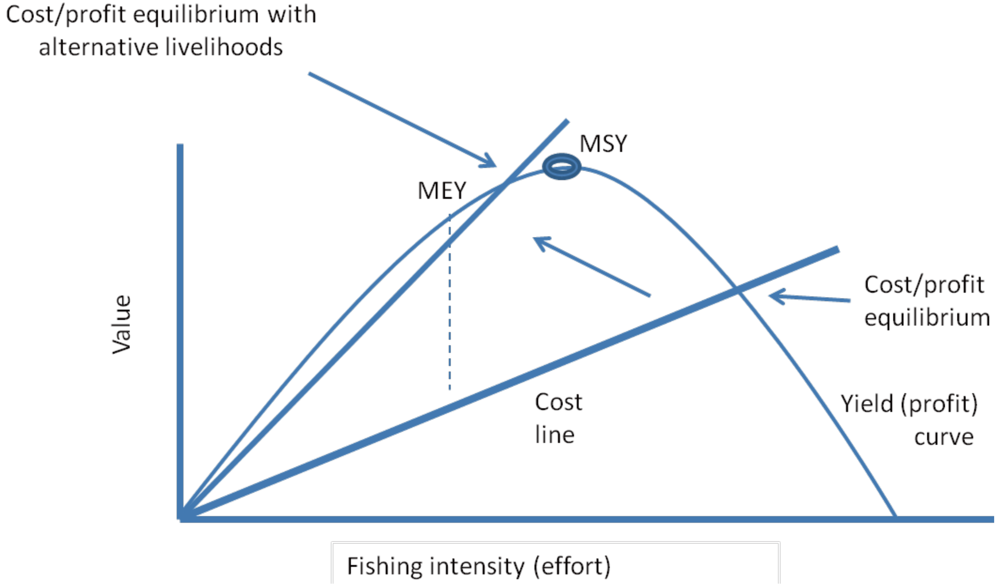
3.2. Catch per Unit Effort
3.3. Proportion of Stock Caught
3.4. Proportion of Catch Reproductive
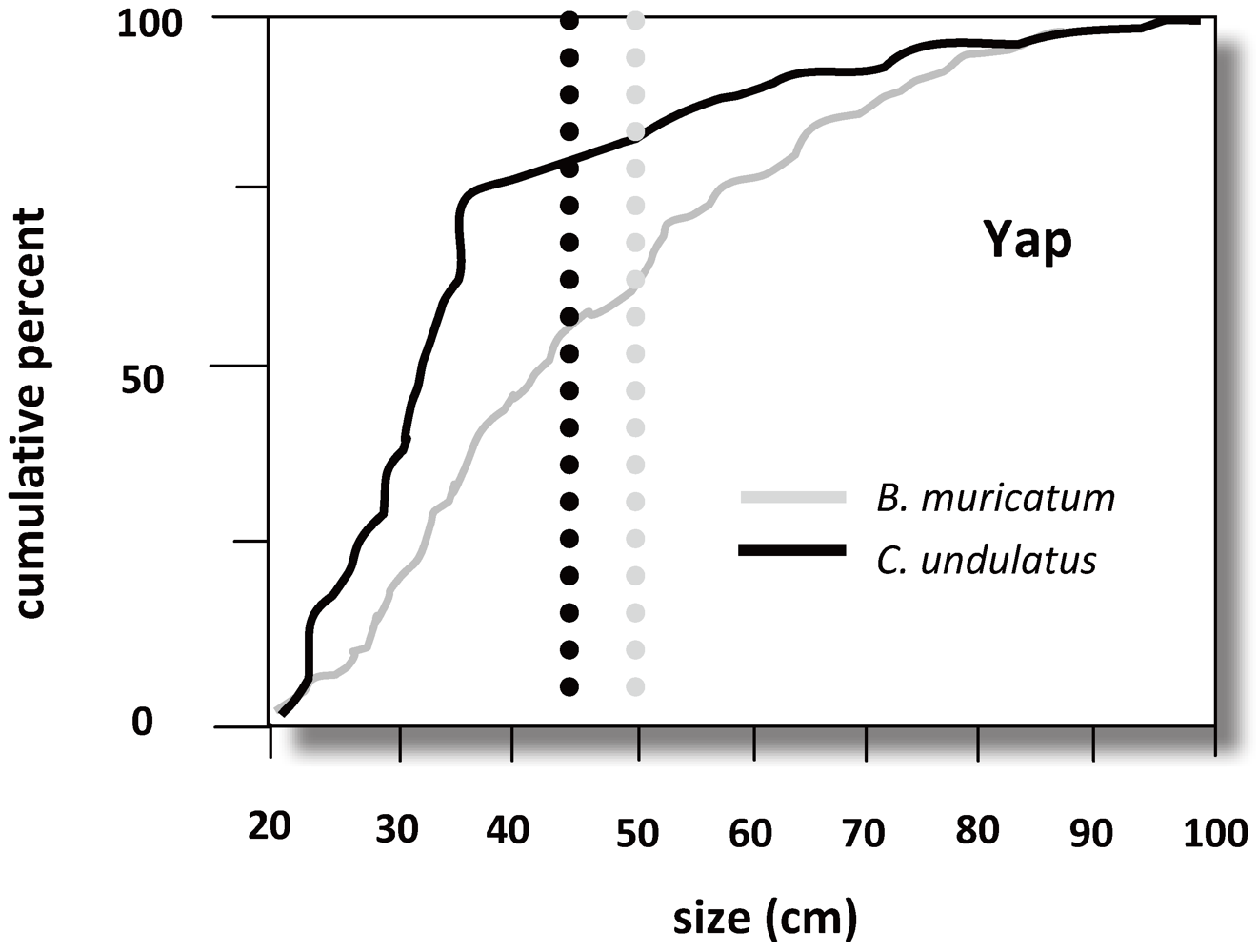
3.5. Trophic Level
3.6. Virgin Stocks
3.7. NTA Control

3.8. Large Fish Abundances
4. Fisheries Management Tools
4.1. Effort and/or Catch
4.2. Incentives, Rights-based Management and Individual Transferable Quotas
4.3. Ecosystem Approach
4.4. Alternative Income
4.5. Spatial Closures: MPAs and NTAs
4.6. Dataless Management and Traditional Ecological Knowledge (TEK)
4.7. Customary Marine Tenure (CMT)
5. Conclusions
Acknowledgments
References
- Brown, B.E. Worldwide death of corals: Natural cyclic events or man-made pollution? Mar. Pollut. Bull. 1987, 18, 9–13. [Google Scholar] [CrossRef]
- Goreau, T. Bleaching and reef community change in Jamaica, 1951–1991. Am. Zool. 1992, 32, 683–695. [Google Scholar]
- Salvat, B. Coral reefs—A challenging ecosystem for human societies. Glob. Enviro. Change 1992, 2, 12–18. [Google Scholar]
- Wilkinson, C.R. Coral reefs of the world are facing widespread devastation: can we prevent this through sustainable management practices? In Proceedings of the 7th International Coral Reef Symposium, Guam, Micronesia, 22–27 June 1992; 1, pp. 11–21.
- Richmond, R.H. Coral reefs: Present problems and future concerns resulting from anthropogenic disturbance. Am. Zool. 1993, 33, 524–536. [Google Scholar]
- Grigg, R.W. Effects of sewage discharge, fishing pressure, and habitat complexity on coral ecosystems and reef fishes in Hawaii. Mar. Ecol. Prog. Ser. 1994, 103, 25–34. [Google Scholar] [CrossRef]
- Hughes, T.P. Catastrophes, phase shifts, and large-scale degradation of a Caribbean coral reef. Science 1994, 265, 1547–1551. [Google Scholar]
- Sebins, K. Biodiversity of coral reefs: What we are loosing and why? Am. Zool. 1994, 34, 115–133. [Google Scholar]
- Jackson, J.B.C. Reefs since Columbus. Coral Reefs 1997, 16, S23–S32. [Google Scholar]
- Hodgson, G. A global assessment of human effects on coral reefs. Mar. Pollut. Bull. 1999, 38, 345–355. [Google Scholar]
- Risk, M.J. Paradise lost: How marine science failed the world’s coral reefs. Mar. Freshw. Res. 1999, 50, 831–837. [Google Scholar]
- Wilkinson, C. Global and local threats to coral reef functioning and existence: Review and predictions. Mar. Freshw. Res. 1999, 50, 867–878. [Google Scholar]
- Birkeland, C. The future of coral reefs. Galaxea JCRS 2000, 2, 12–16. [Google Scholar]
- Barber, R.T.; Hilting, A.K.; Hayes, M.L. The changing health of coral reefs. Hum. Ecol. Risk Assess. 2001, 7, 1255–1270. [Google Scholar]
- Knowlton, N. The future of coral reefs. Proc. Natl. Acad. Sci. USA 2001, 98, 5419–5425. [Google Scholar]
- Friedlander, A.M.; DeMartini, E.E. Contrasts in density, size, and biomass of reef fishes between the northwestern and main Hawaiian Islands: The effects of fishing down apex predators. Mar. Ecol. Prog. Ser. 2002, 230, 253–264. [Google Scholar] [CrossRef]
- McClanahan, T.R. The near future of coral reefs. Environ. Conserv. 2002, 29, 460–483. [Google Scholar]
- Sale, P.F. The science we need to develop for more effective management. In Coral Reef Fisheries, Dynamics and Diversity in a Complex Ecosystem; Sale, P.F., Ed.; Academic Press: London, UK, 2002; pp. 361–376. [Google Scholar]
- Sheppard, C.R.C. Predicted recurrences of mass coral mortality in the Indian Ocean. Nature 2003, 425, 294–297. [Google Scholar]
- Hughes, T.P.; Baird, A.H.; Bellwood, D.R.; Card, M.; Connolly, S.R.; Floke, C.; Grosberg, R.; Hoegh-Guldberg, O.; Jackson, J.B.C.; Kleypas, J.; et al. Climate change, human impacts, and the resilience of coral reefs. Science 2003, 301, 929–933. [Google Scholar]
- Bellwood, D.R.; Hughes, T.P.; Folke, C.; Nystrom, M. Confronting the coral reef crisis. Nature 2004, 429, 827–833. [Google Scholar]
- Birkeland, C. Ratcheting down the coral reefs. Bioscience 2004, 54, 1021–1027. [Google Scholar]
- Robbins, W.D.; Hisano, M.; Connolly, S.R.; Choat, J.H. Ongoing collapse of coral-reef shark populations. Curr. Biol. 2006, 16, 2314–2319. [Google Scholar]
- Wilkinson, C. Status of coral reefs of the world: Summary of threats and remedial action. In Coral Reef Conservation; Côté, I.M., Reynolds, J.D., Eds.; Cambridge University Press: Cambridge, UK, 2006; pp. 3–39. [Google Scholar]
- Hughes, T.P.; Rodrigues, M.J.; Bellwood, D.R.; Ceccarelli, D.; Hoegh-Guldberg, O.; McCook, L.; Moltschaniwskyj, N.; Pratchett, M.G.; Steneck, R.S.; Willis, B. Phase shifts, herbivory, and the resilience of coral reefs to climate change. Curr. Biol. 2007, 17, 1–6. [Google Scholar]
- Newton, K.; Côté, I.M.; Pilling, G.M.; Jennings, S.; Dulvy, N.K. Current and future sustainability of island coral reef fisheries. Curr. Biol. 2007, 17, 655–658. [Google Scholar]
- Carpenter, K.E.; Abrar, M.; Aeby, G.; Aronson, R.; Banks, S.; Bruckner, A.; Chirboga, A.; Cortés, J.; Delbeek, C.; DeVantier, L.; et al. One third of reef buildingcorals face elevated extinction risk from climate change and local impacts. Science 2008, 321, 560–563. [Google Scholar]
- Jackson, J.B.C. Ecological extinction and evolution in the brave new ocean. Proc. Natl. Acad. Sci. USA 2008, 105, 11458–11465. [Google Scholar]
- Knowlton, N.; Jackson, J.B.C. Shifting baselines, local impacts, and global change on coral reefs. PLoS Biol. 2008, 6. [Google Scholar] [CrossRef]
- Lough, J.M. 10th Anniversary review: A changing climate for coral reefs. J. Environ. Monit. 2008, 10, 21–29. [Google Scholar]
- Veron, J.E.N. A Reef in Time; Belknap Press of Harvard University Press: Cambridge, UK, 2008; p. 289. [Google Scholar]
- Fenner, D. The largest reef fish species were gone most places in the world even before scientists knew about it. 2009. Available online: http://independent.academia.edu/DouglasFenner (accessed on 7 March 2012).
- Hardt, M.J. Lessons from the past: The collapse of Jamaican coral reefs. Fish Fish. 2009, 10, 143–158. [Google Scholar]
- McClenachan, L. Documenting loss of large trophy fish from the Florida Keys with historical photographs. Conserv. Biol. 2009, 23, 636–643. [Google Scholar]
- Riegl, B.; Bruckner, A.; Coles, S.L.; Renaud, P.; Dodge, R.E. Coral reefs: Threats and conservation in an era of global change. Ann. N. Y. Acad. Sci. 2009, 1162, 136–186. [Google Scholar]
- Graham, N.A.J.; Spalding, N.D.; Sheppard, C.R.C. Reef shark declines in remote atolls highlight the need for multi-faceted conservation action. Aquat. Conserv. Mar. Fresh. Ecosyst. 2010, 20, 543–548. [Google Scholar]
- Hay, M.E.; Rasher, D.B. Corals in crisis, marine protected areas reduce coral loss, but they are not enough. Scientist 2010, 24, 42–50. [Google Scholar]
- Ward-Paige, C.A.; Mora, C.; Lotze, H.K.; Pattengill-Semmens, C.; McClenachan, L.; Arias-Castro, E.; Myers, R.A. Large-scale absence of sharks on reefs in the greater-Caribbean: A footprint of human pressures. PLoS One 2010, 5. [Google Scholar] [CrossRef]
- Mora, C.; Aburto-Oropeza, O.; Bocos, A.A.; Ayotte, P.M.; Banks, S.; Bauman, A.G.; Beger, M.; Bessudo, S.; Booth, D.J.; Brokovich, E.; et al. Global human footprint on the linkage between biodiversity and ecosystem functioning in reef fishes. PLoS Biol. 2011, 9. [Google Scholar] [CrossRef]
- Gardner, T.A.; Côté, I.M.; Gill, J.A.; Grant, A.; Watkinson, A.R. Long-term region-wide declines in Caribbean corals. Science 2003, 301, 958–960. [Google Scholar]
- Pandolfi, J.M.; Bradbury, R.H.; Sala, E.; Hughes, T.P.; Bjorndal, K.A.; Cooke, R.G.; McArdle, D.; McClenchan, L.; Newman, M.J.H.; Paredes, G.; et al. Global trajectories of the long-term decline of coral reef ecosystems. Science 2003, 301, 955–957. [Google Scholar]
- Pandolfi, J.M.; Jackson, J.B.C.; Baron, N.; Bradbury, R.H.; Guzman, H.M.; Hughes, T.P.; Kappel, C.V.; Micheli, F.; Ogden, J.C.; Possingham, H.P.; et al. Are U.S. coral reefs on the slippery slope to slime? Science 2005, 307, 1725–1726. [Google Scholar]
- Côté, I.M.; Gardner, T.A.; Gill, J.A.; Hutchinson, D.J.; Watkinson, A.R. New approaches to estimating recent ecological changes on coral reefs. In Coral Reef Conservation; Côté, I.M., Reynolds, J.D., Eds.; Cambridge University Press: Cambridge, UK, 2006; pp. 293–313. [Google Scholar]
- Bruno, J.F.; Selig, E.R. Regional decline of coral cover in the Indo-Pacific: Timing, extent, and subregional comparisons. PLoS One 2007, 2. [Google Scholar] [CrossRef]
- Alvarez-Filip, L.; Dulvy, N.K.; Gill, J.A.; Côté, I.M.; Watkinson, A.R. Flattening of Caribbean coral reefs: Region-wide declines in architectural complexity. Proc. R. Soc. Lond. B 2009, 276, 3019–3025. [Google Scholar]
- Paddack, M.J.; Reynolds, J.D.; Aguilar, C.; Appeldoorn, R.S.; Beets, J.; Burkett, E.W.; Chittaro, P.M.; Clarke, K.; Esteves, R.; Fonesca, A.C.; et al. Recent region-wide declines in Caribbean reef fish abundance. Curr. Biol. 2009, 19, 590–596. [Google Scholar]
- Sweatman, H.; Delean, S.; Syms, C. Assessing loss of coral cover on Australia’s Great Barrier Reef over two decades, with implications for longer term-trends. Coral Reefs 2011, 30, 521–531. [Google Scholar]
- Hughes, T.P.; Bellwood, D.R.; Baird, A.H.; Brodie, J.; Bruno, J.F.; Pandolfi, J.M. Shifting baselines, declining coral cover, and the erosion of reef resilience: Comment on Sweatman et al. (2011). Coral Reefs 2011, 30, 653–660. [Google Scholar] [CrossRef]
- Sweatman, H.; Syms, C. Assessing loss of coral cover on the Great Barrier Reef: A response to Hughes et al. (2011). Coral Reefs 2011, 30, 661–664. [Google Scholar] [CrossRef]
- Ridd, P.V. A critique of a method to determine long-term declines of coral reef ecosystems. Energy Environ. 2007, 18, 783–796. [Google Scholar]
- Veron, J.E.N. Corals of the World; Australian Institute of Marine Science: Townsville, Australia, 2000; Volume 1–3. [Google Scholar]
- Birkeland, C. Value of reefs. In Life and Death of Coral Reefs; Birkeland, C., Ed.; Chapman and Hall: New York, NY, USA, 1997; pp. 2–6. [Google Scholar]
- Moberg, F.; Folke, C. Ecological goods and services of coral reef ecosystems. Ecol. Econ. 1999, 29, 215–233. [Google Scholar]
- Burke, L.; Reytar, K.; Spalding, M.; Perry, A. Reefs at Risk Revisited; World Resources Institute: Washington, DC, USA, 2011. [Google Scholar]
- Donner, S.D.; Potere, D. The inequity of the global threat to coral reefs. Bioscience 2007, 57, 214–215. [Google Scholar]
- Hutchings, J.A. Spatial and temporal variation in the density of northern cod and a review of hypotheses for the stock’s collapse. Can. J. Fish. Aquat. Sci. 1996, 53, 943–962. [Google Scholar]
- Myers, R.A.; Hutchings, J.A.; Barrowman, N.J. Why do fish stocks collapse? The example of cod in Atlantic Canada. Ecol. Appl. 1997, 7, 91–106. [Google Scholar]
- Hutchings, J.A. Collapse and recovery of marine fishes. Nature 2000, 406, 882–885. [Google Scholar]
- Mace, P.M. In defense of fishery scientists, single-species models and other scapegoats: Confronting the real problems. Mar. Ecol. Prog. Ser. 2004, 274, 285–291. [Google Scholar]
- Jackson, J.B.C.; Kirby, M.X.; Berger, W.H.; Bjorndal, K.A.; Bostford, L.W.; Bourque, B.J.; Bradbury, R.H.; Cooke, R.; Erlandson, J.; Estes, J.A.; et al. Historical overfishing and the recent collapse of coastal ecosystems. Science 2001, 293, 629–637. [Google Scholar]
- Pauly, D.; Christensen, V.; Guénette, S.; Pitcher, T.J.; Sumaila, U.R.; Walters, C.J.; Watson, R.; Zeller, D. Towards sustainability in world fisheries. Nature 2002, 418, 689–695. [Google Scholar]
- Anderson, S.C.; Flemming, J.M.; Watson, R.; Lotze, H.K. Serial depletion of global sea cucumber fisheries. Fish Fish. 2011, 12, 317–339. [Google Scholar]
- Myers, R.A.; Worm, B. Rapid worldwide depletion of predatory fish communities. Nature 2003, 423, 280–283. [Google Scholar]
- Rosenberg, A.A. Managing to the margins: The overexploitation of fisheries. Front. Ecol. Environ. 2003, 1, 102–106. [Google Scholar]
- Gewin, V. Troubled waters: The future of global fisheries. PLoS Biol. 2004, 2. [Google Scholar] [CrossRef]
- Worm, B.; Barbier, E.B.; Beaumont, N.; Duffy, E.J.; Folke, C.; Halpern, B.S.; Jackson, J.B.C.; Lotze, H.K.; Micheli, F.; Palumbi, S.R.; et al. Impacts of biodiversity loss on ocean ecosystem services. Science 2006, 314, 787–790. [Google Scholar]
- Hilborn, R. Reinterpreting the state of fisheries and their management. Ecosystems 2007, 10, 1362–1369. [Google Scholar]
- Pitcher, T.J. Fisheries managed to rebuild ecosystems? Reconstructing the past to salvage the future. Ecol. Appl. 2001, 11, 601–617. [Google Scholar]
- Rosenberg, A.A.; Swasey, J.H.; Bowman, M. Rebuilding US fisheries: Progress and problems. Front. Ecol. Environ. 2006, 4, 303–308. [Google Scholar]
- Worm, B.; Hilborn, R.; Baum, J.K.; Branch, T.A.; Collie, J.S.; Costello, C.; Fogarty, M.J.; Fulton, E.A.; Hutchings, J.A.; Jennings, S.; et al. Rebuilding global fisheries. Science 2009, 325, 578–585. [Google Scholar]
- Molloy, P.P.; McLean, I.B.; Côté, I.M. Effects of marine reserve age on fish populations: A global meta-analysis. J. Appl. Ecol. 2009, 46, 743–751. [Google Scholar]
- Roberts, C.M. Effects of fishing on the ecosystem structure of coral reefs. Conserv. Biol. 1995, 9, 988–995. [Google Scholar]
- Munro, J.L. The scope of tropical reef fisheries and their management. In Reef Fisheries; Polunin, N.V.C., Roberts, C.M., Eds.; Chapman & Hall: London, UK, 1996; pp. 1–14. [Google Scholar]
- Dulvy, N.K.; Polunin, N.V.C. Using informal knowledge to infer human-induced rarity of a conspicuous reef fish. Anim. Conserv. 2004, 7, 365–374. [Google Scholar]
- Dalzell, P.; Adams, T.J.H.; Polunin, N.V.C. Coastal fisheries in the Pacific Islands. Oceanogr. Mar. Biol. Ann. Rev. 1996, 34, 395–531. [Google Scholar]
- Hoegh-Guldberg, O. Climate change, coral bleaching and the future of the world’s coral reefs. Mar. Freshw. Res. 1999, 50, 839–866. [Google Scholar]
- Veron, J.E.N.; Hoegh-Guldberg, O.; Lenton, T.M.; Lough, J.M.; Obura, D.O.; Pearce-Kelly, P.; Sheppard, C.R.C.; Spalding, M.; Stafford-Smith, M.G.; Rogers, A.D. The coral reef crisis, the critical importance of <350 ppm CO2. Mar. Pollut. Bull. 2009, 58, 1428–1436. [Google Scholar]
- Goreau, T.J.; Hayes, R.L. Coral bleaching and ocean “Hot Spots”. Ambio 1994, 23, 176–180. [Google Scholar]
- Goreau, T.J.; Hayes, R.L.; Strong, A.C. 1997, Tracking South Pacific coral reef bleaching by satellite and field observations. In Proceedings of the 8th International Coral Reef Symposium, Panama, 24–29 June 1997; 2, pp. 1491–1494.
- Berkelmans, R. Time-integrated thermal bleaching thresholds of reefs and their variation on the Great Barrier Reef. Mar. Ecol. Prog. Ser. 2002, 229, 73–82. [Google Scholar]
- Liu, G.; Strong, A.E.; Skirving, W. Remote sensing of sea surface temperatures during 2002 Barrier Reef coral bleaching. EOS Trans. 2003, 84, 137–141. [Google Scholar]
- Goreau, T.J.; Hayes, R.L. Global coral reef bleaching and sea surface temperature trends from satellite-derived Hotspot analysis. World Resour. Rev. 2005, 17, 254–293. [Google Scholar]
- Manzello, D.P.; Berkelmans, R.; Hendee, J.C. Coral bleaching indices and thresholds for the Florida Reef Tract, Bahamas, and St. Croix, US Virgin Islands. Mar. Pollut. Bull. 2007, 54, 1923–1931. [Google Scholar] [CrossRef]
- Wilkinson, C.; Linden, O.; Cesar, H.; Hodgson, G.; Rubens, J.; Strong, A.E. Ecological and socioeconomic impacts of 1998 coral mortality in the Indian Ocean: An ENSO impact and a warning of future change? Ambio 1999, 28, 188–196. [Google Scholar]
- Donner, S.D.; Skirving, W.J.; Little, C.M.; Oppenheimer, M.; Hoegh-Guldberg, O. Global assessment of coral bleaching and required rates of adaptation under climate change. Glob. Change Biol. 2005, 11, 2251–2265. [Google Scholar]
- Donner, S.D. Coping with commitment: Projected thermal stress on coral reefs under future scenarios. PLoS One 2009, 4. [Google Scholar] [CrossRef]
- Fenner, D.; Heron, S. Annual summer mass bleaching of a multi-species coral community in American Samoa. In Proceedings of the 11th International Coral Reef Symposium, Ft. Lauderdale, FL, USA, 7–11 July 2008; pp. 1289–1293.
- Nakamura, T.; van Woesik, R. Water-flow rates and passive diffusion partially explain differential survival of corals during the 1998 bleaching event. Mar. Ecol. Prog. Ser. 2001, 212, 301–304. [Google Scholar]
- Gunderson, L.H. Resilience in theory and practice. Ann. Rev. Ecol. Syst. 2000, 31, 425–439. [Google Scholar]
- West, J.M.; Salm, R.V. Resistance and resilience to coral bleaching: Implications for coral reef conservation and management. Conserv. Biol. 2003, 17, 956–968. [Google Scholar]
- Marshall, P.; Schuttenberg, H. A Reef Manager’s Guide to Coral Bleaching; Great Barrier Reef Marine Park Authority: Townsville, Australia, 2006. [Google Scholar]
- Nyström, M.; Graham, N.A.J.; Lokrantz, J.; Norström, A.V. Capturing the cornerstones of coral reef resilience: Linking theory to practice. Coral Reefs 2008, 27, 795–809. [Google Scholar]
- Seymour, R.M.; Bradbury, R.H. Lengthening reef recovery times from crown-of-thorns outbreaks signal systemic degradation of the Great Barrier Reef. Mar. Ecol. Prog. Ser. 1999, 176, 1–10. [Google Scholar]
- Done, T.J.; de Vantier, L.M.; Turak, E.; Fisk, D.A.; Wakeford, M.; van Woesik, R. Coral growth on three reefs: Development of recovery benchmarks using a space for time approach. Coral Reefs 2010, 29, 815–833. [Google Scholar]
- Carilli, J.E.; Norris, R.D.; Black, B.A.; Walsh, S.M.; McField, M. Local stressors reduce coral resilience to bleaching. PLoS One 2009, 4. [Google Scholar] [CrossRef]
- Carilli, J.E.; Norris, R.D.; Black, B.; Walsh, S.M.; McField, M. Century-scale records of coral growth rates indicate that local stressors reduce coral thermal tolerance threshold. Glob. Change Biol. 2010, 16, 1247–1257. [Google Scholar]
- Fung, T.; Seymour, R.M.; Johnson, C.R. Alternative stable states and phase shifts in coral reefs under anthropogenic stress. Ecology 2011, 92, 967–982. [Google Scholar]
- Sweatman, H. No-take reserves protect coral reefs from predatory starfish. Curr. Biol. 2008, 18, 598–599. [Google Scholar]
- Dulvy, N.K.; Feckleton, R.P.; Polunin, N.V.C. Coral reef cascades and the indirect effects of predator removal by exploitation. Ecol. Lett. 2004, 7, 410–416. [Google Scholar]
- Birkeland, C. Personal Communication. Honolulu, HI, USA, 2011. [Google Scholar]
- Brodie, J.; Fabricius, K.; De'ath, G.; Okaji, K. Are increased nutrient inputs responsible for more outbreaks of crown-of-thorns starfish? An appraisal of the evidence. Mar. Pollut. Bull. 2005, 51, 266–278. [Google Scholar]
- Raymundo, L.J.; Halford, A.R.; Maypa, A.P.; Kerr, A.M. Functionally diverse reef-fish communities ameliorate coral disease. Proc. Natl. Acad. Sci. USA 2009, 106, 17067–17070. [Google Scholar]
- Mumby, P.J.; Harborne, A.R. Marine reserves enhance the recovery of corals on Caribbean reefs. PLoS One 2010, 5. [Google Scholar] [CrossRef]
- Selig, E.R.; Bruno, J.F. A global analysis of the effectiveness of marine protected areas in preventing coral loss. PLoS One 2010, 5. [Google Scholar] [CrossRef]
- Stevenson, C.; Katz, L.S.; Micheli, L.F.; Block, B.; Heiman, K.W.; Perle, C.; Weng, K.; Dunbar, R.; Witting, J. High apex predator biomass on remote Pacific Islands. Coral Reefs 2006, 26, 47–51. [Google Scholar]
- Sandin, S.A.; Smith, J.E.; DeMaartini, E.E.; Dinsdale, E.A.; Donner, S.D.; Friedlander, A.M.; Konotchik, T.; Malay, M.; Maragos, J.E.; Obura, D.; et al. Baselines and degradation of coral reefs in the Northern Line Islands. PLoS One 2008, 3. [Google Scholar] [CrossRef]
- Williams, I.D.; Richards, B.L.; Sandin, S.A.; Baum, J.K.; Schroeder, R.E.; Nadon, M.O.; Zgliczynski, B.; Craig, P.; McIlwain, J.L.; Brainard, R.E. Differences in reef fish assemblages between populated and remote reefs spanning multiple archipelagos across the Central and Western Pacific. J. Mar. Biol. 2011, 2011, 1–14. [Google Scholar]
- Kaufman, L.; Obura, D.; Rohwer, F.; Sala, E.; Sandin, S.; Tschirky, J. Coral Health Index (CHI): Measuring Coral Community Health; Science and Knowledge Division, Conservation International: Arlington, VA, USA, 2011. [Google Scholar]
- Pauly, D.; Christensen, V.; Dalsgaard, J.; Froese, R.; Torres, F. Fishing down marine food webs. Science 1998, 279, 860–863. [Google Scholar]
- Bellwood, D.R.; Hoey, A.S.; Choat, J.H. Limited functional redundancy in high diversity systems: Resilience and ecosystem function on coral reefs. Ecol. Lett. 2003, 6, 281–285. [Google Scholar]
- Sadovy, Y.; Kulbicki, M.; Labrosse, P.; Letourneur, Y.; Lokani, P.; Donaldson, T.J. The humphead wrasse, Cheilinus undulatus: Synopsis of a threatened and poorly known giant coral reef fish. Rev. Fish Biol. Fish. 2003, 13, 327–364. [Google Scholar] [CrossRef]
- Pauly, D. Anecdotes and the shifting baseline syndrome of fisheries. Trend. Ecol. Evol. 1995, 10, 430. [Google Scholar]
- Sheppard, C. The shifting baseline syndrome. Mar. Pollut. Bull. 1995, 30, 766–767. [Google Scholar]
- Ricker, W.E. Production and utilization of fish populations. Ecol. Monogr. 1946, 16, 373–391. [Google Scholar]
- Russ, G.R. Coral reef fisheries: Effects and yields. In The Ecology of Fishes on Coral Reefs; Sale, P.F., Ed.; Academic Press: New York, NY, USA, 1991; pp. 601–635. [Google Scholar]
- Jennings, S.; Kaiser, M.J. The effects of fishing on marine ecosystems. Adv. Mar. Biol. 1998, 34, 201–352. [Google Scholar]
- Jennings, S.; Reynolds, J.D.; Polunin, N.V.C. Predicting the vulnerability of tropical reef fishes to exploitation with phylogenies and life histories. Conserv. Biol. 1999, 13, 1466–1475. [Google Scholar]
- Dulvy, N.K.; Polunin, N.V.C.; Mill, A.C.; Graham, N.A.J. Size structure change in lightly exploited coral reef fish communities: Evidence for weak indirect effects. Can. J. Fish. Aquat. Sci. 2004, 61, 466–475. [Google Scholar]
- Heino, M.; Godo, O.R. Fisheries-induced selection pressures in the context of sustainable fisheries. Bull. Mar. Sci. 2002, 70, 639–656. [Google Scholar]
- Clua, E.; Legendre, P. Shifting dominance among Scarid species on reefs representing a gradient of fishing pressure. Aquat. Living Res. 2008, 21, 339–348. [Google Scholar]
- Scheffer, M.; Carpenter, S.; de Young, B. Cascading effects of overfishing marine systems. Trends Ecol. Evol. 2005, 20, 579–581. [Google Scholar]
- Heithaus, M.R.; Frid, A.; Wirsing, A.J.; Worm, B. Predicting ecological consequences of marine top predator declines. Trends Ecol. Evol. 2007, 23, 202–210. [Google Scholar]
- Estes, J.A.; Terborgh, J.; Brashares, J.S.; Power, M.E.; Berger, J.; Bond, W.J.; Carpenter, S.R.; Essington, T.E.; Holt, R.D.; Jackson, J.B.C.; et al. Trophic downgrading of planet earth. Science 2011, 333, 301–306. [Google Scholar]
- McClanahan, T.R.; Shafir, S.H. Causes and consequences of sea urchin abundance and diversity in Kenyan coral reef lagoons. Oecologia 1990, 83, 32–370. [Google Scholar]
- O’Leary, J.K.; McClanahan, T.R. Trophic cascades result in large-scale coralline algae loss through differential grazer loss. Ecology 2010, 91, 3584–3597. [Google Scholar]
- Graham, N.A.J.; Evans, R.D.; Russ, G.R. The effects of marine reserve protection on the trophic relationships of reef fishes on the Great Barrier Reef. Envir. Conserv. 2003, 30, 200–208. [Google Scholar]
- Pinnegar, J.K.; Polunin, N.V.C.; Francour, P.; Badalamenti, F.; Chemello, R.; Harmelin-Vivian, M.-L.; Hereu, B.; Milazzo, M.; Zabala, M.; D’anna, G.; et al. Trophic cascades in benthic marine ecosystems: Lessons for fisheries and protected-area management. Environ. Conserv. 2000, 27, 179–200. [Google Scholar] [CrossRef]
- Mumby, P.J.; Dahlgren, C.P.; Harborne, A.R.; Kappel, C.V.; Micheli, F.; Brumbaugh, D.R.; Holmes, K.E.; Mendes, J.M.; Broad, K.; Sanchirico, J.N.; et al. Fishing, trophic cascades, and the process of grazing on coral reefs. Science 2006, 311, 98–101. [Google Scholar]
- Stallings, C.D. Indirect effects of an exploited predator on recruitment of coral-reef fishes. Ecology 2008, 89, 2090–2095. [Google Scholar]
- Newman, M.J.H.; Paredes, G.A.; Sala, E.; Jackson, J.B.C. Structure of Caribbean coral reef communities across a large gradient of fish biomass. Ecol. Lett. 2006, 9, 1216–1227. [Google Scholar]
- Aronson, R.B.; Precht, W.F. Conservation, precaution, and Caribbean reefs. Coral Reefs 2006, 25, 441–450. [Google Scholar] [CrossRef]
- Bruno, J.F.; Sweatman, H.; Precut, W.F.; Selig, E.R.; Schutte, V.G.W. Assessing evidence of phase shifts from coral to macroalgal dominance on coral reefs. Ecology 2009, 90, 1478–1484. [Google Scholar]
- Woodley, J.D.; Chornesky, E.A.; Clifford, P.A.; Jackson, J.B.C.; Kaufman, L.S.; Knowlton, N.; Lang, J.C.; Pearson, M.P.; Porter, J.W.; Rooney, M.C.; et al. Hurricane Allen’s impact on Jamaican coral reefs. Science 1981, 214, 749–755. [Google Scholar]
- Lessios, H.A.; Robertson, D.R.; Cubit, D.J. Spread of Diadema mass mortality through the Caribbean. Science 1984, 226, 335–337. [Google Scholar]
- Norström, A.V.; Nyström, M.; Lokrantz, J.; Folke, C. Alternative states on coral reefs: Beyond coral-macroalgal phase shifts. Mar. Ecol. Prog. Ser. 2009, 376, 295–306. [Google Scholar]
- Vargas-Ángel, B.; Asher, J.; Godwin, L.S.; Brainard, R.E. Invasive didemnid tunicate spreading across coral reefs at remote Swains Island, American Samoa. Coral Reefs 2009, 28, 53. [Google Scholar]
- Aronson, R.B.; MacIntyre, I.G.; Wapnick, C.M.; O’Neill, M.W. Phase shifts, alternative states, and the unprecedented convergence of two reef systems. Ecology 2004, 85, 1876–1891. [Google Scholar] [CrossRef]
- Pastorok, R.A.; Bilyard, G.R. Effects of sewage pollution on coral-reef communities. Mar. Ecol. Prog. Ser. 1985, 21, 175–189. [Google Scholar]
- Lapointe, B.E. Nutrient thresholds for bottom-up control of macroalgal blooms on coral reefs in Jamaica and southeast Florida. Limnol. Oceanogr. 1997, 42, 1119–1131. [Google Scholar]
- Hunter, C.L.; Evans, C.W. Coral reefs in Kaneohe Bay, Hawaii: Two centuries of western influence and two decades of data. Bull. Mar. Sci. 1995, 57, 501–515. [Google Scholar]
- De Georges, A.; Goreau, T.J.; Reilly, B. Land-sourced pollution with an emphasis on domestic sewage: Lessons from the Caribbean and implications for coastal development on Indian Ocean and Pacific coral reefs. Sustainability 2010, 2, 2919–2949. [Google Scholar]
- Smith, J.E.; Hunter, C.L.; Smith, C.M. The effects of top-down versus bottom-up control on benthic coral reef community structure. Oecologia 2010, 163, 497–507. [Google Scholar]
- Mora, C. A clear human footprint in the coral reefs of the Caribbean. Proc. Roy. Soc. B 2008, 275, 767–773. [Google Scholar]
- Szmant, A.M. Nutrient enrichment on coral reefs: Is it a major cause of coral reef decline? Estuaries 2002, 25, 743–766. [Google Scholar] [CrossRef]
- Heck, K.L.; Valentine, J.F. The primacy of top-down effects in shallow benthic ecosystems. Estuaries Coasts 2007, 30, 371–381. [Google Scholar]
- Renken, H.; Mumby, P.J. Modeling the dynamics of coral reef macroalgae using a Bayesian belief network approach. Ecol. Mod. 2009, 220, 1305–1314. [Google Scholar]
- Sammarco, P.W. Echinoid grazing as a structuring force in coral communities: Whole reef manipulations. J. Exp. Mar. Biol. Ecol. 1982, 61, 31–55. [Google Scholar]
- Bellwood, D.R.; Hughes, T.P.; Hoey, A.S. Sleeping functional group drives coral-reef recovery. Curr. Biol. 2006, 16, 2434–2439. [Google Scholar]
- Mörk, E.; Sjöö, G.L.; Kautsky, N.; McClanahan, T.R. Top-down and bottom-up regulation of macroalgal community structure on a Kenyan reef. Estuar. Coast. Shelf Sci. 2009, 84, 331–336. [Google Scholar]
- Littler, M.M.; Littler, D.S.; Brooks, B.L. Harmful algae on tropical coral reefs: Bottom-up eutrophication and top-down herbivory. Harmful Algae 2006, 5, 565–585. [Google Scholar]
- McCook, L.J.; Jompa, J.; Diaz-Pulido, G. Competition between corals and algae on coral reefs: A review of evidence and mechanisms. Coral Reefs 2001, 19, 400–417. [Google Scholar]
- Birrell, C.L.; McCook, L.J.; Willis, B.L.; Diaz-Pulido, G.A. Effects of benthic algae on the replenishment of corals and the implications for the resilience of coral reefs. Oceanogr. Mar. Biol. Ann. Rev. 2008, 46, 25–63. [Google Scholar]
- Burkepile, D.E.; Hay, M.E. Herbivore vs. nutrient control of marine primary producers: Context-dependent effects. Ecology 2006, 87, 3128–3139. [Google Scholar] [CrossRef]
- McManus, J.W.; Polsenberg, J.F. Coral-algal phase shifts on coral reefs: Ecological and environmental aspects. Prog. Oceanogr. 2004, 60, 263–279. [Google Scholar]
- Sheppard, C.R.C.; Davy, S.K.; Pilling, G.M. The Biology of Coral Reefs; Oxford University Press: Oxford, UK, 2009; p. 339. [Google Scholar]
- Walsh, S.M. Ecosystem-scale effects of nutrients and fishing on coral reefs. J. Mar. Biol. 2011, 2011, 187248:1–187248:13. [Google Scholar]
- Smith, J.E.; Smith, C.M.; Hunter, C.L. An experimental analysis of the effects of herbivory and nutrient enrichment on benthic community dynamics on a Hawaiian reef. Coral Reefs 2001, 19, 332–342. [Google Scholar]
- Stimson, J.; Conklin, E. Potential reversal of a phase shift: The rapid decrease in the cover of the invasive green macroalga Dictyosphaeria cavernosa Forsskål on coral reefs in Kāne‘ohe Bay, Oahu, Hawai‘i. Coral Reefs 2008, 27, 717–726. [Google Scholar] [CrossRef]
- Hunte, W.; Younglao, D. Recruitment and population recovery of Diadema antillarum (Echinodermata; Echinoidea) in Barbados. Mar. Ecol. Prog. Ser. 1988, 45, 109–119. [Google Scholar] [CrossRef]
- Carpenter, R.C.; Edmunds, P.J. Local and regional scale recovery of Diadema promotes recruitment of scleractinian corals. Ecol. Lett. 2006, 9, 271–280. [Google Scholar] [CrossRef]
- Idjadi, J.A.; Lee, S.C.; Bruno, J.F.; Precht, W.F.; Allen-Requa, L.; Edmunds, P.J. Rapid phase-shift reversal on a Jamaican coral reef. Coral Reefs 2006, 25, 209–211. [Google Scholar]
- Myhre, S.; Acevedo-Gutierrez, A. Recovery of sea urchin Diadema antillarum populations is correlated to increased coral and reduced macroalgal cover. Mar. Ecol Prog. Ser. 2007, 309, 205–210. [Google Scholar]
- Knowlton, N. Thresholds and multiple states in coral reef dynamics. Am. Zool. 1992, 32, 674–682. [Google Scholar]
- Mumby, P.J. Phase shifts and the stability of macroalgal communities on Caribbean coral reefs. Coral Reefs 2009, 28, 761–773. [Google Scholar]
- Hughes, T.P.; Graham, N.A.J.; Jackson, J.B.C.; Mumby, P.J.; Steneck, R.S. Rising to the challenge of sustaining coral reef resilience. Trends Ecol. Evol. 2010, 25, 633–642. [Google Scholar]
- Mumby, P.J.; Hastings, A.; Edwards, H.J. Thresholds and the resilience of Caribbean coral reefs. Nature 2007, 450, 98–101. [Google Scholar]
- Williams, I.D.; Polunin, N.V.C.; Hendrick, V.I. Limits to grazing by herbivorous fishes and the impact of low coral cover on macroalgal abundance on a coral reef in Belize. Mar. Ecol. Prog. Ser. 2001, 222, 187–196. [Google Scholar]
- Ledlie, M.H.; Graham, N.A.J.; Bythell, J.C.; Wilson, S.K.; Jennings, S.; Polunin, N.V.C.; Hardcastle, J. Phase shifts and the role of herbivory in the resilience of coral reefs. Coral Reefs 2007, 26, 641–653. [Google Scholar]
- Green, A.L.; Bellwood, D.R. Monitoring Functional Groups of Herbivorous Reef Fishes as Indicators of Coral Reef Resilience—A Practical Guide for Coral Reef Managers in the Asia Pacific Region; IUCN working group on Climate Change and Coral Reefs, IUCN: Gland, Switzerland, 2009. [Google Scholar]
- Williams, I.; Polunin, N.V.C.; Hendrick, V.J. Limits to grazing by herbivorous fishes and the impact of low coral cover on macroalgal abundance on a coral reef in Belize. Mar. Ecol. Prog. Ser. 2001, 222, 187–196. [Google Scholar]
- McClanahan, T.R.; Hendrick, V.; Rodrigues, M.J.; Polunin, N.V.C. Varying responses of herbivorous and invertebrate feeding fishes to macroalgal reduction on a coral reef. Coral Reefs 1999, 18, 195–203. [Google Scholar]
- Sheppard, C.R.C.; Spalding, M.; Bradshaw, C.; Wilson, S. Erosion vs. recovery of coral reefs after 1998 El Niño: Chagos reefs, Indian Ocean. Ambio 2002, 31, 40–48. [Google Scholar]
- Graham, N.A.J.; Nash, K.L.; Kool, J.T. Coral reef recovery dynamics in a changing world. Coral Reefs 2011, 30, 283–394. [Google Scholar]
- Anthony, K.R.N.; Maynard, J.A.; Diaz-Pulido, G.; Mumby, P.J.; Marshall, P.A.; Cao, L.; Hoegh-Guldberg, O. Ocean acidification and warming will lower coral reef resilience. Glob. Change Biol. 2011, 17, 1798–1808. [Google Scholar]
- Paddack, M.J.; Cown, R.K.; Sponaugle, S. Grazing pressure of herbivorous coral reef fishes on low coral-cover reefs. Coral Reefs 2006, 25, 461–472. [Google Scholar]
- Stockwell, B.; Jadloc, C.R.L.; Abesamis, R.A.; Alcala, A.C.; Russ, G.R. Trophic and benthic responses to no-take marine reserve protection in the Philippines. Mar. Ecol. Prog Ser. 2009, 389, 1–15. [Google Scholar]
- Bellwood, D.R.; Choat, J.H. A functional analysis of grazing in parrotfishes (family Scaridae): The ecological implications. Environ. Biol. Fishes 1990, 28, 189–214. [Google Scholar]
- Clements, K.D.; Raubenheimer, D.; Choat, J.H. Nutritional ecology of marine herbivorous fishes: Ten years on. Funct. Ecol. 2009, 23, 79–92. [Google Scholar]
- Bonaldo, R.M.; Bellwood, D.R. Parrotfish predation on massive Porites on the Great Barrier Reef. Coral Reefs 2011, 30, 259–269. [Google Scholar] [CrossRef]
- Van Woesik, R. Corals’ prolonged struggle against unfavorable conditions. Galaxea JCRS 2009, 11, 53–58. [Google Scholar]
- Ruckelshaus, M.; Klinger, T.; Knowlton, N.; DeMaster, D.P. Marine ecosystem-based management in practice: Scientific and governance challenges. Bioscience 2008, 58, 53–63. [Google Scholar]
- Munday, P.L.; Jones, G.P.; Pratchett, M.S.; Williams, A.J. Climate change and the future for coral reef fishes. Fish Fish. 2008, 9, 261–285. [Google Scholar]
- Bellwood, D.R.; Hoey, A.S.; Ackerman, J.L.; Depczynski, M. Coral bleaching, reef fish community phase shifts and the resilience of coral reefs. Glob. Change Biol. 2006, 12, 1587–1594. [Google Scholar]
- Wilson, S.K.; Fisher, R.; Pratchett, M.S.; Graham, N.A.; Dulvy, N.K.; Turner, R.A.; Cakacaka, A.; Polunin, N.V.C. Exploitation and habitat degradation as agents of change within coral reef fish communities. Glob. Change Biol. 2008, 14, 2796–2809. [Google Scholar]
- Sano, M.; Shimizu, M.; Nose, Y. Long-term effects of destruction of hermatypic corals by Acanthaster planci infestation on reef fish communities at Iriomote Island, Japan. Mar. Ecol. Prog. Ser. 1987, 37, 191–199. [Google Scholar] [CrossRef]
- Roberts, C.M.; Reynolds, D.; Côté, I.M.; Hawkins, J.P. Redesigning coral reef conservation. In Coral Reef Conservation; Côté, I.M., Reynolds, J.D., Eds.; Cambridge University Press: Cambridge, UK, 2006; pp. 515–537. [Google Scholar]
- Graham, N.A.J.; Wilson, S.K.; Jennings, S.; Polunin, N.V.C.; Bijoux, J.P. Dynamic fragility of oceanic coral reef ecosystems. Proc. Natl. Acad. Sci. USA 2006, 103, 8425–8429. [Google Scholar]
- Graham, N.A.J.; Wilson, S.K.; Jennings, S.; Polunin, N.V.C.; Robinson, J.; Bijoux, J.P.; Daw, T.M. Lag effects in the impacts of mass bleaching on coral reef fish, fisheries, and ecosystems. Conserv. Biol. 2007, 21, 1291–1300. [Google Scholar] [CrossRef]
- Jones, G.P.; McCormick, M.I.; Srinivasan, M.; Eagle, J.V. Coral decline threatens fish biodiversity in marine reserves. Proc. Natl. Acad. Sci. USA 2004, 101, 8251–8253. [Google Scholar]
- Pratchett, M.S.; Hoey, A.S.; Wilson, S.K.; Messmer, V.; Graham, N.A.J. Changes in biodiversity and functioning of reef fish assemblages following coral bleaching and coral loss. Diversity 2011, 3, 424–454. [Google Scholar]
- Wilson, S.K.; Graham, N.A.J.; Pratchett, M.S.; Jones, G.P.; Polunin, N.V.C. Multiple disturbances and the global degradation of coral reefs: Are reef fishes at risk or resilient? Glob. Change Biol. 2006, 12, 2220–2234. [Google Scholar] [CrossRef]
- Cinner, J.E.; McClanahan, T.R.; Graham, N.A.J.; Pratchett, M.S.; Wilson, S.K.; Raina, J.-B. Gear-based fisheries management as a potential adaptive response to climate change and coral mortality. J. Appl. Ecol. 2009, 46, 724–732. [Google Scholar]
- Cheal, A.J.; MacNeil, M.A.; Cripps, E.; Emslie, M.J.; Jonker, M.; Schaffelke, B.; Sweatman, H. Coral-macroalgal phase shifts or reef resilience: Links with diversity and functional roles of herbivorous fishes on the Great Barrier Reef. Coral Reefs 2010, 29, 1005–1015. [Google Scholar]
- Edwards, H.J.; Elliott, I.A.; Eakin, C.M.; Irikawa, A.; Madin, J.S.; McField, M.; Morgan, J.A.; van Woesik, R.; Mumby, P.J. How much time can herbivore protection buy for coral reefs under realistic regimes of hurricanes and coral bleaching? Glob. Change Biol. 2011, 17, 2033–2048. [Google Scholar]
- Thrush, S.F.; Hewitt, J.E.; Dayton, P.E.; Coco, G.; Lohrer, A.M.; Norkko, A.; Norkko, J.; Chiantore, M. Forecasting the limits of resilience: Integrating empirical research with theory. Proc. R. Soc. B 2009, 276, 3209–3217. [Google Scholar]
- Dudgeon, S.R.; Aronson, R.B.; Bruno, J.F.; Precht, W.F. Phase shifts and stable states on coral reefs. Mar. Ecol. Prog. Ser. 2010, 413, 201–216. [Google Scholar]
- Fox, H.E.; Pet, J.S.; Dahuri, R.; Caldwell, R.L. Recovery in rubble fields: Long-term impacts of blast fishing. Mar. Pollut. Bull. 2003, 46, 1024–1031. [Google Scholar]
- Jennings, S.; Kaiser, M.J.; Reynolds, J.D. Marine Fisheries Ecology; Blackwell Science: Oxford, UK, 2001. [Google Scholar]
- Allen, G.R. Reef fishes of Milne Bay Province, Papua New Guinea. In A Rapid Marine Biodiversity Assessment of Milne Bay Province, Papua New Guinea- Survey II; Allen, G.R., Kinche, J.P., McKenna, S.A., Seeto, P., Eds.; RAP Bulletin of Biological Assessment Conservation International: Washington, DC, USA, 2003; 29, pp. 46–55. [Google Scholar]
- Polunin, N.V.C.; Roberts, C.M.; Pauly, D. Developments in Tropical Reef Fisheries Science and Management. In Reef Fisheries; Polunin, N.V.C., Roberts, C.M., Eds.; Chapman & Hall: London, UK, 1996; pp. 361–377. [Google Scholar]
- Hilborn, R. The dark side of reference points. Bull. Mar. Sci. 2002, 70, 403–408. [Google Scholar]
- Dalzell, P. Catch Rates, Selectivity and Yields of Reef Fishing. In Reef Fisheries; Polunin, N.V.C., Roberts;, C.M., Eds.; Chapman & Hall: London, UK, 1996; pp. 161–192. [Google Scholar]
- Adams, T.; Dalzell, P.; Farman, R. Status of Pacific Island Coral Reef Fisheries. In Proceedings of the 8th International Coral Reef Symposium, Panama, 24–29 June 1997; 2, pp. 1977–1980.
- Dalzell, P.; Adams, T.J.H. Sustainability and Management of Reef Fisheries in the Pacific Islands. In Proceedings of the 8th International Coral Reef Symposium, Panama, 24–29 June 1997; 2, pp. 2027–2032.
- McClanahan, T.R.; Graham, N.A.J.; MacNeil, M.A.; Muthiga, N.A.; Cinner, J.E.; Bruggemann, J.H.; Wilson, S.K. Critical thresholds and tangible targets for ecosystem-based management of coral reef fisheries. Proc. Natl. Acad. Sci. USA 2011, 104, 17230–17233. [Google Scholar]
- Donaldson, T.J. Phylogeny, reef fish conservation biology, and the live reef fish trade. Fish. Sci. 2002, 68, 143–147. [Google Scholar]
- Medley, P.A.; Gaudian, G.; Wells, S. Coral reef fisheries stock assessment. Rev. Fish Biol. Fish. 1993, 3, 242–285. [Google Scholar]
- Cheung, W.W.L.; Watson, R.; Morato, T.; Pitcher, T.J.; Pauly, D. Intrinsic vulnerability in the global fish catch. Mar. Ecol. Prog. Ser. 2007, 333, 1–12. [Google Scholar]
- Froese, R.; Pauly, D. FishBase. World Wide Web Electronic Publication; Academia Sinica: Taipei, Taiwan, 2010. Available online: http://www.fishbase.org (accessed on 2 March 2012).
- Andrews, K.; Nall, L.; Jeffrey, C.; Pittman, S.; Banks, K.; sBeaver, C.; Bohnsack, J.; Dodge, R.E.; Gilliam, D.; Jaap, W.; et al. The state of coral reef ecosystems of Florida. In The State of Coral Reef Ecosystems of the United States and Pacific Freely Associated States: 2005; Waddell, J., Ed.; NOAA Technical Memorandum NOS NCCOS 11. NOAA/NCCOS Center for Coastal Monitoring and Assessment’s Biogeography Team: Silver Spring, MD, USA, 2005; pp. 150–200. [Google Scholar]
- Ault, J.S.; Smith, S.G.; Meester, G.A.; Luo, J.; Bohnsack, J.A. Site characterization for Biscayne National Park: Assessment of fisheries resources and habitats. In NOAA Technical Memorandum NMFS-SEFSC-468; Miami, FL, USA, 2001; pp. 1–185. [Google Scholar]
- Ault, J.S.; Smith, S.G.; Bohnsack, J.A. Evaluation of average length as an estimator of exploitation status for the Florida coral-reef fish community. ICES J. Mar. Sci. 2005, 62, 417–423. [Google Scholar]
- Ault, J.S.; Smith, S.G.; Luo, J.; Monaco, M.E.; Appeldoorn, R.S. Length-based assessment of sustainability benchmarks for coral reef fishes in Puerto Rico. Environ. Conserv. 2008, 35, 1–11. [Google Scholar]
- Donahue, S.; Acosta, A.; Akins, L.; Ault, J.; Bohnsack, J.; Boyer, J.; Callahan, M.; Causey, B.; Cox, C.; Delaney, J.; et al. The state of coral reef ecosystems of the Florida Keys. In The State of Coral Reef Ecosystems of the United States and Pacific Freely Associated States: 2008; Waddell, J.E., Clarke, A.M., Eds.; NOAA Technical Memorandum NOS NCCOS 73. NOAA/NCCOS Center for Coastal Monitoring and Assessment’s Biogeography Team: Silver Spring, MD, USA, 2008; pp. 161–187. [Google Scholar]
- Doherty, P.J. Variable replenishment and the dynamics of reef fish populations. In Coral Reef Fisheries, Dynamics and Diversity in a Complex Ecosystem; Sale, P.F., Ed.; Academic Press: London, UK, 2002; pp. 327–355. [Google Scholar]
- Hilborn, R. Ecosystem-based fisheries management: The carrot or the stick? Mar. Ecol. Prog. Ser. 2004, 274, 275–278. [Google Scholar]
- McManus, J.W. Social and economic aspects of reef fisheries and their management. In Reef Fisheries; Polunin, N.V.C., Roberts, C.M., Eds.; Chapman & Hall: London, UK, 1996; pp. 249–281. [Google Scholar]
- Ley, J. Personal Communication. St. Petersburgh, FL, USA, 2011. [Google Scholar]
- Friedlander, A.; Aeby, G.; Brainard, R.; Brown, E.; Chaston, K.; Clark, A.; McGowan, P.; Montgomery, T.; Walsh, W.; Williams, I.; et al. The state of the coral reef ecosystems of the main Hawaiian Islands. In The State of Coral Reef Ecosystems of the United States and Pacific Freely Associated States: 2008; Waddell, J.E., Clarke, A.M., Eds.; NOAA Technical Memorandum NOS NCCOS 73. NOAA/NCCOS Center for Coastal Monitoring and Assessment’s Biogeography Team: Silver Spring, MD, USA, 2008; pp. 219–261. [Google Scholar]
- Eggleston, D.B.; Johnson, E.G.; Kellison, G.T.; Nadeau, D.A. Intense removal and non-saturating functional responses by recreational divers on spiny lobster Panulirus argus. Mar. Ecol. Prog. Ser. 2003, 257, 197–207. [Google Scholar] [CrossRef]
- Eggleston, D.B.; Parsons, D.M.; Kellison, G.T.; Plaia, G.R.; Johnson, E.G. Functional response of sport divers to lobsters with application to fisheries management. Ecol. Appl. 2008, 18, 258–272. [Google Scholar]
- Hixon, M.A.; Webster, M.S. Density dependence in reef fish populations. In Coral Reef Fisheries, Dynamics and Diversity in a Complex Ecosystem; Sale, P.F., Ed.; Academic Press: London, UK, 2002; pp. 303–325. [Google Scholar]
- Appledorn, R.S. Model and method in reef fishery assessment. In Reef Fisheries; Polunin, N.V.C., Roberts, C.M., Eds.; Chapman & Hall: London, UK, 1996; pp. 219–248. [Google Scholar]
- Sadovy, Y. Trouble on the reef: The imperative of managing vulnerable and valuable fisheries. Fish Fish. 2005, 6, 167–185. [Google Scholar]
- Knittweis, L.; Wolff, M. Live coral trade impacts on the mushroom coral Heliofungia actiniformis in Indonesia: Potential future management approaches. Biol. Conserv. 2010, 143, 2722–2729. [Google Scholar] [CrossRef]
- McManus, J.W.; Reyes, R.B., Jr.; Mañola, C.L., Jr. Effects of some destructive fishing methods on coral cover and potential rates of recovery. Environ. Manag. 1997, 21, 69–78. [Google Scholar] [CrossRef]
- Claydon, J. Spawning aggregations of coral reef fishes: Characteristics, hypotheses, threats, and management. Oceanogr. Mar. Biol. Ann. Rev. 2004, 42, 265–302. [Google Scholar] [CrossRef]
- Sadovy, Y.; Domeier, M. Are aggregation-fisheries sustainable? Reef fish fisheries as a case study. Coral Reefs 2005, 24, 254–262. [Google Scholar]
- DeMitcheson, Y.S.; Sadovy, Y.; Cornish, A.; Domeier, M.; Colin, P.L.; Russell, M.; Lindeman, K.C. A global baseline for spawning aggregations of reef fishes. Conserv. Biol. 2008, 22, 1233–1244. [Google Scholar]
- Hamilton, R.J.; Potuku, T.; Montambault, J.R. Community-based conservation results in the recovery of reef fish spawning aggregations in the Coral Triangle. Biol. Conserv. 2011, 144, 1850–1858. [Google Scholar]
- Christiansen, V.; Pauly, D. Coral reef and other tropical fisheries. In Encyclopedia of Ocean Sciences; Steele, J.A., Thorpe, S.A., Turekian, K.K., Eds.; Academic Press: San Diego, CA, USA, 2001; Volume 1, pp. 534–538. [Google Scholar]
- Grigg, R.W. Resource management of precious corals: A review and application to shallow water reef building corals. PSZNI Mar. Ecol. 1984, 5, 57–74. [Google Scholar]
- Goffredo, S.; Lasker, H.R. An adaptive management approach to an octocoral fishery based on the Beverton-Holt model. Coral Reefs 2008, 27, 751–761. [Google Scholar]
- Burke, L.; Selig, E.; Spalding, M. Reefs at Risk in Southeast Asia; World Resources Institute: Washington, DC, USA, 2002. [Google Scholar]
- Johannes, R.E. The case for data-less marine resource management: Examples from tropical nearshore fisheries. Trends Ecol. Evol. 1998, 13, 243–246. [Google Scholar]
- Nowlis, J.S. Short- and long-term effects of three fishery management tools on depleted fisheries. Bull. Mar. Sci. 2000, 66, 651–662. [Google Scholar]
- Beddington, J.R.; Agnew, D.J.; Clark, C.W. Current problems in the management of marine fisheries. Science 2007, 316, 1713–1716. [Google Scholar]
- Birkeland, C.; Davis, G. Personal Communication. Honolulu, HI, USA, 2010. [Google Scholar]
- Hensley, R.A.; Sherwood, T.S. An overview of Guam’s inshore fisheries. Mar. Fish. Rev. 1993, 55, 129–138. [Google Scholar]
- Davis, G. Personal Communication. 2011. [Google Scholar]
- Hawhee, J.M. Western Pacific Coral Reef Ecosystem Report; Western Pacific Regional Fishery Management Council: Honolulu, HI, USA, 2006. [Google Scholar]
- Zeller, D.; Booth, G.; Craig, P.; Pauly, D. Reconstruction of coral reef fisheries catches in American Samoa, 1950–2002. Coral Reefs 2007, 25, 144–152. [Google Scholar]
- Sabater, M.G.; Carroll, B.P. Trends in reef fish population and associated fishery after three millennia of resource utilization and a century of socio-economic changes in American Samoa. Rev. Fish. Sci. 2009, 17, 318–335. [Google Scholar]
- Turner, R.A.; Cakacaka, A.; Graham, N.A.J.; Polunin, N.V.C.; Pratchett, M.S.; Stead, S.M.; Wilson, S.K. Declining reliance on marine resources in remote South Pacific societies: Ecological versus socio-economic drivers. Coral Reefs 2007, 26, 997–1008. [Google Scholar]
- Kronen, M.; Magron, F.; McArdle, B.; Vunisea, A. Reef finfishing pressure risk model for Pacific Island countries and territories. Fish.Res. 2010, 101, 1–10. [Google Scholar] [CrossRef]
- Luck, D.; Dalzell, P. Western Pacific Region Reef Fish Trends, a Compendium of Ecological and Fisheries Statistics for Reef Fishes in American Samoa, Hawai’i, and the Mariana Archipelago, in Support of Annual Catch Limit (ACL) Implementation; Report to the Western Pacific Regional Fishery Management Council, Honolulu, HI, USA, 2010. Available online: http://www.wpcouncil.org/library.html (accessed on 2 March 2012).
- Jennings, S.; Polunin, N.V.C. Biased underwater visual census biomass estimates for target-species in tropical reef fisheries. J. Fish Biol. 1995, 47, 733–736. [Google Scholar]
- Edgar, G.J.; Barrett, N.S.; Morton, J.S. Biases associated with the use of underwater visual census techniques to quantify the density and size-structure of fish populations. J. Exp. Mar. Biol. Ecol. 2004, 30, 269–290. [Google Scholar]
- Bozec, Y.-M.; Kulbicki, M.; Laloë, F.; Mou-Tham, G.; Gascuel, D. Factors affecting the detection distances of reef fish: Implications for visual counts. Mar. Biol. 2011, 158, 969–981. [Google Scholar]
- Minte-Vera, C.V.; de Moura, R.L.; Francini-Filho, R.B. Nested sampling: An improved visual-census technique for studying reef fish assemblages. Mar. Ecol. Prog. Ser. 2008, 267, 283–293. [Google Scholar]
- Karnauskas, M.; McClellan, D.B.; Wiener, J.W.; Miller, M.W.; Babcock, E.A. Inferring trends in a small-scale, data-limited tropical fishery based on fishery-independent data. Fish. Res. 2011, 111, 40–52. [Google Scholar]
- Smith, S.G.; Ault, J.S.; Bohnsack, J.A.; Harper, D.E.; Luo, J.; McClellan, D.B. Multispecies survey design for assessing reef-fish stocks, spatially explicit management performance, and ecosystem condition. Fish. Res. 2011, 109, 25–41. [Google Scholar]
- Houk, P. Personal Communication. Saipan, Commonwealth of the Northern Marianas Islands, USA, 2011. [Google Scholar]
- Choat, J.H.; Robertson, D.R. Age-based studies. In Coral Reef Fisheries, Dynamics and Diversity in a Complex Ecosystem; Sale, P.F., Ed.; Academic Press: London, UK, 2002; pp. 57–101. [Google Scholar]
- Rhodes, K.L.; Tupper, M.H. A preliminary market-based analysis of the Pohnpei, Micronesia, grouper (Serranidae: Epinephelinae) fishery reveals unsustainable fishing practices. Coral Reefs 2007, 26, 335–344. [Google Scholar] [CrossRef]
- Houk, P.; Rhodes, K.; Cuetos-Bueno, J.; Lindfield, S.; Fread, V.; McIlwain, J.L. Commercial coral-reef fisheries across Micronesia: A need for improving management. Coral Reefs 2011, 31, 13–26. [Google Scholar]
- Pinca, S.; Kronen, M.; Magron, F.; McArdle, B.; Vigliola, L.; Kulbicki, M.; Andréfouët, S. Relative importance of habitat and fishing in influencing reef fish communities across seventeen Pacific Island Countries and Territories. Fish Fish. 2011. [Google Scholar] [CrossRef]
- Lieske, E.; Myers, R. Coral Reef Fishes, Indo-Pacific and Caribbean, Revised Edition; Princeton University Press: Princeton, NJ, USA, 2001. [Google Scholar]
- Allen, G. Personal Communication. Perth, Australia, 2010. [Google Scholar]
- Babcock, E.A.; MacCall, A.D. How useful is the ratio of the fish density outside versus inside no-take marine reserves as a metric for fishery management control rules? Can. J. Fish. Aquat. Sci. 2011, 68, 343–359. [Google Scholar] [CrossRef]
- Russ, G.R.; Alcala, A.C. Marine reserves: Long-term protection is required for full recovery of predatory fish populations. Oecologia 2004, 138, 622–627. [Google Scholar]
- Russ, G.R.; Stockwell, B.; Alcala, A.C. Inferring versus measuring rates of recovery in no-take marine reserves. Mar. Ecol. Prog. Ser. 2005, 292, 1–12. [Google Scholar]
- McManus, J.W.; Meñez, L.A.B.; Kesner-Reyes, K.N.; Vergara, S.G.; Ablan, M.C. Coral reef fishing and coral-algal phase shifts: Implications for global reef status. ICES J. Mar. Sci. 2000, 57, 572–578. [Google Scholar]
- Hall, S.J.; Mainprize, B. Towards ecosystem-based fisheries management. Fish Fish. 2004, 5, 1–20. [Google Scholar]
- Graham, N.A.J.; Dulvy, N.K.; Jennings, S.; Polunin, N.V.C. Size-spectra as indicators of the effects of fishing on coral reef assemblages. Coral Reefs 2005, 24, 118–124. [Google Scholar]
- Latin, H. Why conservation by legal fiat does not work. In Proceedings of the Colloquium on Global Aspects of Coral Reefs: Health, Hazards, and History, 1993; Ginsburgh, R.N., Ed.; Rosenstiel School of Marine and Atmospheric Science, University of Miami: Miami, FL, USA, 1994; pp. 113–119. [Google Scholar]
- McClanahan, T. Challenges and accomplishments towards sustainable reef fisheries. In Coral Reef Conservation; Côté, I.M., Reynolds, J.D., Eds.; Cambridge University Press: Cambridge, UK, 2006; pp. 147–182. [Google Scholar]
- Russ, G.R. Yet another review of marine reserves as reef fishery management tools. In Coral Reef Fishes: Dynamics and Diversity in a Complex Ecosystem; Sale, P.F., Ed.; Academic Press: San Diego, CA, USA, 2002; pp. 421–443. [Google Scholar]
- Bartholomew, A.; Bohnsack, J.A. A review of catch-and-release angling mortality with implications for no-take reserves. Rev. Fish Biol. Fish. 2005, 15, 129–154. [Google Scholar]
- Rhodes, K.L.; Tupper, M.H.; Wichilmel, C.B. Characterization and management of the commercial sector of the Pohnpei coral reef fishery, Micronesia. Coral Reefs 2008, 27, 443–454. [Google Scholar]
- Palumbi, S.R. Marine Reserves: A Tool for Ecosystem Management and Conservation; Pew Oceans Commission: Arlington, VA, USA, 2002. [Google Scholar]
- Fenner, D. unpublished observations. Pago Pago, American Samoa, 2011. [Google Scholar]
- Hilborn, R.; Punt, A.E.; Orensanz, J. Beyond band-aids in fisheries management: Fixing world fisheries. Bull. Mar. Sci. 2004, 74, 493–507. [Google Scholar]
- Guteirrez, N.L.; Hilborn, R.; Defeo, O. Leadership, social capital and incentives promote successful fisheries. Nature 2011, 470, 386–389. [Google Scholar]
- Costello, C.; Gaines, S.D.; Lynham, J. Can catch shares prevent fisheries collapse? Science 2008, 321, 1678–1681. [Google Scholar] [CrossRef]
- Aswani, S. Customary sea tenure in Oceania as a case of rights-based fishery management: Does it work? Rev. Fish Biol. Fish. 2005, 15, 285–307. [Google Scholar] [CrossRef]
- Branch, T.A. How do individual transferable quotas affect marine ecosystems? Fish Fish. 2009, 10, 39–57. [Google Scholar] [CrossRef]
- Smith, T.; Gibbs, M.; Smith, D. Fishing for more effective incentives. Science 2009, 323, 337–338. [Google Scholar]
- Turnipseed, M.; Crowder, L.B.; Sagarin, R.D.; Roady, S.E. Legal bedrock for rebuilding America’s ocean resources. Science 2009, 324, 183–184. [Google Scholar]
- Pauly, D. Beyond duplicity and ignorance in global fisheries. Sci. Mar. 2009, 73, 215–224. [Google Scholar]
- Zeller, D.; Pauly, D. The future of fisheries: From ‘exclusive’ resource policy to ‘inclusive’ public policy. Mar. Ecol. Prog. Ser. 2004, 274, 295–298. [Google Scholar]
- St. Martin, K.; McCay, B.J.; Murray, G.D.; Johnson, T.R.; Oles, B. Communities, knowledge, and fisheries of the future. Int. J. Glob. Environ. Issues 2007, 7, 221–239. [Google Scholar] [CrossRef]
- Adams, T.J.H. The interface between traditional and modern methods of fishery management in the Pacific Islands. Ocean Coast. Manag. 1998, 40, 127–142. [Google Scholar]
- Link, J.S. What does ecosystem-based fisheries management mean? Fisheries 2002, 27, 18–21. [Google Scholar]
- Pikitch, E.K.; Santora, C.; Babcock, E.A.; Bakun, A.; Bonfil, R.; Conover, D.O.; Dayton, P.; Doukakis, P.; Fluharty, D.; Heneman, B.; et al. Ecosystem-based fishery management. Science 2004, 305, 346–347. [Google Scholar]
- Jennings, S. Indicators to support an ecosystem approach to fisheries. Fish Fish. 2005, 6, 212–232. [Google Scholar]
- Rice, J. Managing fisheries well: Delivering the promises of an ecosystem approach. Fish Fish. 2011, 12, 209–231. [Google Scholar]
- Arkema, K.K.; Abramson, S.C.; Dewsbury, B.M. Marine ecosystem-based management: From characterization to implementation. Front. Ecol. Environ. 2006, 4, 525–532. [Google Scholar]
- Saila, S.B.; Kocic, V.L.; McManus, J.W. Modeling the effects of destructive fishing practices on tropical coral reefs. Mar. Ecol. Prog. Ser. 1993, 94, 51–60. [Google Scholar]
- Jones, R.J.; Steven, A.L. Effects of cyanide on corals in relation to cyanide fishing on reefs. Mar. Freshw. Res. 1997, 48, 517–522. [Google Scholar]
- Edinger, E.N.; Jompa, J.; Limmon, G.V.; Widjatmoko, W.; Risk, M.J. Reef degradation and coral biodiversity in Indonesia: Effects of land-based pollution, destructive fishing practices, and changes over time. Mar. Pollut. Bull. 1998, 36, 617–630. [Google Scholar] [CrossRef]
- D. Fenner, unpublished observations. Guam, 2011
- Friedman, K.; Eriksson, H.; Tardy, E.; Pakoa, K. Management of sea cucumber stocks; patterns ofvulnerability and recovery of sea cucumber stocks impacted by fishing. Fish Fish. 2011, 12, 75–93. [Google Scholar]
- Rapport, D.J.; Costanza, R.; McMichael, A.J. Assessing ecosystem health. Trend. Ecol. Evol. 1998, 13, 397–402. [Google Scholar]
- Brodziak, J.; Link, J. Ecosystem-based fishery management: What is it, and how can we do it? Bull. Mar. Sci. 2002, 70, 589–611. [Google Scholar]
- Vroom, P.S. “Coral dominance”: A dangerous ecosystem misnomer? J. Mar. Biol. 2011, 2011. [Google Scholar] [CrossRef]
- Mangel, M. Trade-offs between fish habitat and fishing mortality and the role of reserves. Bull Mar. Sci. 2000, 66, 663–674. [Google Scholar]
- Birkeland, C. Important roles of natural history in ecology. Galaxea JCRS 2009, 11, 59–66. [Google Scholar]
- Browman, H.I.; Stergiou, K.I. Marine protected areas as a central element of ecosystem-based management: Defining their location, size, and number. Mar. Ecol. Prog. Ser. 2004, 274, 271–272. [Google Scholar]
- McManus, J.W. Tropical marine fisheries and the future of coral reefs: A brief review with emphasis on Southeast Asia. In Proceedings of the 8th International Coral Reef Symposium, Panama, 24–29 June 1997; 1, pp. 129–134.
- Liese, C. Fishery management for artisanal reef fisheries in developing countries: A holistic economic approach. In Proceedings of the 11th International Coral Reef Symposium, Ft. Lauderdale, FL, USA, 7–11 July 2008; pp. 1116–1120.
- Cinner, J.E. Socioeconomic factors that affect artisanal fishers’ readiness to exit a declining fishery. Conserv. Biol. 2008, 23, 124–130. [Google Scholar]
- Cinner, J.E.; McClanahan, T.R.; Daw, T.W.; Graham, N.A.J.; Maina, J.; Wilson, S.K.; Hughes, T.P. Linking social and ecological systems to sustain coral reef fisheries. Curr. Biol. 2009, 19, 206–212. [Google Scholar]
- Agardy, T.; Bridgewater, T.; Crosby, M.P.; Day, J.; Dayton, P.K.; Kenchington, R.; Laffoley, D.; McConney, P.; Murray, P.A.; Parks, J.E.; et al. Dangerous targets? Unresolved issues and ideological clashes around marine protected areas. Aquat. Conserv. Mar. Freshw. Ecosyst. 2003, 13, 353–367. [Google Scholar] [CrossRef]
- Agardy, T. Opportunities and constraints for using marine protected areas to conserve reef ecosystems. In Proceedings of the 9th International Coral Reef Symposium, Bali, Indonesia, 23–27 October 2000; 2, pp. 601–607.
- Bohnsac, J. Maintenance and recovery of reef fishery productivity. In Reef Fisheries; Polunin, N.V.C., Roberts, C.M., Eds.; Chapman & Hall: London, UK, 1996; pp. 283–313. [Google Scholar]
- Williams, M.J. Do fisheries and marine protected areas need each other? Parks 1998, 8, 47–53. [Google Scholar]
- Sobel, J.; Dahlgren, C. Marine Reserves, a Guide to Science, Design, and Use; Island Press: Washington, DC, USA, 2004. [Google Scholar]
- Lester, S.E.; Halpern, B.S. Biological responses in marine no-take reserves versus partially protected areas. Mar. Ecol. Prog. Ser. 2008, 367, 49–56. [Google Scholar]
- Dayton, P.K.; Sala, E.; Tegner, M.J.; Thrush, S. Marine reserves: Parks, baselines, and fisheries enhancements. Bull. Mar. Sci. 2000, 66, 617–634. [Google Scholar]
- Roberts, C.M.; Bohnsack, J.A.; Gell, F.; Hawkins, J.P.; Goodridge, R. Effects of marine reserves on adjacent fisheries. Science 2001, 294, 1920–1923. [Google Scholar]
- Gell, F.R.; Roberts, C.M. Benefits beyond boundaries: The fishery effects of marine reserves. Trends Ecol. Evol. 2003, 18, 448–455. [Google Scholar]
- Halpern, B.S. The impact of marine reserves: Do reserves work and does reserve size matter? Ecol. Appl. 2003, 13, S117–S137. [Google Scholar] [CrossRef]
- Palumbi, S.R.; Gaines, S.D.; Leslie, H.; Warner, R.R. New wave: High-tech tools to help marine reserve research. Front. Ecol. Environ. 2003, 1, 73–79. [Google Scholar]
- Aburto-Oropeza, O.; Erisman, B.; Galland, G.R.; Mascareñas-Osorio, I.; Sala, E.; Ezcurra, E. Large recovery of fish biomass in a no-take marine reserve. PLoS One 2011, 6. [Google Scholar] [CrossRef]
- Halpern, B.S.; Warner, R.R. Marine reserves have rapid and lasting effects. Ecol. Lett. 2002, 5, 361–366. [Google Scholar]
- Micheli, F.; Halpern, B.S.; Botsford, L.W.; Warner, R.R. Trajectories and correlates of community change in no-take marine reserves. Ecol. Appl. 2004, 14, 1709–1723. [Google Scholar]
- Mosqueira, I.; Cote, I.M.; Jennings, S.; Reynolds, J.D. Conservation benefits of marine reserves for fish populations. Anim. Conserv. 2000, 3, 321–332. [Google Scholar]
- Alcala, A.C.; Russ, G.R.; Nillos, P. Collaborative and community-based conservation of coral reefs, with reference to marine reserves in the Philippines. In Coral Reef Conservation; Cambridge University Press: New York, NY, USA, 2006; pp. 392–418. [Google Scholar]
- McClanahan, T.R.; Graham, A.J.; Wilson, A.K.; Letourneur, Y.; Fisher, R. Effects of fishery closure size, age, and history of compliance on coral reef fish communities in the western Indian Ocean. Mar. Ecol. Prog. Ser. 2009, 396, 99–109. [Google Scholar] [CrossRef]
- Halpern, B.S.; Lester, S.E.; Kellner, J.B. Spillover from marine reserves and the replenishment of fished stocks. Environ. Conserv. 2010, 36, 268–276. [Google Scholar]
- Birkeland, C.; Friedlander, A.M. The Importance of Refuges for Reef Fish Replenishment in Hawai’i; Hawaii Audubon Society: Honolulu, HI, USA, 2001. [Google Scholar]
- Birkeland, C.; Dayton, P.K. The importance in fishery management of leaving the big ones. Trends Ecol. Evol. 2005, 20, 356–358. [Google Scholar]
- Palumbi, S.R. Marine reserves and ocean neighborhoods: The spatial scale of marine neighborhoods and their management. Annu. Rev. Environ. Resour. 2004, 29, 31–68. [Google Scholar]
- Christie, M.R.; Tissot, B.M.; Albins, M.A.; Beets, J.P.; Jia, Y.; Ortiz, D.M.; Thompson, S.E.; Hixon, M.A. Larval connectivity in an effective network of marine protected areas. PLoS One 2010, 5. [Google Scholar] [CrossRef]
- Le Quesne, W.J.F.; Hawkins, S.J.; Shepherd, J.G. A comparison of no-take zones and traditional fishery management tools for managing site-attached species with a mixed larval pool. Fish Fish. 2007, 8, 181–195. [Google Scholar]
- Trexler, J.C.; Travis, J. Can marine protected areas restore and conserve stock attributes of reef fishes? Bull. Mar. Sci. 2000, 66, 853–873. [Google Scholar]
- Simberloff, D. No reserve is an island: Marine reserves and nonindigenous species. Bull. Mar. Sci. 2000, 66, 567–580. [Google Scholar]
- Allison, G.W.; Gaines, S.D.; Lubchenko, J.; Possingham, H.P. Ensuring persistence of marine reserves: Catastrophes require adopting an insurance factor. Ecol. Appl. 2003, 13, S8–S24. [Google Scholar]
- Allison, G.W.; Lubchenco, J.; Carr, M.H. Marine reserves are necessary but not sufficient for marine conservation. Ecol. Appl. 1998, 8, S79–S92. [Google Scholar]
- McClanahan, T.R. Is there a future for coral reef parks in poor tropical countries? Coral Reefs 1999, 18, 321–325. [Google Scholar] [CrossRef]
- Sutinin, J.G.; Kuperan, K. A socio-economic theory of regulatory compliance. Int. J. Soc. Econ. 1999, 26, 173–191. [Google Scholar]
- Mora, C.; Andréfouët, S.; Costello, M.J.; Kranenberg, C.; Rollo, A.; Veron, J.; Gaston, K.J.; Myers, R.A. Coral reefs and the global network of marine protected areas. Science 2006, 312, 1750–1751. [Google Scholar]
- Kelleher, G.; Bleakley, C.; Wells, S. A Global Representative System of Marine Protected Areas; The World Bank: Washington, DC, USA, 1996. [Google Scholar]
- Roberts, C.; Hawkins, J.P.; Gell, F.R. The role of marine reserves in achieving sustainable fisheries. Phil. Trans. R. Soc. B 2005, 360, 123–132. [Google Scholar]
- Bohnsack, J.A. A comparison of the short-term impacts of no-take reserves and minimum size limits. Bull. Mar. Sci. 2000, 66, 635–650. [Google Scholar]
- Tyler, E.H.M.; Speight, M.R.; Henderson, P.; Manica, A. Evidence for a depth refuge effect in artisanal coral reef fisheries. Biol. Conserv. 2009, 142, 652–667. [Google Scholar]
- Hastings, A.; Botsford, L.W. Equivalence in yield from marine reserves and traditional fisheries management. Science 1999, 284, 1537–1538. [Google Scholar]
- Game, E.T.; Bode, M.; McDonald-Madden, E.; Grantham, H.S.; Possingham, H.P. Dynamic marine protected areas can improve the resilience of coral reef systems. Ecol. Lett. 2009, 12, 1336–1346. [Google Scholar]
- Kramer, D.L.; Chapman, M.R. Implications of fish home range size and relocation for marine reserve function. Environ. Biol. Fishes 1999, 55, 65–79. [Google Scholar]
- Agardy, T.; Notarbartolo di Sciara, G.; Christie, P. Mind the gap: Addressing the shortcomings of marine protected areas through large scale marine spatial planning. Mar. Policy 2011, 35, 226–232. [Google Scholar]
- Willis, T.J.; Millar, R.B.; Babcock, R.C.; Tolimieri, N. Burdens of evidence and the benefits of marine reserves: Putting Descartes before des horse? Environ. Conserv. 2003, 30, 97–103. [Google Scholar] [CrossRef]
- McClanahan, T.R.; Kaundaara, R. Fishery recovery in a coral reef marine park and its effect on the adjacent fishery. Conserv. Biol. 1996, 10, 1187–1199. [Google Scholar]
- Russ, G.R.; Alcala, A.C. Marine reserves- rates and patterns of recovery and decline of large predatory fish. Ecol. Appl. 1996, 6, 947–961. [Google Scholar]
- Hilborn, R. Faith-based fisheries. Fisheries 2006, 31, 554–555. [Google Scholar]
- Edgar, G.J.; Bustamante, R.H.; Farina, J.M.; Calvopina, M.; Martinez, C.; Toral-Granda, M.V. Bias in evaluating the effects of marine protected areas: The importance of baseline data for the Galapagos Marine Reserve. Environ. Conserv. 2004, 31, 212–218. [Google Scholar]
- Bohnsack, J.A.; Causey, B.; Crosby, M.P.; Griffis, R.B.; Hixon, M.A.; Hourigan, T.F.; Koltes, K.H.; Maragos, J.E.; Simons, A.; Tilmant, J.T. A Rationale for minimum 20–30% no-take protection. In Proceedings of the 9th International Coral Reef Symposium, Bali, Indonesia, 23–27 October 2000; 2, pp. 615–619.
- Roberts, C.M. Selecting marine reserve locations: Optimality versus opportunism. Bull. Mar. Sci. 2000, 66, 581–592. [Google Scholar]
- Foale, S.; Manele, B. Social and political barriers to the use of marine protected areas for conservation and fishery management in Melanesia. Asia Pac. View. 2004, 45, 373–386. [Google Scholar]
- Crawford, B.; Kasmidi, M.; Korompis, F.; Pollnac, R.B. Factors influencing progress in establishing community-based marine protected areas in Indonesia. Coast. Manag. 2006, 34, 39–64. [Google Scholar]
- Hilborn, R.; Stokes, K.; Maguire, J.J.; Smith, T.; Botsford, L.W.; Mangel, M.; Orensanz, J.; Parma, A.; Rice, J.; Bell, J.; et al. When can marine reserves improve fisheries management? Ocean Coast. Manag. 2004, 47, 195–295. [Google Scholar] [CrossRef]
- Game, E.T.; Lipsett-Moore, G.; Hamilton, R.J.; Peterson, N.; Kereseka, J.; Atu, W.; Watts, M.; Possingham, H.P. Informed opportunism for conservation planning in the Solomon Islands. Conserv. Lett. 2011, 4, 38–46. [Google Scholar]
- Cinner, J.E. Designing marine reserves to reflect local socioeconomic conditions: Lessons from long-enduring customary management systems. Coral Reefs 2007, 26, 1035–1045. [Google Scholar]
- Jobbins, G. Tourism and coral-reef-based conservation: Can they coexist? In Coral Reef Conservation; Côté, I.M., Reynolds, J.D., Eds.; Cambridge University Press: Cambridge, UK, 2006; pp. 237–263. [Google Scholar]
- Fenner, D. A healthy Caribbean coral reef assisted by diving tourism. Reef Encount. 2001, 30, 27–28. [Google Scholar]
- Hand, T. An Economic and Social Evaluation of Implementing the Representative areas Program by Rezoning the Great Barrier Reef Marine Park; Great Barrier Reef Marine Park Authority: Townsville, Australia, 2003. [Google Scholar]
- Vianna, G.M.S.; Meekan, M.G.; Pannell, D.; Marsh, S.; Meeuwig, J. Wanted Dead or Alive? The Relative Value of Reef Sharks as a Fishery and an Ecotourism Asset in Palau; Australian Institute of Marine Science, University of Western Australia: Perth, Australia, 2010. [Google Scholar]
- Brander, L.M.; van Beukering, P.; Cesar, H.S.J. The recreational value of coral reefs: A meta-analysis. Ecol. Econ. 2007, 63, 209–218. [Google Scholar]
- Mascia, M.B.; Claus, A. A property rights approach to understanding human displacement from protected areas: The case of marine protected areas. Conserv. Biol. 2008, 23, 16–23. [Google Scholar]
- Sykes, H.; Reddy, C. “Sacred Water”; 10 years of community managed marine protection supported by ecotourism-based income generation at Waitabu Marine Park, Fiji Islands. In Proceedings of the 11th Pacific Intersicience Congress (PSI 2009), Tahiti, French Polynesia, 2–6 March 2009.
- Brunnschweiler, J.M. The Shark Reef Marine reserve: A marine tourism project in Fiji involving local communities. J. Sustain. Tour. 2010, 18, 29–42. [Google Scholar]
- Johannes, R.E. Traditional conservation methods and protected marine areas in Oceania. Ambio 1982, 11, 258–261. [Google Scholar]
- Aswani, S.; Hamilton, R.J. Integrating indigenous ecological knowledge and customary sea tenure with marine and social science in the Rovianna Lagoon, Solomon Islands. Environ. Conserv. 2004, 31, 69–83. [Google Scholar]
- Drew, J.A. Use of traditional ecological knowledge in marine conservation. Conserv. Biol. 2005, 19, 1286–1293. [Google Scholar]
- Cinner, J.; Marnane, M.J.; McClanahan, T.R.; Almany, G.R. Periodic closures as adaptive coral reef management in the Indo-Pacific. Ecol. Soc. 2005, 11. Available online: http://www.ecologyandsociety.org/vol11/iss1/art31/ (accessed 2 March 2012). Article 31.
- Johannes, R.E.; Freeman, M.M.R.; Hamilton, R.J. Ignore fishers’ knowledge and miss the boat. Fish Fish. 2000, 1, 257–271. [Google Scholar]
- Christie, P.; White, A.T. Best practices for improved governance of coral reef marine protected areas. Coral Reefs 2007, 26, 1047–1056. [Google Scholar]
- Hviding, E. Traditional institutions and their role in the contemporary coastal resource management in the Pacific Islands. Naga ICLARM Q. 1991, 14, 3–6. [Google Scholar]
- Cinner, J.E.; Aswani, S. Integrating customary management into marine conservation. Biol. Conserv. 2007, 140, 201–216. [Google Scholar]
- Foale, S.; Cohen, P.; Januchowski-Hartley, S.; Wenger, A.; Macintyre, M. Tenure and taboos: Origins and implications for fisheries in the Pacific. Fish Fish. 2011, 12, 357–369. [Google Scholar]
- Cinner, J.E. Socioeconomic factors influencing customary marine tenure in the Indo-Pacific. Ecol. Soc. 2005, 10, 1–14. [Google Scholar]
- Johannes, R.E. The renaissance of community-based marine resource management in Oceania. Annu. Rev. Ecol. Syst. 2002, 33, 317–340. [Google Scholar]
- Bartlett, C.Y.; Manua, C.; Cinner, J.; Sutton, S.; Jimmy, R.; South, R.; Nilsson, J.; Raina, J. Comparison of outcomes of permanently closed and periodically harvested coral reef reserves. Conserv. Biol. 2009, 23, 1475–1484. [Google Scholar]
- Williams, I. D.; Walsh, W.J.; Miyasaka, A.; Friedlander, A.M. Effects of rotational closure on coral reef fishes in Waikiki-Diamond head fishery management area, Oahu, Hawai. Mar. Ecol. Prog. Ser. 2006, 310, 139–149. [Google Scholar]
- Gerber, L. R.; Botsford, LW.; Hastings, A.; Possingham, H.P.; Gaines, S.D.; Palumbi, S.R.; Andelman, S. Population models for marine reserve design: A retrospective and prospective synthesis. Ecol. Appl. 2003, 13, S47–S64. [Google Scholar]
- Valderrama, D.; Anderson, J.L. Improving utilization of the Atlantic Sea Scallop resource: An analysis of rotational management of fishing grounds. Land Econ. 2007, 83, 86–103. [Google Scholar]
- Ruddle, K. Traditional management of reef fishing. In Reef Fisheries; Polunin, N.V.C., Roberts, C.M., Eds.; Chapman & Hall: London, UK, 1996; pp. 315–335. [Google Scholar]
- McClanahan, T.R.; Marnane, M.J.; Cinner, J.E.; Kiene, W.J. A comparison of marine protected areas and alternative approaches to coral-reef management. Curr. Biol. 2006, 15, 1406–1413. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Fenner, D. Challenges for Managing Fisheries on Diverse Coral Reefs. Diversity 2012, 4, 105-160. https://doi.org/10.3390/d4010105
Fenner D. Challenges for Managing Fisheries on Diverse Coral Reefs. Diversity. 2012; 4(1):105-160. https://doi.org/10.3390/d4010105
Chicago/Turabian StyleFenner, Douglas. 2012. "Challenges for Managing Fisheries on Diverse Coral Reefs" Diversity 4, no. 1: 105-160. https://doi.org/10.3390/d4010105
APA StyleFenner, D. (2012). Challenges for Managing Fisheries on Diverse Coral Reefs. Diversity, 4(1), 105-160. https://doi.org/10.3390/d4010105



