Ecological Processes and Contemporary Coral Reef Management
Abstract
:1. Coral Reefs
2. The Coral Reef Crisis
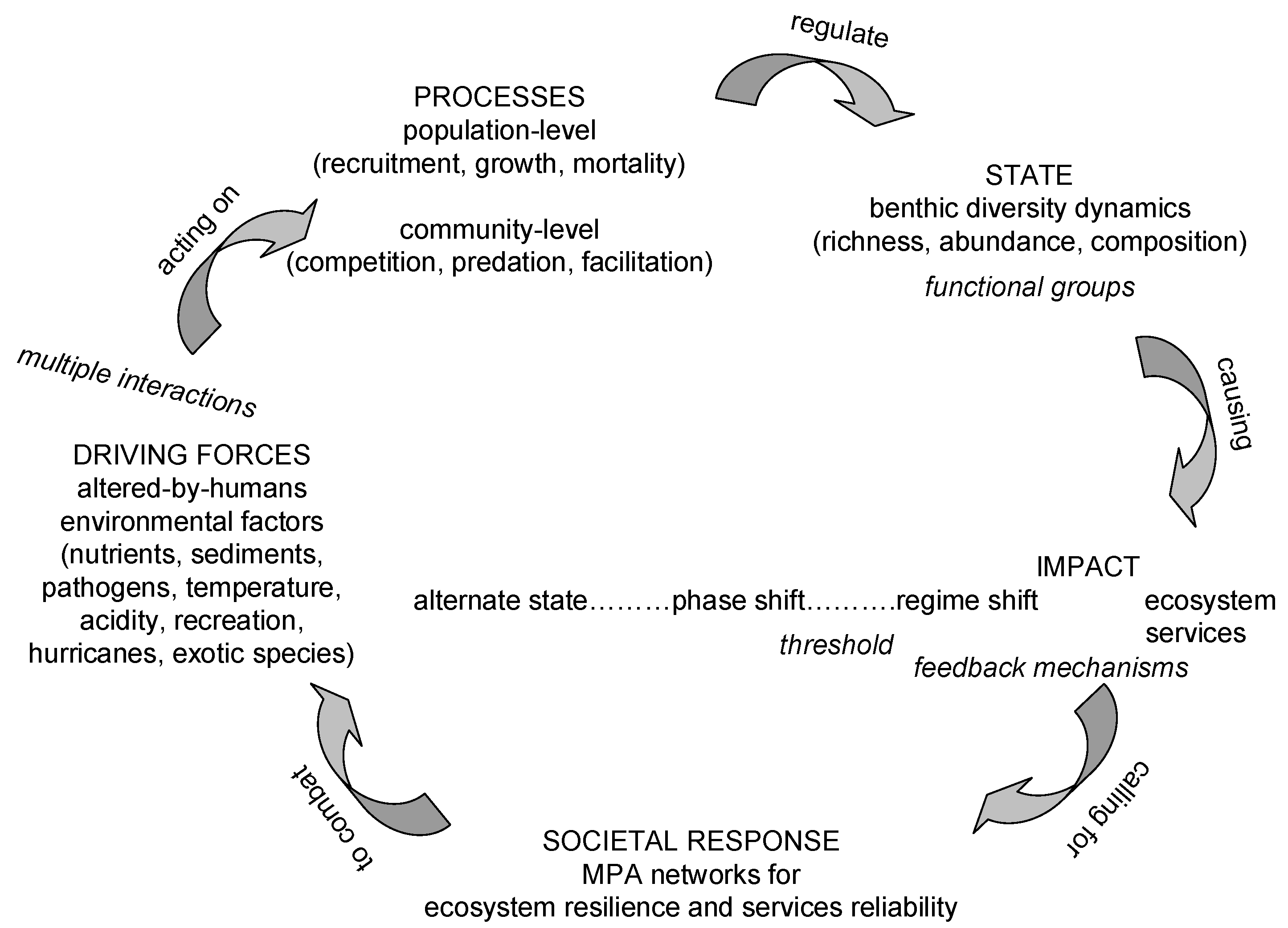
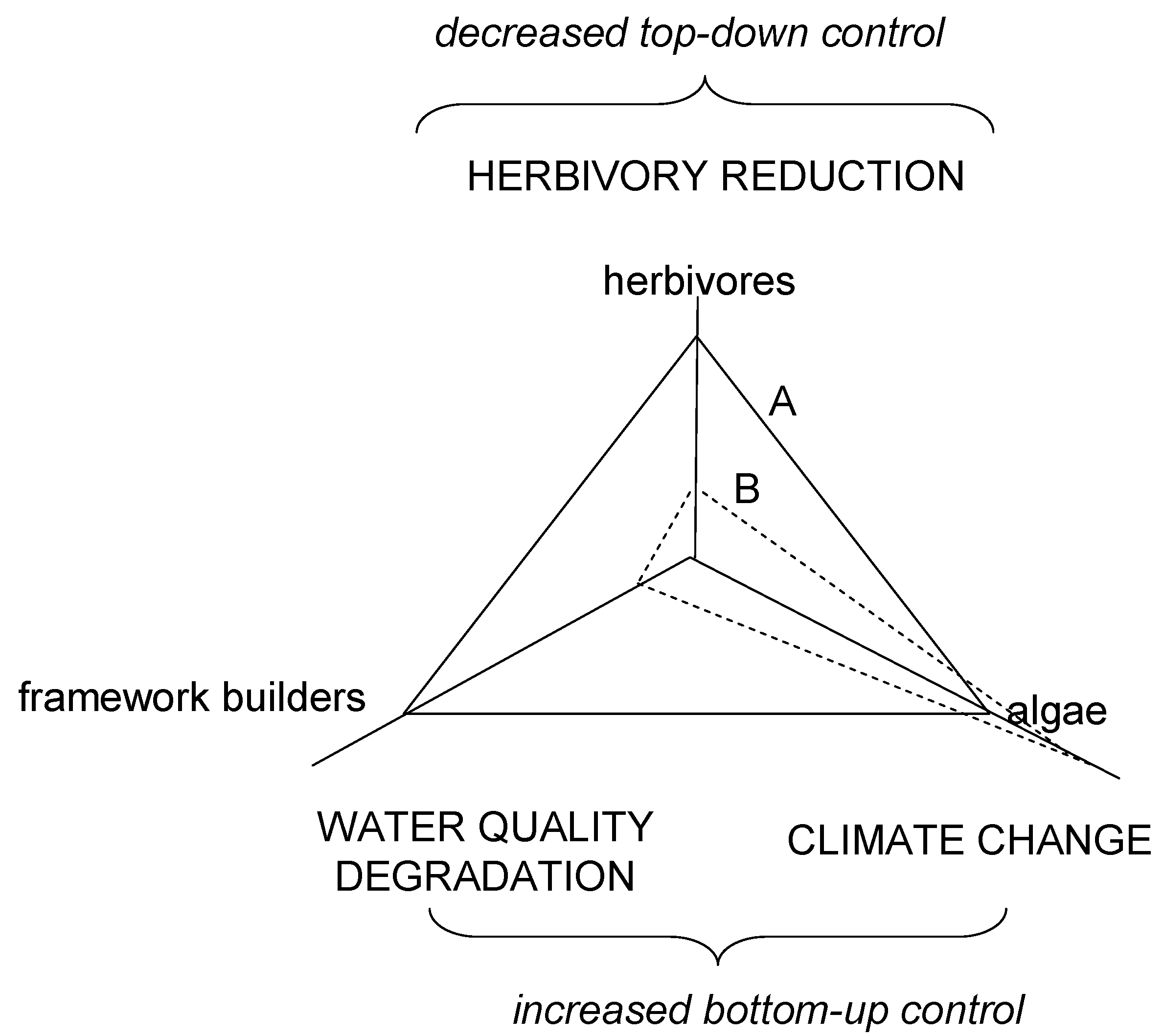
3. Coral Reef Management
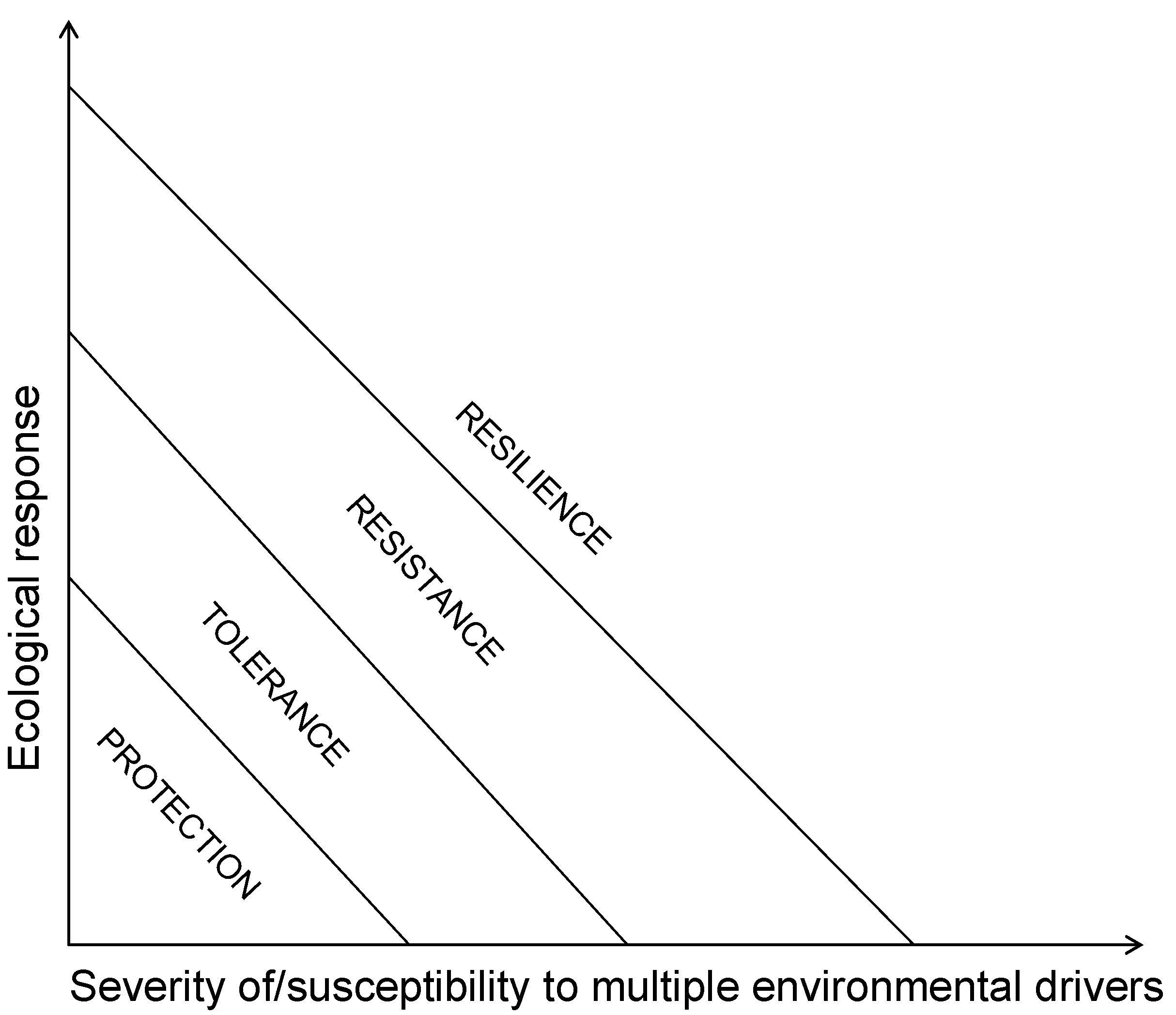
References
- Moberg, F.; Folke, C. Ecological goods and services of coral reef ecosystems. Ecol. Econ. 1999, 29, 215–233. [Google Scholar] [CrossRef]
- Costanza, R.; d’Arge, R.; de Groot, R.; Farber, S.; Grasso, M.; Hannon, B.; Limburg, K.; Naeem, S.; O’Neill, R.V.; Paruelo, J.; Raskin, R.G.; Sutton, P.; van den Belt, M. The value of ecosystem services: putting the issues in perspective. Ecol. Econ. 1998, 25, 67–72. [Google Scholar] [CrossRef]
- Dubinsky, Z. Ecosystems of the World: 25 Coral Reefs; Elsevier Science: Amsterdam, The Netherlands, 1990; p. 550. [Google Scholar]
- Done, T.J.; Ogden, J.C.; Wiebe, W.J. Biodiversity and Ecosystem Function on Coral Reefs. In Bodiversity and Ecosystem Function: a Global Perspective; Mooney, H.A., Cushman, J.H., Medina, E., Sala, O.E., Schulze, E.-D., Eds.; John Wiley and Sons Ltd: New York, NY, USA, 1996; Chapter 15; p. 36. [Google Scholar]
- Bruno, J.F.; Bertness, M.D. Habitat modification and facilitation in Benthic Marine Communities. In Marine Community Ecology; Bertness, M.D., Hay, M.E., Gaines, S.D., Eds.; Sinauer: Sunderland, MA, USA, 2001; p. 17. [Google Scholar]
- Idjadi, J.A.; Edmunds, P.J. Scleractinian corals act as facilitators for other invertebrates on a Caribbean reef. Mar. Ecol. Progr. Ser. 2006, 319, 117–127. [Google Scholar] [CrossRef]
- Fabricius, K.; De’Ath, G. Environmental factors associated with the spatial distribution of crustose coralline algae on the Great Barrier Reef. Coral Reefs 2001, 19, 303–309. [Google Scholar] [CrossRef]
- Bellwood, D.R.; Choat, J.H. A functional analysis of grazing in parrotfishes (family Scaridae): the ecological implications. Environ. Biol. Fish. 1990, 28, 189–214. [Google Scholar] [CrossRef]
- Steneck , R.S. Herbivory on coral reefs: a synthesis. In Vol. 1: Plenary Addressess and Status review, Proceedings of the 6th International Coral Reef Symposium; Choat, J.H., Barnes, D., Borowitzka, M.A., Coll, J.C., Davies, P.J., Flood, P., Hatcher, B.G., Hopley, D., Hutchings, P.A., Kinsey, D., Orme, G.R., Pichon, M., Sale, P.F., Sammarco, P., Wallace, C.C., Wilkinson, C., Wolanski, E., Bellwood, O., Eds.; Brian Clouston Publishing: Canberra, Australia, 1988; pp. 37–49. [Google Scholar]
- McCook, L.J.; Jompa, J.; Diaz-Pulido, G. Competition between corals and algae on coral reefs: a review of evidence and mechanisms. Coral Reefs 2001, 19, 400–417. [Google Scholar] [CrossRef]
- Cvitanovic, C.; Fox, R.J.; Bellwood, D.R. Herbivory by Fishes on the Great Barrier Reef: A Review of Knowledge and Understanding. Unpublished Report to the Marine and Tropical Sciences Research Facility. Reef and Rainforest Research Centre Limited: Cairns, Australia; 33.
- Littler, M.M.; Littler, D.S.; Brooks, B.L. Harmful algae on tropical coral reefs: Bottom-up eutrophication and top-down herbivory. Harmful Algae 2006, 5, 565–585. [Google Scholar] [CrossRef]
- Mumby, P.J.; Edwards, A.J.; Arias-González, J.E.; Lindeman, K.C.; Blackwell, P.G.; Gall, A.; Gorczynska, M.I.; Harborne, A.R.; Pescod, C.L.; Henk, R.; Wabnitz, C.C.C.; Llewellyn, G. Mangroves enhance the biomass of coral reef fish communities in the Caribbean. Nature 2004, 427, 533–536. [Google Scholar] [CrossRef]
- Mumby, P.J.; Hastings, A. The impact of ecosystem connectivity on coral reef resilience. J. Applied Ecol. 2008, 45, 854–862. [Google Scholar] [CrossRef]
- Hughes, T.P.; Connell, J.H. Multiple stressors on coral reefs: A long-term perspective. Limnol. Oceanogr. 1999, 44, 932–940. [Google Scholar] [CrossRef]
- Goreau, T.F.; Goreau, N.I.; Goreau, T.J. Corals and Coral Reefs. Sci. Amer. 1979, 241, 124–136. [Google Scholar] [CrossRef]
- Comparison between Atlantic and Pacific Tropical Marine Coastal Ecosystems: Community Structure, Ecological Processes, and Productivity. Results and Scientific Papers of a Unesco/COMAR Workshop (Suva, Fiji, 24–29 March, 1986); Birkeland, C (Ed.) Unesco Reports in Marine Science 46; UNESCO: Suva, Fiji, 1987.
- Buddemeier, R.W.; Fautin, D.G. Large-scale dynamics: the state of the science, the state of the reef, and the research issues. Coral Reefs 2002, 21, 1–8. [Google Scholar] [CrossRef]
- Johnson, K.G.; Budd, A.F.; Steemann, T.A. Extinction selectivity and ecology of Neogene Caribbean reef corals. Paleobiology 1995, 21, 52–73. [Google Scholar]
- Bellwood, D.R.; Wainwright, P.C. The history and biogeography of fishes on coral reefs. In Coral Reef Fishes. In Coral Reef Fishes: Dynamics and Diversity in a Complex Ecosystem; Sale, P.F., Ed.; Academic Press: San Diego, CA, USA, 2002; p. 27. [Google Scholar]
- Done, T.J. Constancy and change in some Great Barrier Reef coral communities: 1980–1990. American Zoologist 1992, 32, 655–662. [Google Scholar]
- Goreau, T.F.; Goreau, T.F. The ecology of Jamaican coral reefs. 1. Species composition and zonation. Ecology 1959, 40, 67–89. [Google Scholar] [CrossRef]
- Loya, Y. The Red Sea coral Stylophora pistillata is an r strategist. Nature 1976, 259, 478–480. [Google Scholar] [CrossRef]
- Loya, Y. Community structure and species diversity of hermatypic corals at Eilat, Red Sea. Mar. Biol. 1972, 23, 100–123. [Google Scholar] [CrossRef]
- Sammarco, P.W. A Comparison of Some Ecological Processes on Coral Reefs of the Caribbean and the Great Barrier Reef; Unesco Reports in Marine Science 46; Unesco: Paris, France, 1987; pp. 148–187. [Google Scholar]
- Jackson, J.B.C.; Kirby, M.X.; Berger, W.H.; Bjorndal, K.A.; Botsford, L.W.; Bourque, B.J.; Bradbury, R.H.; Cooke, R.; Erlandson, J.; Estes, J.A.; Hughes, T.P.; Kidwell, S.; Lange, C.B.; Lenihan, H.S.; Pandolfi, J.M.; Peterson, C.H.; Steneck, R.S.; Tegner, M.J.; Warner, R.R. Historical overfishing and the recent collapse of coastal ecosystems. Science 2001, 293, 629–638. [Google Scholar] [CrossRef]
- Hughes, T.P. Catastrophes, phase-shifts, and largescale degradation of a Caribbean coral reef. Science 1994, 265, 1547–1551. [Google Scholar]
- Glassom, D.; Zakai, D; Chadwick-Furman, N.E. Coral recruitment: a spatio-temporal analysis along the coastline of Eilat, northern Red Sea. Mar. Biol. 2004, 144, 641–651. [Google Scholar] [CrossRef]
- Pimm, S.L. The complexity and stability of ecosystems. Nature 1984, 307, 321–327. [Google Scholar] [CrossRef]
- Connell, J.H. Diversity in Tropical Rain Forests and Coral Reefs. Science 1978, 199, 1302–1310. [Google Scholar]
- Pearson, R.G. Recovery and decolonisation of coral reefs. Mar. Ecol. Progr. Ser. 1981, 4, 105–122. [Google Scholar] [CrossRef]
- Bythell, J.C.; Hillis-Starr, Z.M.; Rogers, C.S. Local variability but landscape stability in coral reef communities following repeated hurricane impacts. Mar. Ecol. Progr. Ser. 2000, 204, 93–100. [Google Scholar] [CrossRef]
- Connell, J.H. Disturbance and recovery of coral assemblages. Coral Reefs 1997, 16S, 101–113. [Google Scholar] [CrossRef]
- Kaufmann, A.; Fagerstrom, J.A. The Phanero- zoic evolution of reef diversity. In Species Diversity in Ecological Communities: A Historical and Geographical Perspective; Ricklefs, R.E., Schulter, D., Eds.; University of Chicago Press: Chicago, IL, USA, 1993; pp. 315–329. [Google Scholar]
- Wood, R. The changing biology of reef-building. Palaios 1995, 10, 517–529. [Google Scholar] [CrossRef]
- Roberts, C.M. Connectivity and Management of Caribbean Coral Reefs. Science 1997, 278, 1454–1457. [Google Scholar] [CrossRef]
- Palumbi, S.R. Marine speciation on a small planet. Trends Ecol. Evol. 1992, 7, 114–118. [Google Scholar] [CrossRef]
- Benzie, J.A.H. Genetic structure of coral reef organisms: Ghosts of dispersal past. Am. Zool. 1999, 39, 131–145. [Google Scholar]
- Pandolfi, J.M. Coral reef ecology at multiple spatial and temporal scales. Coral Reefs 2002, 21, 13–23. [Google Scholar] [CrossRef]
- Chappell, J. Coral morphology, diversity and reef growth. Nature 1980, 286, 249–252. [Google Scholar] [CrossRef]
- Jackson, J.B.C. Community unity? Science 1994, 264, 1412–1413. [Google Scholar]
- Hughes, T.P.; Baird, A.H.; Dinsdale, E.A.; Moltschaniwskyj, N.A.; Pratchett, M.S.; Tanner, J.E.; Willis, B.L. Patterns of recruitment and abundance of corals along the Great Barrier Reef. Nature 1999, 397, 59–63. [Google Scholar] [CrossRef]
- Van Woesik, R. Processes regulating coral communities. Comment. Theor. Biol. 2002, 7, 201–214. [Google Scholar] [CrossRef]
- Done, T.J. Perspectives on Coral Reefs; Barnes, D.J., Ed.; Australian Institute of Marine Science: Cairns, Australia, 1983; pp. 95–147. [Google Scholar]
- Pandolfi, J.M.; Bradbury, R.H.; Sala, E.; Hughes, T.P.; Bjorndal, K.A.; Cooke, R.G.; McArdle, D.; McClenachan, L.; Newman, M.J.H.; Paredes, G.; Warner, R.R.; Jackson, J.B.C. Global trajectories of the long-term decline of coral reef ecosystems. Science 2003, 301, 955–958. [Google Scholar] [CrossRef]
- The State of Coral Reef Ecosystems of the United States and Pacific Freely Associated States; NOAA Technical Memorandum NOSNCCOS73; NOAA/NCCOS Center for Coastal Monitoring and Assessment’s Biogeography Team: Silver Spring, MD, USA, 2008.
- Carpenter, K.E.; Abrar, M.; Aeby, G.; Aronson, R.B.; Banks, S.; Bruckner, A.; Chiriboga, A.; Cortés, J.; Delbeek, J.C.; DeVantier, L.; Edgar, G.J.; Edwards, A.J.; Fenner, D.; Guzmán, H.M.; Hoeksema, B.W.; Hodgson, G.; Johan, O.; Licuanan, W.Y.; Livingstone, S.R.; Lovell, E.R.; Moore, J.A.; Obura, D.O.; Ochavillo, D.; Polidoro, B.A.; Precht, W.F.; Quibilan, M.C.; Reboton, C.; Richards, Z.T.; Rogers, A.D.; Sanciangco, J.; Sheppard, A.; Sheppard, C.; Smith, J.; Stuart, S.; Turak, E.; Veron, J.E.N.; Wallace, C.; Weil, E.; Wood, E. One-Third of Reef-Building Corals Face Elevated Extinction Risk from Climate Change and Local Impacts. Science 2008, 321, 560–563. [Google Scholar] [CrossRef]
- Gardner, T.A.; Côté, I.M.; Gill, J.A.; Grant, A.; Watkinson, A.R. Long-Term Region-Wide Declines in Caribbean Corals. Science 2003, 301, 958–960. [Google Scholar] [CrossRef]
- Bruno, J.F.; Selig, E.R. Regional Decline of Coral Cover in the Indo-Pacific: Timing, Extent, and Subregional Comparison. PLoS ONE 2007, 8, 1–8. [Google Scholar]
- Hughes, T.P.; Baird, A.H.; Bellwood, D.R.; Card, M.; Connolly, S.R.; Folke, C.; Grosberg, R.; Hoegh-Guldberg, O.; Jackson, J.B.C.; Kleypas, J.; Lough, J.M.; Marshall, P; Nyström, M.; Palumbi, S.R.; Pandolfi, J.M.; Rosen, B.; Roughgarden, J. Climate Change, Human Impacts, and the Resilience of Coral Reefs. Science 2003, 301, 929–933. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O.; Mumby, P.J.; Hooten, A.J.; Steneck, R.S.; Greenfield, P.; Gomez, E.; Harvell, C.D.; Sale, P.F.; Edwards, A.J.; Caldeira, K.; Knowlton, N.; Eakin, C.M.; Iglesias-Prieto, R.; Muthiga, N.; Bradbury, R.H.; Dubi, A.; Hatziolos, M.E. Coral reefs under rapid climate change and ocean acidification. Science 2007, 318, 1737–1742. [Google Scholar] [CrossRef]
- McManus, J.W.; Polsenberg, J.F. Coral-algal phase shifts on coral reefs: ecological and environmental aspects. Prog. Oceanogr. 2004, 60, 263–279. [Google Scholar] [CrossRef]
- Done, T.J. Phase shifts in coral reef communities and their ecological significance. Hydrobiologia 1992, 247, 121–132. [Google Scholar] [CrossRef]
- Ogden, J.C.; Brown, R.A.; Salesky, N. Grazing by the echinoid Diadema antillarum Philippi: Formation of halos around West Indian patch reefs. Science 1973, 182, 715–717. [Google Scholar]
- Hughes, T.P. Catastrophes, phase-shifts, and large-scale degradation of a Caribbean coral reef. Science 1994, 265, 1547–1551. [Google Scholar]
- McClanahan, T.R.; Shafir, S.H. Causes and consequences of sea urchin abundance and diversity in Kenyan coral reef lagoons. Oecologia 1990, 83, 362–370. [Google Scholar]
- Norström, A.V.; Nyström, M.; Lokrantz, J.; Folke, C. Alternative states on coral reefs: beyond coral-macroalgal phase shifts. Mar. Ecol. Progr. Ser. 2009, 376, 295–306. [Google Scholar] [CrossRef]
- Mumby, P.J.; Steneck, R.S. Coral reef management and conservation in light of rapidly evolving ecological paradigms. Trends Ecol. Evol. 2008, 23, 555–563. [Google Scholar] [CrossRef]
- Mumby, P. Phase shifts and the stability of macroalgal communities on Caribbean coral reefs. Coral Reefs 2009, 28, 761–773. [Google Scholar] [CrossRef]
- Knowlton, N. Thresholds and multiple stable states in coral reef community dynamics. Am. Zool. 1992, 32, 674–682. [Google Scholar]
- Scheffer, M.; Carpenter, S.; Foley, J.A.; Folke, C.; Walker, B. Catastrophic shifts in ecosystems. Nature 2001, 413, 591–596. [Google Scholar] [CrossRef]
- Steel, J.H. Regime shifts in the ocean: reconciling observations and theory. Progr. Oceanogr. 2004, 60, 135–141. [Google Scholar] [CrossRef]
- Cesar, H.; Burke, L.; Pet-Soede, L. The Economics of World-Wide Coral Reef Degradation; Cesar Environmental Economics Consulting (CEEC): Arnhem, The Netherlands, 2003. [Google Scholar]
- Bellwood, D.R.; Hughes, T.P.; Folke, C.; Nyström, M. Confronting the coral reef crisis. Nature 2004, 429, 827–833. [Google Scholar] [CrossRef]
- Mora, C. A clear human footprint in the coral reefs of the Caribbean. P. Roy. Soc. B-Biol. Sci. 2008, 275, 767–773. [Google Scholar] [CrossRef]
- Aronson, R.B.; Precht, W.F. Conservation, precaution, and Caribbean reefs. Coral Reefs 2006, 25, 441–450. [Google Scholar] [CrossRef]
- Edmunds, P.J.; Carpenter, R.C. Recovery of Diadema antillarum reduces macroalgal cover andincreases abundance of juvenile corals on a Caribbean reef. Proc. Natl. Acad. Sci. USA 2001, 98, 5067–5073. [Google Scholar] [CrossRef]
- Done, T.J.; Turak, E.; Wakeford, M,; DeVantier, L.; McDonald, A.; Fisk, D. Decadal changes in turbid-water coral communities at Pandora Reef: loss of resilience or too soon to tell? Coral Reefs 2007, 26, 789–805. [Google Scholar] [CrossRef]
- Carpenter, R.C.; Edmunds, P.J. Local and regional scale recovery of Diadema promotes recruitment of scleractinian corals. Ecol. Lett. 2006, 9, 268–277. [Google Scholar]
- Aronson, R.B.; Macintyre, I.G.; Precht, W.F.; Murdoch, T.J.T.; Wapnicki, C.M. The expanding scale of species turnover events on coral reefs in Belize. Ecol. Monogr. 2002, 72, 233–249. [Google Scholar] [CrossRef]
- Knowlton, N. Sea urchin recovery from mass mortality: New hope for Caribbean coral reefs? Proc. Natl. Acad. Sci. USA 2001, 98, 4822–4824. [Google Scholar] [CrossRef]
- Van Nes, E.H.; Scheffer, M. Large species shifts triggered by small forces. Am. Nat. 2004, 164, 255–266. [Google Scholar] [CrossRef]
- Van Nes, E.H.; Scheffer, M. Slow recovery from perturbations as a generic indicator of a nearby catastrophic shift. Am. Nat. 2007, 170, 660–660. [Google Scholar] [CrossRef]
- McClanahan, T.R.; Mutere, J.C. Coral and sea-urchin assemblage structure and interrelationships in Kenyan reef lagoons. Hydrobiologia 1994, 286, 109–124. [Google Scholar] [CrossRef]
- Eakin, C.M. Where have all the carbonates gone? A model comparison of calcium carbonate budgets before and after the 1982–1983 El Niño at Uva Island in the eastern Pacific. Coral Reefs 1996, 15, 109–119. [Google Scholar]
- Hunter, C.L.; Evans, C.W. Coral reefs in Kaneohe Bay, Hawaii—two centuries of western influence and two decades of data. Bull. Mar. Sci. 1995, 57, 501–515. [Google Scholar]
- Idjadi, J.A.; Lee, S.C.; Bruno, J.F.; Precht, W.F.; Allen-Requa, L.; Edmunds, P.J. Rapid phase-shift reversal on a Jamaican coral reef. Coral Reefs 2006, 25, 209–211. [Google Scholar] [CrossRef]
- Nyström, M.; Graham, N.A.J.; Lokrantz, J.; Norström, A.V. Capturing the cornerstones of coral reef resilience: linking theory to practice. Coral Reefs 2008, 27, 795–809. [Google Scholar] [CrossRef]
- Knowlton, N.; Jackson, J.B.C. Shifting Baselines, Local Impacts, and Global Change on Coral Reefs. PLOS Biol. 2008, 6, 215–220. [Google Scholar] [CrossRef]
- Fabricius, K.E. Effects of terrestrial runoff on the ecology of corals and coral reefs: Review and synthesis. Mar. Pollut. Bull. 2005, 50, 125–146. [Google Scholar] [CrossRef]
- Bruno, J.F.; Petes, L.E.; Harvell, C.D.; Hettinger, A. Nutrient enrichment can increase the severity of coral diseases. Ecol. Lett. 2003, 6, 1056–1061. [Google Scholar] [CrossRef]
- Smith, J.E.; Shaw, M.; Edwards, R.A.; Obura, D.; Pantos, O.; Sala, E.; Sandin, S.A.; Smriga, S.; Hatay, M.; Rohwer, F.L. Indirect effects of algae on coral: algae-mediated, microbe-induced coral mortality. Ecol. Lett. 2006, 9, 835–845. [Google Scholar] [CrossRef]
- Szmant, A.M. Editorial Introduction to the special issue of Coral Reefs on “Coral Reef Algal Community Dynamics”. Why are coral reefs world-wide overgrown by algae? “Algae, algae everywhere, and nowhere a bit to eat!”. Coral Reefs 2001, 19, 299–302. [Google Scholar] [CrossRef]
- Burkepile, D.E.; Hay, M.E. Impact of Herbivore Identity on Algal Succession and Coral Growth on a Caribbean Reef. PLoS ONE 2010, 5, 1–9. [Google Scholar]
- Mumby, P.J.; Harborne, A.R.; Williams, J.; Kappel, C.V.; Brumbaugh, D.R.; Micheli, F.; Holmes, K.E.; Dahlgren, C.P.; Paris, C.B.; Blackwell, P.G. Trophic cascade facilitates coral recruitment in a marine reserve. Proc. Natl. Acad. Sci. USA 2007, 104, 8362–8367. [Google Scholar] [CrossRef]
- Kline, D.I.; Kuntz, N.M.; Breitbart, M.; Knowlton, N.; Rohwer, F. Role of elevated organic carbon levels and microbial activity in coral mortality. Mar. Ecol. Progr. Ser. 2006, 314, 119–125. [Google Scholar] [CrossRef]
- Nugues, M.M.; Smith, G.W.; van Hooidonk, R.J.; Seabra, M.I.; Bak, R.P.M. Algal contact as a trigger for coral disease. Ecol. Lett. 2004, 7, 919–923. [Google Scholar] [CrossRef]
- Donner, S.D.; Skirving, W.J.; Little, C.M.; Oppenheimer, M.; Hoegh-Guldberg, O. Global assessment of coral bleaching and required rates of adaptation under climate change. Global Change Biol. 2005, 11, 2251–2265. [Google Scholar] [CrossRef]
- Coles, S.L.; Brown, B.E. Coral Bleaching—Capacity for Acclimatization and Adaptation. Adv. Mar. Biol. 2003, 46, 183–223. [Google Scholar] [CrossRef]
- Baker, A.C.; Starger, C.J.; McClanahan, T.R.; Glynn, P.W. Corals’ adaptive response to climate change. Nature 2004, 430, 741. [Google Scholar] [CrossRef]
- Marshall, P.M.; Schuttenberg, H.Z. A Reef Manager’s Guide to Coral Bleaching; Great Barrier Reef Marine Park Authority: Townsville, Australia, 2006; p. 165. [Google Scholar]
- Fabricius, K.E. Theme section on “Ocean Acidification and Coral Reefs”. Coral Reefs 2008, 27, 455–457. [Google Scholar] [CrossRef]
- Randall, C.J.; Szmant, A.M. Elevated temperature reduces survivorship and settlement of the larvae of the Caribbean scleractinian coral, Favia fragum (Esper). Coral Reefs 2009, 28, 537–545. [Google Scholar] [CrossRef]
- Pörtner, H.O.; Langenbuch, M.; Michaelidis, B. Synergistic effects of temperature extremes, hypoxia, and increases in CO2 on marine animals: From Earth history to global change. J. Geophys. Res. 2005, 110, C09–S10. [Google Scholar]
- Bruno, J.F.; Selig, E.R.; Casey, K.S.; Page, C.A.; Willis, B.L.; Harvell, D.C.; Sweatman, H.; Melendy, A.M. Thermal Stress and Coral Cover as Drivers of Coral Disease Outbreaks. PLOS Biol. 2007, 5, 1–8. [Google Scholar] [CrossRef]
- Jones, G.P.; McCormick, M.I.; Srinivasan, M.; Eagle, J.V. Coral decline threatens fish biodiversity in marine reserves. Proc. Natl. Acad. Sci. USA 2004, 101, 8251–8253. [Google Scholar] [CrossRef]
- Kareiva, P.; Marvier, M. Conserving biodiversity coldspots. Am. Sci. 2003, 91, 344–351. [Google Scholar] [CrossRef]
- Graham, N.A.J.; Wilson, S.K.; Jennings, S.; Polunin, N.V.C.; Robinson, J.; Bijoux, J.P.; Daw, T.M. Lag effects in the impacts of mass coral bleaching on coral reef fish, fisheries, and ecosystems. Conserv. Biol. 2007, 21, 1291–1300. [Google Scholar] [CrossRef]
- Hughes, T.P.; Rodrigues, M.J.; Bellwood, D.R.; Ceccarelli, D.; Hoegh-Guldberg, O.; McCook, L.; Moltschaniwskyj, N.; Pratchett, M.S.; Steneck, R.S.; Willis, B. Phase shifts, herbivory, and the resilience of coral reefs to climate change. Curr. Biol. 2007, 17, 360–365. [Google Scholar]
- Stone, L.; Eilam, E.; Abelson, A.; Llano, M. Modelling cora1 reef biodiversity and habitat destruction. Mar. Ecol. Progr. Ser. 1996, 134, 299–302. [Google Scholar] [CrossRef]
- Perry, R.I.; Philippe, C.; Brander, K.; Jennings, S.; Möllmann, C.; Planque, B. Sensitivity of marine systems to climate and fishing: Concepts, issues and management responses. J. Mar. Systems 2010, 79, 427–435. [Google Scholar] [CrossRef]
- Hughes, T.P.; Bellwood, D.R.; Folke, C.; Steneck, R.S.; Wilson, J. New paradigms for supporting the resilience of marine ecosystems. Trends Ecol. Evol. 2005, 20, 380–386. [Google Scholar] [CrossRef]
- Allison, G.W.; Gaines, S.D.; Lubchenco, J.; Possingham, H.P. Ensuring persistence of marine reserves: catastrophes require adopting an insurance factor. Ecol. Appl. 2003, 13, S8–S24. [Google Scholar] [CrossRef]
- Roberts, C.M.; Andelman, S.; Branch, G.; Bustamante, R.H.; Castilla, J.C.; Dugan, J.; Halpern, B.S.; Lafferty, K.S.; Leslie, H.; Lubchenco, J.; McArdle, D.; Possingham, H.P.; Ruckleshaus, M.; Warner, R.R. Ecological criteria for evaluating candidate sites for marine reserves. Ecol. Appl. 2003, 13, S199–S214. [Google Scholar] [CrossRef]
- Pikitch, E.K.; Santora, C.; Babcock, E.A.; Bakun, A.; Bonfil, R.; Conover, D.O.; Dayton, P.; Doukakis, P.; Fluharty, D.; Heneman, B.; Houde, E.D.; Link, J.; Livingston, P.A.; Mangel, M.; McAllister, M.K.; Pope, J.; Sainsbury, K.J. Ecosystem-based fishery management. Science 2004, 305, 346–347. [Google Scholar] [CrossRef]
- Guerry, A.D. Icarus and Daedalus: conceptual and tactical lessons for marine ecosystem-based management. Front. Ecol. Environ. 2005, 3, 202–211. [Google Scholar] [CrossRef]
- Fernandes, L.; Day, J.; Lewis, A.; Slegers, S.; Kerrigan, B.; Breen, D.; Cameron, D.; Jago, B.; Hall, J.; Lowe, D.; Innes, J.; Tanzer, J.; Chadwick, V.; Thompson, L.; Gorman, K.; Simmons, M.; Barnett, B.; Sampson, K.; De’Ath, G.; Mapstone, B.; Marsh, H.; Possingham, H.; Ball, I.; Ward, T.; Dobbs, K.; Aumend, J.; Slater, D.; Stapleton, K. Establishing representative no-take areas in the Great Barrier Reef: large-scale implementation of theory on marine protected areas. Conserv. Biol. 2005, 19, 1733–1744. [Google Scholar] [CrossRef]
- McClanahan, T.R.; Verheij, E.; Maina, J. Comparing the managementeffectiveness of a marine park and a multiple-use collaborative fisheries management area in East Africa. Aquat. Conserv. 2006, 16, 147–165. [Google Scholar] [CrossRef]
- McClanahan, T.R.; Polunin, N.V.C.; Done, T.J. Ecological States and the Resilience of Coral Reefs. In Resilience and the Behaviour of Large-Scale Systems; Gunderson, L.H., Pritchard, L., Jr., Eds.; Island Press: Washington, DC, USA, 2002; pp. 111–163. [Google Scholar]
- Ehrlich, P.R.; Kennedy, D. Millennium assessment of human behaviour. Science 2005, 309, 562–563. [Google Scholar] [CrossRef]
- Carney, D.; Farrington, J. Natural Resource Management and Institutional Change; Routledge: New York, NY, USA, 1998; p. 132. [Google Scholar]
- Scientific Consensus Statement on Marine Reserves and Marine Protected Areas; American Association for the Advancement of Science (AAAS): Santa Barbara, CA, USA, 2001.
- Mora, C.; Andréfouët, S.; Costello, M.J.; Kranenburg, C.; Rollo, A.; Veron, J.; Gaston, K.J.; Myers, R.A. Coral Reefs and the Global Network of Marine Protected Areas. Science 2006, 312, 1750–1751. [Google Scholar] [CrossRef]
- Nyström, M.; Folke, C.; Moberg, F. Coral reef disturbance and resilience in a human-dominated environment. Trends Ecol. Evol. 2000, 15, 413–417. [Google Scholar] [CrossRef]
- Nyström, M.; Folke, C. Spatial resilience of coral reefs. Ecosystems 2001, 4, 406–417. [Google Scholar] [CrossRef]
- Adger, N.; Hughes, T.P.; Folke, C.; Carpenter, S.R.; Rockström, J. Social-ecological resilience to coastal disasters. Science 2005, 309, 1036–1039. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Folke, C. Ecology for transformation. Trends Ecol. Evol. 2006, 21, 309–315. [Google Scholar] [CrossRef]
- Andersen, T.; Carstensen, J.; Hernández-García, E.; Duarte, C.M. Ecological thresholds and regime shifts: approaches to identification. Trends Ecol. Evol. 2008, 24, 49–57. [Google Scholar]
- deYoung, B.; Barange, M.; Beaugrand, G.; Harris, R.; Perry, R.I.; Scheffer, M.; Werner, F. Regime shifts in marine ecosystems: detection, prediction and management. Trends Ecol. Evol. 2008, 23, 402–409. [Google Scholar] [CrossRef]
- Schuttenberg, H.Z.; Corrigan, C.; McLeod, L.; Marshall, P.; Setiasih, N.; Obura, D.; Hoegh-Guldberg, O.; Causey, B.; Drew, M.; Hansen, L.; Grimsditch, G.; West, J.; Skeat, A.; Eakin, M.; McCook, L.; Crawford, M.; Kramer, P.; Campbell, S. Building resilience into coral reef management: Key findings & recommendations. In Global Problems Local Solutions, Proceedings of the 3rd International Tropical Marine Ecosystems Management Symposium, Cozumel, Mexico, 16–20 October 2006; ICRI and ICRAN: Cambridge, UK, 2006; p. 8. [Google Scholar]
- Smith, L.D.; Devlin, M.; Haynes, D.; Gilmour, J.P. A demographic approach to monitoring the health of coral reefs. Mar. Pollut. Bull. 2004, 51, 399–407. [Google Scholar]
- Done, T.J.; DeVantier, L.M.; Turak, E.; Fisk, D.A.; Wakeford, M.; van Woesik, R. Coral growth on three reefs: development of recovery benchmarks using a space for time approach. Coral Reefs 2010, (in press). [Google Scholar]
- Roberts, C.M. Ecological advice for the global fisheries crisis. Trends Ecol. Evol. 1997, 12, 35–38. [Google Scholar] [CrossRef]
- Pauly, D.; Christensen, V.; Guénette, S.; Pitcher, T.J.; Sumaila, U.R.; Walters, C.J.; Watson, R.; Zeller, D. Towards sustainability in world fisheries. Nature 2002, 418, 689–695. [Google Scholar] [CrossRef]
- Harris, J.A.; Hobbs, R.J.; Higgs, E.; Aronson, J. Ecological restoration and global climate change. Restor. Ecol. 2006, 14, 170–176. [Google Scholar] [CrossRef]
- Sherman, K.; Duda, A.M. Large marine ecosystems: an emerging paradigm for fishery sustainability. Fisheries 1999, 24, 15–26. [Google Scholar] [CrossRef]
- Establishing Marine Protected Area Networks—Making It Happen; IUCN-WCPA, National Oceanic and Atmospheric Administration and The Nature Conservancy: Washington, DC, USA, 2008; p. 118.
- Heller, N.E.; Zavaleta, E.S. Biodiversity management in the face of climate change: A review of 22 years of recommendations. Biol. Conserv. 2009, 142, 14–32. [Google Scholar] [CrossRef]
- Hulme, P.E. Adapting to climate change: is there scope for ecological management in the face of a global threat? J. Applied Ecol. 2005, 42, 784–794. [Google Scholar] [CrossRef]
- Threatened Elkhorn and Staghorn Corals (Acropora sp.); NOAA Fisheries: Silver Spring, MD, USA, November 25 2009.
- McClanahan, T.R.; Cinner, J.E.; Maina, J.; Graham, N.A.; Daw, T.M.; Stead, S.M.; Wamukota, A.; Brown, K.; Ateweberhan, M.; Venus, V.; Polunin, N.V.C. Conservation action in a changing climate. Conserv. Lett. 2008, 1, 53–59. [Google Scholar]
- Suffling, R.; Scott, D. Assessment of climate change effects on Canada’s national park system. Environ. Monit. Assess. 2002, 74, 117–139. [Google Scholar] [CrossRef]
- McLachlan, J.; Hellmann, J.; Schwartz, M. A framework for debate of assisted migration in an era of climate change. Conserv. Biol. 2007, 21, 297–302. [Google Scholar] [CrossRef]
- Brown, I. Modelling future landscape change on coastalfloodplains using a rule-based GIS. Environ. Modell. Softw. 2006, 21, 1479–1490. [Google Scholar] [CrossRef]
- Millar, C.I.; Stephenson, N.L.; Stephens, S.L. Climate changeand forests of the future: managing in the face of uncertainty. Ecol. Appl. 2007, 17, 2145–2151. [Google Scholar] [CrossRef]
- McLeod, L. Integrating Resilience Into MPA Design. In Global Problems Local Solutions, Proceedings of the 3rd International Tropical Marine Ecosystems Management Symposium, Cozumel, Mexico, 16–20 October 2006; ICRI and ICRAN: Cambridge, UK, 2006; p. 8. [Google Scholar]
- Camargo, C.; Maldonado, J.H.; Alvarado, E.; Moreno-Sánchez, R.; Mendoza, S.; Manrique, N.; Mogollón, A,; Osorio, J.D.; Grajales, A.; Sánchez, J.A. Community involvement in management for maintaining coral reef resilience and biodiversity in southern Caribbean marine protected areas. Biodivers. Conserv. 2009, 18, 935–956. [Google Scholar] [CrossRef]
- Stern, M.J. The power of trust: toward a theory of local opposition to neighboring protected areas. Society Nat. Resources 2008, 21, 859–875. [Google Scholar] [CrossRef]
- Christie, P.; White, A.T. Best practices for improved governance of coral reef marine protected areas. Coral Reefs 2007, 26, 1047–1056. [Google Scholar] [CrossRef]
- Clark, R.B. Marine Pollution; Oxford University Press: Oxford, UK, 2001; p. 248. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Dikou, A. Ecological Processes and Contemporary Coral Reef Management. Diversity 2010, 2, 717-737. https://doi.org/10.3390/d2050717
Dikou A. Ecological Processes and Contemporary Coral Reef Management. Diversity. 2010; 2(5):717-737. https://doi.org/10.3390/d2050717
Chicago/Turabian StyleDikou, Angela. 2010. "Ecological Processes and Contemporary Coral Reef Management" Diversity 2, no. 5: 717-737. https://doi.org/10.3390/d2050717
APA StyleDikou, A. (2010). Ecological Processes and Contemporary Coral Reef Management. Diversity, 2(5), 717-737. https://doi.org/10.3390/d2050717



