Mineral Licks as Diversity Hotspots in Lowland Forest of Eastern Ecuador
Abstract
: Mineral licks are sites where a diverse array of mammals and birds consume soil (geophagy) or drink water, likely for mineral supplementation. The diversity of species that visit such sites makes them important for conservation, particularly given that hunters often target animals at licks. Use of mineral licks varies among species, with frugivores among the most common visitors but there is considerable temporal and spatial variation in lick use both within and among species. Camera traps triggered by heat and motion were used to document use of mineral licks by birds and non-volant mammals over a four-year period at a lowland forest site in eastern Ecuador. We obtained 7,889 photographs representing 23 mammal species and 888 photographs representing 15 bird species. Activity (photographs/100 trap-days) at the four licks varied from 89 to 292 for mammals and from six to 43 for birds. Tapirs (Tapirus terrestris), peccaries (Pecari tajacu, Tayassu pecari), deer (Mazama americana), and pacas (Cuniculus paca) were the most frequent mammal visitors; guans (Pipile pipile) and pigeons (Columba plumbea) were the most common birds. Use of licks varied diurnally and seasonally but patterns of use varied among species and sites. Mineral licks provide an important resource for many species but further studies are needed to determine the precise benefit(s) obtained and how benefits may vary with diet and other factors, such as rainfall.1. Introduction
Many mammals and birds, particularly frugivores and other herbivores, visit mineral licks to consume soil (geophagy) or drink water [1-6]. Among the suggested benefits are mineral supplementation, detoxification of plant secondary compounds, and alleviation of digestive disorders [7-13]. Mineral licks often occur along river banks [14,15] but also are found within forests, well away from rivers [6,16-19]. In the Neotropics, such sites are visited by a variety of birds (e.g., guans, pigeons, parrots) and mammals (e.g., bats, primates, rodents, ungulates) [1,5,14,17-22].
Geophagy likely has multiple causes that may vary geographically, seasonally, and taxonomically [11,23] leading to temporal and spatial variation in use of mineral licks [1,17-19]. Diurnal variation in lick use reflects differences among species in general activity patterns (i.e., diurnal versus nocturnal species) and also may be a response to weather, threat of predation, and variation in foraging and ranging behavior [14,16-18,21]. Given that soil or water from mineral licks may provide a dietary supplement of some type [3,9,13,22,24], changes in diet may alter the need for geophagy, leading to seasonal shifts in use of mineral licks (e.g., increased use during the dry season; ([13,25], but see [23]). Stage of reproductive activity also may influence use of mineral licks [1,7,8]. Sodium, for example, may be more available in mineral licks than in other areas and may be particularly sought after during lactation [7-9].
Species composition of visitors and frequency of visits may differ from one lick to the next [18,19]. Such variation may reflect differences in mineral composition of the soil or water among different sites [26] or differences among species in habitat preferences and availability of licks in different habitats [18]. Distances individuals have to travel to access different licks, foraging or ranging behavior of troops or herds, or topographic features that make some licks more accessible or, perhaps, safer from predators [18,27] also might affect the types of species and numbers of visits to different licks.
In lowland forests of eastern Ecuador, mineral licks (also known as saladeros) are visited by a wide range of species but use of specific licks can vary both among species and over time [19,20]. Here, we examine use of four mineral licks by non-volant mammals and birds to gain a better idea of the importance of such sites. (Although licks at the study site [22,24] and elsewhere in the Neotropics [9,10] are known to be visited by large numbers of bats, we do not include bats in this study, for two reasons. First, camera traps used in this study were not suitable for recording bat activity (i.e., digital cameras did not have a sufficiently rapid response time) so that numbers of bats captured in photographs would not necessarily be a good representation of bat activity. Second, although some individual bats could be identified to species, this was not true in most cases.) We were particularly interested in how use of licks varied throughout the year (i.e., in relation to changes in rainfall) and whether that variation differed among species. Further, we ask whether different licks are visited by the same suite of species or whether there is significant variation in use of sites among species.
Mineral licks can be an important resource for local people, as they may be used as hunting sites. Thus, a better appreciation of the use of such sites by mammals and birds, and how that use varies both temporally and spatially, may help in developing arguments for restricting hunting in some regions. This may be particularly important in areas, such as much of lowland eastern Ecuador, where oil exploration or development activities may lead to greater accessibility of areas to hunters.
2. Methods
2.1. Study Area
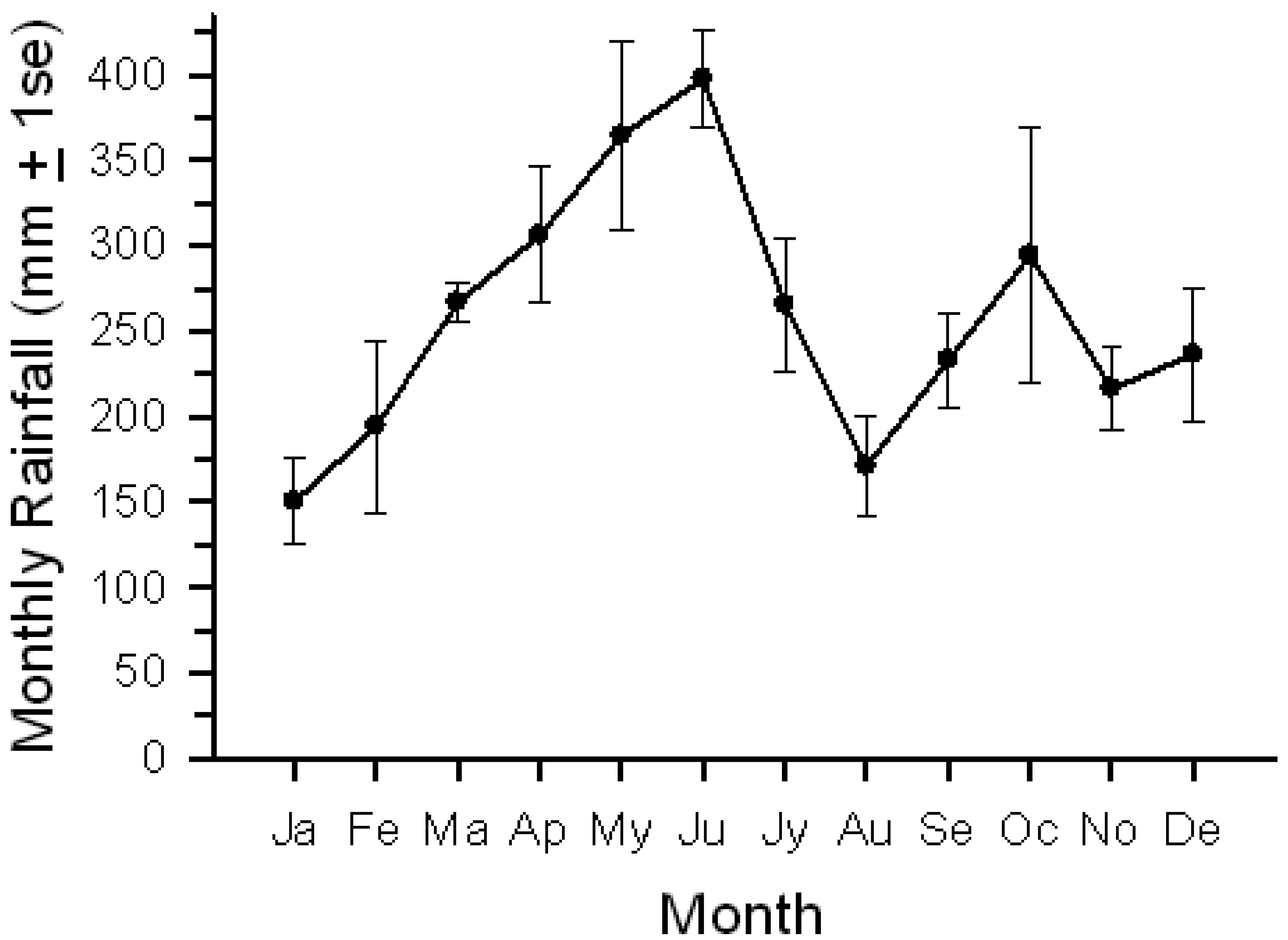
We conducted our research at Tiputini Biodiversity Station (TBS), Orellana Province, Ecuador (∼0°37′ S, 76°10′ W, 190–270 m asl). TBS was founded in 1994 by the Universidad San Francisco de Quito (USFQ) on a tract of undisturbed lowland rainforest within the ∼1.7-million ha Yasuní Biosphere Reserve, one of the most biologically diverse regions of the world [28]. The reserve also is home to indigenous groups, such as the Waorani and Kichwa, who depend on these forests for much or all of their subsistence. The station and nearby areas contain a variety of habitats including terra firme and várzea forest, palm swamps and other wetlands, as well as areas of succession that follow treefalls, windthrows, or other natural disturbances. The mean annual precipitation at Yasuní Research Station, approximately 30 km WSW of TBS, is about 3,100 mm [29]. Rainiest months are from April through June; January and August are relatively dry, with January often very dry (personal observations) (Figure 1).
We documented use of four licks [Chorongo, Harpia, Harpia Parcela (HP), Puma] located in terra firme forest. Licks were located ∼0.5 km (Chorongo) to ∼1.5 km (Harpia, Puma) north of the Tiputini River. Farthest distance between licks (Puma, HP) was ∼2.5 km. Soil was continually moist at Puma and Chorongo but flowing water was not always present. Water was typically present at Harpia and HP; after heavy rains, water covered most of the ground surface, often with a noticeable flow. Chorongo (0°38.072′ S; 76°8.880′ W) was located at the start of a small drainage, in an area with relatively steep slopes on three sides (approximately 6–8 m high); the lick was approximately 10–12 m across. HP (0°37.599′ S; 76°8.333′ W) was in a small drainage with gentle slopes, approximately 10–12 m across at the camera-trap location. Puma (0°37.435′ S; 76°9.626′ W) also was located at the start of a drainage, but slopes were much lower (2–3 m) than at Chorongo; the lick site was approximately 7–9 m across at the widest point. Harpia (0°37.482′ S; 76°8.890′ W) was the largest site, with gentle slopes leading into a wide drainage approximately 12–15 m across.
2.2. Camera Trapping
We used both digital (Snapshot Sniper, LLC) and film-based (Highlander Photoscout™, PTC Technologies) camera traps triggered by an infrared motion-and-heat detector to document activity at licks. We placed cameras at edges of each site, ∼0.75–1 m above ground surface. Cameras remained continuously activated (except when batteries failed or other malfunctions occurred); date and time were automatically stamped on each photograph. We set cameras with a minimum time between photographs of 5 min. We checked cameras at approximately 7 to 10-day intervals to replace film, batteries, memory sticks, and desiccant. Rolls of film (36 exposure, 400 ASA, Kodak Gold) often were used up within one week. All images were labeled with location, camera, date, time, and species.
2.3. Analyses
We summarized images by species, hour, and date. We classified photographs as belonging to independent records if more than 30 min had elapsed between consecutive photographs of the same species at a given location. Other studies have used 5 min as an interval [30], 30 min [6,31,32], or 1 h [18,33]. We used 30 min as a cut-off because visits by herds of Tayassu pecari (white-lipped peccary) typically were the longest of any species at a given site, but most visits were less than 30 min. When herds did remain in front of cameras for over 30 min, all photographs of the visit were counted as one. Activity at lick sites was evaluated in terms of percentage of photographs or photographs per 100 trap-days. We calculated number of trap-days from the time the camera was placed in operation to the time the film was replaced (i.e., starting a new sequence) or last photograph was taken (based on the date and time stamp on the photographs). We classified records by hour, starting at midnight, to examine diurnal patterns of activity and by month to examine seasonal variation.
Patterns of activity were sufficiently well marked in most cases that statistical tests were not needed or applied, following recommendations about overuse of statistical tests [34,35]. When appropriate, we compared observed numbers of records to expected numbers using chi-squared tests [36]. To compare use by hour, we based expected values on an even distribution during 24 hours. To compare use across months, we calculated expected numbers based on number of trap-days cameras were operational during each month (summed across years) so expected values for visits/month were standardized by sample effort. We compared use between species and sites with contingency table tests (i.e., comparing number of photos by hour, month, or site) and correlation (Pearson's) analyses. For the latter, we correlated numbers of visits between species by month, with months summed across years (i.e., N = 12). We used correlation analyses to examine the relationship between monthly use of licks and average monthly rainfall. Data were checked for assumptions of normality (Shapiro–Wilk test) and homogeneity of variances (Levene's test) before parametric tests were applied. All analyses were conducted using STATISTIX Ver. 8.0 [37].
3. Results
3.1. Overview
After eliminating photographs with no identifiable animal or only with people, we had 16,152 photographs; 751 photographs did not have the full date and time information and were not used except to help generate complete species lists. After eliminating photographs not separated by at least 30 min per species at a given site, we were left with 888 photographs of 15 bird species (10 families; Table 1) and 7,889 photographs of 23 non-volant mammal species (15 families; Table 2). In addition, there were at least five species of bats (Sturnira magna, Sturnira lilium, Artibeus lituratus, Platyrrhinus sp., Vampyressa sp.) that are not included in totals for species or numbers of photographs.
In contrast to the results from lick sites, we recorded approximately 3,200 photographs of 29 mammal species in camera traps set at 10–12 locations along trails in the same area (unpubl. data). Particularly noteworthy species that were recorded along trails but that were rarely if ever photographed at mineral licks included Myrmecophaga tridactyla (giant anteater), Priodontes maximus (giant armadillo), Atelocynus microtis (short-eared dog), Puma concolor (puma), Panthera onca (jaguar), and Mazama gouazoubira (grey brocket deer). Among birds, Psophia crepitans (grey-winged trumpeter) accounted for the great majority of approximately 1,200 photographs of birds along trails but were recorded only six times at saladeros. Overall, total activity levels were considerably higher at licks than along trails.
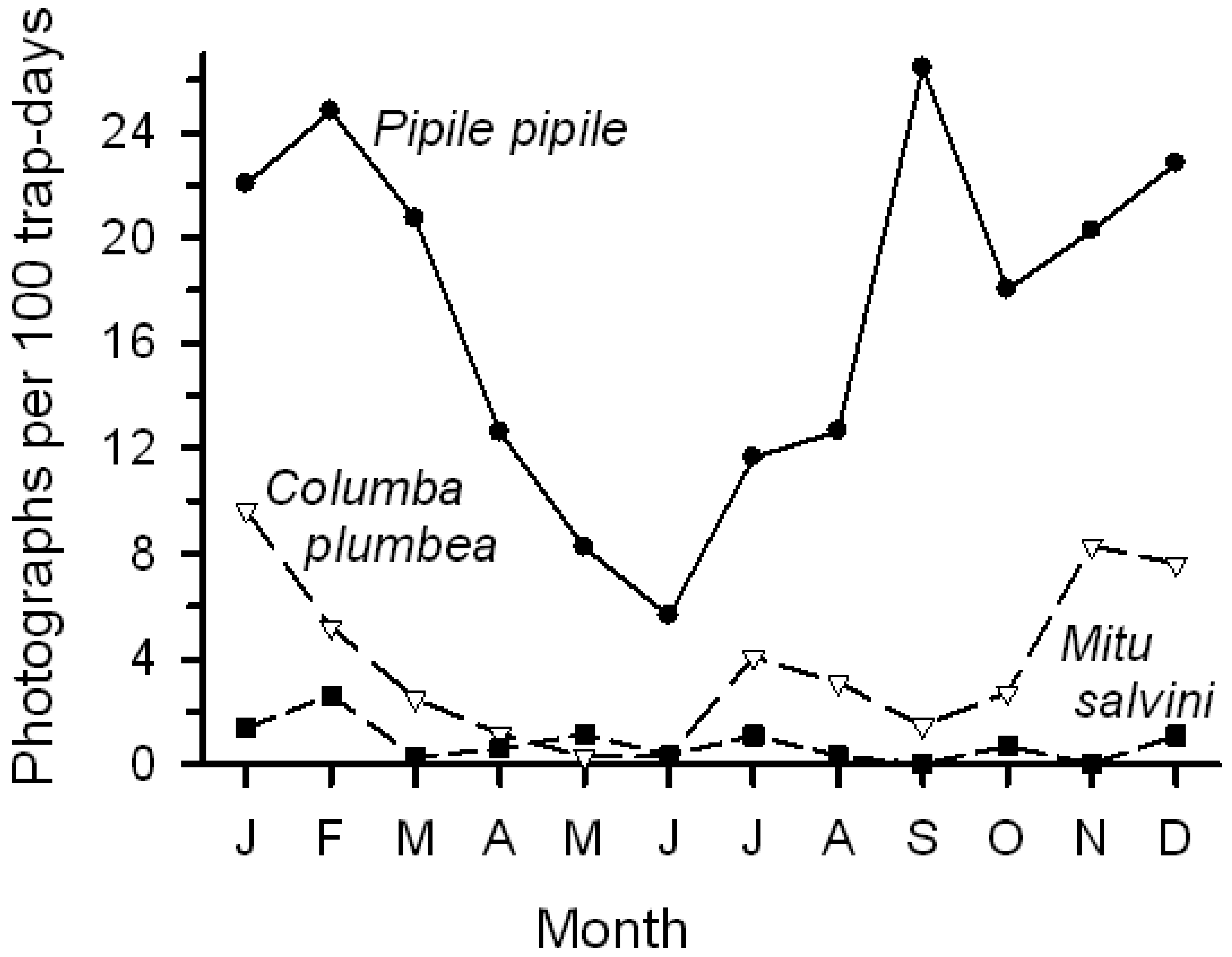
Activity (number of photographs/100 trap-days) varied among species, among sites, and within species among sites. Bird visits were most frequent at Chorongo, much lower at HP, and intermediate at the other sites (Table 1). Number of photographs likely underestimates visits by birds that either moved too fast to be recorded or that were too small to be detected. Total visits largely reflected visits by Pipile pipile (common names of birds in Table 1) and Columba plumbea. The piping-guan was a frequent visitor to all four sites, although it was most common at Chorongo (88% of photos; Table 1). In contrast, the pigeon was recorded at only three sites (not at HP) and was the most common visitor to Harpia (69% of photos). Of the remaining 13 species, only Mitu salvini was recorded with any regularity.
In contrast to birds, visitation rate of mammals was, overall, highest at Harpia and lowest at Chorongo but activity varied markedly among species and sites (Table 2). Four ungulates (Tapirus terrestris, Mazama americana, Tayassu pecari, Pecari tajacu; common names in Table 2) accounted for ∼70 to 94 percent of all visits. M. americana or T. terrestris was the most frequent visitor at all sites. T. pecari activity varied the most among sites, with Chorongo and HP visited infrequently (Table 2). Cuniculus paca and Dasyprocta fuliginosa also were common visitors, particularly to Chorongo where they accounted for ∼17 and 10 percent, respectively, of total visits. Thus, although all sites were visited by many of the same species, both birds and mammals, substantial differences existed among sites in number and frequency of visits by a given species.
3.2. Diurnal Activity Patterns
Total bird activity peaked during the middle of the day (1100–1400 h) and was low before 0800 h and after 1800 h. Activity largely reflected visits by P. pipile and C. plumbea, both of whom showed strong peaks of activity around the middle of the day, although visits by the guan peaked somewhat earlier and stayed at higher levels somewhat longer compared to the pigeon (Figure 2); differences between species were not, however, significant (χ2 = 11.4, df = 10, P < 0.32).
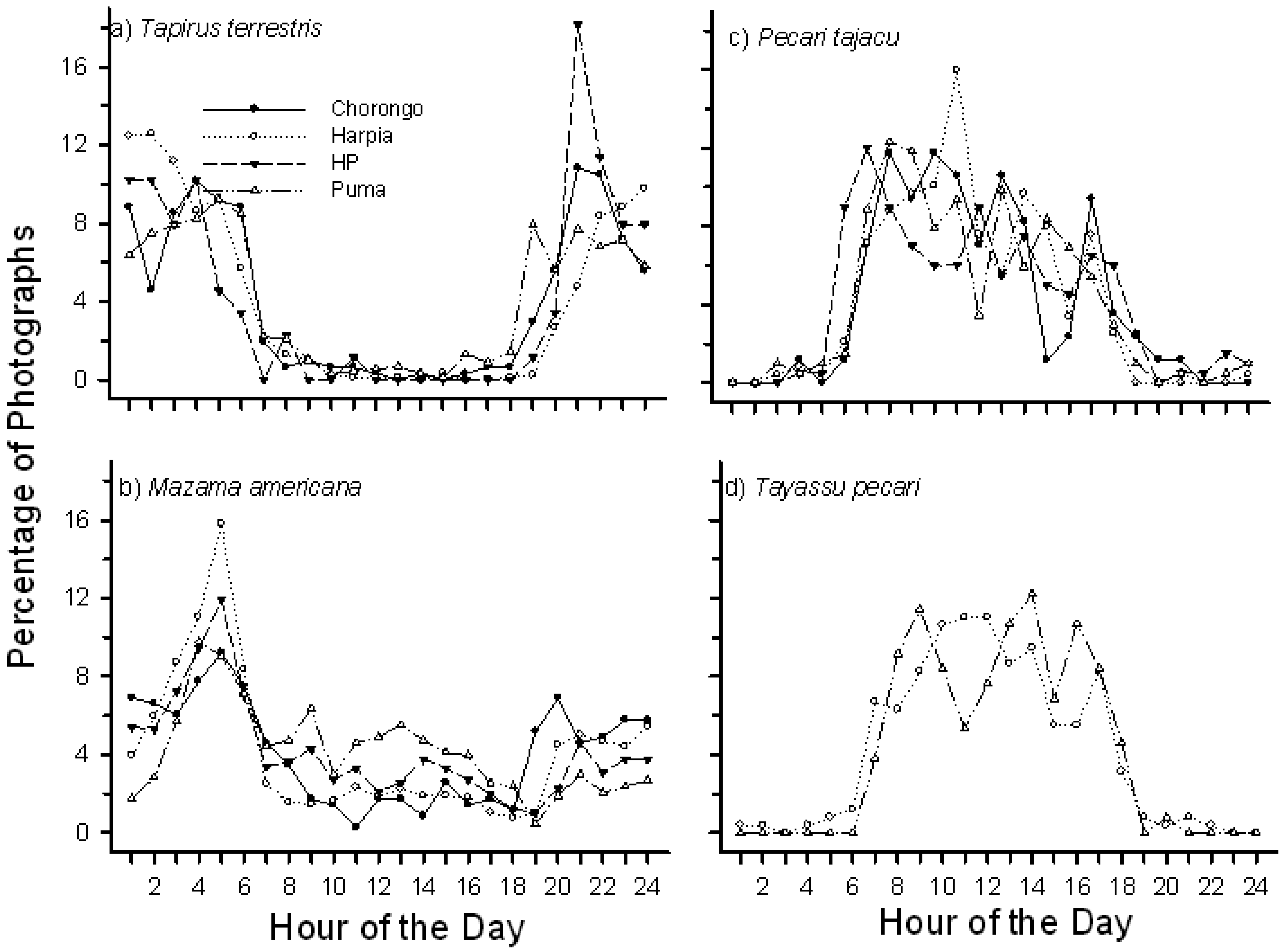
Mammals were active at mineral licks throughout the day and night but activity was greatest between 2100 h and 0600 h, with a strong peak in number of photographs at 0500 h. Most species displayed well-defined activity patterns. T. terrestris, for example, were primarily active at night, with number of photographs increasing rapidly after 1800 h and declining rapidly after 0600 h (Figure 3A). Peak activity levels varied somewhat among sites with, for example, a strong peak in numbers of photographs around 2100 h at HP but a longer, less pronounced peak activity period from 2400 h to about 0300 h at Harpia. M. americana, in contrast, showed a pronounced peak of activity around 0500 h at all sites (Figure 3B), decreasing but consistent activity throughout the day, and an increased activity again after 1800 h. In contrast to tapir and deer, the two peccaries rarely visited licks during the night (Figure 3C, 3D). P. tajacu, the smaller species, had more visits during the night and showed pronounced activity from about 0600 h to about 0800 h at three of the four sites; activity remained high but gradually decreased during the day. T. pecari visits were more pronounced later in the morning. The latter species made few visits to the two smallest sites, HP and Chorongo (Table 2). The two large rodent species, Cuniculus paca and Dasyprocta fuliginosa, had largely non-overlapping activity patterns; activity of the former species was highest from about 2100 h to 0200 h whereas Dasyprocta showed a gradually increasing activity during the day with a peak around 1800 h (Figure 4). Overall, diurnal activity patterns were well defined among species and generally comparable, with some variation, among sites within a species.
3.2. Seasonal Activity Patterns
Birds, as a group, showed a more pronounced seasonal pattern of lick use than did mammals. Bird activity (photographs/100 trap-days) steadily declined from January into June before increasing again; mammal activity varied among months but generally remained relatively high. The change in bird activity was closely but inversely related to changes in rainfall (Figure 1; r = −0.79, P < 0.005); total mammal activity showed no relationship with rainfall. Number of visits by the two common bird visitors declined from early in the year to June, followed by an increase (Figure 5); activity levels were negatively correlated with rainfall (Pipile, r = −0.70, P < 0.02; Columba, r = −0.73, P < 0.01).
Seasonal use of licks by mammals varied strongly both among species and among sites (Figure 6). T. terrestris activity was greatest and comparable at two saladeros (Puma, Harpia), although patterns of use diverged during the latter months of the year. In contrast, activity at the smaller sites (Chorongo, HP) was low but comparable throughout the year (Figure 6A). M. americana showed a more complex pattern. Visitation rates were generally lower but consistent at Chorongo throughout the year, in contrast to HP, where activity was high during most of the year, with a peak in July. Visits to Harpia and Puma generally increased from January through March and then declined during the middle months of the year (Figure 6B). P. tajacu activity varied substantially among sites (Figure 6C). Visits to Harpia and HP fluctuated but showed a generally increasing trend throughout the year. In contrast, there was a sharp increase in visits to Puma from January to April, followed by a sharp decline in visits for the rest of the year; visits to Chorongo were fewer but also showed a peak in the earlier part of the year. T. pecari activity was greatest during the first and last few months of the year, but showed large variation in visit rates. Visits to Chorongo and HP remained low throughout the year (Figure 6D). Both large rodents showed a decline in activity from early in the year (not shown) although Cuniculus paca activity remained higher than Dasyprocta fuliginosa throughout most of the year.
4. Discussion
Mineral licks in lowland forest of eastern Ecuador are an important resource for many species of mammals and birds, as shown by the number of photographs obtained at four different lick sites within Tiputini Biodiversity Station. Species recorded at these licks were primarily frugivorous (e.g., primates, tapir, peccaries, guans, pigeons) or herbivorous (e.g., deer), a pattern seen in most previous studies in the Neotropics and elsewhere [1,2,4,6,12,18,22,40].
Many animals visited all four mineral licks both at night and during the day but there were clear differences among species in diurnal patterns of visits. Seasonal variation in use of licks also was noted although patterns of use were somewhat less consistent compared to diurnal patterns. Although all four sites were in relatively close proximity (<3 km apart at farthest distance) in terra firme forest, there were clear differences among the sites both in species composition of visitors and in patterns of activity of individual species.
Diurnal patterns of activity seen in this study largely match previous accounts [18]: tapirs were primarily nocturnal, peccaries primarily diurnal, deer mostly nocturnal but with regular daytime activity as well. Contrasting patterns of activity of some species, such as Cuniculus paca and Dasyprocta fuliginosa, might reflect division of resources, as suggested by Tobler et al. [18] but a clear understanding of such patterns would require more detailed knowledge of their diets and local patterns of habitat use. Diurnal patterns of activity may also reflect use of different habitats. T. terrestris at this site was active at mineral licks throughout the night, with less activity during the day. Similarly, T. terrestris was active throughout the night at a site in Peru [18]. In contrast, Tapirus pinchaque (mountain tapir) exhibited a bimodal activity pattern along trails in forest, with peaks from 0500–0700 h and from 1800–2000 h, but activity at a salt lick peaked from 0100–0300 h and from 1200–1500 h [17].
Patterns of movement or ranging behavior also might affect timing of lick use. Ateles belzebuth, for example, uses specific travel routes during daily activity; routes may remain stable across years [41]. Thus, timing of use of different mineral licks might be influenced by locations of such licks along different travel routes. Use of such routes also might help explain why some licks are used more than others. Changes in weather also may influence use of licks; as noted by Brightsmith [14], parrots were more active at clay licks on sunny days than on rainy ones.
Changes in use of mineral licks among months might be in response to seasonal movements or shifts in habitat use, changes in levels of reproductive activity (particularly by females), or changes in diets. Changes in movement may alter spatial availability of some sites and changes in reproduction or diet may alter the need for mineral supplementation, one of the suggested reasons for use of mineral licks. The two species of peccaries that occur at Tiputini, T. pecari and P. tajacu, showed different seasonal patterns in use of mineral licks. P. tajacu, the smaller species, occurs in smaller herds and moves over relatively small areas; use of licks remained relatively high throughout the year. In contrast, the larger species, T. pecari, occurs in large herds (often >100 individuals, personal observations) that are known to move over large distances on a seasonal basis, perhaps in relation to changes in seasonal and spatial availability of fruit [42]. Movement of herds away from Tiputini during the wetter periods may account for the greatly reduced use of mineral licks during some parts of the year.
Reproductive activity may increase the need for mineral supplementation, particularly by females [7,8], and could lead to changes in seasonal use of mineral licks. Use of mineral licks by bats in Ecuador and Peru was strongly biased towards females, with reproductive individuals predominating among captures [1,22]. In addition to sodium, licks also may have provided a source of calcium for pregnant or lactating females. Similarly, female elephants in Zimbabwe spent more time at mineral licks and consumed more soil than did males, perhaps to supplement their sodium intake [25].
Seasonal variation in use of mineral licks may reflect changes in diet that accompany changes in rainfall [13]. For example, a switch in diet to include a greater proportion of leaves, which occurred during the wet to dry season transition, led to an increase in geophagy in Alouatta belzebul (red-handed howler monkey) [43]. Alouatta seniculus and Ateles belzebuth both showed an increased use of mineral licks at Tiputini during the drier months [19]. Similarly, use of mineral licks by birds, primarily Pipile pipile, showed a strong negative correlation with rainfall. In contrast, seasonal variation in activity levels of T. pinchaque [17] showed no correlation with rainfall, as was true for use of licks by most mammals at Tiputini. Clearly, a variety of factors influences seasonal changes in use of such sites.
Changes in activity were noticeable among mineral licks for a variety of different species but we do not know how individual behavioral changes might have influenced overall patterns of use. Tobler et al. [18] pointed out that tapirs, for example, may travel long distances (>10 km) from terra firme forest to reach mineral licks in várzea. Licks within TBS are all within range of individual movements of many species, so it would be instructive to know whether different individuals, herds, or troops change patterns of use among sites or use different licks at different seasons or times of the day. Changes in behavior might, for example, account for some of the variation in use of mineral licks shown by primates at Tiputini. Alouatta seniculus and Ateles belzebuth were recorded many times at two of the four sites studied here (Puma, Harpia) but use of Puma by the former species ended after 2005 [19], suggesting a change in spatial patterns of the group(s) using that site. A. belzebuth was recorded only twice at a third site (Chorongo) suggesting that either the site was not suitable or that it was not along regular foraging routes of the species. The general absence from two of the four sites suggests that factors other than diet influence patterns of use.
Use of mineral licks has often been associated with diets that are low in some essential nutrients, such as sodium [25,26,44], one of the most limiting nutrients to vertebrates in many areas of the Neotropics [15]. Fruits may be low in sodium, which may help explain why frugivores are among the most frequent visitors to mineral licks [1,9]. Yet, not all frugivores are equally likely to visit licks. In this study, Cuniculus paca was a frequent nocturnal visitor and Dasyprocta fuliginosa a common diurnal visitor; both species feed on fallen fruits and seeds [42] fitting the general pattern of use by frugivores. Yet, Myoprocta pratti, also frugivorous, was an infrequent visitor to licks but was commonly photographed along trails (unpubl. data), suggesting that a frugivorous diet does not necessarily lead to use of licks for mineral supplementation.
Among birds, Pipile pipile, Columba plumbea, and Mitu salvini are all primarily frugivorous, which may partially explain their use of mineral licks. The less frequent use by M. salvini may reflect the fact that its diet, although primarily fruits, also contains many other items, including insects and soil away from licks [45,46]. On the other hand, Penelope jacquacu, although common in the study area and photographed regularly along trails, was rarely photographed at licks, despite a largely frugivorous diet. Similarly Nothocrax urumutum (nocturnal curassow) was not photographed at licks and other pigeons and doves were much less common than C. plumbea. Clay licks along rivers that are used by parrots, macaws, parakeets, and other species may be rich in sodium [14] accounting for their use. Such sites are present along the Tiputini River as well, and attract large numbers of psittacids (pers. obs.). In this study, although mineral licks well away from the river were visited by guans, pigeons, and other species, there was only one photograph of a psittacid (Ara macao). Thus, use of different lick sites likely reflects factors other than mineral content.
If soils at mineral licks are richer in necessary compounds than are soils at other locations [9,15,21,47] this could explain why such sites see concentrations of activity. Evidence is mixed about whether lick soils at Tiputini are different in terms of mineral content than are soils in other parts of the forest. Studies on use of mineral licks by bats at Tiputini [22,24] suggest that lick soils are more mineral-rich than soils at control locations in the forest (C. Voigt, pers. comm.). Yet, a separate study, also at Tiputini, found no differences in sodium content in lick soils when compared to random locations in the forest (G. Jaramillo, unpubl. undergraduate thesis, Universidad San Francisco de Quito). In some cases, differences in mineral content among mineral licks might explain why some sites are used more than others [26]. Use of the four licks in this study varied both within and among species, which suggests that use of different licks might be associated with different costs or benefits, or that species differ in their needs for dietary supplementation. A lack of significant differences in mineral content among ten licks examined at Tiputini (J. Fabara, unpubl. undergraduate thesis, Universidad San Francisco de Quito) suggests, however, that use of particular sites may be related to factors other than mineral content.
Accessibility of mineral lick sites may influence composition of species using different sites, as well as temporal variation in that use. Tobler et al. [18] studied use of mineral licks located in várzea forest by five species of ungulates and suggested that use of such sites (or lack thereof) might depend on preferred habitats of the different species. Species typically found in terra firme forest might be more restricted in their access to licks in várzea. In their study, Mazama gouazoubira was never recorded at licks and they suggested this was because of its preference for terra firme. In our study, we also did not record M. gouazoubira at any of the four sites, even though the species was recorded in cameras located along trails in terra firme forest, relatively close to the licks studied here (unpubl. data). M. gouazoubira is rarely encountered at Tiputini when compared to M. americana and, thus, as for any rare species, we caution against drawing firm conclusions regarding spatial or temporal patterns of abundance for such species.
As Tobler et al. [18] pointed out, however, spatial proximity is not the only factor determining use, as P. tajacu was an infrequent visitor to sites in várzea even though it was common there. At Tiputini, P. tajacu is common in terra firme and was a frequent visitor to all four sites. T. pecari, often associated with várzea forest [42] was commonly observed and photographed in terra firme at TBS and was a frequent visitor to two of the four licks. The sites that were visited on a regular basis were the two largest, which may help explain the preference of those sites by T. pecari, whose herds are considerably larger than those of P. tajacu ([42]; personal observations). T. pecari herds range widely over the region and their general absence from licks during the middle of the year may reflect changes in distribution of herds over a wider area.
Mineral licks may have ecological importance beyond mineral supplementation. In Congo, for example, elephants create well-defined trails leading to mineral licks [48]; such trails can be used as travel routes by other species, including predators. Similarly, mineral licks in lowland forest in Ecuador often have many trails leading to them. Although licks may be visited by many different species, there were few records of Leopardus pardalis or Puma concolor in this study, and none of Panthera onca; all three predators are common in the study area and feed on many of the species that use the licks.
4. Conclusions
Mineral licks in Ecuador and elsewhere are important components in the ecology of many different species. As a consequence, relatively small areas of the forest may play a crucial role in maintaining populations of species and, hence, the overall diversity of a region. Given that licks may differ in physical and chemical characteristics, the composition of species visiting a particular lick may vary over time and from one lick to another. Thus, it is important to ensure that protected areas maintain a diverse array of such sites. Mineral licks also can be important in the lives of indigenous (and other) peoples. The concentration of animal activity at licks, including many species that are important food items for many people (e.g., ungulates, primates, large birds), can make licks focal points for hunters ([49], as cited in [18]; personal observations) with potentially negative consequences for local populations of favored prey. Clearly, preservation of sites such as Tiputini Biodiversity Station, which are not affected by hunters, can provide important ecological and conservation benefits to a wide range of species.
This study also illustrates the value of camera traps for research, education, and conservation. By operating cameras throughout the year, it is possible to gain a much better appreciation of the spatial and temporal variation in use of such sites, information potentially valuable for management. Similarly, inclusion of local people in such projects may increase the likelihood of positive outcomes, as been true in this study. Photographs derived from this and related projects can and have been disseminated to local schools in the forms of talks and posters; many presentations have been given to governmental and non-governmental organizations; and photographs can be made available on web sites. All such educational activities have the potential to further efforts at conservation. After all, “a picture is worth a thousand words.”



| Family | Common Name, Scientific Name | Chorongo | Harpia | HP | Puma |
|---|---|---|---|---|---|
| 1,214 | 1,006 | 818 | 931 | ||
| Tinamidae | great tinamou, Tinamus major | 0.1 (1) | |||
| Ardeidae | rufescent tiger-heron, Tigrisoma lineatum | 0.1 (1) | |||
| Ardeidae | cocoi heron, Ardea cocoi | 0.1 (1) | |||
| Accipitridae | black-faced hawk, Leucopternis melanops | 0.1 (1) | |||
| Cracidae | Spix's guan, Penelope jacquacu | 0.2 (3) | 0.2 (2) | ||
| Cracidae | common piping-guan, Pipile pipile | 37.7 (458) | 2.6 (26) | 4.4 (36) | 16.6 (155) |
| Cracidae | Salvin's curassow, Mitu salvini | 1.0 (12) | 1.0 (10) | 0.9 (7) | 0.4 (4) |
| Eurypygidae | sunbittern, Eurypyga helias | 0.1 (1) | |||
| Psophiidae | gray-winged trumpeter, Psophia crepitans | 0.2 (2) | 0.2 (2) | 0.1 (1) | |
| Columbidae | plumbeous pigeon, Columba plumbea | 3.5 (43) | 8.0 (80) | 3.2 (30) | |
| Columbidae | gray-fronted dove, Leptotila rufaxilla | 0.7 (6) | |||
| Columbidae | ruddy quail-dove, Geotrygon montana | 0.3 (3) | |||
| Psittacidae | scarlet macaw, Ara macao | 0.1 (1) | |||
| Cuculidae | rufous-vented ground-cuckoo, Neomorphus geoffroyi | 0.1 (1) | |||
| Parulidae | buff-rumped warbler, Basileuterus fulvicauda | 0.1 (1) | |||
| Total | 42.8 (520) | 12.2 (123) | 6.4 (52) | 20.7 (193) |
| Family | Common Name, Scientific Name | Chorongo | Harpia | HP | Puma |
|---|---|---|---|---|---|
| 1,214 | 1006 | 818 | 931 | ||
| Didelphidae | common opossum, Didelphis marsupialis | 0.7 (8) | |||
| Myrmecophagidae | southern tamandua, Tamandua tetradactyla | 0.1 (1) | 0.1 (1) | ||
| Megalonychidae | Hoffmann's two-toed sloth, Choloepus hoffmanni | 0.1 (1) | |||
| Dasypodidae | giant armadillo, Priodontes maximus | 0.2 (2) | |||
| Dasypodidae | nine-banded armadillo, Dasypus novemcinctus | 1.1 (13) | 0.2 (2) | 1.5 (14) | |
| Cebidae | Venezuelan red howler monkey, Alouatta seniculus | 3.0 (30) | 0.9 (8) | ||
| Cebidae | white-fronted spider monkey, Ateles belzebuth | 0.1 (1) | 9.9 (100) | 0.4 (4) | |
| Procyonidae | crab-eating racoon, Procyon cancrivorus | 0.2 (2) | 0.2 (2) | 0.3 (3) | |
| Procyonidae | South American coati, Nasua nasua | 0.1 (1) | 0.4 (4) | ||
| Felidae | ocelot, Leopardus pardalis | 0.1 (1) | 0.2 (2) | 1.0 (8) | 0.1 (1) |
| Felidae | margay, Leopardus wiedii | 0.1 (1) | |||
| Felidae | puma, Puma concolor | 0.1 (1) | |||
| Tapiridae | South American tapir, Tapirus terrestris | 25.1 (305) | 85.4 (860) | 10.8 (88) | 97.7 (910) |
| Tayassuidae | collared peccary, Pecari tajacu | 7.0 (85) | 23.7 (238) | 24.4 (200) | 21.8 (203) |
| Tayassuidae | white-lipped peccary, Tayassu pecari | 0.5 (6) | 25.3 (254) | 3.1 (25) | 14.5 (135) |
| Cervidae | South Am. red brocket deer, Mazama americana | 28.6 (347) | 115.0 (1,157) | 161.9 (1,324) | 67.9 (632) |
| Sciuridae | northern Amazon red squirrel, Sciurus igniventris | 0.1 (1) | 0.3 (3) | ||
| Erethizontidae | Brazilian porcupine, Coendou prehensilis | 0.1 (1) | |||
| Cuniculidae | lowland paca, Cuniculus paca | 15.1 (183) | 24.0 (241) | 8.2 (67) | 6.0 (56) |
| Dasyproctidae | black agouti, Dasyprocta fuliginosa | 8.6 (105) | 4.0 (40) | 0.5 (4) | 5.4 (50) |
| Dasyproctidae | green acouchi, Myoprocta pratti | 1.3 (16) | 0.2 (2) | 0.2 (2) | |
| Echimyidae | spiny rats, Proechimys spp. | 0.5 (6) | 0.7 (7) | 1.1 (9) | 1.7 (16) |
| Echimyidae | dark spiny tree-rat, Echimys saturnus | 1.1 (9) | |||
| Total | 88.8 (1,078) | 291.6 (2,935) | 212.7 (1,740) | 219.7 (2,045) |
Acknowledgments
We thank the many staff and volunteers who helped check the cameras, particularly Rene Torres, Franklin Narvaez, Ramiro San Miguel, and Jose Macanilla. We also appreciate the help of Consuelo de Romo in facilitating our work at Tiputini and the many staff that helped to make working there such a pleasure. Support for this study was provided by National Geographic Society, Universidad San Francisco de Quito, Tiputini Biodiversity Station, and University of Missouri–St. Louis. We thank Gary Kohout (Snapshot Sniper, LLC), for his help in maintaining the digital cameras.
References
- Bravo, A.; Harms, K.E.; Stevens, R.S.; Emmons, L.H. Collpas: Activity hotspots for frugivorous bats (Phyllostomidae) in the Peruvian Amazon. Biotropica 2008, 40, 203–210. [Google Scholar]
- Diamond, J.; Bishop, K.D.; Gilardi, J.D. Geophagy in New Guinea birds. Ibis 1999, 141, 181–193. [Google Scholar]
- Dominy, N.J.; Davoust, E.; Minekus, M. Adaptive function of soil consumption: An in vitro study modeling the human stomach and small intestine. J. Exper. Biol. 2004, 207, 319–324. [Google Scholar]
- Kreulen, D.A. Lick use by large herbivores: a review of benefits and banes of soil consumption. Mammal Rev. 1985, 15, 107–123. [Google Scholar]
- Krishnamani, R.; Mahaney, W.C. Geophagy among primates: Adaptive significance and ecological consequences. Anim. Behav. 2000, 59, 899–915. [Google Scholar]
- Matsubayashi, H.; Lagam, P.; Majalap, N.; Tangah, J.; Sukon, J.R.A.; Kitayama, K. Importance of natural licks for the mammals in Bornean inland tropical rain forests. Ecol. Res. 2007, 22, 742–748. [Google Scholar]
- Atwood, T.C.; Weeks, H.P., Jr. Sex- and age-specific patterns of mineral lick use by white-tailed deer (Odocoileus virginianus). Amer. Midl. Natur. 2002, 148, 289–296. [Google Scholar]
- Atwood, T.C.; Weeks, H.P., Jr. Sex-specific patterns of mineral lick preference in white-tailed deer. Northeast Nat. 2003, 10, 409–414. [Google Scholar]
- Bravo, A.; Harms, K.E.; Stevens, R.S.; Emmons, L.H. Puddles created by geophagous mammals are potential mineral sources for frugivorous bats (Stenodermatinae) in the Peruvian Amazon. J. Trop. Ecol. 2010, 26, 173–184. [Google Scholar]
- Bravo, A.; Harms, K.E.; Emmons, L.H. Preference for collpa water by frugivorous bats (Artibeus): an experimental approach. Biotropica 2010, 42, 276–280. [Google Scholar]
- Davies, A.G.; Baillie, I.C. Soil-eating by red leaf monkeys (Presbytis rubicunda) in Sabah, northern Borneo. Biotropica 1988, 20, 252–258. [Google Scholar]
- Klaus, G.; Klaus-Hugi, C.; Schmid, B. Geophagy by large mammals at natural licks in the rain forest of the Dzanga National Park, Central African Republic. J. Trop. Ecol. 1998, 14, 829–839. [Google Scholar]
- Mahaney, W.C.; Aufreiter, A.; Hancock, R.G.V. Mountain gorilla geophagy: A possible seasonal behavior for dealing with the effects of dietary changes. Int. J. Primatol. 1995, 16, 475–488. [Google Scholar]
- Brightsmith, D.J. Effects of weather on parrot geophagy in Tambopata, Peru. Wilson Bull. 2004, 116, 134–145. [Google Scholar]
- Emmons, L.H.; Stark, N.M. Elemental composition of a natural mineral lick in Amazonia. Biotropica 1979, 11, 311–313. [Google Scholar]
- Clayton, L.; MacDonald, D.W. Social organization of the babirusa (Babyrousa babyrussa) and their use of salt licks in Sulawesi, Indonesia. J. Mammal. 1999, 80, 1147–1157. [Google Scholar]
- Lizcano, D.J.; Cavelier, J. Daily and seasonal activity of the mountain tapir (Tapirus pinchaque) in the Central Andes of Colombia. J. Zool. 2000, 252, 429–435. [Google Scholar]
- Tobler, M.W.; Carrillo-Percastegui, S.E.; Powell, G. Habitat use, activity patterns and use of mineral licks by five species of ungulate in south-eastern Peru. J. Trop. Ecol. 2009, 25, 261–270. [Google Scholar]
- Blake, J.G.; Guerra, J.; Mosquera, D.; Torres, R.; Loiselle, B.A.; Romo, D. Use of mineral licks by white-bellied spider monkeys (Ateles belzebuth) and red howler monkeys (Alouatta seniculus) in eastern Ecuador. Int. J. Primatol. 2010, 31, 471–483. [Google Scholar]
- Blake, J.G.; Mosquera, D.; Guerra, J.; Romo, D. New locality records and the first photographs of living Echimys saturnus (dark tree rat, Echimyidae) from eastern Ecuador. Ecotropica 2010, 16, 141–144. [Google Scholar]
- Lizcano, D.J.; Cavelier, J. Chemical characteristics of salt licks and feeding habits of mountain tapir (Tapirus pinchaque) in the Central Andes of Colombia. Maztozool. Neotrop. 2004, 11, 193–201. [Google Scholar]
- Voigt, C.C.; Dechmann, D.K.N.; Bender, J.; Rinehart, B.J.; Michener, R.H.; Kunz, T.H. Mineral licks attract neotropical seed-dispersing bats. Res. Lett. Ecol. 2007. 1155/2007/34212. [Google Scholar]
- Setz, E.Z.F.; Enzweiler, J.; Solferini, V.N.; Amêndola, M.P.; Berton, R.S. Geophagy in the golden-faced saki monkey (Pithecia pithecia chrysocephala) in the Central Amazon. J. Zool. 1999, 247, 91–103. [Google Scholar]
- Voigt, C.C.; Capps, K.A.; Dechmann, D.K.N.; Michener, R.H; Kunz, T.H. Nutrition or detoxification: Why bats visit mineral licks of the Amazonian rainforest. PLoS ONE 2008, 3, e2011. [Google Scholar]
- Holdø, R.M.; Dudley, J.P.; McDowell, L.R. Geophagy in the African elephant in relation to availability of dietary sodium. J. Mammal. 2002, 83, 652–664. [Google Scholar]
- Abrahams, P.W. The chemistry and mineralogy of three savanna lick soils. J. Chem. Ecol. 1999, 25, 2215–2228. [Google Scholar]
- Izawa, K. Soil-eating by Alouatta and Ateles. Int. J. Primatol. 1993, 14, 229–242. [Google Scholar]
- Bass, M.S.; Finer, M.; Jenkins, C.N.; Kreft, H.; Cisneros-Heredia, D.F.; McCracken, S.F.; Pitman, N.C.A.; English, P.H.; Swing, K.; Villa, G.; Di Fiore, A.; Voigt, C.C.; Kunz, T.H. Global conservation significance of Ecuador's Yasuní National Park. PLoS ONE 2010, 5, e8767. [Google Scholar]
- Estacíon Científica Yasuní Escuela de Ciencias Biológicas, Pontificia Universidad Católica del Ecuador. Available online: http://www.biologia.puce.edu.ec/natura.php?c=337 (accessed on 7 March 2010).
- Srbek-Araujo, A.C.; Chiarello, A.G. Is camera-trapping an efficient method for surveying mammals in Neotropical forests? A case study in south-eastern Brazil. J. Trop. Ecol. 2005, 21, 121–125. [Google Scholar]
- Datta, A.; Anand, M.O.; Naniwadekar, R. Empty forests: Large carnivore and prey abundance in Namdapha National Park, north-east India. Biol. Conserv. 2008, 141, 1429–1435. [Google Scholar]
- Dinata, Y.; Nugroho, A.; Haidir, I.A.; Linkie, M. Camera trapping rare and theatened avifauna in west-central Sumatra. Bird Conserv. Int. 2008, 18, 30–37. [Google Scholar]
- Bowkett, A.E.; Rovero, F.; Marshall, A.R. The use of camera-trap data to model habitat use by antelope species in the Udzungwa Mountain forests, Tanzania. Afr. J. Ecol. 2007, 46, 479–487. [Google Scholar]
- Cherry, S. Statistical tests in publications of The Wildlife Society. Wildl. Soc. Bull. 1998, 26, 947–953. [Google Scholar]
- Johnson, D.H. The insignificance of statistical significance testing. J. Wildl. Manage. 1999, 63, 763–772. [Google Scholar]
- Whitlock, M.C.; Schluter, D. The Analysis of Biological Data; Roberts and Company Publishers: Greenwood Village, CO, USA, 2009; p. 704. [Google Scholar]
- Analytical Software. Statistix 8; Analytical Software: Tallahassee, FL, USA, 2003. [Google Scholar]
- Ridgely, R.S.; Greenfield, P.J. The Birds of Ecuador, Vol. II; Cornell University Press: Ithaca, NY, USA, 2001; p. 768. [Google Scholar]
- Wilson, D.E.; Reeder, D. Mammal Species of the World. A Taxonomic and Geographic Reference, 3rd ed.; Johns Hopkins University Press: Baltimore, ML, USA, 2005; p. 2142. [Google Scholar]
- Symes, C.T.; Hughes, J.C.; Mack, A.L.; Marsden, S.J. Geophagy in birds of Crater Mountain Wildlife Management Area, Papua New Guinea. J. Zool. 2005, 268, 87–96. [Google Scholar]
- Di Fiore, A.; Suarez, S.A. Route-based travel and shared routes in sympatric spider and wooly monkeys: cognitive and evolutionary implications. Anim. Cognit. 2007, 10, 317–329. [Google Scholar]
- Emmons, L.H.; Feer, F. Neotropical Rainforest Mammals. A Field Guide, 2nd ed.; The University of Chicago Press: Chicago, IL, USA, 1997; p. 396. [Google Scholar]
- De Souza, L.L.; Ferrari, S.F.; Da Costa, M.L.; Kern, D.C. Geophagy as a correlate of folivory in red-handed howler monkeys (Alouatta belzebul) from eastern Brazilian Amazonia. J. Chem. Ecol. 2002, 28, 1613–1621. [Google Scholar]
- Brightsmith, D.; Muñoz-Najar, R. Avian geophagy and soil characteristics in southeastern Peru. Biotropica 2004, 36, 534–543. [Google Scholar]
- Jiménez, I. Understanding Vertebrate Frugivores through Foraging Theory. PhD Thesis, University of Missouri–St. Louis, St. Louis, MO, USA, 2005. [Google Scholar]
- Santamaría, M.; Franco, A.M. Frugivory of Salvin's Curassow in a rainforest of the Colombian Amazon. Wilson Bull. 2007, 112, 473–481. [Google Scholar]
- Mahaney, W.C.; Milner, M.W.; Sanmugadas, K.; Hancock, R.G.V.; Aufreiter, S.; Wrangham, S.; Wrangham, R.; Pier, H.W. Analysis of geophagy soils in Kibale Forest, Uganda. Primates 1997, 38, 159–176. [Google Scholar]
- Blake, S.; Inkamba-Nkulu, C. Fruit, minerals, and forest elephant trails: Do all roads lead to Rome? Biotropica 2004, 36, 392–401. [Google Scholar]
- Montenegro, O.L. Natural Licks as Keystone Resources for Wildlife and People in Amazonia. PhD Thesis, University of Florida, Gainesville, FL, USA, 2004. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Blake, J.G.; Mosquera, D.; Guerra, J.; Loiselle, B.A.; Romo, D.; Swing, K. Mineral Licks as Diversity Hotspots in Lowland Forest of Eastern Ecuador. Diversity 2011, 3, 217-234. https://doi.org/10.3390/d3020217
Blake JG, Mosquera D, Guerra J, Loiselle BA, Romo D, Swing K. Mineral Licks as Diversity Hotspots in Lowland Forest of Eastern Ecuador. Diversity. 2011; 3(2):217-234. https://doi.org/10.3390/d3020217
Chicago/Turabian StyleBlake, John G., Diego Mosquera, Jaime Guerra, Bette A. Loiselle, David Romo, and Kelly Swing. 2011. "Mineral Licks as Diversity Hotspots in Lowland Forest of Eastern Ecuador" Diversity 3, no. 2: 217-234. https://doi.org/10.3390/d3020217
APA StyleBlake, J. G., Mosquera, D., Guerra, J., Loiselle, B. A., Romo, D., & Swing, K. (2011). Mineral Licks as Diversity Hotspots in Lowland Forest of Eastern Ecuador. Diversity, 3(2), 217-234. https://doi.org/10.3390/d3020217




