Projecting Range Shifts of Hippophae neurocarpa in China Under Future Climate Change Using CMIP6 Models
Abstract
1. Introduction
2. Materials and Methods
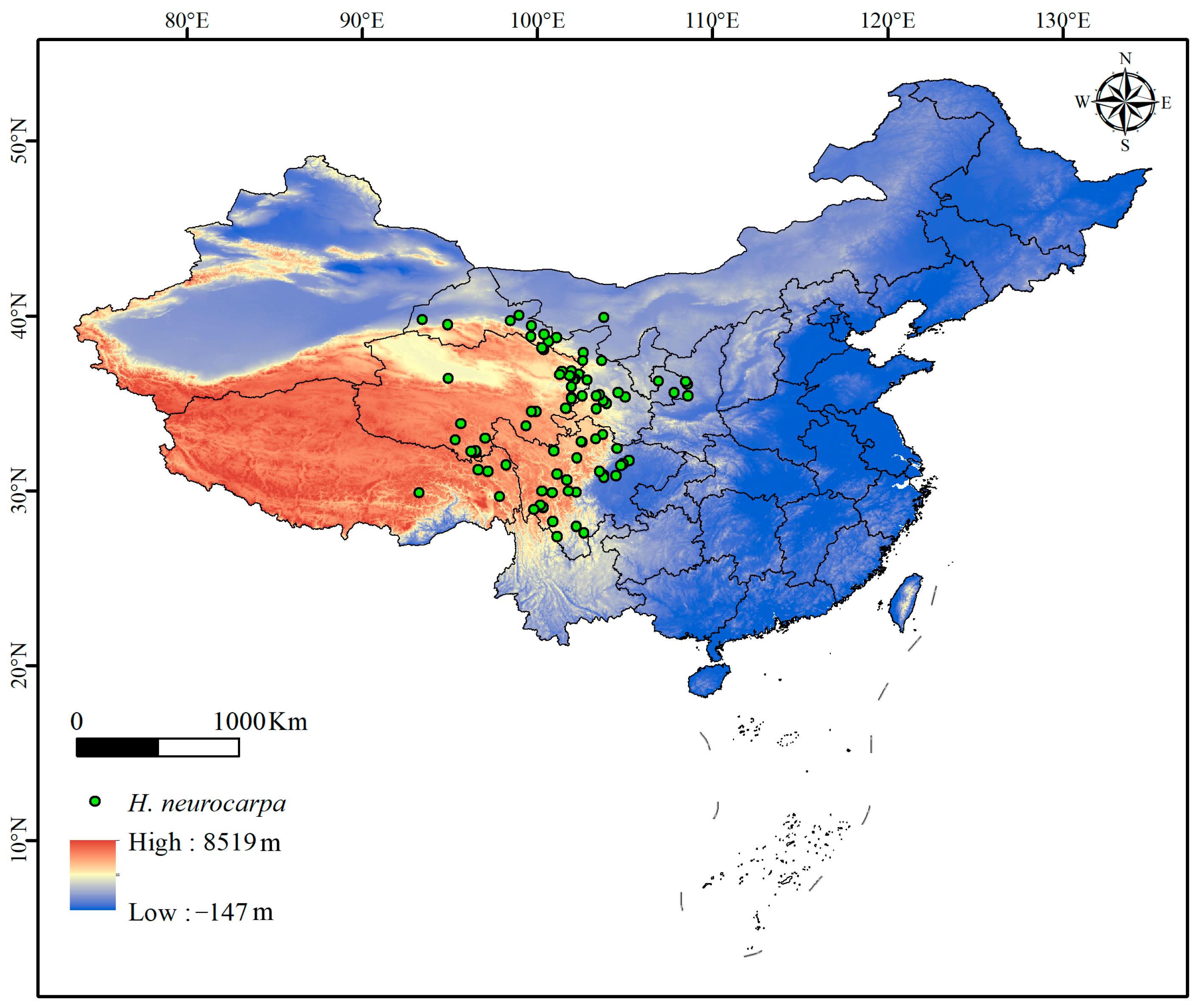
2.1. Species Occurrence Data
2.2. Screening and Processing of the Environmental Variables
2.3. MaxEnt Model Setting
2.4. Shift of Suitable Habitat Distribution Center
3. Results
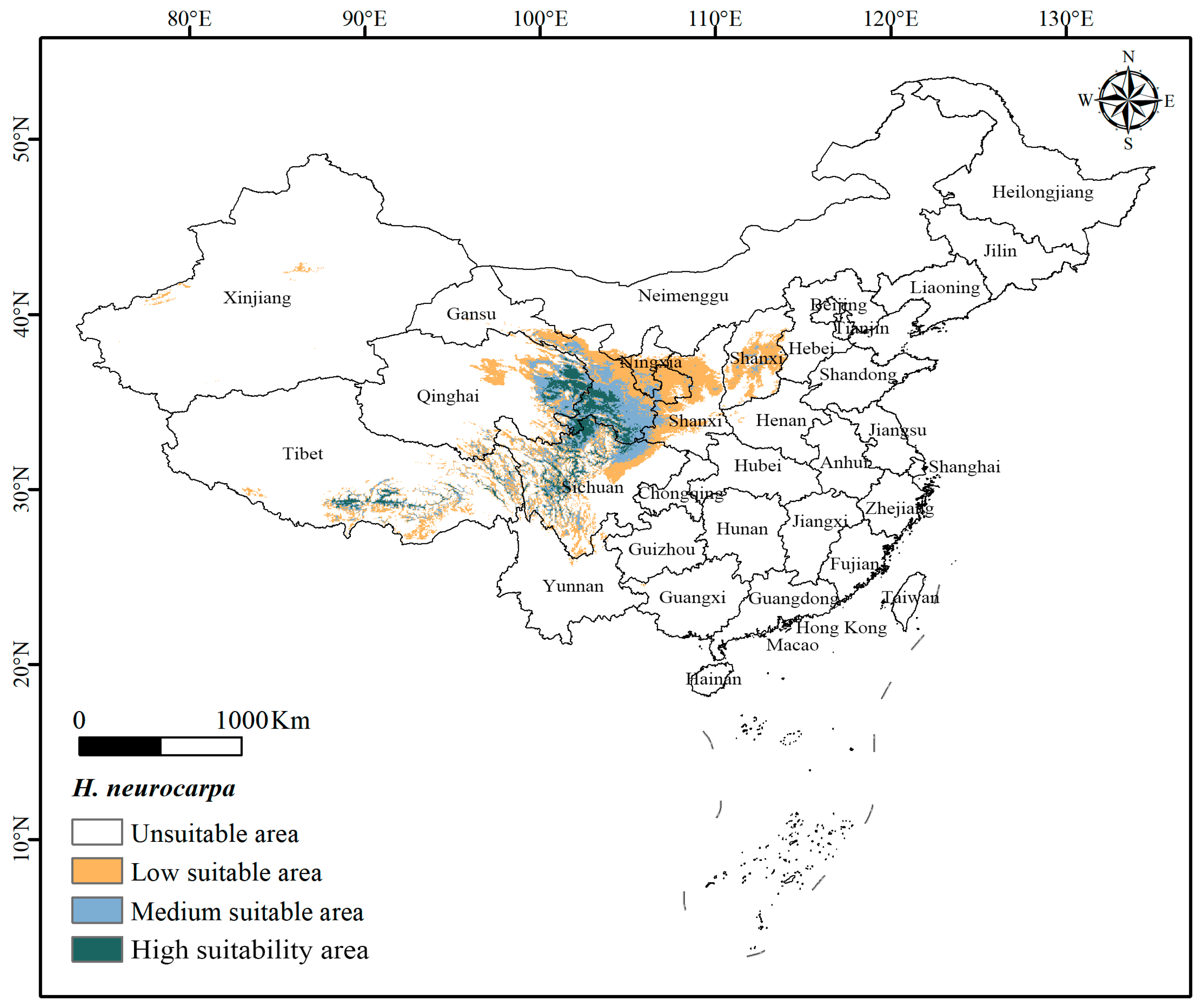
3.1. Potentially Suitable Distribution Areas of H. neurocarpa Under Current Climate
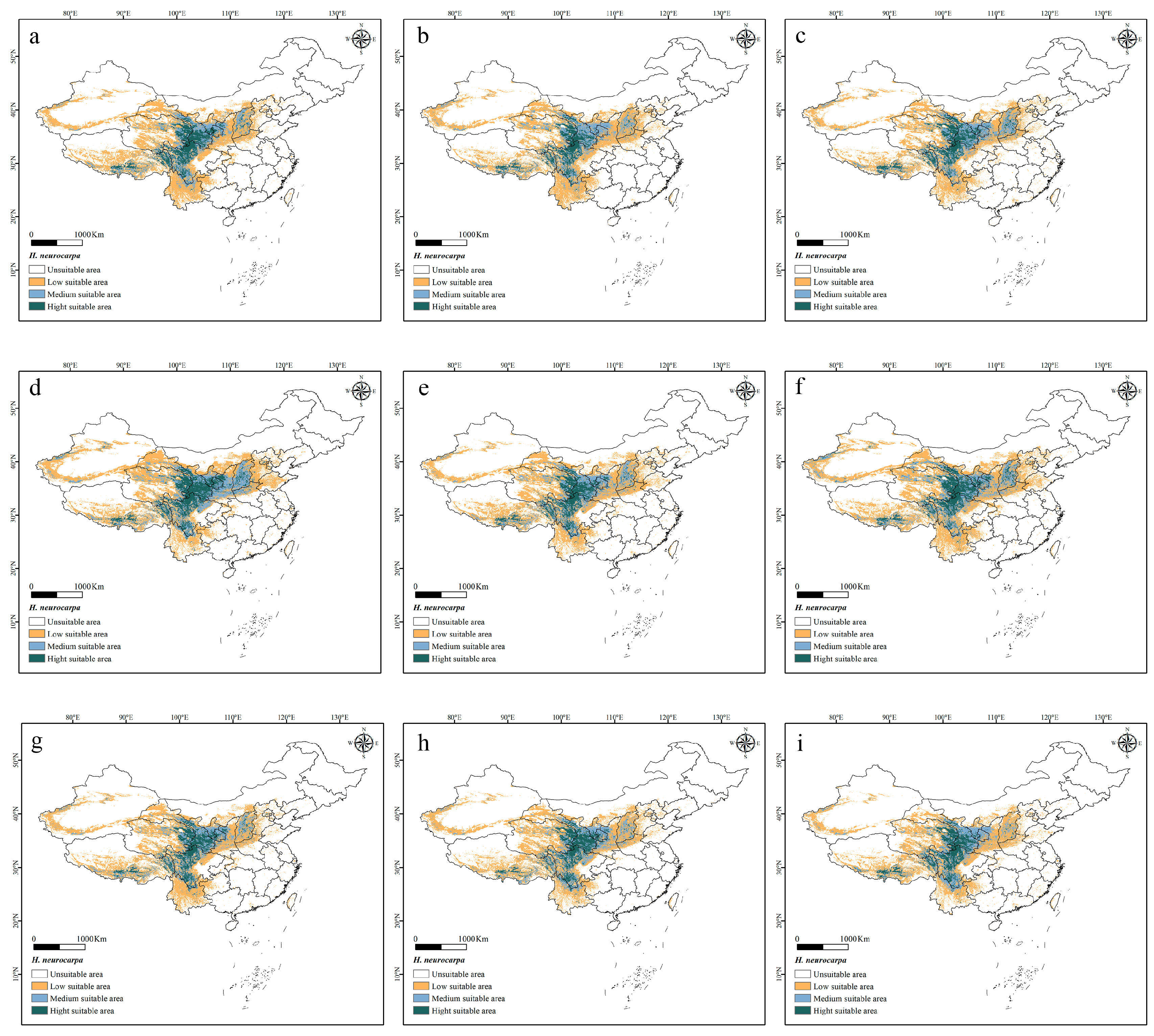
3.2. Future Range Dynamics of H. neurocarpa Under Climate Change Scenarios
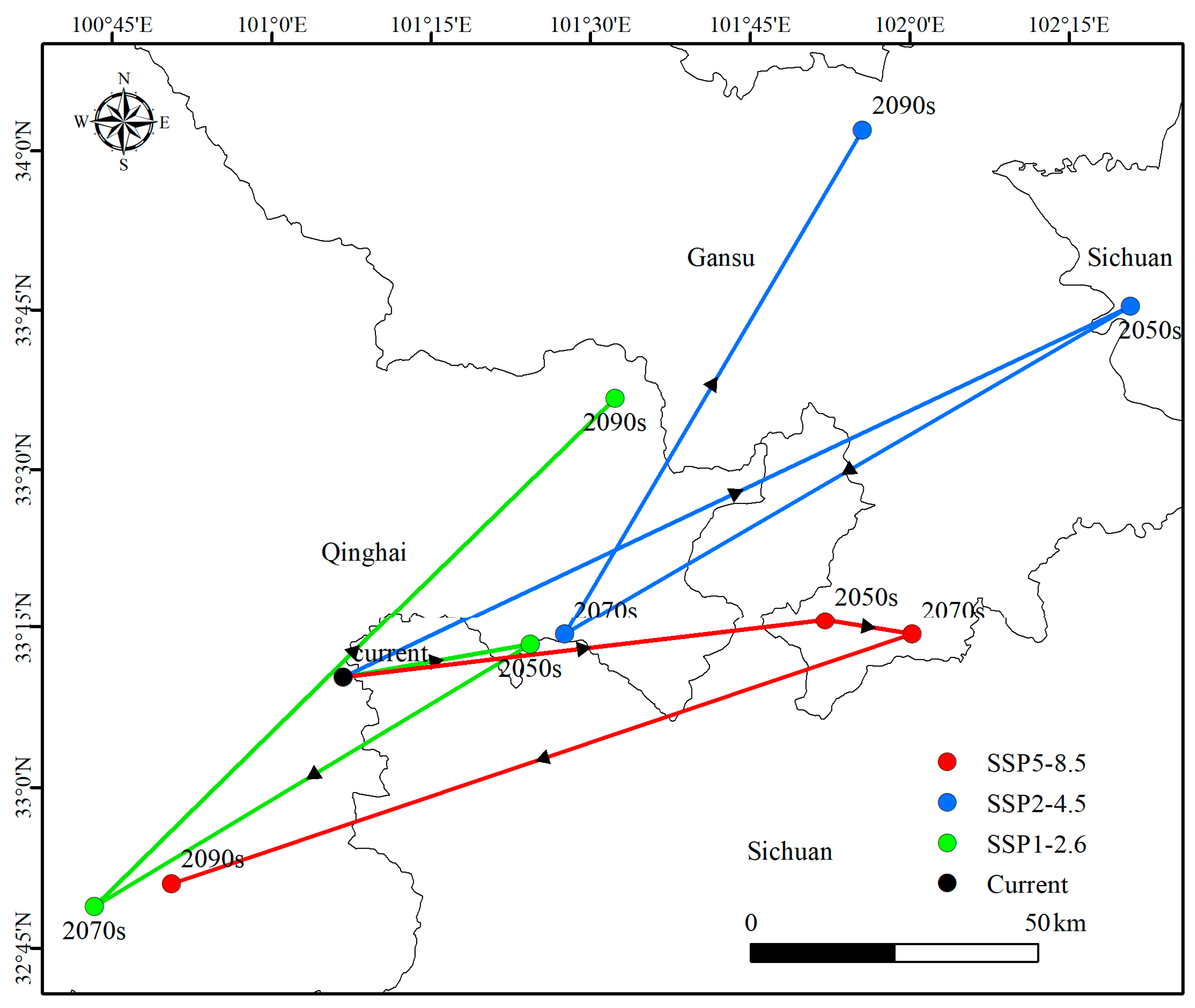
3.3. Centroid Changes in Potential Distribution
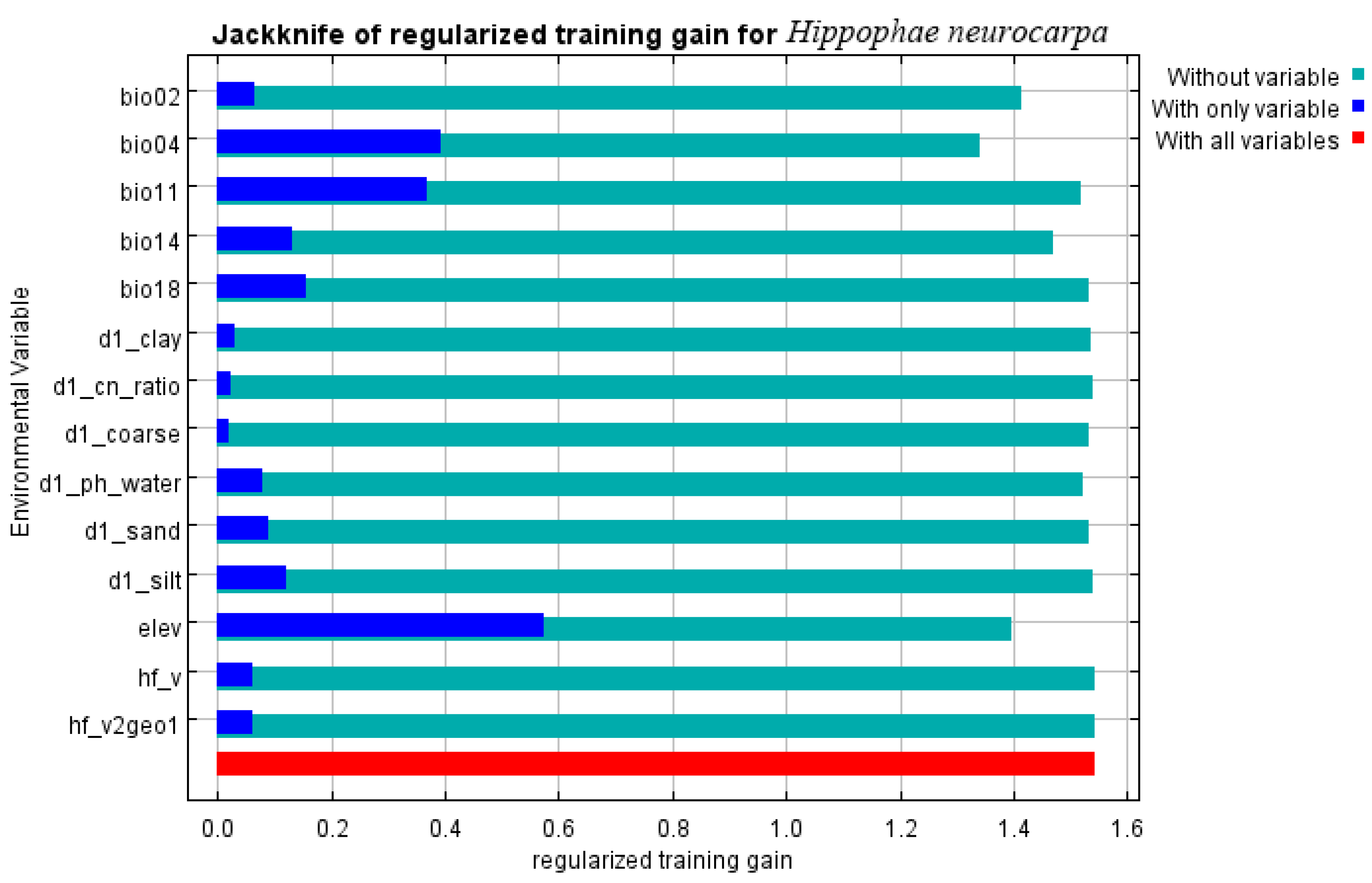
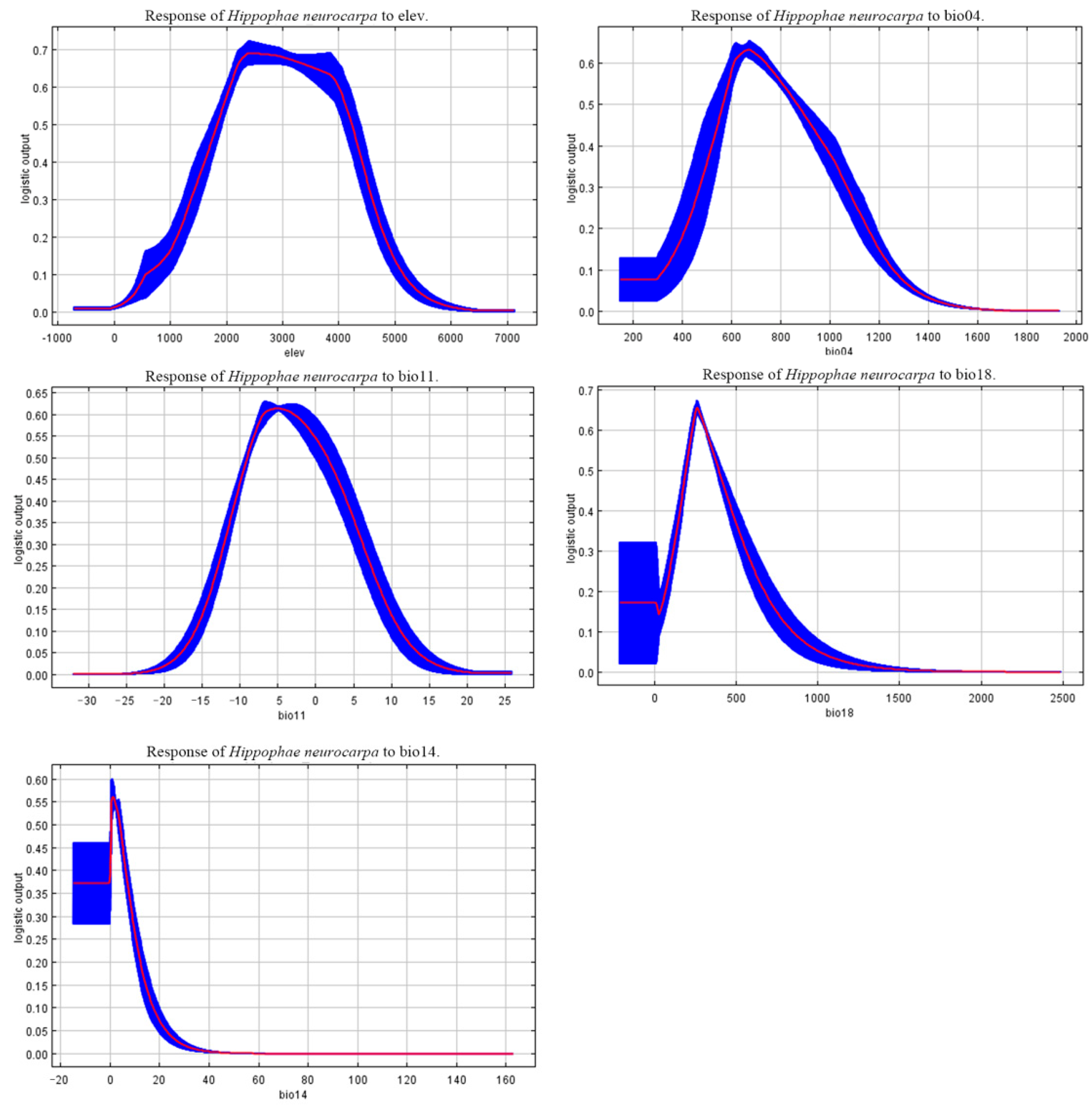
3.4. Relationships Between the Distribution of H. neurocarpa and Bioclimatic Variables
4. Discussion
4.1. Advantages and Limitations of the Model
4.2. Model Limitations
4.3. Main Environmental Factors Affecting the Distribution of H. neurocarpa
4.4. Impact of Climate Change on the Potential Distribution of H. neurocarpa
5. Conclusions
- Key Environmental Factors: Elevation, precipitation, and temperature are the critical environmental factors influencing the spatial and temporal distributions of H. neurocarpa. The suitable environmental thresholds include elevations of 1800–4200 m asl, mean temperatures of the coldest quarter between −8 °C and 2 °C, and precipitation of the warmest quarter ranging from 220 mm to 385 mm. H. neurocarpa is primarily distributed in the Qinghai-Tibet Plateau and its surrounding high-altitude regions in China.
- Future Climate Scenarios: Under future climate scenarios, the suitable distribution areas of H. neurocarpa are expected to expand. By the 2090s, under the SSP2-4.5 scenario, the total suitable area will have expanded the most, increasing by 11.64%. With rising greenhouse gas emissions, the suitable distributions of H. neurocarpa will gradually shift from lower latitudes to higher latitudes.
- Strengths and Limitations of This Study: The strength of this study lies in the selection of environmental factors, which extend beyond the climatic and topographic factors associated with H. neurocarpa. By incorporating comprehensive conditions such as land use and human disturbance, the study provides a more comprehensive evaluation of the species’ current and future potential distributions of this species. However, this study is subject to unavoidable constraints. These include sampling limitations and an unintentional reliance of our data on GBIF and literature sources. We conclude by urging and inviting other researchers to complement our efforts by filling the gaps in this study and providing constructive contributions to help pave the way forward.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviation
| GBIF | Global Biodiversity Information Facility |
References
- Wang, A.; Schluetz, F.; Liu, J. Molecular evidence for double maternal origins of the diploid hybrid Hippophae goniocarpa (Elaeagnaceae): MATERNAL ORIGINS OF Hippophae GONIOCARPA. Bot. J. Linn. Soc. 2008, 156, 111–118. [Google Scholar] [CrossRef]
- Nan, J.B.; Chen, B.Z.; Lin, L.; Zhao, Y.W. Phenotypic Diversity and Germination Characteristics of Hippophae in the Qinghai-Tibet Plateau. Seed 2019, 38, 25–30. [Google Scholar] [CrossRef]
- Olas, B. The beneficial health aspects of sea buckthorn (Elaeagnus rhamnoides (L.) A.Nelson) oil. J. Ethnopharmacol. 2018, 213, 183–190. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, Z.; Zhou, H.; Bai, Z.; Sun, K. The Study on Sea Buckthorn (Genus Hippophae L.) Fruit Reveals Cell Division and Cell Expansion to Promote Morphogenesis. Plants 2023, 12, 1005. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Hu, N.; Dong, Q.; Wang, H.; Wang, Y. Complete chloroplast genome sequences of Hippophae neurocarpa. Mitochondrial DNA Part B 2019, 4, 2048–2049. [Google Scholar] [CrossRef]
- Kou, Y.X.; Wu, Y.X.; Jia, D.R.; Li, Z.H.; Wang, Y.J. Range expansion, genetic differentiation, and phenotypic adaption of Hippophaë neurocarpa (E laeagnaceae) on the Qinghai–Tibet Plateau. J. Syst. Evol. 2014, 52, 303–312. [Google Scholar] [CrossRef]
- Meng, L.H.; Yang, H.L.; Wu, G.L.; Wang, Y.J. Phylogeography of Hippophae neurocarpa (Elaeagnaceae) inferred from the chloroplast DNA trnL-F sequence variation. J. Syst. Evol. 2008, 46, 32–40. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2022: Impacts, Adaptation and Vulnerability; Cambridge University Press: Cambridge, UK, 2022. [Google Scholar]
- Fu, C.; Peng, Y.; Yang, F.; He, Z.; Ali, H.; Xu, D. Potentially suitable geographical area for Colletotrichum acutatum under current and future climatic scenarios based on optimized MaxEnt model. Front. Microbiol. 2024, 15, 1463070. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species Distribution Models: Ecological Explanation and Prediction Across Space and Time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Zimmermann, N.E.; Edwards, T.C.; Graham, C.H.; Pearman, P.B.; Svenning, J. New trends in species distribution modelling. Ecography 2010, 33, 985–989. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y. Applying various algorithms for species distribution modelling. Integr. Zool. 2013, 8, 124–135. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of Species Distributions with Maxent: New Extensions and a Comprehensive Evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Warren, D.L.; Seifert, S.N.; Stohlgren, T.J. Ecological niche modeling in Maxent: The importance of model complexity and the performance of model selection criteria. Ecol. Appl. 2011, 21, 335–342. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, J.; Ren, G.; Zhao, K.; Wang, X. Global potential distribution prediction of Xanthium italicum based on Maxent model. Sci. Rep. 2021, 11, 16545. [Google Scholar] [CrossRef]
- Gao, X.; Lin, F.; Li, M.; Mei, Y.; Li, Y.; Bai, Y.; He, X.; Zheng, Y. Prediction of the potential distribution of a raspberry (Rubus idaeus) in China based on MaxEnt model. Sci. Rep. 2024, 14, 24438. [Google Scholar] [CrossRef]
- Ramampiandra, E.C.; Scheidegger, A.; Wydler, J.; Schuwirth, N. A comparison of machine learning and statistical species distribution models: Quantifying overfitting supports model interpretation. Ecol. Model. 2023, 481, 110353. [Google Scholar] [CrossRef]
- Zhang, F.-G.; Liang, F.; Wu, K.; Xie, L.; Zhao, G.; Wang, Y. The potential habitat of Angelica dahurica in China under climate change scenario predicted by Maxent model. Front. Plant Sci. 2024, 15, 1388099. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, B.; Meng, Y.; Jia, Y.; Xu, Q.; Pan, Y. The Influence of Climate Change on the Distribution of Hibiscus mutabilis in China: MaxEnt Model-Based Prediction. Plants 2024, 13, 1744. [Google Scholar] [CrossRef]
- Fu, C.; Qian, Q.; Deng, X.; Zhuo, Z.; Xu, D. Prediction and Analysis of the Global Suitable Habitat of the Oryctes rhinoceros (Linnaeus, 1758) (Coleoptera: Scarabaeidae) Based on the MaxEnt Model. Insects 2024, 15, 774. [Google Scholar] [CrossRef]
- Wen, X.; Fang, G.; Chai, S.; He, C.; Sun, S.; Zhao, G.; Lin, X. Can ecological niche models be used to accurately predict the distribution of invasive insects? A case study of Hyphantria cunea in China. Ecol. Evol. 2024, 14, e11159. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Xiao, N.; Shen, M.; Li, J. Comparison between optimized MaxEnt and random forest modeling in predicting potential distribution: A case study with Quasipaa boulengeri in China. Sci. Total Environ. 2022, 842, 156867. [Google Scholar] [CrossRef]
- Ali, F.; Khan, N.; Khan, A.M.; Ali, K.; Abbas, F. Species distribution modelling of Monotheca buxifolia (Falc.) A. DC.: Present distribution and impacts of potential climate change. Heliyon 2023, 9, e13417. [Google Scholar] [CrossRef]
- Robinson, N.M.; Nelson, W.A.; Costello, M.J.; Sutherland, J.E.; Lundquist, C.J. A Systematic Review of Marine-Based Species Distribution Models (SDMs) with Recommendations for Best Practice. Front. Mar. Sci. 2017, 4, 421. [Google Scholar] [CrossRef]
- Zhao, Y.; Deng, X.; Xiang, W.; Chen, L.; Ouyang, S. Predicting potential suitable habitats of Chinese fir under current and future climatic scenarios based on Maxent model. Ecol. Inform. 2021, 64, 101393. [Google Scholar] [CrossRef]
- Duan, R.-Y.; Kong, X.-Q.; Huang, M.-Y.; Fan, W.-Y.; Wang, Z.-G.; Hernandez-Lemus, E. The Predictive Performance and Stability of Six Species Distribution Models. PLoS ONE 2014, 9, e112764. [Google Scholar] [CrossRef]
- Moreno, R.; Zamora, R.; Molina, J.R.; Vasquez, A.; Herrera, M.Á. Predictive modeling of microhabitats for endemic birds in South Chilean temperate forests using Maximum entropy (Maxent). Ecol. Inform. 2011, 6, 364–370. [Google Scholar] [CrossRef]
- Hayat, U.; Shi, J.; Wu, Z.; Rizwan, M.; Haider, M.S. Which SDM Model, CLIMEX vs. MaxEnt, Best Forecasts Aeolesthes sarta Distribution at a Global Scale under Climate Change Scenarios? Insects 2024, 15, 324. [Google Scholar] [CrossRef]
- Hashim, A.M.; Alharbi, B.M.; Abdulmajeed, A.M.; Elkelish, A.; Hozzein, W.N.; Hassan, H.M. Oxidative Stress Responses of Some Endemic Plants to High Altitudes by Intensifying Antioxidants and Secondary Metabolites Content. Plants 2020, 9, 869. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Dong, M.; Li, M.; Jin, L.; Paré, P.W. Light-Induced Flavonoid Biosynthesis in Sinopodophyllum hexandrum with High-Altitude Adaptation. Plants 2023, 12, 575. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Deswal, R. Comparative proteome profiling of seabuckthorn leaves from low altitude ‘Sikkim’ and high altitude ‘Himachal Pradesh’ Himalayan region hints towards differential stress adaptive responses. J. Proteins Proteom. 2021, 12, 125–141. [Google Scholar] [CrossRef]
- Suren, H. Research on the Growth Characteristics and Management of Mongolian Seabuckthorn in Mongolia. Master’s Thesis, Inner Mongolia Agricultural University, Hohhot, China, 2021. [Google Scholar]
- Gan, T.; Liu, Q.; Xu, D.; He, Z.; Zhuo, Z. Assessing the Impact of Climate Change on Hippophae neurocarpa in China Using Biomod2 Modeling. Agriculture 2025, 15, 722. [Google Scholar] [CrossRef]
- Li, S.; Liu, X.; Shi, P.; Liu, J.; Zhu, P.; Liu, R.; Xing, L.; Luo, X.; Zhao, H.; Zheng, Y.; et al. Changes in Ginkgo biloba L.’s Habitat Due to Climate Change in China. Forests 2024, 15, 2260. [Google Scholar] [CrossRef]
- Fu, C.; Li, M.; Tian, C.; Song, Y.; Yi, X.; Wang, X. Prediction of potential suitable regions of Prunus pseudocerasus based on MaxEnt model under climate change. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2024, 48, 235–242. [Google Scholar] [CrossRef]
- Liu, L.; Guan, L.; Zhao, H.; Huang, Y.; Mou, Q.; Liu, K.; Chen, T.; Wang, X.; Zhang, Y.; Wei, B.; et al. Modeling habitat suitability of Houttuynia cordata Thunb (Ceercao) using MaxEnt under climate change in China. Ecol. Inform. 2021, 63, 101324. [Google Scholar] [CrossRef]

| Abbreviation | Climate Variables | Unit |
|---|---|---|
| Bio01 | Annual mean temperature | °C |
| Bio02 | Mean diurnal range | °C |
| Bio03 | Isothermality (bio2/bio7) (×100) | |
| Bio04 | Temperature Seasonality (standard deviation × 100) | |
| Bio05 | Max temperature of warmest month | °C |
| Bio06 | Min temperature of coldest month | °C |
| Bio07 | Temperature annual range (bio5–bio6) | °C |
| Bio08 | Mean temperature of wettest quarter | °C |
| Bio09 | Mean temperature of driest quarter | °C |
| Bio10 | Mean temperature of warmest quarter | °C |
| Bio11 | Mean temperature of coldest quarter | °C |
| Bio12 | Annual precipitation | mm |
| Bio13 | Precipitation of wettest month | mm |
| Bio14 | Precipitation of driest month | mm |
| Bio15 | Precipitation seasonality (Coefficient of variation) | |
| Bio16 | Precipitation of wettest quarter | mm |
| Bio17 | Precipitation of driest quarter | mm |
| Bio18 | Precipitation of warmest quarter | mm |
| Bio19 | Precipitation of coldest quarter | mm |
| Elev | Altitude (elevation above sea level) (m) | m |
| Slope | Slope | ° |
| Aspect | Aspect | rad |
| d1_bsat | Soil base saturation | % |
| d1_clay | Cation exchange capacity of clayey layer soils | Mol/kg |
| d1_cn_ratio | Carbon to nitrogen ratio | - |
| d1_elec_cond | Conductivity | S/m |
| d1_org_carbon | Organic carbon content | - |
| d1_ph_water | pH (chemistry) | Mol/L |
| d1_sand | Sand content | % |
| d1_silt | Silt content | % |
| d1_swr | Soil moisture status | θg |
| d1_total_n | Total nitrogen | mg/L |
| d1_usda | Classification of soil texture | - |
| gm_lc_v3 | Type of land cover | - |
| gm_ve_v2 | Percentage of vegetation cover | % |
| hf_v2geo1 | Human footprint and anthropogenic impact index | - |
| Variable | Percent Contribution | Permutation Importance |
|---|---|---|
| Elev (m) | 9.9 | 9.2 |
| Bio11 (°C) | 17.8 | 2.9 |
| Bio18 (mm) | 4.7 | 1 |
| Bio14 (mm) | 13 | 10 |
| Bio04 | 16.5 | 60.9 |
| Bio02 (°C) | 3.5 | 10.2 |
| d1_clay | 1.5 | 0.5 |
| d1_cn_ratio | 0.5 | 0 |
| d1_coarse | 0.6 | 0.4 |
| d1_ph_water | 1.7 | 2.2 |
| d1_sand | 1.4 | 0.8 |
| d1_silt | 2.4 | 0.2 |
| hf_v2geo1 | 0.1 | 0 |
| hf_v | 6.4 | 1.6 |
| Decade Scenarios | Predicted Area (×104 km2) | Comparison with Current Distribution (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Low Habitat Suitability | Medium Habitat Suitability | High Habitat Suitability | Total Area | Low Habitat Suitability | Medium Habitat Suitability | High Habitat Suitability | Total Area | |
| Current | 171.65 | 60.06 | 36.95 | 268.66 | - | - | - | - |
| 2050s-SSP1-2.6 | 176.22 | 55.96 | 55.96 | 288.14 | 2.66 | −6.82 | 51.44 | 7.25 |
| 2070s-SSP1-2.6 | 167.56 | 60.76 | 31.59 | 259.90 | −2.38 | 1.16 | −14.52 | −3.26 |
| 2090s-SSP1-2.6 | 157.48 | 59.31 | 36.85 | 253.64 | −8.25 | −1.25 | −0.28 | −5.59 |
| 2050s-SSP2-4.5 | 178.62 | 70.43 | 49.78 | 298.82 | 4.06 | 17.27 | 34.70 | 11.23 |
| 2070s-SSP2-4.5 | 169.87 | 59.69 | 38.54 | 268.10 | −1.04 | −0.61 | 4.29 | −0.21 |
| 2090s-SSP2-4.5 | 189.74 | 66.62 | 43.57 | 299.93 | 10.54 | 10.93 | 17.90 | 11.64 |
| 2050s-SSP5-8.5 | 164.97 | 54.92 | 37.53 | 257.42 | −3.89 | −8.56 | 1.56 | −4.18 |
| 2070s-SSP5-8.5 | 156.51 | 57.75 | 38.26 | 252.51 | −8.82 | −3.84 | 3.52 | −6.01 |
| 2090s-SSP5-8.5 | 143.75 | 55.46 | 36.35 | 235.56 | −16.25 | −7.66 | −1.62 | −12.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, B.; Peng, Y.; Xu, D. Projecting Range Shifts of Hippophae neurocarpa in China Under Future Climate Change Using CMIP6 Models. Diversity 2025, 17, 609. https://doi.org/10.3390/d17090609
Zhu B, Peng Y, Xu D. Projecting Range Shifts of Hippophae neurocarpa in China Under Future Climate Change Using CMIP6 Models. Diversity. 2025; 17(9):609. https://doi.org/10.3390/d17090609
Chicago/Turabian StyleZhu, Bing, Yaqin Peng, and Danping Xu. 2025. "Projecting Range Shifts of Hippophae neurocarpa in China Under Future Climate Change Using CMIP6 Models" Diversity 17, no. 9: 609. https://doi.org/10.3390/d17090609
APA StyleZhu, B., Peng, Y., & Xu, D. (2025). Projecting Range Shifts of Hippophae neurocarpa in China Under Future Climate Change Using CMIP6 Models. Diversity, 17(9), 609. https://doi.org/10.3390/d17090609








