The CC-Bio Project: Studying the Effects of Climate Change on Quebec Biodiversity
Abstract
:1. Introduction
2. Methods
2.1. Research Structure
2.2. Scientific Approach
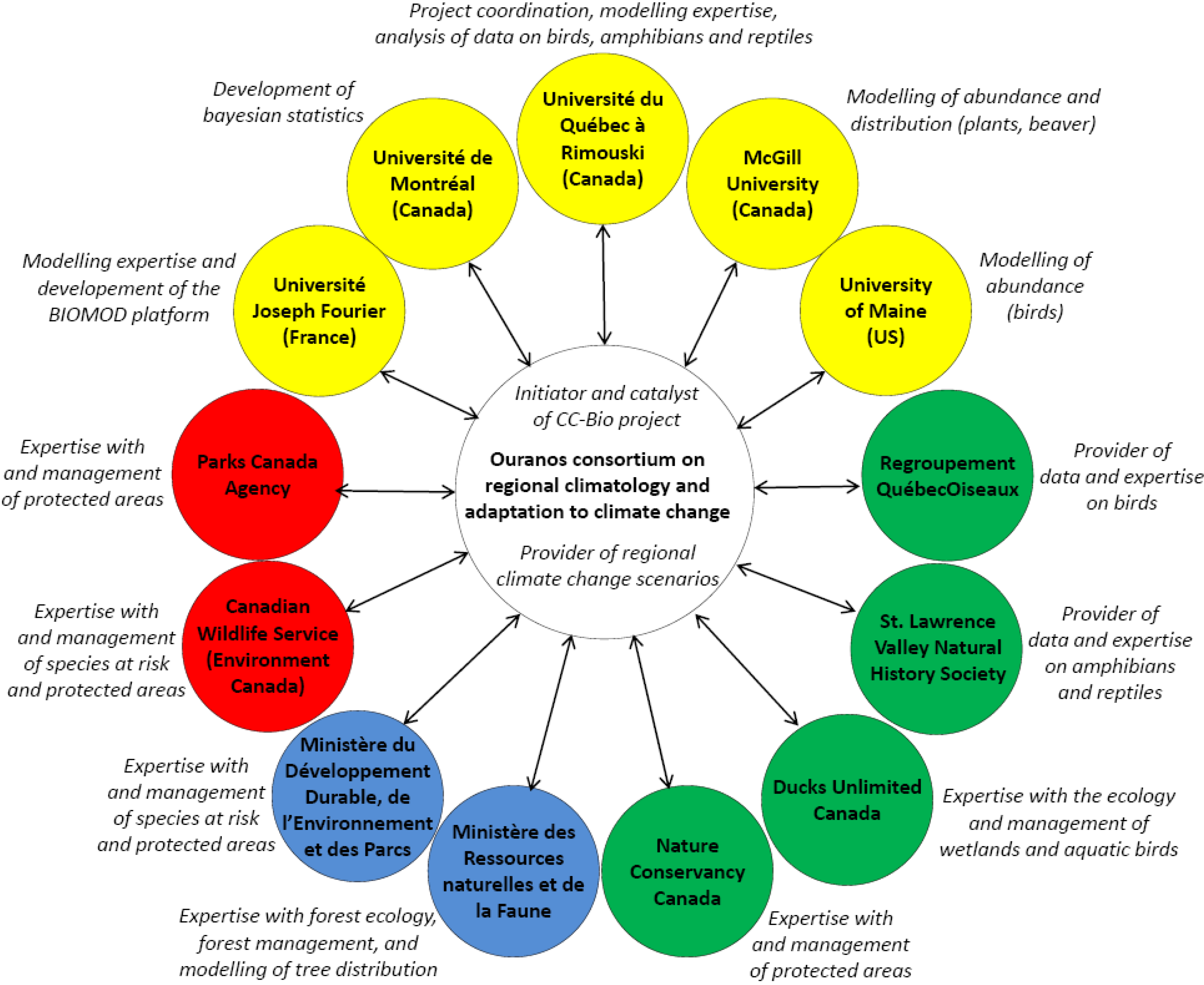
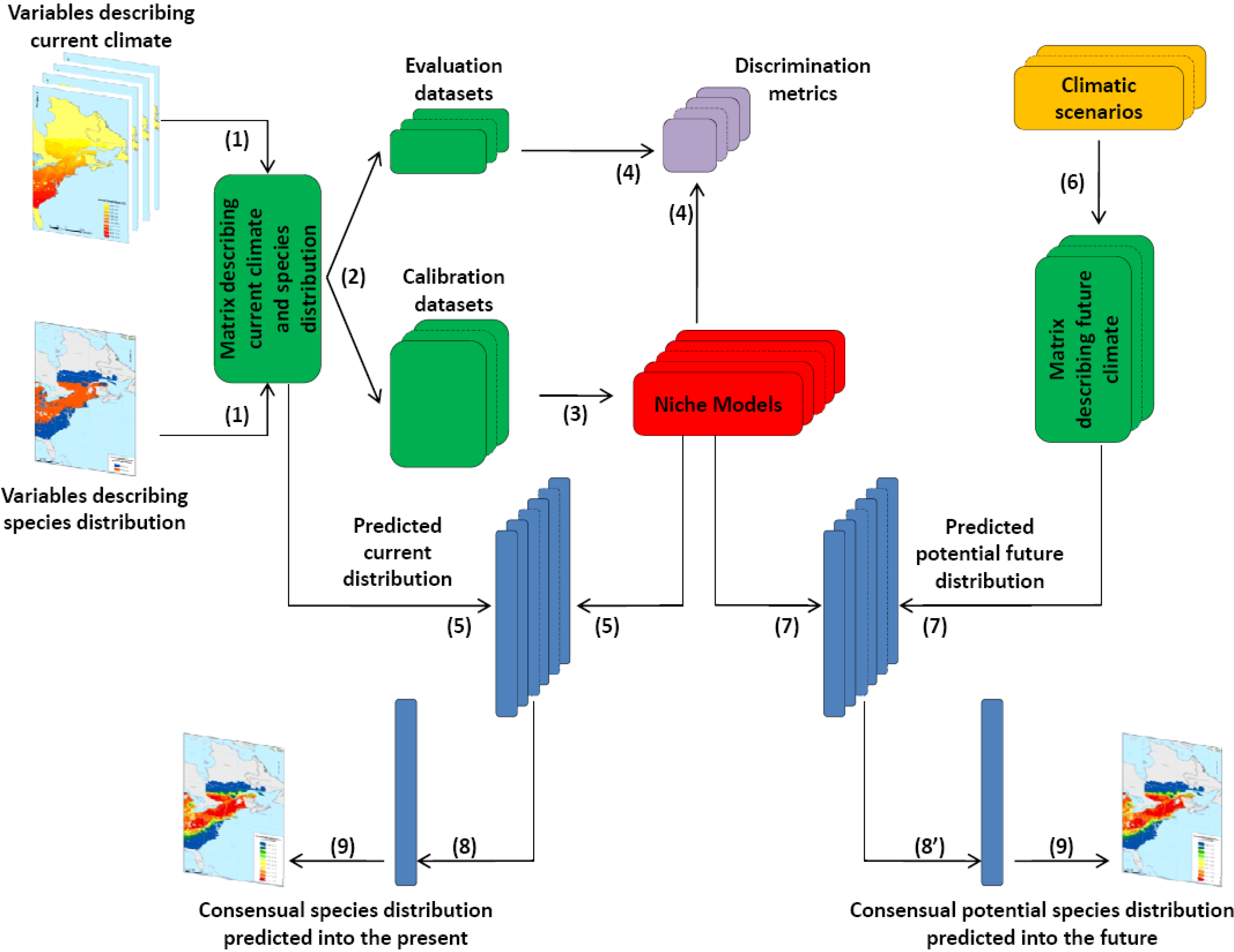
2.3. Sources of Data Used in the Project
3. Outlook of Expected Results
| Type of data | Name of database | Reference |
|---|---|---|
| Biodiversity | ||
| Birds | Étude des Populations d’Oiseaux du Québec (ÉPOQ) | http://www.quebecoiseaux.org/index.php?option=com_content&view=article&id=196&Itemid=103 |
| Birds | Breeding Bird Survey (BBS) | http://www.pwrc.usgs.gov/BBS/index.html |
| Amphibians, Reptiles | Atlas des Amphibiens et Reptiles du Québec (AARQ) | http://www.atlasamphibiensreptiles.qc.ca/ |
| Amphibians | National Amphibian Atlas | http://www.pwrc.usgs.gov/naa |
| Amphibians | Atlantic Canada Conservation Data Center | http://www.accdc.com/Products/Publicdata.html |
| Beaver | - | Jarema et al. [55] |
| Trees |
| |
| Vascular plants | Actaea database | http://cc-bio.uqar.ca/publications/ActaeaReport.pdf |
| Wetlands | - | Ménard et al. [61] |
| Threatened and vulnerable species | Centre de données sur le patrimoine naturel du Québec (CDPNQ) | http://www.cdpnq.gouv.qc.ca/index-en.htm |
| Climate | ||
| Current climate | Anusplin USDA Forest Service data | http://forest.moscowfsl.wsu.edu/climate/ |
| Historical climate | Adjusted Historical Canadian Climate Data (AHCCD) | http://www.cccma.bc.ec.gc.ca/hccd/data/temperature/tempdata_e.shtml |
| Future climate | Canadian Regional Climate Model (CRCM4) | http://www.ouranos.ca/ |
| Future climate | Global Climate Models | http://www-pcmdi.llnl.gov/ipcc/about_ipcc.php |
| Soil variables | ||
| Soil (Quebec) | SIEF | http://www.mrnf.gouv.qc.ca/forets/connaissances/connaissances-inventaire-cartes-sief-temporaires.jsp |
| Soil (USA) | USDA SSURGO | http://soils.usda.gov/survey/geography/ssurgo/ |
3.1. The Biological Importance of Climate Change in Quebec
3.2. Potential Response of Quebec Biodiversity to Climate Change: Towards a Northern Biodiversity Paradox
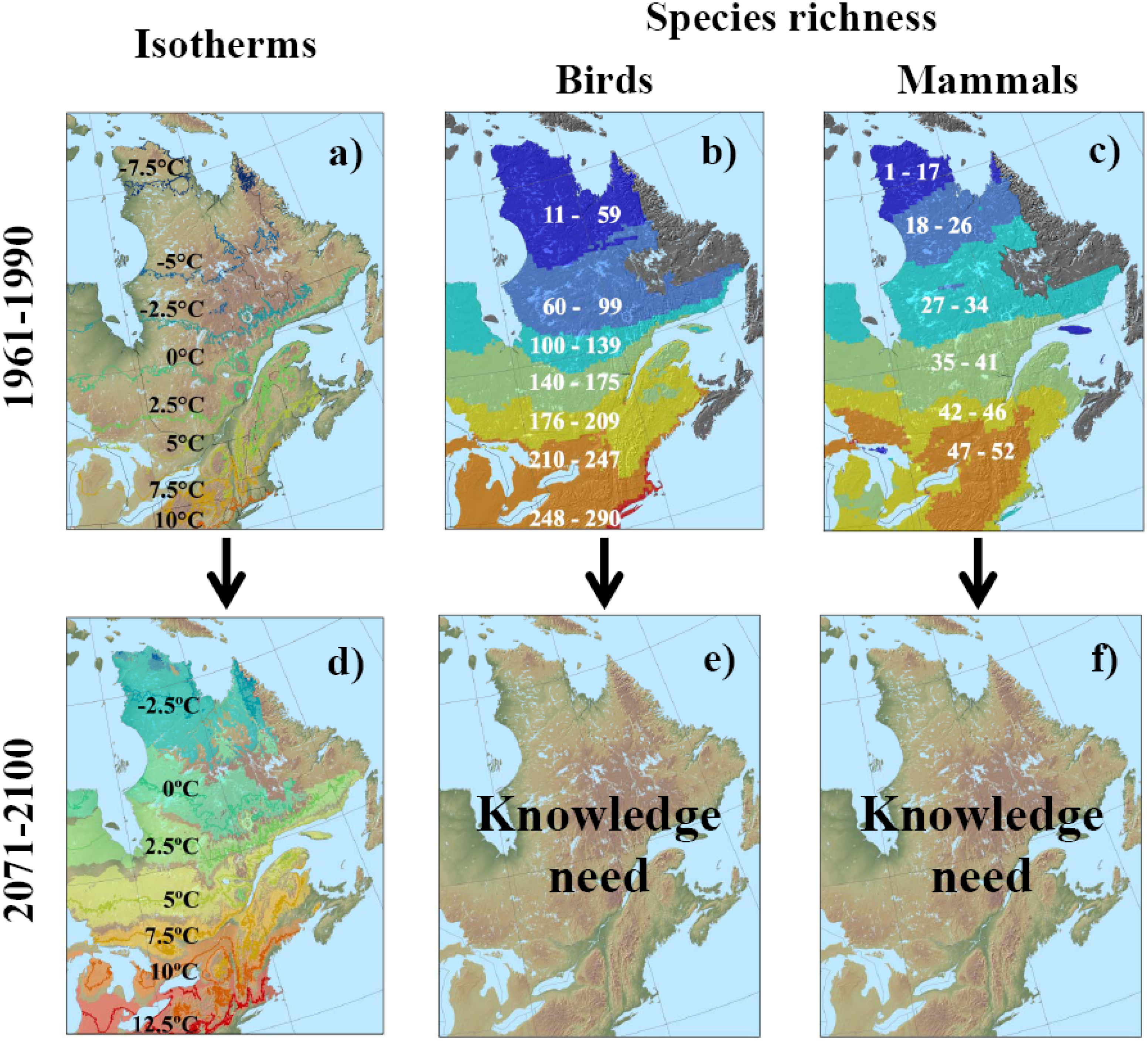
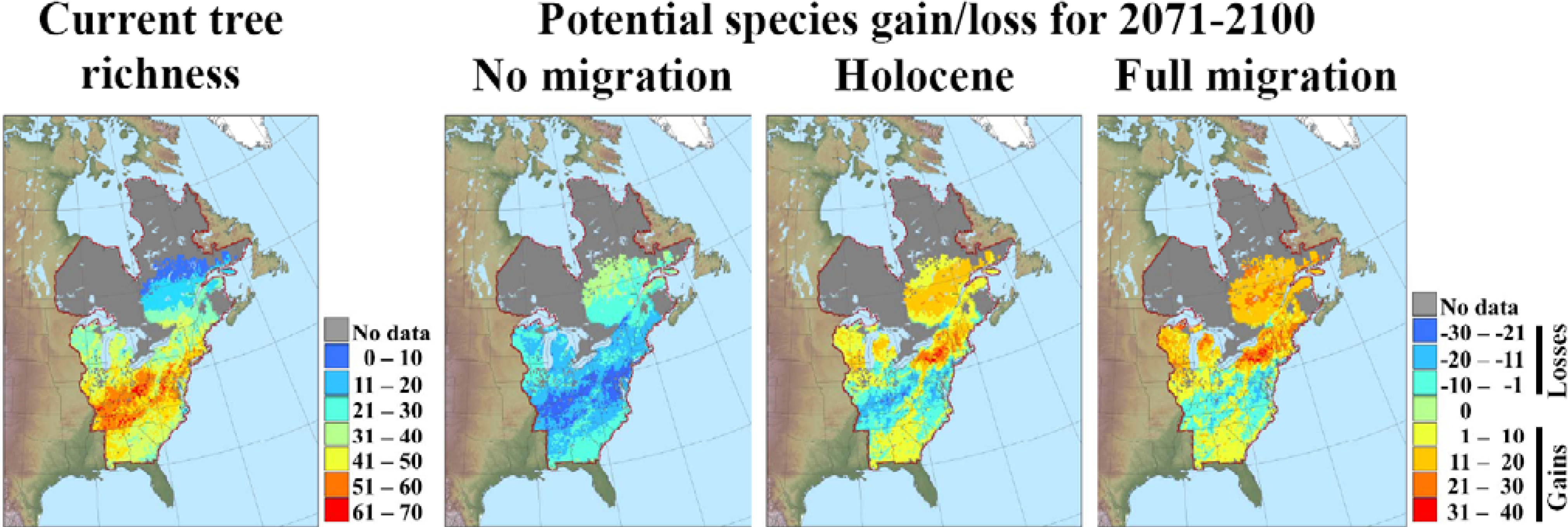
| Taxa | QC | NY | VT | NH | ME | NB | ON | Res. (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Breeding birds | 233 | 230 | 184 | 181 | 197 | 179 | 241 | 57 (24%) | a |
| Mammals | 75 | 96 | 55 | 78 | 79 | 74 | 91 | 30 (40%) | b |
| Amphibians | 21 | 32 | 21 | 21 | 17 | 16 | 22 | 14 (67%) | c |
| Reptiles 1 | 16 | 32 | 19 | 18 | 16 | 7 | 24 | 24 (150%) | c |
| Odonata 2 | 139 | 185 | 135 | 153 | 159 | 129 | 170 | 70 (50%) | d |
| Trees | 164 | 302 | 184 | 184 | 200 | 123 | 258 | 188 (115%) | e |
| Vascular plants 3 | 2,855 | 3,267 | 2,007 | 1,965 | 2,155 | 1,550 | 2,412 | 1,821 (62%) | e |
| Area | 1,542 | 141 | 25 | 24 | 86 | 73 | 1,076 | f |
4. Expected Conservation Impacts
| Adaptation strategy | Suitability for Quebec |
|---|---|
| Land and water protection and management | |
| 1. Increase extent of protected areas | Currently undertaken in northern Quebec where protected areas are scarce and human density is low. Little room is available in the southern part of Quebec (<50º Lat. N) where human density and demand for land are high and where biodiversity and presence of species at risk reach their peak in the province [62]. A target of 12% of protected area has been set for 2015 in Quebec (the 2009 figure is 8.12%, [80], but this may be insufficient to conserve some taxonomic groups [81,82]. |
| 2. Improve representation and replication within protected-area networks to conserve multiple examples of each ecosystem type | Same as #1, but more knowledge is needed to predict how ecosystem types will be reorganized through time as climate changes, and how decisions about representation made now will remain valid in the future. |
| 3. Improve management of existing protected areas to offset some of the effects of climate change (e.g., build dikes to protect some coastal sites from sea-level rise) | This strategy might potentially prove useful but a gap analysis is first needed to identify the protected areas most vulnerable to climate change, and to determine the management tools that could offset the effects of climate change. |
| 4. Design new natural areas and restoration sites to maximize resilience of natural systems to climate-change effects (e.g., establish protected area networks along elevational gradients to allow species to shift distributions along these gradients) | The strong latitudinal gradient in temperatures found in Quebec suggests that species migrations will occur mostly along a south-north or southwest-northeast axis. Therefore, spatial configuration of protected areas and corridors should favor connectivity along these axes. This raises important challenges for conservation in the agricultural parts of Quebec [80]. Also, although altitudinal gradients are less important in Quebec than in other Canadian provinces (like British Columbia), some regions with strong altitudinal gradients and high biodiversity value have already been identified (e.g., Chic-Chocs area) and should deserve special attention. |
| 5. Protect movement corridors, stepping stones, and refugia to direct protection efforts toward areas deemed essential for climate-induced species redistribution | See # 4. |
| 6. Manage and restore ecosystem function rather than focusing on specific components (species or assemblages) | Ecosystem-based management approaches are progressively implemented in Quebec to manage some ecosystem types such as forests and oceans. However, the complexity of this approach requires a long implementation time, a high level of support and information sharing from federal, provincial and local decision bodies, and a strong involvement of all stakeholders involved in natural resource management. |
| 7. Improve the matrix by increasing landscape permeability to species movement | An important strategy that must be developed in southern Quebec, where the landscape is severely fragmented by urbanization and agriculture. This strategy must be weighed, however, against the costs generated by the facilitation of the immigration of unwanted species coming from the south. |
| Direct Species Management | |
| 8. Focus conservation resources on species that might become extinct | This strategy was implemented in 1989 through the Loi sur les espèces menacées ou vulnérables (Quebec) and in 2002 through the Species At Risk Act (Canada). However, financial resources and political support are often lacking for adequate action, and lack of coordination between the institutions responsible for conservation of resources prevents the strategy from being fully efficient. |
| 9. Translocate species at risk of extinction from sites becoming unsuitable due to climate change to sites more favorable to their continued existence | Early debate has emerged in Quebec regarding this strategy, with both strong proponents and opponents. The acceptability and effectiveness of this strategy is likely to be case specific. Decisions will need to rely on detailed cost-benefit analyses involving complex assessments of potential ecological risks and sufficient data about population dynamics. |
| 10. Establish captive populations of species that would otherwise go extinct | In a context of limited resources, this might be an interesting tool in cases of extreme necessity, but must not be seen as a viable option in the long-term because of prohibitive costs (except perhaps in the case of ex situ conservation of plants, if this is considered as part of strategy # 10). |
| 11. Reduce pressures on species from sources other than climate change | This is the main goal of currently-existing conservation strategies implemented in Quebec, but habitat loss and fragmentation are still the most likely causes of extinction or extirpation for some taxa (e.g., reptiles, amphibians). Most species at risk are located in the south of the province, where land tenure is mostly private and protected areas are scarce. |
| Monitoring and Planning | |
| 12. Evaluate and enhance monitoring programs for wildlife and ecosystems | Ways to enhance biodiversity monitoring programs in the context of climate change are currently being analyzed by the Quebec government, in collaboration with academic researchers. However, the costs involved in biodiversity monitoring in a large area with low population density such as the province of Quebec can be prohibitive. Improved support to and better coordination of the efforts of naturalists (citizen science) must be considered. |
| 13. Incorporate predicted climate-change impacts into species and land-management plans, programs, and activities | The existence of a boundary organization such as Ouranos (see text) which is in relation with data users, planners, and decision-makers, and the current implementation of new research projects through the Quebec Plan d’Action sur les Changements Climatiques should help to implement this strategy in Quebec. |
| 14. Develop dynamic landscape conservation plans that explicitly address the climate adaptation needs of wildlife and biodiversity at a landscape scale | See # 13. However, the perceived poor economic benefits of conserving biodiversity represent a strong obstacle to fully implementing this strategy. |
| 15. Ensure wildlife and biodiversity needs are considered as part of the broader societal adaptation process, which targets mainly human health, infrastructure needs, and economically important resources | See # 14. |
| Law and Policy | |
| 16. Review and modify existing laws, regulations, and policies regarding wildlife and natural resource management, which were designed for the conservation of “static” biodiversity | Not to be implemented in the short term, since additional knowledge on the effects of climate change on biodiversity, as well as massive collaboration between stakeholders are first required. |
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgements
References and Notes
- Berteaux, D.; Reale, D.; McAdam, A.G.; Boutin, S. Keeping pace with fast climate change: Can arctic life count on evolution? Integr. Comp. Biol. 2004, 44, 140–151. [Google Scholar] [CrossRef]
- Bradshaw, W.E.; Holzapfel, C.M. Evolutionary response to rapid climate change. Science 2006, 312, 1477–1478. [Google Scholar] [CrossRef]
- Birks, H.J.B. Holocene Isochrone maps and patterns of tree-spreading in the British Isles. J. Biogeogr. 1989, 16, 503–540. [Google Scholar] [CrossRef]
- Huntley, B. European post-glacial forests: compositional changes in response to climatic change. J. Veg. Sci. 1990, 1, 507–518. [Google Scholar] [CrossRef]
- Lovejoy, T.E.; Hannah, L. Climate Change and Biodiversity; Yale University Press: New Haven, CT, USA and London, UK, 2004. [Google Scholar]
- Walther, G.R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.C.; Fromentin, J.M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological responses to recent climate change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef]
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar]
- Root, T.L.; Price, J.T.; Hall, K.R.; Schneider, S.H.; Rosenzweig, C.; Pounds, J.A. Fingerprints of global warming on wild animals and plants. Nature 2003, 421, 57–60. [Google Scholar] [CrossRef]
- Thomas, J.A. Monitoring change in the abundance and distribution of insects using butterflies and other indicator groups. Phil. Trans. R. Soc. B 2005, 360, 339–357. [Google Scholar] [CrossRef]
- Walther, G.R.; Berger, S.; Sykes, M.T. An ecological ‘footprint’ of climate change. Proc. R. Soc. B 2005, 272, 1427–1432. [Google Scholar] [CrossRef]
- Parmesan, C. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 637–669. [Google Scholar] [CrossRef]
- Walther, G.R.; Roques, A.; Hulme, P.E.; Sykes, M.T.; Pysek, P.; Kühn, I.; Zobel, M.; Bacher, S.; Botta-Dukát, Z.; Bugmann, H.; Czúcz, B.; Dauber, J.; Hickler, T.; Jarošík, V.; Kenis, M.; Klotz, S.; Minchin, D.; Moora, M.; Nentwig, W.; Ott, J.; Panov, V.E.; Reineking, B.; Robinet, C.; Semenchenko, V.; Solarz, W.; Thuiller, W.; Vilà, M.; Vohland, K.; Settele, J. Alien species in a warmer world: Risks and opportunities. Trends Ecol. Evol. 2009, 24, 686–693. [Google Scholar] [CrossRef]
- IPCC Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2007.
- Lyons, S.K. A quantitative assessment of the range shifts of Pleistocene mammals. J. Mammal. 2003, 84, 385–402. [Google Scholar] [CrossRef]
- Humphries, M.M.; Thomas, D.W.; Speakman, J.R. Climate-mediated energetic constraints on the distribution of hibernating mammals. Nature 2002, 418, 313–316. [Google Scholar] [CrossRef]
- de Blois, S.; Domon, G.; Bouchard, A. Landscape issues in plant ecology. Ecography 2002, 25, 244–256. [Google Scholar] [CrossRef]
- Thuiller, W.; Lavorel, S.; Araújo, M.B. Niche properties and geographical extent as predictors of species sensitivity to climate change. Global Ecol. Biogeogr. 2005, 14, 347–358. [Google Scholar] [CrossRef]
- Berteaux, D.; Stenseth, N.C. Measuring, understanding and projecting the effects of large-scale climatic variability on mammals. Climate Res. 2006, 32, 95–97. [Google Scholar] [CrossRef]
- Thuiller, W. Biodiversity: Climate change and the ecologist. Nature 2007, 448, 550–552. [Google Scholar]
- Root, T.L.; Schneider, S.H. Conservation and climate change: The challenges ahead. Conserv. Biol. 2006, 20, 706–708. [Google Scholar]
- Kerr, J.T. Butterfly species richness patterns in Canada: Energy, heterogeneity, and the potential consequences of climate change. Conserv. Ecol. 2001, 5, 10. [Google Scholar]
- Clark, J.S.; Grimm, E.C.; Lynch, J.; Mueller, P.G. Effects of Holocene climate change on the C4 grassland/woodland boundary in the Northern Plains, USA. Ecology 2001, 82, 620–636. [Google Scholar]
- Hadly, E.A.; Maurer, B.A. Spatial and temporal patterns of species diversity in montane mammal communities of western North America. Evol. Ecol. Res. 2001, 3, 477–486. [Google Scholar]
- Nelson, D.M.; Hu, F.S.; Tian, J.; Stefanova, I.; Brown, T.A. Response of C3 and C4 plants to middle-Holocene climatic variation near the prairie–forest ecotone of Minnesota. Proc. Natl. Acad. Sci. USA 2004, 101, 562–567. [Google Scholar] [CrossRef]
- Lemieux, C.J.; Scott, D.J. Climate change, biodiversity conservation and protected area planning in Canada. Can. Geogr. 2005, 49, 384–397. [Google Scholar] [CrossRef]
- Yagouti, A.; Boulet, G.; Vincent, L.; Vescovi, L.; Mekis, É. Observed Changes in Daily Temperature and Precipitation Indices for Southern Québec, 1960–2005. Atmosphere-Ocean 2008, 46, 243–256. [Google Scholar] [CrossRef]
- DesJarlais, C.; Allard, M.; Bélanger, D.; Blondlot, A.; Bouffard, A.; Bourque, A.; Chaumont, D.; Gosselin, P.; Houle, D.; Larrivée, C.; Lease, N.; Pham, A.T.; Roy, R.; Savard, J.P.; Turcotte, R.; Villeneuve, C. Savoir s’Adapter aux Changements Climatiques; Ouranos: Montréal, Canada, 2010. [Google Scholar]
- Hulme, M.; Sheard, N. Climate Change Scenarios for Canada; Climatic Research Unit: Norwich, UK, 1999. [Google Scholar]
- Guston, D.H. Boundary organizations in environmental policy and science: An introduction. Sci. Technol. Hum. Val. 2001, 26, 399–408. [Google Scholar] [CrossRef]
- Austin, M.P.; Cunningham, R.B.; Berry, P.M. New approaches to direct gradient analysis using environmental scalars and statistical curve-fitting procedures. Vegetatio 1984, 55, 11–27. [Google Scholar]
- Guisan, A.; Zimmermann, N.E. Predictive habitat distribution models in ecology. Ecol. Model. 2000, 135, 147–186. [Google Scholar] [CrossRef]
- Guisan, A.; Thuiller, W. Predicting species distribution: offering more than simple habitat models. Ecol. Lett. 2005, 8, 993–1009. [Google Scholar] [CrossRef]
- Austin, M.P.; Belbin, L.; Meyers, J.A.; Doherty, M.D.; Luoto, M. Evaluation of statistical models used for predicting plant species distributions: Role of artificial data and theory. Ecol. Model. 2006, 199, 197–216. [Google Scholar] [CrossRef]
- Araújo, M.B.; Pearson, R.G.; Thuiller, W.; Erhard, M. Validation of species-climate impact models under climate change. Glob. Change Biol. 2005, 11, 1504–1513. [Google Scholar]
- Martínez-Meyer, E. Climate change and biodiversity: some considerations in forecasting shifts in species' potential distributions. Biodivers. Inform. 2005, 2, 42–55. [Google Scholar]
- Heikkinen, R.K.; Luoto, M.; Araujo, M.B.; Virkkala, R.; Thuiller, W.; Sykes, M.T. Methods and uncertainties in bioclimatic envelope modelling under climate change. Prog. Phys. Geog. 2006, 30, 751–777. [Google Scholar] [CrossRef]
- Thuiller, W.; Midgley, G.F.; Hugues, G.O.; Bomhard, B.; Drew, G.; Rutherford, M.C.; Woodward, F.I. Endemic species and ecosystem sensitivity to climate change in Namibia. Glob. Change Biol. 2006, 12, 759–776. [Google Scholar]
- Pearson, R.G.; Dawson, T.P. Predicting the impacts of climate change on the distribution of species: are bioclimate envelope models useful? Global Ecol. Biogeogr. 2003, 12, 361–371. [Google Scholar] [CrossRef]
- Hampe, A. Bioclimate envelope models: What they detect and what they hide. Global Ecol. Biogeogr. 2004, 13, 469–471. [Google Scholar] [CrossRef]
- Midgley, G.F.; Hughes, G.O.; Thuiller, W.; Rebelo, A.G. Migration rate limitations on climate change-induced range shifts in Cape Proteaceae. Diversity Distrib. 2006, 12, 555–562. [Google Scholar] [CrossRef]
- Hughes, G.O.; Thuiller, W.; Midgley, G.F.; Collins, K. Environmental change hastens the demise of the critically endangered riverine rabbit (Bunolagus monticularis). Biol. Conserv. 2008, 141, 23–34. [Google Scholar] [CrossRef]
- Engler, R.; Guisan, A. MigClim: Predicting plant distribution and dispersal in a changing climate. Diversity Distrib. 2009, 15, 590–601. [Google Scholar] [CrossRef]
- Rose, N.; Burton, P.J. Using bioclimatic envelopes to identify temporal corridors in support of conservation planning in a changing climate. For. Ecol. Manage. 2009, 258, S64–S74. [Google Scholar] [CrossRef]
- Berteaux, D.; Humphries, M.M.; Krebs, C.J.; Lima, M.; McAdam, A.G.; Pettorelli, N.; Réale, D.; Saitoh, T.; Tkadlec, E.; Weladji, R.B.; Stenseth, N.C. Constraints to projecting the effects of climate change on mammals. Climate Res. 2006, 32, 151–158. [Google Scholar] [CrossRef]
- Thuiller, W. Patterns and uncertainties of species' range shifts under climate change. Glob. Change Biol. 2004, 10, 2020–2027. [Google Scholar] [CrossRef]
- Araújo, M.B.; New, M. Ensemble forecasting of species distributions. Trends Ecol. Evol. 2006, 22, 42–47. [Google Scholar] [CrossRef]
- Raftery, A.E.; Madigan, D.; Hoeting, J.A. Bayesian Model Averaging for Linear Regression Models. J. Am. Stat. Assoc. 1997, 92, 179–191. [Google Scholar] [CrossRef]
- Marmion, M.; Parviainen, M.; Luoto, M.; Heikkinen, R.K.; Thuiller, W. Evaluation of consensus methods in predictive species distribution modelling. Diversity Distrib. 2009, 15, 59–69. [Google Scholar] [CrossRef]
- Thuiller, W.; Lafourcade, B.; Engler, R.; Araújo, M.B. BIOMOD—A platform for ensemble forecasting of species distributions. Ecography 2009, 32, 369–373. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2009. [Google Scholar]
- Ellison, E. Bayesian inference in ecology. Ecol. Lett. 2004, 7, 509–520. [Google Scholar] [CrossRef]
- Clark, J.S. Why environmental scientists are becoming Bayesians. Ecol. Lett. 2005, 8, 2–14. [Google Scholar] [CrossRef]
- Wintle, B.A.; McCarthy, M.A.; Volinsky, C.T.; Kavanagh, R.P. The use of Bayesian Model averaging to better represent uncertainty in ecological models. Conserv. Biol. 2003, 17, 1579–1590. [Google Scholar] [CrossRef]
- Araújo, M.B.; Thuiller, W.; Pearson, R.G. Climate warming and the decline of amphibians and reptiles in Europe. J. Biogeogr. 2006, 33, 1712–1728. [Google Scholar] [CrossRef]
- Jarema, S.I.; Samson, J.; McGill, B.J.; Humphries, M.M. Variation in abundance across a species’ range predicts climate change responses in the range interior will exceed those at the edge: A case study with North American beaver. Glob. Change Biol. 2009, 15, 508–522. [Google Scholar]
- Fischlin, A.; Midgley, G.F.; Price, J.T.; Leemans, R.; Gopal, B.; Turley, C.; Rounsevell, M.D.A.; Dube, O.P.; Tarazona, J.; Velichko, A.A. Ecosystems, their properties, goods, and services. In Climate Change 2007: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Parry, M.L., Canziani, O., Palutikof, J.P., van der Linden, P.J., Hanson, C.E., Eds.; Cambridge University Press: Cambridge, UK, 2007; pp. 211–272. [Google Scholar]
- Young, B.E.; Byers, E.; Gravuer, G.; Redder, A. Guidelines for using the NatureServe Climate Change Vulnerability Index; NatureServe: Arlington, VA, USA, 2009. [Google Scholar]
- von Storch, H. Climate research and policy advice: Scientific and cultural constructions of knowledge. Environ. Sci. Pol. 2009, 12, 741–747. [Google Scholar] [CrossRef]
- West, J.M.; Julius, S.H.; Kareiva, P.; Enquist, C.; Lawler, J.J.; Petersen, B.; Johnson, A.E.; Shaw, M.R. U.S. natural resources and climate change: concepts and approaches for management adaptation. Environ. Manage. 2009, 44, 1001–1021. [Google Scholar] [CrossRef]
- Mawdsley, J.R.; O'Malley, R.; Ojima, D.S. A Review of climate-change adaptation strategies for wildlife management and biodiversity conservation. Conserv. Biol. 2009, 23, 1080–1089. [Google Scholar]
- Ménard, S.; Darveau, M.; Imbeau, L.; Lemelin, L.V. Méthode de Classification des Milieux Humides du Québec Boréal à Partir de la Carte Écoforestière du 3e Inventaire Décennal. Rapport Technique Q2006-3; Canards Illimités: Québec, Canada, 2006. [Google Scholar]
- Tardif, B.; Lavoie, G.; Lachance, Y. Québec Biodiversity Atlas—Threatened or Vulnerable Species; Gouvernement du Québec, Ministère du développement durable, du patrimoine écologique et des parcs, Direction du développement durable, du patrimoine écologique et des parcs: Québec, Canada, 2005. [Google Scholar]
- Rockwell, D. Nature of North America; Berkley (TRD): New York, NY, USA, 2002. [Google Scholar]
- Loarie, S.R.; Duffy, P.B.; Hamilton, H.; Asner, G.P.; Field, C.B.; Ackerly, D.D. The velocity of climate change. Nature 2009, 462, 1052–1055. [Google Scholar] [CrossRef]
- Ridgely, R.S.; Allnutt, T.F.; Brooks, T.; McNicol, D.K.; Mehlman, D.W.; Young, B.E.; Zook, J.R. Digital Distribution Maps of the Birds of the Western Hemisphere; NatureServe: Arlington, VA, USA, 2007; Version 3.0. [Google Scholar]
- Patterson, B.D.; Ceballos, G.; Sechrest, W.; Tognelli, M.F.; Brooks, T.; Luna, L.; Ortega, P.; Salazar, I.; Young, B.E. Digital Distribution Maps of the Mammals of the Western Hemisphere; NatureServe: Arlington, VA, USA, 2007; Version 3.0. [Google Scholar]
- ESRI. ArcGIS 9.3; ESRI Inc.: Redlands, Canada, 2008. [Google Scholar]
- Global Biodiversity Outlook 3; Secretariat of the Convention on Biological Diversity: Montreal, Canada, 2010.
- Morin, X.; Thuiller, W. Comparing niche- and process-based models to reduce prediction uncertainty in species range shifts under climate change. Ecology 2009, 90, 1301–1313. [Google Scholar] [CrossRef]
- Matthews, S.N.; O'Connor, R.J.; Iverson, L.R.; Prasad, A.M. Atlas of Climate Change Effects in 150 Birds Species of the Eastern United States; US Department of Agriculture, Forest Service, Northeastern Research Station: Newton Square, PA, USA, 2004. [Google Scholar]
- Kerr, J.T.; Packer, L. The impact of climate change on mammal diversity in Canada. Environ. Monit. Assess. 1998, 49, 263–270. [Google Scholar] [CrossRef]
- Ogden, N.H.; Bigras-Poulin, M.; Hanincová, K.; Maarouf, A.; O’Callaghan, C.J.; Kurtenbach, K. Projected effects of climate change on tick phenology and fitness of pathogens transmitted by the North American tick Ixodes scapularis. J. Theor. Biol. 2008, 254, 621–632. [Google Scholar]
- Iverson, L.R.; Schwartz, M.W.; Prasad, A.M. Potential colonization of newly available tree-species habitat under climate change: an analysis for five eastern US species. Landscape Ecol. 2004, 19, 787–799. [Google Scholar] [CrossRef]
- Malmsheimer, R.W.; Hefferman, P.; Brink, S.; Crandall, D.; Deneke, F.; Galik, C.; Gee, E.; Helms, J.A.; McClure, N.; Mortimer, M.; Ruddell, S.; Smith, M.; Steward, J. Forest Management Solutions for Mitigating Climate Change in the United States. J. Forest. 2008, 106, 115–117. [Google Scholar]
- Marinissen, J.C.Y.; Bosch, F. Colonization of new habitats by earthworms. Oecologia 1992, 91, 371–376. [Google Scholar] [CrossRef]
- Jobin, B.; DesGranges, J.L.; Boutin, C. Population trends in selected species of farmland birds in relation to recent developments in agriculture in the St. Lawrence Valley. Agric. Ecosyst. Environ. 1996, 57, 103–116. [Google Scholar]
- Roy, V.; de Blois, S. Evaluating hedgerow corridors for the conservation of native forest herb diversity. Biol. Conserv. 2008, 141, 298–307. [Google Scholar]
- Krebs, C.J.; Berteaux, D. Problems and pitfalls in relating climate variability to population dynamics. Climate Res. 2006, 32, 143–149. [Google Scholar] [CrossRef]
- Brisson, J.; Paradis, E.; Bellavance, M.E. New evidence of common reed (Phragmites australis) sexual reproduction in Eastern Canada: a consequence of the recent global warming? Rhodora 2008, 110, 225–230. [Google Scholar] [CrossRef]
- Brassard, F.; Bouchard, A.R.; Boisjoly, D.; Poisson, F.; Bazoge, A.; Bouchard, M.A.; Lavoie, G.; Tardif, B.; Bergeron, M.; Perron, J.; Balej, R.; Blais, D. Portrait du Réseau d’Aires Protégées au Québec, Période 2002/2019; Gouvernement du Québec, ministère du Développement durable, de l’Environnement et des Parcs, Direction du patrimoine écologique et des parcs: Québec, Canada, 2010. [Google Scholar]
- Wiersma, Y.F.; Nudds, T.D. Conservation targets for viable species assemblages in Canada: Are percentage targets appropriate? Biodivers. Conserv. 2006, 15, 4555–4567. [Google Scholar] [CrossRef]
- Wiersma, Y.F.; Nudds, T.D. Efficiency and effectiveness in representative reserve design in Canada: The contribution of existing protected areas. Biol. Conserv. 2009, 142, 1639–1646. [Google Scholar] [CrossRef]
- MEA (Millenium Ecosystem Assessment). Ecosystems and Human Well-Being: Biodiversity Synthesis; World Resources Institute: Washington, DC, USA, 2005. [Google Scholar]
- Diamond, J.M. "Normal" extinctions of isolated populations. In Extinctions; Nitecki, M.H., Ed.; Chicago University Press: Chicago, MI, USA, 1984; pp. 191–246. [Google Scholar]
- Steffen, W.; Burbidge, A.A.; Hugues, L.; Kitching, R.; Lindenmayer, D.; Musgrave, W.; Stafford Smith, M.; Werner, P.A. Australia's Biodiversity and Climate Change; CSIRO Publishing: Collingwood, Australia, 2009. [Google Scholar]
- Cramer, W. Advanced terrestrial ecosystem analysis and modelling. Reg. Environ. Change 2008, 8, 89–90. [Google Scholar] [CrossRef]
- Settele, J.; Hammen, V.C.; Hulme, P.E.; Karlson, U.; Klotz, S.; Kotarac, M.; Kunin, W.E.; Marion, G.; O'Connor, M.; Petanidou, T.; Peterson, K.; Potts, S.G.; Pritchard, H.; Pysek, P.; Rounsevell, M.; Spangenberg, J.; Steffan-Dewenter, I.; Sykes, M.T.; Vighi, M.; Zobel, M.; Kuhn, I. ALARM: Assessing Large scale environmental Risks for biodiversity with tested Methods. Gaia 2005, 14, 69–72. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Berteaux, D.; Blois, S.d.; Angers, J.-F.; Bonin, J.; Casajus, N.; Darveau, M.; Fournier, F.; Humphries, M.M.; McGill, B.; Larivée, J.; et al. The CC-Bio Project: Studying the Effects of Climate Change on Quebec Biodiversity. Diversity 2010, 2, 1181-1204. https://doi.org/10.3390/d2111181
Berteaux D, Blois Sd, Angers J-F, Bonin J, Casajus N, Darveau M, Fournier F, Humphries MM, McGill B, Larivée J, et al. The CC-Bio Project: Studying the Effects of Climate Change on Quebec Biodiversity. Diversity. 2010; 2(11):1181-1204. https://doi.org/10.3390/d2111181
Chicago/Turabian StyleBerteaux, Dominique, Sylvie de Blois, Jean-François Angers, Joël Bonin, Nicolas Casajus, Marcel Darveau, François Fournier, Murray M. Humphries, Brian McGill, Jacques Larivée, and et al. 2010. "The CC-Bio Project: Studying the Effects of Climate Change on Quebec Biodiversity" Diversity 2, no. 11: 1181-1204. https://doi.org/10.3390/d2111181
APA StyleBerteaux, D., Blois, S. d., Angers, J.-F., Bonin, J., Casajus, N., Darveau, M., Fournier, F., Humphries, M. M., McGill, B., Larivée, J., Logan, T., Nantel, P., Périé, C., Poisson, F., Rodrigue, D., Rouleau, S., Siron, R., Thuiller, W., & Vescovi, L. (2010). The CC-Bio Project: Studying the Effects of Climate Change on Quebec Biodiversity. Diversity, 2(11), 1181-1204. https://doi.org/10.3390/d2111181




