Evidence in Support of the Kelp Conveyor Hypothesis
Abstract
1. Introduction
1.1. Kelp Rafting Violates Isolation by Distance
1.2. The Setting of Haida Gwaii
1.3. The Kelp Conveyor Hypothesis
2. Materials and Methods
2.1. Species Selection
2.2. Sample Collection
2.3. Data Analyses
3. Results
4. Discussion
4.1. Gene Flow Across Barriers
4.2. Community-Level Impacts
4.3. Uneven Sampling Sizes & Low Diversity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Fraser, C.; Nikula, R.; Waters, J. Oceanic rafting by a coastal community. Proc. R. Soc. 2011, 278, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, C.-S. Floating kelps in Patagonian Fjords: An important vehicle for rafting invertebrates and its relevance for biogeography. Mar. Biol. 2012, 159, 2035–2049. [Google Scholar] [CrossRef]
- Avila, C.; Angulo-Preckler, C.; Martín-Martín, R.P.; Figuerola, B.; Griffiths, H.J.; Waller, C.L. Invasive marine species discovered on non–native kelp rafts in the warmest Antarctic island. Sci. Rep. 2020, 10, 1639. [Google Scholar] [CrossRef]
- Smith, S.D.A. Kelp rafts in the Southern Ocean. Glob. Ecol. Biogeogr. 2002, 11, 67–69. [Google Scholar] [CrossRef]
- Craw, D.; Waters, J. Long distance kelp-rafting of rocks around southern New Zealand. J. Geol. Geophys. 2018, 61, 428–443. [Google Scholar] [CrossRef]
- Foster, M.; Schiel, D. The Ecology of Giant Kelp Forests in California: A Community Profile. In US Fish & Wildlife Service Biological Report; The U.S. Fish and Wildlife Service (FWS): Falls Church, VA, USA, 1985; Volume 85, p. 152. [Google Scholar]
- Bushing, W.W. Biogeographic and ecological implications of kelp rafting as a dispersal vector for marine invertebrates. In The Fourth California Islands Symposium: Update on the Status of Resources; Halvorson, W.L., Maender, G.J., Eds.; Santa Barbara Museum of Natural History: Santa Barbara, CA, USA, 1994; pp. 103–110. [Google Scholar]
- Hobday, A.J. Persistence and transport of fauna on drifting kelp (Macrocystis pyrifera (L.) C. Agardh) rafts in the Southern California Bight. J. Exp. Mar. Biol. Ecol. 2000, 253, 75–96. [Google Scholar] [CrossRef]
- Wright, S. Isolation by distance. Genetics 1943, 28, 114–138. [Google Scholar] [CrossRef]
- Durrant, H.M.S.; Burridge, C.P.; Kelaher, B.P.; Barrett, N.S.; Edgar, G.J.; Coleman, M.A. Implications of macroalgal isolation by distance for networks of marine protected areas. Conserv. Biol. 2013, 28, 438–445. [Google Scholar] [CrossRef]
- Santelices, B. Patterns of reproduction, dispersal and recruitment in seaweeds. In Oceanography and Marine Biology Annual Review; Barnes, M., Ed.; Aberdeen University Press: Aberdeen, Scotland, 1990; Volume 28, pp. 177–276. [Google Scholar]
- Kumagai, N.H.; Molinos, J.G.; Yamano, H.; Takao, S.; Fujii, M.; Yamanaka, Y. Ocean currents and herbivory drive macroalgae-to-coral community shift under climate warming. Proc. Natl. Acad. Sci. USA 2018, 115, 8990–8995. [Google Scholar] [CrossRef]
- De Jode, A.; David, R.; Haguenauer, A.; Cahill, A.; Erga, Z.; Guillemain, D.; Sartoretto, S.; Rocher, C.; Marjorie, S.; Le Gall, L.; et al. From seascape ecology to population genomics and back. Spatial and ecological differentiation among cryptic species of the red algae Lithophyllum stictiforme/L. cabiochiae, main bioconstructors of coralligenous habitats. Mol. Phylogenet. Evol. 2019, 137, 104–113. [Google Scholar] [CrossRef]
- Gaylord, B.; Reed, D.; Raimondi, P.; Washburn, L.; McLean, S. A physically based model of macroalgal spore dispersal in the wave and current-dominated nearshore. Ecology 2002, 83, 1239–1251. [Google Scholar] [CrossRef]
- Van den Hoek, C. The possible significance of long-range dispersal for the biogeography of seaweeds. Helgol. Mar. Res. 1987, 41, 261–272. [Google Scholar] [CrossRef]
- Macaya, E.C.; Lopez, B.; Tala, F.; Tellier, F.; Thiel, M. Float and raft: Role of buoyant seaweeds in the phylogeography and genetic structure of non-buoyant associated flora. In Seaweed Phylogeography: Adaptation and Evolution of Seaweeds Under Environmental Change; Hu, Z.M., Fraser, C., Eds.; Springer: Dordrecht, The Netherlands, 2016; pp. 97–130. [Google Scholar]
- Calder, J.A.; Taylor, R.L. Flora of the Queen Charlotte Islands, Part I, Systematics of Vascular Plants; Research Branch, Canada, Department of Agriculture Monograph No. 4 Queen’s Printer: Ottawa, ON, Canada, 1968. [Google Scholar]
- Warner, B.C.; Mathewes, R.W.; Clague, J.J. Ice-free conditions on the Queen Charlotte Islands, British Columbia, at the height of the late Wisconsin glaciation. Science 1982, 218, 675–677. [Google Scholar] [CrossRef]
- Shafer, A.B.A.; Cullingham, C.I.; Côté, S.D.; Coltman, D.W. Of glaciers and refugia: A decade of study sheds new light on the phylogeography of northwestern North America. Mol. Ecol. 2010, 19, 4589–4621. [Google Scholar] [CrossRef]
- Keever, C.C.; Sunday, J.; Puritz, J.B.; Addison, J.A.; Toonen, R.J.; Grosberg, R.K.; Hart, M.W. Discordant distribution of populations and genetic gariation in a sea star with high dispersal potential. Evolution 2009, 63, 3214–3227. [Google Scholar] [CrossRef]
- Saunders, G.W. Long distance kelp rafting impacts seaweed biogeography in the northeast Pacific: The kelp conveyor hypothesis. J. Phycol. 2014, 50, 968–974. [Google Scholar] [CrossRef] [PubMed]
- Schwartzlose, R.A.; Reid, J.L. Near-shore circulation in the California Current. Calif. Mar. Res. Comm. CalCOFI Rept. 1972, 16, 57–65. [Google Scholar]
- Penrose, L.W. Analysis of the inshore California Current System off Central California using naval oceanographic office survey data from 1997 to 2002. Master’s Thesis, Naval Postgraduate School, Monterey CA, USA, September 2012. [Google Scholar]
- Thomson, R.E.; Krassovski, M.V. Poleward reach of the California undercurrent extension. J. Geophys. Res. 2010, 15, C09027. [Google Scholar] [CrossRef]
- Dayton, P.K.; Tegner, M.J. Catastrophic storms, El Niño, and patch stability in a Southern California kelp community. Science 1984, 224, 283–285. [Google Scholar] [CrossRef] [PubMed]
- Rothäusler, E.; Gómez, I.; Hinojosa, I.A.; Karsten, U.; Tala, F.; Thiel, M. Effect of temperature and grazing on growth and reproduction of floating Macrocystis spp. (Phaeophyceae) along a latitudinal gradient. J. Phycol. 2009, 45, 547–549. [Google Scholar] [CrossRef]
- Hobday, A.J. Abundance and dispersal of drifting kelp Macrocystis pyrifera rafts in the Southern California Bight. Mar. Ecol. Prog. Ser. 2000, 195, 101–116. [Google Scholar] [CrossRef]
- Saunders, G.W.; McDevit, D.C. Methods for DNA barcoding photosynthetic protists emphasizing the macroalgae and diatoms. In DNA Barcodes: Methods and Protocols; Kress, W.J., Erickson, D.L., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 207–222. [Google Scholar]
- Saunders, G.W.; Moore, T.E. Refinements for the amplification and sequencing of red algal DNA barcode and Red ToL phylogenetic markers: A summary of current primers, profiles and strategies. Algae 2013, 28, 31–43. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Drummond, A. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R-Project. Available online: https://www.R-project.org (accessed on 6 May 2025).
- Chao, A. Non-parametric estimation of the number of classes in a population. Scand. J. Stat. 1984, 11, 265–270. [Google Scholar]
- Cayuela, L.; Gotelli, N.J.; Colwell, R.K. Ecological and biogeographic null hypotheses for comparing rarefaction curves. Ecol. Monogr. 2015, 85, 437–455. [Google Scholar] [CrossRef]
- Winter, D. Mmod: An R library for the calculation of population differentiation statistics. Mol. Ecol. Res. 2012, 12, 1158–1160. [Google Scholar] [CrossRef]
- Galarza, J.A.; Carreras-Carbonell, J.; Macpherson, E.; Pascual, M.; Roques, S.; Turner, G.F.; Rico, C. The influence of oceanographic fronts and early-life-history traits on connectivity among littoral fish species. Proc. Natl. Acad. Sci. USA 2009, 106, 1473–1478. [Google Scholar] [CrossRef]
- Magnell, B.A.; Bray, N.A.; Winant, C.D.; Greengrove, C.L.; Largier, J.; Borchardt, J.F.; Bernstein, R.L.; Dorman, C.E. Convergent shelf flow at Cape Mendocino. Oceanography 1990, 4, 4–11. [Google Scholar] [CrossRef]
- Barth, J.A.; Pierce, S.D.; Smith, R.L. A separating coastal upwelling jet at Cape Blanco, Oregon and its connection to the California Current System. Deep Sea Res. Part II 2000, 47, 783–810. [Google Scholar] [CrossRef]
- Nikula, R.; Spencer, H.G.; Waters, J.M. Passive rafting is a powerful driver of transoceanic gene flow. Biol. Lett. 2013, 9, 20120821. [Google Scholar] [CrossRef]
- Leese, F.; Agrawal, S.; Held, C. Long-distance island hopping without dispersal stages: Transportation across major zoographic barriers in a Southern Ocean isopod. Sci. Nat. 2010, 97, 583–594. [Google Scholar] [CrossRef]
- Guillemin, M.; Valero, M.; Tellier, F.; Macaya, E.; Destombe, C.; Faugeron, S. Phylogeography of seaweeds in the South East Pacific: Complex Evolutionary Processes Along a Latitudinal Gradient. In Seaweed Phylogeography; Hu, Z.-M., Fraser, C., Eds.; Springer: Dordrecht, The Netherlands, 2016; pp. 251–277. [Google Scholar]
- Kelly, R.P.; Palumbi, S.R. Genetic structure among 50 species of the northeastern Pacific rocky intertidal community. PLoS ONE 2010, 5, e8594. [Google Scholar] [CrossRef]
- Merilees, B. Records of the Red Abalone in British Columbia. Vic. Nat. 2008, 63, 11. [Google Scholar]
- Sloan, N.A.; McDevit, D.C.; Saunders, G.W. Further to the occurrence of red abalone, Haliotis rufescens, in British Columbia. Can. Field Nat. 2010, 124, 238. [Google Scholar] [CrossRef]
- Hobday, A.J.; Tegner, M.J. The Warm and the Cold: Influence of Temperature and Fishing on Local Population Dynamics of Red Abalone. Rep. Calif. Coop. Ocean. Fish. Investig. 2002, 43, 74–96. [Google Scholar]
- Saunders, G.W.; Brooks, C.M. Metabarcoding extends the distribution of Porphyra corallicola (Bangiales) into the Arctic while revealing novel species and patterns for Conchocelis stages in the Canadian flora. Diversity 2023, 15, 677. [Google Scholar] [CrossRef]
- Drew, K. Conchocelis-phase in the life-history of Porphyra umbilicalis (L.) Kütz. Nature 1949, 164, 748–749. [Google Scholar] [CrossRef]
- Martinez, E. The conchocelis-phase of porphyra (Rhodophyta) in the intertidal of San-Juan island, Washington, USA. Phycologia 1990, 29, 391–395. [Google Scholar] [CrossRef]
- Yang, M.Y.; Kim, M.S. Phylogeography of economic seaweeds Chondrus (Gigartinales, Rhodophyta) in the Northwest Pacific based on rbcL and COI-5P genes. Algae 2022, 37, 135–147. [Google Scholar] [CrossRef]
- Brooks, C.M.; Saunders, G.W. First record of Scinaia cf. johnstoniae (Nemaliales, Rhodophyta) in Gwaii Haanas, British Columbia. BioInvasions Rec. 2021, 10, 270–276. [Google Scholar] [CrossRef]

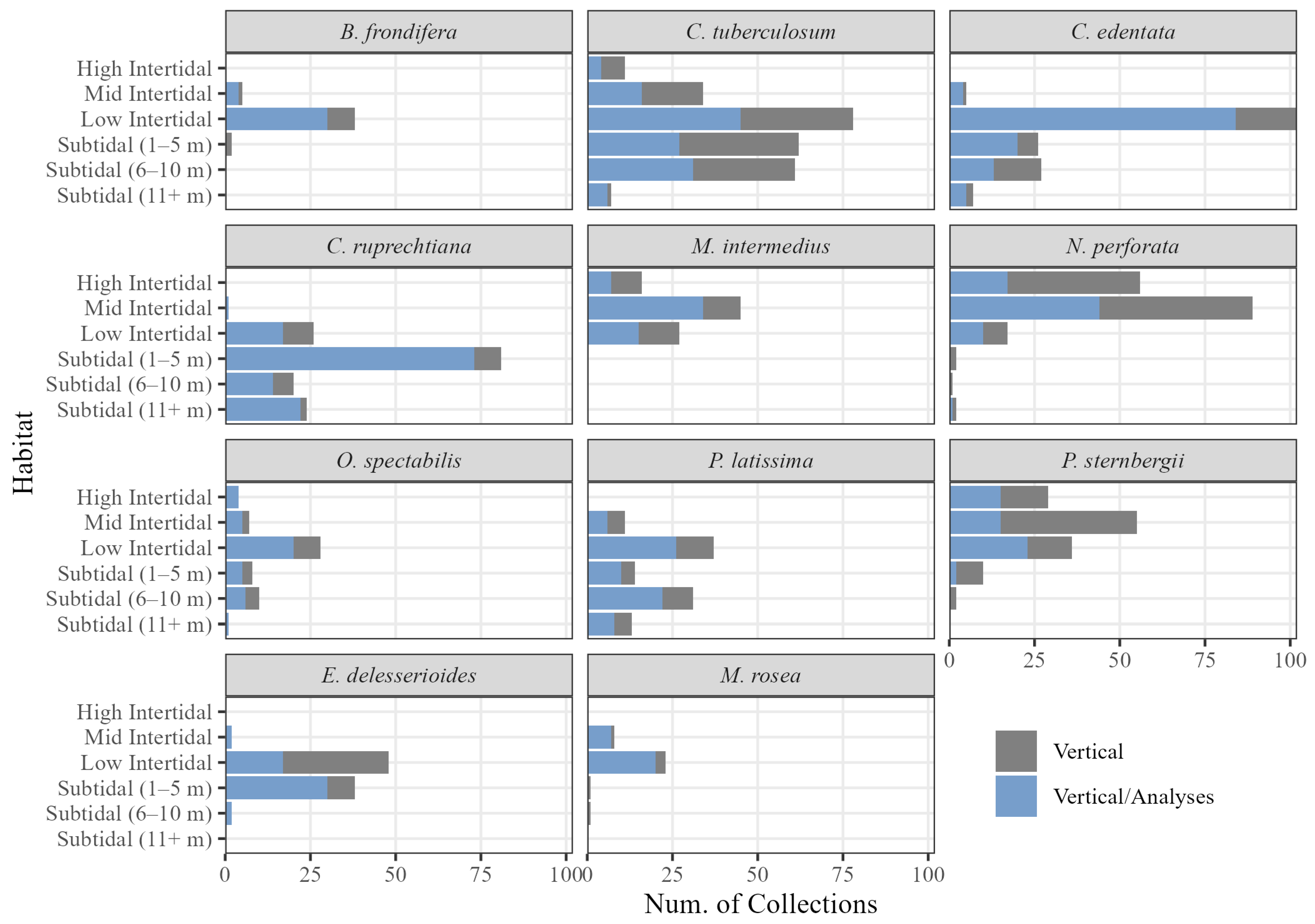
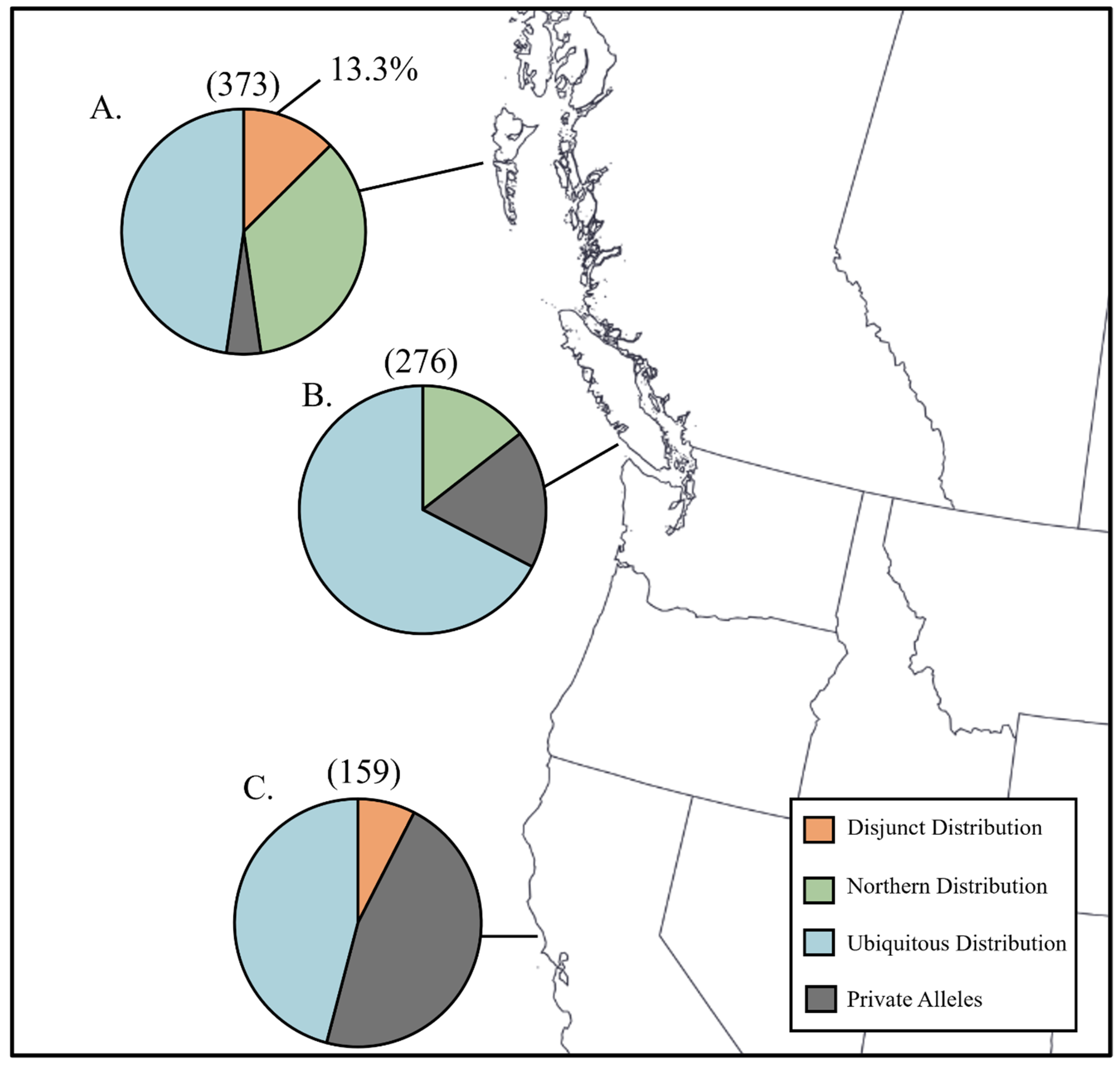

| Species | California | Haida Gwaii | S. Vancouver Isl. |
|---|---|---|---|
| Bossiella frondifera | 12 | 16 | 6 |
| Calliarthron tuberculosum | 28 | 49 | 54 |
| Callophyllis edentata | 15 | 70 | 48 |
| Cryptopleura ruprechtiana | 30 | 84 | 20 |
| Erythrophyllum delesserioides | 8 | 32 | 12 |
| Mastocarpus intermedius | 9 | 30 | 17 |
| Mazzaella rosea | 11 | 8 | 8 |
| Neoporphyra perforata | 12 | 16 | 44 |
| Osmundea spectabilis | 13 | 19 | 9 |
| Polyneura latissima | 12 | 40 | 21 |
| Prionitis sternbergii | 9 | 9 | 37 |
| Species | California | Haida Gwaii | S. Vancouver Isl. |
|---|---|---|---|
| Bossiella frondifera | 4/5 | 3/4 | 2/2 |
| Calliarthron tuberculosum | 2/2 | 4/4 | 5/7 |
| Callophyllis edentata | 5/5 | 3/3 | 1/1 |
| Cryptopleura ruprechtiana | 5/5 | 2/2 | 3/3 |
| Erythrophyllum delesserioides | 6/8 | 3/3 | 2/2 |
| Mastocarpus intermedius | 2/2 | 2/2 | 4/4 |
| Mazzaella rosea | 5/7 | 3/4 | 1/1 |
| Neoporphyra perforata | 4/6 | 2/2 | 4/4 |
| Osmundea spectabilis | 5/6 | 2/2 | 4/5 |
| Polyneura latissima | 3/3 | 7/11 | 6/9 |
| Prionitis sternbergii | 5/5 | 1/1 | 1/1 |
| Species | Haida Gwaii–California | S. Vancouver Isl.–California | Haida Gwaii–S. Vancouver Isl. |
|---|---|---|---|
| Bossiella frondifera | 0.0000143 * | 0.0000299 * | 0.0000254 * |
| Calliarthron tuberculosum | 0.0000054 | 0.0000033 | 0.0000554 * |
| Callophyllis edentata | 0.0000210 * | 0.0000908 * | 0.0000530 * |
| Cryptopleura ruprechtiana | 0.0000449 * | 0.0000901 * | 0.0000290 * |
| Erythrophyllum delesserioides | 0.0000066 * | 0.0002481 * | 0.0005711 * |
| Mastocarpus intermedius | 0.0000624 * | 0.0000453 * | 0.0001304 * |
| Mazzaella rosea | 0.0000058 | 0.0000249 * | 0.0000103 * |
| Neoporphyra perforata | 0.0000374 * | 0.0000450 * | 0.0000472 * |
| Osmundea spectabilis | 0.0000200 | 0.0000679 | <0.00001 |
| Polyneura latissima | 0.0000451 * | 0.0000666 * | 0.0000071 |
| Prionitis sternbergii | 0.0000440 * | 0.0000663 * | <0.00001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brooks, C.M.; Saunders, G.W. Evidence in Support of the Kelp Conveyor Hypothesis. Diversity 2025, 17, 629. https://doi.org/10.3390/d17090629
Brooks CM, Saunders GW. Evidence in Support of the Kelp Conveyor Hypothesis. Diversity. 2025; 17(9):629. https://doi.org/10.3390/d17090629
Chicago/Turabian StyleBrooks, Cody M., and Gary W. Saunders. 2025. "Evidence in Support of the Kelp Conveyor Hypothesis" Diversity 17, no. 9: 629. https://doi.org/10.3390/d17090629
APA StyleBrooks, C. M., & Saunders, G. W. (2025). Evidence in Support of the Kelp Conveyor Hypothesis. Diversity, 17(9), 629. https://doi.org/10.3390/d17090629






