Direct and Indirect Effects of Climate Change on Amphibian Populations
Abstract
:1. Introduction
2. Discussion
2.1. Climate Perspective
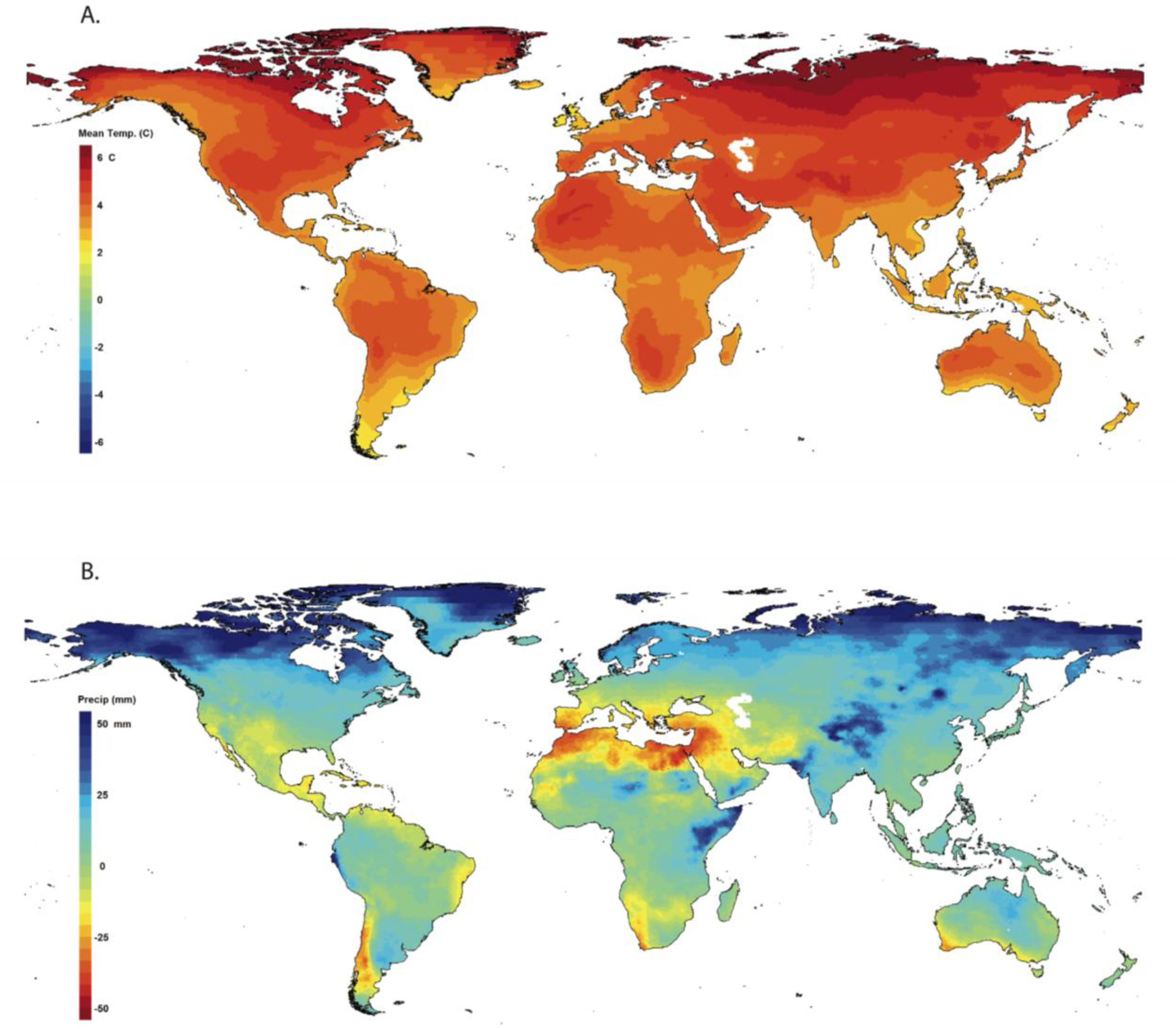
2.2. Direct Effects of Climate Change on Amphibians
2.2.1. Climate change, extreme weather patterns and amphibians
2.2.2. Range shifts
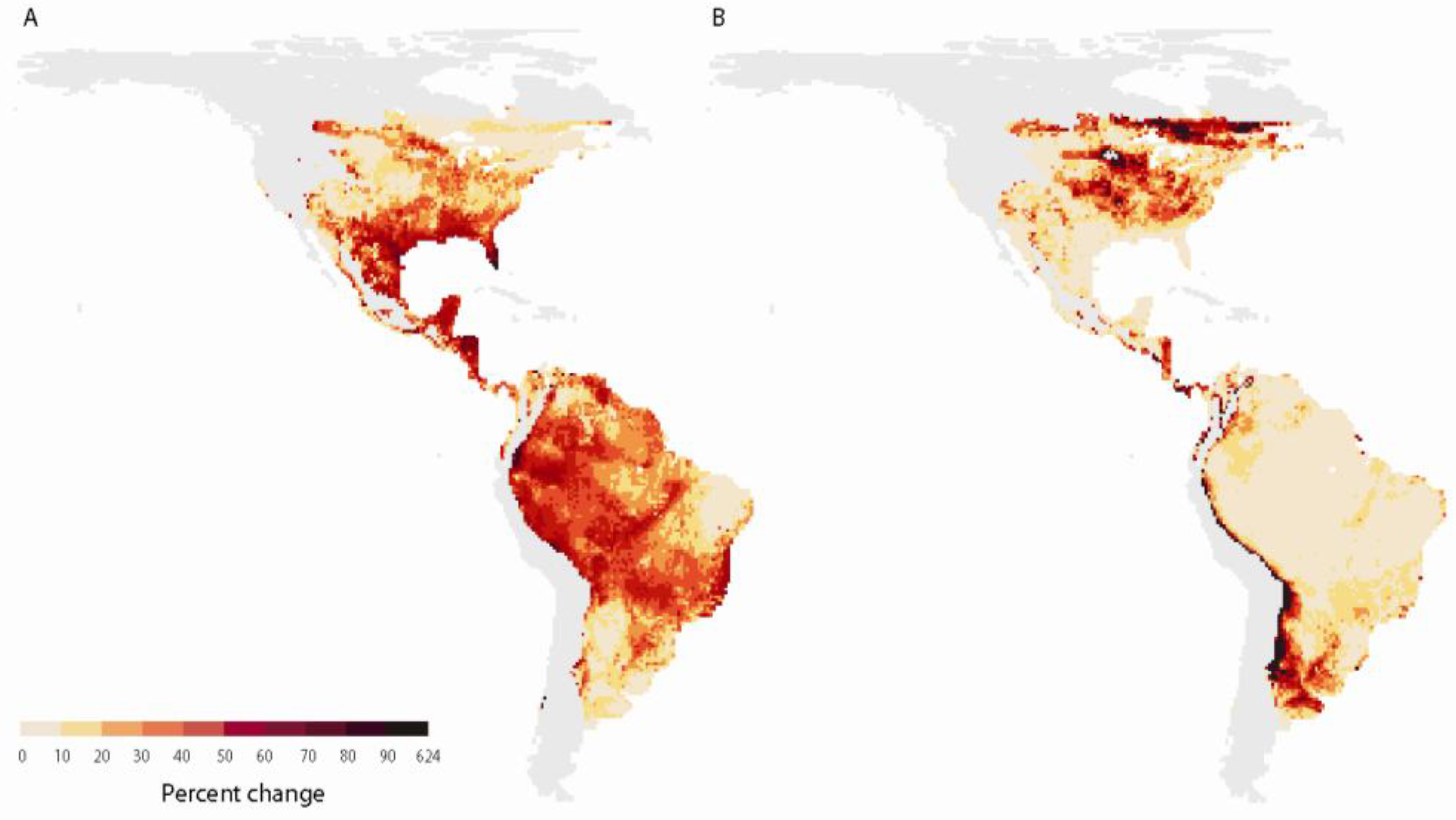
2.2.3. Effects of climate change on survival
2.2.4. Effects of climate change on reproduction
2.2.5. Effects of climate change on development
2.2.6. Effects of climate change on behavior
2.3. Indirect Effects of Climate Change on Amphibians
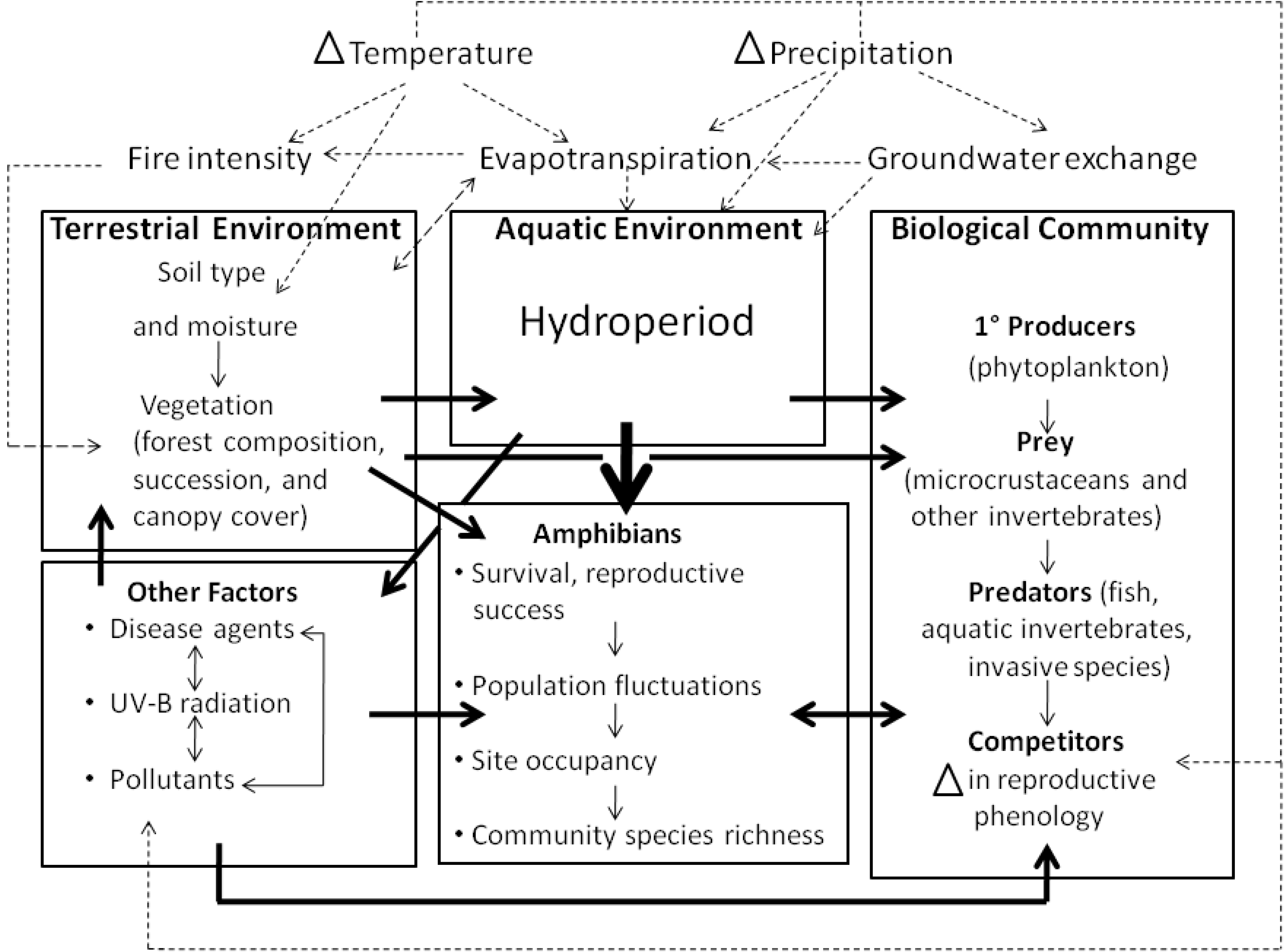
2.3.1. Habitat change
2.3.2. Food availability
2.3.3. Community changes
2.4. Interactions with Other Stressors
2.4.1. Emerging diseases
2.4.2. Immunity
2.4.3. Emerging amphibian diseases
2.4.4. UV-B radiation
2.4.5. Air-borne contaminants
3. Conclusions
Acknowledgements
References
- Lawton, J.H.; May, R.M. Extinction Rates; Oxford University Press: Oxford, UK, 1995. [Google Scholar]
- Wilson, E.O. The Diversity of Life; Harvard University Press: Cambridge, MA, USA, 1992. [Google Scholar]
- Houlahan, J.E.; Findlay, C.S.; Schmidt, B.R.; Meyer, A.H.; Kuzmin, S.L. Quantitative evidence for global amphibian population declines. Nature 2000, 404, 752–755. [Google Scholar] [CrossRef]
- Stuart, S.N.; Chanson, J.S.; Cox, N.A.; Young, B.E.; Rodrigues, A.S.L.; Fischmann, D.L.; Waller, R.W. Status and trends of amphibian declines and extinctions worldwide. Science 2004, 306, 1783–1786. [Google Scholar] [CrossRef]
- Lannoo, M. Amphibian Declines: The Conservation Status of United States Species; University California Press: Berkeley, CA, USA, 2005. [Google Scholar]
- Mendelson, J.R., III; Lips, K.R.; Gagliardo, R.W.; Rabb, G.B.; Collins, J.P.; Diffendorfer, J.E.; Daszack, P.; Ibáñez, R.; Zippel, K.C.; Lawson, D.P.; Wright, K.M.; Stuart, S.N.; Gascon, C.; Silva, H.R.; Burrowes, P.A.; Joglar, R.L.; La Marca, E.; Lötters, S.; Perez, L.H.; Weldon, C.; Hyatt, A.; Rodriguez-Mahecha, J.V.; Hunt, S.; Robertson, H.; Lock, B.; Raxworthy, C.J.; Frost, D.R.; Lacy, R.C.; Alfrod, R.A.; Campbell, J.A.; Parra-Olea, G.; Bolaños, F.; Domingo, J.J.C.; Halliday, T.; Murphy, J.B.; Wake, M.H.; Coloma, L.A.; Kuzmin, S.L.; Price, M.S.; Howell, K.M.; Lau, M.; Pethiyagoda, R.; Boone, M.; Lannoo, M.J.; Blaustein, A.R.; Dobson, A.; Griffiths, A.; Crump, M.L.; Wake, D.B.; Brodie, E.D.J. Confronting amphibian declines and extinctions. Science 2006, 313, 48. [Google Scholar] [CrossRef]
- Bielby, J.; Cooper, N.; Cunningham, A.A.; Garner, T.W.J.; Purvis, A. Predicting susceptibility to rapid declines in the world’s frogs. Conserv. Lett. 2008, 1, 82–90. [Google Scholar] [CrossRef]
- Pounds, J.A.; Fogden, M.P.L.; Savage, J.M.; Gorman, G.C. Test of null models for amphibian declines on a tropical mountain. Conserv. Biol. 1997, 11, 1307–1322. [Google Scholar] [CrossRef]
- Pounds, J.A.; Fogden, M.P.L.; Campbell, J.H. Biological responses to climate change on a tropical mountain. Nature 1999, 398, 611–615. [Google Scholar] [CrossRef]
- Wake, D.B.; Vredenburg, V.T. Are we in the midst of the sixth mass extinction? A review from the world of amphibians. Proc. Natl. Acad. Sci. USA 2008, 105, 11466–11473. [Google Scholar] [CrossRef]
- Blaustein, A.R.; Kiesecker, J.M. Complexity in conservation: Lessons from the global decline of amphibian populations. Ecol. Lett. 2002, 5, 597–608. [Google Scholar] [CrossRef]
- van der Leun, J.C.; Tang, X.; Tevini, M. Environmental Effects of Ozone Depletion 1998 Assessment; Elsevier: Lausanne, Switzerland, 1998. [Google Scholar]
- Peters, R.L; Lovejoy, T.E. Global Warming and Biological Diversity; Yale University Press: New Haven, CT, USA, 1992. [Google Scholar]
- Reaser, J.K.; Blaustein, A.R. Repercussions of global change. In Status and Conservation of North American Amphibians; Lannoo, M., Ed.; University of California Press: Berkeley, CA, USA, 2005; pp. 60–63. [Google Scholar]
- Cockell, C.S.; Blaustein, A.R. Ecosystems, Evolution and Ultraviolet Radiation; Springer: New York, NY, USA, 2001. [Google Scholar]
- IPCC. Climate change 2007: the physical science basis. In Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L., Eds.; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Lawler, J.J.; Shafer, S.L.; White, D.; Kareiva, P.; Maurer, E.P.; Blaustein, A.R.; Bartlein, P.J. Projected climate-induced faunal change in the western hemisphere. Ecology 2009, 90, 588–597. [Google Scholar] [CrossRef]
- Andrady, A.; Aucamp, P.J.; Bais, A.; Ballaré, C.L.; Björn, L.O.; Bornman, J.F.; Caldwell, M.; Cullen, A.P.; Erickson, D.J.; de Gruijl, F.R.; Häder, D.P.; Ilyas, M.; Kulandaivelu, G.; Kumar, H.D.; Longstreth, J.; McKenzie, R.L.; Norval, M.; Paul, N.; Redhwi, H.H.; Smith, R.C.; Solomon, K.R.; Sulzberger, B.; Takizawa, Y.; Tang, X.; Teramura, A.H.; Torikai, A.; van der Leun, J.C.; Wilson, S.R.; Worrest, R.C.; Zepp, R.G. Environmental effects of ozone depletion and its interactions with climate change: progress report, 2008. Photochem. Photobiol Sci. 2009, 8, 13–22. [Google Scholar] [CrossRef]
- Ovaska, K. Vulnerability of amphibians in Canada to global warming and increased ultraviolet radiation. Amphibians in decline: Canadian studies of a global problem, society for the study of amphibians and reptiles. Herpetol. Conserv. 1997, 1, 206–225. [Google Scholar]
- Kiesecker, J.M.; Blaustein, A.R.; Belden, L.K. Complex causes of amphibian population declines. Nature 2001, 410, 681–684. [Google Scholar] [CrossRef]
- Tevini, M. UV-B Radiation and Ozone Depletion: Effects on Humans, Animals, Plants, Microorganisms, and Materials; Lewis Publishers: Boca Raton, FL, USA, 1993. [Google Scholar]
- Schindler, D.W.; Curtis, P.J.; Parker, B.R.; Stainton, M.P. Consequences of climate warming and lake acidification for UV-B penetration in North American boreal lakes. Nature 1996, 379, 705–708. [Google Scholar] [CrossRef]
- Kerr, J.B.; McElroy, C.T. Evidence for large upward trends of ultraviolet-B radiation linked to ozone depletion. Science 1993, 262, 1032–1034. [Google Scholar]
- Herman, J.R.; Bhartia, P.K.; Ziemke, J.; Ahmad, Z.; and Larko, D. UV-B increases (1979-1992) from decreases in total ozone. Geophys. Res. Lett. 1996, 23, 2117–2120. [Google Scholar] [CrossRef]
- Middleton, E.M.; Herman, J.R.; Celarier, E.A.; Wilkinson, J.W.; Carey, C.; Rusin, R.J. Evaluating ultraviolet radiation exposure with satellite data at sites of amphibian declines in Central and South America. Conserv. Biol. 2001, 15, 914–929. [Google Scholar] [CrossRef]
- Donnelly, M.A.; Crump, M.L. Potential effects of climate change on two Neotropical amphibian assemblages. Clim. Change 1998, 39, 541–561. [Google Scholar] [CrossRef]
- Alford, R.A.; Richards, S.J. Global amphibian declines: a problem in applied ecology. Ann. Rev. Ecol. Syst. 1999, 30, 133–165. [Google Scholar] [CrossRef]
- Alexander, M.A.; Eischeid, J.K. Climate variability in regions of amphibian declines. Conserv. Biol. 2001, 15, 930–942. [Google Scholar] [CrossRef]
- Blaustein, A.R.; Hatch, A.C.; Belden, L.K.; Scheessele, E.; Kiesecker, J.M. Global change: challenges facing amphibians. In Amphibian Conservation; Semlitsch, R.D., Ed.; Smithsonian Press: Washington, DC, USA, 2003; pp. 187–198. [Google Scholar]
- Carey, C.; Alexander, M.A. Climate change and amphibian declines: Is there a link? Divers. Distribut. 2003, 9, 111–121. [Google Scholar] [CrossRef]
- McMenamin, S.K.; Hadley, E.A.; Wright, C.K. Climatic change and wetland desiccation cause amphibian decline in Yellowstone National Park. Proc. Natl. Acad. Sci. USA 2008, 105, 16988–16993. [Google Scholar] [CrossRef]
- Heyer, W.R.; Rand, A.S.; Goncalvez da Cruz, C.A.; Peixoto, O.L. Decimations, extinctions and colonizations of frog populations in southeast Brazil and their evolutionary implications. Biotropica 1988, 20, 230–235. [Google Scholar] [CrossRef]
- Weygoldt, P. Changes in the composition of mountain stream frog communities in the Atlantic Mountains of Brazil: Frogs as indicators of environmental deteriorations? Neotrop. Fauna & Environ. 1989, 24, 249–255. [Google Scholar]
- Crump, M.L.; Hensley, F.R.; Clark, K.L. Apparent decline of the golden toad: Underground or extinct? Copeia 1992, 1, 413–420. [Google Scholar]
- Blaustein, A.R.; Belden, L.K.; Olson, D.H.; Green, D.L.; Root, T.L.; Kiesecker, K.M. Amphibian breeding and climate change. Conserv. Biol. 2001, 15, 1804–1809. [Google Scholar] [CrossRef]
- Bustamante, M.R.; Ron, S.R.; Coloma, L.A. Cambios en la diversidad en siete communidades de anuros en los Andes de Ecuador. Biotropica 2005, 37, 180–189. [Google Scholar] [CrossRef]
- Raxworthy, C.J.; Pearson, R.G.; Rabibisoa, N.; Rakotondrazafy, A.M.; Ramanamanjato, J.-B.; Raselimanana, A.P.; Wu, S.; Nussbaum, R.A.; Stone, D.A. Extinction vulnerability of tropical montane endemism from warming and upslope displacement: a preliminary appraisal for the highest massif in Madagascar. Glob. Change Biol. 2008, 14, 1703–1720. [Google Scholar] [CrossRef]
- Parmesan, C. Ecological and evolutionary responses to recent climate change. Ann. Rev. of Ecol. and Syst. 2006, 37, 637–669. [Google Scholar] [CrossRef]
- Thomas, C.D.; Cameron, A.; Green, R.E. Extinction risk from climate change. Nature 2004, 427, 145–148. [Google Scholar] [CrossRef] [Green Version]
- Thuiller, W.; Lavorel, S.; Araújo, M.B.; Sykes, M.T.; Prentice, I.C. Climate change threats to plant diversity in Europe. Proc. Natl. Acad. Sci. USA 2005, 102, 8245–8250. [Google Scholar]
- Lovejoy, T.E.; Hannah, L. Climate Change and biodiversity; Yale University Press: New Haven, CT, USA, 2005. [Google Scholar]
- Araújo, M. B.; Thuiller, W.; Pearson, R.G. Climate warming and the decline of amphibians and reptiles in Europe. J. Biogeo. 2006, 33, 1712–1728. [Google Scholar] [CrossRef]
- Pearson, R.G.; Dawson, T.P. Predicting the impacts of climate change on the distribution of species: are climate envelope models useful? Glob. Ecol. Biogeo. 2003, 12, 361–371. [Google Scholar] [CrossRef]
- Lawler, J.J.; White, D.; Neilson, R.P.; Blaustein, A.R. Predicting climate-induced range shifts: model differences and model reliability. Glob. Change Biol. 2006, 12, 1568–1584. [Google Scholar] [CrossRef]
- Lawler, J.J.; Shafer, S.L.; Bancroft, B.A.; Blaustein, A.R. Projected climate impacts for the amphibians of the western hemisphere. Conserv. Biol. 2010, 24, 38–50. [Google Scholar] [CrossRef]
- Rome, L.C.; Stevens, E.D.; John-Alder, H.B. Temperature and thermal acclimation and physiological function. In Environmental Physiology of the Amphibia; Feder, M.E., Burggren, W.W., Eds.; University of Chicago Press: London, UK, 1992; pp. 183–205. [Google Scholar]
- Hillyard, S.D. Behavioral, molecular and integrative mechanisms of amphibian osmoregulation. J. Exper. Zool. 1999, 283, 662–674. [Google Scholar] [CrossRef]
- Brooks, R.T. Potential impacts of global climate change on the hydrology and ecology of ephemeral freshwater systems of the forests of the northeastern United States. Clim. Change 2009, 95, 469–483. [Google Scholar] [CrossRef]
- Rios-López, N. Effects of increased salinity on tadpoles of two anurans from a Caribbean coastal wetland in relation to their natural abundance. Amphibia-Reptilia 2008, 29, 7–18. [Google Scholar] [CrossRef]
- Kundzewicz, Z.W.; Mata, L.J.; Arnell, N.W.; Döll, P.; Kabat, P.; Jiménez, B.; Miller, K.A. Freshwater Resources and Their Management. Climate Change 2007: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Fourth. In Assessment Report of the Intergovernmental Panel on Climate Change; Oki, T., Sen, Z., Shiklomanov, I.A., Parry, M.L., Canziani, O.F., Palutikof, J.P., van der Linden, P.J., Hanson, C.E., Eds.; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- IPCC. Assessment of Observed Changes and Responses in Natural and Managed Systems. In Climate Change 2007: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Rosenzweig, C., Casassa, G., Karoly, D.J., Imeson, A., Liu, C., Menzel, A., Rawlins, S., Root, T.L., Seguin, B., Tryjanowski, P., Parry, M.L., Canziani, O.F., Palutikof, J.P., van der Linden, P.J., Hanson, C.E., Eds.; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Reading, C.J. Linking global warming to amphibian declines through its effect on female body condition and survivorship. Oecologia 2007, 151, 125–131. [Google Scholar] [CrossRef]
- Burrowes, P.A.; Joglar, R.L.; Green, D.L. Potential causes for amphibian declines in Puerto Rico. Herpetologica 2004, 60, 141–154. [Google Scholar] [CrossRef]
- Daszak, P.; Scott, D.E.; Kilpatrick, A.M.; Faggioni, C.; Gibbons, J.W.; Porter, D. Amphibian population declines at Savannah River Site are linked to climate, not chytridiomycosis. Ecology 2005, 86, 3232–3237. [Google Scholar] [CrossRef]
- Carey, C.; Heyer, W.R.; Wilkinson, J.; Alford, R.A.; Arntzen, J.W.; Halliday, T.; Hungerford, L.; Lips, K.R.; Middelton, E.M.; Orchard, S.A.; Rand, A.S. Amphibian declines and environmental change: Use of remote-sensing data to identify environmental correlates. Conser. Biol. 2001, 15, 903–913. [Google Scholar] [CrossRef]
- Beebee, T.J.C. Amphibian breeding and climate. Nature 1995, 374, 219–220. [Google Scholar] [CrossRef]
- Kusano, T.; Inoue, M. Long-term trends toward earlier breeding of Japanese amphibians. J. Herp. 2008, 42, 608–614. [Google Scholar] [CrossRef]
- Chadwick, E.A.; Slater, F.M.; Ormerod, S.J. Inter- and intraspecific differences in climatically mediated phenological change in coexisting Triturus species. Glob. Change Biol. 2006, 12, 1069–1078. [Google Scholar] [CrossRef]
- Gibbs, J.P.; Breisch, A.R. Climate warming and calling phenology of frogs near Ithaca, New York, 1900−1999. Conserv. Biol. 2001, 15, 1175–1178. [Google Scholar] [CrossRef]
- Hartel, T. Weather conditions, breeding date and population fluctuation in Rana dalmantina from central Romania. Herpetol. J. 2008, 18, 1–5. [Google Scholar] [CrossRef]
- Vaira, M. Annual variation of breeding patterns of the toad, Melanophryniscus rubriventris (Vellard, 1947). Amphibia-Reptilia 2005, 26, 193–199. [Google Scholar] [CrossRef]
- Richter-Boix, A.; Llorente, G.A.; Montori, A. Breeding phenology of an amphibian community in a Mediterranean area. Amphibia-Reptilia 2006, 27, 549–559. [Google Scholar] [CrossRef]
- Mills, N.E.; Barnhart, M.C. Effects of hypoxia on embryonic development in two Ambystoma and two Rana species. Physiol. Biochem. Zool. 1999, 72, 179–188. [Google Scholar] [CrossRef]
- Wassersug, R.J.; Seibert, E.A. Behavioral responses of amphibian larvae to variation in dissolved oxygen. Copeia 1975, 1975, 86–103. [Google Scholar] [CrossRef]
- Eggert, C. Sex determination: the amphibian models. Reprod. Nutr. Dev. 2004, 44, 539–549. [Google Scholar] [CrossRef]
- Duellman, W.E.; Trueb, L. Biology of Amphibians; Johns Hopkins University Press: Baltimore, MD, USA, 1986. [Google Scholar]
- Volpe, E.P. Embryonic temperature tolerance and rate of development of Bufo valliceps. Physiol. Zool. 1957, 30, 164–175. [Google Scholar]
- Vonesh, J.R.; De la Cruz, O. Complex life cycles and density dependence: assessing the contribution of egg mortality to amphibian declines. Oecologia 2002, 133, 325–333. [Google Scholar] [CrossRef]
- Biek, R.; Funk, W.C.; Maxell, B.A.; Mills, L.S. What is missing in amphibian decline research: Insights from ecological sensitivity analysis. Conserv. Biol. 2002, 16, 728–734. [Google Scholar] [CrossRef]
- Broomhall, S.D. Egg temperature modifies predator avoidance and the effects of the insecticide endosulfan on tadpoles of an Australian frog. J. Appl. Ecol. 2004, 41, 105–113. [Google Scholar] [CrossRef]
- Govindarajulu, P.P.; Anholt, B.R. Interaction between biotic and abiotic factors determines tadpole survival rate under natural conditions. Ecoscience 2006, 13, 413–421. [Google Scholar] [CrossRef]
- Álvarez, D.; Nicieza, A.G. Effects of temperature and food quality on anuran larval growth and metamorphosis. Funct. Ecol. 2002, 16, 640–648. [Google Scholar] [CrossRef]
- Loman, J. Temperature genetic and hydroperiod effects on metamorphosis of brown frogs Rana arvalis and R. temporaria in the field. J. Zool. 2002, 258, 115–129. [Google Scholar] [CrossRef]
- Morand, A.; Joly, P.; Grolet, O. Phenotypic variation in metamorphosis in five anuran species along a gradient of stream influence. C.R. Acad. Sci. Paris Life Sciences 1997, 320, 645–652. [Google Scholar]
- Buchholz, D.R.; Hayes, T.B. Larval period comparison for the spadefoot toads Scaphiopus couchii and Spea multiplicata (Pelobatidae: Anura). Herpetologica 2000, 56, 455–468. [Google Scholar]
- Voss, S.R. Relationship between stream order and length of larval period in the salamander Eurycea wilderae. Copeia 1993, 1993, 736–742. [Google Scholar] [CrossRef]
- Browne, R.K.; Edwards, D.L. The effect of temperature on the growth and development of the endangered green and golden bell frog (Litoria aurea). J. Thermal Biol. 2003, 28, 295–299. [Google Scholar] [CrossRef]
- Berven, K.A.; Gill, D.E.; Smith-Gill, S.J. Countergradient selection in the Green Frog, Rana clamitans. Evolution 1979, 33, 609–623. [Google Scholar] [CrossRef]
- Beachy, C.K. Effects of larval growth history on metamorphosis in a stream-dwelling salamander (Desmognathus ochrophaeus). J. Herpetol. 1995, 29, 375–382. [Google Scholar] [CrossRef]
- Hickerson, C.M.; Barker, E.L.; Beachy, C.K. Determinants of metamorphic timing in the black-bellied salamander, Desmognathus quadramaculatus. Southeast. Nature. 2005, 4, 33–50. [Google Scholar] [CrossRef]
- Wilbur, H.M.; Collins, J.P. Ecological aspects of amphibian metamorphosis. Science 1973, 182, 1305–1314. [Google Scholar]
- Werner, E.E. Amphibian metamorphosis: growth rate, predation risk, and the optimal size at transformation. Amer. Nat. 1986, 128, 319–341. [Google Scholar]
- John-Alder, H.B.; Morin, P.J. Effects of larval density on jumping ability and stamina in newly metamorphosed Bufo woodhousii fowleri. Copeia 1990, 1, 856–860. [Google Scholar] [CrossRef]
- Goater, C.P.; Semlitsch, R.D.; Bernasconi, M.V. Effects of body size and parasite infection on the locomotory performance of juvenile toads. Bufo bufo. Oikos 1993, 66, 129–136. [Google Scholar] [CrossRef]
- Beck, C.W.; Congdon, J.D. Effects of age and size at metamorphosis on performance and metabolic rates of southern toad, Bufo terrestris, metamorphs. Funct. Ecol. 2000, 14, 32–38. [Google Scholar] [CrossRef]
- Gervasi, S.S.; Foufopoulos, J. Costs of plasticity: responses to desiccation decrease post-metamorphic immune function in a pond-breeding amphibian. Funct. Ecol. 2008, 22, 100–108. [Google Scholar]
- Downie, J.R.; Bryce, R.; Smith, J. Metamorphic duration: an under-studied variable in frog life histories. Biol. J. Linn. Soc. 2004, 83, 261–272. [Google Scholar] [CrossRef]
- Pough, F.H.; Taigen, T.L.; Stewart, M.L.; Brussard, P.F. Behavioral modification of evaporative water loss by a Puerto Rican frog. Ecology 1983, 64, 244–252. [Google Scholar] [CrossRef]
- Chan-McLeod, A.C.A. Factors affecting the permeability of clearcuts to red-legged frogs. J. Wild. Manag. 2003, 67, 663–671. [Google Scholar] [CrossRef]
- Roe, A.W.; Grayson, K.L. Terrestrial movements and habitat use of juvenile and emigrating adult Eastern red-spotted newts, Notophthalmus viridescens. J. Herpetol. 2008, 42, 22–30. [Google Scholar] [CrossRef]
- Ray, C. Vital limits and rates of desiccation in salamanders. Ecology 1958, 39, 75–83. [Google Scholar] [CrossRef]
- Wells, K.D. The Ecology and Behavior of Amphibians; The University of Chicago Press: Chicago, IL, USA, 2007. [Google Scholar]
- Karl, T.R.; Knight, R.W. Secular trends of precipitation amount, frequency, and intensity in the United States. Bull. Am. Meteorol. Soc. 1998, 79, 231–241. [Google Scholar] [CrossRef]
- National Assessment Synthesis Team. Climate Change Impacts on the United States: The Potential Consequences of Climate Variability and Change; U.S. Global Change Research Program: Washington, DC, USA, 2000. [Google Scholar]
- Brooks, R.T. Weather-related effects on woodland vernal pool hydrology and hydroperiod. Wetlands 2004, 24, 104–114. [Google Scholar] [CrossRef]
- Lake, P.S. Ecological effects of perturbation by drought in flowing waters. Freshwater Biol. 2003, 48, 1161–1172. [Google Scholar] [CrossRef]
- Semlitsch, R.D. Relationship of pond drying to the reproductive success of the salamander Ambystoma talpoideum. Copeia 1987, 1, 61–69. [Google Scholar] [CrossRef]
- Dodd, C.K., Jr. Cost of living in an unpredictable environment: the ecology of striped newts Notophthalmus perstriatus during a prolonged drought. Copeia 1993, 1, 605–614. [Google Scholar] [CrossRef]
- Dodd, C.K., Jr. The effects of drought on population structure, activity, and orientation of toads (Bufo quercicus and B. terrestris) at a temporary pond. Ethol. Ecol. Evol. 1994, 6, 331–349. [Google Scholar] [CrossRef]
- Richter, S.C.; Young, J.E.; Johnson, G.N.; Seigel, R.A. Stochastic variation in reproductive success of a rare frog, Rana sevosa: implications for conservation and for monitoring amphibian populations. Biol. Conserv. 2003, 111, 171–177. [Google Scholar] [CrossRef]
- Palis, J.G.; Aresco, M.J.; Kilpatrick, S. Breeding biology of a Florida population of Ambystoma cingulatum (Flatwoods salamander) during a drought. Southeast. Nat. 2006, 5, 1–8. [Google Scholar]
- Taylor, B.E.; Scott, D.E.; Gibbons, J.W. Catastrophic reproductive failure, terrestrial survival, and persistence of the marbled salamander. Conserv. Biol. 2006, 20, 792–801. [Google Scholar] [CrossRef]
- Patla, D.A.; Peterson, C.R.; Corn, P.S. Amphibian decline in Yellowstone National Park. Proc. Natl. Acad. Sci. USA 2009, 106, 22. [Google Scholar] [CrossRef]
- Michener, W.K.; Blood, E.R.; Bildstein, K.L.; Brinson, M.M.; Gardner, L.R. Climate change, hurricanes and tropical storms, and rising sea level in coastal wetlands. Ecol. Apps 1997, 7, 770–801. [Google Scholar] [CrossRef]
- Schriever, T.A.; Ramspott, J.; Crother, B.I.; Fontenot, C.L., Jr. Effects of hurricanes Ivan, Katrina, and Rita on a southeastern Louisiana herpetofauna. Wetlands 2009, 29, 112–122. [Google Scholar] [CrossRef]
- Palis, J.G. Element stewardship abstract: flatwoods salamander (Ambystoma cingulatum Cope). Nat. Areas J. 1996, 16, 49–54. [Google Scholar]
- Dodd, C.K.; Barichivich, W.J.; Johnson, S.A.; Staiger, J.S. Changes in a northwestern Florida gulf coast herpetofaunal community over a 28-y period. Am. Midl. Nat. 2007, 158, 29–48. [Google Scholar] [CrossRef]
- Spotila, J.R. Role of temperature and water in the ecology of lungless salamanders. Ecol. Monogr. 1972, 42, 95–124. [Google Scholar] [CrossRef]
- Bernardo, J.; Spotila, J.R. Physiological constraints on organismal response to global warming: mechanistic insights from clinally varying populations and implications for assessing endangerment. Biol. Lett. 2006, 2, 135–139. [Google Scholar] [CrossRef] [Green Version]
- Stroh, C.L.; DeSteven, D.; Guntenspergen, G.R. Effect of climate fluctuations on long-terms vegetation dynamics in Carolina Bay wetlands. Wetlands 2008, 28, 17–27. [Google Scholar] [CrossRef]
- Kloeppel, B.D.; Clinton, B.D.; Vose, J.M.; Cooper, A.R. Drought impacts on tree growth and mortality of southern Appalachian forests. In Climate Variability and Ecosystem Response at Long-Term Ecological Research Sites; Greenland, D., Goodin, D.G., Smith, R.C., Eds.; Oxford University Press: New York, NY, USA, 2003. [Google Scholar]
- Halverson, M.A.; Skelly, D.K.; Kiesecker, J.M.; Freidenburg, L.K. Forest mediated light regime linked to amphibian distribution and performance. Oecologia 2003, 134, 360–364. [Google Scholar]
- Binckley, C.A.; Resetarits, W.J., Jr. Effects of forest canopy on habitat selection in treefrogs and aquatic insects: implications for communities and metacommunities. Oecologia 2007, 153, 951–958. [Google Scholar] [CrossRef]
- Roznik, E.A.; Johnson, S.A. Canopy closure and emigration by juvenile gopher frogs. J. Wildlife Manag. 2009, 73, 260–268. [Google Scholar] [CrossRef]
- Rubbo, M.J.; Kiesecker, J.M. Leaf litter composition and community structure: translating regional species changes into local dynamics. Ecology 2004, 85, 2519–2525. [Google Scholar] [CrossRef]
- Williams, B.K.; Rittenhouse, T.A.G.; Semlitsch, R.D. Leaf litter input mediates tadpole performance across forest canopy treatments. Oecologia 2008, 155, 377–384. [Google Scholar] [CrossRef]
- Kirkman, L.K. Impacts of fire and hydrological regimes on vegetation in depressional wetlands of southeastern USA. In Fire in Wetlands: A Management Perspective. Proceedings of the Tall Timbers Fire Ecology Conference, No. 19; Cerulean, S.I., Engstrom, R.T., Eds.; Tall Timbers Research Station: Tallahassee, FL, USA, 1995. [Google Scholar]
- Huxman, T.E.; Wilcox, B.P.; Breshears, D.D.; Scott, R.L.; Snyder, K.A.; Small, E.E.; Hultine, K.; Pockman, W.T.; Jackson, R.B. Ecohydrological implications of woody plant encroachment. Ecology 2005, 86, 308–319. [Google Scholar] [CrossRef]
- Altig, R.; Whiles, M.R.; Taylor, C.L. What do tadpoles really eat? Assessing the trophic status of an understudied and imperiled group of consumers in freshwater habitats. Freshwater Biol. 2007, 52, 386–395. [Google Scholar] [CrossRef]
- Meyer, J.L.; Sale, M.J.; Mulholland, P.K.; Hoff, N.L. Impacts of climate change on aquatic ecosystem functioning and health. J. Am. Water Res. Assoc. 1999, 35, 1373–1386. [Google Scholar] [CrossRef]
- Schindler, D.W. Widespread effects of climatic warming on freshwater ecosystems in North America. Hydrol. Process. 1997, 11, 1043–1067. [Google Scholar] [CrossRef]
- IPCC. Climate change and water. In Technical Paper of the Intergovernmental Panel on Climate Change; Bates, B.C., Kundzewicz, Z.W., Wu, S., Palutikof, J.P., Eds.; Secretariat: Geneva, Switzerland, 2008. [Google Scholar]
- Taylor, B.E.; Estes, R.A.; Pechmann, J.H.K.; Semlitsch, R.D. Trophic relations in a temporary pond: larval salamanders and their microinvertebrate prey. Can. J. Zool. 1988, 66, 2191–2198. [Google Scholar] [CrossRef]
- Leeper, D.A.; Taylor, B.E. Abundance, biomass and production of aquatic invertebrates in Rainbow Bay, a temporary wetland in South Carolina, USA. Arch. Hydrobiol. 1998, 143, 335–362. [Google Scholar]
- Durance, I.; Ormerod, S.J. Climate change effects on upland stream macroinvertebrates over a 25-year period. Glob. Change Biol. 2007, 13, 942–957. [Google Scholar] [CrossRef]
- Batzer, D.P.; Wissinger, S.A. Ecology of insect communities in nontidal wetlands. Ann. Rev. Entomol. 1996, 41, 75–100. [Google Scholar] [CrossRef]
- Brooks, R.T. Annual and seasonal variation and the effects of hydroperiod on benthic macroinvertebrates of seasonal forest (“vernal”) ponds in Central Massachusetts, USA. Wetlands 2000, 20, 707–715. [Google Scholar] [CrossRef]
- Taylor, B.E.; Leeper, D.A.; McClure, M.A.; DeBiase, A.E. Carolina Bays: Ecology of aquatic invertebrates and perspectives on conservation. In Invertebrates in Freshwater Wetlands of North America: Ecology and Management; Batzer, D.P., Rader, R.B., Wissinger, S.A., Eds.; John Wiley & Sons: New York, NY, USA, 1999; pp. 167–197. [Google Scholar]
- Jaeger, R.G. Fluctuations in prey availability and food limitation for a terrestrial salamander. Oecologia 1980, 44, 335–341. [Google Scholar] [CrossRef]
- Jaeger, R.G.; Wicknick, J.A.; Griffis, M.R.; Anthony, C.D. Socioecology of a terrestrial salamander: juveniles enter adult territories during stressful foraging periods. Ecology 1995, 76, 533–543. [Google Scholar]
- Walton, B.M. Salamanders in forest-floor food webs: environmental heterogeneity affects the strength of top-down effects. Pedobiologia 2005, 49, 381–393. [Google Scholar] [CrossRef]
- Milanovich, J.R.; Trauth, S.E.; Saugey, D.A.; Jordan, R.R. Fecundity, reproductive ecology, and influence of precipitation on clutch size in the Western Slimy Salamander (Plethodon albagula). Herpetologica 2006, 62, 292–301. [Google Scholar] [CrossRef]
- Scott, D.E.; Fore, M.R. The effect of food limitation on lipid levels, growth, and reproduction in the marbled salamander, Ambystoma opacum. Herpetologica 1995, 51, 462–471. [Google Scholar]
- Harris, R.N.; Ludwig, P.M. Resource level and reproductive frequency in female four-toed salamanders, Hemidactylium scutatum. Ecology 2004, 85, 1585–1590. [Google Scholar] [CrossRef]
- Cunningham, H.R.; Rissler, L.J.; Apodaca, J.J. Competition at the range boundary in the slimy salamander: using reciprocal transplants for studies on the role of biotic interactions in spatical distributions. J. Anim. Ecol. 2009, 78, 52–62. [Google Scholar] [CrossRef]
- Alford, R.A.; Wilbur, H.M. Priority effects in experimental pond communities: competition between Bufo and Rana. Ecology 1985, 66, 1097–1105. [Google Scholar] [CrossRef]
- Wilbur, H.M.; Alford, R.A. Priority effects in experimental pond communities: responses of Hyla to Bufo and Rana. Ecology 1985, 66, 1106–1114. [Google Scholar] [CrossRef]
- Alford, R.A. Variation in predator phenology affects predator performance and prey community composition. Ecology 1989, 70, 206–219. [Google Scholar] [CrossRef]
- Morin, P.J.; Lawler, S.P.; Johnson, E.A. Ecology and breeding phenology of larval Hyla andersonii: the disadvantages of breeding late. Ecology 1990, 71, 1590–1598. [Google Scholar] [CrossRef]
- Lawler, S.P.; Morin, P.J. Temporal overlap, competition, and priority effects in larval anurans. Ecology 1993, 74, 174–182. [Google Scholar] [CrossRef]
- Simberloff, D. Global climate change and introduced species in United States forests. Sci. Total Environ. 2000, 262, 253–261. [Google Scholar] [CrossRef]
- Kats, L.B.; Ferrer, R.P. Alien predators and amphibian declines: review of two decades of science and the transition to conservation. Divers. Distrib. 2003, 9, 99–110. [Google Scholar] [CrossRef]
- Rödder, D.; Weinsheimer, F. Will future anthropogenic climate change increase the potential distribution of the alien invasive Cuban treefrog (Anura: Hylidae)? J. Nat. Hist. 2009, 43, 1207–1217. [Google Scholar] [CrossRef]
- Kearney, M.; Phillips, B.L.; Tracy, C.R.; Christian, K.R.; Betts, G.; Porter, W.P. Modeling species distributions without using species distributions: the cane toad in Australia under current and future climates. Ecography 2008, 31, 423–434. [Google Scholar] [CrossRef]
- Ficetola, G.F.; Thuiller, W.; Miaud, C. Prediction and validation of the potential global distribution of a problematic alien invasive species—the American bullfrog. Divers. Distrib. 2007, 13, 476–485. [Google Scholar] [CrossRef]
- Morin, P.J. Predatory salamanders reverse the outcome of competition among three species of anuran tadpoles. Science 1981, 212, 1284–1286. [Google Scholar]
- Fauth, J.E. Identifying potential keystone species from field data—an example from temporary ponds. Ecol. Lett. 1999, 2, 36–43. [Google Scholar]
- Fauth, J.E.; Resetarits, W.J., Jr. Interactions between the salamander Siren intermedia and the keystone predator Notophthalmus viridescens. Ecology 1991, 72, 827–838. [Google Scholar] [CrossRef]
- Smith, K.G. Keystone predators (eastern newts, Notophthalmus viridescens) reduce the impacts of an aquatic invasive species. Oecologia 2006, 148, 342–349. [Google Scholar] [CrossRef]
- Harvell, C.D.; Mitchell, C.; Ward, J.; Altizer, S.; Dobson, A.; Ostfeld, R.; Samuel, M. Climate warming and disease risks for terrestrial and marine biota. Science 2002, 296, 2158–2162. [Google Scholar] [CrossRef]
- Kilpatric, A.M.; Briggs, C.J.; Daszak, P. The ecology and impact of chytridiomycosis: an emerging disease of amphibians. Trends in Ecol. Evol. 2010, 25, 109–118. [Google Scholar] [CrossRef]
- Richards-Zawacki, C.L. Thermoregulatory behavior affects prevalence of chytrid fungal infection in a wild population of Panamanian golden frogs. Proc. Roy. Soc. B. 2010, 277, 519–528. [Google Scholar] [CrossRef]
- Green, G.; Cohen, N. Effect of temperature on serum complement levels in the leopard frog, Rana pipiens. Develop. Comp. Immunol. 1977, 1, 59–64. [Google Scholar] [CrossRef]
- Maniero, G.D.; Carey, C. Changes in selected aspects of immune function in the leopard frog, Rana pipiens, associated with exposure to cold. J. Comp. Physiol. B 1997, 167, 256–263. [Google Scholar] [CrossRef]
- Matutte, B.; Storey, K.B.; Knoop, F.C.; Conlon, J.M. Induction of synthesis of an antimicrobial peptide in the skin of the freeze-tolerant frog, Rana sylvatica, in response to environmental stimuli. FEBS Lett. 2000, 483, 135–138. [Google Scholar] [CrossRef]
- Wright, R.K.; Cooper, E.L. Temperature effects of ectotherm immune response. Develop. Comp. Immunol. 1991, 5, 117–122. [Google Scholar]
- Cooper, E.L.; Wright, R.K.; Klembau, A.E.; Smith, C.T. Hibernation alters the frog’s immune system. Cryobiology 1992, 29, 616–631. [Google Scholar] [CrossRef]
- Bly, J.E.; Clem, L.W. Temperature-mediated processes in teleost immunity: in vitro immunosuppression induced by in vivo low temperature in channel catfish. Vet. Immunol. Immunopathol. 1991, 28, 365–377. [Google Scholar] [CrossRef]
- Cone, R.E.; Marchalonis, J.J. Cellular and humoral aspects of the influence of environmental temperature on the immune response of poikilothermic vertebrates. J. Immunol. 1972, 108, 952–957. [Google Scholar]
- Le Morvan, C.; Deschaux, P.; Troutaud, D. Effects and mechanisms of environmental temperature on carp (cyprinus carpio) anti-DNP antibody response and non-specific cytotoxic cell activity: A kinetic study. Develop. Comp. Immunol. 1996, 20, 331–340. [Google Scholar] [CrossRef]
- Hardie, L.J.; Fletcher, T.C.; Secombes, C.J. Effect of temperature on macrophage activation and the production of macrophage activating factor by rainbow trout (Oncorhynchus mykiss) leucocytes. Develop. Comp. Immunol. 1994, 18, 57–66. [Google Scholar]
- Le Morvan, C.; Clerton, P.; Deschaux, P.; Troutaud, D. Effects of environmental temperature on macrophage activities in carp. Fish Shellfish Immunol. 1997, 7, 209–212. [Google Scholar] [CrossRef]
- Raffel, T.R.; Rohr, J.R.; Kiesecker, J.M.; Hudson, P.J. Negative effects of changing temperature on amphibian immunity under field conditions. Funct. Ecol. 2006, 20, 819–828. [Google Scholar] [CrossRef]
- Collazos, M.E.; Barriga, C.; Ortega, E. Effect of high summer temperatures upon granulocyte phagocytic function of the tench (Tinca Tinca, L.). Comp. Immunol. Microbiol. Infect. Dis. 1995, 18, 115–121. [Google Scholar] [CrossRef]
- Daszak, P.A.; Cunningham, A.; Hyatt, A.D. Infectious disease and amphibian population declines. Divers. Distrib. 2003, 9, 141–150. [Google Scholar]
- Skerratt, L.F.; Berger, L.; Speare, R.; Cashins, S.; McDonald, K.R.; Phillott, A.D.; Hines, H.B.; Kenyon, N. Spread of chytridiomycosis has caused the rapid global decline and extinction of frogs. EcoHealth 2007, 4, 125–134. [Google Scholar] [CrossRef]
- Rödder, D.; Kielgast, J.; Bielby, J.; Schmidtlein, S.; Bosch, J.; Garner, T.W.K.; Veith, M.; Walker, S.; Fisher, M.C.; Lötters, S. Global amphibian extinction risk assessment for the panzootic chytrid fungus. Diversity 2009, 1, 52–66. [Google Scholar]
- Burdon, J.; Elmqvist, T. Selective sieves in the epidemiology of Melampsora lini. Plant Pathol. 1996, 45, 933–943. [Google Scholar] [CrossRef]
- Dwyer, G.; Elkinton, J.S. Using simple models to predict virus epizootics in gypsy moth populations. J. Anim. Ecol. 1993, 62, 1–11. [Google Scholar]
- Blaustein, A.R.; Johnson, P.T.J. Prevalence, causes, and implications of amphibian deformities. Front. Ecol. Environ. 2003, 1, 87–94. [Google Scholar]
- Johnson, P.T.J.; Chase, J.M.; Dosch, K.L.; Hartson, R.B.; Gross, J.A.; Larson, D.J.; Sutherland, D.R.; Carpenter, S.R. Aquatic eutrophication promotes pathogenic infection in amphibians. Proc. Natl. Acad. Sci. 2007, 104, 15781–15786. [Google Scholar] [CrossRef]
- Johnson, M.L.; Berger, L.; Philips, L.; Speare, R. Fungicidal effects of chemical disinfectants, UV light, desiccation and heat on the amphibian chytrid Batrachochytrium dendrobatidis. Dis. Aquat. Organ. 2003, 57, 255–260. [Google Scholar] [CrossRef]
- Kriger, K.M. Lack of evidence for the drought-linked chytridiomycosis hypothesis. J. Wild. Dis. 2009, 45, 537–541. [Google Scholar] [CrossRef]
- Lampo, M.; Rodríguez-contreras, A.; La Marca, E.; Daszak, P. A chytridiomycosis epidemic and a severe dry season precede the disappearance of Atelopus species from the Venezuelan Andes. Herpetol. J. 2006, 16, 395–402. [Google Scholar]
- Longcore, J.E.; Pessier, A.P.; Nichols, D.K. Batrachochytrium dendrobatidis gen. et sp. nov., a chytrid pathogenic to amphibians. Mycologia 1999, 91, 219–227. [Google Scholar] [CrossRef]
- Piotrowski, J.S.; Annis, S.L.; Longcore, J.E. Physiology of Batrachochytrium dendrobatidis, a chytrid pathogen of amphibians. Mycologia 2004, 96, 9–15. [Google Scholar] [CrossRef]
- Seimon, T.A.; Seimon, A.; Daszak, P.; Halloys, S.R.P.; Schloegel, L.M.; Aguilar, C.A.; Sowell, P.; Hyatt, A.D.; Konecky, B.; Simmons, J.E. Upward range extension of Andean anurans and chytridiomycosis to extreme elevations in response to tropical deglaciation. Glob. Change Biol. 2007, 13, 288–299. [Google Scholar] [CrossRef]
- Pounds, A.; Bustamante, M.R.; Coloma, L.A.; Consuegra, J.A.; Fogden, M.P.L.; Foster, P.M.; La Marca, E.; Masters, K.L.; Merino-Viteri, A.; Puschendorf, R.; Ron, S.R.; Sánchez-Azofeifa, G.A.; Still, C.J.; Young, B.E. Widespread amphibian extinctions from epidemic disease driven by global warming. Nature 2006, 439, 161–167. [Google Scholar] [CrossRef]
- Kupferberg, S.J.; Catenazzi, A.; Lunde, K.; Lind, A.J.; Palen, W.J. Parasitic Copepod (Lernaea cyprinacea) Outbreaks in Foothill Yellow-legged Frogs (Rana boylii) Linked to Unusually Warm Summers and Amphibian Malformations in Northern California. Copeia 2009, 1, 529–537. [Google Scholar]
- Blaustein, A.R.; Dobson, A. Extinction: A message from the frogs. Nature 2006, 439, 143–144. [Google Scholar] [CrossRef]
- Bosch, J.; Carrascal, L.M.; Duran, L.; Walker, S.; Fisher, M.C. Climate change and outbreaks of amphibian chytridiomycosis in a montane area of Central Spain; is there a link? Proc. Roy. Soc. (B) 2006, 274, 253–260. [Google Scholar]
- D’Amen, M.; Bombi, P. Global warming and biodiversity: evidence of climate-linked amphibian declines in Italy. Biol. Conserv. 2009, 142, 3060–3067. [Google Scholar] [CrossRef]
- Drew, A.; Allen, E.J.; Allen, L.J.S. Analysis of climatic and geographic factors affecting the presence of chytridiomycosis in Australia. Dis. Aquat. Organ. 2006, 68, 245–250. [Google Scholar] [CrossRef]
- Lips, K.R.; Diffendorfer, J.; Mendelson, J.R., III; Sears, M.W. Riding the wave: Reconciling the roles of disease and climate change in amphibian declines. Plos Biol. 2008, 6, 441–454. [Google Scholar]
- Lampo, M.; Sánchez, D.; Nava-González, F.; García, C.Z.; Acevedo, A. Wavelike epidemics in Venezuela? Plos Bio. 2008. [Google Scholar]
- Parmesan, C.; Singer, M.C. Amphibian extinctions: Disease not the whole story. Plos Biol. 2008. [Google Scholar]
- Rohr, J.R.; Raffel, T.R.; Romansic, J.M.; McCallum, H.; Hudson, P.J. Evaluating the links between climate, disease spread, and amphibian declines. Proc. Natl. Acad. Sci. USA 2008, 105, 17436–17441. [Google Scholar]
- Alford, R.A.; Bradfield, K.S.; Richards, S.J. Global warming and amphibian losses. Nature 2007, 447, E3–E4. [Google Scholar] [CrossRef]
- Di Rosa, I.; Simoncelli, F.; Fagotti, A.; Pascolini, R. The proximate cause of frog declines? Nature 2007, 447, E4–E5. [Google Scholar] [CrossRef]
- Pounds, J.A.; Bustamante, M.R.; Coloma, L.A. Pounds et al. reply. Nature 2007, 447, E5–E6. [Google Scholar] [CrossRef]
- Bancroft, B.A.; Baker, N.J.; Blaustein, A.R. A meta-analysis of the effects of ultraviolet B radiation and other stressors on survival in amphibians. Conser Biol. 2008, 22, 987–996. [Google Scholar] [CrossRef]
- Blaustein, A.R.; Kiesecker, J.M.; Chivers, D.P.; Hokit, D.G.; Marco, A.; Belden, L.K.; Hatch, A. Effects of ultraviolet radiation on amphibians: Field experiments. Am. Zool. 1998, 38, 799–812. [Google Scholar]
- Nagl, A.M.; Hofer, R. Effects of ultraviolet radiation on early larval stages of the Alpine newt, Triturus alpestris, under natural and laboratory conditions. Oecologia 1997, 110, 514–519. [Google Scholar] [CrossRef]
- Blaustein, A.R.; Chivers, D.P.; Kats, L.B.; Kiesecker, J.M. Effects of ultraviolet radiation on locomotion and orientation in roughskin newts (Taricha granulosa). Ethology 2000, 108, 227–234. [Google Scholar]
- Kats, L.B.; Kiesecker, J.M.; Chivers, D.P.; Blaustein, A.R. Effects of UV-B on antipredator behavior in three species of amphibians. Ethology 2000, 106, 921–932. [Google Scholar] [CrossRef]
- Belden, L.K.; Wildy, E.L.; Blaustein, A.R. Growth, survival, and behaviour of larval long-toed salamanders (Ambystoma macrodactylum) exposed to ambient levels of UV-B radiation. J. Zool. (London) 2000, 251, 473–479. [Google Scholar] [CrossRef]
- Pahkala, M.; Laurila, A.; Merilä, J. Ambient ultraviolet-B radiation reduces hatchling size in the common frog Rana temporaria. Ecography 2000, 23, 531–538. [Google Scholar] [CrossRef]
- Pahkala, M.; Laurila, A.; Merilä, J. Carry-over effects of ultraviolet-B radiation on larval fitness in Rana temporaria. Proc. Roy. Soc. London B 2001, 268, 1699–1706. [Google Scholar] [CrossRef]
- Smith, G.R.; Waters, M.A.; Rettig, J.E. Consequences of embryonic UV-B exposure for embryos and tadpoles of the plains leopard frog. Conserv. Biol. 2000, 14, 1903–1907. [Google Scholar] [CrossRef]
- Worrest, R.D.; Kimeldorf, D.J. Distortions in amphibian development induced by ultraviolet-B enhancement (290−310 nm) of a simulated solar spectrum. Photochem. Photobiol. 1976, 24, 377–382. [Google Scholar] [CrossRef]
- Hays, J.B.; Blaustein, A.R.; Kiesecker, J.M.; Hoffman, P.D.; Pandelova, I.; Coyle, I.C.; Richardson, T. Developmental responses of amphibians to solar and artificial UV-B sources: A comparative study. Photochem. Photobiol. 1996, 64, 449–456. [Google Scholar] [CrossRef]
- Blaustein, A.R.; Kiesecker, J.M.; Chivers, D.P.; Anthony, R.G. Ambient UV-B radiation causes deformities in amphibian embryos. Proc. Natl. Acad. Sci. USA 1997, 94, 13735–13737. [Google Scholar] [CrossRef]
- Bruggeman, D.J.; Bantle, J.A.; Goad, C. Linking teratogenesis, growth, and DNA photodamage to artificial ultraviolet-B radiation in Xenopus laevis larvae. Environ. Toxicol. Chem. 1998, 17, 2114–2121. [Google Scholar] [CrossRef]
- Fite, K.V.; Blaustein, A.R.; Bengston, L.; Hewitt, H.E. Evidence of retinal light damage in Rana cascadae: a declining amphibian species. Copeia 1998, 1, 906–914. [Google Scholar]
- Croteau, M.C.; Davidson, M.A.; Lean, D.R.S.; Trudeau, V.L. Global increases in ultraviolet B radiation: Potential impacts on amphibian development and metamorphosis. Physiol. Biochem. Zool. 2008, 81, 743–761. [Google Scholar] [CrossRef]
- Kiesecker, J.M.; Blaustein, A.R. Synergism between UV-B radiation and a pathogen magnifies amphibian embryo mortality in nature. Proc. Natl. Acad. Sci. USA 1995, 92, 11049–11052. [Google Scholar] [CrossRef]
- Long, L.E.; Saylor, L.S.; Soule, M.E. A pH/UV-B synergism in amphibians. Conserv. Biol. 1995, 9, 1301–1303. [Google Scholar]
- Merilä, J.; Pahkala, M.; Johanson, U. Increased ultraviolet-B radiation, climate change and latitudinal adaptation-a frog perspective. Annales Zool. Fenn. 2000, 37, 129–134. [Google Scholar]
- Van Uitregt, V.O.; Wilson, R.S.; Franklin, C.E. Cooler temperatures increase sensitivity to ultraviolet B radiation in embryos and larvae of the frog Limnodynastes peronii. Glob. Change Biol. 2007, 13, 1114–1121. [Google Scholar] [CrossRef]
- Searle, C.L.; Belden, L.K.; Bancroft, B. A.; Han, B.A.; Biga, L.M.; Blaustein, A.R. Experimental examination of the effects of ultraviolet-B radiation in combination with other stressors on frog larvae. Oecologia 2009, 162, 237–245. [Google Scholar]
- Tilman, D.; Fargione, J.; Wolff, B. Forecasting agriculturally driven global environmental change. Science 2001, 292, 281–284. [Google Scholar] [CrossRef]
- Davidson, C.; Shaffer, H.B.; Jennings, M.R. Declines of the California red-legged frog: Climate, UV-B, habitat, and pesticides hypotheses. Ecol. App. 2001, 11, 464–479. [Google Scholar] [CrossRef]
- Davidson, C.; Knapp, R. Multiple stressors and amphibian declines: dual impacts of pesticides and fish on yellow-legged frogs. Ecol. App. 2007, 17, 587–597. [Google Scholar] [CrossRef]
- Wright, R.F.; Schindler, D.W. Interaction of acid rain and global changes: effects on terrestrial and aquatic ecosystems. Water Air Soil Poll. 1995, 85, 89–99. [Google Scholar] [CrossRef]
- Zabik, J.M.; Seiber, J.N. Atmospheric transport of organophosphate pesticides from California’s Central Valley to the Sierra Nevada mountains. J. Environ. Qual. 1993, 22, 80–90. [Google Scholar] [CrossRef]
- Aston, L.S.; Seiber, J.N. Fate of summertime airborne organophosphate pesticide residues in the Sierra Nevada Mountains. J. Environ. Qual. 1997, 26, 1483–1492. [Google Scholar] [CrossRef]
- Davidson, C. Declining downwind: Amphibian population declines in California and historical pesticide use. Ecological Applications 2004, 14, 1892–1902. [Google Scholar] [CrossRef]
- Hageman, K.J.; Simonich, S.L.; Campbell, D.H.; Wilson, G.R.; Landers, D.H.; Dunson, W.A.; Wyman, R.L. Atmospheric deposition of current-use and historic-use pesticides in snow at national parks in the western United States. Environ. Sci. Technol. 2006, 40, 3174–3180. [Google Scholar] [CrossRef]
- Kiesecker, J.M. pH induced growth reduction and its effects on predator-prey interactions between Ambystoma tigrinum and Pseudacris triseriata. Ecol. App. 1996, 6, 1325–1331. [Google Scholar]
- Pounds, J.A.; Crump, M.L. Amphibian declines and climate disturbance: the case of the golden toad and the harlequin frog. Conserv. Biol. 1994, 8, 72–85. [Google Scholar]
- Sparling, D.W.; Fellers, G.M.; McConnell, L.L. Pesticides and amphibian population declines in California, USA. Environ. Tox. Chem. 2001, 20, 1591–1595. [Google Scholar]
- Blaustein, A.R.; Alford, R.A.; Harris, R.N. The value of well designed experiments in studying diseases with special reference to amphibians. EcoHealth 2009, in press. [Google Scholar]
- Maurer, E.P.; Adam, J.C.; Wood, A.W. Climate model based consensus on the hydrologic impacts of climate change to the Rio Lempa basin of Central America. Hydrol. Earth Sys. Sci. 2009, 13, 183–194. [Google Scholar] [CrossRef]
- Girvetz, E.; Zganjar, C.; Raber, G.; Shafer, S.L.; Maurer, E.P.; Kareiva, P.; Lawler, J.J. Applied climate-change analysis. PLoS ONE 2009, 4, e8320. [Google Scholar]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Blaustein, A.R.; Walls, S.C.; Bancroft, B.A.; Lawler, J.J.; Searle, C.L.; Gervasi, S.S. Direct and Indirect Effects of Climate Change on Amphibian Populations. Diversity 2010, 2, 281-313. https://doi.org/10.3390/d2020281
Blaustein AR, Walls SC, Bancroft BA, Lawler JJ, Searle CL, Gervasi SS. Direct and Indirect Effects of Climate Change on Amphibian Populations. Diversity. 2010; 2(2):281-313. https://doi.org/10.3390/d2020281
Chicago/Turabian StyleBlaustein, Andrew R., Susan C. Walls, Betsy A. Bancroft, Joshua J. Lawler, Catherine L. Searle, and Stephanie S. Gervasi. 2010. "Direct and Indirect Effects of Climate Change on Amphibian Populations" Diversity 2, no. 2: 281-313. https://doi.org/10.3390/d2020281
APA StyleBlaustein, A. R., Walls, S. C., Bancroft, B. A., Lawler, J. J., Searle, C. L., & Gervasi, S. S. (2010). Direct and Indirect Effects of Climate Change on Amphibian Populations. Diversity, 2(2), 281-313. https://doi.org/10.3390/d2020281



