First Insights into the Mitochondrial DNA Diversity of the Italian Sea-Slater Across the Strait of Sicily
Abstract
1. Introduction
2. Materials and Methods
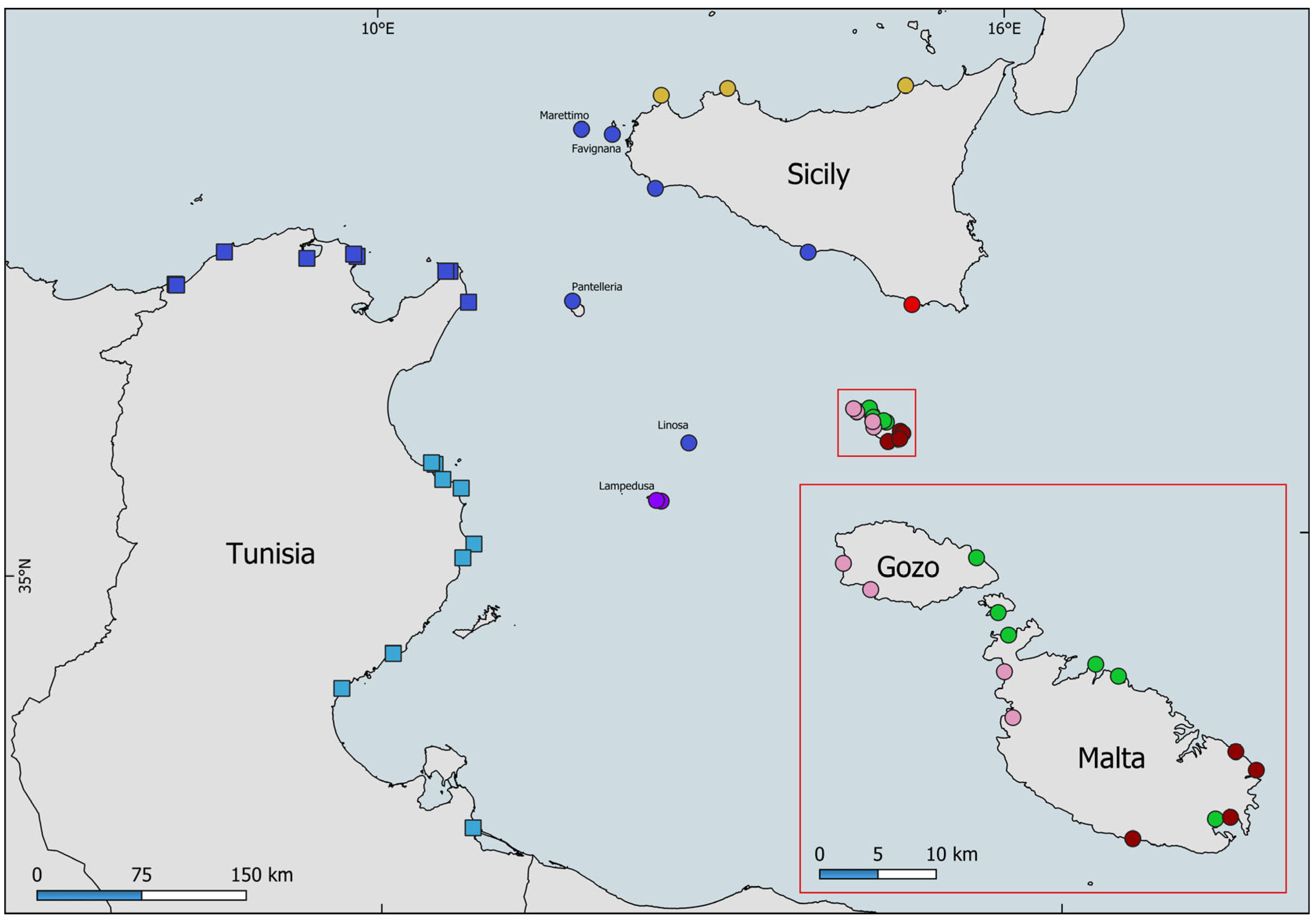

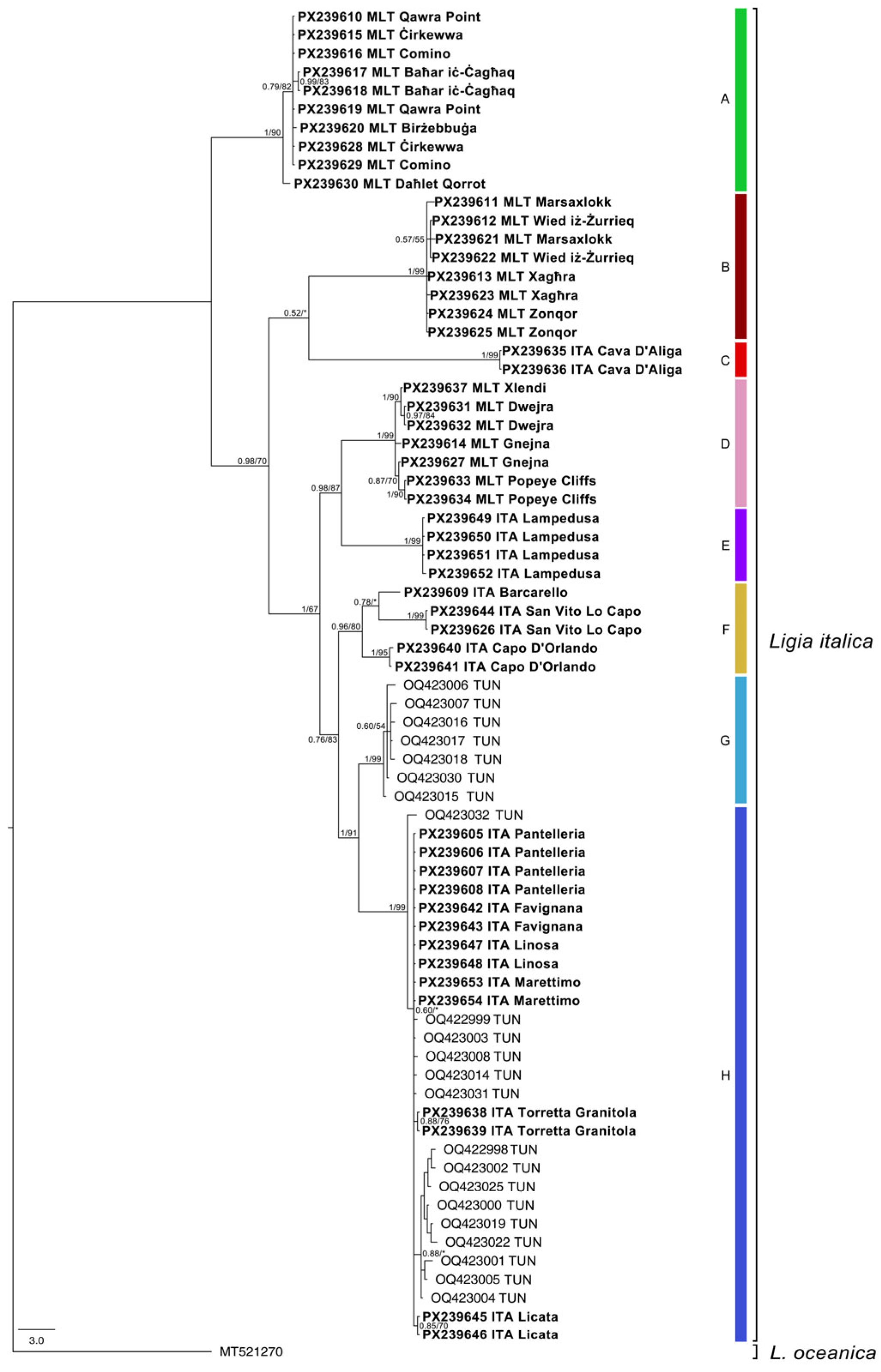
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boyko, C.B.; Campos-Filho, I.S.; Hadfield, K.A.; Hughes, T.; Merrin, K.L.; Ota, Y.; Poore, G.C.B. World List of Marine, Freshwater and Terrestrial Isopod Crustaceans Database. Available online: https://www.marinespecies.org/isopoda/ (accessed on 6 August 2025).
- Schmalfuss, H. Terrestrial Isopods (Crustacea: Isopoda) from the Caucasus Region. 5. Cylisticus Schnitzler, Parcylisticus Verhoeff, Cylisticoides n. gen. Stuttg. Beitr. Zur Naturkunde Ser. A 2003, 647, 1–38. [Google Scholar]
- Vandel, A. Isopodes Terrestres (Première Partie); Faune de France; Lechevalier P: Paris, France, 1960; Volume 64. [Google Scholar]
- Edney, E.B. Woodlice and the Land Habitat. Biol. Rev. 1954, 29, 185–219. [Google Scholar] [CrossRef]
- Morris, R.H.; Abbott, D.P.; Haderlie, E.C. Intertidal Invertebrates of California; Stanford University Press: Stanford, CA, USA, 1980. [Google Scholar]
- Schiller, C.; Fielder, D.R.; Brown, I.W.; Obed, A. Reproduction, Early Life-History and Recruitment. In The Coconut Crab: Aspects of Birgus latro Biology and Ecology in Vanuatu; Brown, I.W., Fielder, D.R., Eds.; ACIAR Monograph 8: Canberra, Australia, 1991; pp. 13–15. [Google Scholar]
- Lucrezi, S.; Schlacher, T.A. The Ecology of Ghost Crabs. Oceanogr. Mar. Biol. Annu. Rev. 2014, 52, 201–256. [Google Scholar] [CrossRef]
- Vecchioni, L.; Marrone, F.; Deidun, A.; Adepo-Gourene, B.; Froglia, C.; Sciberras, A.; Bariche, M.; Burak, A.Ç.; Foka-Corsini, M.; Arculeo, M. DNA Taxonomy Confirms the Identity of the Widely-Disjunct Mediterranean and Atlantic Populations of the Tufted Ghost Crab Ocypode cursor (Crustacea: Decapoda: Ocypodidae). Zool. Sci. 2019, 36, 322–329. [Google Scholar] [CrossRef]
- Warburg, M.R. Evolutionary Biology of Land Isopods; Springer: Berlin/Heidelberg, Germany, 1993. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, J.; Wang, S.; He, D.; Zhao, L. Influences of Desiccation, Submergence, and Salinity Change on Survival of Ligia cinerascens (Crustacea, Isopoda): High Potential Implication for Inland Migration and Colonization. Hydrobiologia 2016, 772, 277–285. [Google Scholar] [CrossRef]
- Carefoot, T.H.; Taylor, B.E. Ligia: A Prototypal Terrestrial Isopod. In Crustacean Issues 9: Terrestrial Isopod Biology; Scharam, F.R., Balkema, A.A., Eds.; Routledge: London, UK, 1995; pp. 40–70. [Google Scholar]
- Tsai, M.-L.; Dai, C.F.; Chen, H.-C. Desiccation Resistance of Two Semiterrestrial Isopods, Ligia exotica and Ligia taiwanensis (Crustacea) in Taiwan. Biochem. Physiol. 1998, 119, 361–367. [Google Scholar] [CrossRef]
- Hurtado, L.A.; Mateos, M.; Santamaria, C.A. Phylogeography of Supralittoral Rocky Intertidal Ligia Isopods in the Pacific Region from Central California to Central Mexico. PLoS ONE 2010, 5, e11633. [Google Scholar] [CrossRef]
- Santamaria, C.A. Molecular Taxonomy of Endemic Coastal Ligia Isopods from the Hawaiian Islands: Re-Description of L. hawaiensis and Description of Seven Novel Cryptic Species. PeerJ 2019, 7, e7531. [Google Scholar] [CrossRef]
- Santamaria, C.A.; Mateos, M.; Taiti, S.; DeWitt, T.J.; Hurtado, L.A. A Complex Evolutionary History in a Remote Archipelago: Phylogeography and Morphometrics of the Hawaiian Endemic Ligia Isopods. PLoS ONE 2013, 8, e85199. [Google Scholar] [CrossRef]
- Greenan, T.M.; Griffiths, C.L.; Santamaria, C.A. Molecular Approaches Uncover Cryptic Diversity in Intertidal Ligia Isopods (Crustacea, Isopoda, Ligiidae) across the Southern Africa Coastline. PeerJ 2018, 6, e4658. [Google Scholar] [CrossRef]
- Laifi-Necibi, N.; Amor, N.; Merella, P.; Mohammed, O.B.; Medini, L. DNA Barcoding Reveals Cryptic Species in the Sea Slater Ligia italica (Crustacea, Isopoda) from Tunisia. Mitochondrial DNA A DNA Mapp. Seq. Anal. 2025, 35, 1–11. [Google Scholar] [CrossRef]
- UNEP-MAP-RAC/SPA. Sicily Channel/Tunisian Plateau: Topography, Circulation and Their Effects on Biological Components; Würtz, M., Cebrian, D., Requena, S., Eds.; RAC/SPA: Tunis, Tunisia, 2015. [Google Scholar]
- Guariento, L.A.; Devincenzo, U.; Schifani, E.; Russo, R.; Moretto, E.; Sarà, M. Rediscovery of the Enigmatic Solifuges (Arachnida: Solifugae) at Lampedusa Island (Italy). Eur. Zool. J. 2018, 85, 201–209. [Google Scholar] [CrossRef]
- Colomba, M.; Lo Verde, G.; Liberto, F.; Gregorini, A.; Sparacio, I. Molecular and Biometric Data on Carabus (Macrothorax) morbillosus Fabricius, 1792 (Coleoptera, Carabidae) from Mid Mediterranean Areas. Zookeys 2022, 2022, 119–134. [Google Scholar] [CrossRef]
- Faraone, F.P.; Melfi, R.; Di Nicola, M.R.; Giacalone, G.; Lo Valvo, M. The Genetic Identity of the Only Italian Population of the Genus Macroprotodon Guichenot, 1850 on the Island of Lampedusa, Sicily. Vertebr. Zool. 2020, 70, 235–240. [Google Scholar] [CrossRef]
- Senczuk, G.; Harris, D.J.; Castiglia, R.; Litsi Mizan, V.; Colangelo, P.; Canestrelli, D.; Salvi, D. Evolutionary and Demographic Correlates of Pleistocene Coastline Changes in the Sicilian Wall Lizard Podarcis wagleriana. J. Biogeogr. 2019, 46, 224–237. [Google Scholar] [CrossRef]
- Schifani, E.; Prebus, M.M.; Alicata, A. Integrating Morphology with Phylogenomics to Describe Four Island Endemic Species of Temnothorax from Sicily and Malta (Hymenoptera, Formicidae). Eur. J. Taxon. 2022, 833, 143–179. [Google Scholar] [CrossRef]
- Thake, M.A. The Biogeography of the Maltese Islands, Illustrated by the Clausiliidae. J. Biogeogr. 1985, 12, 269–287. [Google Scholar] [CrossRef]
- Muscarella, C.; Baragona, A. The Endemic Fauna of the Sicilian Islands. Biodivers. J. 2017, 8, 249–278. [Google Scholar]
- Sparacio, I.; Muscarella, C.; Di Giulio, A.; Ruzzier, E. Description of a New Rhizotrogus Latreille, 1825 (Scarabaeidae, Melolonthinae), from the Island of Pantelleria (Sicily Channel, Italy). Biodivers. Data J. 2025, 13, e154423. [Google Scholar] [CrossRef]
- Nardi, G.; Mifsud, D. The Bostrichidae of the Maltese Islands (Coleoptera). Zookeys 2015, 481, 69–108. [Google Scholar] [CrossRef]
- Papežík, P.; Sciberras, A.; Benovics, M.; Sciberras, J.; Deidun, A.; Mikulíček, P. Far from Home: Tracing the Origin of Non-Native Water Frogs (Genus Pelophylax) in Malta by Molecular Markers. Biol. Invasions 2024, 26, 1045–1059. [Google Scholar] [CrossRef]
- QGIS Development Team QGIS Geographic Information System. Open-Source Geospatial Foundation Project. Available online: http://www.qgis.org/ (accessed on 6 August 2025).
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA Primers for Amplification of Mitochondrial Cytochrome c Oxidase Subunit 1 from Diverse Metazoan Invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Richterich, P. Estimation of Errors in “Raw” DNA Sequences: A Validation Study. Genome Res. 1998, 8, 251–259. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Suleski, M.; Sanderford, M.; Sharma, S.; Tamura, K. MEGA12: Molecular Evolutionary Genetic Analysis Version 12 for Adaptive and Green Computing. Mol. Biol. Evol. 2024, 41, msae263. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. Mrbayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New Algorithms and Methods to Estimate Maximum-Likelihood Phylogenies: Assessing the Performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef]
- Markow, T.A.; Pfeiler, E. Mitochondrial DNA Evidence for Deep Genetic Divergences in Allopatric Populations of the Rocky Intertidal Isopod Ligia occidentalis from the Eastern Pacific. Mol. Phylogenet Evol. 2010, 56, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Khalaji-Pirbalouty, V.; Santamaria, C.A.; Puniamoorthy, J.; Mendoza, J.C.E. Description of a New Species of Ligia Fabricius, 1798 (Crustacea: Isopoda: Ligiidae) from Samoa Based on Morphological and Molecular Data, with Notes on L. vitiensis Dana, 1853. Raffles Bull. Zool. 2025, 73, 276–292. [Google Scholar] [CrossRef]
- Fourdrilis, S.; Backeljau, T. Highly Polymorphic Mitochondrial DNA and Deceiving Haplotypic Differentiation: Implications for Assessing Population Genetic Differentiation and Connectivity. BMC Evol. Biol. 2019, 19, 92. [Google Scholar] [CrossRef] [PubMed]
- Sabatelli, S.; Ruspantini, P.; Cardoli, P.; Audisio, P. Underestimated Diversity: Cryptic Species and Phylogenetic Relationships in the Subgenus Cobalius (Coleoptera: Hydraenidae) from Marine Rockpools. Mol. Phylogenet Evol. 2021, 163, 107243. [Google Scholar] [CrossRef]
- Vecchioni, L.; Arculeo, M.; Cottarelli, V.; Marrone, F. Range-Wide Phylogeography and Taxonomy of the Marine Rock Pools Dweller Tigriopus fulvus (Fischer, 1860) (Copepoda, Harpacticoida). J. Zool. Syst. Evol. Res. 2021, 59, 839–857. [Google Scholar] [CrossRef]
- Vecchioni, L.; Marrone, F.; Rodilla, M.; Belda, E.J.; Arculeo, M. An Account on the Taxonomy and Molecular Diversity of a Marine Rock-Pool Dweller, Tigriopus fulvus (Copepoda, Harpacticoida). Cienc. Mar. 2019, 45, 59–75. [Google Scholar] [CrossRef]
- Yin, J.; Pan, D.; He, C.; Wang, A.; Yan, J.; Sun, H. Morphological and Molecular Data Confirm Species Assignment and Dispersal of the Genus Ligia (Crustacea: Isopoda: Ligiidae) along Northeastern Coastal China and East Asia. Zool. J. Linn. Soc. 2013, 169, 362–376. [Google Scholar] [CrossRef]
- Agius, J.; Miceli, M.; Spatola, D. A Geological Overview of the Maltese Archipelago with Reference to the Area of Sliema. Xjenza Online Sci. J. Malta Chamb. Sci. 2023, 11, 53–69. [Google Scholar] [CrossRef]
- Salvi, D.; Schembri, P.J.; Sciberras, A.; Harris, D.J. Evolutionary History of the Maltese Wall Lizard Podarcis filfolensis: Insights on the “Expansion-Contraction” Model of Pleistocene Biogeography. Mol. Ecol. 2014, 23, 1167–1187. [Google Scholar] [CrossRef]
- Colomba, M.S.; Gregorini, A.; Cilia, D.P.; Liberto, F.; Reitano, A.; Sparacio, I. Molecular Studies on the Genus Muticaria Lindholm, 1925 (Pulmonata, Clausiliidae) from the Maltese Islands. Biodivers. J. 2019, 10, 517–526. [Google Scholar] [CrossRef]
- Reyes-Suarez, N.C.; Cook, M.S.; Gačić, M.; Paduan, J.D.; Drago, A.; Cardin, V. Sea Surface Circulation Structures in the Malta-Sicily Channel from Remote Sensing Data. Water 2019, 11, 1589. [Google Scholar] [CrossRef]
- Burgio, E.; Catalisano, A.; Salvo, G.; Zava, B. Primo Ritrovamento Di Vertebrati Fossili Nell’isola di Lampedusa (Sicilia). Il Nat. Sicil. 1997, 21, 229–236. [Google Scholar]
- Massa, B. Arthropoda Di Lampedusa, Linosa e Pantelleria (Canale Di Sicilia, Mar Mediterraneo). Il Nat. Sicil. 1995, 19, 1–909. [Google Scholar]
- Nicolosi, G.; Pantini, P.; Devincenzo, U.; Guariento, L.A.; Italiano, V.; Zanca, L.; Sarà, M.; Isaia, M. New Data on Spiders (Arachnida, Araneae) from the Islands of the Strait of Sicily (Southern Italy) with Taxonomic Notes on Poecilochroa loricata Kritscher, 1996 (Araneae, Gnaphosidae) and Eight New Records for Europe. Eur. Zool. J. 2024, 91, 1009–1034. [Google Scholar] [CrossRef]
- Pasta, S.; Garfi, G.; Silvestre Gristina, A.; Marcenò, C.; Guarino, R.; Sparacio, I.; Muscarella, C.; Giacalone, G.; Badalamenti, E.; La Mantia, T.; et al. L’ultima Spiaggia. Il Declino Degli Ecosistemi Dunali Della Sicilia Negli Ultimi 50 Anni—The Last Beach. The Decline of Sicily’s Dune Ecosystems over the Last 50 Years. In Le Dune Costiere—Valore Ambientale, Paesaggistico Ed Economico Risorsa Da Proteggere e Preservare. Monografie Di Geologia Ambientale; Stragapede, F., Ed.; Direzione generale Educazione, Ricerca e istituti Culturali del Ministero della cultura: Roma, Italy, 2024. [Google Scholar]
- Highsmith, R.C. Floating and Algal Rafting as Potential Dispersal Mechanisms in Brooding Invertebrates. Mar. Ecol. Prog. Ser. 1985, 25, 169–179. [Google Scholar] [CrossRef]
- Thiel, M.; Gutow, L. The Ecology of Rafting in the Marine Environment. I. the Floating Substrata. Oceanogr. Mar. Biol. Annu. Rev. 2005, 42, 181–264. [Google Scholar] [CrossRef]
- Raupach, M.J.; Bininda-Emonds, O.R.P.; Knebelsberger, T.; Laakmann, S.; Pfaender, J.; Leese, F. Phylogeographical Analysis of Ligia oceanica (Crustacea: Isopoda) Reveals Two Deeply Divergent Mitochondrial Lineages. Biol. J. Linn. Soc. 2014, 112, 16–30. [Google Scholar] [CrossRef]
- Kim, G.; Kim, H.; Mun, S.; Choi, E.H.; Nguyen, A.D.; Hwang, U.W. Molecular Population Genetics and Phylogeographic Studies of Ligia exotica and Ligia cinerascens in East Asia. Front. Mar. Sci. 2023, 10, 1260171. [Google Scholar] [CrossRef]
- Guarino, R.; Pasta, S. Sicily: The Island That Didn’t Know to Be an Archipelago. Berichte Der Reinhold-Tuxen-Ges. 2018, 30, 133–148. [Google Scholar]
| Country | Locality | Latitude | Longitude | Sampling Date | COI A.N. | Clade |
|---|---|---|---|---|---|---|
| MLT | Malta, Baħar iċ-Ċagħaq | 35.9496 | 14.4464 | 27/09/2024 | PX239617-PX239618 | A |
| MLT | Malta, Qawra Point | 35.9599 | 14.4254 | 27/09/2024 | PX239610, PX239619 | A |
| MLT | Malta, Birżebbuġa | 35.8325 | 14.5315 | 28/09/2024 | PX239620 | A |
| MLT | Comino | 36.0052 | 14.3351 | 02/10/2024 | PX239616, PX239629 | A |
| MLT | Malta, Ċirkewwa | 35.9869 | 14.3439 | 02/10/2024 | PX239615, PX239628 | A |
| MLT | Gozo, Daħlet Qorrot | 36.0494 | 14.3171 | 16/10/2024 | PX239630 | A |
| MLT | Malta, Marsaxlokk | 35.8331 | 14.5458 | 28/09/2024 | PX239611, PX239621 | B |
| MLT | Malta, Wied iż-Żurrieq | 35.8208 | 14.4521 | 28/09/2024 | PX239612, PX239622 | B |
| MLT | Malta, Xagħra | 35.8846 | 14.5542 | 30/09/2024 | PX239613, PX239623 | B |
| MLT | Malta, Zonqor | 35.8692 | 14.5727 | 30/09/2024 | PX239624-PX2396245 | B |
| ITA | Sicily, Cava D’Aliga | 36.7174 | 14.7015 | 13/10/2024 | PX239635-PX2396356 | C |
| MLT | Malta, Gnejna | 35.9216 | 14.3441 | 01/10/2024 | PX239614, PX239627 | D |
| MLT | Gozo, Dwejra | 36.0507 | 14.1901 | 16/10/2024 | PX239631-PX2396312 | D |
| MLT | Gozo, Xlendi | 36.0289 | 14.2149 | 16/10/2024 | PX239637 | D |
| MLT | Malta, Popeye Cliffs | 35.9583 | 14.3383 | 18/10/2024 | PX239633-PX239634 | D |
| ITA | Lampedusa, Cala Croce | 35.5011 | 12.5933 | 27/05/2025 | PX239651-PX239652 | E |
| ITA | Lampedusa, Punta Sottile | 35.4951 | 12.6307 | 27/05/2025 | PX239649-PX23964950 | E |
| ITA | Sicily, San Vito Lo Capo | 38.1796 | 12.7333 | 19/09/2011 | PX239626, PX239644 | F |
| ITA | Sicily, Capo D’Orlando | 38.1655 | 14.7482 | 02/11/2024 | PX239640-PX2396401 | F |
| ITA | Sicily, Barcarello | 38.2092 | 13.2819 | 01/06/2019 | PX239609 | F |
| ITA | Pantelleria, Punta Bue Marino | 36.8378 | 11.9632 | 01/05/2018 | PX239605-PX2396058 | H |
| ITA | Sicily, Torretta Granitola | 37.5667 | 12.6608 | 01/11/2024 | PX239638-PX2396389 | H |
| ITA | Favignana, Calafumere | 37.9331 | 12.3215 | 11/12/2024 | PX239642-PX239643 | H |
| ITA | Sicily, Licata | 37.1012 | 13.8834 | 04/02/2025 | PX239645-PX2396456 | H |
| ITA | Marettimo, Scugghiazzu | 37.9721 | 12.0700 | 11/05/2025 | PX239653-PX2396534 | H |
| ITA | Linosa, Molo Mannarazza | 35.8748 | 12.8649 | 01/06/2025 | PX239647-PX2396478 | H |
| Within Groups | Between Groups | |||||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | ||
| A | 0.004 | |||||||
| B | 0.003 | 0.161 | ||||||
| C | 0.000 | 0.170 | 0.176 | |||||
| D | 0.006 | 0.142 | 0.132 | 0.179 | ||||
| E | 0.001 | 0.150 | 0.139 | 0.171 | 0.090 | |||
| F | 0.041 | 0.135 | 0.127 | 0.177 | 0.089 | 0.092 | ||
| G | 0.005 | 0.137 | 0.128 | 0.171 | 0.087 | 0.089 | 0.063 | |
| H | 0.006 | 0.150 | 0.132 | 0.175 | 0.096 | 0.102 | 0.076 | 0.055 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faraone, F.P.; Vecchioni, L.; Sciberras, A.; Di Gangi, A.; Deidun, A. First Insights into the Mitochondrial DNA Diversity of the Italian Sea-Slater Across the Strait of Sicily. Diversity 2025, 17, 622. https://doi.org/10.3390/d17090622
Faraone FP, Vecchioni L, Sciberras A, Di Gangi A, Deidun A. First Insights into the Mitochondrial DNA Diversity of the Italian Sea-Slater Across the Strait of Sicily. Diversity. 2025; 17(9):622. https://doi.org/10.3390/d17090622
Chicago/Turabian StyleFaraone, Francesco Paolo, Luca Vecchioni, Arnold Sciberras, Antonella Di Gangi, and Alan Deidun. 2025. "First Insights into the Mitochondrial DNA Diversity of the Italian Sea-Slater Across the Strait of Sicily" Diversity 17, no. 9: 622. https://doi.org/10.3390/d17090622
APA StyleFaraone, F. P., Vecchioni, L., Sciberras, A., Di Gangi, A., & Deidun, A. (2025). First Insights into the Mitochondrial DNA Diversity of the Italian Sea-Slater Across the Strait of Sicily. Diversity, 17(9), 622. https://doi.org/10.3390/d17090622






