Abstract
The fossiliferous Dursunlu Lignite Quarry (DLQ) is highlighted prominently in the archeological and paleontological literature because of the study of Pleistocene fauna and lithic artifacts, being considered the oldest Paleolithic site in Türkiye. Although the fauna and flora assemblage from DLQ are reasonably well known, taxonomic studies devoted to some groups, such as ostracods, mollusks, reptiles, and amphibians, have never been carried out. Here, we describe, illustrate, and study the taxonomic composition and ecological implications of the unpublished material of said groups, together with the aquatic plants and fish, recovered from six samples taken from the palustrine and peat bog facies of the sedimentary sequence. In addition, the recovered charophytes and cyprinids refine our taxonomical knowledge of both aquatic plants and fish. Our results concur with previous paleoenvironmental inferences based on flora and fauna composition—with DLQ representing a very shallow eutrophic lake with a dense palustrine vegetation belt during the cold (glacial) stage of the late Early Pleistocene—as well as highlight the study of all available groups as pivotal for better understanding the paleolake biota. We further conclude that the wetland areas of Dursunlu and surrounding steppe areas appear to have been an excellent environment for sporadic settlement of hominins during the Early Pleistocene, given the availability of food resources and easy access to water.
1. Introduction
1.1. The Hominin-Bearing Site of Dursunlu Lignite Quarry
1.1.1. Historical Background
The fossil site of Dursunlu Lignite Quarry (DLQ) in the Konya Province, Central Anatolia region, Ilgın Basin (Figure 1) has stood out in the study of Pleistocene mammals from Eurasia and lithic artifacts, being considered the oldest known Paleolithic site (Early Pleistocene) in Türkiye [1,2]. It was discovered in 1986 ([3]; and references therein) during a mapping project of the General Directorate of Mineral Research and Exploration of Turkey and gained more importance with the discovery of human artifacts by the latter team, together with the Ankara University (Türkiye) and the University of California, Berkeley (USA) within the project “Vertebrate Fossils of Turkey” in 1993–1994 [4]. The DLQ was opened and abandoned several times during the 1980s and early 90s because of the low quality of coal, until it was declared as a “protected area” in 1994. Although it used as a power plant in 2024, it is currently not in service and was filled with groundwater and wetlands (e.g., [3,5,6]).
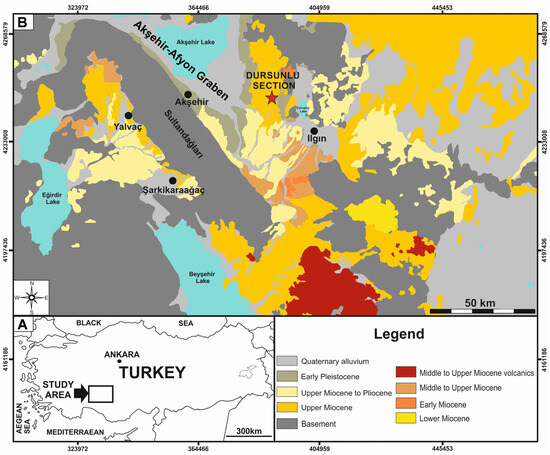
Figure 1.
(A) Geographic location of the Ilgın Basin within Türkiye. (B) Simplified geological scheme of the study area: the red star denotes the location of the studied section. Modified from (Koopman [7]: Figure 3) and (Demirci et al. [5]: Figure 2).
Mammal remains, assigned to proboscideans, artiodactyls, perissodactyls, and carnivorans, were found by the miners in 1986 from the bioturbated, gastropod-rich clays and silt layers, as well as from the overlying lignite layer, also called upper lignite [3,4]. Moreover, small mammals, such as eulipotyphlans, lagomorphs, and rodents, were recovered from the clay layer by wet sieving more than ten tons of sediment [4]. Additional remains of mammals, reptiles, amphibians, birds, and fish were subsequently recovered during 1993 and 1994, and reported in several publications (e.g., [2,4,8,9,10]). Paleontological studies conducted at DLQ increased significantly when several fragments of mammal bones were found associated with lithic artifacts, including chipped stone tools [1]. This not only attested the presence of hominin at that site, but further, they represented the oldest evidence of hominins ever found in Türkiye. The significance of the site grew further during the next years, with the recovery of more than 100 modified lithic artifacts produced with the bipolar technique, including quartz flakes, as well as cut-marked bones [2,11,12]. The first palynological studies were published by Yavuz-Işık [13], indicating a pollen community dominated by different kinds of herbs, abundant arboreal components, and moist-adapted aquatic plants, which suggested a humid-temperate climate during the Early Pleistocene. More recently, a Ph.D. thesis focused on the revision of the Miocene-Pleistocene ostracod fauna from Southwest Anatolia and recovered up to 18 ostracod taxa from Dursunlu Formation [14]. Similarly, Demirci et al. [5] studied the aquatic plant assemblage (mainly charophytes) along with gastropods and bivalves recovered from 50 samples taken from all exposed strata at the DLQ (about 18 m thick). These authors also conducted a sedimentological analysis in order to assess the environmental evolution of the paleolake. Subsequently, fish remains (cyprinids and cobitids) from the same samples were published by Blanco-Lapaz et al. [6]. Finally, Yavuz et al. [3] restudied the pollen assemblage of freshwater algae, herbs, hydrophytes ferns, and Pinus, together performing a reconstruction of the paleovegetation and paleoenvironmental conditions.
1.1.2. Chronological Background and Aims
Recovered macromammals from DLQ are diverse, including felids, bovids, cervids, and equids. Ünay et al. [4] mentioned faunal similarities of the Pleistocene faunas of Monte Peglia (Italy) and Les Valerots (France) with DLQ, and more specifically they used the presence of the vole Allophaiomys nutiensis to assign an age of ca. 0.9 Ma to the latter. Howell et al. [9] revised the entire micromammal assemblages from DLQ under a strictly biostratigraphic approach, concluding that the presence of the arvicolid rodents A. nutiensis and Lagurus arankae fit well with zone MNQ-20. Güleç et al. [2] went a step further and combined both magnetostratigraphic—two 50 m cores— and biostratigraphic data. In fact, they correlated the sedimentary sequence of DLQ to the reverse polarity of the upper Matuyama chron (0.773–0.99 Ma) but further proposed an interpolated age (0.85 to 0.90 Ma) for the lithic assemblage bearing layer (LAL) based on the micromammal assemblage.
Despite many detailed studies on fish, birds, macro- and micromammals, pollen, and aquatic plants, other groups from DLQ have received little (i.e., seeds and ostracods) to no (reptiles, amphibians, and mollusks) attention. Here, we study the above-mentioned groups to update the faunal record and complete the paleoenvironmental reconstruction of the DLQ during the cold (glacial) stage of the late Early Pleistocene (Günz glacial period).
2. Geographical and Geological Setting
The DLQ is located on the Lycaonian Plateau (31°46′10.7″ N, 38°22′38.3″ E), on the northern front of the Taurus Mountains (Figure 1), and more specifically, about 1.5 km southwest of the homonymous village in the Ilgın district, Konya province (Central Anatolia, Türkiye). As stated by Koçyiğit et al. [15], the western half of Central Anatolia is dominated by a series of horst and graben structures bounded by active normal faults. Among them, the Akşehir-Afyon Graben is one of the most important active rifts, which is W–NW to E–SE-oriented, and bordered North–South by the Emir and Sultan Mountains, respectively. This graben contains both Eber and Akşehir Lakes, as well as sedimentary infills ranging from Miocene to Quaternary. From a geological viewpoint, the DLQ is placed on the eastern border of the Isparta Angle and further represents the Southeast extension of the larger Akşehir-Afyon Basin [7].
In the Dursunlu area, the bedrock basement of the Akşehir Basin consists of Early Cambrian–Late Cretaceous metavolcanic (quartzite, schist, and ophiolites) and metasedimentary (marble, dolomite, and nodular cherty limestones) rocks (e.g., [3,16,17,18,19]). The Akşehir-Afyon Basin is filled by two main sedimentary sequences, Miocene and Pleistocene in age, respectively, separated by an angular unconformity. During the Miocene, alluvial fans of the Belekler (conglomerates, sandstones, siltstones, and mudstones) and Aşağıçiğil (mainly coal, marls, conglomerates, sandstones, siltstones, and mudstones) Formations were deposited discordantly on the older Paleozoic/Mesozoic deposits ([20]; and references therein). An alternation of coals (with up to three m-thick layers) with claystones, marls, and mudstones (Dursunlu Fm.; Figure 2A–C) overlies the Miocene alluvial fan deposits [21]: this formation has a total thickness of 320 m and is composed of fine-grained horizons and peatlands littered with vertebrate and mollusk remains. Quaternary sedimentation in the Akşehir-Afyon Basin terminates with the uppermost colluvial and alluvial sediments.
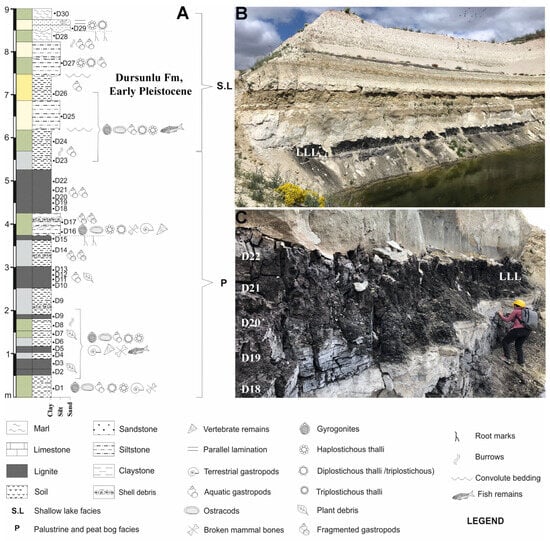
Figure 2.
(A) Stratigraphic section of the first nine m at DLQ (SE of Ilgın Basin, Türkiye). (B) Field photo showing the palustrine (where the largest lignite layer is denoted by LLL), peat bog, and lake facies; (C) detailed field photo of the bottom of the stratigraphic column (between four and six m) showing the samples D18–D22. D1 indicates sample 1: the same applies for D2 until D30.
The six samples studied herein belong to the Dursunlu Formation [21], including the upper lignite member also described by Güleç et al. [2]. According to Demirci et al. [5], the entire sequence exposed in the DLQ consists of two facies associations: the base is formed by approximately six m of organic-rich siltstones/claystones (dark brown to grayish layers with root marks: Figure 2B,C; samples D1–D23), which are rich in invertebrate, vertebrate, and floral remains (i.e., palustrine and peat bog facies = also corresponding to lithic assemblage bearing layer); and the upper part of the section, from 6 to 18 m (samples D24–D50 in [5]: Figure 2), which consists of light gray-yellowish fossiliferous marls, marly siltstones, and a limestone bed (Figure 2B) with abundant charophyte flora, ostracods, and mollusk remains.
3. Material and Methods
The samples studied were extracted from soft rocks (marls, siltstones, and claystones) in the DLQ: coordinates 38.37519130 N, 31.77109127 E (Figure 1A,B). Six samples (D2–D3, D16–D17, and D22–D23), each weighing 3 kg, were extracted and processed to recover the material of interest: flora (including seeds), arthropods, and mollusks in the Paleontology Laboratory of the Department of Geological Engineering, Hacettepe University (Ankara, Türkiye), and in the Department of Earth and Ocean Dynamics (Faculty of Earth Sciences), University of Barcelona (Catalonia, Spain), and vertebrate remains in the Paleontology Unit, Department of Geology, Universitat Autònoma de Barcelona (Catalonia, Spain). Soft rocks were disaggregated in a solution of 12 L of water, hydroxide peroxide (150 mL), and sodium carbonate (7 g) for 72 h. Once the clayed component had been dissolved, sediments of the different samples were washed with a regulated stream of running water in sieves of varying mesh sizes (2, 1, 0.5, 0.35, and 0.2 mm). All fossils were sorted under a light binocular microscope. The most complete gyrogonites, seeds, and fish bones were selected and photographed using a scanning electron microscope (Quanta 200 at the Centres Científics i Tecnològics, Universitat de Barcelona: CCiTUB; Tescan Gaia3 + at Hacettepe University: HÜNİTEK), whereas photos of selected herpetofauna and mollusk specimens were mainly taken with a Leica M80 stereomicroscope (Wetzlar, Germany) and a Nikon D7500 camera (Tokyo, Japan). All illustrated remains presented here are currently housed in the Ege University Natural History Museum (Izmir, Türkiye) under the catalogue numbers EUNHM PV–KID-1-45 (KID = Konya, Ilgın, Dursunlu). To estimate whether the mollusk taxa composition was accurately sampled, conducted rarefaction analyses for the assemblages of four of the six samples (D2, D16, D17, D22) using R v4.3.2 [22] with the iNEXT v3.0.1 package was performed [23] and 500 calculation knots. For bivalves, the higher number of left or right valves was used as the number of specimens.
Taxonomic analysis follows the systematics of Horn af Rantzien [24] and Feist et al. [25] for gyrogonites, Neubauer et al. [26] for mollusks, Schönhuth et al. [27] and Küçuk et al. [28] for cyprinids, Dubois et al. [29] for anurans, de Broin [30] for turtles, and Szyndlar [31] for snakes.
4. Results
The Floral and Faunal Assemblage
The following sections report on the fossil flora, arthropods, mollusks, and vertebrates identified in the faunal assemblages of samples D2, D16–D17, and D22–D23 (Table 1).

Table 1.
List of fossil taxa of flora, invertebrates (arthropods and mollusks), and vertebrates (teleosts, anurans, testudines, and squamates) recovered at Dursunlu site. ‘•’ Denotes which taxa were previously reported in other publications, whereas ‘This work’ denotes the taxa described in this article.
Charophytes: Up to three gyrogonite species of the genus Chara (Characeae) have been found in the sample D-2. Chara hispida gyrogonites are large and prolate to perprolate in shape (Figure 3A–C: EUNHM PV–KID-1). The morphometrical parameters and the presence of a psilocharoid-type apex allow us to refer them to genus Chara, and more particularly, to C. hispida on the basis of the tapering base and periapical thinning and swollen apex (e.g., [32,33]; and references therein). The second species is attributed to Chara vulgaris (Figure 3D–G: EUNHM PV–KID-2) based on having a smaller size, prolate shape, pointed and psilocharoid apex, a rounded and tapered base, and a higher number of convolutions in lateral view (see [5,32]). The third species fits well with Chara globularis (Figure 3H–J: EUNHM PV–KID-3) since it displays a characteristic prominent and protruding psilocharoid apex, forming a hat-like shape. The base is slightly pointed and shows a large pore within a shallow pentagonal funnel [5,34]. These charophyte taxa have been recently described and depicted in detail by Demirci et al. [5] in the same stratigraphical column from Dursunlu.

Figure 3.
Remains of aquatic plants from DLQ (Ilgın Basin, Türkiye). (A–C) Gyrogonites of Chara hispida (EUNHM PV–KID-1) from sample D2. (A) Apical view; (B) lateral view; (C) basal view. (D–G) Gyrogonites of Chara vulgaris (EUNHM PV–KID-2) from sample D2: (D) apical view; (E,F) lateral views; (G) basal view. (H–J) Gyrogonites of Chara globularis (EUNHM PV–KID-3) from sample D2: (H) apical view; (I) lateral view; (J) basal view. (K,L) Lateral views of seeds attributed to Najas marina s. str. (EUNHM PV–KID-4) from sample D17. (M,N) Lateral views of seeds of Zannichellia palustris (EUNHM PV–KID-5) from sample D17.
From a paleobiogeographic viewpoint, Chara hispida probably originated during the Late Miocene in Western Europe and expanded quickly towards Asia during the latest Miocene–Pliocene. This species became fully cosmopolitan during the Quaternary (e.g., [35,36]), thriving in lakes in Europe (Spain and Poland: [37]), the Middle East (Western Türkiye: [38]), Africa (Morocco, Sudan: [39,40]), and South America (Argentina: [35]). Currently, it is a common taxon growing in permanent lakes in Europe, Siberia, and North Africa [36]. In turn, C. vulgaris is a cosmopolitan taxon that thrives in a wide array of shallow freshwater habitats (see [36,41]; and references therein). The fossil record suggests it originated during the Late Miocene in Western Europe as well and achieved a cosmopolitan distribution during the Pleistocene (see [33]). Pleistocene populations of this species have been found in lacustrine deposits in Eastern Europe (Cluj Basin, Romania: [42]), Western Türkiye (Söke region: Tuncer and Tunoğlu [43]), India (Kashmir and Bangong Co Basin: [44]), Africa (Chad, Algeria, and Sudan: [36]), and Argentina (Puna Plateau: [35]). Likewise, C. globularis appeared during the late Neogene, when its geographic distribution was restricted to the Northern Hemisphere. This species has been reported in the Late Miocene–Pliocene of the Middle East (Bekaa Valley, Lebanon: [45]), India (Haiti, Guajart, and Jammu: [34]) and North America (Kansas, USA: [46]). However, its Pleistocene record is limited to the West of Türkiye (Söke area: [43]).
Other aquatic plants: Seeds from two taxa of aquatic plants, belonging to the genera Najas and Zannichellia, have also been recovered from these deposits. EUNHM PV–KID-4 (Figure 3K,L) is a longer seed measuring more than 2 mm. Najas marina is an aquatic, annual, dioecious plant with notable morphological variability, especially in leaf shape, spine presence, and seed size (e.g., [47,48]. It typically has slender, branching, submerged stems with leaves that possess marginal teeth on the sheaths (see [47]; and references therein). The species adapts well to diverse freshwater habitats [48]. The revision of 29 herbarium specimens and recent field collections confirmed that all samples from the study area belong to Najas marina s. str. (Figure 3K,L), based on key morphological traits [47,48]. Despite size variations, the features fall within the species’ known range. New populations were discovered in the Sharypovskii District, extending the species’ known range 240–250 km northwest in Southern Siberia [49]. These new localities are endorheic post-glacial lakes developed on Devonian and Neogene formations, underscoring the long-term persistence of N. marina s.str. in the region [49]. Najas marina is a submerged macrophyte found in Turkish wetlands such as Lakes Mogan, Eber, and Beyşehir (e.g., [50,51]). It thrives in shallow, nutrient-rich freshwater habitats with stable sediments and high light penetration. It supports water clarity, sedimentation, and aquatic biodiversity, and indicates ecological status through changes in its distribution ([50]; and references therein).
EUNHM PV–KID-5 (Figure 3M,N) is a seed with a morphological appearance resembling a bean pod and measuring around 2 mm length. Zannichellia is a genus of submerged aquatic plants living in freshwater to brackish habitats [52,53]. According to Van Vierssen [52], Zannichellia palustris occurs in Northern to Western Europe, tolerates up to 3.5% salinity, and requires cold water for germination. It has short shoots, narrow leaves, and grows in shallow, temporary freshwater environments [52,53]. In the Baltic region, it is found in both brackish and freshwater environments [52]. Zannichellia palustris has been observed in several lakes across Türkiye, including Işıklı, Gökgöl, Eğirdir, Eber, Gölhisar, and Beyşehir ([51]; and references therein). It has a broad and subcosmopolitan distribution across temperate, subtropical, and tropical regions, including Europe, Asia Minor, Northern Africa, and parts of North America. It thrives in lakes, ponds, and slow-moving waters, demonstrating significant ecological flexibility (e.g., [49,52,53]).
Ostracods. In this study, ostracod fauna (Cyprididae, Ilyocyprididae, and Candonidae) of the palustrine and coal-bearing lower parts of the Dursunlu Formation are determined by three samples (D2, D16, and D17): six ostracod taxa belonging to six genera were identified. The most abundant and diverse ostracod assemblage was recovered in the sample D2: Heterocypris salina (53 valves), Ilyocypris cf. bradyi (52 valves), Neglecandona angulata (33 valves and two carapaces), Prionocypris zenkeri (16 valves), Cyclocypris sp. (one valve), and Pseudocandona sp. (one valve).
Heterocypris salina. The valves, particularly the dorsal margins, exhibit a sub-triangular shape in lateral view (e.g., [54,55,56,57]). The left valve overlaps the right valve. Posteroventral and anteroventral rims of the right valve are covered by some small denticles (see [14]) that are apparent along the latter (Figure 4A,B: EUNHM PV–KID-6).
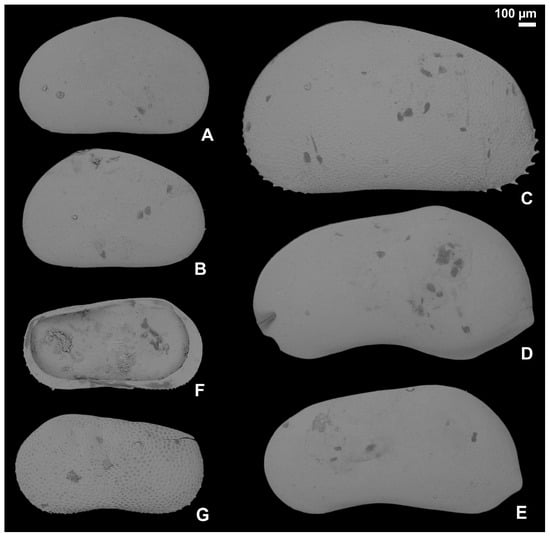
Figure 4.
Ostracod carapaces recovered from samples D2–D3 and D16–D17, DLQ (Ilgın Basin, Türkiye). All images are external views of the carapaces, except for F, which is in internal view. S. (A,B) Heterocypris salina (EUNHM PV–KID-6): (A), external view of the right valve (l: 1094 µm; h: 680 µm); (B) external view of the left valve (l: 1057 µm; h: 689 µm). (C) Prionocypris zenkeri (EUNHM PV–KID-7): external view of the left valve (l: 1705 µm; h: 996 µm). (D,E) Neglecandona angulata (EUNHM PV–KID-8): (D) external view of the left valve, male (l: 1617 µm; h: 891 µm); (E) external view of the left valve, female (l: 1487 µm; h: 743 µm). (F,G) Ilyocypris cf. bradyi (EUNHM PV–KID-9): (F) internal view of the left valve (l: 1085 µm; h: 574 µm); (G) external view of the left valve (l: 1032 µm; h: 533 µm). Abbreviations: l = length, h = height.
Prionocypris zenkeri. The valves are claviform in lateral view. Well-developed 7–8 bigger denticles are arranged in a row along the posteroventral margin of the left valve, whereas the anteroventral margin displays comparatively smaller ones (e.g., [14,55,58]). These diagnostic characteristics make it simple to distinguish this salient species from other taxa (Figure 4C: EUNHM PV–KID-7).
Neglecandona angulata. The valves are elongated in lateral view and exhibit sexually dimorphic variations in both lateral and dorsal views (e.g., [58,59]). Lobe-like expansion, the diagnostic character of the species, is visible in the posteroventral margin of the adult left valves [58,59,60,61]. Moreover, particularly in adult forms, the posterior area of the valve surfaces is reticulated (Figure 4D,E: EUNHM PV–KID-8).
Ilyocypris cf. bradyi. The valves are sub-rectangular in lateral view (e.g., [14,54,56,62]). The dorsomedian part of both valves contains two vertically oriented sulci (grooves). The anterior and posterior margins have a few tiny denticles, with the latter being particularly noticeable [54,62]. Although it is unclear, marginal ripplets of the left valve resemble bradyi-type ripples. According to the above-mentioned features, they are here referred to I. cf. bradyi (Figure 4F,G: EUNHM PV–KID-9).
Cyclocypris sp. and Pseudocandona sp.: Due to the limited number of forms obtained, detailed diagnoses enabling the way for species-rank identification could not be performed. Therefore, these are left in open nomenclature.
The new ostracod fauna studied here from DLQ resembles those determined by Tuncer [14], who thoroughly examined this group but from laterally equivalent levels of our samples of the same Dursunlu Formation. Unlike sample D2, the abundance of H. salina in D16 level strikingly decreases, being represented by only two valves and one carapace. The rest of taxa are composed by I. cf. bradyi (38 valves and two carapaces), N. angulata (29 valves and one carapace), and P. zenkeri (14 valves). As for the diversity and abundance of ostracod assemblage in D17 sample, it is relatively low: the most abundant species is P. zenkeri (14 valves of adults and larger juveniles), while species common in other samples, like N. angulata and H. salina, become extremely rare (two and one valves respectively, in this sample).
The earliest known records of H. salina are from the Early Miocene sequences of Anatolia [63] and surrounding regions. Particularly, the species became common in Pleistocene-Holocene deposits of Central and Southwestern Anatolia and in the Pleistocene region (e.g., [43,54,57,62,64]). The living representatives of the species are worldwide distributed, particularly in the Northern Hemisphere [60]. The fossil forms of N. angulata became prevalent since Late Pliocene-Pleistocene in the Mediterranean region [61] and have been also documented in several Pliocene-Quaternary strata from Central and Southwestern Anatolia [58,59]. Recent occurrences of the species are observed in the Palearctic zone [60]. Ilyocypris bradyi was previously reported from numerous Neogene-Quaternary sequences of Mediterranean and Balkan areas [64,65]. In Central and Southwestern Anatolia, it is known from Neogene [14,56] and particularly Pleistocene-Holocene [43,54,57,58,62] deposits. The living forms of this taxon are also widely distributed, like H. salina [60]. The oldest record of P. zenkeri in Europe is reported from the Lower Pleistocene strata of the Liri Valley, Italy (see [14]; and references therein). On the other hand, this species is significantly more common in the Anatolian fossil record and was discovered from Upper Miocene–Pleistocene [14,55] and Holocene [58] deposits. The distribution of the recent representatives is relatively narrow and reported in the Palearctic like N. angulata [60].
Mollusks. The mollusk fauna was recovered from samples D2, D16–D17, and D22. It is moderately diverse, containing at least 17 taxa, including 12 freshwater gastropods, four terrestrial gastropods, and at least one freshwater bivalve. The complete list of studied material is given in Supplementary Table S1. Most shells are juvenile specimens or fragments, sometimes not allowing the identification at the genus or species rank. All taxa that could be identified are still living in Europe and Asia Minor today. Since most of them are well-known taxa, we refrain from a detailed systematic assessment, but instead briefly summarize notes on taxonomy.
Two valvatids could be identified, i.e., the widespread European species Valvata cristata (Figure 5A: EUNHM PV–KID-10) and the Turkish endemic Valvata cf. kebapcii (Figure 5B: EUNHM PV–KID-11). The latter species resembles Valvata macrostoma, but the characteristic, weak angulation at the aperture suggests V. kebapcii. It is worth noting that V. cf. kebapcii represents the second most common taxon found at the Dursunlu site. Bithyniids are represented by a high number of apices and opercula with a subcentral nucleus (Figure 5C: EUNHM PV–KID-12): not a single complete shell could be obtained. The morphology suggests that all specimens may belong to a single species, which is here classified cautiously as Bithynia s.l. sp. A rather unusual find is the record of a minute, pseudamnicoline-like hydrobiid from sample D2, here attributed tentatively to the genus Tefennia (Figure 5D,E: EUNHM PV–KID-13 and EUNHM PV–KID-14). This genus is so far only known from a single extant species, Tefennia tefennica, endemic to the headwaters of a spring system in the Tefenni depression 60 km SW of Burdur [66]. Classification of hydrobiids is exceptionally difficult and, for extant material, primarily based on molecular and soft anatomical data. The identification of our material as Tefennia relies on the strong morphological similarity with an unpublished extant shell from Western Anatolia referred to this genus based on anatomical and molecular data (D. Delicado, pers. comm. in 2024). Further research is needed to substantiate this attribution. Two species of lymnaeids are recorded at Dursunlu, but both are represented by juvenile specimens only. The first taxon exhibits a broader spire and is here, tentatively attributed to the genus Radix (Figure 5G: EUNHM PV–KID-16). The second one has a more elongated shell resembling the genera Galba or Stagnicola, but without more complete shells, we provisionally place the taxon in open nomenclature (Figure 5F: EUNHM PV–KID-15) as an indeterminate member of the family.
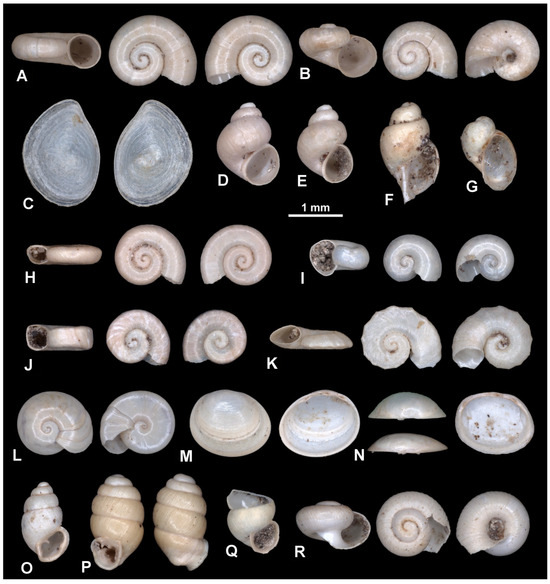
Figure 5.
Representatives of the mollusk taxa retrieved from sample D2 from DLQ (Ilgın Basin, Türkiye). (A) Valvata cristata (EUNHM PV–KID-10). (B) Valvata cf. kebapcii (EUNHM PV–KID-11). (C) Opercula of Bithynia s.l. sp. (EUNHM PV–KID-12). (D,E) ?Tefennia sp. (EUNHM PV–KID-13 and EUNHM PV–KID-14). (F) Lymnaeidae gen. et sp. indet. (EUNHM PV–KID-15). (G) ?Radix s.l. sp. (EUNHM PV–KID-16). (H) Anisus sp. (EUNHM PV–KID-17). (I) ?Gyraulus sp. (EUNHM PV–KID-18). (J) Planorbidae gen. et sp. indet. (EUNHM PV–KID-19). (K) Armiger crista (EUNHM PV–KID-20). (L) Segmentina nitida (EUNHM PV–KID-21). (M) Euglesa sp. (EUNHM PV–KID-22) right valve, convex form. (N) Euglesa sp. (EUNHM PV–KID-23) left valve, low-convex form. (O) Carychium minimum (EUNHM PV–KID-24). (P) Vertigo angustior (EUNHM PV–KID-25). (Q) Truncatellina sp. (EUNHM PV–KID-26). (R) Vallonia cf. enniensis (EUNHM PV–KID-27).
Planorbidae are the dominant gastropod family at Dursunlu, represented by at least five taxa. Of these, only common Armiger crista (Figure 5K: EUNHM PV–KID-20) and rare Segmentina nitida (Figure 5L: EUNHM PV–KID-21) could be identified to the species rank. In addition, one species of Anisus, one ?Gyraulus sp., and one Planorbidae gen. et sp. indet. were found. Anisus sp. is characterized by a rather slow increase in whorl diameter and pronounced convexity above the whorl center (Figure 5H: EUNHM PV–KID-17). It resembles juveniles of Anisus spirorbis, but the shell is more flattened and the ratio between shell height and width is less than in typical A. spirorbis. ?Gyraulus sp. shows an inflated early teleoconch whorl with spiral striae (Figure 5I: EUNHM PV–KID-18), which is found in several living species of the genus. Juvenile Planorbarius shows a similar morphology but has a usually immersed umbilical side. For the fifth taxon, even the genus could not be identified with certainty. The comparatively fast whorl expansion rate, and broadly rounded to subangulate whorl cross-section, matches several extant species of Planorbis and Gyraulus (Figure 5J: EUNHM PV–KID-19). Terrestrial gastropods were only detected in sample D2. Among them is a single specimen of Carychium minimum (Figure 5O: EUNHM PV–KID-24). It differs from its congener Carychium tridentatum in being bulkier. The sinistral Vertigo angustior is the most common terrestrial species (Figure 5P: EUNHM PV–KID-25). It can be distinguished from the generally similar Vertigo pusilla by its smaller and narrower shell, the presence of 5–6 apertural teeth that do not almost fill the aperture and a long and lamella-like palatal tooth. Truncatellina is represented by a single fragment of the last whorl containing the aperture (Figure 5Q: EUNHM PV–KID-26). The general shape as well as the delicate riblets suggest placement in Truncatellina. Finally, a taxon of Vallonia was detected that is tentatively identified as Vallonia cf. enniensis (Figure 5R: EUNHM PV–KID-27). The type and frequency of the ribs as well as the width of the umbilicus match well with V. enniensis. Another similar species is Vallonia chinensis, which is, however, geographically an unlikely candidate [67,68]. The common species Vallonia costata has fewer and typically stronger ribs. Valvata asiatica differs in a broader umbilicus, while Vallonia tenuilabris has a higher spire [69]. Moreover, the species V. enniensis has frequently been reported in the fossil record [70].
Among freshwater bivalves, several juvenile valves of the sphaeriid Euglesa sp. were found. The specimens cover a large range of variability and may include more than a single species; however, due to their juvenile state, identification is difficult. Specimens with a more angulate morphology, slender hinge, and low convexity (Figure 5N: EUNHM PV–KID-23) are similar to Euglesa pseudosphaerium, as described and illustrated by Gittenberger et al. [71]. This species is also known from other Pleistocene Turkish localities [72]. A more convex specimen (Figure 5M: EUNHM PV–KID-22) may represent Euglesa obtusalis.
Fishes. Up to eight fish remains belonging to four taxa (one unidentified Teleostei plus three cyprinids) have been identified in Dursunlu samples (D2, D3, D16, and D23), including a vertebra and pharyngeal bones. The single vertebra coming from the sample D3 displays an amphicoelous morphology and both neural arches are broken off. Although the vertebral morphology is compatible with that of Cyprinidae, due to its poor preservation, here, it is attributed to a Teleostei indet. (Figure 6A: EUNHM PV–KID-28). Three isolated and broken pharyngeal teeth were recovered from samples D2, D3, and D16 (Figure 6B–D: EUNHM PV–KID-29, EUNHM PV–KID-30, and EUNHM PV–KID-31). All of them are elongated and compressed mesiodistally on their entire length. The tip of the tooth is a short, sharp, and hook-directed mesial. The grinding surface is narrow and serrated with wrinkled surface patterns. The teeth have an elongated, narrow, labial grinding surface, serrated with marked wrinkled surface patterns. In two teeth (Figure 6B,D), several small bumps with rounded tips, which decrease in size towards the basal margin of the tooth, are discerned. In all recovered teeth, both the neck and the pedicle are not preserved. On the basis of the above-mentioned features, these pharyngeal teeth are referred to the cyprinid genus Squalius, which is widely distributed in the Western Palearctic, and more specifically in Türkiye (e.g., [6,73,74]). Regarding the third taxon, three isolated and broken teeth were recovered from samples D3 (Figure 6E–G: EUNHM PV–KID-32, EUNHM PV–KID-33, and EUNHM PV–KID-34). All available teeth are slim and compressed throughout their entire mesiodistal axis. The grinding surface is elongated and knife-shaped: in three specimens, a short hook is preserved (Figure 6F–H). The neck is poorly developed and slightly curves inward below the grinding surface. According to Çiçek et al. [74], up to fourteen species of the genus Chondrostoma are present in the Türkiye fossil record, of which eleven are endemic. Lastly, one isolated and broken pharyngeal tooth of Barbus is available (Figure 6H: EUNHM PV–KID-35). The tooth crown is slightly narrower than the tooth base, being the latter compressed at the foot-crown boundary (Figure 6H). It has a robust crown with a hook on its top, and a smooth grinding surface. According to Çiçek et al. [74]; and references therein), ten endemic species of the genus Barbus are present in Türkiye.
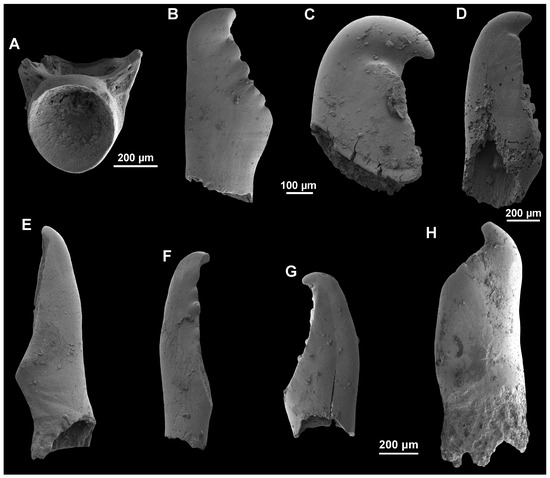
Figure 6.
Fish remains recovered from samples D2–D3, D16, and D23 from DLQ (Ilgın Basin, Türkiye). (A) Partial vertebra of Teleostei indet. (EUNHM PV–KID-28) from D3. (B–D) Isolated pharyngeal teeth of Squalius sp. (EUNHM PV–KID-29, EUNHM PV–KID-30 and EUNHM PV–KID-31) from D2 (see also [6]; Figure 3B), D3 (see also [6]; Figure 3C3), and D16 (see also [6]; Figure 3M), respectively. (E–G) Isolated pharyngeal teeth of Chondrostoma sp. (EUNHM PV–KID-32, EUNHM PV–KID-33 and EUNHM PV–KID-34) from D3 (see also [6]; Figure 3C1–C2). (H) Isolated pharyngeal teeth of Barbus sp. (EUNHM PV–KID-35) from D23 (see also [6]; Figure 3N).
Amphibians. This clade is represented by six remains that belong to three taxa (see Table 1), all of them recovered from sample D16. Due to the fragmentary nature of the bones, they have only been identified between the order and family level. Anurans are represented by one partial maxilla, EUNHM PV–KID-36, which is small and rather lightly built (Figure 7A,B). In particular, this maxilla cannot pertain to a bufonid, given the presence of teeth (in contrast to toothless maxillae in toads; [75]). Since this element is quite fragmentary and poorly significative for a more precise identification, it is here referred to Anura indet. Anurans are previously identified in Plio-Pleistocene deposits of Türkiye, and more specifically in Central Anatolia (e.g., [76,77]). EUNHM PV–KID-37 (Figure 7C–G) is a middle sized and moderately robust procoelous trunk vertebra. The centrum is well individualized and distinctly dorsoventrally compressed. The neural canal is wide. The transverse processes are robust, directed laterally and located posteriorly to the prezygapophyses. The dorsal surface of the neural arch is wide and rather short, with a strongly concave anterior margin. The arch displays a sharp carina neuralis, originating a very short point posteriorly. The large and well-extended zygapophyses are tilted dorsally with an angle of about 30°. The prezygapophyseal facets are circular, whereas the postzygapophyseal ones are subelliptical. According to Bailon [75], the attribution of this vertebra to a bufonid is supported by: procoelous morphology; short neural arch; transverse processes located posterior to the prezygapophyses; well individualized centrum; and overall robust structure. Isolated vertebrae of these anurans are not diagnostically significant enough for a more precise identification at the genus rank, because of a general homogeneous morphology. In Europe, bufonids are known since the Early Miocene (MN4) of Spain, whereas in Türkiye, the oldest remains were reported from the Pliocene locality of Çalta [78,79].
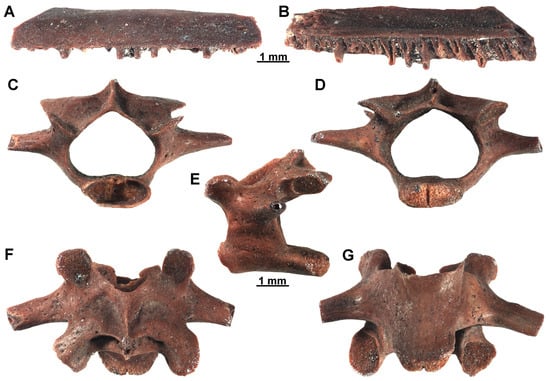
Figure 7.
Indeterminate anuran and bufonid remains recovered from sample D16, DLQ (Ilgın Basin, Türkiye). (A,B) Partial maxilla (EUNHM PV–KID-36) of Anura indet. in lateral (A) and medial (B) views. (C–G) Trunk vertebra (EUNHM PV–KID-37) of Bufonidae indet. in anterior (C), posterior (D), left lateral (E), dorsal (F), and ventral (G) views.
Specimen EUNHM PV–KID-38 is a slender angular bone, with a well-developed processus coronoideus. The process is wide, rounded, and almost vertically oriented (Figure 8A,B). Specimen EUNHM PV–KID-39, a coracoid, is lightly built and straight, very narrow at mid-length, but expanding towards the medial and lateral ends (Figure 8C,D). The pars epicoracoidalis is strongly more expanded in anteroposteriorly length than the pars glenoidalis, being more than twice the width of the latter. The first ilium is very small and lightly built, probably representing a very young individual (EUNHM PV–KID-40: Figure 8E,F). A second ilium (EUNHM PV–KID-41) is notably larger and much more robust (Figure 8G,H). All two ilia are fragmentary, but they clearly display the presence of a dorsal crest (always damaged), a dorsal tubercle (poorly marked in the small ilium, but well distinct in the largest one), a supraacetabular fossa, a wide acetabular fossa with a raised anteroventral portion of the acetabular rim, and a smooth medial surface of the ilial body with no evidence of an interiliac tubercle or groove. The supraacetabular fossa is much deeper in the largest ilium, compared to the smallest one. The angle between the dorsal tubercle and the short dorsal acetabular expansion is very obtuse in the small ilium. The same feature cannot be evaluated in the largest one as both the tubercle and the expansion are almost completely broken off (Figure 8G,H: EUNHM PV–KID-41). The largest ilium is the only specimen in which the morphology of the ilioischiatic juncture can be observed. The latter is rather narrow in the posterior view. All these remains show ranid features. As far as the angular and the coracoid are concerned, diagnostically significant are the vertical processus coronoideus and the strongly expanded pars epicoracoidalis, respectively [75]. The two ilia are comparable in morphology to the genus Rana, but they are referred tentatively to Ranidae indet. We avoid an attribution to brown frogs, though. The obtuse angle in the two small individuals may indeed suggest Rana affinities, but this feature is similar in very young green frogs [80]. Adding to this, the narrow ilioischiatic juncture of the largest specimen may also hint towards Rana for this other specimen [81], but identification to genus rank of such a fragmentary fossil would be far from confident in our opinion (especially without clear information on the dorsal tubercle/acetabular expansion angle).
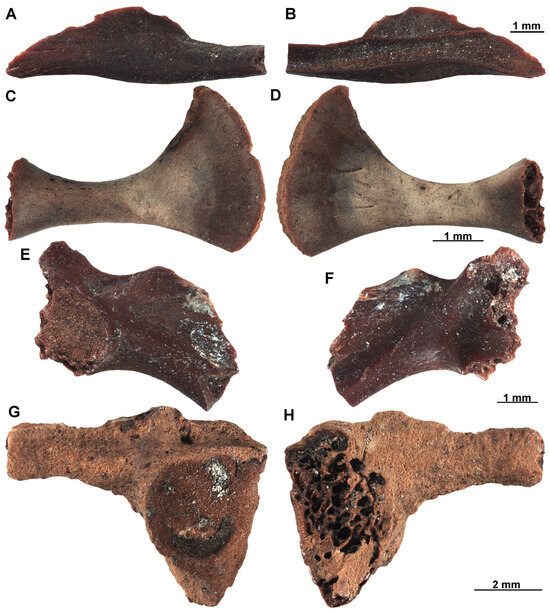
Figure 8.
Ranid remains recovered from sample D16, DLQ (Ilgın Basin, Türkiye). (A,B) Partial angular (EUNHM PV–KID-38) of Ranidae indet. in ventral (A) and dorsal (B) views. (C,D) Coracoid (EUNHM PV–KID-39) of Ranidae indet. in dorsal (C) and ventral (D) views. (E,F) Partial ilium (EUNHM PV–KID-40) of Ranidae indet. in lateral (E) and medial (F) views. (G,H) Partial ilium (EUNHM PV–KID-41) in ventral (G), and medial (H) views.
Reptiles. A single shell fragment of a testudinoid (EUNHM PV–KID-42) coming from D16 sample is available (Figure 9A,B). This specimen corresponds most likely to a portion of plastron as it is lightly built, but it is difficult to confirm this with certainty. The maximum width of the preserved plate fragment is 4,5 cm. As a rule, both geoemydids and testudinids have dermal scutes, which can or not be ornamented (e.g., [82,83]); however, the external portion of this preserved shell is smooth and without ornamentation, and, further, it is not crossed by any sulcus (Figure 9A). Nonetheless, on the basis of the minimal thickness of the plate, observed in EUNHM PV–KID-42, we refer it to cf. Geoemydidae indet. Another reptilian specimen (EUNHM PV–KID-43) consists of a notably small and poorly preserved procelous trunk vertebra. Only the centrum and the right wall of the neural arch (with the right prezygapophysis) are preserved (Figure 9C: EUNHM PV–KID-43). The posterior condyle broke off during manipulation, but it is still available as very small, separated bone fragments. The centrum is anteroposteriorly elongated and dorsoventrally compressed. The ventral surface of the centrum is gently convex and displays no evident ventral keel. The right synapophysis is dorsoventrally elongated. The prezygapophysis is slightly tilted dorsally in anterior view and ovoid in dorsal view. This isolated vertebra can be ascribed to Squamata indet. Referral to both snakes and amphisbaenians can be discarded given the morphology of the synapophysis (i.e., not separated into para- and diapophysis as in most snakes and not massive and rounded as in amphisbaenians). The dorsoventrally elongated shape is more comparable with that of a lizard, to which this vertebra here is tentatively referred. The small size, the compressed centrum, as well as the gently convex ventral surface are somehow reminiscent of vertebrae of scincids, such as Ophiomorus [84], suggesting that this fossil may belong to a taxon related to this group of lizards. However, we herein deem this potential identification to be too speculative, given the very poor preservation of the specimen and absence of more informative material. Therefore, this vertebra is only referred to as an indeterminate lizard (Squamata indet.).
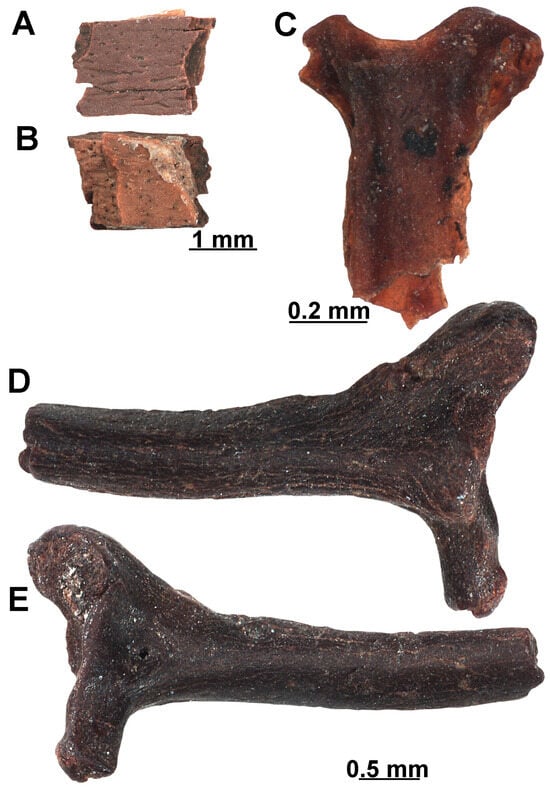
Figure 9.
Turtles and squamate remain recovered from sample D16, DLQ (Ilgın Basin, Türkiye). (A,B) Shell fragment (EUNHM PV–KID-42) of cf. Geoemydidae indet. in dorsal (A) and ventral (B) views. (C) Trunk vertebra (EUNHM PV–KID-43) of Squamata indet. in ventral view. (D,E) Partial rib of Serpentes indet. (EUNHM PV–KID-44) of Serpentes indet. in anterior (D), and posterior (E) views.
Snakes are represented by a single small partial rib (EUNHM PV–KID-44; Figure 9D,E) and one middle trunk vertebra (EUNHM PV–KID-45; Figure 10A–F). EUNHM PV–KID-44 is an elongated element, medially curved, and with its distal end broken. Given the poor information provided by this bone, here, we refer it to an indeterminate snake i.e., Serpentes indet. The incomplete procoelous vertebra (EUNHM PV–KID-45; Figure 10A–F) is rather small and almost complete, with elongated centrum. The neural spine and hypapophysis are not preserved and the diapophyses are also slightly damaged. In the lateral view, the broken-off neural spine rose at the posterior end of suboval zygosphenal facets. Large lateral foramina are situated close under the rather sharp interzygapophyseal ridges. Synapophyses are well divided into para- and diapophyses, with the latter situated slightly behind the former. The short parapophyseal processes are directed anteriorly rather than antero-ventrally. Subcentral ridges are long and moderately arched dorsally. The preserved base of the hypapophysis starts to rise behind the posterior level of the diapophyses. In dorsal view, the zygosphene has two sharp and short lateral lobes oriented anteriorly and a wide, but indistinct median lobe. The prezygapophyseal articular facets are subtriangular in outline. Only the left prezygapophyseal process is preserved; it is about as long as the prezygapophyseal facet and oriented anterolaterally. The epizygapophyseal ridges are moderately developed. The caudal notch is rather deep. In the ventral view, the vertebral centrum is triangular. Subcentral grooves are relatively deep and small; marked subcentral foramina are present close to the base of the hypapophysis and situated at the midlength of the subcentral grooves. The anterior keel of the thin hypapophysis is triangular. The postzygapophyseal articular facets are roughly circular in outline. In the anterior view, the neural arch is moderately vaulted, with slightly developed epizygapophyseal ridges. The neural canal is rounded with two short lateral sinuses. The zygosphene is thin and nearly straight. Prezygapophyses are horizontal, with prezygapophyseal facets situated high above the base of the neural canal. The obtuse parapophyseal processes are oriented medially. On the sides of the subcircular cotylar rim, there are small, yet well visible paracotylar foramina. In caudal view, small zygantral foramina are situated within the rather deep zygantrum. The condyle is suborbicular. Measurements are as follows: central length (cl) = 3.72 mm; narrow anterior width (naw) = 2.61 mm; cl/naw: or = 1.42. The sole snake vertebra from Dursunlu undisputedly displays an hypapophysis. Because of the rather deep subcentral ridges, it can be excluded that it comes from the anterior trunk section of a colubrid snake. The vaulted neural arch and horizontal prezygapophyses in anterior view also excludes its classification as a viperid snake. This vertebra could be easily attributed to Natrix, if the two most important structures, neural spine and hypapophysis, were not broken. Therefore, the vertebra is attributed rather as cf. Natrix sp. on the basis of the following combination of features: (1) the vertebral centrum is elongated; (2) the hypapophysis is present with triangular anterior keel; (3) parapophyseal processes are directed anteriorly; (4) vaulted neural arch with rather indistinct, yet present epizygapophyseal ridges; (5) subcentral ridges are strong and marked; and (6) neural arch is moderately vaulted [85,86,87]. Two species of Natrix can be considered survivors through the Pliocene-Pleistocene transition: Natrix longivertebrata and Natrix rudabanyaensis. Specifically, N. longivertebrata, which is documented mainly from Hungary and Poland [85,88,89], shares with the Dursunlu specimen a narrow zygosphene, well delimited subcentral ridges, and a broad triangular anterior hypapophyseal keel. However, EUNHM PV–KID-45 differs in less prominent centrum length, a sharper prezygapophyseal process, and shorter parapophyseal processes. Natrix rudabanyaensis, known mostly from Hungary, but also from Romania and Greece [90,91,92], shares with EUNHM PV–KID-45 the elongated vertebral centrum, long prezygapophyseal processes, and moderately developed subcentral ridges. EUNHM PV–KID-45 differs in a more vaulted neural arch with less prominent epizygapophyseal ridges, and horizontal prezygapophyses with triangular facets. It is up to discussion if Natrix aff. rudabanyaensis from the Greek locality of Maramena (Miocene-Pliocene transition; [92]) could share affinities with Natrix occurrences from the Pleistocene of Anatolia, such as the one from Dursunlu. From these Greek specimens, EUNHM PV–KID-45 differs in shorter parapophyseal processes, pezygapophyseal facets being triangular in outline, smaller subcentral foramina, and wider lateral sinuses of the neural canal. During the Pleistocene, two species of Natrix are reported from Eastern Europe: N. natrix ([31,93,94,95,96,97]; and references therein) and N. tessellata [81,85]. EUNHM PV–KID-45 differs from Natrix natrix in a longer prezygapophyseal process, less prominent lateral lobes of the zygosphene, and shorter parapophyseal processes [31,81], while Natrix tessellata has shorter prezygapophyseal processes and smaller diapophyses [85]. The studied vertebra closely resembles Natrix sp. from the Turkish Pasinler Basin, but in contrast to that, it has a straight zygosphene and a cotyle without subcotylar tubercles [98]. In lateral view, it differs from Natrix cf. natrix from the Emirkaya-2 locality only in straighter subcentral ridges [76]. While the preserved long prezygapophyseal process differs from most of the Pleistocene Natrix taxa, it is not possible to attribute the vertebra beyond (tentatively) the genus rank as the highly diagnostic hypapophysis and neural spine are broken. Several snake taxa have been described from the Pleistocene localities of Türkiye. Specifically, from the Early Pleistocene, the only properly documented snake taxon is Natrix sp. from the Pasinler Basin [98]. Alternatively, the Middle Pleistocene locality of Emirkaya-2 provides the richest snake assemblage, including the following taxa: Scolecophidia indet., Coluber caspius (now referred to as Dolichophis caspius; see [99]), Coluber (s.l.) sp., Elaphe cf. quatuorlineata (Elaphe cf. E. quatuorlineata sensu [76]), cf. Telescopus sp., Colubridae indet., Natrix cf. natrix (sensu [76]) and Vipera sp. [76]. Also of Middle Pleistocene age, locality Yenisarbademli yielded only a fragmentary vertebra each of Natricinae indet. and Colubrinae indet. [100].
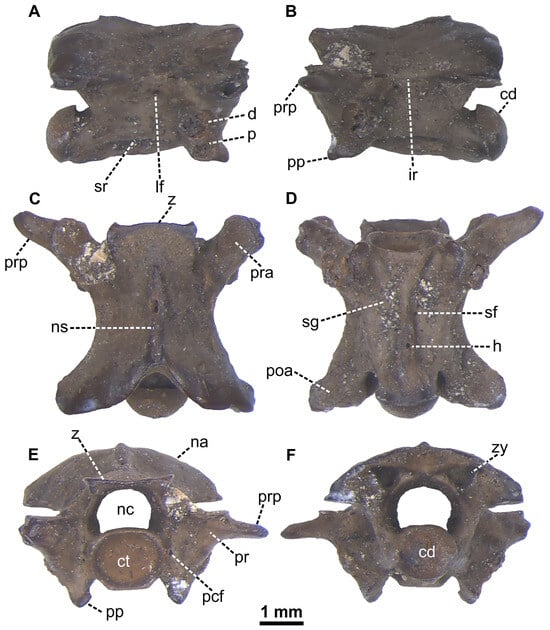
Figure 10.
Cf. Natrix sp. remains recovered from sample D16, DLQ (Ilgın Basin, Türkiye). (A–F) Trunk vertebra (EUNHM PV–KID-45) in right lateral (A), left lateral (B), dorsal (C), ventral (D), anterior (E), and posterior (F) views. Abbreviations: cd, condyle; ct, cotyle; d, diapophysis; h, hypapophysis; ir, interzygapophyseal ridge; lf, lateral foramen; na, neural arch, nc, neural canal; ns, neural spine; p, parapophysis; pcf, paracotylar foramen; poa, postzygapophyseal articular facet; pp, parapophyseal process; pr, prezygapophysis; pra, prezygapophyseal articular facet; prp, prezygapophyseal process; sf, subcentral foramen; sg, subcentral groove; sr, subcentral ridge; z, zygosphene; zy, zygantrum.
5. Discussion
5.1. Paleoenvironmental Reconstruction of Dursunlu Paleolake
Most of the identified taxa from DLQ are still present in Türkiye and Central Anatolia and; therefore, we use an actualistic approach to infer conditions that prevailed in the lake during the Early Pleistocene. The presence of well-preserved gyrogonites of Chara hispida, C. vulgaris, and C. globularis not only confirms those remains were deposited in situ but further denotes a fully lacustrine conditions of the studied section. It is noteworthy that both C. vulgaris and C. globularis, display wide ecological amplitude, thriving in many kinds of shallow aquatic environments from small eutrophic temporary ponds to oligotrophic permanent lakes, at depths of up to one meter (see [5]; and references therein). Among the charophyte flora, the most remarkable and informative taxa is C. hispida, mainly recovered from samples D2–D3 and D22–D23 of the stratigraphical column, which indicates that stable oligohaline and meso-oligotrophic conditions prevailed in these intervals [5,6]. According to Van Vierssen [52], Zannichellia palustris is a fresh-brackish water cosmopolitan taxon, which commonly lives in eutrophic temporary water bodies, and seeds of this taxon germinate at relatively low temperatures (8–12 °C). The abundance of macrophyte seeds, such as Zannichellia palustris and Najas marina, the abundance of planorbid gastropods, the high organic content, and the strongest decrease in charophytes in the middle part of the series (D17), denotes very shallow and highly eutrophic conditions in a fully palustrine context. Such reconstruction is also reinforced by the fragments of halophytic plants (reeds) and the presence of rootlet marks at the base of the lignite intervals.
Regarding ostracods, the presence of Heterocypris salina and Neglecandona angulata throughout all samples supports a palustrine paleoenvironment, but more specifically, the abundance of those ostracods in the lower samples (i.e., D2–D3) would suggest shallow water conditions and very little saline. In contrast, the ostracod fauna recovered from middle levels (samples D16–D17) is dominated by Ilyocypris cf. bradyi: N. angulata and H. salina are poorly represented in these samples, which implies almost zero salinity conditions. Although it is difficult to assess with confidence the conditions of the paleolake in detail for D16-D17 samples, it seems that the prevalence of P. zenkeri and I. cf. bradyi in the assemblage may indicate that the stream recharges to the basin were still effective. The ostracod assemblage presented here resembles those studied by Tuncer [14], who thoroughly examined a similar fauna coming from laterally equivalent levels of the Dursunlu Formation.
As for mollusks, Valvata cristata is a calciphile species known from springs, slowly flowing waters, lakes, ponds and, rarely, temporary water bodies. It prefers eutrophic habitats with rich vegetation, muddy substrate, and well-oxygenated water [70,101]. Valvata cf. kebapcii, which is the second-most common species found at DLQ, prefers to inhabit shallow streams and brooks with lush vegetation and sandy substrate [102]. Considering the uncertainty of the systematic classification and the wide range of environments extant bithyniids dwell in, we refrain from inferring any ecological preference for this taxon. Though lymnaeid snails occur in all types of habitats, they prefer richly vegetated waters [101]. Planorbids are the dominant gastropod family at Dursunlu, but species classification was possible only in two cases: Armiger crista and Segmentina nitida. The former dwells today in lentic, permanent water bodies with rich vegetation, often on the leaves of water plants or at the vegetated fringes of lakes [70], whereas the latter is known to live in lentic waters such as ponds, pools, ditches, and lakes with rich vegetation and high humic contents throughout most of the Palearctic [70,101]. The terrestrial snail Carychium minimum favors wet habitats, such as swamps, river plain woods, and wet meadows, and usually occurs near silent water bodies on stones, plants, tree branches, or submerged wood. It does not tolerate drought but can withstand flooding periods [70]. Vertigo angustior is a small terrestrial and calciphile species typically found in litter. It occurs in a variety of environments, ranging from open dry habitats and sandy dunes in coastlands with low vegetation, to areas with high humidity or permanently moist, open habitats [70]. The genus Truncatellina is known from a variety of habitats but is much more common in dry, often calcareous environments [70]. The species Vallonia enniensis dwells today in humid and wet habitats in lowlands, on meadows, and on calcareous swamps, and sunny hills with springs in the highlands [70]. Among freshwater bivalves, today, both E. pseudosphaerium and E. obtusalis live in swamps or lentic waters with abundant vegetation, sometimes even occurring together [70].
From a mollusk diversity point of view (Figure 11, ST1), the number of taxa generally decreases from the oldest to the youngest sample. While in sample D2, almost all taxa were recorded, samples D16, D17, and D22 each contain a smaller subset. The rarefaction analysis indicates that only D17 captures the mollusk fauna well, while for D2 and D16, one additional species each may be recovered with more intensive sampling. Note, however, that the sphaeriids may already include more than a single species. For D22, the rarefaction curve was even steeper: according to the analysis, approximately three more species may be found with doubling the sampling effort.
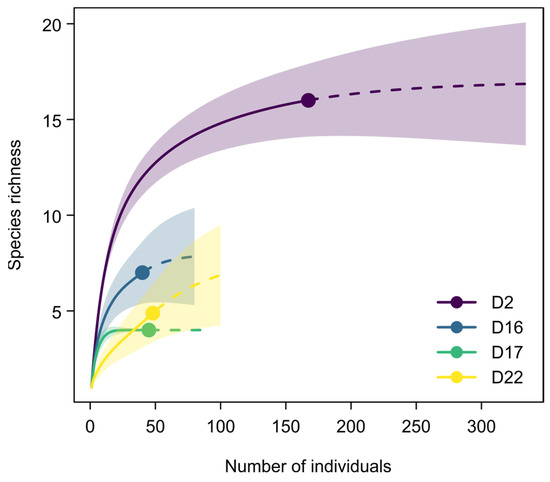
Figure 11.
Rarefaction analysis of the mollusk samples (D2, D16, D17, and D22) from DLQ.
Sample D2 is dominated by pulmonate taxa (Planorbidae, Lymnaeidae), followed by truncatelloids (Hydrobiidae, Bithyniidae), valvatids, and sphaeriids. Especially, the pulmonates, valvatids, and sphaeriids indicate the presence of a richly vegetated habitat. The pulmonates and sphaeriids furthermore point towards a permanent, lentic environment. Valvata cristata indicates well-oxygenated water, and Valvata cf. kebapcii and ?Tefennia sp. suggest at least some water movement. It may be noted that studied samples contain many juvenile and fragmented shells, which signals of transport, and further suggests that taxa may not have all dwelled in the same habitats. In any case, the rather pristine shell matter makes a bias from reworking unlikely. The fringes of the water body were inhabited by terrestrial snails: Vertigo angustior, Truncatellina sp., and Vallonia enniensis suggest the presence of calcareous soils near the water. The remaining samples consist of similar assemblages. All four samples (D2, D16, D17, D22) contain Bithynia s.l. sp. and Valvata cf. kebapcii, along with few planorbids and sphaeriids. In the case of D16, rare lymnaeids also occur. Despite being much less diverse assemblages, the paleoecological conditions were probably similar to D2. Apart from absent hydrobiids and terrestrial snails, the same groups (bithyniids, pulmonates, valvatids, and sphaeriids) are present. The lack of Valvata cristata may point towards poorly oxygenated waters, whereas the absence of terrestrial snails perhaps indicates deposition further away from the shore. Note that compared to the other samples, shells in sample D22 are poorly preserved, possibly indicating some transport or reworking. In any case, the mollusk association is congruent with a mixture of palustrine and shallow lake facies at DLQ.
The fish assemblage recovered from the six samples is low diverse, including three genera Cyprinidae (Squalius, Chondrostoma, and Barbus) and the unidentified remains of another teleost taxa. The three cyprinind genera are still present in Türkiye and Central Anatolia in particular (Figure 12). The fish composition from the bottom of the sequence fits well with palustrine freshwater environment, following previous interpretations based on charophytes and fish [5,6]. The presence of Squalius and Chondrostoma, together with the family Cobitidae reported by Blanco-Lapaz et al. ([6]; samples D4, D6–D9), would suggest the presence of some water movement (e.g., fast/slow-moving waters). The same authors further reported in the lowermost samples (i.e., D6–D7) the genera Barbus (here in sample D23) and Capoeta: the former are generalist and benthonic but prefer fast-flowing rivers or streams with sandy and pebbly bottoms and well-oxygenated waters, whereas the latter preferred either slow-flowing or oxygen rich waters (e.g., [28,103,104]).

Figure 12.
Life reconstruction of the most relevant flora and fauna from the Early Pleistocene site of DLQ. Reconstruction artwork by Roc Olivé.
Due to the low precision to which remains were identified, anurans and lizards provide only limited paleoecological information. Today, amphibians inhabit most types of freshwater bodies—such as rivers, lakes, swamps, and ponds—as they need water to lay their eggs and/or for other physiological needs. As a rule, frogs always need to be near water to keep the skin moist, while toads spend more time out of the water and have adapted more efficiently to thrive in drier environments. However, bufonids and ranids of the genus Rana include species with wide ecological preferences (e.g., [105]) and so they can only be used to confirm the presence of water bodies in Dursunlu. Affinities of the lizard vertebra with Ophiomorus skinks may support the presence of rocky microenvironments in the surroundings of the lake, where extant Western species of this lizard genus live [84,106,107]. However, this cannot be stated with certainty given that preservation of the fossil hampers a confident identification even at genus rank. Moreover, most geoemydids are classified as semiaquatic turtles and only leave the water for a few hours to either thermoregulate or lay their eggs. However, some species of this family show some ecological flexibility (e.g., genera Rhinoclemmys, Cuora, and Geoemyda) and could be considered semiterrestrial, which implies that they spend a significant amount of time on land as well, mainly for feeding ([108,109,110]; and references therein). Though members of the genus Natrix spends time thermoregulating on land or diving in shallow water, this group of natricines is well adapted to wet areas, such as rivers, lakes, ponds, and wetlands (Figure 12), as they depend on them for both food (mainly fish and amphibians) and reproduction (e.g., [111]). The fact that only amphibians and reptiles have been recovered in sample D16 reinforces the idea that this level was deposited under extremely shallow conditions, and particularly at the lake margins. In this paleoenvironment, extensive stands of halophytic plants would have thrived, forming local peat swamps, being ideal for the proliferation of herpetofauna, mollusks, and fish fingerlings.
5.2. Potential Resources for Homo During the Early Pleistocene
During the Early Pleistocene, the area of Dursunlu had two peculiarities: a high elevation and an unfavorable climate. As pointed out by Kuhn et al. [12], the high elevation (i.e., altitudes above 1000 m above sea level) of the most productive sites of Central Anatolia, such as KD3, Dursunlu, and Göllüdağ, was an important challenge for early hominins in Eurasia during the Early Pleistocene (see also [112]). Thereby, attempts by hominins to inhabit the Dursunlu area at the time would have been affected by the continental climate of Central Anatolia with harsh winters at those elevations and even more so during glacial periods. Previous studies (e.g., [113]) have suggested that sites in Anatolia would not have been an inhospitable environment for the early hominins who dispersed from Africa and Asia (see also [112,114,115]).
The steparian conditions around wetland areas of Dursunlu appear to have been an excellent environment to support sporadic settlement of hominins, who would make stone tools and obtain food resources [1,2,8]. One may assume that early hominins at Dursunlu have exploited alluvial plains and nearby areas that provided access to quartz rocks, enabling tool production and supporting a mobile foraging subsistence strategy, i.e., scavenging, gathering, and small to medium-scale hunting (e.g., [116,117,118]). Although more than a hundred stone tools have been recovered at Dursunlu, this sample of artifacts does not provide a complete account of the tool-making technologies and, therefore, does not allow to confirm the idea of a continuous settlement in Central Anatolia [1,2,8,112].
As far as we know, many African and Eurasian Lower Paleolithic sites are located near freshwater sources, serving as potential meeting points or hotspots during the early dispersal routes of hominins (e.g., [119,120]). Early hominins certainly exploited available resources provided by the lacustrine system of Dursunlu, offering with clear and well-oxygenated water and at the same time attracting potential prey [1,2,5,6,8,121]. Even though detailed taphonomical analyses are required, the herpetofauna and fish recovered from Dursunlu could have been part of the potential resource for early Homo during the Early Pleistocene. Particularly, fossils recovered from sample D17 were deposited at the lake margins under extremely shallow conditions, further indicating a very short transportation of the fish and some herpetofauna remains [5,6]. Although there is no evidence of reptile and amphibian consumption by early humans in Dursunlu, the extensive stands of halophytic plants and peat swamp environments would have facilitated the capture of these animals. Previous authors also reported on the consumption of turtles in other Eurasian and African Early Pleistocene contexts, such as testudinids (e.g., [122]), geoemydids (e.g., [123]), trionychids (e.g., [124]), and pelomedusids (e.g., [125]). While the consumption of turtles by hominins is considered a rather sporadic event, small animals—i.e., birds, reptiles, amphibians, fishes, and others—inhabiting the surrounding areas of the Dursunlu paleolake would have been an important component of small game, and more specially, during the cold (glacial) stage of the late Early Pleistocene (Günz glaciation) when large prey and carrion would have become scarce.
6. Conclusions
The DLQ assemblage stands out as one of the most abundant and diversified floras and faunas from the Early Pleistocene documented thus far in Central Anatolia (Türkiye), enhancing our understanding of the past ecosystem of the Dursunlu paleolake and surrounding steppe areas during the Günz glaciation.
The charophyte association recovered from the six samples herein studied coming from palustrine and peat bog facies, includes three (Chara hispida, Ch. vulgaris, and Ch. globularis) of the 11 taxa already reported in previous studies. Two taxa of seeds have been identified (i.e., Najas marina and Zannichellia palustris), which refine our knowledge of aquatic plants from the paleolake. Ostracods are scarcely represented in DLQ by six taxa, which resemble those studied in Tuncer’s PhD thesis: Heterocypris salina, Prionocypris zenkeri, Neglecandona angulata, Ilyocypris cf. bradyi, Cyclocypris sp., and Pseudocandona sp. On the other hand, mollusks are moderately diverse (17 taxa), including one freshwater bivalve, four terrestrial gastropods, and 12 freshwater gastropods. Fish are underrepresented with only four taxa (Teleostei indet., Squalius sp., Chondostroma sp. and Barbus sp.); however, this is most probably due to the few samples included in the present study. Among amphibians, Anura indet., Bufonidae indet., and Rana sp. have been identified. The reptile assemblage, in turn, comprises cf. Geoemydidae indet., Squamata indet, Serpentes indet., and cf. Natrix sp.
The fauna and flora reported from DLQ in our study is in line with the Early Pleistocene age, estimated based on the rodent assemblage. The ecological requirements of flora, ostracods, mollusks, fish, amphibians, and reptiles presented here are congruent with previous paleoenvironmental inferences, indicating a very shallow eutrophic lake with a dense palustrine vegetation belt at the margins, surrounded by steppe areas, under glaciation conditions in the Ilgın Basin, Central Anatolia, Türkiye. The present study further adds new data to the potential role played by the Dursunlu wetland and the surrounding steppes for sporadic settlements of hominins during the Early Pleistocene: the paleolake would have provided easy access to water and a variety of food resources, such as the fish, amphibians, and reptiles described here for the first time.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d17090631/s1, Table S1: Composition of the studied mollusk assemblages at DLQ. The numbers for Bithynia refer to number and apices and opercula, respectively; only opercula (including fragments) containing nucleus were counted. For Euglesa, the higher number of left or right valves was used to calculate the total number of specimens.
Author Contributions
Conceptualization, À.H.L., E.D., and J.S.; methodology, À.H.L., E.D., and J.S.; software, T.A.N.; validation, À.H.L., E.D., and J.S.; formal analysis, À.H.L. and T.A.N.; investigation, À.H.L., V.P., E.D., A.V., T.A.N., A.T., M.I., À.B.-L., K.A.V.-P., and J.S.; resources, À.H.L., E.D., T.A.N., and J.S.; data curation, À.H.L., E.D., T.A.N., and J.S.; writing—original draft preparation, À.H.L., V.P., E.D., A.V., T.A.N., A.T., M.I., and J.S.; writing—review and editing, À.H.L., V.P., E.D., A.V., T.A.N., A.T., M.I., À.B.-L., K.A.V.-P., and J.S.; visualization, À.H.L., V.P., E.D., and J.S.; supervision, À.H.L.; project administration, À.H.L., V.P., E.D., T.A.N., and J.S.; funding acquisition, À.H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research is funded by the postdoctoral grant ‘Programa Postdoctoral Beatriu de Pinós de la Secretaria d’Universitats i Recerca del Departament d’Empresa i Coneixement de la Generalitat de Catalunya’ (2019 BP 00154 À.H.L. and 2021 BP 00038 to A.V.) and the Generalitat de Catalunya (CERCA Program). This paper is part of projects Bap Hacettepe University (Ankara/Türkiye), code ID: 18910, FHD-2021-18910; International Research Fellowship Program of TUBITAK 2214-A, fellowship code: 1059B142100601; Project IBERINSULA, code: PID2020-113912GB-100/AEI/10.13039/501100011033; and Project IBERCAFO, code: PID2024-159218NB-I00 of the Spanish Research Agency (AEI) and the European Regional Development Fund (ERDF) and the project “Geologia Sedimentària” (2022 SGR 00349) of the AGAUR (Catalan Autonomous Government); R+D+I projects PID2020-117289GB-100/AEI/10.13039/501100011033 and PID2020-117118GB-I00/AEI/10.13039/501100011033, funded by the Agencia Estatal de Investigación of the Spanish Ministerio de Ciencia e Innovación. This study was also supported by the Specific Research Project MUNI/A/1576/2024 of the Faculty of Science at Masaryk University in Brno (VP, MI).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
All studied specimens have a repository number and are available at the Ege University Natural History Museum (Izmir, Türkiye).
Acknowledgments
We thank the editor and three anonymous reviewers for revisions which ultimately led to the improvement of this work. T.A.N. is grateful to Ruud Bank, Diana Delicado, Eike Neubert, and Maxim Vinarski for the comments and discussion regarding mollusk taxonomy. E.D. also thanks Cemal Tunoğlu of Hacettepe University. The authors thank the Konya Şeker Factory for offering the access to the Dursunlu coal mine and for its help and kind assistance during fieldtrips.
Conflicts of Interest
Author Elvan Demirci was employed by the company MSA Geological Engineering. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Güleç, E.; Howell, F.C.; White, T. Dursunlu—A new Lower Pleistocene artifact-bearing locality in southern Anatolia. In Hominid Evolution: Lifestyles and Survival Strategies; Ullrich, H., Ed.; Archaea: Gelsenkirchen, Germany, 1999; pp. 349–364. [Google Scholar]
- Güleç, E.; White, T.; Kuhn, S.; Özer, I.; Sagır, M.; Yımaz, H.; Howel, F.C. The Lower Pleistocene lithic assemblage from Dursunlu (Konya), central Anatolia, Turkey. Antiquity 2009, 83, 11–22. [Google Scholar] [CrossRef]
- Yavuz, N.; Saraç, G.; Ilgar, A.; Tuncay, E.; Bozkurt, A.; Ergen, A. Palynology of the Lower Palaeolithic Dursunlu hominin site, Central Anatolia, Türkiye. Mediterr. Geosci. Rev. 2024, 6, 639–650. [Google Scholar] [CrossRef]
- Ünay, E.; Karabıyıkoğlu, M.; Kazancı, N.; Saraç, G. The Dursunlu open cast mine. In Volume of Abstracts and Excursion Guide. INTER-INQUA Colloquium on Milankovitch and Plio-Pleistocene Vegetation Successions from 2.6 to 0.9, Ankara, Türkiye, 29 March–1 April 1997; Leroy, S.A., Ravazzi, C., Eds.; IQUA: Milano, Italy, 1997; pp. 69–74. [Google Scholar]
- Demirci, E.; Sanjuan, J.; Tunoğlu, C. Early Pleistocene charophyte flora from Dursunlu (Ilgın Basin, Turkey): Paleoecological implications. Rev. Palaeobot. Palynol. 2023, 311, 104848. [Google Scholar] [CrossRef]
- Blanco-Lapaz, À.; Luján, À.H.; Demirci, E.; Sanjuan, J. Early Pleistocene freshwater fish from Dursunlu (Ilgın Basin, south-western Türkiye): Implications for early hominin dispersals out of Africa. Quat. Environ. Hum. 2024, 2, 100029. [Google Scholar] [CrossRef]
- Koopman, M. A Fault Kinematic and Geomorphological Study of the Late Cenozoic Ilgın Basin, Central Anatolia, Türkiye. Master’s Thesis, Utrecht University, Utrecht, The Netherlands, 2011. [Google Scholar]
- Louchart, A.; Mourer-Chauvite, C.; Güleç, E.; Howell, F.C.; White, T.D. L’avifaune de Dursunlu Turquie, Pleistocene inferieur: Climat, environnement et biogeographie. Comptes Rendus L’académie Sci. Ser. IIA Earth Planet. Sci. 1998, 327, 341–346. [Google Scholar] [CrossRef]
- Howell, F.C.; White, T.D.; Güleç, E.; Saraç, G.; Curtis, G.H. Dursunlu, Lower Pleistocene Faunal and Archeological Locality, Konya Basin, Anatolia (Turkey). In Los Homínidos y su Entorno en el Pleistoceno Inferior de Eurasia Actas del Congreso Internacional de Paleontología Humana; Gibert, J., Sánchez, F., Gibert, L., Ribot, F., Eds.; Dialnet: La Rioja, Spain, 1999; pp. 459–468. [Google Scholar]
- Güleç, E.; Howell, F.C.; White, T.; Karabıyıkoğlu, M. Anadolu’da İlk İnsan İzleri: Dursunlu Alt Paleolitik Buluntu Yeri. Ank. Üniversitesi Dil ve Tar. Coğrafya Fakültesi Antropoloji Derg. 2002, 15, 79–90. [Google Scholar]
- Saraç, G. Anadolu’nun bilinen en eski sakinleri. Mavi Gezegen 2001, 4, 12–17. [Google Scholar]
- Kuhn, S.L.; Dinçer, B.; Balkan-Atlı, N.; Erturaç, M.K. Paleolithic occupations of the Göllü Dağ, Central Anatolia, Turkey. J. Field Archaeol. 2015, 40, 581–602. [Google Scholar] [CrossRef]
- Yavuz-Işık, N.; Saraç, G.; Ünay, E.; de Bruijn, H. Palynological analysis of neogene mammal sites of Turkey-vegetational and climatic implications. Yerbilimleri 2011, 32, 105–120. [Google Scholar]
- Tuncer, A. Ostracoda Taxonomy and Biostratigraphy in the Yalvaç and Ilgın Continental Neogene Basins (Southwest Anatolia): Ostracoda-Based Paleoenvironmental and Paleoclimatic Approaches. Ph.D. Thesis, Hacettepe University, Ankara, Türkiye, 2020. (In Turkish). [Google Scholar]
- Koçyiğit, A.; Ünay, E.; Saraç, G. Episodic graben formation and extensional neotectonic regime in West Central Anatolia and the Isparta Angle: A case study in the Aksehir-Afyon graben, Turkey. Geol. Soc. Lond. Spec. Publ. Vol. 2000, 173, 405–421. [Google Scholar] [CrossRef]
- Özgül, N.; Bölükbaşı, S.; Alkan, H.; Öztaş, Y.; Korucu, M. Sultandağları-Sandıklı-Homa-Akdağ yöresinin Jeolojisi; Report No: 3028; Türkiye Petrolleri (TPAO) Anonim Ortaklığı: Ankara, Türkiye, 1991.
- Göncüoğlu, M.C.; Çapkınoğlu, Ş.; Gürsu, S.; Noble, P.; Turhan, N.; Tekin, U.K.; Okuyucu, C.; Göncüoğlu, Y. The Mississippian in the Central and Eastern Taurides (Turkey): Constraints on the tectonic setting of the Tauride-Anatolide Platform. Geol. Carpathica 2007, 58, 427–442. [Google Scholar]
- Güngör, T. Kinematics of the Central Taurides during Neotethys closure and collision, the nappes in the Sultan Mountains, Turkey. Int. J. Earth Sci. 2013, 102, 1381–1402. [Google Scholar] [CrossRef]
- Ergen, A.; Bozkurt, A.; Ilgar, A.; Tuncay, E.; Doğan, A. Sultandağları’nın Jeolojisi ve Jeodinamik Evrimi. In Proceedings of the Maden Tetkik ve Arama Genel Müdürlüğü, Ankara, Türkiye, 15 April 2021; Report No: 13958. 241p. [Google Scholar]
- Ilgar, A.; Ergen, A.; Bozkurt, A.; Tuncay, E. Sedimentology and Miocene-Pliocene depositional evolution of the stream-dominated alluvial fan deposits at circum-Sultandağları region. Bull. Miner. Res. Explor. 2022, 168, 43–66. [Google Scholar] [CrossRef]
- Umut, M.; Karabıyıkoğlu, M.; Saraç, G.; Bulut, V.; Demirci, A.R.; Erkan, M.; Kurt, Z.; Metin, S.; Özgönül, E. Tuzlukçu-Ilgın-Doğanhisar-Doğanbey (Konya ili) ve Dolayının Jeolojisi; Report No: 8246; Maden Tetkik Arama Genel Müdürlüğü: Ankara, Türkiye, 1987; 38p. [Google Scholar]
- R Core Team. A Language and Environment for Statistical Computing; Version 4.3.2; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.r-project.org/ (accessed on 1 October 2024).
- Hsieh, T.C.; Ma, K.H.; Chao, A. iNEXT: iNterpolation and Extrapolation for Species Diversity. R Package Version 3.0.1. Available online: http://CRAN.R-project.org/package=iNEXT (accessed on 1 October 2024).
- Horn af Rantzien, H. An annotated check-list of genera of fossil Charophyta. Micropaleontology 1956, 2, 243–256. [Google Scholar] [CrossRef]
- Feist, M.; Grambast-Fessard, N.; Guerlesquin, M.; Karol, K.; Lu, H.; Mc-Court, R.M.; Wang, Q.; Shenzen, Z. Treatise on Invertebrate Paleontology. Part B. Protoctista 1. Charophyta; The University of Kankas: Kansas, CO, USA, 2005; p. 170. [Google Scholar]
- Neubauer, T.A. The fossil record of freshwater Gastropoda–A global review. Biol. Rev. 2024, 99, 177–199. [Google Scholar] [CrossRef]
- Schönhuth, S.; Vukic, J.; Sanda, R.; Yang, L.; Mayden, R.L. Phylogenetic relationships and classification of the Holarctic family Leuciscidae (Cypriniformes: Cyprinoidei). Mol. Phylogenet. Evol. 2018, 127, 781–799. [Google Scholar] [CrossRef]
- Küçük, F.; Çiftçi, Y.; Güçlü, S.S.; Mutlu, A.G.; Turan, D. Taxonomic review of the Chondrostoma (Teleostei, Leuciscidae) species from inland waters of Turkey: An integrative approach. Zoosyst. Evol. 2023, 99, 1–13. [Google Scholar] [CrossRef]
- Dubois, A.; Ohler, A.; Pyron, R.A. New concepts and methods for phylogenetic taxonomy and nomenclature in zoology, exemplified by a new ranked cladonomy of recent amphibians (Lissamphibia). Megataxa 2021, 5, 1–738. [Google Scholar] [CrossRef]
- de Broin, F. Contribution à l’étude des chéloniens. Chéloniens continentaux du Crétacé et du Tertiaire de France. Mémoires Muséum Natl. D’histoire Nat. 1977, 38, 1–366. [Google Scholar]
- Szyndlar, Z. Fossil snakes from Poland. Acta Zool. Cracoviensia 1984, 28, 3–156. [Google Scholar]
- Groumpou, M.; Sanjuan, J.; Koukouvelas, I.; Nikolakopoulos, K.; Iliopoulos, G. Subrecent charophyte flora from the Pheneos palaeolake (Greece): Palaeoecological implications. Rev. Palaeobot. Palynol. 2023, 318, 104973. [Google Scholar] [CrossRef]
- Sanjuan, J.; Matamoros, D.; Casanovas-Vilar, I.; Vicente, A.; Moreno-Bedmar, J.A.; Holmes, J.; Martín-Closas, C. Palaeoecology of Middle Miocene charophytes from the Vallès–Penedès and Vilanova basins (Catalonia, Spain). Hist. Biol. 2023, 35, 1665–1685. [Google Scholar] [CrossRef]
- Singh, N.A.; Singh, N.P.; Sharma, K.M.; Patnaik, R.; Tiwari, R.P.; Sehgal, R.K.; Kumar, V.; Wazir, W.A.; Singh, Y.P.; Choudhary, D. First report on late Miocene (Tortonian: ~11–10 Ma) charophyte gyrogonites from Tapar, Kachchh District, Gujarat State, western India. Proc. Indian Natl. Sci. Acad. 2022, 88, 439–455. [Google Scholar] [CrossRef]
- García, A. Quaternary charophytes from Salina del Bebedero, Argentina: Their relation with extant taxa and palaeolimnological significance. J. Paleolimnol. 1999, 21, 307–323. [Google Scholar] [CrossRef]
- Soulié-Märsche, I. Diversity of Quaternary aquatic environments in NE Africa as shown by fossil Charophytes. In Geoscientific Research in Northeast Africa; CRC Press: Boca Raton, FL, USA, 2017; pp. 575–579. [Google Scholar]
- Anadón, P.; Julià, R.; De Deckker, P.; Rosso, J.C.; Soulié-Märsche, I. Contribución de la paleolimnología del Pleistoceno inferior de la Cuenca de Baza (sector Orce-Venta Micena). Paleontol. I Evol. Memòria 1987, 1, 35–72. [Google Scholar]
- Barinova, S.; Romanov, R.; Solak, C.N. New record of Chara hispida (L.) Hartm. (Streptophyta: Charophyceae, Charales) from the Işıklı Lake (Turkey) and critical checklist of Turkish charophytes. Nat. Resour. Conserv. 2014, 2, 33–42. [Google Scholar] [CrossRef]
- Soulié-Märsche, I. Charophytes as lacustrine biomarkers during the Quaternary in North Africa. J. Afr. Earth Sci. 1991, 12, 341–351. [Google Scholar] [CrossRef]
- Détriché, S.; Bréhéret, J.G.; Soulié-Märsche, I.; Karrat, L.; Macaire, J.J. Late Holocene water level fluctuations of Lake Afourgagh (Middle-Atlas Mountains, Morocco) inferred from charophyte remains. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2009, 283, 134–147. [Google Scholar] [CrossRef]
- Rey-Boissezon, A.; Joye, D.A. Habitat requirements of charophytes—Evidence of species discrimination through distribution analysis. Aquat. Bot. 2015, 120, 84–91. [Google Scholar] [CrossRef]
- Baciu, C.; Feist, M. Les charophytes Oligocènes du nord-ouest de la Transylvanie (Roumanie). Acta Palaeontol. Rom. 1999, 2, 27–29. [Google Scholar]
- Tuncer, A.; Tunoğlu, C. Geç Erken-Orta Miyosen Yaşlı Söke Formasyonu’nun Ostrakod Faunası ve Paleoortamsal Karakteristikleri, Söke Havzası, Aydın/Batı Anadolu. Yerbilimleri 2015, 36, 97–120. [Google Scholar] [CrossRef][Green Version]
- Fan, H.; Gasse, F.; Huc, A.; Li, Y.; Sifeddine, A.; Soulié-Märsche, I. Holocene environmental changes in bangong co basin (Western Tibet). Part 3: Biogenic remains. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1996, 120, 65–78. [Google Scholar] [CrossRef]
- Sanjuan, J.; Alqudah, M. Charophyte flora from the Miocene of Zahle (Beeka Valley, Lebanon). Biostratigraphic, palaeoenvronmental and palaeobiogeographical implications. Geodiversitas 2018, 40, 195–209. [Google Scholar] [CrossRef]
- Daily, F.K. Some observations on the occurrence and distribution of the Characeae of Indiana. Proc. Indiana Acad. Sci. 1958, 68, 95–107. [Google Scholar]
- Viinikka, Y. The role of U-type exchanges in the differentiation of karyotypes in Najas marina. Hereditas 1977, 86, 91–101. [Google Scholar] [CrossRef]
- Triest, L. Electrophoretic polymorphism and divergence in Najas marina L. (Najadaceae): Molecular markers for individuals, hybrids, cytodemes, lower taxa, ecodemes and conservation of genetic diversity. Aquat. Bot. 1989, 33, 301–380. [Google Scholar] [CrossRef]
- Efimov, D.Y.; Pimenov, A.V.; Bobrov, A.A. Najas marina (Hydrocharitaceae) in Southern Middle Siberia: Refinds after a Century-Old Recess. Inland Water Biol. 2023, 16, 1178–1184. [Google Scholar] [CrossRef]
- Şanal, M.; Köse, B.; Coşkun, T.; Demir, N. Mogan Gölü’nde Sucul Makrofitlere Göre Ekolojik Kalitenin Tahmini. Iğdır Üniversitesi fen Bilim. Derg. 2015, 5, 51–55. [Google Scholar]
- Özdal, I.; Çetinkaya, O. Aquatic Plant Hotspots of Türkiye: The Lakes Region Habitats, Macrophytes, Biodiversity, Usage, Threats and Problems. Acta Aquat. Turc. 2024, 20, 267–286. [Google Scholar] [CrossRef]
- Van Vierssen, W. The ecology of communities dominated by Zannichellia taxa in western Europe. I. Characterization and autecology of the Zannichellia taxa. Aquat. Bot. 1982, 12, 103–155. [Google Scholar] [CrossRef]
- Haynes, R.R.; Les, D.H.; Holm-Nielsen, L.B. Zannichelliaceae. Flowering Plants—Dicotyledons. In The Families and Genera of Vascular Plants; Kubitzki, K., Ed.; Springer: Berlin/Heidelberg, Germany, 1998; Volume 4. [Google Scholar]
- Beker, K.; Tunoğlu, C.; Ertekin, I.K. Pliocene—Lower Pleistocene ostracoda fauna from İnsuyu Limestone (Karapinar-Konya/Central Turkey) and its paleoenvironmental implications. Geol. Bull. Turk. 2008, 51, 1–31. [Google Scholar]
- Freels, D. Limnische Ostrakoden aus Jungtertiär und Quartär der Türkei. Geol. Jahrb. Reihe B 1980, 39, 1–172. [Google Scholar]
- Kayseri-Özer, M.S.; Karadenizli, L.; Akgün, F.; Oyal, N.; Saraç, G.; Şen, Ş.; Tunoğlu, C.; Tuncer, A. Palaeoclimatic and palaeoenvironmental interpretations of the late Oligocene, Late Miocene–early Pliocene in the Çankırı-Çorum Basin. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2017, 467, 16–36. [Google Scholar] [CrossRef]
- Tuncer, A.; Tunoğlu, C. Early Pleistocene (Calabrian) Ostracoda assemblage and paleoenvironmental characteristics of the Fevzipaşa Formation, Western Anatolia. Micropaleontology 2015, 61, 69–83. [Google Scholar] [CrossRef]
- Külköylüoğlu, O.; Akdemir, D.; Yavuzatmaca, M.; Yilmaz, O. A checklist of recent non-marine Ostracoda (Crustacea) of Turkey with three new records. Rev. Hydrobiol. 2015, 8, 20–33. [Google Scholar]
- Tuncer, A.; Karayiğit, A.İ.; Oskay, R.G.; Tunoğlu, C.; KayseriÖzer, M.S.; Gümüş, B.A.; Bulut, Y.; Akbulut, A. A multi-proxy record of palaeoenvironmental and palaeoclimatic conditions during Plio-Pleistocene peat accumulation in the eastern fank of the Isparta Angle: A case study from the Şarkikaraağaç coalfeld (Isparta, SW Central Anatolia). Int. J. Coal Geol. 2023, 265, 104149. [Google Scholar] [CrossRef]
- Meisch, C.; Scharf, B.; Fuhrmann, R.; Thiéry, A. Neglecandona altoides (Petkovski, 1961) nov. comb. and the genus Neglecandona Krstić, 2006 (Crustacea, Ostracoda, Candonidae). Bull. Société Nat. Luxemb. 2019, 121, 237–264. [Google Scholar]
- Spadi, M.; Gliozzi, E.; Medici, M.C. Piacenzian–Gelasian non-marine ostracods from the Dunarobba Fossil Forest (Tiberino Basin, Umbria, central Italy). Pap. Palaeontol. 2019, 5, 391–413. [Google Scholar] [CrossRef]
- Tuncer, A.; Tunoğlu, C.; Aydar, E.; Yilmaz, İ.Ö.; Gümüş, B.A.; Şen, E. Holocene paleoenvironmental evolution of the Acıgöl paleo maar lake (Nevşehir, Central Anatolia). Mediterr. Geosci. Rev. 2019, 1, 255–269. [Google Scholar] [CrossRef]
- Darbaş, G.; Demircan, H. Ostracoda assemblages and palaeoenvironmental characteristics of the Soma Formation (Late Miocene-Pliocene), İvrindi–NW Balıkesir, Turkey. IOP Conf. Ser. Earth Environ. Sci. 2017, 95, 032018. [Google Scholar] [CrossRef]
- Mischke, S.; Ginat, H.; Al-Saqarat, B.; Almogi-Labin, A. Ostracods from water bodies in hyperarid Israel and Jordan as habitat and water chemistry indicators. Ecol. Indic. 2012, 14, 87–99. [Google Scholar] [CrossRef]
- Floroiu, A.; Stoica, M.; Vasiliev, I.; Krijgsman, W. Maeotian/Pontian ostracods in the Badislava? Topolog area (South carpathian foredeep-Romania). GeoEcoMarina 2011, 17, 237–244. [Google Scholar]
- Schütt, H.; Yildirim, Z.A. A new freshwater snail from Turkey, resembling the genus Lyhnidia Hadžišče 1956 (Mollusca: Gastropoda: Prosobranchia: Hydrobiidae). Arch. Für Molluskenkd. 2003, 132, 97–103. [Google Scholar] [CrossRef]
- Sysoev, A.V.; Schileyko, A.A. Land Snails and Slugs of Russia and Adjacent Countries; Pensoft Publishers: Sofia, Bulgaria, 2009; Volume 87, p. 454. [Google Scholar]
- White, D.; Preece, R.C.; Shchetnikov, A.A.; Parfitt, S.A.; Dlussky, K.G. A Holocene molluscan succession from floodplain sediments of the upper Lena River (Lake Baikal region), Siberia. Quat. Sci. Rev. 2008, 27, 962–987. [Google Scholar] [CrossRef]
- Gerber, J. Revision der Gattung Vallonia Risso 1826 (Mollusca: Gastropoda: Valloniidae). In Schriften zur Malakozoologie aus dem Haus der Natur; Hemmen: Luxembourg, 1996; Volume 8, pp. 1–227. [Google Scholar]
- Welter-Schultes, F.W. European Non-Marine Molluscs, a Guide for Species Identification; Planet Poster Editions: Göttingen, Germany, 2012; 679p. [Google Scholar]
- Gittenberger, E.; Janssen, A.W.; Kuijper, W.J.; Kuiper, J.G.J.; Meijer, T.; van der Velde, G.; De Vries, J.N. De Nederlandse zoetwatermollusken. In Recente en Fossiele Weekdieren uit Zoet en Brak Water—Nederlandse Fauna 2, 2nd ed.; Nationaal Natuurhistorisch Museum Naturalis, KNNV Uitgeverij & EIS-Nederland: Leiden, The Nederland, 2004; p. 288. [Google Scholar]
- Becker-Platen, J.D.; Kuiper, J.G.J. Sphaeriiden (Mollusca, Lamelli–branchia) aus dem Känozoikum der Türkei. Geol. Jahrb. B 1979, 33, 159–185. [Google Scholar]
- Ozulug, M.; Freyhof, J. Revision of the genus Squalius in Western and Central Anatolia, with a description of four new species (Teleostei: Cyprinidae). Ichthyol. Explor. Freshw. 2011, 22, 107–148. [Google Scholar]
- Çiçek, E.; Sungur, S.; Fricke, R. Freshwater lampreys and fishes of Turkey; a revised and updated annotated checklist 2020. Zootaxa 2020, 4809, 241–270. [Google Scholar] [CrossRef]
- Bailon, S. Différenciation Ostéologique des Anoures (Amphibia, Anoura) de France. In Fiches d’Ostéologie Animale Pour L’archéologie; Desse, V.J., Desse-Berset, N., Eds.; Série C: Varia; Centre de Recherches Archéologiques-CNRS: Paris, France, 1999; pp. 1–38. [Google Scholar]
- Venczel, M.; Sen, S. Pleistocene amphibians and reptiles from Emirakaya-2, Turkey. Herpetol. J. 1994, 4, 159. [Google Scholar]
- Syromyatnikova, E.; Mayda, S.; Tesakov, A. Late Miocene amphibians and reptiles: New insight into the pre-Messinian herpetofaunas in Turkey. Hist. Biol. 2022, 34, 1964–1971. [Google Scholar] [CrossRef]
- Bailon, S.; Hossini, S. Les plus anciens Bufonidae (Amphibia, Anura) d’Europe: Les espèces du Miocène français. Ann. Paléontol. 1999, 76, 121–132. [Google Scholar]
- Rage, J.C.; Sen, S. Les amphibiens et les reptiles du Pliocène supérieur de Çalta (Turquie). Géologie Méditerranéenne 1976, 3, 127–134. [Google Scholar] [CrossRef]
- Villa, A.; Blain, H.A.; van den Hoek Ostende, L.W.; Delfino, M. Fossil amphibians and reptiles from Tegelen (Province of Limburg) and the early Pleistocene palaeoclimate of The Netherlands. Quat. Sci. Rev. 2018, 187, 203–219. [Google Scholar] [CrossRef]
- Gleed-Owen, C.P. Quaternary Herpetofaunas of the British Isles: Taxonomic Descriptions, Palaeoenvironmental Reconstructions, and Biostratigraphic Implications. Ph.D. Thesis, Coventry University, Coventry, UK, 1998. [Google Scholar]
- Luján, À.H.; Delfino, M.; Casanovas-Vilar, I.; Alba, D.M. Taxonomy of subgenus Temnoclemmys Bergounioux, 1958 (Testudines: Geoemydidae: Ptychogasterinae) based on new material from the Vallès-Penedès Basin (NE Iberian Peninsula). CR Palevol 2014, 13, 277–295. [Google Scholar] [CrossRef]
- Luján, À.H.; Delfino, M.; Robles, J.M.; Alba, D.M. The Miocene tortoise Testudo catalaunica Bataller, 1926, and a revised phylogeny of extinct species of genus Testudo (Testudines: Testudinidae). Zool. J. Linn. Soc. 2016, 178, 312–342. [Google Scholar] [CrossRef]
- Camaiti, M.; Villa, A.; Wencker, L.C.; Bauer, A.M.; Stanley, E.L.; Delfino, M. Descriptive osteology and patterns of limb loss of the European limbless skink Ophiomorus punctatissimus (Squamata, Scincidae). J. Anat. 2019, 235, 313–345. [Google Scholar] [CrossRef]
- Szyndlar, Z. A review of Neogene and Quaternary snakes of central and eastern Europe. Part 2: Natricinae, Elapidae, Viperidae. Estud. Geológicos 1991, 47, 237–266. [Google Scholar] [CrossRef]
- Ivanov, M.; Böhme, M. Snakes from Griesbeckerzell (Langhian, Early Badenian), North Alpine Foreland Basin (Germany), with comments on the evolution of snake faunas in Central Europe during the Miocene Climatic Optimum. Geodiversitas 2011, 33, 411–449. [Google Scholar] [CrossRef]
- Ivanov, M.; Čerňanský, A.; Bonilla-Salomón, I.; Luján, À.H. Early Miocene squamate assemblage from the Mokrá-Western Quarry (Czech Republic) and its palaeobiogeographical and palaeoenvironmental implications. Geodiversitas 2020, 42, 343–376. [Google Scholar] [CrossRef]
- Venczel, M. Anurans and squamates from the Lower Pliocene (MN 14) Osztramos A locality (Northern Hungary). Fragm. Palaeontol. Hung. 2001, 19, 79–90. [Google Scholar]
- Rage, J.-C.; Szyndlar, Z. Natrix longivertebrata from the European Neogene, a Snake with One of the Longest Known Stratigraphic Ranges. In Neues Jahrbuch für Geologie und Paläontologie–Monatshefte; E. Schweizerbart: Stuttgart, Germany, 1986; pp. 56–64. [Google Scholar]
- Szyndlar, Z. Snake fauna from the Late Miocene of Rudabánya. Palaeontogr. Ital. 2005, 90, 31–52. [Google Scholar]
- Venczel, M.; Ştiucă, E. Late middle Miocene amphibians and squamate reptiles from Tauţ, Romania. Geodiversitas 2008, 30, 731–763. [Google Scholar]
- Georgalis, G.L.; Villa, A.; Ivanov, M.; Vasilyan, D.; Delfino, M. Fossil amphibians and reptiles from the Neogene locality of Maramena (Greece), the most diverse European herpetofauna at the Miocene/Pliocene transition boundary. Palaeontol. Electron. 2019, 22, 1–99. [Google Scholar] [CrossRef]
- Lister, A.M.; McGlade, J.; Stuart, J.A. The Early Middle Pleistocene vertebrate fauna from Little Oakley, Essex. Philos. Trans. R. Soc. Lond. B 1990, 328, 359–385. [Google Scholar]
- Holman, J.A. Pleistocene Amphibians and Reptiles in Britain and Europe; Oxford University Press (OUP): Oxford, UK, 1998; pp. 1–265. [Google Scholar]
- Blain, H.-A.; Rubio-Jara, S.; Panera, J.; Uribelarrea, D.; Laplana, C.; Herráez, E.; Pérez-González, A. A new middle Pleistocene (Marine Oxygen Isotope Stage 6) cold herpetofaunal assemblage from the central Iberian Peninsula (Manzanares Valley, Madrid). Quat. Res. 2017, 87, 499–515. [Google Scholar] [CrossRef]
- Harvati, K.; Darlas, A.; Bailey, S.E.; Rein, T.R.; El Zaatari, S.; Fiorenza, L.; Kullmer, O.; Psathi, E. New Neanderthal remains from Mani peninsula, Southern Greece: The Kalamakia Middle Paleolithic cave site. J. Hum. Evol. 2013, 64, 486–499. [Google Scholar] [CrossRef] [PubMed]
- Villa, A.; Blain, H.A.; Delfino, M. The Early Pleistocene herpetofauna of Rivoli Veronese (Northern Italy) as evidence for humid and forested glacial phases in the Gelasian of Southern Alps. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2018, 490, 393–403. [Google Scholar] [CrossRef]
- Vasilyan, D.; Schneider, S.; Bayraktutan, M.S.; Şen, Ş. Early Pleistocene freshwater communities and rodents from the Pasinler Basin (Erzurum Province, north-eastern Turkey). Turk. J. Earth Sci. 2014, 23, 293–307. [Google Scholar] [CrossRef]
- Uetz, P.; Freed, P.; Aguilar, R.; Hošek, J. The Reptile Database. 2022. Available online: https://reptile-database.reptarium.cz (accessed on 9 November 2022).
- Syromyatnikova, E.; Tesakov, A.; Mayda, S.; Kaya, T.; Saraç, G. Plio-Pleistocene amphibians and reptiles from Central Turkey: New faunas and faunal records with comments on their biochronological position based on small mammals. Foss. Impr. 2019, 75, 343–358. [Google Scholar] [CrossRef]
- Glöer, P. The Freshwater Gastropods of the West-Palaearctis. Volume I. Fresh- and Brackish Waters Except Spring and Subterranean Snails. Identification Key, Anatomy, Ecology, Distribution. Privately Published. 2019, p. 399. Available online: https://www.malaco.de/Sonderdrucke/WPal.pdf (accessed on 1 September 2024).
- Odabași, D.A.; Glöer, P.; Yildirim, M.Z. The Valvata species of Turkey with a description of Valvata kebapcii n. sp. (Mollusca: Valvatidae). Ecol. Montenegrina 2015, 2, 135–142. [Google Scholar] [CrossRef]
- Doadrio, I.; Perea, S.; Garzón-Heydt, P.; González, J.L. Ictiofauna Contintental Española. Bases para su Seguimiento; Ministerio de Medio Ambiente y Medio Rural y Marino, Ed.; DG Medio Natural y Política Forestal: Madrid, Spain, 2011; 616p.
- Güçlü, S.S.; Küçük, F.; Turan, D.; Çiftçi, Y.; Mutlu, A.G. A new Chondrostoma species from the Büyük Menderes River Basin, Turkey (Teleostei: Cyprinidae). Zool. Middle East 2018, 64, 315–321. [Google Scholar] [CrossRef]
- Speybroeck, J.; Beukema, W.; Bok, B.; Van Der Voort, J. Field Guide to the Amphibians and Reptiles of Britain and Europe; Bloomsbury Publishing: London, UK, 2016; 432p. [Google Scholar]
- Anderson, S.C.; Leviton, A.E. A review of the genus Ophiomorus (Sauria: Scincidae), with descriptions of three new forms. Proc. Calif. Acad. Sci. 1966, 33, 499–534. [Google Scholar]
- Greer, A.E.; Wilson, G.D.F. Comments on the scincid lizard genus Ophiomorus, with a cladistic analysis of the species. Hamadryad 2001, 26, 261–271. [Google Scholar]
- Marmi, J.; Luján, À.H. An overview of the threatened phylogenetic diversity of living testudines based on a review of the complex evolutionary history of turtles. In Turtles Anatomy, Ecology and Conservation; Cosgrove, M.J., Roe, S.A., Eds.; Nova Science Publishers: New York, NY, USA, 2012; pp. 117–150. [Google Scholar]
- Steen, D.A.; Gibbs, J.P.; Buhlmann, K.A.; Carr, J.L.; Compton, B.W.; Congdon, J.D.; Doody, J.S.; Godwin, J.C.; Holcomb, K.L.; Jackson, D.R.; et al. Terrestrial habitat requirements of nesting freshwater turtles. Biol. Conserv. 2012, 150, 121–128. [Google Scholar] [CrossRef]
- Luján, À.H.; Čerňanský, A.; Bonilla-Salomón, I.; Březina, J.; Ivanov, M. Fossil turtles from the early Miocene localities of Mokrá-Quarry (Burdigalian, MN4), South Moravian Region, Czech Republic. Geodiversitas 2021, 43, 691–707. [Google Scholar] [CrossRef]
- Kornilev, Y.V.; Popgeorgiev, G.; Plachiyski, D.; Dyugmedzhiev, A.; Mladenov, V.; Andonov, K.; Lukanov, S.; Vacheva, E.; Slavchev, M.; Naumov, B. Distribution of the grass snake (Natrix natrix) and dice snake (N. tessellata) in Bulgaria. Hist. Nat. Bulg. 2023, 45, 239–254. [Google Scholar] [CrossRef]
- Dinçer, B. The Lower Paleolithic in Türkiye: Anatolia and Hominin Dispersals Out of Africa. In Paleoanthropology of the Balkans and Anatolia: Human Evolution and Its Context Evolution and Its Context; Harvati, K., Roksandic, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 213–228. [Google Scholar]
- Vislobokova, I.A.; Agadzhanyan, A.K.; Lopatin, A.V. The case of Trlica TRL11-10 (Montenegro): Implications for possible early hominin dispersals into the Balkans in the middle of the Early Pleistocene. Quat. Int. 2020, 554, 15–35. [Google Scholar] [CrossRef]
- Slimak, L. Implantations humaines et exploitation des obsidiennes en Anatolie centrale durant le Pléistocène. In Paléorient; CNRS Editins: Paris, France, 2004; pp. 7–20. [Google Scholar]
- Roosevelt, C.H.; Dinçer, B.; Luke, C.; Çilingiroğlu, Ç. A Lower Paleolithic assemblage from western Anatolia: The lithics from Bozyer. Quat. Int. 2019, 522, 66–84. [Google Scholar] [CrossRef]
- Mosquera, M.; Ollé, A.; Saladie, P.; Cáceres, I.; Huguet, R.; Rosas, A.; Villalaín, J.; Carrancho, A.; Bourlès, D.; Braucher, R.; et al. The Early Acheulean technology of Barranc de la Boella (Catalonia, Spain). Quat. Int. 2016, 393, 95–111. [Google Scholar] [CrossRef]
- Barsky, D.; Titton, S.; Sala-Ramos, R.; Bargalló, A.; Grégoire, S.; Saos, T.; Serrano-Ramos, A.; Oms, O.; Solano García, J.A.; Toro-Moyano, I.; et al. The significance of subtlety: Contrasting lithic raw materials procurement and use patterns at the oldowan sites of Barranco León and Fuente Nueva 3 (Orce, Andalusia, Spain). Front. Earth Sci. 2022, 10, 893776. [Google Scholar] [CrossRef]
- Kuhn, S.L. Paleolithic Archaeology in Turkey. Evol. Anthropol. Issues News Rev. 2022, 11, 198–210. [Google Scholar] [CrossRef]
- Antón, S.C.; Leonard, W.R.; Robertson, M.L. An ecomorphological model of the initial hominid dispersal from Africa. J. Hum. Evol. 2002, 43, 773–785. [Google Scholar] [CrossRef]
- Zohar, I.; Alperson-Afil, N.; Goren-Inbar, N.; Prévost, M.; Tütken, T.; Sisma-Ventura, G.; Hershkovitz, I.; Najorka, J. Evidence for the cooking of fish 780,000 years ago at Gesher Benot Ya’aqov, Israel. Nat. Ecol. Evol. 2022, 6, 2016–2028. [Google Scholar] [CrossRef]
- Saraç, G.; Maden Tetkik ve Arama Genel Müdürlügü. Türkiye Omurgali Fosil Yataklari; MTA Derleme Rapor N° 10609; Jeoloji Kütüphane N° 637; Jeoloji Etütleri Dairesi: Ankara, Türkiye, 2003; p. 138. [Google Scholar]
- Blasco, R.; Blain, H.A.; Rosell, J.; Díez, J.C.; Huguet, R.; Rodríguez, J.; Arsuaga, J.L.; de Castro, J.M.B.; Carbonell, E. Earliest evidence for human consumption of tortoises in the European Early Pleistocene from Sima del Elefante, Sierra de Atapuerca, Spain. J. Hum. Evol. 2011, 61, 503–509. [Google Scholar] [CrossRef]
- Richards, M.P.; Karavanić, I.; Pettitt, P.; Miracle, P. Isotope and faunal evidence for high levels of freshwater fish consumption by Late Glacial humans at the Late Upper Palaeolithic site of Šandalja II, Istria, Croatia. J. Archaeol. Sci. 2015, 61, 204–212. [Google Scholar] [CrossRef]
- Karl, H.V. Human consumption of turtles of the Homo rudolfensis site Uraha (Malawi, East Africa). Archaeofauna 2012, 21, 267–279. [Google Scholar] [CrossRef]
- Sampson, C.G. Tortoise remains from a later Stone Age rock shelter in the Upper Karoo, South Africa. J. Archaeol. Sci. 1998, 25, 985–1000. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).