3.1. Taxonomy
Isoperla myersi Szczytko & Kondratieff, 2015
Paddle stripetail
Isoperla myersi [
4] (p. 162)—holotype (USNM, USNMENT 01882902, examined), USA, New York, Ulster Co., Big Indian Hollow—new species description, adult key
Isoperla holochlora: [
26] (p. 311)—larval description, adult illustrations
Isoperla holochlora: [
27] (p. 199)—larval key
Isoperla myersi: [
28] (p. 6)—color illustrations
Isoperla powhatan: [
4] (p. 199)—holotype (USNM, USNMENT 01882907, examined), USA, Virginia, Prince William County, Catharpin Creek—new species description,
syn. nov.
Isoperla powhatan: [
28] (p. 84)—color illustrations
Isoperla holochlora—light form: [
29] (p. 19)—larval description
Isoperla powhatan: [
29] (p. 31)—larval description
Isoperla powhatan: [
5] (p. 164)—new state record (North Carolina)
Isoperla powhatan: [
10] (p. 24)—new state record (Kentucky)
Isoperla powhatan: [
30] (p. 449)—watershed record (North Carolina)
Isoperla myersi: [
31] (p. 46)—geographic atlas (New York)
Isoperla myersi: [
32]—electronic catalogue
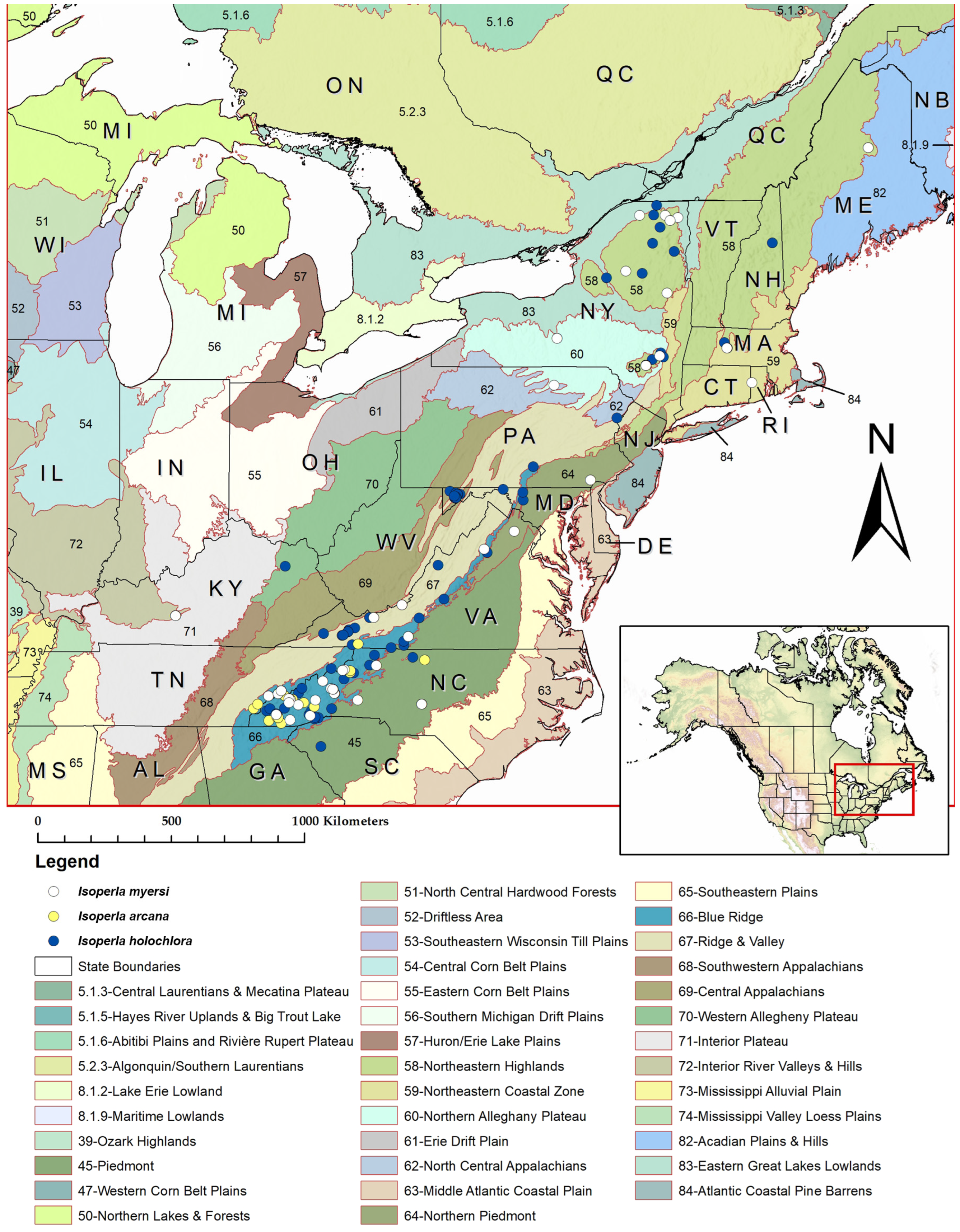
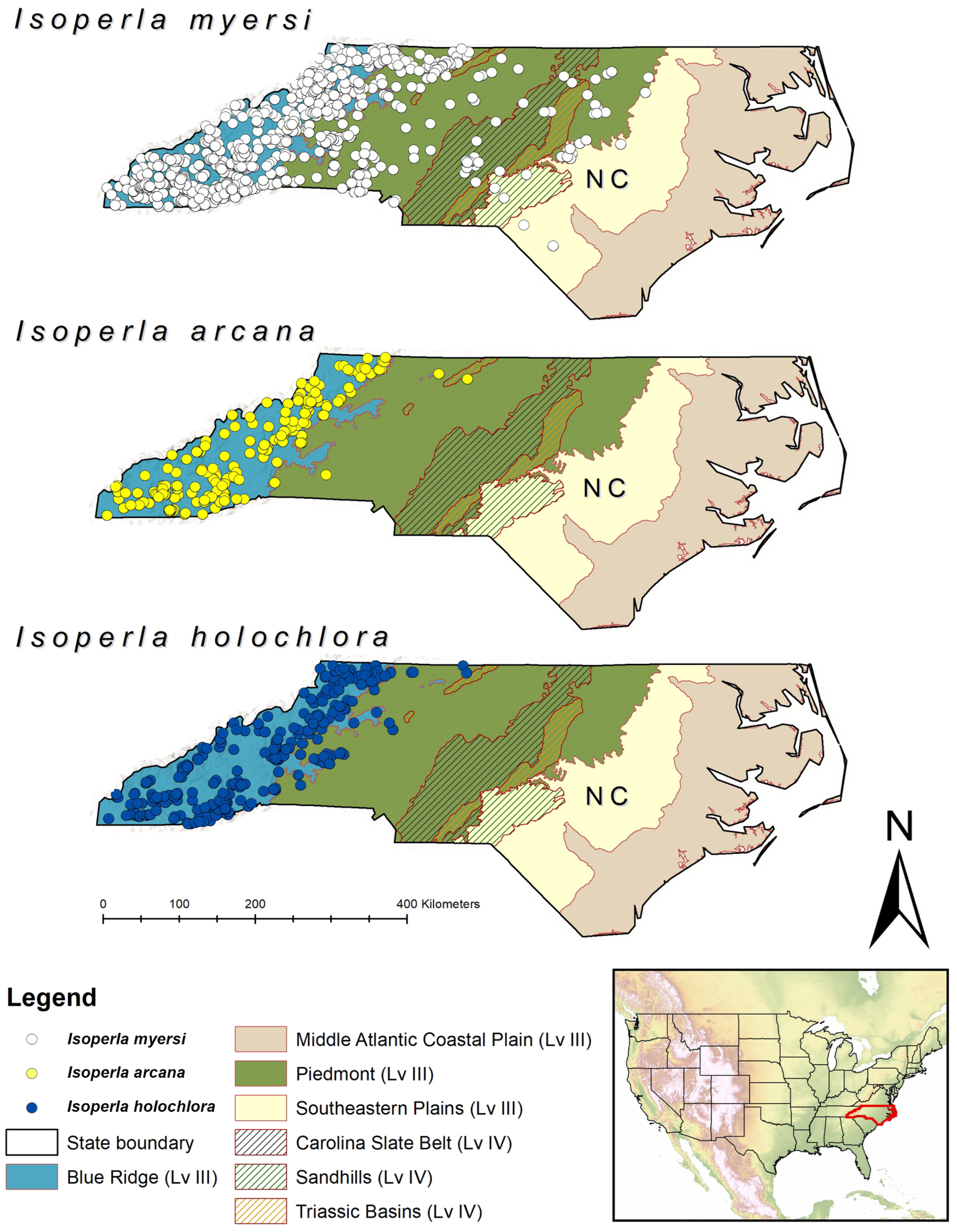
Distribution. USA: Kentucky, Maine, Massachusetts, New York, North Carolina, Pennsylvania, Rhode Island, Tennessee, Virginia (
Figure 1)
Material examined. USA,
Kentucky,
Edmonson Co., Mill Branch, Mill Branch Trail, Mammoth Cave National Park, 37.21548, −86.13983, 21 May 2019, 1 male, T.C. McRoberts (WKUC); same location but 24 May 2019, 1 male, 1 female, S.A. Grubbs (WKUC); same location but 28 May 2019, UV light, 36 males, 14 females, S.A. Grubbs, T.C. McRoberts (WKUC).
Maine,
Piscataquis Co., Roaring Brook, Mount Katahdin, 45.93422, −68.86088, 26 August 1939, 4 males, T.H Frison, T.H. Frison Jr. (INHS Insect Collection 876015); same data, but 6 females (INHS Insect Collection 876018).
Massachusetts,
Hampshire Co., Cadwell Creek, 1.7 km SSE Knights Corner, 42.34752, −72.38308, 232 m asl, Site 2, em. trap W3, 30 June 2009, 1 male, 1 female (INHS Insect Collection 1545084); same location and date, but 1 male (INHS Insect Collection 1545095); tributary Cadwell Creek, 1.9 km SSE Knights Corner, 42.34537, −72.38226, 244 m asl, Site 1, em. trap R2, 1 July 2009, 1 male, A.H. Roy (INHS Insect Collection 1545083).
New York,
Clinton Co., Bradley Brook, crossing Rt. 374, Dannemora, 44.74850, −73.92599, 29 April 2024 (emerged 23 June 2024), 1 male, 1 exuvia, L. Myers (LCRI 063); small stream, jct. Rt. 3 and Akey Rd., Cadyville, black light trap, 44.70710, −73.60910, 11 July 2019, 1 male, L. Micheels (LCRI 358); tributary to True Brook, Clark Hill Rd., Saranac, 44.65580, −73.80220, Malaise trap, 27 May—4 June 2012, 1 male, T. Mihuc (LCRI 359–360);
Franklin Co., Alburg Brook, CR-5, S. Dickinson, 44.74667 −74.56422, 29 April 2024 (emerged 20 May 2024), 1 male, 1 female, 2 exuvia, L. Myers (LCRI 361–363);
Herkmer Co., Bald Mountain Brook, Rondaxe Rd., 43.75175, −74.91208, 15 August 2023, 1 male, L. Myers (LCRI 364);
Tompkins Co., tributary to Taughannock Creek, Taughannock State Park, 42.52874, −76.66258, PNH-2023-211, 11 July 2023, 1 male, P.N. Hogan (INHS Insect Collection 1523737); same locality data, PNH-2024-250, 4 July 2024, 4 males, 2 females, P.N. Hogan (PNHC);
Ulster Co., [tributary Esopus Creek], Big Indian Hollow, Oliverea Road, 42.02830, −74.40890, 27 May 2009, 1 male, L. Myers, B.C. Kondratieff (holotype; USNMENT 01882902); tributary Esopus Creek, Co. Rd. 47, 42.02802, −74.40835, 7 June 2024, 8 larvae, C. Verdone (NCDWR); same but 23 June 2024, 7 males, 3 females, 4 larvae, C. Verdone (NCDWR).
North Carolina,
Avery Co., Cranberry Creek, US 19E, 36.12471, −81.97538, 17 June 2020 (emerged 20 June 2024), 1 male, 1 exuvium, C. Verdone, V. Holland, E. Fleek (NCDWR); Blevins Creek, SR 1361, 36.13817, −81.92942, 17 June 2020, 1 male, C. Verdone, V. Holland, E. Fleek (NCDWR);
Buncombe Co., Bill Moore Creek, SR 3439, 35.521111, −82.656944, 14 May 2002, 8 larvae, D. Lenat (NCDWR);
Cleveland Co., Bald Knob Creek, SR 1627, 35.523416, −81.59971, 29 April 2019, 1 male, 1 exuvium, C. Verdone, S. Beaty, L. Housely (NCDWR);
Jackson Co., Balsam, 35.42667, −83.08528, 24 March 1938, 4 larvae, H.H. Ross, B.D. Burks (INHS Plecoptera 3182);
Macon Co., Cullasaja River, Peeks Creek Rd., 35.12152, −83.28492, 29 May 2019, 6 males, 4 females, 2 exuviae, C. Verdone, S. Beaty, V. Holland (NCDWR); Whiteoak Creek, SR 1310, 35.22781, −83.62773, 25 July 2019, 17 males, 27 females, 10 exuviae, 16 larvae, C. Verdone, S. Beaty, E. Fleek (NCDWR);
McDowell Co., Curtis Creek, SR 1227 fishing access, 35.65570, −82.17877, 8 May 2019, 2 larvae, C. Verdone, S. Beaty, E. Fleek (NCDWR);
Montgomery Co., Barnes Creek, Ophir Rd., 35.43861, −79.99888, 2 May 2013, 1 female, 1 exuvium, D. Lenat, S. Beaty (NCDWR); same but 7 May 2014 (emerged 19 May 2014) 1 male, 1 exuvium, D. Lenat (NCDWR); same but 2 May 21 May 2019, 2015, 3 males, 1 female, 4 exuviae, S. Beaty, D. Lenat (NCDWR);
Swain Co., Cherokee, 35.47417, −83.31500, 26 June 1938, 1 male, W.C. Stehr (INHS Plecoptera 3173); Mingo Creek, off Big Cove Rd, below falls, 35.53409, −83.27641, 13 May 2021, 2 males, 2 females, C. Verdone, R. Tetreault (NCDWR); Oconoluftee River, Smokemont Campground, Great Smoky Mountains National Park, 35.55298, −83.30956, 28 May 1934, 5 larvae, T.H. Frison, H.H. Ross (INHS Insect Collection 876025);
Transylvania Co., Catheys Creek, SR 1338, 35.20750, −82.78056, 21 May 1987, 11 larvae, D. Penrose, D. Lenat (NCDWR);
Wilkes Co., tributary Mulberry Creek, SR 1002, 36.21900, −81.13600, 5 May 2015, 1 larva, S. Beaty, M. Walters, E. Fleek (NCDWR);
Yancey Co., Bald Creek, SR 1399, 35.74298, −82.21127, 25 May 2004, 7 larvae, W. Crouch, E. Fleek, B. Prusha (NCDWR). Big Lost Cove Creek, FS 472, Pisgah National Forest, 35.74298, −82.21127, 29 August 2019, 1 female, M.L. Metzger, G.J. Kratina (WKUC); Neals Creek, FS 472, Pisgah National Forest, 35.74176, −82.21492, 29 August 2019, 1 female, M.L. Metzger, G.J. Kratina (WKUC); Lower Creek, Buncombe Trail, Pisgah National Forest, 35.75564, −82.26803, 30 August 2019, 1 female, M.L. Metzger, G.J. Kratina (WKUC).
Pennsylvania,
Bradford Co., Holcomb Falls, Leroy Mt. Rd., 41.66074, −76.70036, 23 June 2025, 2 males, 1 female, R.E. DeWalt (INHS Insect Collection 1545135).
Rhode Island,
Kent Co., Moosup River, Rte. 14, 2 km NW Greene, Nicholas Farm Wildlife Management Area, 41.70713, −71.76158, 30 June 2006, 1 male, S.A. Grubbs (WKUC).
Tennessee,
Blount Co., Cades Cove, Great Smoky Mountains National Park, 35.60806, −83.82639, 13 June 1940, 1 female, 1 exuvium, T.H. Frison, C.O. Mohr, A.W. Hawkins (INHS Plecoptera 3187); same data, but 1 female, 1 exuvium (INHS Plecoptera 3188); same data, but 62 larvae, 1 exuvium (INHS Insect Collection 876026);
Sevier Co., Elkmont, Great Smoky Mountains National Park, 35.65361, −83.58056, 12 May 1935, 3 females, H.H. Ross (INHS Insect Collection 876024); same data, but 12 June 1935, 3 males (INHS Plecoptera 3168); Gatlinburg, 35.71420, −83.51220, 19 June 1940, 1 male, 1 exuvium, T.H. Frison, C.O. Mohr, A.W. Hawkins (INHS Insect Collection 877157); same data, but 1 female, 1 exuvium (INHS Insect Collection 876027); same data, but 25 June 1940, 2 females, 2 exuviae (INHS Insect Collection 875997); LeConte Creek, Gatlinburg, 35.71417, −83.51028, 13 June 1938, 4 larvae, 1 exuvium, T.H. Frison, T.H. Frison Jr. (INHS Plecoptera 3201); same data, but 16 June 1938, 2 larvae (INHS Plecoptera 3198); same location, but 14 May 1939, 8 larvae, T.H. Frison, H.H. Ross (INHS Plecoptera 3204) Little Pigeon River, Gatlinburg, 35.71417, −83.51028, 12 June 1935, 1 female, 1 exuvium, H.H. Ross (INHS Insect Collection 876038); same location, but 14 June 1940, 5 larvae, T.H. Frison (INHS Plecoptera 3190); Little River; Elkmont, 35.654447, −83.579716, 17 June 1938, 10 larvae, 1 exuvium, T.H. Frison; T.H. Frison Jr. (INHS Plecoptera 3192); same data, but 1 larva (INHS Plecoptera 3193).
Virginia,
Bland Co., Wolf Creek, Rte. 614 picnic area, 37.18026, −81.19496, 3 May 2019 (emerged 30 May 2019), 1 male, C. Verdone, D. Fuller, S. Beaty (NCDWR);
Giles Co., White Rocks Branch, White Rocks Campground, 37.43106, −80.49279, 4 May 2019, 1 male, 1 exuvium, 1 larva, S. Beaty, C. Verdone, D. Fuller (NCDWR); same but 16 May 2022, 2 males, S. Beaty, C. Verdone, V. Holland (NCDWR); same but 17 May 2022, 1 male, 1 female, 2 exuviae, S. Beaty, C. Verdone, V. Holland (NCDWR);
Madison Co., Big Meadows, Shenandoah National Park, 38.52639, −78.44000, 20 March 1938, 5 larvae, H.H. Ross, B.D. Burks (INHS Plecoptera 3177);
Patrick Co., Little Rock Castle Creek, footbridge to trail, ca. 55 m upstream confluence Rock Castle Creek, 36.80763, −80.33106, 19 July 2007, J.L. Robinson, C.R. Parker (BLRI 23184);
Prince William Co., Catharpin Creek, Jackson Hollow Campground area, Bull Run Mountain, 38.87813, −77.68918, 28 June–13 July 2012, Malaise trap, 1 male, D.R. Smith (holotype; USNMENT 01882907).
Figure 1.
Distribution of the
Isoperla holochlora group species overlaid on USEPA Level III Ecoregions. Records include examined material, three literature sources [
4,
30,
33], and BOLD specimen data included in the mtCOI molecular phylogenetic analyses. Canada: NB = New Brunswick; ON = Ontario; QC = Quebec; USA: AL = Alabama; CT = Connecticut; DE = Delaware; GA = Georgia; IL = Illinois; IN = Indiana; KY = Kentucky; MA = Massachusetts; MD = Maryland; ME = Maine; MI = Michigan; MS = Mississippi; NC = North Carolina; NH = New Hampshire; NJ = New Jersey; NY = New York; OH = Ohio; RI = Rhode Island; SC = South Carolina; TN = Tennessee; VA = Virginia; VT = Vermont; WI = Wisconsin; WV = West Virginia.
Figure 1.
Distribution of the
Isoperla holochlora group species overlaid on USEPA Level III Ecoregions. Records include examined material, three literature sources [
4,
30,
33], and BOLD specimen data included in the mtCOI molecular phylogenetic analyses. Canada: NB = New Brunswick; ON = Ontario; QC = Quebec; USA: AL = Alabama; CT = Connecticut; DE = Delaware; GA = Georgia; IL = Illinois; IN = Indiana; KY = Kentucky; MA = Massachusetts; MD = Maryland; ME = Maine; MI = Michigan; MS = Mississippi; NC = North Carolina; NH = New Hampshire; NJ = New Jersey; NY = New York; OH = Ohio; RI = Rhode Island; SC = South Carolina; TN = Tennessee; VA = Virginia; VT = Vermont; WI = Wisconsin; WV = West Virginia.
Figure 2.
Isoperla myersi, tributary Esopus Creek, Ulster Co., New York, USA, adult male habitus, dorsal.
Figure 2.
Isoperla myersi, tributary Esopus Creek, Ulster Co., New York, USA, adult male habitus, dorsal.
Amended descriptions—adult male, adult female, and egg. These updated descriptions are purposely brief so as to not duplicate the 2015 revision [
4] and instead focus on notable variation. The descriptions and associated images provided herein should be used in conjunction with Szczytko and Kondratieff [4, their figures 32.1–32.6 under
I. myersi and 42.1–42.12 under
I. powhatan; 28, their figures 31.1–31.5 under
I. myersi and 40.1–40.9 under
I. powhatan].
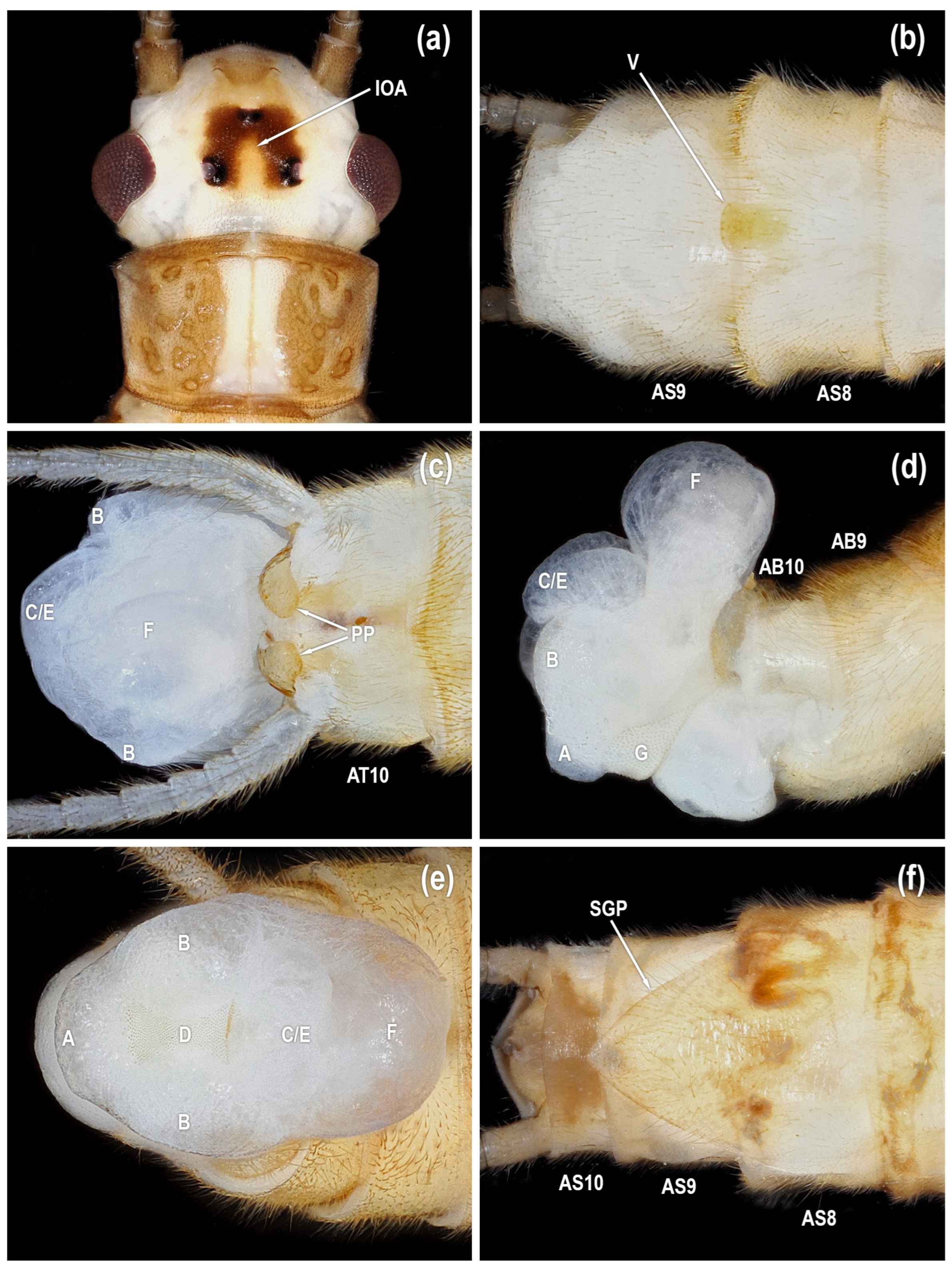
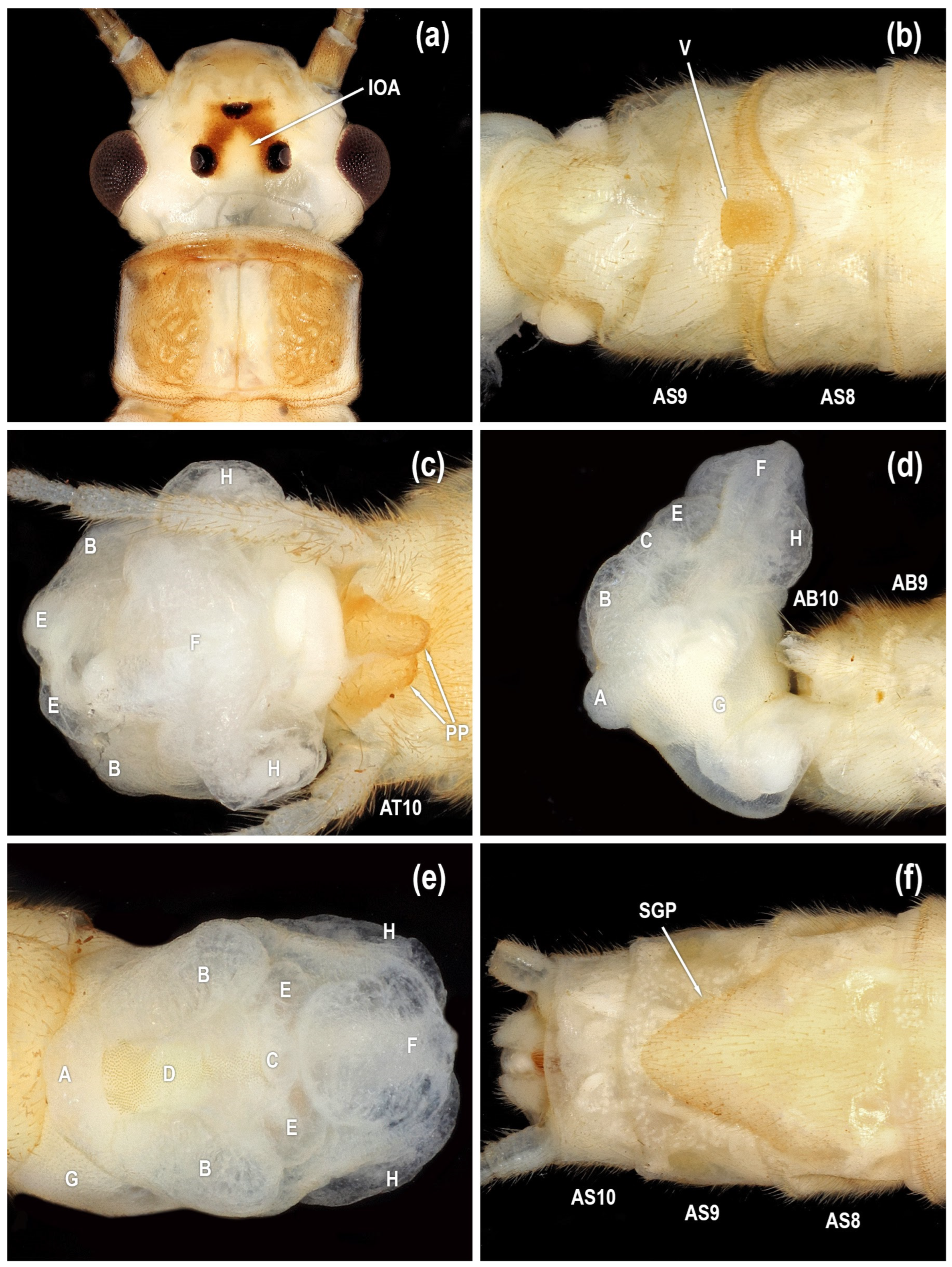
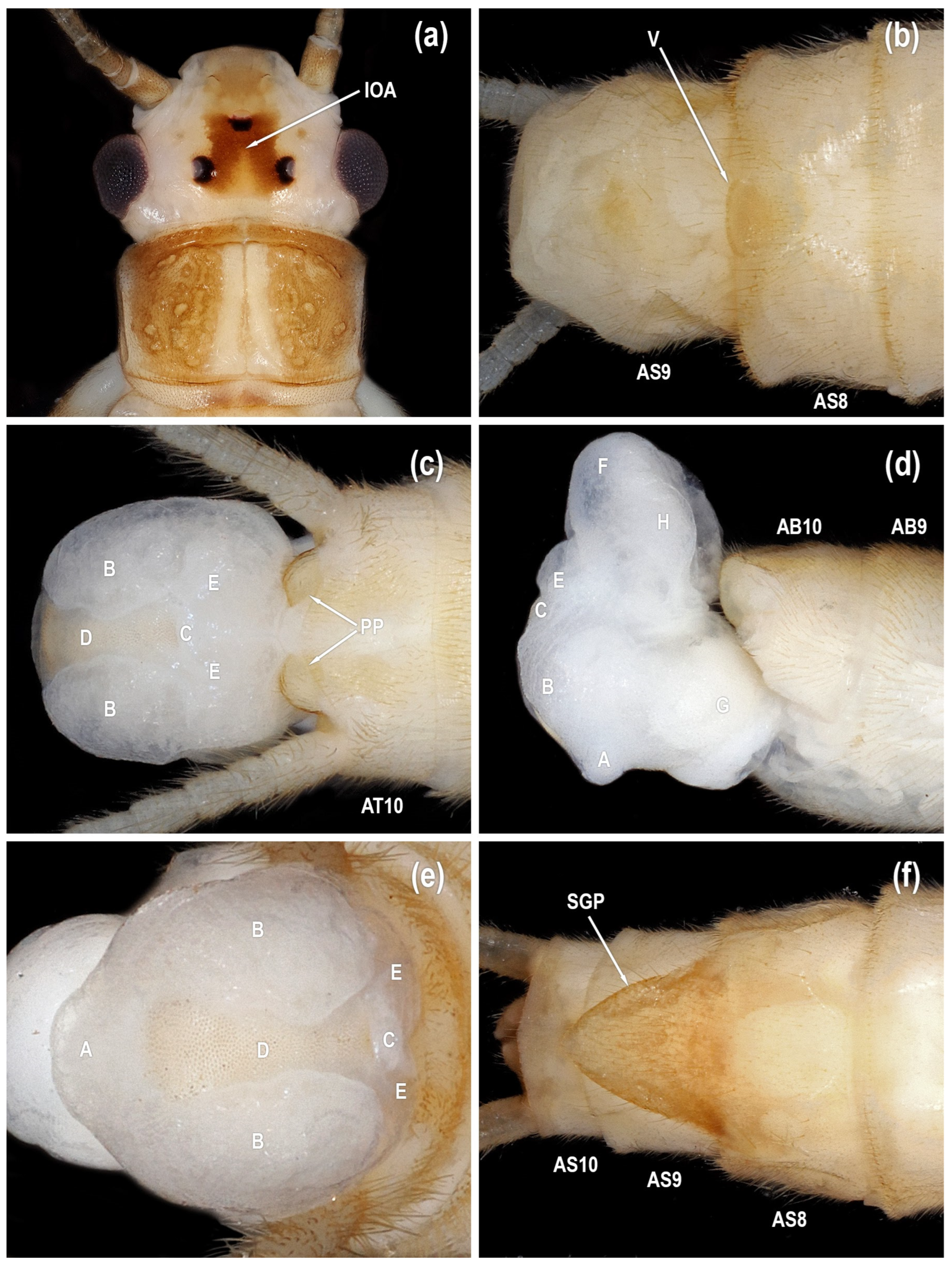
Male. Head pattern variable; interocellar area pale with broad dark stripes connecting median ocellus with lateral ocelli (IOA in
Figure 2,
Figure 3a,
Figure 4a and
Figure 6a); or interocellar area nearly completely dark (IOA in
Figure 7a).
Tibial callus usually darkened (TC in
Figure 10d). Mid-dorsal abdominal stripe usually absent. Vesicle rounded posteriorly, barely exceeding posterior margin of sternum 8 (V in
Figure 3b,
Figure 4b,
Figure 5b,
Figure 6b and
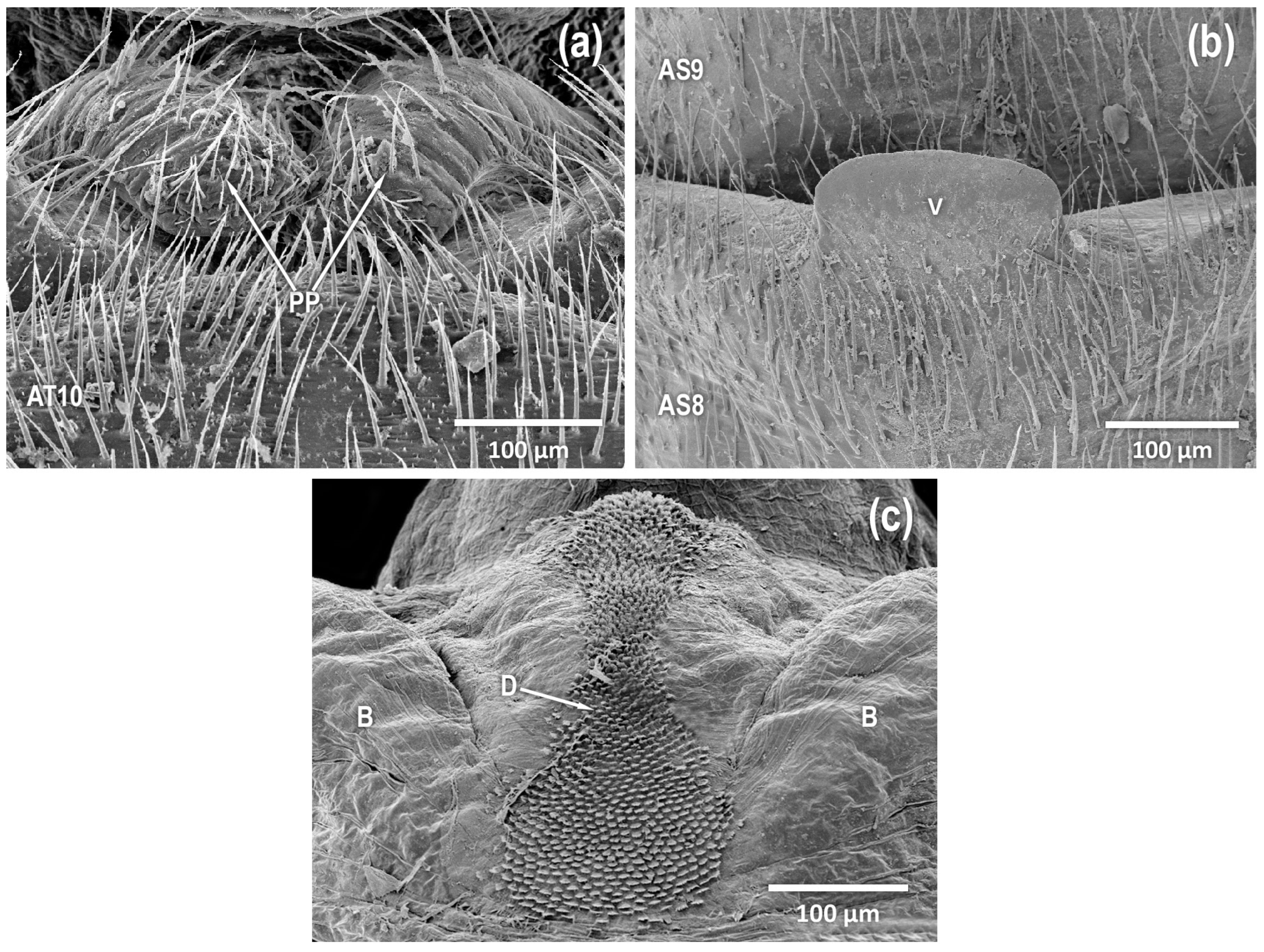
Figure 7b). Paraproct dorsoventrally flattened with broadly rounded apex, with symmetrical proximal and distal margins (PP in
Figure 3c,
Figure 4c,
Figure 5a,
Figure 6c and
Figure 7c). Aedeagus membranous with a posteroventral lobe (A in
Figure 3d,e,
Figure 4d,e,
Figure 6d,e and
Figure 7d,e), paired posterolateral lobes (B in
Figure 3c–e,
Figure 4c–e,
Figure 5c,
Figure 6c–e and
Figure 7c–e), posteromesal lobe (C in
Figure 4d,e and
Figure 6c–e), paired submedial posteromesal lobes (E in
Figure 4c–e and
Figure 6c–e), which may be contiguous with posteromesal lobe (C, E in
Figure 3c–e and
Figure 7c–e), recessed posteromesal spine patch (D in
Figure 3e,
Figure 4e,
Figure 5c,
Figure 6c,e and
Figure 7e), large dorsal lobe (F in
Figure 3c–e,
Figure 4c–e,
Figure 6d and
Figure 7c,d), pair of large anteromedian lobes (H in
Figure 4c–e and
Figure 6d), and a wide mesal band of stout golden brown spinulae (G in
Figure 3d,
Figure 4d,
Figure 6d and
Figure 7d).
Female. General body color and external morphology similar to male. Subgenital plate triangular, lateral margins evenly convex from base to apex, length 0.75–0.90X width (SGP in
Figure 3f,
Figure 4f,
Figure 6f and
Figure 7f). Free portion of subgenital plate not exceeding posterior margin of sternum 9; or produced to the posterior margin of sternum 10.
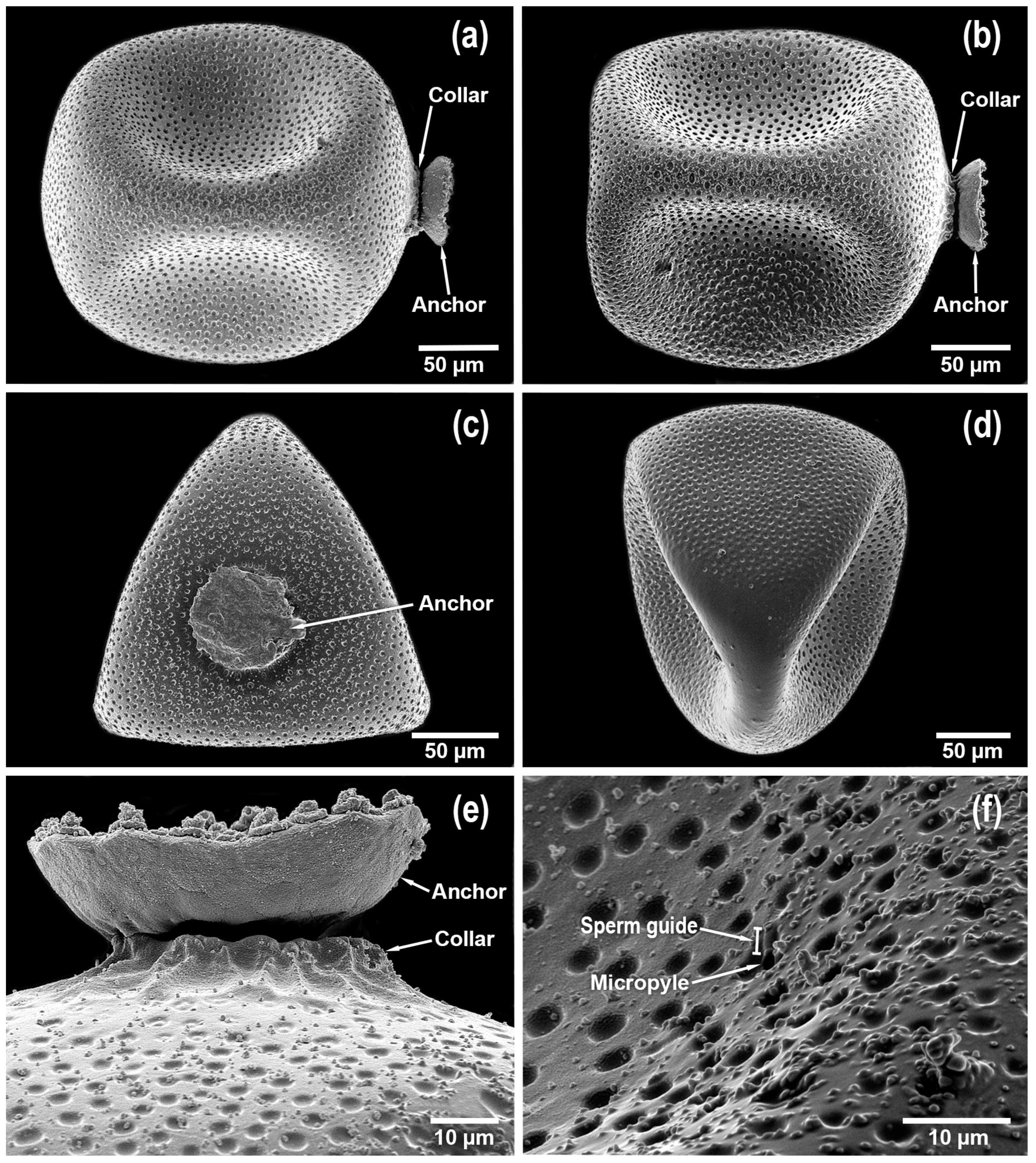
Egg. Color cream, nearly transparent. General shape subcircular (
Figure 8a), but some eggs are subtruncate anteriorly (
Figure 8b); distinctly triangular in cross-section (
Figure 8c,d). Chorionic surface covered with numerous shallow pits (
Figure 8a–f); eclosion line absent. Eggs of Midwestern
I. myersi are similar to those of Northeastern
I. myersi [under
I. powhatan in 4, their figures 42.9–42.12], namely in color, size, shape, and chorionic characteristics. There is one notable difference. The collar of midwestern
I. myersi is scarcely raised above the chorionic surface (
Figure 8a,b,e), whereas the collar of northeastern
I. myersi is well developed and flanged laterally [4, their figures 42.9–42.10]. In addition, there is an inverted disc-shaped anchor present on most eggs of midwestern
I. myersi (
Figure 8a–c,e) that is not present on the scanned egg presented in Szczytko and Kondratieff [4, their figures 42.9–42.10]. Micropyles located near the anterior 1/3, arranged singularly, sperm guides tunnel-shaped and visible (
Figure 8f).
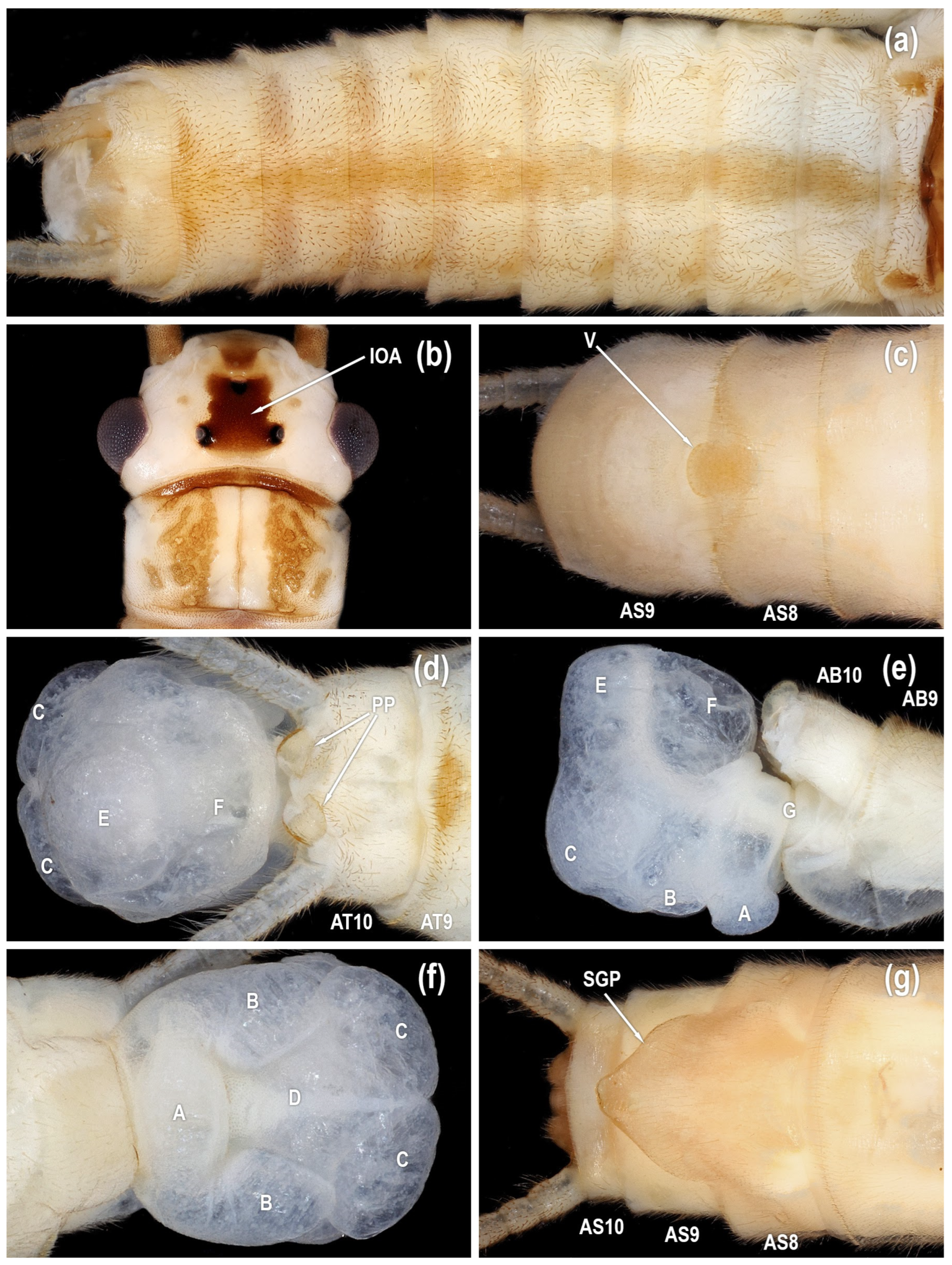
Figure 3.
Isoperla myersi, tributary Esopus Creek, Ulster Co., New York, USA, adults. (a) male head, dorsal. (b) male sterna, ventral. (c) male terminalia, dorsal. (d) male terminalia, lateral. (e) male terminalia, caudal. (f) female sterna, ventral [posteroventral lobe (A), abdominal segment (AB), abdominal sterna (AS), abdominal tergum (AT), paired posterolateral lobes (B), posteromesal lobe (C), recessed posteromesal spine patch (D), paired submedial posteromesal lobes (E), dorsal lobe (F), mesal band of spinulae (G), interocellar area (IOA), paraproct (PP), subgenital plate (SGP), vesicle (V)].
Figure 3.
Isoperla myersi, tributary Esopus Creek, Ulster Co., New York, USA, adults. (a) male head, dorsal. (b) male sterna, ventral. (c) male terminalia, dorsal. (d) male terminalia, lateral. (e) male terminalia, caudal. (f) female sterna, ventral [posteroventral lobe (A), abdominal segment (AB), abdominal sterna (AS), abdominal tergum (AT), paired posterolateral lobes (B), posteromesal lobe (C), recessed posteromesal spine patch (D), paired submedial posteromesal lobes (E), dorsal lobe (F), mesal band of spinulae (G), interocellar area (IOA), paraproct (PP), subgenital plate (SGP), vesicle (V)].
Figure 4.
Isoperla myersi, Mill Branch, Edmonson Co., Kentucky, USA, adults. (a) male head, dorsal. (b) male sterna, ventral. (c) male terminalia, dorsal. (d) male terminalia, lateral. (e) male terminalia, ventral. (f) female sterna, ventral [posteroventral lobe (A), abdominal segment (AB), abdominal sterna (AS), abdominal tergum (AT), paired posterolateral lobes (B), posteromesal lobe (C), recessed posteromesal spine patch (D), paired submedial posteromesal lobes (E), dorsal lobe (F), mesal band of spinulae (G), paired anteromedian lobes (H), interocellar area (IOA), paraproct (PP), subgenital plate (SGP), vesicle (V)].
Figure 4.
Isoperla myersi, Mill Branch, Edmonson Co., Kentucky, USA, adults. (a) male head, dorsal. (b) male sterna, ventral. (c) male terminalia, dorsal. (d) male terminalia, lateral. (e) male terminalia, ventral. (f) female sterna, ventral [posteroventral lobe (A), abdominal segment (AB), abdominal sterna (AS), abdominal tergum (AT), paired posterolateral lobes (B), posteromesal lobe (C), recessed posteromesal spine patch (D), paired submedial posteromesal lobes (E), dorsal lobe (F), mesal band of spinulae (G), paired anteromedian lobes (H), interocellar area (IOA), paraproct (PP), subgenital plate (SGP), vesicle (V)].
Figure 5.
Isoperla myersi, Mill Branch, Edmonson Co., Kentucky, USA, adult male. (a) paraprocts, dorsal. (b) vesicle, ventral. (c) aedeagus, posterior [abdominal sterna (AS), abdominal tergum (AT), paired posterolateral lobes (B), recessed posteromesal spine patch (D), paraproct (PP), vesicle (V)].
Figure 5.
Isoperla myersi, Mill Branch, Edmonson Co., Kentucky, USA, adult male. (a) paraprocts, dorsal. (b) vesicle, ventral. (c) aedeagus, posterior [abdominal sterna (AS), abdominal tergum (AT), paired posterolateral lobes (B), recessed posteromesal spine patch (D), paraproct (PP), vesicle (V)].
New description—last instar larva. 6.1–8.8 mm (
n = 15). Body small and slender with contrasting body pattern (
Figure 9a and
Figure 10a). Brown clothing hairs present on most of body.
Dorsum of head (
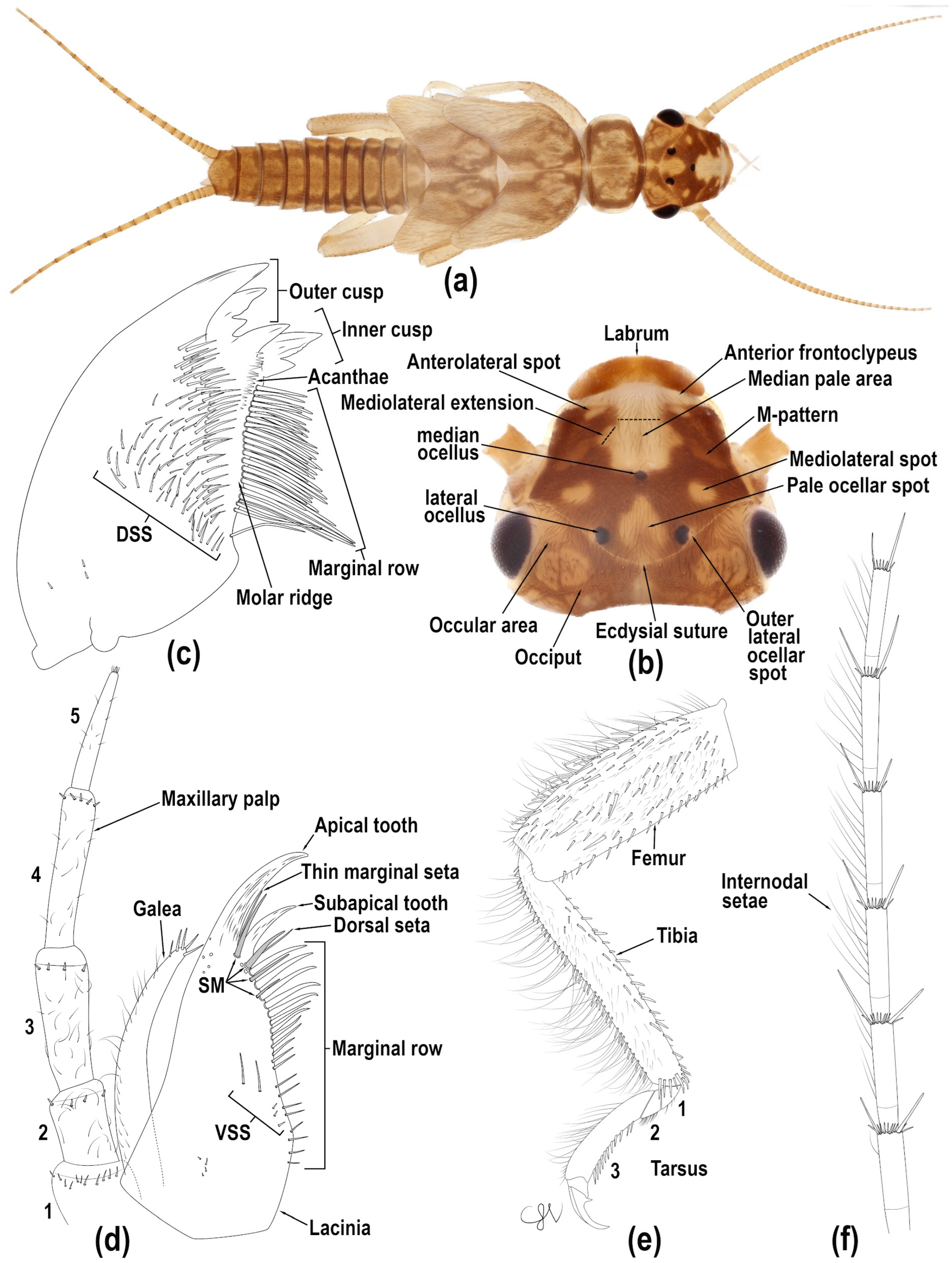
Figure 9b). Ground color pale yellow with brown mask and brown clothing hairs. Labrum pale medially, brown laterally. Antennal scape and pedicel light brown. Flagellum with 35–38 antennomeres (n = 4), light brown proximally and brown distally. Anterior frontoclypeus pale. Frontoclypeus brown laterally and pale medially with two, often enclosed, pale, oval anterolateral spots. Median pale area about 1/3 head width and extends from the anterior frontoclypeus to frons, touching the anterior ocellus; broadly open anteriorly, with truncate, anterolaterally directed, mediolateral extensions (1/2 or less median pale area anterior width) that intersect the pale M-pattern. Lateral arms of M-pattern faint, extending posterolaterally from median pale area of frons. A pair of enclosed oval mediolateral pale spots anterior to lateral epicranial suture arms. Interocellar area with an irregular oval pale ocellar spot, usually confluent with epicranial suture and typically not touching ocelli. Outer lateral ocellar pale area variously developed. Ocular area pale anteriorly and mottled with brown pigment posteromedially. Occiput brown posterior to epicranial suture and junction of the epicranial stem and suture; pale area over posterior extent of epicranial stem. Occiput with socketed setae mostly along brown mottled areas.
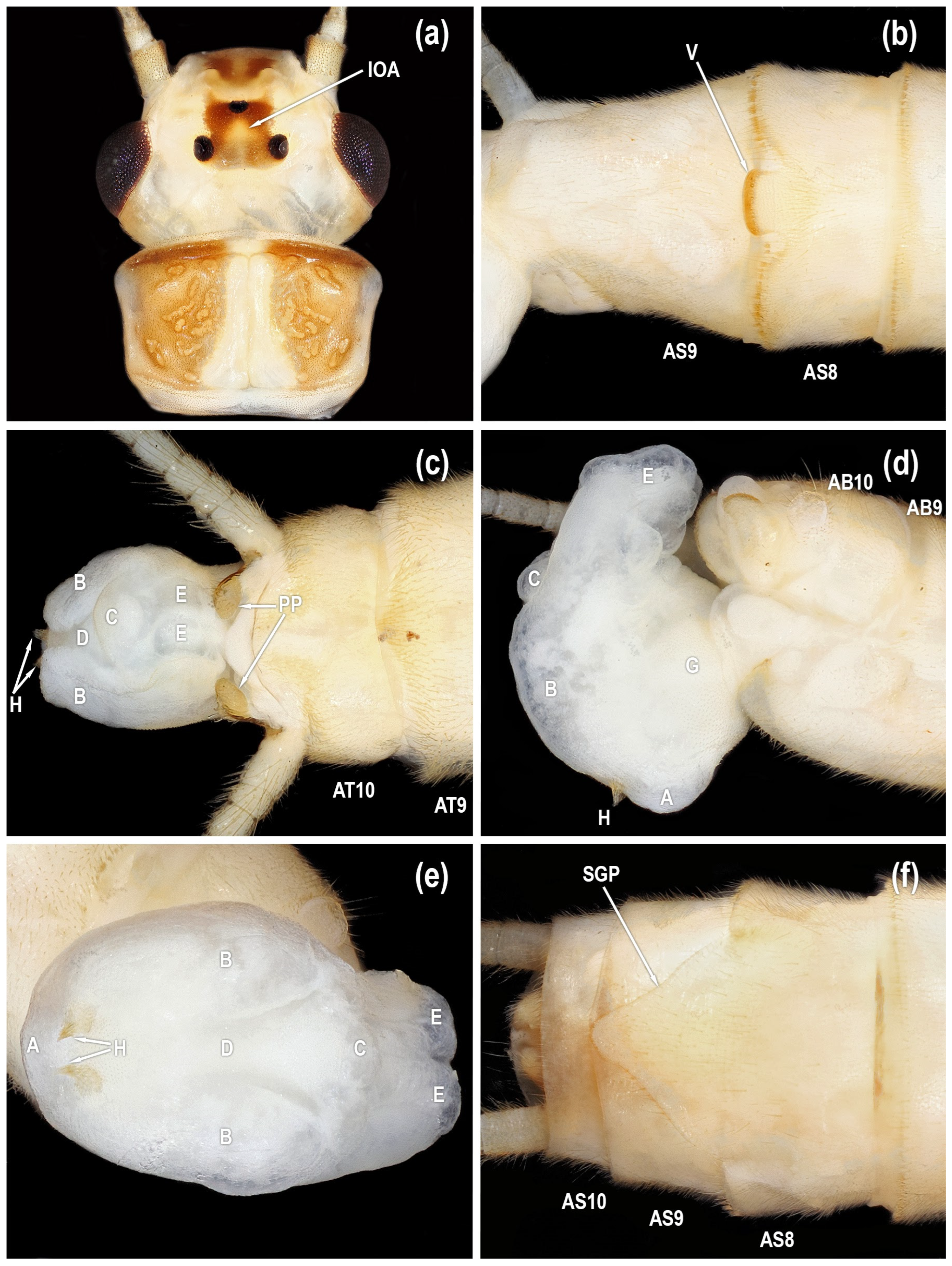
Figure 6.
Isoperla myersi, White Oak Creek, Macon Co., North Carolina, USA, adults. (a) male head, dorsal. (b) male sterna, ventral. (c) male terminalia, dorsal. (d) male terminalia, lateral. (e) male terminalia, caudal. (f) female sterna, ventral [posteroventral lobe (A), abdominal segment (AB), abdominal sterna (AS), abdominal tergum (AT), paired posterolateral lobes (B), posteromesal lobe (C), recessed posteromesal spine patch (D), paired submedial posteromesal lobes (E), dorsal lobe (F), mesal band of spinulae (G), paired anteromedian lobes (H), interocellar area (IOA), paraproct (PP), subgenital plate (SGP), vesicle (V)].
Figure 6.
Isoperla myersi, White Oak Creek, Macon Co., North Carolina, USA, adults. (a) male head, dorsal. (b) male sterna, ventral. (c) male terminalia, dorsal. (d) male terminalia, lateral. (e) male terminalia, caudal. (f) female sterna, ventral [posteroventral lobe (A), abdominal segment (AB), abdominal sterna (AS), abdominal tergum (AT), paired posterolateral lobes (B), posteromesal lobe (C), recessed posteromesal spine patch (D), paired submedial posteromesal lobes (E), dorsal lobe (F), mesal band of spinulae (G), paired anteromedian lobes (H), interocellar area (IOA), paraproct (PP), subgenital plate (SGP), vesicle (V)].
Figure 7.
Isoperla myersi, (a–e). Wolf Creek, Bland Co., Virginia, USA, adults. (a) male head, dorsal. (b) male sterna, ventral. (c) male terminalia, dorsal. (d) male terminalia, lateral. (e) male terminalia, dorsal. (f) White Rock Branch, Giles Co., Virginia, female sterna, ventral [posteroventral lobe (A), abdominal segment (AB), abdominal sterna (AS), abdominal tergum (AT), paired posterolateral lobes (B), posteromesal lobe (C), recessed posteromesal spine patch (D), paired submedial posteromesal lobes (E), dorsal lobe (F), mesal band of spinulae (G), interocellar area (IOA), paraproct (PP), subgenital plate (SGP), vesicle (V)].
Figure 7.
Isoperla myersi, (a–e). Wolf Creek, Bland Co., Virginia, USA, adults. (a) male head, dorsal. (b) male sterna, ventral. (c) male terminalia, dorsal. (d) male terminalia, lateral. (e) male terminalia, dorsal. (f) White Rock Branch, Giles Co., Virginia, female sterna, ventral [posteroventral lobe (A), abdominal segment (AB), abdominal sterna (AS), abdominal tergum (AT), paired posterolateral lobes (B), posteromesal lobe (C), recessed posteromesal spine patch (D), paired submedial posteromesal lobes (E), dorsal lobe (F), mesal band of spinulae (G), interocellar area (IOA), paraproct (PP), subgenital plate (SGP), vesicle (V)].
Figure 8.
Isoperla myersi, Mill Branch, Edmonson Co., Kentucky, USA, egg. (a) habitus. (b) habitus. (c) posterior pole. (d) anterior pole. (e) collar and anchor. (f) chorion, micropyle, and sperm guide.
Figure 8.
Isoperla myersi, Mill Branch, Edmonson Co., Kentucky, USA, egg. (a) habitus. (b) habitus. (c) posterior pole. (d) anterior pole. (e) collar and anchor. (f) chorion, micropyle, and sperm guide.
Maxilla (
Figure 9d). Lacinia bidentate, inner margin sinuous. Long thin marginal seta between apical and subapical tooth present, and often a shorter seta present at base of subapical tooth. Inner margin with 9–12 stout, socketed marginal setae below subapical tooth, setae progressively thinner and shorter towards base. Inner margin below stout marginal setae with 8–10 thin marginal setae. Submarginal row of four stout, socketed setae below apical tooth. Middle of ventral palm face with two long socketed setae. Ventral face of palm adjacent to thin marginal setae with 3–4 short, socketed setae. Middle of ventral face with 3–4 short, socketed setae near basal 1/4. Length of striated apical tooth of lacinia approximately 0.4× palm length and 0.75× basal palm width. Subapical tooth approximately 0.6× length of the apical tooth. Galea approximately 0.8× lacinial palm length, with a sparse outer row of thin setae and tipped with 3–5 long apical spines. Maxillary palp approximately 1.4× length of lacinia. Segments 1–4 successively longer, segment five slightly shorter than segment three. Palp segments 1–4 with transverse row of spinous setae at apex. Segment five tipped with one sensilla and four thin setae.
Mandibles. Right mandible (
Figure 9c) bicuspid, outer cusp with three teeth, apical tooth largest, basal tooth smallest, tooth serrations present on inner margin of apical tooth. Ventral face of mandible with an unorganized patch of socketed setae extending from near inner basal margin to base of the outer apical tooth. Inner cusp with three teeth, middle tooth longest and largest; row 15–16 of small sharply pointed acanthae at base of inner apical tooth, molar ridge with a dense row of socketed spine-like marginal setae; setae longer and thinner towards base of mandible. Dorsal surface of mandible also with band of stout socketed setae extending from near inner basal margin to the inner apical tooth. Left mandible similar to right mandible except comb-like row of sharply pointed acanthae at base of inner cusp replaced by a dense brush of short spinous setae from basal tooth on inner cusp to molar ridge.
Thorax (
Figure 9a and
Figure 10a). Pronotum width 1.8× length. Pronotum covered with brown clothing hairs and encircled with brown pigment; lateral flanges pale. Interior of pronotal disk pale to light brown with brown mottling. Pronotum edged with a closely set row of short, stout, socketed setae with long stiff, socketed setae interspersed on the anterior and posterior margins and corners. Mesonotum and metanotum with a pair conspicuous brown longitudinal stripes that are divergent anteriorly, convergent medially and divergent posteriorly; wing pads pale-yellow. Lateral margins of mesonotum and metanotum edged with a closely set row of short, stout, socketed setae, with intervening long, stiff socketed setae. Lateral aspect of thorax pale yellow with light-brown spicules and with trochantins brown to dark brown at apex. Thoracic sterna pale-yellow, without distinctive markings. Furcasternal pits conspicuous, Y-stem arms inconspicuous. Mesosternal Y-stem conspicuous, inconspicuous on prosternum and metasternum. All thoracic sterna with light-brown spicules and light-brown, medial clothing hairs.
Legs (
Figure 9e). Coxa and trochanter pale-yellow to light-brown and with light-brown clothing hairs and a few stout, socketed setae; edged with short, stiff, socketed setae. Anterior and posterior faces of femur covered with brown clothing hairs and a few scattered, long, stiff, socketed setae. Anterior face of femur with a glabrous longitudinal pale-yellow stripe. Ventral margins of the anterior and posterior femoral faces edged with a row of short, stiff, socketed setae. Venter of femur pale, membranous. The dorsal femoral margin with a row of long, stiff, socketed setae. Femur, tibia and tarsi with brown clothing hairs and a dorsal fringe of hair-like setae. Tibia with two outer longitudinal rows of short, stout, socketed setae; outer margin pale-yellow. Inner tibial margin with scattered, long, stiff, socketed setae. Tarsi pale-yellow to light brown with a comb-like ventral row of stiff socketed setae. Two claws darker apically, moderately long and gradually curved.
Abdomen (
Figure 9a and
Figure 10a). Terga light brown, covered with brown clothing hairs and scattered, stout, socketed setae. Posterior margins of terga with a transverse row of long, stiff, socketed setae. Two sub-lateral brown stripes and often a mid-dorsal brown stripe extend from tergum-1 to the midpoint of tergum-10. Sub-lateral brown stripes wider at anterior and posterior margins of respective tergites and usually bordered by pale pigment on both inner and outer margins. Abdominal segments light brown in lateral aspect. Abdominal sterna pale-yellow with light-brown clothing hairs. Posterior margins of sterna with a transverse row of long, stiff, socketed setae. Intersegmental membranes with light-brown spicules. Paraproct triangular, pointed, 1.4× as long as basal width. Cercus with up to 21 flagella. Cercus light brown; each cercal segment with an apical circlet of spines and bearing one long ventral spine about 1/2 length of respective segment. Last 5–8 apical flagella with a dorsal fringe of internodal hair-like setae (
Figure 9f).
Figure 9.
Isoperla myersi, tributary Esopus Creek, Ulster Co., New York, USA, larva. (a) habitus, dorsal. (b) head, dorsal. (c) right mandible, ventral. (d) right lacinia, ventral. (e) right fore-femur, anterior. (f) right cercus, apical segments, lateral [dorsal stout seta (DSS), submarginal seta (SM), ventral stout seta (VSS)].
Figure 9.
Isoperla myersi, tributary Esopus Creek, Ulster Co., New York, USA, larva. (a) habitus, dorsal. (b) head, dorsal. (c) right mandible, ventral. (d) right lacinia, ventral. (e) right fore-femur, anterior. (f) right cercus, apical segments, lateral [dorsal stout seta (DSS), submarginal seta (SM), ventral stout seta (VSS)].
Diagnosis. Isoperla myersi can be distinguished in adult males by the following combination of characters: the lateral ocelli are connected to the anterior ocellus by broad dark stripes that extend laterally beyond the anterior ocellus, the interocellar area is usually pale and open posteriorly, though in some specimens this area may be only slightly lighter in color (IOA in
Figure 2,
Figure 3a,
Figure 4a,
Figure 6a and
Figure 7a). The tibial callus is usually darkened (TC in
Figure 10d). The paraproct has a broadly rounded apex, with symmetrical proximal and distal margins (PP in
Figure 3c,
Figure 4c,
Figure 5a,
Figure 6c and
Figure 7c). The aedeagus bears a posteromesal spine patch that is paddle-shaped or rectangular in outline (D in
Figure 3e,
Figure 4e,
Figure 5c,
Figure 6c,e and
Figure 7e). The abdomen typically lacks a dusky mid-dorsal stripe. Adult females are unique in possessing a subgenital plate with lateral margins that are convex from base to apex (SGP in
Figure 3f,
Figure 4f,
Figure 6f and
Figure 7f), a darkened tibial callus (TC in
Figure 10d), and a generally absent mid-dorsal abdominal stripe.
Larvae can be separated from other species by the following characters: the median pale area on the frons is broadly open anteriorly, with broad, truncate, and anterolaterally directed mediolateral extensions that intersect a pale M-pattern (
Figure 9b). The interocellar area contains an irregular, oval pale spot that is usually confluent with the epicranial suture and typically does not contact the ocelli (
Figure 9b). The abdomen displays two sub-lateral brown stripes and often a mid-dorsal brown stripe that extends from tergum-1 to the midpoint of tergum 10 (
Figure 9a and
Figure 10a). The lateral stripes are widest at the anterior and posterior margins of each tergite (
Figure 9a and
Figure 10a).
Biological notes. An analysis of nearly 1200 NCBAB benthic records from 826 unique locations in North Carolina shows that
I. myersi is very common throughout the Blue Ridge, with sporadic occurrences further eastward into the Piedmont and Southeastern Plains USEPA Level III Ecoregions (
Figure 11). These data are available on request (
https://www.deq.nc.gov/about/divisions/water-resources/water-sciences-section/biological-assessment-branch) (accessed on 6 August 2025). This species frequently occurs sympatrically with the two other species in the
I. holochlora group (
I. arcana,
I. holochlora), which are also widespread in the southern Blue Ridge. It is currently unknown what types of reproductive isolation keep these three lineages separate.
Larvae of I. myersi inhabit a range of aquatic habitats, from small headwater streams to relatively large rivers, and are typically the only Isoperla larvae collected in summer benthic samples in North Carolina. The North Carolina Biotic Index value calculated for I. myersi is 0.1, indicating an exceptionally low pollution tolerance (NCBAB unpublished data). Benthic larval data indicate the species exhibits a univoltine life cycle. Adults have been collected from late April through late August.
One notable finding is the large series collected from Mammoth Cave National Park using an ultraviolet light. This method was much more effective than traditional beating sheets or sweep nets. This supports previous observations that adults of several
Isoperla species tend to climb or fly into tall trees [
4], making them difficult to capture with standard aerial netting techniques.
Conservation notes. Isoperla myersi was only known from the type locality when this species was designated as an RSGCN [
34]. In light of the new synonym proposed herein,
Isoperla myersi is more widespread than previously recognized, which is not unexpected since this species was described only one decade ago and from only two individuals. Now that
I. myersi encompasses a much broader range in the eastern USA from North Carolina north to Maine, plus the single locality further westward in central Kentucky (
Figure 1 and
Figure 11), we anticipate that National (N) and Global (G) ranks of N5/G5 (= Secure) will be applied to
I. myersi. We also expect that additional records will emerge both from institutional collections and from new field sampling efforts in several eastern U.S. states. This is especially likely considering that the species shows no strong affinity for any specific USEPA Level III Ecoregion, but does appear to show a general affinity to Appalachian and sub-Appalachian topography (
Figure 1).
Figure 10.
Isoperla holochlora group species. (a–c) larval habitus, dorsal. (a) Isoperla myersi, White Oak Creek, Macon Co., North Carolina, USA. (b) Isoperla arcana, tributary Bradley Creek, Henderson Co., North Carolina, USA. (c) Isoperla holochlora, Middle Fork Reddies River, Wilkes Co., North Carolina, USA. (d–f) adult female right hind leg. (d) White Rock Branch, Giles Co., Virginia, USA. (e) Wine Spring Creek, Macon, Co., North Carolina, USA. (f) Middle Fork Reddies River, Wilkes Co., North Carolina, USA [tibial callus (TC)].
Figure 10.
Isoperla holochlora group species. (a–c) larval habitus, dorsal. (a) Isoperla myersi, White Oak Creek, Macon Co., North Carolina, USA. (b) Isoperla arcana, tributary Bradley Creek, Henderson Co., North Carolina, USA. (c) Isoperla holochlora, Middle Fork Reddies River, Wilkes Co., North Carolina, USA. (d–f) adult female right hind leg. (d) White Rock Branch, Giles Co., Virginia, USA. (e) Wine Spring Creek, Macon, Co., North Carolina, USA. (f) Middle Fork Reddies River, Wilkes Co., North Carolina, USA [tibial callus (TC)].
Figure 11.
Distribution of the Isoperla holochlora group species in North Carolina (NC) overlaid on US Environmental Protection Agency Level Level III and IV Ecoregions. Occurrence data collected by the North Carolina Biological Assessment Branch.
Figure 11.
Distribution of the Isoperla holochlora group species in North Carolina (NC) overlaid on US Environmental Protection Agency Level Level III and IV Ecoregions. Occurrence data collected by the North Carolina Biological Assessment Branch.
Isoperla arcana Beaty, Holland & Lenat, 2017
Secret stripetail
Isoperla nr.
holochlora: [
29] (p. 41)—larval description
Isoperla arcana [
4] (p. 143)—holotype (USNM; not examined), USA, North Carolina, McDowell Co., Curtis Creek—new species description
Isoperla arcana/
holochlora: [
30] (p. 449)—watershed record (North Carolina)
Isoperla arcana: [
32]—electronic catalogue
Distribution. USA: North Carolina, Tennessee, Virginia (
Figure 1)
Material examined. USA, North Carolina, Avery Co., Cranberry Creek, US 19E, 36.12471, −81.97538, 17 June 2020, C. Verdone, V. Holland, E. Fleek, 1 female (NCDWR); Little Wilson Creek, Edgemont Rd., 36.083364, −81.791353, 29 April 2016, S. Beaty, V. Holland, E. Fleek, 1 male (NCDWR); Clay Co., Fires Creek, Bristol Campground, 35.10785, −83.81056, 26 May 2018, C. Verdone, 2 males (NCDWR); Haywood Co., Tom Creek, Hwy 215, 35.36228, −82.92464, 22 May 2021, C. Verdone, 1 male, 1 female (NCDWR); tributary Raccoon Creek, off Pipen Ln., 35.47038, −82.94412, 8 May 2012, S. Beaty, M. Walters, D. Black, 1 male, 1 exuvium (NCDWR); tributary Bradley Creek, Yellow Gap Rd., 35.38462, −82.68639, 7 May 2018, C. Verdone, 1 larva (NCDWR); Henderson Co., tributary Bradley Creek, Yellow Gap Rd., 35.38462, −82.68639, 7 May 2018, C. Verdone, 1 larva (NCDWR); Macon Co., Robin Branch, FSR 69, 35.17067, −83.58215, 16 May 2014, V. Holland, 1 male, 1 exuvium (NCDWR); Thomas Branch, Upper Nantahala Rd., 35.05140, −83.51128, 26 May 2018, C. Verdone, 2 larvae (NCDWR); tributary Wine Spring Creek, FS Rd. 711, 35.17410, −83.60670, 29 May 2019, C. Verdone, S. Beaty, V. Holland, 1 female, 1 exuvium, 2 larvae (NCDWR), same but 23 May 2023, C. Verdone, V. Holland, M. Green, 1 male (NCDWR); Wine Spring Creek, FS Rd. 711, 35.17466, −83.60575, 29 May 2019, (emerged 6 June 2019) C. Verdone, S. Beaty, V. Holland, 1 female, 1 exuvium (NCDWR); McDowell Co., tributary Curtis Creek, off SR 1227, 35.65580, −82.17140, 9 April 2019, S. Beaty, V. Holland, C. Verdone, E. Fleek, 2 females, 2 exuvium (NCDWR); same but 30 April 2019, S. Beaty, V. Holland, C. Verdone, E. Fleek, 1 female, 1 exuvium (NCDWR); Rockingham Co., Brushy Creek, SR 2321, 36.33583, −79.92583, 17 May 2007, S. Beaty, E. Fleek, T. MacPhearson, 1 larva (NCDWR); Swain Co., Mingo Creek, off Big Cove Rd, below falls, 35.53409, −83.27641, 13 May 2021, C. Verdone, R. Tetreault, 1 male (NCDWR); Moody Branch, Bunches Creek Rd., 35.54124, −83.22181, 7 May 2021, C. Verdone, R. Tetreault, 1 male (NCDWR); same but 10 April 2023, C. Verdone, C. Hoover, 1 male, 1 exuvium (NCDWR); Newfound Gap, 35.61111–83.42500, 28 May 1934 2 males, 1 female, T.H. Frison (INHS Plecoptera 3184); Watauga Co., Boone Fork, Grandfather Mountain State Park, 36.11869, −81.78310, 24 May 2023, C. Verdone, V. Holland, M. Green, 5 males, 1 female (NCDWR). Tennessee, Monroe Co., tributary Jake Best Creek, Cold Spring Rd., 35.44139, −84.08820, 27 May 2018, C. Verdone, 1 male (NCDWR); tributary Laurel Creek, Turkey Creek Rd., 35.34753, −84.19225, 27 May 2018, C. Verdone, 1 male (NCDWR); Sevier Co., Fighting Creek, Fighting Creek Gap, 35.67216–83.579492, 15 May 1939, 2 males, T.H. Frison, H.H. Ross (INHS Insect Collection 876003); Fighting Creek, Gatlinburg, 35.68948–83.53821, 27 May 1934, 2 males, T.H. Frison (INHS Insect Collection 876009). Virginia, Smyth Co., Daves Branch, Rte 600, 36.65463, −81.58420, 23 May 2021, C. Verdone, 1 female (NCDWR).
Figure 12.
Isoperla arcana, adults. (a–c) Tom Creek, Haywood Co., North Carolina, USA. (a) male abdominal terga, dorsal. (b) male head, dorsal. (c) male sterna, ventral. (d–f) Whiteoak Creek, Macon Co., North Carolina, USA. (d) male terminalia, dorsal. (e) male terminalia, lateral. (f) male terminalia, ventral. (g) Tom Creek, Haywood Co., North Carolina, USA, female sterna, ventral [posteroventral lobe (A), abdominal segment (AB), abdominal sterna (AS), abdominal tergum (AT), paired posterolateral lobes (B), paired posteromesal lobes (C), recessed posteromesal spine patch (D), dorsal lobe (E), anterodorsal lobe (F), mesal band of spinulae (G), interocellar area (IOA), paraproct (PP), subgenital plate (SGP), vesicle (V)].
Figure 12.
Isoperla arcana, adults. (a–c) Tom Creek, Haywood Co., North Carolina, USA. (a) male abdominal terga, dorsal. (b) male head, dorsal. (c) male sterna, ventral. (d–f) Whiteoak Creek, Macon Co., North Carolina, USA. (d) male terminalia, dorsal. (e) male terminalia, lateral. (f) male terminalia, ventral. (g) Tom Creek, Haywood Co., North Carolina, USA, female sterna, ventral [posteroventral lobe (A), abdominal segment (AB), abdominal sterna (AS), abdominal tergum (AT), paired posterolateral lobes (B), paired posteromesal lobes (C), recessed posteromesal spine patch (D), dorsal lobe (E), anterodorsal lobe (F), mesal band of spinulae (G), interocellar area (IOA), paraproct (PP), subgenital plate (SGP), vesicle (V)].
Isoperla holochlora Klapálek, 1923
Pale stripetail
Isoperla holochlora: [
35] (p. 28)—syntype series, USA, Georgia—new species description (RBINS; examined)
Isoperla holochlora: [
36] (p. 147)—lectotype designated—female illustration (RBINS; examined)
Isoperla holochlora: [
37] (p. 406)—catalogue
Isoperla holochlora: [
38] (p. 246)—catalogue
Isoperla holochlora: [
39] (p. 147)—checklist (Massachusetts)
Isoperla holochlora: [
40] (p. 246)—checklist (Virginia)
Isoperla holochlora: [
41] (p. 44)—checklist (Delaware)
Isoperla holochlora: [
42] (p. 51)—checklist (West Virginia)
Isoperla holochlora: [
43] (p. 69)—checklist (Maine)
Isoperla holochlora: [
44] (p. 392)—checklist (USA)
Isoperla holochlora: [
45] (p. 28)—checklist (Virginia)
Isoperla holochlora: [
46] (p. 123)—adult ecology, checklist (Maryland)
Isoperla holochlora: [
47] (p. 286)—larval ecology
Isoperla holochlora: [
48] (p. 1)—checklist (Nova Scotia)
Isoperla holochlora: [
49] (p. 1262)—checklist (New Brunswick)
Isoperla holochlora: [
50] (p. 259)—checklist (Pennsylvania)
Isoperla holochlora: [
51] (p. 81)—checklist (Maryland)
Isoperla holochlora: [
52] (p. 40)—checklist (Abrams Creek, Great Smoky Mountains National Park)
Isoperla holochlora: [
53] (p. 6)—checklist (Kentucky)
Isoperla holochlora: [
54] (p. 171)—checklist (Great Smoky Mountains National Park)
Isoperla holochlora: [
55] (p. 161)—checklist (West Virginia)
Isoperla holochlora: [
56] (p. 94)—checklist (New York)
Isoperla holochlora: [
57] (p. 9)—faunal assessment and checklist (Ohio)
Isoperla holochlora: [
58] (p. 7)—checklist (Ohio)
Isoperla holochlora: [
4] (p. 109)—adult descriptions, illustrations, key
Isoperla holochlora: [
28] (p. 44)—color photographs
Isoperla holochlora: [
59] (p. 170)—checklist (Kentucky)
Isoperla holochlora—dark form: [
29] (p. 21)—larval description
Isoperla holochlora: [
60] (p. 7)—geographic atlas (Ohio)
Isoperla holochlora: [
5] (p. 163)—checklist (North Carolina)
Isoperla holochlora: [
61] (p. 26)—checklist (Virginia)
Isoperla holochlora: [
62] (p. 44)—checklist (Georgia)
Isoperla holochlora: [
63] (p. 73)—checklist (Ohio)
Isoperla holochlora: [
64] (p. 78)—checklist (Maryland)
Isoperla holochlora: [
65] (p. 28)—checklist (Alabama)
Isoperla holochlora: [
33] (p. 400)—elevation patterns, checklist (Maryland)
Isoperla holochlora: [
31] (p. 44)—geographic atlas (New York)
Isoperla holochlora: [
32]—electronic catalogue
Distribution. Canada: New Brunswick, Nova Scotia, Quebec;
USA: Alabama, Connecticut, Delaware, Georgia, Kentucky, Maine, Maryland, Massachusetts, New Hampshire, New York, North Carolina, Ohio, Pennsylvania, South Carolina, Tennessee, Virginia, West Virginia (
Figure 1).
Figure 13.
Isoperla holochlora, Little River, Transylvania Co., North Carolina, USA. adults. (a) male head, dorsal. (b) male sterna, ventral. (c) male terminalia, dorsal. (d) male terminalia, lateral. (e) male terminalia, ventral. (f) female sterna, ventral [posteroventral lobe (A), abdominal segment (AB), abdominal sterna (AS), abdominal tergum (AT), paired posterolateral lobes (B), posteromesal lobe (C), recessed posteromesal spine patch (D), paired dorsal lobes (E), mesal band of spinulae (G), paired long posterobasal spine clusters (H), interocellar area (IOA), paraproct (PP), subgenital plate (SGP), vesicle (V)].
Figure 13.
Isoperla holochlora, Little River, Transylvania Co., North Carolina, USA. adults. (a) male head, dorsal. (b) male sterna, ventral. (c) male terminalia, dorsal. (d) male terminalia, lateral. (e) male terminalia, ventral. (f) female sterna, ventral [posteroventral lobe (A), abdominal segment (AB), abdominal sterna (AS), abdominal tergum (AT), paired posterolateral lobes (B), posteromesal lobe (C), recessed posteromesal spine patch (D), paired dorsal lobes (E), mesal band of spinulae (G), paired long posterobasal spine clusters (H), interocellar area (IOA), paraproct (PP), subgenital plate (SGP), vesicle (V)].
Figure 14.
Isoperla holochlora, Georgia, USA, lectotype female. (a) Type labels. (b) Left, hind tibia. (c) head and pronotum, dorsal. (d) abdominal sterna, ventral [abdominal sterna (AS), Interocellar area (IOA), subgenital plate (SGP), tibial callus (TC)]. Photo credits: Royal Belgian Institute for Natural Sciences: Tim Laebens (photographer), Jérôme Constant (Plecoptera collection manager), Wouter Dekoninck (entomological collections curator).
Figure 14.
Isoperla holochlora, Georgia, USA, lectotype female. (a) Type labels. (b) Left, hind tibia. (c) head and pronotum, dorsal. (d) abdominal sterna, ventral [abdominal sterna (AS), Interocellar area (IOA), subgenital plate (SGP), tibial callus (TC)]. Photo credits: Royal Belgian Institute for Natural Sciences: Tim Laebens (photographer), Jérôme Constant (Plecoptera collection manager), Wouter Dekoninck (entomological collections curator).
Material examined. USA, Massachusetts, Hampshire Co., Cadwell Creek, Packardsville Rd. at PL-2 gate Quabbin Reservoir, 42.34649, −72.37889, 24 June 2025, R.E. DeWalt, 5 males, 5 females (INHS Insect Collection 1545139); Roaring Brook, Shutesbury Rd., 42.44918, −72.44649, 25 June 2025, R.E. DeWalt, 1 female (INHS Insect Collection 1545136). New Hampshire, Coos Co., Cutler River, Pinkham Notch Trailhead, White Mountains National Forest, 44.25880, −71.25449, 4 August 2021, L.W. Myers, 1 female (LCRI 374). New York, Clinton Co., tributary to True Brook, Clark Hill Rd., Saranac, 44.65580, −73.80220, 21 June 2014, L. Zajac, 2 males (LCRI 373); Essex Co., seep to North Fork Boquet River, crossing Rt. 73, 44.10820, −73.69777, 27 June 2007, L.W. Myers, B.C. Kondratieff, 1 male (LCRI 372); Franklin Co., Dutton Brook, Rt. 3 nr. Saranac Lake, 44.24950, −74.23810, 27 June 2007, L.W. Myers, B.C. Kondratieff, 2 males, 1 female (LCRI 367); Allen Brook, East Rd., N. of Thayer Corners, 44.92820, −74.14170, 30 June 2008, L.W. Myers, 1 male, 2 females (LCRI 368); Oregon Brook, Mud Pond Road, 44.53920, −74.05390, 15 August 2008, L.W. Myers, 1 male, 1 female (LCRI 369); Greene Co., Dutcher Creek, Maple Lawn Rd., 42.25713, −74.04012, 24 June 2007, L.W. Myers, B.C. Kondratieff, 1 male (LCRI 371); Lewis Co., Mill Creek, Turin Road, 43.63188, −75.39333, 29 June 2007, L.W. Myers, B.C. Kondratieff, 2 males, 1 female (LCRI 370). North Carolina, Alleghany Co., spring, Blue Ridge Parkway, NE side of Doughton Rec. Area (BLRI), 36.43147, −81.18011, 26 May 2016, C. Verdone, B. Kondratieff, 1 female (CSUIC); Caldwell Co., Thunderhole Creek, Rte 1366, 36.07324, −81.69975, 14 May 2017, C. Verdone, B. Kondratieff, 1 male (CSUIC); Graham Co., Snowbird Creek, FS Rd. 75, Wilson Cabin, 35.27367, 83.90501, 11 May 2022, C. Verdone, 1 male, (NCDWR); Snowbird Creek, Snowbird School Rd., 35.30361, −83.87347, 11 May 2022, C. Verdone, 4 males, 4 females, (NCDWR); Mountain Creek, SR 1214, 35.35272, −83.78316, 11 May 2022, C. Verdone, 1 male, 1 female, (NCDWR); Haywood Co., Cataloochee Creek, Old Cataloochee Turnpike at Bridge (GSMNP), 35.66695, −83.07280, 25 May 2016, C. Verdone, B. Kondratieff, 1 male, 2 females (CSUIC); tributary Cold Spring Creek, Cold Springs Rd., 35.76342, −82.98767, 27 May 2021, C. Verdone, 1 male (NCDWR); Henderson Co., Green River Headwaters, off SR 1106, 35.16420, −82.58065, 21 May 2021, C. Verdone, 1 female (NCDWR); Macon Co., Cold Spring Creek, jct. Cold Spring Creek Rd. & FR 711, 35.22323, −83.60403, 15 May 2017, C. Verdone, B. Kondratieff, 2 males, 5 females (CSUIC); Otter Creek, Otter Creek Rd., 35.24687, −83.62884, 15 May 2017, C. Verdone, B. Kondratieff, 1 male, 2 females (CSUIC); McDowell Co., North Fork Catawba River, Hwy 221, 35.95203, −81.94435, 14 May 2017, C. Verdone, B. Kondratieff, 1 female (CSUIC); Polk Co., Panther Creek, SR 1156, 35.35193, −82.24326, 5 April 2023, C. Verdone, V. Holland, D. Harwood, 1 female, 1 exuvium, (NCDWR); Stokes Co., Mill Creek, SR 2018, 36.38680, −80.22729, 21 April 2021, C. Verdone, S. Beaty, V. Holland, 1 male, 1 female (NCDWR); Swain Co., Alarka Creek, Alarka Rd. E of Eagles Roost Rd., 35.35773, −83.42802, 15 May 2017, C. Verdone, B. Kondratieff, 1 female, (CSUIC); Newfound Gap, 35.61111, −83.42500, 25 May 1934, 1 female, T.H. Frison (INHS Plecoptera 3183); Oconoluftee River, Smokemont Campground, Great Smoky Mountains National Park, 35.55298, −83.30956, 28 May 1934, 1 male, 1 exuvium, T.H. Frison, H.H. Ross (INHS Plecoptera 3196); same data, but 21 larvae, (INHS Insect Collection 876025); Transylvania Co., Cedar Rock Creek, SR 1338, 35.22820, −82.80904, 2 May 2024, C. Verdone, S. Beaty, E. Fleek, 4 larvae (NCDWR); Davidson River, US 276, 35.28881, −82.76285, 28 April 2021, C. Verdone, S. Beaty, V. Holland, 3 males, 2 female (NCDWR); Little River, Off US 276, 35.14675, −82.67143, 28 April 2021, C. Verdone, S. Beaty, V. Holland, J. DeBerardinis, 2 male, female, 2 exuvia (NCDWR); Cannon Creek, Rich Mountain Rd., 35.17432, −82.66481, 23 May 2023, C. Verdone, V. Holland, M. Green, 2 female (NCDWR); Wilkes Co., Middle Fork Reddies River, SR 1559, 36.23206, −81.30067, 28 April 2025, C. Verdone, 1 male, 2 female, 3 exuvia, 4 larvae (NCDWR). Pennsylvania, Monroe Co., Marshalls Creek, 41.04289, −75.12774, 1 June 1935, 1 female, 1 exuvium, W.V. Harmer (INHS Insect Collection 875998). South Carolina, Anderson Co., E. Trib. Rock Creek, N of S-4-670, [34.58289, −82.51308], 3–4 April 1980, 4 males, 1 female (NCDWR). Tennessee, Sevier Co., Elkmont, Great Smoky Mountains National Park, 35.65361, −83.58056, 12 June 1935, 2 females, H.H. Ross (INHS Insect Collection 876024); Unicoi Co., Indian Creek, Hwy 107, 36.16153, −82.25130, 17 May 2017, C. Verdone, B. Kondratieff, 1 male (CSUIC). Virginia, Bedford Co., Battery Creek, FR 951, 37.55194, −79.44059, 17 June 2016, C. Verdone, 1 female (CSUIC); Carroll Co., Stewarts Creek, Rte 696, 36.58083, −80.76437, 30 May 2016, C. Verdone, B. Kondratieff, 2 females (CSUIC); Highland Co., Bullpasture River, Bullpasture Gorge Parking Area, 38.21400, −79.59200, 9 June 2017, B. Kondratieff, 1 female (CSUIC); Madison Co., Rapidan River, Co. Rd. 649 (SHEN), 38.46256, −78.36535, 2 June 2016, C. Verdone B. Kondratieff, S. Roble, 1 male, 1 female (CSUIC); Patrick Co., Dan River, Rte 648, 36.62230, −80.44497, 11 May 2017, C. Verdone, B. Kondratieff, 1 male, 1 female (CSUIC); Little Rock Castle Creek, Rte 605 Rocky Knob Rec Area BLRI, 36.80772, −80.33114, 9 May 2017, C. Verdone, B. Kondratieff, 1 female (CSUIC); Mayberry Creek, BLRI ~ 0.25 mi S of Rte 634, 36.70842, −80.44395, 9 May 2017, C. Verdone, B. Kondratieff, 7 larvae (CSUIC); Roanoke Co., spring tributary to Metz Run, Blue Ridge Parkway (BLRI), 37.17599, −80.06296, 31 May 2016, C. Verdone, B. Kondratieff, 1 female (CSUIC); Scott Co., Little Stony Creek, Rte 72, Hanging Rock Rec Area, 36.86028, −82.44692, 6 May 2017, C. Verdone, B. Kondratieff, S. Roble, 1 female (CSUIC); Smyth Co., tributary Tumbling Creek, Tumbling Creek Rd. (above fee area D), 36.93718, −81.81480, 23 May 2016, C. Verdone, B. Kondratieff, 1 male (CSUIC); Tumbling Creek, Tumbling Creek Rd. (Ray Bottoms), 36.94069, −81.80773, 23 May 2016, C. Verdone, B. Kondratieff, 1 male (CSUIC); Tazewell Co., Cove Creek, Co. Rd. 662, off Hwy 61, 37.17837, −81.29900, 27 May 2016, C. Verdone, B. Kondratieff, 1 male, 3 females (CSUIC); Cove Creek, Rte 662, 37.17898, −81.30067, 7 May 2017, C. Verdone, B. Kondratieff, S. Roble, 5 males, 5 females (CSUIC); Little Tumbling Creek, Rte 607, Little Valley access, 36.97765, −81.66656, 6 May 2017, C. Verdone, B. Kondratieff, S. Roble, 1 female (CSUIC); spring nr. spring house, Cove Creek, Rte 622, 37.17989, −81.30067, 7 May 2017, C. Verdone, B. Kondratieff, S. Roble, 1 female (CSUIC); Washington Co., Brumley Creek, FR 689 (Baptist Camp), 36.82327, −81.98497, 24 May 2016, C. Verdone, B. Kondratieff, B. Evans, 2 females (CSUIC); same but 4 May 2017, C. Verdone, B. Kondratieff, 2 male (CSUIC); spring tributary to Tumbling Creek, Tumbling Creek Rd., 2nd bridge, 36.90318, −81.84122, 5 May 2017, C. Verdone, B. Kondratieff, S. Roble, 1 larva (CSUIC); Tumbling Creek, Tumbling Creek Rd., 36.89408, −81.84314, 23 May 2016, C. Verdone, B. Kondratieff, 4 females (CSUIC); Tumbling Creek, Tumbling Creek Rd. (2nd bridge), 36.90336, −81.84026, 23 May 2016, C. Verdone, B. Kondratieff, 1 males, 3 females (CSUIC); Whiterock Branch, Rte 689, 36.83047, −81.95962, 4 May 2017, C. Verdone, B. Kondratieff, 1 male, 1 female (CSUIC); Whitetop Creek, Hwy 58, Straight Branch Picnic Area, 36.64316, −81.73969, 8 May 2017, C. Verdone, B. Kondratieff, 4 males, 10 females (CSUIC).
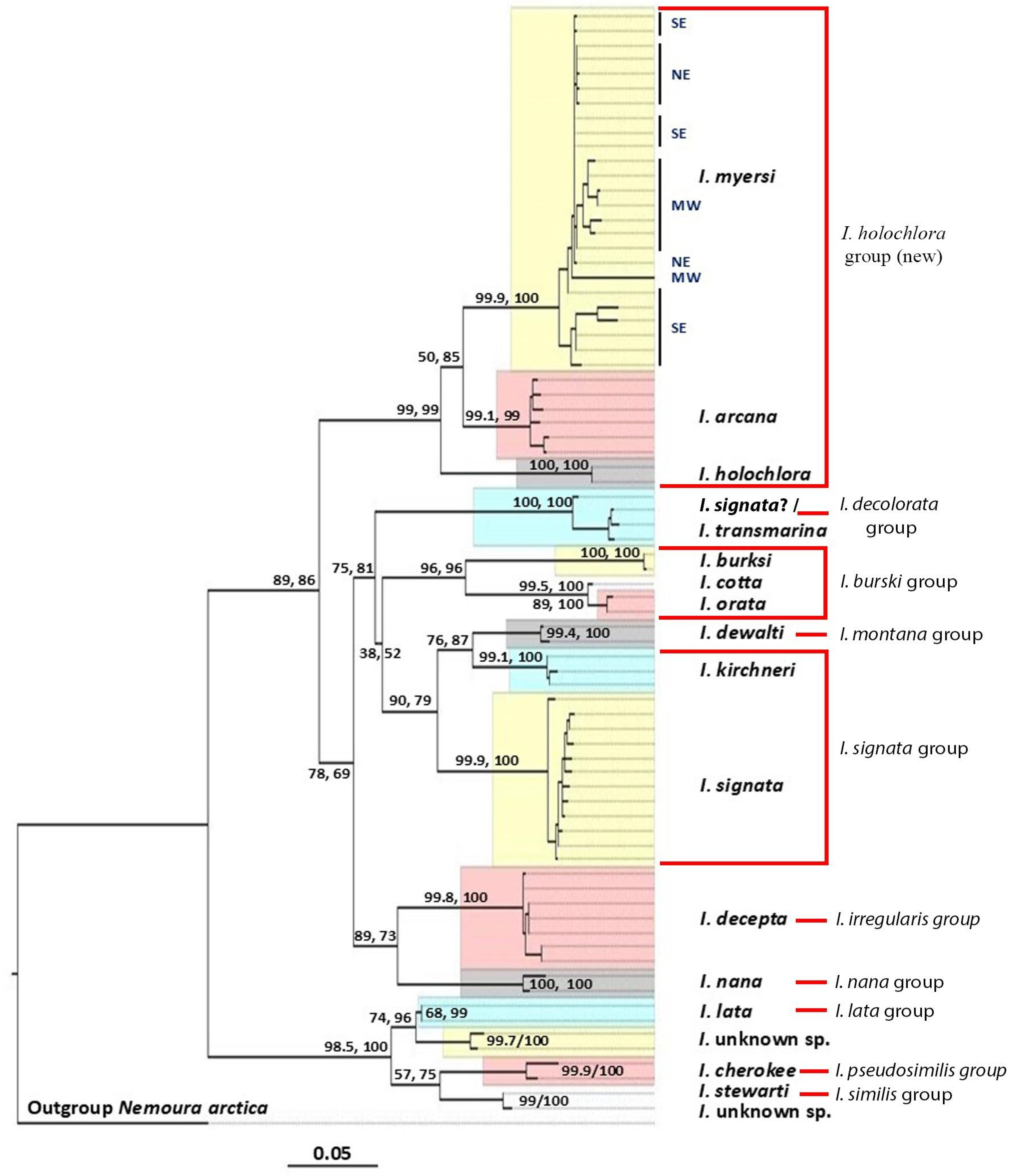
Isoperla holochlora group
The
Isoperla holochlora group includes
I. holochlora, I. arcana and
I. myersi. Isoperla myersi and
I. powhatan syn. nov. were previously placed in the
I. bilineata group based on their yellow habitus, head pattern, and the membranous aedeagus of adult males [
4].
Isoperla holochlora was formerly assigned to the
I. signata (Banks, 1902) group due to the presence of paired aedeagal spine plates [
4]. However, we found that these three species form a distinct phylogenetic group and share many morphological similarities.
Adult males of these species exhibit an interocellar area marked either by dark bars connecting the anterior and lateral ocelli or by a nearly completely dark interocellar area (IOA in
Figure 2,
Figure 3a,
Figure 4a,
Figure 6a,
Figure 7a,
Figure 12b,
Figure 13a and
Figure 14c). They also possess flattened paraprocts (PP in
Figure 3c,
Figure 4c,
Figure 5a,
Figure 6c,
Figure 7c,
Figure 12d and
Figure 13c), a vesicle that barely extends beyond the posterior margin of sternum-8 (V in
Figure 3b,
Figure 4b,
Figure 5b,
Figure 6b,
Figure 7b,
Figure 12c and
Figure 13b) and an aedeagus with a recessed posteromesal spine patch, a posteroventral lobe (D in
Figure 3e,
Figure 4e,
Figure 5c,
Figure 6c,e,
Figure 7e,
Figure 12f and
Figure 13c,e), and paired posterolateral lobes (B in
Figure 3c–e,
Figure 4c–e,
Figure 5c,
Figure 6c–e,
Figure 7c–e,
Figure 12e,f and
Figure 13c–e). Females are characterized by a triangular subgenital plate (SGP in
Figure 3f,
Figure 4f,
Figure 6f,
Figure 7f,
Figure 12g,
Figure 13f and
Figure 14d), which often appears remarkably similar across the group. Larvae display a pair of lateral abdominal stripes and may also bear a narrow medial stripe (
Figure 9a and
Figure 10a–c).
Diagnoses. Due to the many overlapping adult features, the larva provides the most reliable distinguishing characteristics. In
I. myersi, the median pale area on the larval head is broadly open anteriorly, and the mediolateral extensions are truncate and relatively broad, comprising about half the width of the median pale area, and the interocellar spot is confluent with the epicranial suture (
Figure 9a,b and
Figure 10a). In contrast,
I. holochlora has a median pale area that is narrow and closed anteriorly; its mediolateral extensions are narrower and about equal in width to the anterior median pale area expanse; the mediolateral extension apices are either acute or rounded, and the interocellar spot in this species is closed (
Figure 10c). In
I. arcana, the frons lacks a prominent medial pale area but displays a distinct M-shaped pattern (
Figure 10b), providing a clear basis for differentiation from the other two species.
When larvae are not available, males with everted aedeagi, along with external morphological features, offer the next best method for species identification.
Isoperla holochlora is readily distinguished by the presence of a pair of long posterobasal spine clusters at the base of the recessed posteromesal spine patch (H in
Figure 13c–e), structures that are absent on
I. myersi and
I. arcana. Additionally,
I. holochlora typically lacks a mid-dorsal abdominal stripe and exhibits a pale tibial callus demarcated by a thin dark line (TC in
Figure 10f and
Figure 14b).
The aedeagi of
I. myersi and
I. arcana are similar in overall appearance, both possessing a recessed posteromesal spine patch (D in
Figure 3e,
Figure 4e,
Figure 5c,
Figure 6c,e,
Figure 7e and
Figure 12f). However, paraproct morphology and coloration patterns are useful in differentiating between the two. For
I. myersi, the interocellar region usually includes a pale intervening area posteromedially (IOA in
Figure 2,
Figure 3a,
Figure 4a and
Figure 6a), the tibial callus is generally darkened (TC in
Figure 10d), and the abdomen typically lacks a mid-dorsal stripe. The paraproct in this species has a broadly rounded apex, with symmetrical proximal and distal margins (PP in
Figure 3c,
Figure 4c,
Figure 5a,
Figure 6c and
Figure 7c). In contrast, males of
I. arcana usually have an interocellar area completely filled with dark pigment (IOA in
Figure 12b). The tibial callus is usually pale and demarcated by a thin dark line (TC in
Figure 10e), the abdomen often features a mid-dorsal stripe (
Figure 12a), and the paraproct has an acute apex with asymmetrical margins (PP in
Figure 12d).
Females of this group, like those of many stonefly species, are the most difficult to identify. The subgenital plate of all three species is triangular and resembles that of
I. dicala Frison, 1942 [8, their figure 44]. However, species within this group can be distinguished by the presence of a well-developed head pattern (IOA in
Figure 2,
Figure 3a,
Figure 4a,
Figure 6a,
Figure 7a,
Figure 12b,
Figure 13a and
Figure 14c). Among the three,
I. myersi females are the most readily recognized by their typical combination of features, which includes a subgenital plate with convex lateral margins (SGP in
Figure 3f,
Figure 4f,
Figure 6f and
Figure 7f), a darkened tibial callus (TC in
Figure 10d), and the absence of a mid-dorsal abdominal stripe. In
I. arcana and
I. holochlora, the subgenital plates are often nearly identical, usually with sinuous lateral margins (SGP in
Figure 12g,
Figure 13f and
Figure 14d), and both species generally have a pale tibial callus (TC in
Figure 10e,f and
Figure 14b). The only character for separating females is the presence or absence of a mid-dorsal abdominal stripe, which is usually present in
I. arcana (
Figure 12a) and typically absent in
I. holochlora.
Taxonomic notes. Although larval characters provide the most reliable means of distinguishing
I. myersi from other closely related species, confusion has persisted regarding its identity, which has long been mistakenly associated with
I. holochlora. Frison [
26] reported rearing
I. holochlora from larvae that are presently indistinguishable from those of
I. myersi (his figure 86). At that time, the diagnostic value of aedeagal characters was not yet recognized, and species identifications relied solely on external morphology (his figure 85). Frison [
26] compared his reared specimens to type material, including the lectotype female [
36] (
Figure 14a–d) and determined them to be conspecific. Later, Hitchcock [
27] reproduced Frison’s larval illustration of
I. holochlora in his manual on the stoneflies of Connecticut, USA. This illustration was widely used by monitoring programs for the identification of
I. holochlora until Beaty [
29] made available a photographic guide to
Isoperla larvae from North Carolina.
Beaty [
29] introduced a new larval form of
I. holochlora, referred to as the “dark form”. From this larval habitus, Beaty [
29] reared male specimens whose aedeagi matched the description of
I. holochlora provided in Szczytko and Kondratieff [
4], but also continued to recognize Frison’s [
26] larva as the “light form” of
I. holochlora. However, no adult specimens have ever been reared from the “light form” that matches
I. holochlora either morphologically or genetically. Therefore, we must conclude that the Frison [
26] illustration actually represents
I. myersi and that the Beaty [
29] “dark form” corresponds to the true larva of
I. holochlora. In addition, Beaty [
29] also provided larval descriptions of
I. powhatan syn. nov. and
I. nr.
holochlora (=
I. arcana), which he grouped as the
I. holochlora complex.