Abstract
Understanding microhabitat preferences of endangered species and the drivers involved in this selection are crucial for understanding their ecology and implementing conservation actions. This issue gains more importance with amphibians, which are known to be constrained by specific environmental conditions and are among the most threatened organisms globally. We assessed shelter preference of Speleomantes strinatii in three different contiguous habitats (mixed broadleaf forest, transitional mixed-chestnut and a chestnut forest used both for fruit and coppice), located at different distances from an Apennine first-order stream. We placed 22 plots in these three habitats and searched for salamanders under the potential available shelters (logs and rocks). Using a Bayesian generalized linear mixed-effects model, we assessed the role of distance from watercourse, shelter type and area in salamanders’ microhabitat selection. As expected, salamanders were mostly found in the plots near the stream. However, stream distance seemed to not be a crucial driver of amphibians’ detection under a shelter. Indeed, salamanders increasingly used wood shelters at greater distances from the stream relative to rocks, suggesting that logs seemed to compensate for the distance from the stream. In the managed habitat, trunks and branches cut during coppicing or chestnut harvest, as well as naturally fallen wood, were often left on the ground, thereby increasing the availability of shelter for salamanders. Our findings highlight how properly managed forests may enable the persistence of forest salamanders, even in human-exploited environments, representing a cost-effective solution for maintaining soil and forest biodiversity.
1. Introduction
Microhabitat selection plays a fundamental role in the ecology of species with narrow physiological requirements, such as amphibians. Amphibians, and lungless salamanders (Plethodontidae) in particular, are dependent on specific environmental conditions, such as high air humidity and reduced temperature variations [1,2,3]. Lungless salamanders are active on the forest floor during rainy periods, sheltering under rocks and logs, on humid rockfaces and in the talus along streams [1,4]. Consequently, plethodontids generally display low vagility rates and high site fidelity [5,6], selecting those patches where soil moisture is higher [1,7]. In forest habitats, plethodontid salamanders are capable to actively select a specific type of microhabitat, depending on environmental conditions (e.g., soil humidity, shelter availability, aspect), prey distribution and social processes [7,8,9]. An important role in microhabitats selection is also played by experience and familiarity of salamanders with available shelters [10], confirming how behavioral adaptations are relevant in salamanders’ ecology. In addition to these elements, availability and size of shelters can also be a key factor for salamanders’ persistence in forest habitats [11,12,13]. Terrestrial salamanders tend to favor larger cover objects because they retain soil moisture better than smaller ones, buffering against extreme environmental fluctuations, while providing larger supply of invertebrate prey [14,15]. These patterns demonstrate how organisms develop behavioral adaptations, which involve physiological and morphological changes, in response to environmental factors that may impact their persistence [16,17,18]. Efficient behavioral responses (e.g., minimizing predation risk, ensuring access to resources and finding mates) allow animals to maximize their fitness, allowing them to persist in space and time [19]. In this context, assessing how individuals select their microhabitat is fundamental, especially for species that are highly constrained by narrow physiological requirements.
Despite a relatively vast knowledge of which factors are critical in forest-floor salamanders’ microhabitat choice, the information regarding the role of microhabitat type is minimal and only refers to north American plethodontids [13]. In this context, we evaluated if shelter type (rock or log) and size may influence shelter selection under environmental pressures (i.e., increasing distance from the stream and shelter availability) in a terrestrial European plethodontid salamander. We expected that salamanders’ distribution should be strongly influenced by stream distance, with a preference for larger shelters and logs, as distance from stream increases. The results obtained will be useful for providing practical information for correct forest management and potentially important from a conservation point of view within the recent climate change context. Indeed, terrestrial salamanders are increasingly exposed to both environmental pressures, such as drought and habitat alteration, and sanitary risks, including the spread of emerging infectious diseases (e.g., Batrachochytrium salamandrivorans), which further threaten their persistence [20,21].
2. Materials and Methods
2.1. Study Species and Site
Strinati’s cave salamander, Speleomantes strinatii (Aellen 1958), is a fully terrestrial plethodontid characterized by direct development, distributed in SE France and NW Italy [22]. The species is found in the talus and the leaf litter, on wet rocky outcrops in mixed broadleaf woodlands and riparian habitats and in underground environments (e.g., caves, crevices and the interstitial superficial system) [22,23]. This lungless salamander is usually active on the forest floor during or immediately after periods of rainfall with mild air temperatures [7], feeding on a large variety of invertebrate prey [24].
The study area is located in northern Italy, across the Apennine Mountain range of Liguria region, at an altitude of 350 m a.s.l. in the municipality of Mezzanego. The site, situated on the left bank of Sturla Stream, is characterized by three distinct forest units: a mixed broadleaf deciduous forest (e.g., Ostrya carpinifolia, Fraxinus ornus, Castanea sativa, Acer campestre), an abandoned sweet chestnut (Castanea sativa Mill., 1768) forest, partially used also as coppice, and a transition zone between the two, characterized by the presence of abandoned terraces (Figure 1). Both the transition and the terraced area showed signs of past anthropic management; however, the latter had been abandoned more recently (40 vs. 10 years). This is confirmed also by the presence not only of naturally dead wood, but also of trunks and branches cut during coppicing or chestnut harvest in the last decade. The mixed deciduous forest is situated along a first-order tributary stream, while the chestnut forest is placed at a distance of at least 60 m.
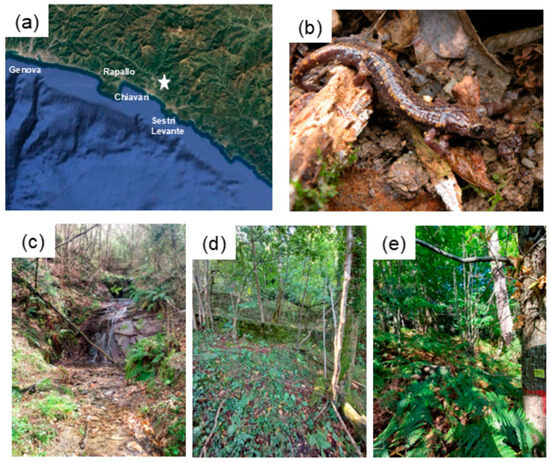
Figure 1.
Map depicting the location of the sampling site in Liguria (Northern Italy), indicated with a white star (a), specimen of Speleomantes strinatii found under a shelter during the study (b) and the three forest units located at increasing distances from the stream (c–e).
2.2. Sampling Design
We randomly placed, individually marked and GPS-positioned 22 square plots, each one measuring 16 m2 (4 m sides), at a minimum distance from each other of 5 m, and so divided: 10 plots in mixed forest, 5 in transitional one, and 7 in chestnut orchard. For each plot, we counted all the shelters available to cave salamanders, distinguishing them into two types (i.e., log and rock) and measuring their length and width or diameter.
During autumn (12 to 24 October 2022 and 1 to 7 November 2023) and spring (14 March to 14 April 2023 and 30 March to 11 April 2024), the same observer visited the site, during daytime, in favorable weather conditions for salamander activity (e.g., during or after rainfall). During each survey, the observer searched each plot, checking the leaf litter, inspecting rock crevices with an electric flashlight, and lifting rocks and deadwood shelters. Animals were sexed and aged based on body-size [25] and the presence of a conspicuous mental gland, which is characteristic of reproductive males [22]. Salamanders were captured by hand, weighted, measured (snout-vent-length—SVL) and photographed on the ventral side [6,26], thus allowing their subsequent identification using Wild-ID software (version 1.0) [27] and the avoidance of pseudo-replication during data analysis (i.e., recaptures were excluded from analyses). After the process and shelter measurement, salamanders were released at their capture location. Before and during each sampling occasion we followed the procedures for mitigating the spreading of fungal diseases [28].
2.3. Data Analysis
We evaluated the effects of distance from the stream, shelter type and area (i.e., the estimated surface of litter covered by the shelter), and their interaction on the probability of shelter use by salamanders, using a Bayesian generalized linear mixed-effects model (GLMM). We assumed shelter use as the response variable, following a Bernoulli distribution and modeled it through a logit link function. Distance from the stream, shelter type and area were assumed as predictors, while plot identity was included as a random intercept to account for repeated measurements within plots. Continuous predictors were standardized prior to analysis. Posterior distributions were summarized using the median and 90% credible intervals (CI). We considered an effect to be supported when its 90% CI did not include zero and reported the posterior probability of the effect being positive (β > 0) as a measure of effect certainty. Predictive accuracy of models was assessed using Leave-One-Out cross-validation (LOO) [29]. The analysis was performed using the brms package [30] in the R environment. In the case of significant interaction between shelter type or area and distance from the stream, we assessed whether this pattern was driven by differences in shelter availability farther or closer to the stream. Increase in availability with distance means that usage patterns reflect shelter availability (i.e., salamanders exploit the most abundant shelter type), while the absence of significant relationship would mean that shelters are actively selected by salamanders, regardless of their availability. Individual measures of body mass and SVL were used to calculate the Scaled Mass Index (SMI) of salamanders to assess their body condition depending on shelter and habitat types. The SMI is based on the relationship between body mass and a linear predictor of body size, accounting for allometric growth [31]. This condition index has proven to be highly reliable to assess energy reserve and predictor of fat, protein, or lean mass content in salamanders [32]. After checking for normality, differences between groups were assessed using one-way ANOVA and eventually Tukey’s pairwise test.
3. Results
We captured 54 individual salamanders (26 males, 23 females and 5 sub-adults) of which 7 were captured twice (i.e., 61 total captures). Captures were made in all three forest habitats and only one salamander was not found under a shelter but active in the leaf litter. Mean used shelter area was 0.091 m2 ± 0.05 SD for rocks and 0.33 m2 ± 0.2 for logs. Salamanders used logs and rocks as shelter for a total of 28 (20 in the chestnut, six in transitional and two in mixed forest) and 25 (one in the chestnut, one in transitional and 23 in mixed forest; see Table S1) occasions, respectively. In the study area, used logs had significant larger covering surface than used rocks (Mann–Whitney U test: U = 44, Nlogs = 28; Nrocks = 25; p < 0.001).
Model comparison showed that the model including an interaction between distance from the stream and shelter type had the best predictive performance among the candidate models (LOOIC = 93.32). Models accounting for distance and shelter type nevertheless showed similar support and their differences in expected log predictive density from best model were small and within one standard error (Table 1).

Table 1.
Comparison between candidate models based on Leave-One-Out information criterion (looic). Difference in expected log predictive density (elpd_diff) together with the relative standard error (se_diff), core measure of model fit (elpd_loo), effective number of parameters (p_loo) and relative model weight (Weight) are also reported.
We found strong evidence for a positive interaction between distance from the stream and shelter type (log), with a posterior mean of 2.89 (90% CI: 0.62–5.81). Posterior probability was 98.6%, suggesting that salamanders increasingly used trunk shelters at greater distances from the stream relative to rocks (Figure 2). On the other hand, there was no clear preference between rocks and logs in the plots closer to the stream (posterior mean: −1.55; 90% CI: −4.07–0.51).
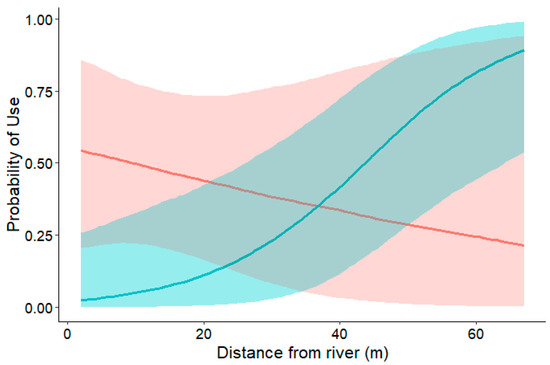
Figure 2.
Probability of use of logs (blue line) and rocks (red line) with increasing distance. Dashed area represents 90% Bayesian confidence intervals.
We did not find strong evidence that log availability increased with distance from the stream (estimate = 0.38, SE = 0.27, t = 1.43, p = 0.18), suggesting that the observed increase in use of wood shelters farther from the stream was not availability-driven (Figure S1).
We did not detect any difference regarding SMI either regarding shelter (F [1, 48] = 0.11, p = 0.74) and habitat type (F [2, 48] = 0.62, p = 0.54; Figure 3).
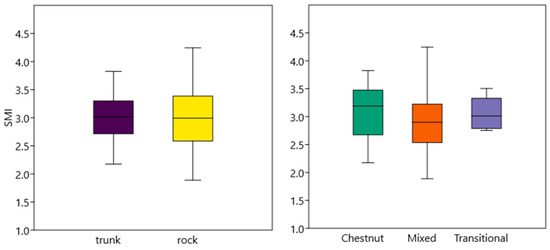
Figure 3.
Scaled Mass Index of cave salamanders in the different shelters and habitats. Whiskers represent standard error.
4. Discussion
Our expectations were only partially confirmed by the outcomes obtained. The probability of shelter use in Speleomantes strinatii was mainly driven by the interaction between shelter type and distance from the stream. Salamanders showed a stronger preference for logs over rocks at greater distances from the watercourse, whereas no clear preference emerged near the stream (Figure 2). In contrast, shelter area and stream distance alone did not significantly explain microhabitat choice. Consequently, our findings are partly consistent with previous studies reporting that salamanders tend to select objects that provide greater coverage (i.e., have larger areas) when they are farther from surface waters or on drier substrates [11,33]. Therefore, logs seemed to compensate for the constraints related to the distance from the stream (i.e., reduced humidity). This hypothesis is also supported by the fact that closer to the stream there was no preference for one of the two shelter types. The explanation of this trend seems quite straightforward: wood is a porous and hygroscopic material, less prone to extreme fluctuations of humidity and temperature than rocks [34].
Contrary to our expectations, the choice of microhabitat did not seem to be influenced by the distance from the stream (posterior mean: −0.61; 90% CI: −2.69–1.25), as well as the shelter area (Figure S2). Although unexpected, this pattern may be explained by the active selection of logs far from the stream, while the effect of area is probably buffered by the absence of shelter preference near the watercourse. Both elements further highlight the importance of woody debris for the maintenance and conservation of forest amphibian’s communities [8,35]. At the same time, microhabitat selection and spatial ecology of individuals are driven by a more complex combination of both ecological and social interactions than our study could capture. For instance, shelter use may be influenced by prey availability since larger woody debris can host richer invertebrate communities [18]. In addition, intraspecific interactions including resource competition, individual personality, and demographic structure [1,18] may play a role in how individuals distribute among shelters. Individual personality traits, including boldness or exploratory behavior, could further shape fine-scale microhabitat use [36]. The investigation of these processes accounting also for environmental drivers would provide a more integrative understanding of habitat use in S. strinatii and terrestrial amphibians in general.
Also, body condition of salamanders did not seem to be influenced either by shelter or habitat type. Despite the potential lower environmental suitability, the salamanders found further away from the stream had energy reserves comparable to those found closer to the stream. This result reinforces the hypothesis that trunks, as well as being a refuge, provide a range of prey similar to that found in the most optimal areas for salamanders.
In any case, our findings confirm how retaining downed or deadwood on soil, as performed in sustainable anthropic activities and managed forests, is a good practice for maintaining forest communities, including amphibians. This practice has already been demonstrated to be effective for favoring the persistence of terrestrial salamanders [37,38]. Targeted management operations, such as selective tree felling, forest thinning or retention of downed dead wood (DDW) or coarse woody debris (CWD), may represent a cost-effective solution for maintaining soil and forest biodiversity while enhancing the environmental carrying capacity of forest ecosystems [39,40]. Such activities must be preceded by analyses providing reliable estimates based both on the demographic structure of the focal populations and on the specific characteristics of the environment (e.g., habitat-dependence analysis) [39]. In the context of climate change, retaining and managing dead wood on the forest floor in temperate habitats represents a key component of silvicultural practices. As Mediterranean regions face increasingly extreme droughts and temperatures, maintaining microhabitats that protect against desiccation (e.g., logs, branches and stumps) can help support local biodiversity and critical ecological functions. The importance of dead wood as a structural and functional element of forest ecosystems is widely emphasized by European forest management policies, including the EU Forest Strategy and National Biodiversity Action Plans [41]. In the face of a changing climate, promoting DDW and CWD retention is consistent with the general objectives of preserving ecosystem services, bolstering soil health, and protecting the complexity of microhabitats [42,43,44].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d17090632/s1, Figure S1: Relationship between mean number of available woody shelters and distance from the watercourse; Figure S2: Distribution of shelters’ surface coverage for logs (a) and rocks (b) respectively. Bars represent 95% confidence intervals; Table S1: Summary table regarding the available refuges and their use in the three forest habitats located at different distances from the stream, provided in meters (m) between brackets.
Author Contributions
Conceptualization, G.R., A.C. and S.S.; methodology, G.R., A.C. and S.S.; data analysis, G.R.; writing—original draft preparation, G.R., A.C. and S.S.; writing—review and editing, G.R., A.C. and S.S. All authors have read and agreed to the published version of the manuscript.
Funding
G.R. fulfilled his Ph.D. at the University of Genoa and is funded by the “National Recovery and Resilience Plan” (NRRP—Spoke 2; CUP D33C22000960007, Project title “National Biodiversity Future Center”—NBFC). A.C. is funded by the Italian National Operational Programme “Research and Innovation” (PON–Ricerca e Innovazione, tematica GREEN; CUP N. D31B21008270007), and S.S. is funded by the University of Genoa (FRA-2018).
Institutional Review Board Statement
Permits for capture, handling and stomach flushing of salamanders in the present study were issued by the Ital Ministry of Environment—authorization # 0039130 of 15 April 2021.
Data Availability Statement
The data presented in this study are available from the corresponding author upon reasonable request.
Acknowledgments
We are grateful to Agnese Pessagno and Geordie Biffoni for their help during the field activities.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jaeger, R.G. Moisture as a Factor Influencing the Distributions of Two Species of Terrestrial Salamanders. Oecologia 1971, 6, 191–207. [Google Scholar] [CrossRef]
- Wake, D.B. Adaptive Radiation of Salamanders in Middle American Cloud Forests. Ann. Mo. Bot. Gard. 1987, 74, 242–264. [Google Scholar] [CrossRef]
- Farallo, V.R.; Miles, D.B. The Importance of Microhabitat: A Comparison of Two Microendemic Species of Plethodon to the Widespread P. cinereus. Copeia 2016, 104, 67–77. [Google Scholar] [CrossRef]
- Petranka, J.W. Salamanders of the United States and Canada; Smithsonian Institution Press: Washington, DC, USA, 1998. [Google Scholar]
- Sutherland, C.; Royle, J.A.; Linden, D.W. oSCR: A Spatial Capture–Recapture R Package for Inference about Spatial Ecological Processes. Ecography 2019, 42, 1459–1469. [Google Scholar] [CrossRef]
- Rosa, G.; Costa, A.; Salvidio, S. Moving in the Dark: Enlightening the Spatial Population Ecology of European Cave Salamanders. Popul. Ecol. 2025, 1–10. [Google Scholar] [CrossRef]
- Rosa, G.; Salvidio, S.; Costa, A. European Plethodontid Salamanders on the Forest Floor: Testing for Age-Class Segregation and Habitat Selection. J. Herpetol. 2022, 56, 27–33. [Google Scholar] [CrossRef]
- Costa, A.; Crovetto, F.; Salvidio, S. European Plethodontid Salamanders on the Forest Floor: Local Abundance Is Related to Fine-Scale Environmental Factors. Herpetol. Conserv. Biol. 2016, 11, 344–349. [Google Scholar]
- Moore, S.J.; Nicholson, K.E. Beneath the Leaf-Litter: Can Salamander Personality Influence Forest-Floor Dynamics? Herpetologica 2021, 77, 209–218. [Google Scholar] [CrossRef]
- Rosa, G.; Salvidio, S.; Costa, A. The Role of Familiarity in Shelter Site Fidelity: Insights from a Mesocosm Experiment with a Plethodontid Salamander. Ethol. Ecol. Evol. 2024, 36, 616–626. [Google Scholar] [CrossRef]
- Keen, W.H. Influence of Moisture on the Activity of a Plethodontid Salamander. Copeia 1984, 1984, 684–688. [Google Scholar] [CrossRef]
- Kluber, M.R.; Olson, D.H.; Puettmann, K.J. Downed Wood Microclimates and Their Potential Impact on Plethodontid Salamander Habitat in the Oregon Coast Range. Northwest Sci. 2009, 83, 25–34. [Google Scholar] [CrossRef]
- Hill, S.; Johnson, I.; Kennedy, C.; Kennedy, I.; Mullins, C.; Roark, M.; Salazar, O.; Still, K.; Smith, W.H. Cover Object Availability and Preferences by Woodland Salamanders (Genus Plethodon) on Surface-Mined and Unmined Habitats in the Virginia Coalfields. Catesbeiana 2021, 41, 43–56. [Google Scholar]
- Grover, M.C. Determinants of Salamander Distributions along Moisture Gradients. Copeia 2000, 2000, 156–168. [Google Scholar] [CrossRef]
- Jaeger, R.G.; Wicknick, J.A.; Griffis, M.R.; Anthony, C.D. Socioecology of a Terrestrial Salamander: Juveniles Enter Adult Territories during Stressful Foraging Periods. Ecology 1995, 76, 533–543. [Google Scholar] [CrossRef]
- Scheffers, B.R.; Edwards, D.P.; Diesmos, A.; Williams, S.E.; Evans, T.A. Microhabitats Reduce Animal’s Exposure to Climate Extremes. Glob. Change Biol. 2014, 20, 495–503. [Google Scholar] [CrossRef]
- Farallo, V.R.; Muñoz, M.M.; Uyeda, J.C.; Miles, D.B. Scaling between Macro-to Microscale Climatic Data Reveals Strong Phylogenetic Inertia in Niche Evolution in Plethodontid Salamanders. Evolution 2020, 74, 979–991. [Google Scholar] [CrossRef]
- Lunghi, E.; Mammola, S.; Martínez, A.; Hesselberg, T. Behavioural Adjustments Enable the Colonization of Subterranean Environments. Zool. J. Linn. Soc. 2024, 201, 549–559. [Google Scholar] [CrossRef]
- Morris, D.W. Adaptation and Habitat Selection in the Eco-Evolutionary Process. Proc. R. Soc. B Biol. Sci. 2011, 278, 2401–2411. [Google Scholar] [CrossRef] [PubMed]
- Milanovich, J.R.; Peterman, W.E.; Nibbelink, N.P.; Maerz, J.C. Projected Loss of a Salamander Diversity Hotspot as a Consequence of Projected Global Climate Change. PLoS ONE 2010, 5, e12189. [Google Scholar] [CrossRef] [PubMed]
- Dondero, L.; Allaria, G.; Rosa, G.; Costa, A.; Ficetola, G.F.; Cogoni, R.; Grasselli, E.; Salvidio, S. Threats of the Emerging Pathogen Batrachochytrium salamandrivorans (Bsal) to Italian Wild Salamander Populations. Acta Herpetol. 2023, 18, 3–9. [Google Scholar] [CrossRef]
- Lanza, B. Speleomantes Strinatii (Aellen, 1958). Fauna D’italia 2007, 42, 152–156. [Google Scholar]
- Manenti, R. Dry Stone Walls Favour Biodiversity: A Case-Study from the Appennines. Biodivers. Conserv. 2014, 23, 1879–1893. [Google Scholar] [CrossRef]
- Costa, A.; Romano, A.; Rosa, G.; Salvidio, S. Weighted Individual-Resource Networks in Prey–Predator Systems: The Role of Prey Availability on the Emergence of Modular Structures. Integr. Zool. 2022, 17, 115–127. [Google Scholar] [CrossRef]
- Ficetola, G.F.; Pennati, R.; Manenti, R. Spatial Segregation among Age Classes in Cave Salamanders: Habitat Selection or Social Interactions? Popul. Ecol. 2013, 55, 217–226. [Google Scholar] [CrossRef]
- Renet, J.; Leprêtre, L.; Champagnon, J.; Lambret, P. Monitoring Amphibian Species with Complex Chromatophore Patterns: A Non-Invasive Approach with an Evaluation of Software Effectiveness and Reliability. Herpetol. J. 2019, 29, 13–22. [Google Scholar] [CrossRef]
- Bolger, D.T.; Morrison, T.A.; Vance, B.; Lee, D.; Farid, H. A Computer-assisted System for Photographic Mark–Recapture Analysis. Methods Ecol. Evol. 2012, 3, 813–822. [Google Scholar] [CrossRef]
- Thomas, V.; Wang, Y.; Rooij, P.V.; Verbrugghe, E.; Baláž, V.; Bosch, J.; Cunningham, A.A.; Fisher, M.C.; Garner, T.W.J.; Gilbert, M.J.; et al. Mitigating Batrachochytrium salamandrivorans in Europe. Amphibia-Reptilia 2019, 40, 265–290. [Google Scholar] [CrossRef]
- Bürkner, P.-C.; Gabry, J.; Vehtari, A. Efficient Leave-One-out Cross-Validation for Bayesian Non-Factorized Normal and Student-t Models. Comput. Stat. 2021, 36, 1243–1261. [Google Scholar] [CrossRef]
- Bürkner, P.-C. Advanced Bayesian Multilevel Modeling with the R Package Brms. arXiv 2017, arXiv:1705.11123. [Google Scholar]
- Peig, J.; Green, A.J. New Perspectives for Estimating Body Condition from Mass/Length Data: The Scaled Mass Index as an Alternative Method. Oikos 2009, 118, 1883–1891. [Google Scholar] [CrossRef]
- MacCracken, J.G.; Stebbings, J.L. Test of a Body Condition Index with Amphibians. J. Herpetol. 2012, 46, 346–350. [Google Scholar] [CrossRef]
- Southerland, M.T. The Effects of Variation in Streamside Habitats on the Composition of Mountain Salamander Communities. Copeia 1986, 1986, 731–741. [Google Scholar] [CrossRef]
- Thybring, E.E.; Fredriksson, M. Wood and Moisture. In Springer Handbook of Wood Science and Technology; Niemz, P., Teischinger, A., Sandberg, D., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 355–397. ISBN 978-3-030-81315-4. [Google Scholar]
- Rittenhouse, T.A.G.; Harper, E.B.; Rehard, L.R.; Semlitsch, R.D. The Role of Microhabitats in the Desiccation and Survival of Anurans in Recently Harvested Oak–Hickory Forest. Copeia 2008, 2008, 807–814. [Google Scholar] [CrossRef]
- Brehm, A.M.; Mortelliti, A. Land-use Change Alters Associations between Personality and Microhabitat Selection. Ecol. Appl. 2021, 31, e02443. [Google Scholar] [CrossRef]
- Romano, A.; Costa, A.; Salvidio, S.; Menegon, M.; Garollo, E.; Tabarelli de Fatis, K.; Miserocchi, D.; Matteucci, G.; Pedrini, P. Forest Management and Conservation of an Elusive Amphibian in the Alps: Habitat Selection by the Golden Alpine Salamander Reveals the Importance of Fine Woody Debris. For. Ecol. Manag. 2018, 424, 338–344. [Google Scholar] [CrossRef]
- Blomquist, S.M. A Multi-Scale Assessment of Habitat Selection and Movement Patterns by Northern Leopard Frogs (Lithobates [Rana] pipiens) in a Managed Forest. Herpetol. Conserv. Biol. 2009, 4, 142–160. [Google Scholar]
- Indermaur, L.; Schmidt, B.R. Quantitative Recommendations for Amphibian Terrestrial Habitat Conservation Derived from Habitat Selection Behavior. Ecol. Appl. 2011, 21, 2548–2554. [Google Scholar] [CrossRef][Green Version]
- Otto, C.R.V.; Kroll, A.J.; McKenny, H.C. Amphibian Response to Downed Wood Retention in Managed Forests: A Prospectus for Future Biomass Harvest in North America. For. Ecol. Manag. 2013, 304, 275–285. [Google Scholar] [CrossRef]
- European Commission; Joint Research Centre. Mapping and Assessment of Primary and Old-Growth Forests in Europe; Publications Office: Luxembourg, 2021. [Google Scholar]
- Nordén, B.; Ryberg, M.; Götmark, F.; Olausson, B. Relative Importance of Coarse and Fine Woody Debris for the Diversity of Wood-Inhabiting Fungi in Temperate Broadleaf Forests. Biol. Conserv. 2004, 117, 1–10. [Google Scholar] [CrossRef]
- Siitonen, J. Forest Management, Coarse Woody Debris and Saproxylic Organisms: Fennoscandian Boreal Forests as an Example. Ecol. Bull. 2001, 49, 11–41. [Google Scholar]
- Manning, A.D.; Cunningham, R.B.; Lindenmayer, D.B. Bringing Forward the Benefits of Coarse Woody Debris in Ecosystem Recovery under Different Levels of Grazing and Vegetation Density. Biol. Conserv. 2013, 157, 204–214. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).