Morphometric, Phenological, and Nutritional Characterization of Five Wild Bean Species from Durango, Mexico
Abstract
1. Introduction
2. Materials and Methods
2.1. Germplasm and Its Management
2.2. Growing Conditions
2.3. Morphometric Characterization
2.4. Phenological Characterization
2.5. Nutritional Composition Analysis
2.6. Analysis of Results
3. Results
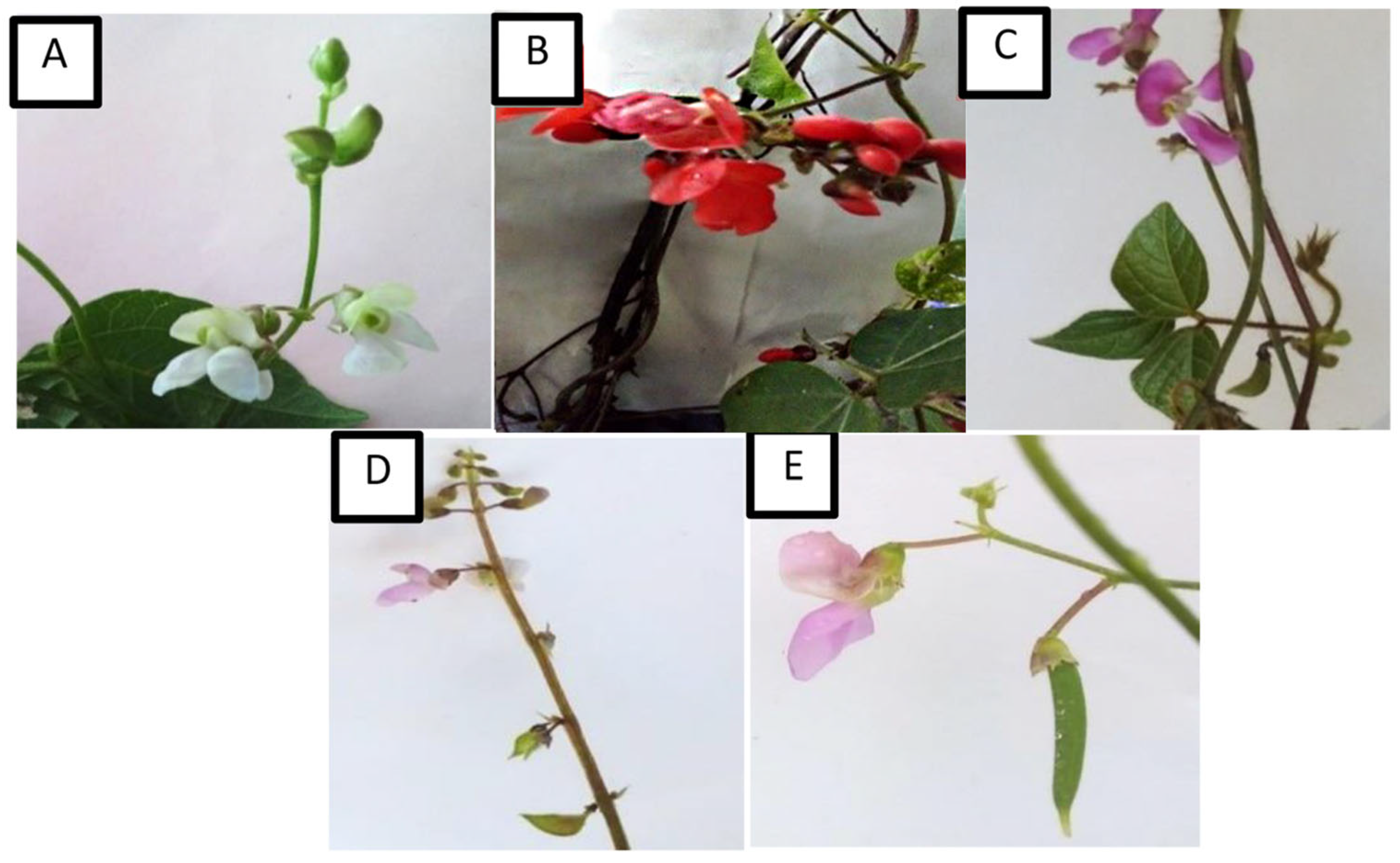
3.1. Morphometric Characterization of Qualitative Variables
3.2. Characterization in the Leaflets, Petiole, and Steam
3.3. Characterization in the Pods
3.4. Characterization in Seeds
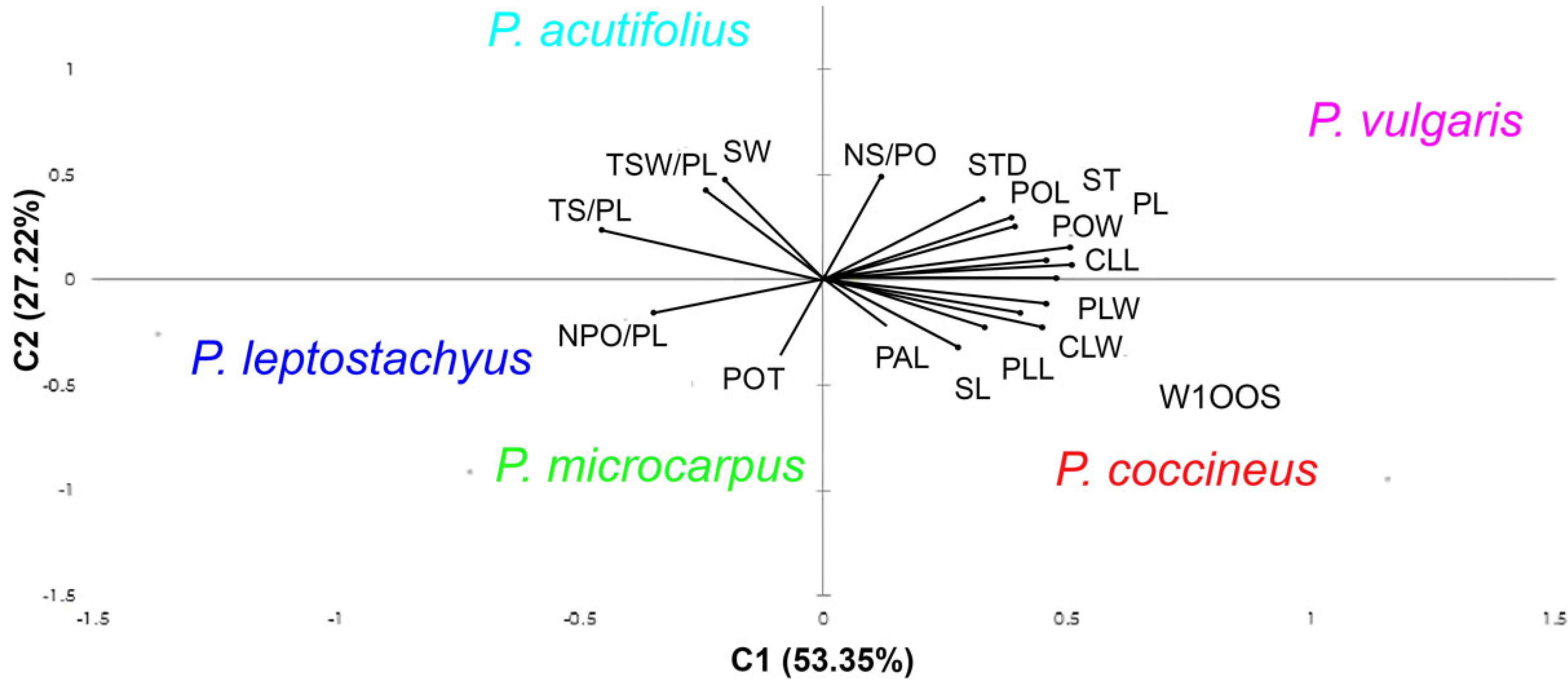
3.5. Principal Component Analysis and Clustering Analysis of Quantitative Variables in Wild Phaseolus
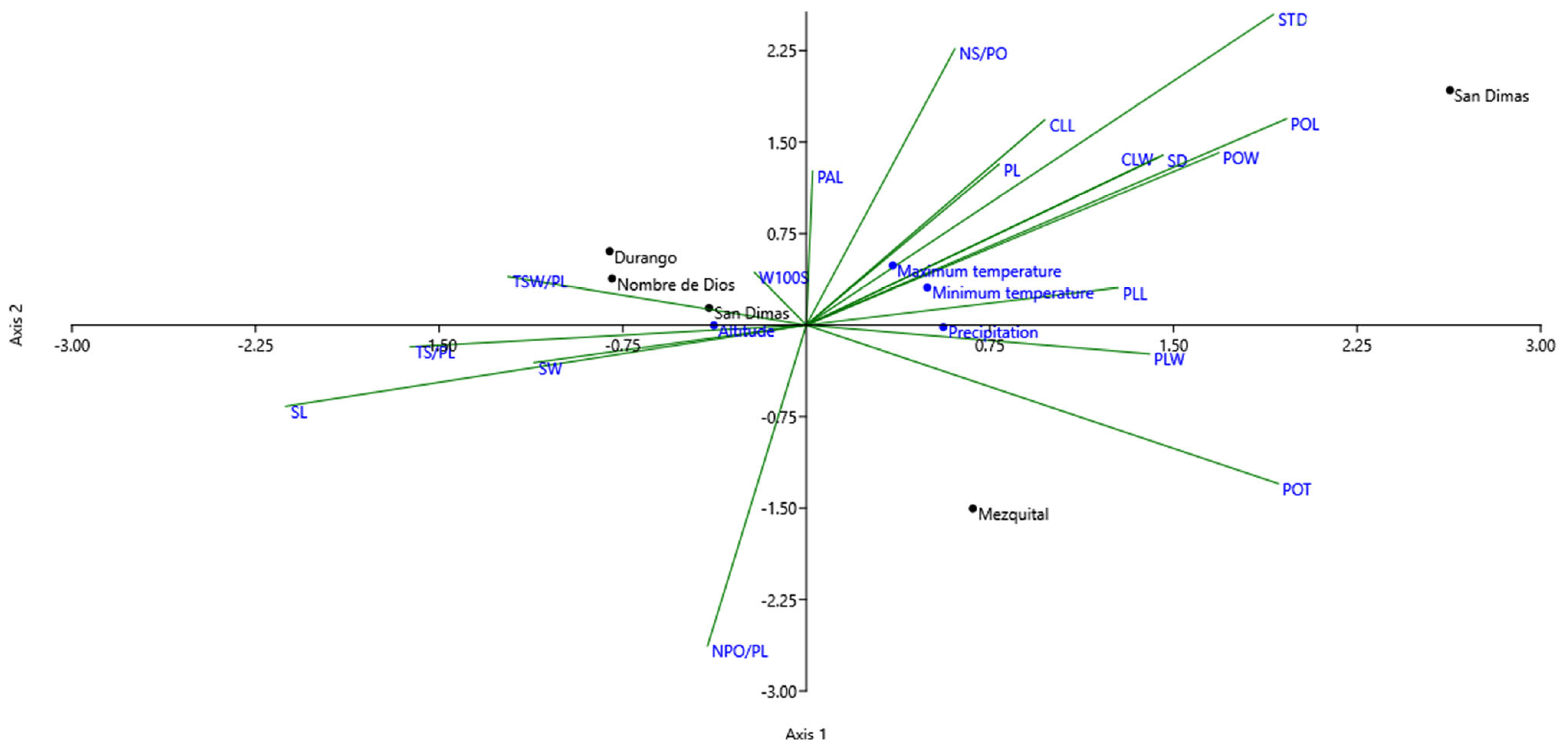
3.6. Canonical Correspondence Analysis (CCA) Showing Relationship Between Morphometric Characteristics and Environmental Variables of Provenance
3.7. Analysis of the Coefficient of Variation
3.8. Phenological Characterization
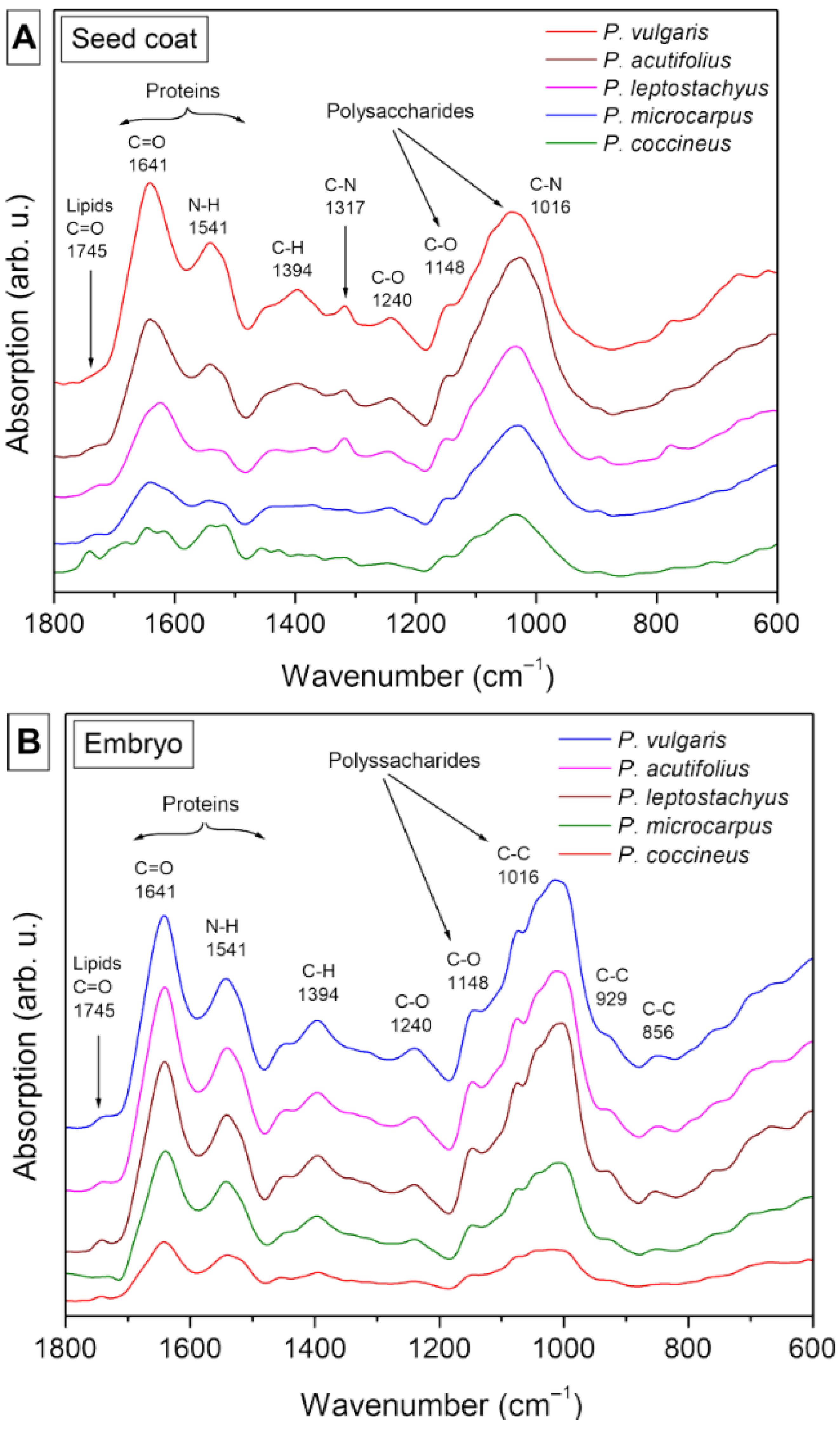
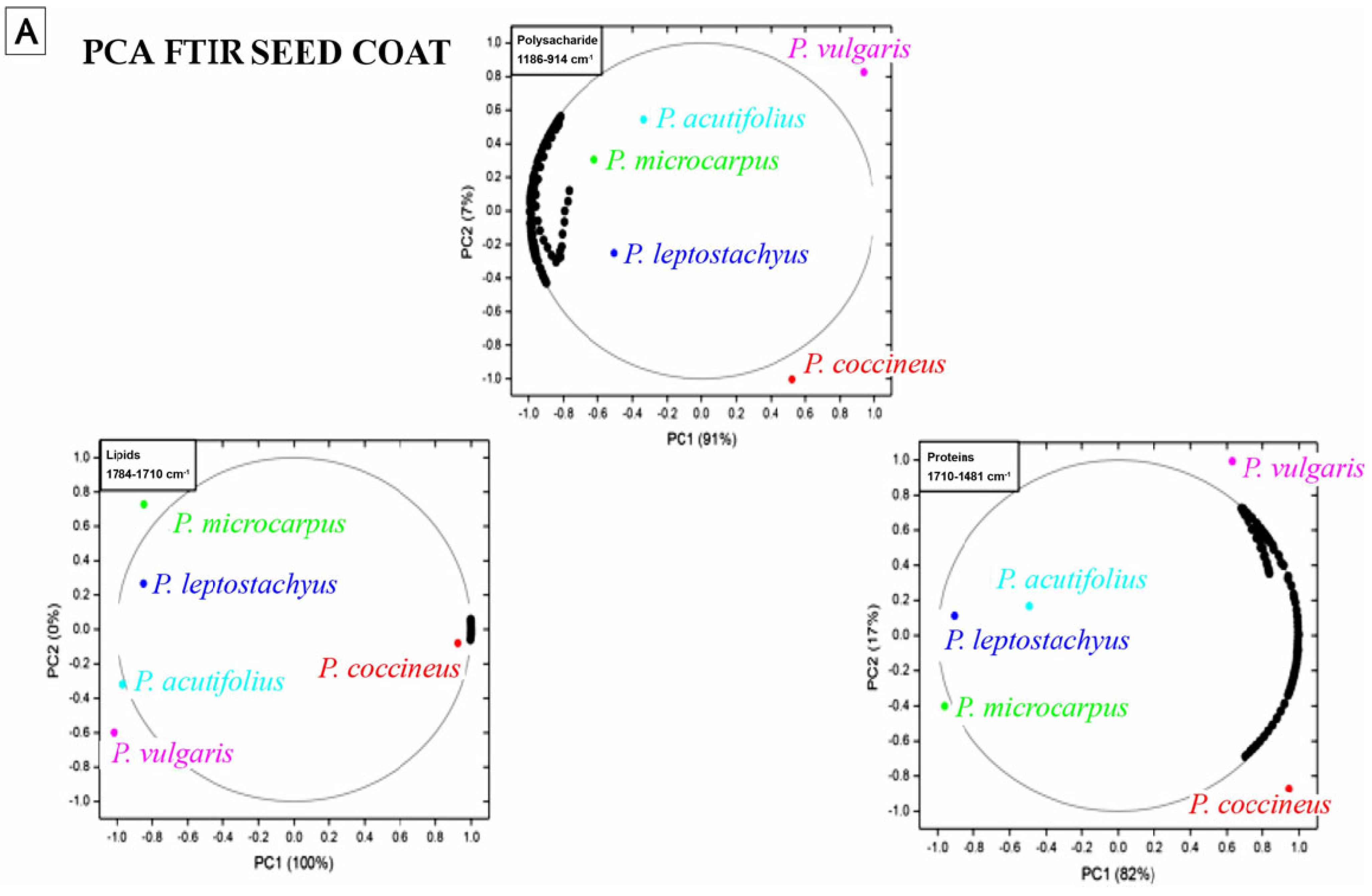
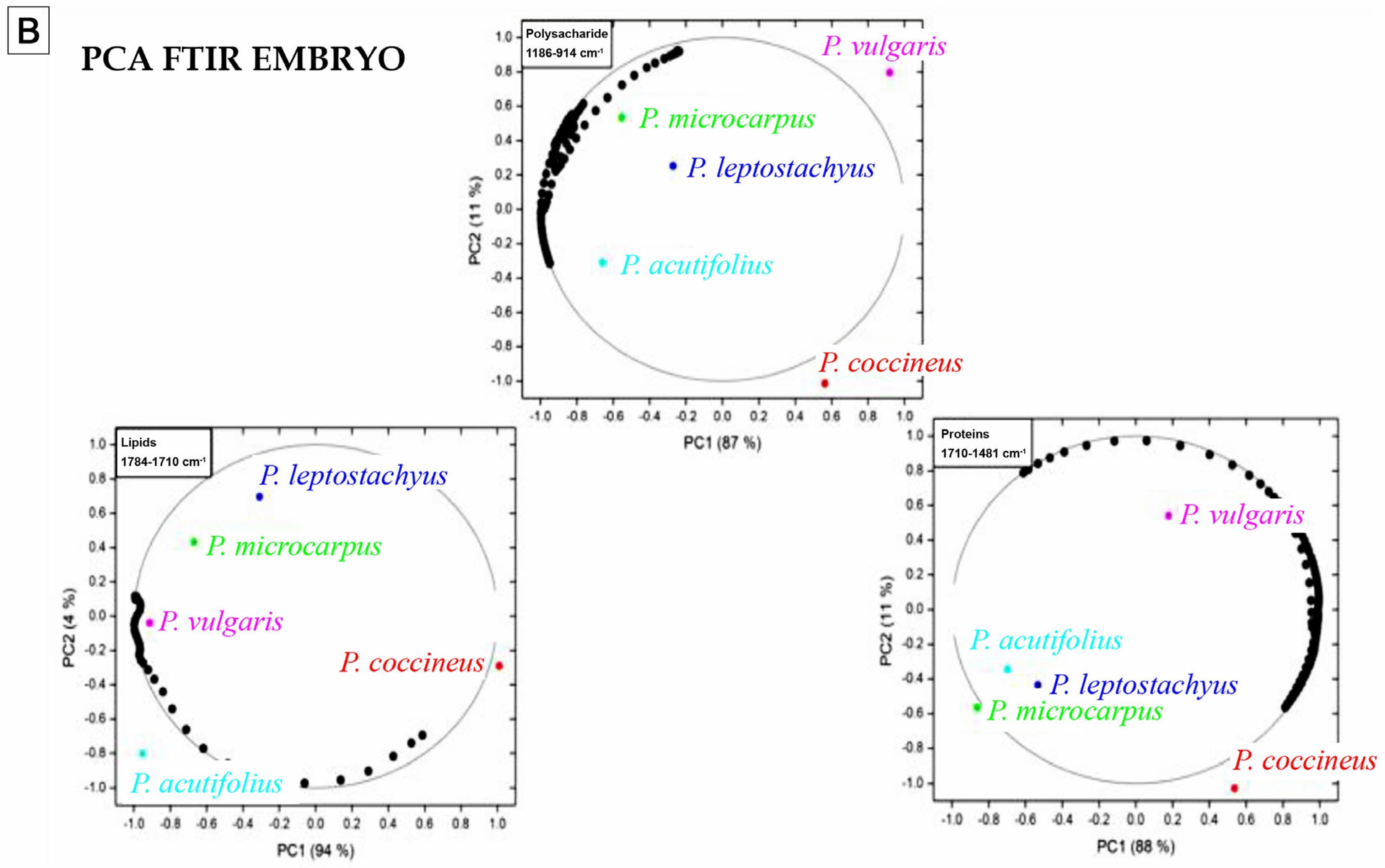
3.9. Nutritional Composition Analysis
4. Discussion
4.1. Morphometric Characterization in Qualitative Variables
4.2. Morphometric Characterization of Leaflets, Petiole, and Stem
4.3. Morphometric Characterization in the Legume Pods
4.4. Morphometric Characterization in Seeds
4.5. Principal Component Analysis and Clustering Analysis of Quantitative Variables in Wild Phaselous
4.6. Canonical Correspondence Analysis (CCA) Showing Relationship Between Morphometric Characteristics and Environmental Variables of Provenance
4.7. Analysis of the Coefficient of Variation
4.8. Phenological Characterization
4.9. Nutritional Composition Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arias, J.D.; Chavero, A.; Olavarría, E.; Vigil, J.M.; Zárate, J. México a Través de Los Siglos. In Compendio de la obra de Vicente Riva Palacio; Océano: Barcelona, España, 1999; p. 272. [Google Scholar]
- Maphosa, Y.; Jideani, V.A. The role of legumes in human nutrition. In Functional Food—Improve Health Through Adequate Food; Hueda, M.C., Ed.; IntechOpen: London, UK, 2017; p. 318. [Google Scholar] [CrossRef]
- Singh, A.; Schöb, C.; Iannetta, P.P.M. La fijación de nitrógeno por frijol común en mezclas de cultivos se ve influenciada por la tasa de crecimiento de las especies asociadas. BMC Plant Biol. 2023, 23, 253. [Google Scholar] [CrossRef]
- Villarreal-Villagran, V.H.; Muruaga-Martínez, J.S.; Vargas-Vázquez, M.L.P.; Mayek-Pérez, N.; Hérnandez-Delgado, S. Relaciones filogenéticas de especies de Phaseolus de México con base en marcadores de ADN cloroplástico. Polibotánica 2022, 27, 35–51. [Google Scholar] [CrossRef]
- Delgado-Salinas, A.; Gama-López, S. Diversidad y distribución de los frijoles silvestres en México. Rev. Digit. Univ. 2015, 16, 1–8. [Google Scholar]
- Bitocchi, E.; Rau, D.; Bellucci, E.; Rodríguez, M.; Murgia, M.L.; Gioia, T.; Santo, D.; Nanni, L.; Attene, G.; Papa, R. Beans (Phaseolus spp.) as a model for understanding crop evolution. Front. Plant Sci. 2017, 8, 722. [Google Scholar] [CrossRef]
- González-Elizondo, M.S.; González-Elizondo, M.; Márquez-Linares, M.A. Vegetación y Ecorregiones de Durango; Instituto Politécnico Nacional-Plaza y Valdés: Mexico City, Mexico, 2007; p. 220. [Google Scholar]
- González-Elizondo, M.; González-Elizondo, S.; Herrera-Arrieta, Y. Listados Florísticos de México. IX. Flora de Durango; Instituto de Biología, Universidad Nacional Autónoma de México: Mexico City, Mexico, 1991; p. 52. [Google Scholar]
- Rzedowsky, J. Vegetación de México, México: Limusa, 1978, p. 432—“Vegetación Potencial 1:4000 000. IV.8.2. Atlas Nacional de México” [en Línea]. 1990, vol. II. México: Instituto de Geografía, UNAM. Available online: http://www.conabio.gob.mx/informacion/gis/ (accessed on 6 September 2025).
- Mahuk, G.S.; Jara, C.; Cajiao, C.; Beebe, S. Source of resistance to angular leaf spot (Phaeoisariopsis griseola) in common bean core collection, wild Phaseolus vulgaris and secondary gene pool. Euphytica 2003, 130, 303–313. [Google Scholar] [CrossRef]
- Espinosa-Alonso, L.G.; Lygin, A.; Widholm, J.M.; Valverde, M.E.; Paredes-López, O. Polyphenols in wild and weedy Mexican common beans (Phaseolus vulgaris L.). J. Agric. Food Chem. 2006, 54, 4436–4444. [Google Scholar] [CrossRef]
- Baez-González, A.D.; Acosta-Díaz, E.; Padilla-Ramírez, J.S.; Almeyda-León, I.H.; Zavala-García, F. Estado de conservación de Once Especies de Frijol Silvestre (Phaseolus spp. Fabaceae) en el Noreste de México. Rev. Mex. Cienc. Agríc. 2019, 10, 417–429. [Google Scholar] [CrossRef]
- Nuñez-Colín, C.A.; Escobedo-López, D. Caracterización de germoplasma vegetal: La piedra angular en el estudio de los recursos fitogenéticos. Acta Agríc. Pecu. 2015, 1, 1–6. [Google Scholar]
- Vidal-Barahona, A.; Lagunes-Espinoza, L.C.; Valadez-Moctezuma, E.; Ortiz-García, C. Variabilidad morfológica y molecular de cultivares criollos y mejorados de frijol común en Tabasco, México. Rev. Fitotec. Mex. 2006, 29, 273–281. [Google Scholar] [CrossRef]
- Meza-Vázquez, K.E.; Lépiz-Ildefonso, R.; López-Alcocer, J.J.; Morales-Rivera, M.M. Caracterización morfológica y fenológica de especies silvestres de frijol (Phaseolus). Rev. Fitotec. Mex. 2015, 38, 17–28. [Google Scholar] [CrossRef]
- Acosta-Díaz, E.; Hernández-Torres, I.; Amador-Ramírez, M.D.; Padilla-Ramírez, J.S.; Zavala-García, F.; Baez-González, A. Collection and characterization of wild species of Phaseolus (Fabaceae) in northeastern Mexico for ex situ conservation. Plant Ecol. Evol. 2016, 148, 119–127. [Google Scholar] [CrossRef]
- Lépiz Ildefonso, R.; López Alcocer, J.J.; Sánchez González, J.J.; Santacruz Ruvalcaba, F.; Nuño Romero, R.; Rodríguez Guzmán, E. Características morfológicas de formas cultivadas, silvestres e intermedias de frijol común de hábito trepador. Rev. Fitotec. Mex. 2010, 33, 21–28. [Google Scholar] [CrossRef]
- Peña-Valdivia, C.B.; García-Nava, R.; Aguirre, R.J.R.; Trejo, C. The effects of high temperature on dormancy and hypocotyl-root growth of wild common bean (Phaseolus vulgaris L.). Seed Sci. Technol. 2002, 30, 231–248. [Google Scholar]
- Lot, A.; Chiang, F. Manual de Herbario: Administración y Manejo de Colecciones Técnicas de Recolección y Preparación de Ejemplares Botánicos; Consejo Nacional de la Flora de México, A.C.: Ciudad de México, Mexico, 1986; p. 142. [Google Scholar]
- Instituto Nacional de Estadística y Geografía. Aspectos Geográficos. Available online: https://www.inegi.org.mx/contenidos/app/areasgeograficas/resumen/resumen_10.pdf (accessed on 6 September 2025).
- Pavela, R. History, presence and perspective of using plant extracts as commercial botanical insecticides and farm products for protection against insects—A review. Plant Protect. Sci. 2016, 52, 229–241. [Google Scholar] [CrossRef]
- Al-Shuraym, A. The impact of the onion-garlic extracts to control date palm aphids in Saudi Arabia. J. Saudi Soc. Agric. Sci. 2022, 21, 546–551. [Google Scholar] [CrossRef]
- Fernández, F.; Gepts, P.; López, M. Etapas de desarrollo en la planta de frijol. In Frijol: Investigación y Producción; Lopez, M., Fernandez, F., Schoonhoven, A., Eds.; CIAT: Palmira, Colombia, 1991; pp. 61–78. [Google Scholar]
- Franco, T.L.; Hidalgo, R. Análisis Estadístico de Datos de Caracterización Morfológica de Recursos Fitogenéticos; Boletín Técnico No. 8; Instituto Internacional de Recursos Fitogenéticos (IPGRI): Cali, Colombia, 2003; pp. 11–25. [Google Scholar]
- Ligarreto, M.G.A.; Martínez, W.O. Variabilidad genética en frijol común (Phaseolus vulgaris L.). Análisis de variables morfológicas y agronómicas cuantitativas. Agron. Colomb. 2002, 19, 69–80. [Google Scholar]
- Mendelsoh, R.; Mantsch, H.H. Protein-Lipid Interactions; Watts, A., De Pont, J.J.H.H.M., Eds.; Elsevier: Amsterdam, The Netherlands, 1986; pp. 321–349. [Google Scholar]
- Tamm, L.K.; Tatulian, S.A. Infrared spectroscopy of proteins and peptides in lipid bilayers. Q. Rev. Biophys. 1997, 30, 365–429. [Google Scholar] [CrossRef]
- Arrondo, J.L.R.; Goñi, F.M. Structure and dynamics of membrane proteins as studied by infrared spectroscopy. Prog. Biophys. Mol. Bio. 1999, 72, 367–405. [Google Scholar] [CrossRef]
- Ruíz Salazar, R.; Hernández Delgado, S.; Vargas Vázquez, M.L.P.; Mayek Pérez, N. Estado actual de los recursos genéticos de Phaseolus coccineus (Fabaceae) en México. Bol. Soc. Argent. Bot. 2021, 56, 289–305. [Google Scholar] [CrossRef]
- Freytag, G.F.; Debouck, D.G. Taxonomy, Distribution, and Ecology of the Genus Phaseolus (Leguminosae–Papilionoideae) in North America, México and Central America; Sida Botanical Miscellany 23; Botanical Research Institute of Texas: Forth Worth, TX, USA, 2002; p. 9. [Google Scholar]
- Reverte, S.; Retana, J.; Gómez, J.M.; Bosch, J. Pollinators show flower colour preferences but flowers with similar colours do not attract similar pollinators. Ann. Bot. 2016, 2, 249–257. [Google Scholar] [CrossRef]
- Payró de la Cruz, E.; Gepts, P.; Colunga Garcia, M.P.; Zizumbo Villareal, D. Spatial distribution of genetic diversity in wild populations of Phaseolus vulgaris L. from Guanajuato and Michoacán, México. Genet. Resour. Crop Evol. 2005, 52, 589–599. [Google Scholar] [CrossRef]
- Díaz, A.M.; Caldas, G.V.; Blair, M.W. Concentrations of condensed tannins and anthocyanins in common bean seed coats. Food Res. Int. 2010, 43, 595–601. [Google Scholar] [CrossRef]
- Salinas-Moreno, Y.; Rojas-Herrera, L.; Sosa-Montes, E.; Pérez-Herrera, P. Composición de antocianinas en variedades de frijol negro (Phaseolus vulgaris L.) cultivadas en México. Agrociencia 2005, 39, 385–394. [Google Scholar]
- Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP). Available online: http://www.inifapcirne.gob.mx/Biblioteca/Publicaciones/976.pdf (accessed on 6 September 2025).
- Salinas-Ramírez, N.; Escalante-Estrada, J.S.; Rodríguez-González, M.T.; Sosa-Montes, E. Yield, nutritional quality and profitability of green beans of rainfed in San Pablo Ixayoc, Mexico. Rev. Chapingo. Ser. Hortic. 2013, 19, 333–342. [Google Scholar] [CrossRef]
- García, M.; Watson, C.E. Herencia de la resistencia al acame de raíces en maíz dulce (Zea mays L). Rev. Científica UDO Agrícola 2003, 3, 24–33. [Google Scholar]
- Lagunes-Fortiz, E.; Villanueva-Verduzco, C.; Lagunes-Fortiz, E.R.; Zamora-Macorra, E.; Ávila-Alistac, N.; Villanueva-Sánchez, E. La densidad de siembra en el crecimiento de la verdolaga. Rev. Mex. Cienc. Agríc. 2021, 12, 1–13. [Google Scholar] [CrossRef]
- Gepts, P.L.; Debouck, D.G. Origin, domestication, and evolution of the common bean (Phaseolus vulgaris L.). In Common Beans: Research for Crop Improvement; Schoonhoven, A.V., Voysest, O., Eds.; CAB International y CIAT: Wallingford, UK, 1991; pp. 7–43. [Google Scholar]
- Díaz, M.F. Crecimiento de la vaina y semillas del frijol. Turrialba 1990, 40, 553–561. [Google Scholar]
- Oliker, M.; Poljakoff-Mayber, A.; Mayer, A.M. Changes in weight, nitrogen accumulation, respiration and photosynthesis during growth and development of seeds and pods of Phaseolus vulgaris. Am. J. Bot. 1978, 65, 366–371. [Google Scholar] [CrossRef]
- Nitsch, J.P. Plant Hormones in the Development of Fruits. Q. Rev. Biol. 1952, 27, 33–57. [Google Scholar] [CrossRef]
- Suge, H.; Flores, G.D.M. Changes of gibberellins during seed and pericarp development in common bean with special reference to abortion. J. Crop Sci. 1977, 46, 371–378. [Google Scholar] [CrossRef][Green Version]
- Skene, K.G.; Carr, D.J. A quantitative study of the gibberellin content of seeds of Phaseolus vulgaris at different stages in their development. Aust. J. 1961, 14, 1–13. [Google Scholar] [CrossRef]
- Alcántara Cortés, S.J.; Acero Godoy, J.; Alcántara Cortés, J.D.; Sánchez Mora, R.M. Principales reguladores hormonales y sus interacciones en el crecimiento vegetal. Nova 2019, 17, 109–129. [Google Scholar] [CrossRef]
- Granados, A.R.; Ortega-Delgado, M.L.; Zárate, G. Influencia del peso seco y contenido de nitrógeno de los órganos de la planta en el rendimiento y contenido de proteína del grano de frijol Phaseolus vulgaris L. Chapingo 1987, 54, 47–52. [Google Scholar]
- Nabhan, G.P.; Felger, R.S. Teparis in southwestern North America. Econ. Bot. 1978, 32, 3–19. [Google Scholar] [CrossRef]
- Fenner, M. Environmental influences of seed size and composition. Hortic. Rev. 1992, 13, 183–213. [Google Scholar]
- Eriksson, O. Seed size variation and its effect on germination and seedling performance in the clonal herb Convallaria majalis. Acta Oecol. 1999, 20, 61–66. [Google Scholar] [CrossRef]
- Pitelka, L.F.; Thayer, M.E.; Hansen, S.B. Variation in achene weight in Aster acuminatus. Can. J. Bot. 1983, 61, 1415–1420. [Google Scholar] [CrossRef]
- Meyer, S.E. Ecological correlates of achene mass variation in Chrysothamnus nauseosus (Asteraceae). Am. J. Bot. 1997, 84, 471–477. [Google Scholar] [CrossRef]
- Susko, D.J.; Lovett-Doust, L. Patterns of seed mass variation and their effects on seedling traits in Alliaria petiolata (Brassicaceae). Am. J. Bot. 2000, 87, 56–66. [Google Scholar] [CrossRef]
- Kulandaivelu, R.; Morachan, Y.B. Influencia del clima sobre el rendimiento de vainas y el crecimiento de maní. Turrialba 1983, 33, 332–334. [Google Scholar]
- Zizumbo-Villarreal, D.; Colunga-García Marín, P.; Payró de la Cruz, E.; Delgado-Valerio, P.; Gepts, P. Population structure and evolutionary dynamics of wild-weedy-domesticated complexes of common bean in a Mesoamerican region. Crop Sci. 2005, 45, 1073–1083. [Google Scholar] [CrossRef]
- Enriquez, A.G. Mejoramiento Genético Sobre Otros Factores Limitantes de la Producción de Frijol, Diferentes de Enfermedades e Insectos; CR Centro Agronómico Tropical de Investigación y Enseñanza (CATIE), Departamento de Cultivos y Suelos Tropicales Costa Rica: Turrialba, Costa Rica, 1977; pp. 15–17. [Google Scholar]
- Tapia, B.H. Mejoramiento Varietal de Frijol en Nicaragua; Instituto de Ciencias Agropecuarias (ICSA): Managua, Nicuaraga, 1987; pp. 4–11. [Google Scholar]
- Lépiz Ildefonso, R.; Ramírez Delgadillo, R. Los Parientes Silvestres del Frijol Común en el Occidente de México; Universidad de Guadalajara: Guadalajara, Mexico, 2010; p. 17. [Google Scholar]
- Ambika, S.; Manonmani, V.; Somasundar, G. Review on effect of seed size on seedling vigor and seed yield. Res. J. Seed Sci. 2014, 7, 31–38. [Google Scholar] [CrossRef]
- Nakamura, R.R. Seed abortion and seed size variation within fruit of Phaseolus vulgaris: Pollen donor and resource limitation effects. Am. J. Bot. 1988, 75, 1003–1010. [Google Scholar] [CrossRef]
- Venable, D.L. Size-number trade-offs and the variation of seed size with plant resource status. Am. Natur. 1992, 140, 287–304. [Google Scholar] [CrossRef]
- Matilla, A.J. Desarrollo y germinación de las semillas. In Fundamentos de Fisiología Vegetal; Azcón-Bieto, J., Talón, M., Eds.; Mc Graw Hill. E.U.: Maidenhead, UK, 2008; pp. 537–558. [Google Scholar]
- Sicard, D.; Nanny, L.; Porfiri, O.; Bulfon, D.; Papa, R. Genetic diversity of Phaseolus vulgaris and P. coccineus landraces in central Italy. Plant Breed. 2005, 124, 464–472. [Google Scholar]
- Berrocal-Ibarra, S.; Ortíz Cereceres, J.; Peña-Valdivia, C.B. Yield components, harvest index and leaf area efficiency of a sample of wild population and a domesticated variant of the common bean Phaseolus vulgaris. S. Afr. J. Bot. 2002, 68, 205–211. [Google Scholar] [CrossRef]
- Flores de la Cruz, M.J.; García Esteva, A.; García Nava, J.R.; Kohashi Shibata, J.; Ybarra Moncada, M.C. Diferencias fenológicas, morfológicas y de componentes del rendimiento entre una forma silvestre y una domesticada de frijol común. Rev. Mex. Cienc. Agríc. 2018, 9, 137–149. [Google Scholar] [CrossRef][Green Version]
- Schwanitz, F. The Origin of Cultivated Plants; Harvard University Press: Cambridge MA, USA, 1966; p. 175. [Google Scholar]
- Parker, K.C. Seed crop characteristics and minimum reproductive size of organ pipe cactus (Stenocereus thurberi) in southern Arizona. Madroño 1987, 34, 294–303. [Google Scholar][Green Version]
- Rocha, O.J.; Stephenson, A.G. Effects of nonrandom seed abortion on progeny performance in Phaseolus coccineus L. Evolution 1991, 45, 1198–1208. Evolution 1991, 45, 1198–1208. [Google Scholar][Green Version]
- Stephenson, A.G. Flower and fruit abortion: Proximate cause and ultimate functions. Ann. Rev. Ecol. Syst. 1981, 12, 253–279. [Google Scholar] [CrossRef]
- García-Urióstegui, A.; Garcia-Nava, J.R.; Uscanga-Mortera, E.; García-Esteva, A.; Kohashi-Shibata, J.; Garcia de los Santos, G. Rendimiento, biomasa y fenología de la cruza entre fríjol silvestre y domesticado. Rev. Mex. Cienc. Agríc. 2025, 16, 1–11. [Google Scholar] [CrossRef]
- Búrquez, A.; Sarukhán, J. Biología floral de poblaciones silvestres y cultivadas de Phaseolus coccineus L. II. Sistemas reproductivos. Bol. Soc. Bot. Méx. 1984, 46, 3–12. [Google Scholar] [CrossRef]
- Kendall, D.A.; Smith, B.D. The pollinating efficiency of honeybee and bumblebee visits to flowers of the runner bean Phaseolus coccineus L. J. Appl. Ecol. 1976, 13, 749–752. [Google Scholar] [CrossRef]
- Stephenson, A.G.; Winsor, J.A.; Schlichting, C.D. Evidence for non-random fertilization in the common zucchini, Cucurbita pepo. In Reproduction in Higher Plants; Cresti, M., Gori, P., Pacini, E., Eds.; Springer: Berlin/Heidelberg, Germany, 1988; pp. 333–338. [Google Scholar]
- Kaushal, N.; Awasthi, R.; Gupta, K.; Gaur, P.; Siddique, K.H.; Nayyar, H. Heat-stress-induced reproductive failures in chickpea (Cicer arietinum) are associated with impaired sucrose metabolism in leaves and anthers. Funct. Plant Biol. 2013, 40, 1334–1349. [Google Scholar] [CrossRef] [PubMed]
- Jurado, E.; Westoby, M. Seedling growth in relation to seed size among species of arid Aust. J. Ecol. 1992, 80, 407–416. [Google Scholar] [CrossRef]
- Reyes Martínez, A. Variabilidad Genética de Frijol (Phaseolus vulgaris L.) Cultivado Y Silvestre en el Estado de Durango. Ph.D. Thesis, Instituto Politécnico Nacional CIIDIR, Oaxaca, Mexico, 2014. [Google Scholar]
- Stearns, S.C.; Koella, J.C. The evolution of phenotypic plasticity in life history traits: Predictions for norms of reaction for age and size at maturity. Evolution 1986, 40, 893–913. [Google Scholar]
- Stenseth, N.C.; Mysterud, A.; Ottersen, G.; Hurrell, J.W.; Chan, K.S.; Lima, M. Ecological effects of climate fluctuations. Science 2002, 23, 1292–1296. [Google Scholar] [CrossRef]
- Walther, G.R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.C.; Fromentin, J.M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological responses to recent climate change. Nature 2022, 28, 389–395. [Google Scholar] [CrossRef]
- Matesanz, S.; Gianoli, E.; Valladares, F. Global change and the evolution of phenotypic plasticity in plants. Ann. N. Y. Acad. Sci. 2010, 2, 35–55. [Google Scholar] [CrossRef]
- Nicotra, A.B.; Atkin, O.K.; Bonser, S.P.; Davidson, A.M.; Finnegan, E.J.; Mathesius, U.; Poot, P.; Purugganan, M.D.; Richards, C.L.; Valladares, F.; et al. Plant phenotypic plasticity in a changing climate. Trends Plant Sci. 2010, 15, 684–692. [Google Scholar] [CrossRef]
- Quero, J.L.; Villar, R.; Marañón, T.; Murillo, A.; Zamora, R. Plastic response to light and water in four Mediterranean Quercus species (Fagaceae). Rev. Chil. Hist. Nat. 2008, 81, 373–385. [Google Scholar]
- Zavala, M.A.; Espelta, J.M.; Caspersen, J.; Retana, J. Interspecific differences in sampling performance with respect to light and aridity gradients in Mediterranean pine-oak forests: Implications for species coexistence. Can. J. For. Res. 2011, 41, 1432–1444. [Google Scholar] [CrossRef]
- Andivia, E.; Fernández, M.; Vazquez-Piqué, J.; Alejano, R. Two provenances of Quercus ilex ssp. ballota (Desf) Samp. nursery seedlings have different response to frost tolerance and autumn fertilization. Eur. J. For. Res. 2012, 131, 1091–1101. [Google Scholar] [CrossRef]
- Vargas Vázquez, M.L.P.; Muruaga Martínez, J.S.; Alejandre Iturbide, G.; Mayek Pérez, N. Variabilidad fenológica de una población de frijol Patol (Phaseolus coccineus L.) nativo de Durango, México. Rev. Mex. Cienc. Agríc. 2015, 6, 2121–2127. [Google Scholar] [CrossRef][Green Version]
- Orduño-Cruz, A.; Troyo-Dieguéz, E. Morfología y Desarrollo del Frijol Tepari Phaseolus acutifolius; Gray, A., Ed.; Centro de Investigaciones Biológicas del Noreste. S.C. Unidad-Guerrero Negro: Guerrero Negro, Mexico, 2003; p. 10. [Google Scholar]
- Forero Hernández, D.C.; Hormaza Martínez, P.A.; Moreno Caicedo, L.P.; Ruiz Romero, R. Generalidades Sobre la Morfología y Fenología de la Palma de Aceite; Publicación del Centro de Investigación en Palma de Aceite (Cenipalma): Bogota, Colombia, 2012; p. 124. [Google Scholar]
- Pérez Herrera, P.; Acosta Gallegos, J.A. Permeabilidad de la testa y la porción micrópilo-Hilio en semilla de frijol silvestre y cultivado. Rev. Fitotec. Mex. 2002, 25, 57–63. [Google Scholar] [CrossRef]
- Quiroz-Sodi, M.; Mendoza-Díaz, S.; Hernández-Sandoval, L.; Carrillo-Ángeles, I. Characterization of the secondary metabolites in the seeds of nine native bean varieties (Phaseolus vulgaris and P. coccineus) from Queretaro, México. Bot. Sci. 2018, 96, 650–661. [Google Scholar] [CrossRef]
- Vargas-Vázquez, M.L.P.; Uscanga-Mortera, E.; Padilla-Chacón, D.; Vibrans, H.; Kohashi-Shibata, J.; Miranda-Colín, S.; Yañez-Jiménez, P. Biomass and carbohydrate partitioning in seeds and seedlings of domesticated and wild Phaseolus coccineus L. Bot. Sci. 2020, 98, 366–376. [Google Scholar] [CrossRef]
- Sánchez-Chávez, E.; Férnandez-Valeriano, A.F. Estudio de las propiedades físico-químicas y calidad nutricional en distintas variedades de frijol consumidas en México. Nova Sci. 2017, 9, 133–148. [Google Scholar] [CrossRef][Green Version]



| Species | Locality | Voucher | Latitude | Longitude | Altitude | Precipitation | Maximum Temperature | Minimum Temperature | Vegetation |
|---|---|---|---|---|---|---|---|---|---|
| N | W | (m) | (mm) | (°C) | (°C) | ||||
| P. vulgaris L. | San Dimas | 60673 | 23°40′ | 105°47′ | 1546 | 1050 | 27.0 | −7 | LDF |
| P. coccineus L. | San Dimas | 57557 | 23°39′ | 105°47′ | 2383 | 1050 | 27.0 | −7 | TF |
| P. leptostachyus Benth. | Durango | 57559 | 24°2′ | 104°20′ | 1851 | 500.0 | 31.4 | −3 | XS |
| P. microcarpus Mart. | Mezquital | 57558 | 24°2′ | 104°16′ | 1464 | 391.8 | 42.4 | −2 | SS |
| P. acutifolius A. Gray. | Nombre de Dios | 57567 | 23°56′ | 104°18′ | 1798 | 446.6 | 36.7 | −3.5 | XS |
| Species | P. vulgaris | P. coccineus | P. leptostachyus | P. microcarpus | P. acutifolius |
|---|---|---|---|---|---|
| Qualitative Variables | |||||
| GF | Epigeal | Hypogeal | Hypogeal | Epigeal | Epigeal |
| GH | Indeterminate | Indeterminate | Determinate | Indeterminate | Indeterminate |
| LM | Whole | Whole | Whole | Whole | Whole |
| LV | Closed | Reticulated | Open | Open | Closed |
| LS | Ovate | Rhombic | Roped | Roped | Lanceolate |
| LC | Light green | Dark green | Dark green | Light green | Dark green |
| SC | Green | Purple | Green | Green | Green |
| WC | White | Red | Intense lilac | Light lilac | Light lilac |
| BC | White | Red | Intense lilac | Light lilac | Light lilac |
| CMP | Opaque green | Light brown | Dark brown | Light brown | Dark brown |
| CP | Weak | Very weak | Half | Absent | Half |
| OPA | Curved upward | Curved upward | Curved upward | Curved upward | Curved upward |
| PT | Smooth | Rough | Rough | Smooth | Rough |
| PP | Light | Light | Predominant | Light | Light |
| SPP | Absent | Light | Absent | Prominent | Light |
| SS | Kidney | Square | Spherical | Triangular | Rectangular |
| SC | Brown and cream | Black and gray | Black and brown | Gray | Black and gray |
| CAHS | Black and cream | Black | Black and brown | Light brown | Black and gray |
| SZ | Small | Small | Small | Small | Small |
| ST | Smooth | Smooth | Smooth | Smooth | Smooth |
| Species | P. vulgaris | P. coccineus | P. leptostachyus | P. microcarpus | P. acutifolius |
|---|---|---|---|---|---|
| Quantitative Variables | |||||
| 1 | 4.00 ± 0.25 c | 4.38 ± 0.36 c | 1.71 ± 0.13 a | 3.37 ± 0.16 b | 3.51 ± 0.10 b |
| 2 | 3.43 ± 0.45 cd | 3.80 ± 0.56 d | 1.33 ± 0.15 a | 3.20 ± 0.12 bc | 2.74 ± 0.28 b |
| 3 | 6.44 ± 0.29 c | 6.72 ± 0.51 d | 4.03 ± 0.35 a | 4.05 ± 0.44 a | 5.40 ± 0.51 b |
| 4 | 5.82 ± 0.32 d | 6.21 ± 0.47 d | 3.52 ± 0.43 b | 3.31 ± 0.38 b | 2.70 ± 0.23 a |
| 5 | 2.52 ± 0.18 a | 2.81 ± 0.20 a | 1.36 ± 0.15 a | 1.51 ± 0.21 a | 2.28 ± 0.18 a |
| 6 | 2.62 ± 0.30 a | 2.34 ± 0.18 a | 2.16 ± 0.26 a | 2.22 ± 0.37 a | 2.63 ± 0.21 a |
| 7 | 6.25 ± 1.09 d | 3.62 ± 0.74 b | 1.77 ± 0.56 a | 3.08 ± 1.02 b | 4.95 ± 0.34 bc |
| 8 | 0.57 ± 0.14 ab | 0.51 ± 0.19 ab | 0.37 ± 0.10 a | 0.44 ± 0.23 ab | 0.52 ± 0.05 ab |
| 9 | 2.60 ± 1.05 bc | 2.28 ± 1.42 b | 1.77 ± 0.52 a | 3.06 ± 0.44 c | 2.07 ± 0.58 a |
| 10 | 0.79 ± 0.30 ab | 1.21 ± 0.63 b | 0.25 ± 0.13 a | 0.18 ± 0.12 a | 0.82 ± 0.63 ab |
| 11 | 6.95 ± 1.89 d | 2.16 ± 0.59 b | 3.85 ± 1.11 c | 1.00 ± 0.0 a | 7.59 ± 0.86 d |
| 12 | 0.61 ± 0.36 a | 4.31 ± 1.83 c | 1.64 ± 1.27 b | 1.59 ± 0.98 b | 4.20 ± 0.42 c |
| 13 | 0.95 ± 0.30 a | 2.96 ± 1.80 bc | 1.50 ± 0.96 ab | 1.51 ± 0.39 ab | 3.33 ± 0.52 c |
| 14 | 2.80 ± 0.19 b | 2.10 ± 1.15 b | 1.79 ± 0.65 a | 1.65 ± 1.69 a | 2.23 ± 0.33 b |
| 15 | 3.34 ± 0.32 ab | 8.71 ± 0.44 b | 1.23 ± 0.13 a | 1.26 ± 0.06 a | 2.53 ± 0.22 a |
| 16 | 17.88 ± 4.64 a | 16.88 ± 7.94 a | 104.33 ± 56.89 c | 266.88 ± 35.74 d | 90.77 ± 49.14 b |
| 17 | 121.66 ± 22.38 b | 15.11 ± 4.34 a | 594.77 ± 90.03 e | 262.44 ± 100.55 c | 489.44 ± 67.78 d |
| 18 | 4.14 ± 00.78 ab | 1.30 ± 0.40 a | 6.78 ± 1.54 b | 3.58 ± 1.32 ab | 13.72 ± 1.68 c |
| Variables | CV | |
|---|---|---|
| PLL | 3.3 ± 1.00 | 30.30 |
| PLW | 2.88 ± 0.96 | 33.61 |
| CLL | 5.30 ± 1.28 | 24.16 |
| CLW | 4.30 ± 1.58 | 36.88 |
| PL | 2.06 ± 0.64 | 31.20 |
| STD | 2.39 ± 0.22 | 9.06 |
| POL | 3.93 ± 1.72 | 43.75 |
| POW | 0.48 ± 0.07 | 15.52 |
| POT | 2.36 ± 0.49 | 21.04 |
| PAL | 0.65 ± 0.43 | 65.72 |
| NS/PO | 4.26 ± 2.87 | 67.40 |
| SL | 1.69 ± 1.50 | 88.72 |
| SW | 1.52 ± 1.12 | 74.27 |
| ST | 2.11 ± 0.44 | 21.13 |
| W100S | 3.40 ± 3.09 | 90.72 |
| NPO/PL | 103.04 ± 98.52 | 95.62 |
| TS/PL | 296.67 ± 243.47 | 82.06 |
| TSW/PL | 5.88 ± 4.78 | 81.33 |
| Species | Germination VO | Emergency V1 | Primordial Leaves V2 | Trifoliate First Leaves V3 | Trifoliate Third Leaves V4 |
|---|---|---|---|---|---|
| P. vulgaris | 2 | 2 | 5 | 16 | 23 |
| P. coccineus | 3 | 5 | 10 | 23 | 32 |
| P. leptostachyus | 3 | 2 | 5 | 12 | 23 |
| p. microcarpus | 2 | 3 | 8 | 18 | 27 |
| P. acutifolius | 3 | 4 | 5 | 13 | 22 |
| Species | Pre-Flowering R5 | Flowering R6 | Pod Formation R7 | Pod Filling R8 | Maturation R9 | Number of Days |
|---|---|---|---|---|---|---|
| P. vulgaris | 52 | 58 | 67 | 85 | 121 | 163 |
| P. coccineus | 49 | 61 | 75 | 99 | 136 | 171 |
| P. leptostachyus | 40 | 46 | 54 | 67 | 95 | 133 |
| p. microcarpus | 44 | 53 | 63 | 100 | 128 | 138 |
| P. acutifolius | 35 | 40 | 51 | 74 | 102 | 128 |
| Frequency (cm−1) | Functional Group and Vibration Mode | Seed Coat | Embryo |
|---|---|---|---|
| 1745 | C=O extension (ester carbonyl) | X | X |
| 1641 | C=C extension, C=O extension (amide), N–H folding (amide I) | X | X |
| 1541 | C=C extension (aromatic), N–H folding (amide II), C–N extension. | X | X |
| 1454 | CH3 lipids/proteins y COO− of proteins (amide III) | X | X |
| 1394 | CH3 lipids/proteins y COO− of proteins (amide III) | X | X |
| 1317 | C–N polysaccharides | X | |
| 1240 | C–O extension, in-plain C–H folding (aromatic), aliphatic C–O extension P=O extension (aliphatic) (amide III) | X | X |
| 1148 | C–O extension, C–N extension (aliphatic), in-plain C–H folding (aromatic), aliphatic C–O extension (polysaccharides) | X | X |
| 929 | Vibrations C–O y C–C of polysaccharides | X | |
| 856 | Vibrations C–O y C–C of polysaccharides | X |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Téllez-Mazzocco, D.; Herrera-Arrieta, Y.; Alejandre-Iturbide, G.; Correa-Ramírez, M.M.; Rojas-López, M.; Bermúdez-Torres, K. Morphometric, Phenological, and Nutritional Characterization of Five Wild Bean Species from Durango, Mexico. Diversity 2025, 17, 645. https://doi.org/10.3390/d17090645
Téllez-Mazzocco D, Herrera-Arrieta Y, Alejandre-Iturbide G, Correa-Ramírez MM, Rojas-López M, Bermúdez-Torres K. Morphometric, Phenological, and Nutritional Characterization of Five Wild Bean Species from Durango, Mexico. Diversity. 2025; 17(9):645. https://doi.org/10.3390/d17090645
Chicago/Turabian StyleTéllez-Mazzocco, Denisse, Yolanda Herrera-Arrieta, Gabriel Alejandre-Iturbide, Miguel Mauricio Correa-Ramírez, Marlon Rojas-López, and Kalina Bermúdez-Torres. 2025. "Morphometric, Phenological, and Nutritional Characterization of Five Wild Bean Species from Durango, Mexico" Diversity 17, no. 9: 645. https://doi.org/10.3390/d17090645
APA StyleTéllez-Mazzocco, D., Herrera-Arrieta, Y., Alejandre-Iturbide, G., Correa-Ramírez, M. M., Rojas-López, M., & Bermúdez-Torres, K. (2025). Morphometric, Phenological, and Nutritional Characterization of Five Wild Bean Species from Durango, Mexico. Diversity, 17(9), 645. https://doi.org/10.3390/d17090645








