Italian Common Bean Landraces: History, Genetic Diversity and Seed Quality
Abstract
:1. Domestication and Dissemination Pathways of Common Bean
2. Common Bean Introduction in Italy

3. Italian Landraces and Safeguard Actions
| Code | Collection year | Local name | Donor | Plant and seed description |
|---|---|---|---|---|
| VIR 2096 | 1924 | Fagiolo Burro Rampicante | Ingegnoli, Milan | climbing bean; very late maturity; sphaericus x ellipticus; seeds large, dark-violet to black |
| VIR 2279 | 1925 | Raparino Nano | Instituto Superiore Abburo, Bologna | bush bean; very late maturity; green pod; ellipticus; seeds pale reddish-brown with red stippling |
| VIR 2284 | 1925 | Raparino Gigante | Instituto Superiore Abburo Bologna | semi-climbing bean; late maturity; ellipticus; seeds pale reddish-brown with red stippling |
| VIR 3559 | 1926 | Fagiolo Metis | Ingegnoli, Rome | bush bean; middle maturity; flowers white; seeds bicolored: half is white and half black |
| VIR 3560 | 1926 | Fagiolo Cento per uno | Ingegnoli, Rome | bush bean; middle maturity; pods flat; seeds small, pale brown |
| VIR 3562 | 1926 | Regino Nano | Ingegnoli, Rome | late maturity; plant with curly top; pods green with striped purple; seeds pale reddish-brown with red stippling |
| VIR 3563 | 1926 | Fagiolo | Orvieto, Umbria | bush bean; late maturity; flowers pale pink; green pod; seeds buff |
| VIR 3564 | 1926 | No name | Orvieto, Umbria | semi-climbing bean; late maturity; flowers white; seeds small, white |
| VIR 3565 | 1926 | No name | Orvieto, Umbria | semi-climbing bean; late maturity; flowers white; green pods; seeds slightly flattened, white |
| VIR 5009 | 1926 | Fagioli scritti | Lucca, Tuscany | bush bean; late maturity; flowers pale pink; pods green and flat; seeds pale reddish-brown with pink stippling; productivity medium |
| VIR 5010 | 1926 | Fagioli rossi scritti | Lucca, Tuscany | bush bean; late maturity; flowers pink; green pod; seeds reddish-brown with purple stippling; low productivity |
| VIR 5105 | 1926 | Fagiolo Bianco comune | unknown | semi-climbing bean; very late maturity; flowers pink; seeds flat, white |
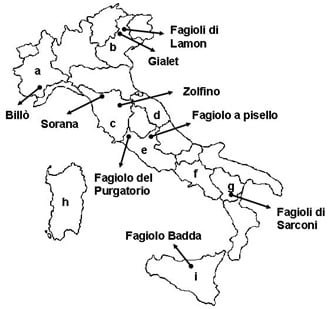
4. Characterization and Evaluation of Italian Common Bean Landraces
4.1. Phenotypic Variation
| Region | N. pop | Coat without pattern | Coat with pattern | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| White | Brown pale to dark | Yellow | Black | Other | bicolor | Borlotto type | Pattern around hilum | |||
| Abruzzo | 10 | 3 | 1 | 4 | 2 | [28,47,49] | ||||
| Basilicata | 65 | 18 | 11 | 6 | 10 | 16 | 4 | [30,37,117] | ||
| Calabria | 1 | 1 | [118,119] | |||||||
| Campania | 3 | 2 | 1 | [67,102] | ||||||
| Friuli | 4 | 2 | 2 | [56] | ||||||
| Lazio | 19 | 6 | 4 | 1 | 1 | 1 | 1 | 5 | [28,49,53,119] | |
| Liguria | 2 | 1 | 1 | [119] | ||||||
| Marche | 23 | 8 | 4 | 1 | 3 | 7 | [120] | |||
| Piedmont | 5 | 1 | 1 | 3 | [119] | |||||
| Sicily | 4 | 3 | 1 | [100,119] | ||||||
| Tuscany | 24 | 11 | 2 | 1 | 1 | 1 | 2 | 4 | 2 | [59,121] |
| Veneto | 8 | 1 | 1 | 1 | 5 | [34,54,122,123] | ||||
| Total | 168 | 51 | 25 | 5 | 3 | 9 | 19 | 46 | 10 | |
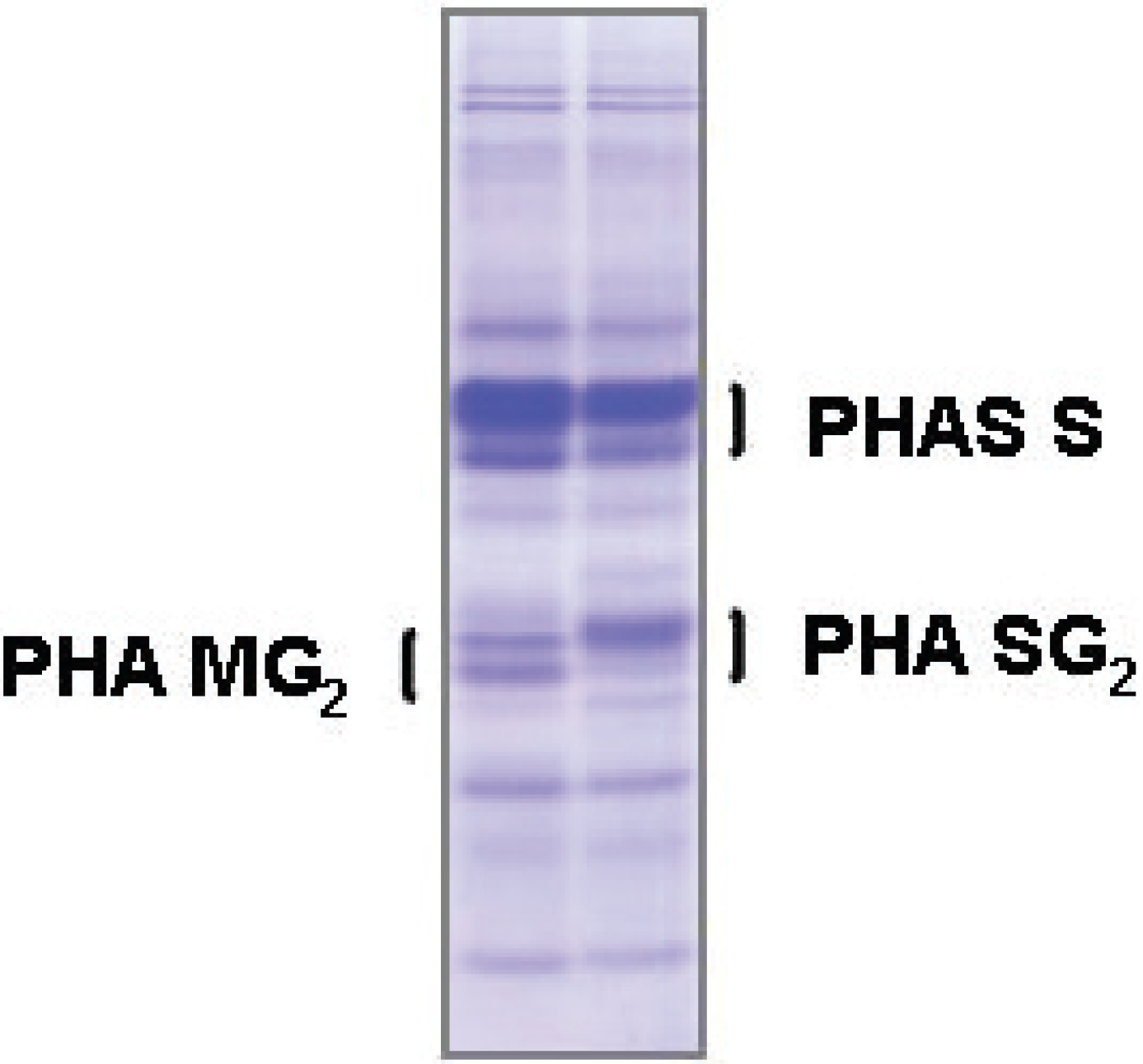
4.2. Seed Storage Proteins as Biochemical Markers
| Region | N pop | Phaseolin type | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T | C | T+C | A | H | Mix Andean | S | Mix Andean- Mesoamerican | |||
| Abruzzo | 9 | 1 | 1 | 1 | 3 | 3 | [ 49] | |||
| Basilicata | 66 | 18 | 38 | 8 | 2 | [ 45,46,104] | ||||
| Calabria | 4 | 3 | 1 | [ 118] | ||||||
| Campania | 98 | 89 | 9 | [ 57,67] | ||||||
| Lazio | 17 | 4 | 1 | 1 | 4 | 3 | 2 | 2 | [ 34,49] | |
| Piedmont | 5 | 1 | 3 | 1 | [ 34,50] | |||||
| Sardinia | 73 | 21 | 51 | 1 | [ 66] | |||||
| Sicily | 28 | 3 | 17 | 1 | 7 | * | ||||
| Tuscany | 1 | 1 | [ 104] | |||||||
| Veneto | 12 | 4 | 5 | 3 | [ 34,54,104,122] | |||||
| Total | 313 | 49 | 119 | 89 | 2 | 4 | 8 | 31 | 8 | |
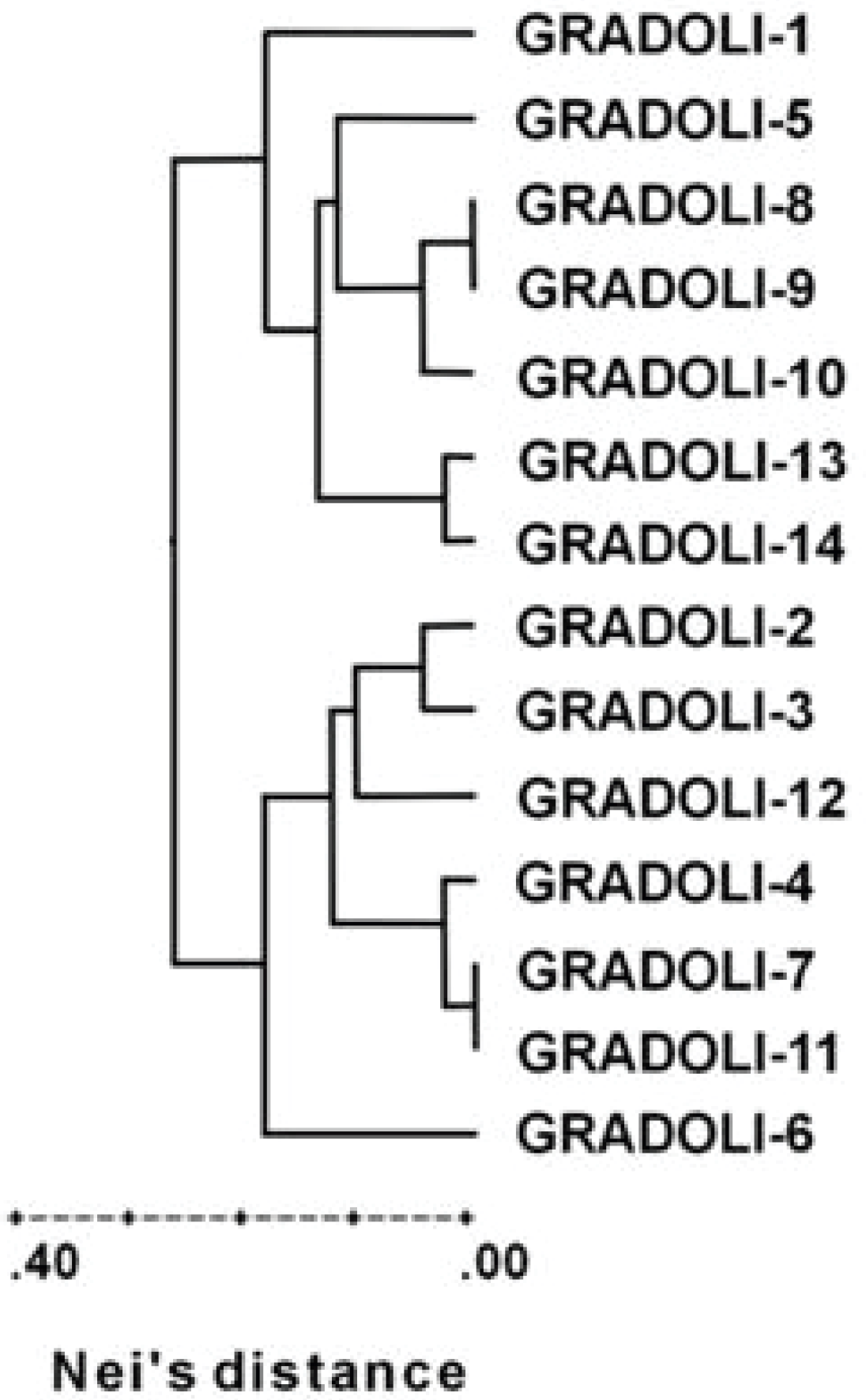
4.3. Molecular Diversity
4.4. Seed Quality
| Local name | Cultivation region | Protein (% dm) | Ref. |
|---|---|---|---|
| Northern Italy | |||
| Billò | Piedmont | 27.4 | [50] |
| Bianco di Bagnasco | Piedmont | 23.2 | [27] |
| Spagnolet nano | Veneto | 21.8–23.8 | [27] |
| Gialet | Veneto | 26.1–27.8 | [123] |
| Bala rossa | Veneto | 24.5 | [54] |
| Bonei | Veneto | 24.2 | [54] |
| Lamon | Veneto | 28.0 | [108] |
| Central Italy | |||
| Zolfino | Tuscany | 21.5 | [124] |
| Fagiolo del Purgatorio | Lazio | 21.4–27.6 | [27] |
| Fagiolo romanesco | Lazio | 26.8 | [125] |
| Fagiolo regina | Lazio | 27.7 | [125] |
| Fagiolina arsolana | Lazio | 23.6–27.3 | [125] |
| Cioncone | Lazio | 24.4–25.9 | [125] |
| Pallini | Lazio | 23.4 | [125] |
| A pisello peligni | Abruzzo | [125] | |
| Pane aquilano | Abruzzo | [125] | |
| Pane peligni | Abruzzo | [125] | |
| Sourthern Italy | |||
| Bianco di Rotonda | Basilicata | 26.6 | [34] |
| Ciuoto | Basilicata | 20.8 | [34] |
| Verdolino | Basilicata | 22.2 | [34] |
| Bianco del Pollino | Calabria | 23.6–27.2 | [118] |
| Badda bianco, Badda nero and Monaco Mussu Niuru | Sicily | 26.8–27.1 | [108,126] |
5. Conclusions
Acknowledgements
References
- Kaplan, L.; Lynch, T.F. Phaseolus (Fabaceae) in archaeology: AMS radiocarbon dates and their significance for pre-colombian agriculture. Econ. Bot. 1999, 53, 261–272. [Google Scholar] [CrossRef]
- Gepts, P.; Kmiecik, K.; Pereira, P.; Bliss, F.A. Dissemination pathways of common bean (Phaseolus vulgaris Fabaceae) deduced from phaseolin electrophoresis variability. I The Americas. Econ. Bot. 1988, 42, 73–85. [Google Scholar] [CrossRef]
- Singh, S.P. Patterns of variation in cultivated common bean (Phaseolus vulgaris Fabaceae). Econ. Bot. 1989, 43, 39–57. [Google Scholar] [CrossRef]
- Singh, S.P.; Gepts, P.; Debouck, D.G. Races of common bean (Phaseolus vulgaris Fabaceae). Econ. Bot. 1991a, 45, 379–396. [Google Scholar] [CrossRef]
- Singh, S.P.; Gutierrez, J.A.; Molina, A.; Urrea, C.; Gepts, P. Genetic diversity in cultivated common bean. II Marker-based analysis of morphological and agronomic traits. Crop Sci. 1991b, 31, 23–29. [Google Scholar] [CrossRef]
- Gepts, P.; Osborn, T.C.; Rashka, K.; Bliss, F.A. Phaseolin protein variability in wild forms and landraces of common bean (Phaseolus vulgaris L.): evidence for multiple centers of domestication. Econ. Bot. 1986, 40, 451–468. [Google Scholar] [CrossRef]
- Gepts, P.; Bliss, F.A. Dissemination pathways of common bean (Phaseolus vulgaris Fabaceae) deduced from phaseolin electrophoresis variability. II Europe and Africa. Econ. Bot. 1988, 42, 86–104. [Google Scholar] [CrossRef]
- Koenig, R.L.; Singh, S.P.; Gepts, P. Novel phaseolin types in wild and cultivated common bean (Phaseolus vulgaris Fabaceae). Econ. Bot. 1990, 44, 50–60. [Google Scholar] [CrossRef]
- Koenig, R.L.; Gepts, P. Allozyme diversity in wild Phaseolus vulgaris: further evidence for two major centers of genetic diversity. Theor. Appl. Genet. 1989, 78, 809–817. [Google Scholar]
- Debouck, D.G.; Toro, O.; Paredes, O.M.; Johnson, W.C.; Gepts, P. Genetic diversity and ecological distribution of Phaseolus vulgaris (Fabaceae) in northwestern south America. Econ. Bot. 1993, 47, 408–423. [Google Scholar] [CrossRef]
- Gepts, P. Origin and evolution of common bean: past events and recent trends. HortSci. 1998, 33, 1124–1130. [Google Scholar]
- Mumba, L.E.; Galwey, N.W. Compatibility between wild and cultivated common bean (Phaseolus vulgaris L.) genotypes of the Mesoamerican and Andean gene pools: Evidence from the inheritance of quantitative characters. Euphytica 1999, 108, 105–119. [Google Scholar] [CrossRef]
- Pallottini, L.; Garcia, E.; Kami, J.; Barcaccia, G.; Gepts, P. The genetic anatomy of a patented yellow bean. Crop Sci. 2004, 44, 968–977. [Google Scholar] [CrossRef]
- Birri, F.; Coco, C. Cade a Fagiolo; La Grafica & Stampa: Vicenza, Italy, 2000; p. 184. [Google Scholar]
- Zeven, A.C. The introduction of the common bean (Phaseolus vulgaris L.) into Western Europe and the phenotypic variation of dry beans collected in the Netherlands in 1946. Euphytica 1997, 94, 319–328. [Google Scholar] [CrossRef]
- Krell, K.; Hammer, K. 500 jahre gartenbohne (Phaseolus vulgaris L.) in Europa. Botanik, einführungsgeschichte und genetische ressourcen; Schriften VEN 7: Cremlingen-Schandelah, Germany, 2008; p. 100. [Google Scholar]
- McClean, P.E.; Myers, J.; Hammond, J.J. Coefficient of parentage and cluster analysis of North American dry bean cultivars. Crop Sci. 1993, 33, 190–197. [Google Scholar] [CrossRef]
- Albala, K. Phaseolus vulgaris: Mexico and the World. In Beans. A History; Berg Publishers: Oxford, UK, 2007; pp. 127–190. [Google Scholar]
- Hammer, K.; Knupfer, H.; Laghetti, G.; Perrino, P. Seeds from the Past. A Catalogue of Crop Germplasm in South Italy and Sicily; Italgrafica Sud: Bari, Italy, 1992; p. 173. [Google Scholar]
- Polegri, L.; Negri, V. Molecular markers for promoting agro-biodiversity conservation: a case study from Italy. How cowpea landraces were saved from extinction. Genet. Resour. Crop Evol. 2010. [Google Scholar] [CrossRef]
- Piola Caselli, C. Il regno vegetale. Agricoltura—frutticoltura—orticoltura. In Le origini degli alimenti e la loro conservazione nel mondo; Museo Europeo, Museo Lombardo di Storia dell’Agricoltura: Lodi, Italy, 1995. [Google Scholar]
- Comes, O. Del fagiolo comune: storia, filogenesi, qualità sospettata tossicità e sistemazione delle sue razze ovunque coltivate. Atti Ist. Incoraggiamento di Napoli 1910, 61, 75–145. [Google Scholar]
- Guzzini, D.; Gherardi, E. Il Fagiolo; R.E.D.A.: Roma, Italy, 1936. [Google Scholar]
- Zanini, E. Varietà ed ibridi di fagiolo coltivati in Sicilia. Studio biologico e sistematico. Giornale di Scienze Naturali ed Economiche 1949, 46, 1–52. [Google Scholar]
- Hammer, K.; Knupfer, H.; Laghetti, G.; Perrino, P. Seeds from the Past. A Catalogue of Crop Germplasm in Central and North Italy; Italgrafica Sud: Bari, Italy, 1999; p. 254. [Google Scholar]
- Bellati, G.P. Risposte del Comizio Agrario di Feltre; Feltre (Bl), Italy, 1896. [Google Scholar]
- Lioi, L.; Piergiovanni, A.R.; Soressi, G.P.; Nigro, C.; Tamietti, G.; Turina, M.; Campion, B. Caratterizzazione, selezione, risanamento e valutazione di cultivar locali di fagiolo comune (Phaseolus vulgaris L.). Italus Hortus 2007, 14, 31–40. [Google Scholar]
- Piergiovanni, A.R.; Lioi, L. La conservazione on farm: importanza della caratterizzazione e valutazione degli agro-ecotipi di fagiolo. Agroindustria 2007, 6, 29–36. [Google Scholar]
- Belletti, P.; Quagliotti, L. Conservazione di germoplasma ortivo in Piemonte. Sementi Elette 1996, 42, 3–7. [Google Scholar]
- Masi, P.; Figliuolo, G.; Spagnoletti Zeuli, P.L. Landraces of bean (Phaseolus vulgaris L.) collected in Basilicata, Italy. Plant Genet. Res. Newslett. 1999, 119, 51–55. [Google Scholar]
- Negri, V.; Tosti, N. Phaseolus genetic diversity maintained on-farm in central Italy. Genet. Resour. Crop Evol. 2002, 49, 511–520. [Google Scholar] [CrossRef]
- Negri, V.; Tosti, N. Genetic diversity within a common bean landrace of potential economic value: its relevance for on-farm conservation and product certification. J. Genet Breed. 2002, 56, 113–118. [Google Scholar]
- Silveri, D.D.; Dalla Ragione, I.; Porfiri, O.; Torricelli, R.; Tosti, N.; Veronesi, F. Collecting, evaluation and conservation of plant genetic resources in the Abruzzo region, central Italy. Plant Genet. Resour. Newslett. 2002, 129, 36–43. [Google Scholar]
- Lioi, L.; Piergiovanni, A.R.; Pignone, D.; Puglisi, S.; Santantonio, M.; Sonnante, G. Genetic diversity of some surviving on-farm Italian common bean (Phaseolus vulgaris L.) landraces. Plant Breed. 2005, 124, 576–581. [Google Scholar] [CrossRef]
- Piergiovanni, A.R.; Laghetti, G. The common bean landraces from Basilicata (Southern Italy): an example of integrated approach applied to genetic resources management. Genet. Resour. Crop Evol. 1999, 46, 47–52. [Google Scholar] [CrossRef]
- Piotto, B.; Giacanelli, V.; Ercole, S. (a cura di). La conservazione ex situ della biodiversità delle specie vegetali spontanee e coltivate in Italia. Stato dell’arte, criticità e azioni da compiere. Manuali e linee guida ISPRA 52/2010.
- Piergiovanni, A.R.; Brandi, M.; Cerbino, D.; Olita, G.; Perrino, P.; Laghetti, G. Gli agro-ecotipi di fagiolo (Phaseolus vulgaris L.) di Sarconi e Rotonda (Basilicata, Italy); Laghetti, G., Piergiovanni, A.R., Eds.; BGM: Matera, Italy, 2000; p. 150. [Google Scholar]
- Dalla Ragione, I.; Porfiri, O.; Silveri, D.D.; Torricelli, R.; Veronesi, F. Le risorse genetiche autoctone della regione Abruzzo: un patrimonio da valorizzare; Arti Grafiche Galvan: Avezzano, Italy, 2004; pp. 90–92. [Google Scholar]
- Filella, F.; Brunori, A.; Convertini, M.; Bruno, M.; Tarditi, E. Recupero e caratterizzazione degli ecotipi di fagioli individuati sul territorio della regione Calabria. In Proceedings of VIII Congresso Nazionale “La biodiversità—una risorsa per sistemi multifunzionali”, Lecce, Italy, 21–23 April 2008; Arti Grafiche Favia: Modugno, Italy; p. 175.
- Spizzo, A. I fagioli di Pradumbli. In La biodiversità coltivata. Storie di persone, piante e agricoltura tradizionale tra Friuli e Carinzia; Università di Udine: Udine, Italy, 2008; pp. 143–147. [Google Scholar]
- Breda, N. I Fagioli scritti e l’occhio della Fava. In Biodiversità coltivata nel Parco Nazionale delle Dolomiti Bellunesi; Grafiche Crivellari: Ponzano Veneto, Italy, 2006; pp. 81–117. [Google Scholar]
- IBPGR. Descriptors for Phaseolus Vulgaris; Rome, Italy, 1982. [Google Scholar]
- Venora, G.; Grillo, O.; Ravalli, C.; Cremonini, R. Identification of Italian landraces of bean (Phaseolus vulgaris L.) using an image analysis system. Sci. Hort. 2009, 121, 410–418. [Google Scholar] [CrossRef]
- Dell’Aquila, A. Towards new computer imaging techniques applied to seed quality testing and sorting. Seed Sci. Tech. 2007, 35, 519–538. [Google Scholar]
- Limongelli, G.; Laghetti, G.; Perrino, P.; Piergiovanni, A.R. Variation of seed storage proteins in landraces of common bean (Phaseolus vulgaris L.) from Basilicata, southern Italy. Euphytica 1996, 92, 393–399. [Google Scholar]
- Piergiovanni, A.R.; Cerbino, D.; Brandi, M. The common bean populations from Basilicata (southern Italy). An evaluation of their variation. Genet. Resour. Crop Evol. 2000, 47, 489–495. [Google Scholar] [CrossRef]
- Piergiovanni, A.R.; Taranto, G.; Pignone, D. Diversity among common bean populations from the Abruzzo region (Central Italy): a preliminary inquiry. Genet. Resour. Crop Evol. 2000, 47, 467–470. [Google Scholar] [CrossRef]
- Piergiovanni, A.R.; Cerbino, D.; Della Gatta, C. Diversity in seed quality traits of common bean populations from Basilicata (Southern Italy). Plant Breed. 2000, 119, 513–516. [Google Scholar] [CrossRef]
- Piergiovanni, A.R.; Taranto, G.; Losavio, F.P. Common bean (Phaseolus vulgaris L.) landraces from Abruzzo and Lazio regions (central Italy). Genet. Resour. Crop Evol. 2006, 53, 313–322. [Google Scholar] [CrossRef]
- Piergiovanni, A.R.; Taranto, G.; Losavio, F.P.; Lioi, L. Gli agro-ecotipi di fagiolo (Phaseolus vulgaris L.) del cuneese. Primi risultati di un’indagine multidisciplinare. Italus Hortus 2006, 13, 485–487. [Google Scholar]
- Quagliotti, L.; Portis, E.; Lisa, V.; Dellavalle, G. Raccolta di germoplasma ortivo in Piemonte. Recenti risultati. Sementi Elette 1998, 44, 11–19. [Google Scholar]
- Turchi, R. Il Fagiolo zolfino. In Proceedings of Congress: Il germoplasma della Toscana: tutela e valorizzazione, Firenze, Italy, 2000; pp. 15–17.
- Agenzia Regionale per lo Sviluppo e Innovazione dell’Agricoltura del Lazio. Prodotti tipici e tradizionali del Lazio. Available online: http://www.arsial.it/portalearsial/prd_tipici/Default.asp (acccessed May 2010).
- Piergiovanni, A.R.; Taranto, G.; Losavio, F.P.; Sanson, S. The agro-ecotypes of common bean (Phaseolus vulgaris L.) from Val Belluna (Veneto region). In Proceedings of the XLVIII Italian Society of Agricultural Genetics Annual Congress, Lecce, Italy, 15–18 September 2004; TorGraf: Galatina, Italy; pp. 173–174.
- Sanson, S. Fagiolo Giale. In : Biodiversità coltivata nel Parco Nazionale delle Dolomiti Bellunesi; Grafiche Crivellari: Ponzano Veneto, Italy, 2006; pp. 178–179. [Google Scholar]
- Foschiani, A.; Miceli, F.; Vischi, M. Assessing diversity in common bean (Phaseolus vulgaris L.) accessions at phenotype and molecular level: a preliminary approach. Genet. Resour. Crop Evol. 2009, 56, 445–453. [Google Scholar] [CrossRef]
- Gioia, T.; Ierardi, G.; Montesano, G.; Viggiani, F.; Spagnoletti Zeuli, P.L.; Logozzo, G. Raccolta, caratterizzazione, valorizzazione e tutela di ‘tipi morfologici’ di fagiolo (Phaseolus vulgaris L.) per l’ottenimento di produzioni tipiche certificate nel parco del Cilento. In Proceedings of 4th Convegno Nazionale sulle Piante Mediterranee, Marina di Nova Siri, Italy, 7–10 October 2009; Tipografia Grafica Sud: Policoro, Italy; p. 75.
- Sicard, D.; Nanni, L.; Porfiri, O.; Bulfon, D.; Papa, R. Genetic diversity of Phaseolus vulgaris L. and Phaseolus coccineus L. landraces in central Italy. Plant. Breed. 2005, 124, 464–472. [Google Scholar] [CrossRef]
- Baldanzi, M.; Pardini, G. Descrizione della varietà locale di fagiolo “Piattella pisana” o “San Michele”. Agricoltura Ricerca. 2002, 189, 59–72. [Google Scholar]
- Tallarico, R.; Tesi, R.; Chisci, G. Caratterizzazione e valutazione del fagiolo “Turco della Garfagnana” (Phaseolus vulgaris L.). In Proceedings of National Congress: Il fagiolo fresco in Italia: stato attuale e prospettive, Potenza, Italy, 1994; pp. 91–100.
- Santangelo, E.; Mazzuccato, A.; Picarella, M.E.; Mosconi, P.; Lioi, L.; Soressi, G.P. Caratterizzazione del “Fagiolo del Purgatorio di Gradoli” (VT). Italus Hortus 2006, 13, 496–502. [Google Scholar]
- Falcinelli, M.; Negri, V. Utilizzazione e valorizzazione delle antiche varietà locali nell’ambito della conservazione delle risorse genetiche vegetali. Sementi Elette 1998, 44, 5–9. [Google Scholar]
- Logozzo, G.; Donnoli, R.; Macaluso, L.; Papa, R.; Knüpffer, H.; Spagnoletti Zeuli, P.L. Analysis of the contribution of Mesoamerican and Andean gene pools to European common bean (Phaseolus vulgaris L.) germplasm and strategies to establish a core collection. Genet. Resour. Crop Evol. 2007, 54, 1763–1779. [Google Scholar] [CrossRef]
- Lioi, L. Geographical variation of phaseolin in an old world collection of Phaseolus vulgaris. Seed Sci. Technol. 1989, 17, 317–324. [Google Scholar]
- Tiranti, B.; Logozzo, G.; Spagnoletti Zeuli, P.L.; Negri, V. Genetic diversity in a common bean (Phaseolus vulgaris L.) ex situ collection of Italian landraces. In Proceedings of the 18th Eucarpia Genetic Resources Section Meeting, Piestany, Slovakia, 2007.
- Angioi, S.A.; Rau, D.; Rodriguez, M.; Logozzo, G.; Desiderio, F.; Papa, R.; Attene, G. Nuclear and chloroplast microsatellite diversity in Phaseolus vulgaris L. from Sardinia (Italy). Mol. Breed. 2009, 23, 413–429. [Google Scholar]
- Mennella, G.; Onofaro Sanajà, V.; D’Alessandro, A.; Milone, M.; Perrone, D. HPLC analyses of seed storage proteins reveal polymorphism in Italian common bean (Phaseolus vulgaris L.) ecotypes. Euphytica 2003, 134, 85–95. [Google Scholar] [CrossRef]
- Rodiño, A.P.; Santalla, M.; De Ron, A.M.; Singh, S.P. A core collection of common bean from the Iberian Peninsula. Euphytica 2003, 131, 165–175. [Google Scholar] [CrossRef]
- Laghetti, G.; Piergiovanni, A.R.; Sonnante, G.; Lioi, L.; Pignone, D. The Italian lentil genetic resources: a worthy basic tool for breeders. Europ. J. Plant Sci. Biotech. 2008, 2, 48–59. [Google Scholar]
- Zeven, A.C.; Waninge, J.; Van Hintum, Th.; Singh, S.P. Phenotypic variation in a core collection of common bean (Phaseolus vulgaris L.) in the Netherlands. Euphytica 1999, 109, 93–106. [Google Scholar] [CrossRef]
- Rodiño, A.P.; Santalla, M.; Gonzales, A.M.; De Ron, A.M.; Singh, S.P. Novel genetic variation in common bean from the Iberian Peninsula. Crop Sci. 2006, 46, 2540–2546. [Google Scholar] [CrossRef]
- Brown, J.W.S.; Osborn, T.C.; Bliss, F.A.; Hall, T.C. Bean lectins: Part 1: Relationships between agglutinating activity and electrophoretic variation in the lectin-containing G2/albumin seed proteins of French bean (Phaseolus vulgaris L.). Theor. Appl. Genet. 1982, 62, 263–271. [Google Scholar]
- Brown, J.W.S.; Bliss, F.A.; Hall, T.C. Linkage relationships between genes controlling seed proteins in French bean. Theor. Appl. Genet. 1981, 60, 251–259. [Google Scholar] [CrossRef]
- Gepts, P. A Middle American and an Andean common bean gene pool. In Genetic Resources of Phaseolus Beans: Their Maintenance, Domestication, Evolution and Utilization; Gepts, P., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1988; pp. 375–390. [Google Scholar]
- Lioi, L. Electrophoretic variation and geographical distribution of the seed protein phytohemagglutinin in cultivated Phaseolus vulgaris L. J. Genet. Breed. 1991, 45, 97–102. [Google Scholar]
- Lioi, L.; Soressi, G.P. Caratterizzazione della popolazione locale “Fagiolo del Purgatorio” di Gradoli (VT) mediante parametri fenotipici, produttivi, biochimico-nutrizionali e genetico-molecolari. Italus Hortus 2006, 13, 496–502. [Google Scholar]
- Ellegren, H. Microsatellites: simple sequences with complex evolution. Nat. Rev. Genet. 2004, 5, 435–445. [Google Scholar] [CrossRef]
- Yu, K.; Park, S.J.; Poysa, V. Abundance and variation of microsatellite DNA sequences in beans (Phaseolus and Vigna). Genome 1999, 42, 27–34. [Google Scholar] [CrossRef]
- Yu, K.; Park, S.J.; Poysa, V.; Gepts, P. Integration of simple sequence repeat (SSR) markers into a molecular linkage map of common bean (Phaseolus vulgaris L.). J. Hered. 2000, 91, 429–434. [Google Scholar] [CrossRef]
- Guerra-Sanz, J.M. New SSR markers of Phaseolus vulgaris from sequence databases. Plant Breed. 2004, 123, 87–89. [Google Scholar] [CrossRef]
- Caixeta, E.T.; Borém, A.; Kelly, J.D. Microsatellite markers for common bean. Ann. Rep. Bean Improv. Coop. 2003, 46, 157–158. [Google Scholar]
- Gaitán-Solís, E.; Duque, M.C.; Edwards, K.J.; Tohme, J. Microsatellite repeats in common bean (Phaseolus vulgaris): isolation, characterization, and cross-species amplification in Phaseolus ssp. Crop Sci. 2002, 42, 2128–2136. [Google Scholar] [CrossRef]
- Yaish, M.W.F.; Perez de la Vega, M. Isolation of (GA)n microsatellite sequences and description of a predicted MADS-box sequence isolated from common bean (Phaseolus vulgaris L.). Genet. Mol. Biol. 2003, 3, 337–342. [Google Scholar] [CrossRef]
- Buso, G.S.C.; Amaral, Z.P.S.; Brondani, R.P.V.; Ferreira, M.E. Microsatellite markers for the common bean Phaseolus vulgaris. Mol. Ecol. Notes 2006, 6, 252–254. [Google Scholar] [CrossRef]
- Hanai, L.Z.; de Campos, T.; Camargo, L.E.A.; Benchimol, L.L.; de Souza, A.P.; Melotto, M.; Carbonell, S.A.L.; Chioratto, A.F.; Consoli, L.; Formighieri, E.F.; Siqueira, M.V.B.M.; Tsai, S.M.; Vieira, M.L.C. Development, characterization, and comparative analysis of polymorphism at common bean SSR loci isolated from genic and genomic sources. Genome 2007, 50, 266–277. [Google Scholar] [CrossRef]
- Blair, M.W.; Muñoz Torres, M.; Pedraza, F.; Giraldo, M.C.; Buendía, H.F.; Hurtado, N. Development of microsatellite markers for common bean (Phaseolus vulgaris L.) based on screening of non-enriched, small-insert genomic libraries. Genome 2009, 52, 772–782. [Google Scholar] [CrossRef]
- Blair, M.W.; Pedraza, F.; Buendía, H.F.; Gaitán-Solís, E.; Beebe, S.E.; Gepts, P.; Tohme, J. Development of a genome-wide anchored microsatellite map for common bean (Phaseolus vulgaris L.). Theor. Appl. Genet. 2003, 107, 1362–1374. [Google Scholar] [CrossRef]
- Grisi, M.C.M.; Blair, M.W.; Gepts, P.; Brondani, C.; Pereira, P.A.A.; Brondani, R.P.V. Genetic mapping of a new set of microsatellite markers in a reference common bean (Phaseolus vulgaris) population BAT93 x Jalo EEP558. Genet. Mol. Res. 2007, 6, 691–706. [Google Scholar]
- Métais, I.; Hamon, B.; Jalouzot, R.; Peltier, D. Structure and level of genetic diversity in various bean types evidenced with microsatellite markers isolated from a genomic enriched library. Theor. Appl. Genet. 2002, 104, 1346–1352. [Google Scholar] [CrossRef]
- Gómez, O.J.; Blair, M.W.; Frankow-Lindberg, B.E.; Gullberg, U. Molecular and phenotypic diversity of common bean landraces from Nicaragua. Crop Sci. 2004, 44, 1412–1418. [Google Scholar] [CrossRef]
- Blair, M.W.; Giraldo, M.C.; Buendía, H.F.; Tovar, E.; Duque, M.C.; Beebe, S.E. Microsatellite marker diversity in common bean. (Phaseolus vulgaris L.). Theor. Appl. Genet. 2006, 113, 100–109. [Google Scholar] [CrossRef]
- Díaz, L.M.; Blair, M.W. Race structure within the Mesoamerican gene pool of common bean (Phaseolus vulgaris L.) as determined by microsatellite markers. Theor. Appl. Genet. 2006, 114, 143–154. [Google Scholar] [CrossRef]
- Blair, M.W.; Muñoz Torres, M.; Giraldo, M.C.; Pedraza, F. Development and diversity of Andean-derived, gene-based microsatellites for common bean (Phaseolus vulgaris L.). BMC Plant Biol. 2009, 9, 100. [Google Scholar]
- Masi, P.; Spagnoletti Zeuli, P.L.; Donini, P. Development and analysis of multiplex microsatellite markers sets in common bean (Phaseolus vulgaris L.). Mol. Breed. 2003, 11, 303–313. [Google Scholar] [CrossRef]
- Grassi, F.; Labra, M.; Minuto, L. Molecular diversity in Ligurian local races of common bean (Phaseolus vulgaris L.). Plant Biosyst. 2006, 140, 17–20. [Google Scholar] [CrossRef]
- Tiranti, B.; Negri, V. Selective microenvironmental effects play a role in shaping genetic diversity and structure in a Phaseolus vulgaris L. landrace: implications for on-farm conservation. Mol. Ecol. 2007, 16, 4942–4955. [Google Scholar] [CrossRef]
- Beebe, S.; Rengifo, E.; Gaitan, M.C.; Duque, M.C.; Tohme, J. Diversity and origin of Andean landraces of common bean. Crop Sci. 2001, 41, 854–862. [Google Scholar] [CrossRef]
- Papa, R.; Acosta, J.; Delgado-Salinas, A.; Gepts, P. A genome-wide analysis of differentiation between wild and domesticated Phaseolus vulgaris from Mesoamerica. Theor. Appl. Genet. 2005, 111, 1147–1158. [Google Scholar] [CrossRef]
- Paniconi, G.; Gianfilippi, F.; Picarella, M.E.; Mosconi, P.; Mazzuccato, A. Caratterizzazione del fagiolo ‘Badda’ di Polizzi Generosa (Palermo). In Proceedings II National Congress “La biodiversità—una risorsa per sistemi multifunzionali”, Lecce, Italy, 21–23 April 2008.
- Lioi, L.; Campion, B.; Piergiovanni, A.R. Genetic diversity and technological traits of a common bean landrace from Sicily (Italy): Fagiolo a Badda. In Meeting of the International Consortium Phaseomics V, Pontevedra, Spain, 21–23 May 2009; Idea Grafica: Pontevedra, Spain; p. 72.
- Avitabile, A.; Pentangelo, A.; Giordano, I.; Di Mauro, A.; Rao, R. Caratterizzazione bioagronomica e molecolare dell’ecotipo fagiolo di Controne. In Proceedings of 5th Biodiversity National Congress, Caserta, Italy, 1999; pp. 471–475.
- Zaccardelli, M.; Torre, R.; Campanile, F.; Giordano, I. Molecular characterization of the bean (Phaseolus vulgaris L.) ecotype “Fagiolo occhio nero di Oliveto Citra”. In Proceedings of the XLVIII Italian Society of Agricultural Genetics Annual Congress, Lecce, Italy, 15–18 September 2004.
- Del Piano, L.; Capone, C.; Sorrentino, C.; Abet, M.; Sicignano, M.; Enotrio, T. ISSR and SSR analysis of the common bean (Phaseolus vulgaris L.) ecotype “fagiolo di Controne”. In Proceedings of the LIII Italian Society of Agricultural Genetics Annual Congress, Torino, Italy, 2009.
- Marotti, I.; Bonetti, A.; Minelli, M.; Catizone, P.; Dinelli, G. Characterization of some Italian common bean (Phaseolus vulgaris L.) landraces by RAPD, semi-random and ISSR molecular markers. Genet. Resour. Crop Evol. 2007, 54, 175–188. [Google Scholar] [CrossRef]
- Negri, V.; Tiranti, B. Molecular analysis for ex situ and on-farm conservation of common bean (Phaseolus vulgaris L.) Italian germplasm. In The role of Biotechnology, Torino, Italy, 2005.
- Tosti, N.; Torricelli, R.; Negri, V. Uso dei marcatori AFLP nello studio di germoplasma di fagiolo. In Proceedings of 5th Biodiversity National Congress, Caserta, Italy, 1999; pp. 628–632.
- Perazzini, R.; Leonardi, D.; Ruggeri, S.; Alesiani, D.; D’Arcangelo, G.; Canini, A. Characterisation of Phaseolus vulgaris L. landraces cultivated in Central Italy. Plant Food Hum Nutr. 2008, 63, 211–218. [Google Scholar] [CrossRef]
- Carbonaro, M. Le proprietà nutrizionali del Phaseolus vulgaris L.: caratterizzazione del fagiolo di Lamon IGP. Agroindustria 2007, 6, 37–40. [Google Scholar]
- Russo, G.; Messina, B.; Campanella, V.; Miceli, C. Progetto “Piano per la produzione di proteine vegetali in Sicilia” recupero e valorizzazione del Fagiolo Badda di Polizzi. Risultati Preliminari. In Proceedings of VIII National Congress “La biodiversità – una risorsa per sistemi multifunzionali”, Lecce, Italy, 21–23 April 2008.
- Gupta, Y.P. Antinutritional and toxic factors in food legumes: a review. Plant Food Hum. Nutr. 1987, 37, 201–228. [Google Scholar] [CrossRef]
- Piergiovanni, A.R.; Pignone, D. Effect of year-to-year variation and genotype on trypsin inhibitor level in common bean (Phaseolus vulgaris L.) seeds. J. Sci. Food Agric. 2003, 83, 473–476. [Google Scholar] [CrossRef]
- Romani, A.; Vignolini, P.; Galardi, C.; Mulinacci, N.; Benedettelli, S.; Heimler, D. Germplasm characterisation of Zolfino landraces (Phaseolus vulgaris L.) by flavonoid content. J. Agric Food Chem. 2004, 52, 3838–3842. [Google Scholar] [CrossRef]
- Dinelli, G.; Sonetti, A.; Minelli, M.; Marotti, I.; Catione, P.; Mazzanti, A. Content of flavonols in Italian bean (Phaseolus vulgaris L.) ecotypes. Food Chem. 2006, 99, 105–114. [Google Scholar] [CrossRef]
- Cardador-Martinez, A.; Loarca-Pina, G.; Oomah, B.D. Antioxidant activity in common bean (Phaseolus vulgaris L.). J. Agric Food Chem. 2002, 50, 6975–6980. [Google Scholar] [CrossRef]
- Heimler, D.; Vignolini, P.; Dini, M.G.; Romani, A. Rapid test to assess the antioxidant activity of Phaseolus vulgaris L. dry beans. J. Agric Food Chem. 2005, 53, 3053–3056. [Google Scholar] [CrossRef]
- Piergiovanni, A.R. Kinetic of water adsorption in common bean. Considerations on the suitability of Peleg’s model for describing bean hydration. J. Food Proc. Preserv. 2010, in press. [Google Scholar]
- Conforti, M.; Fascetti, S.; Catalano, M.; Enza, R.; Mele, G. Valorizzazione del fagiolo di Pignola e del fagiolo di Muro Lucano: Caratterizzazione morfologica dei semi e delle piante. In Proceedings of 4th Convegno Nazionale sulle Piante Mediterranee, Marina di Nova Siri, Italy, 7–10 October 2009; Tipografia Grafica Sud: Policoro, Italy; p. 58.
- Lioi, L.; Piergiovanni, A.R. Qualità della granella. In Il fagiolo poverello bianco dell’area del pollino. Tecnica di coltivazione e qualità della granella; Agenzia Regionale per lo Sviluppo e per i Servizi in Agricoltura (ARSSA), Ed.; Grafica Pollino: Castrovillari, Italy, 2003; pp. 10–13. [Google Scholar]
- ILCB. The web site of Italian landraces of common bean. Available online: http://ilcb.altervista.org/.
- Piccinini, M.; Petrini, A.; Fuselli, D.; Antonelli, M.; Fagioli. Progetto di sperimentazione e recupero di produzioni Agricole e agroalimentari; Progetto GAL Sibilla, Ed.; Scocco & Gabrielli: Tolentino, Italy, 2005. [Google Scholar]
- Agenzia Regionale per lo Sviluppo e l’Innovazione nel Settore Agricolo-Forestale. Banca dati in linea del germoplasma autoctono toscano. Razze e varietà locali. Tutela e valorizzazione. Available online: http://germoplasma.arsia.toscana.it/Germo/modules.php?op=modload&name=MESI_Menu&file=Manager&act=D_1:@201&act2=L&&EROSGEN=-1&ATTRIBUTO=6 (accessed May 2010).
- Crocetta, G.; De Vero, L.; Sommariva, G.P.; Bollini, R.; Campion, B. Valorizzazione del fagiolo di Lamon. Informatore Agrario 2004, 22, 53–56. [Google Scholar]
- Piergiovanni, A.R.; Taranto, G.; Sanson, S. Fagiolo ‘Gialet’. Una popolazione autoctona della Val Belluna. In Proceedings of VI Biodiverity National Congress, Valenzano, Italy, 6–7 September 2001; Volume 3. pp. 974–976.
- Spagnoletti Zeuli, P.L.; Baser, N.; Riluca, M.; Laghetti, G.; Logozzo, G.; Masi, P.; Molinari, S.; Negri, V.; Olita, G.; Tiranti, B.; Veronesi, F. Valorizzazione e certificazione di agro-ecotipi italiani di fagiolo (Phaseolus vulgaris). In Proceedings of Conferenza Nazionale: Ecotipi vegetali italiani: una preziosa risorsa di variabilità genetica, Rome, Italy, 6–7 October 2004; p. 15.
- Piergiovanni, A.R.; Taranto, G.; Losavio, F.P.; Pignone, D. Local populations of Phaseolus vulgaris L. and P. coccineous L. from Central Italy. I: Valle Peligna and Val Aniene. In Proceedings of the XLVI Italian Society of Agricultural Genetics Annual Congress, Giardini Naxos, Italy, 18–21 September 2002.
- Carbonaro, M.; Aguzzi, A. Caratterizzazione biochimico-nutrizionale del seme di alcune cultivar locali di fagiolo. In Il Fagiolo; Parisi, B., Campion, B., Eds.; PROM: Italy, 2010; in press. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an Open Access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Piergiovanni, A.R.; Lioi, L. Italian Common Bean Landraces: History, Genetic Diversity and Seed Quality. Diversity 2010, 2, 837-862. https://doi.org/10.3390/d2060837
Piergiovanni AR, Lioi L. Italian Common Bean Landraces: History, Genetic Diversity and Seed Quality. Diversity. 2010; 2(6):837-862. https://doi.org/10.3390/d2060837
Chicago/Turabian StylePiergiovanni, Angela R., and Lucia Lioi. 2010. "Italian Common Bean Landraces: History, Genetic Diversity and Seed Quality" Diversity 2, no. 6: 837-862. https://doi.org/10.3390/d2060837
APA StylePiergiovanni, A. R., & Lioi, L. (2010). Italian Common Bean Landraces: History, Genetic Diversity and Seed Quality. Diversity, 2(6), 837-862. https://doi.org/10.3390/d2060837