Diversity and Seasonal Variation in Live Baits Caught in Hann Bay, Dakar, Senegal
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling Strategy
2.3. Data Analysis
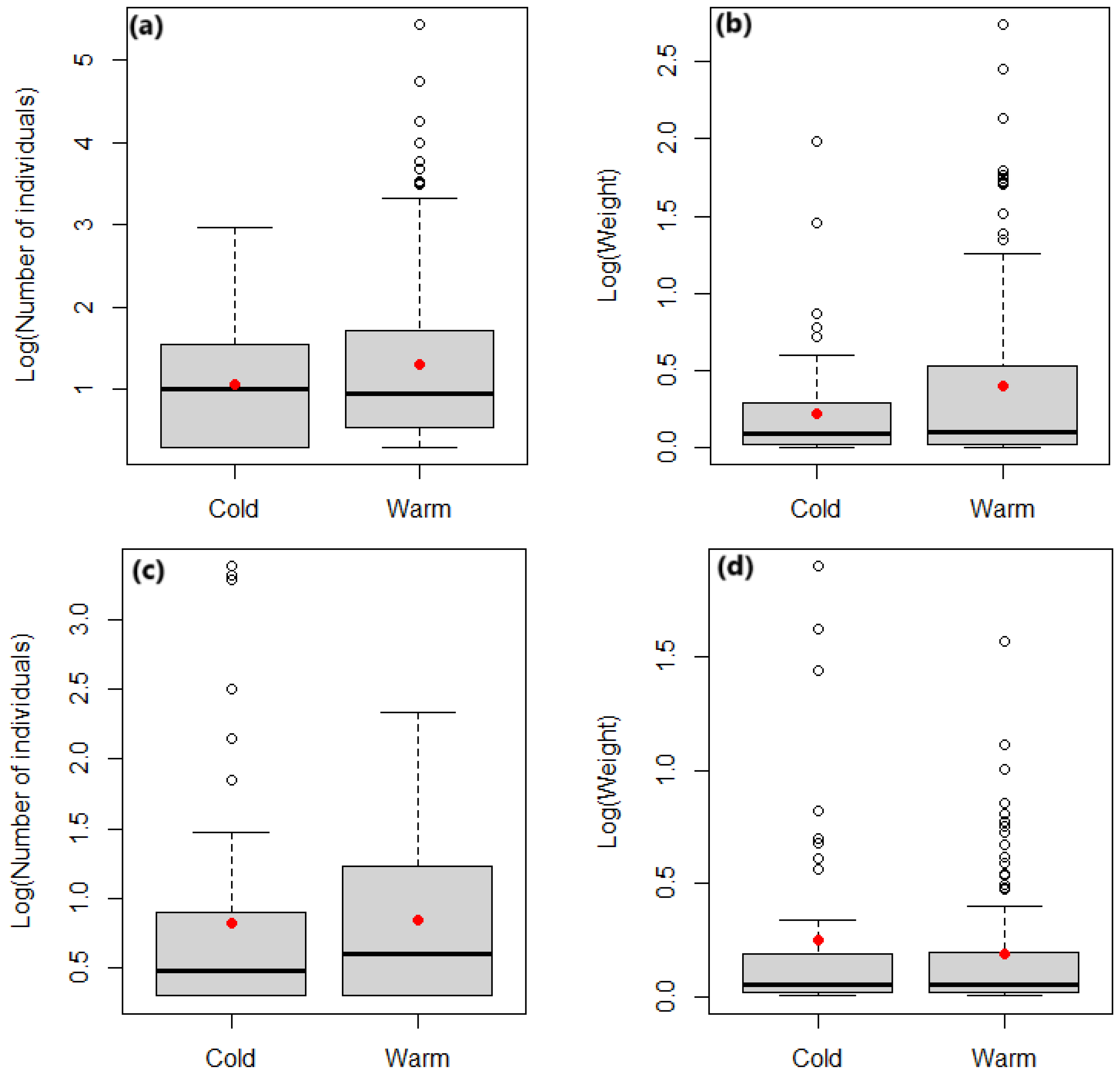
3. Results
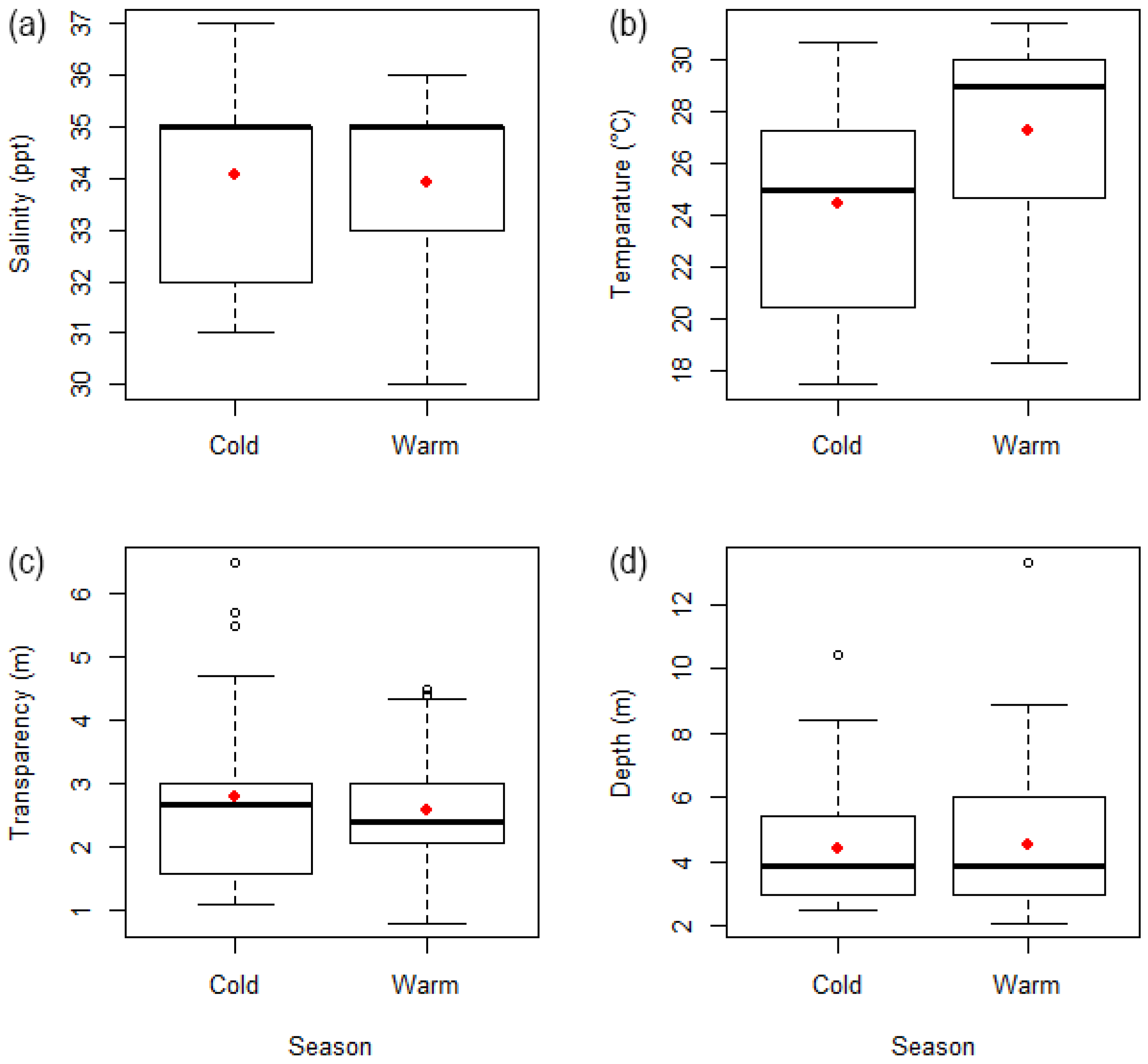
3.1. Aquatic Environment of Hann Bay
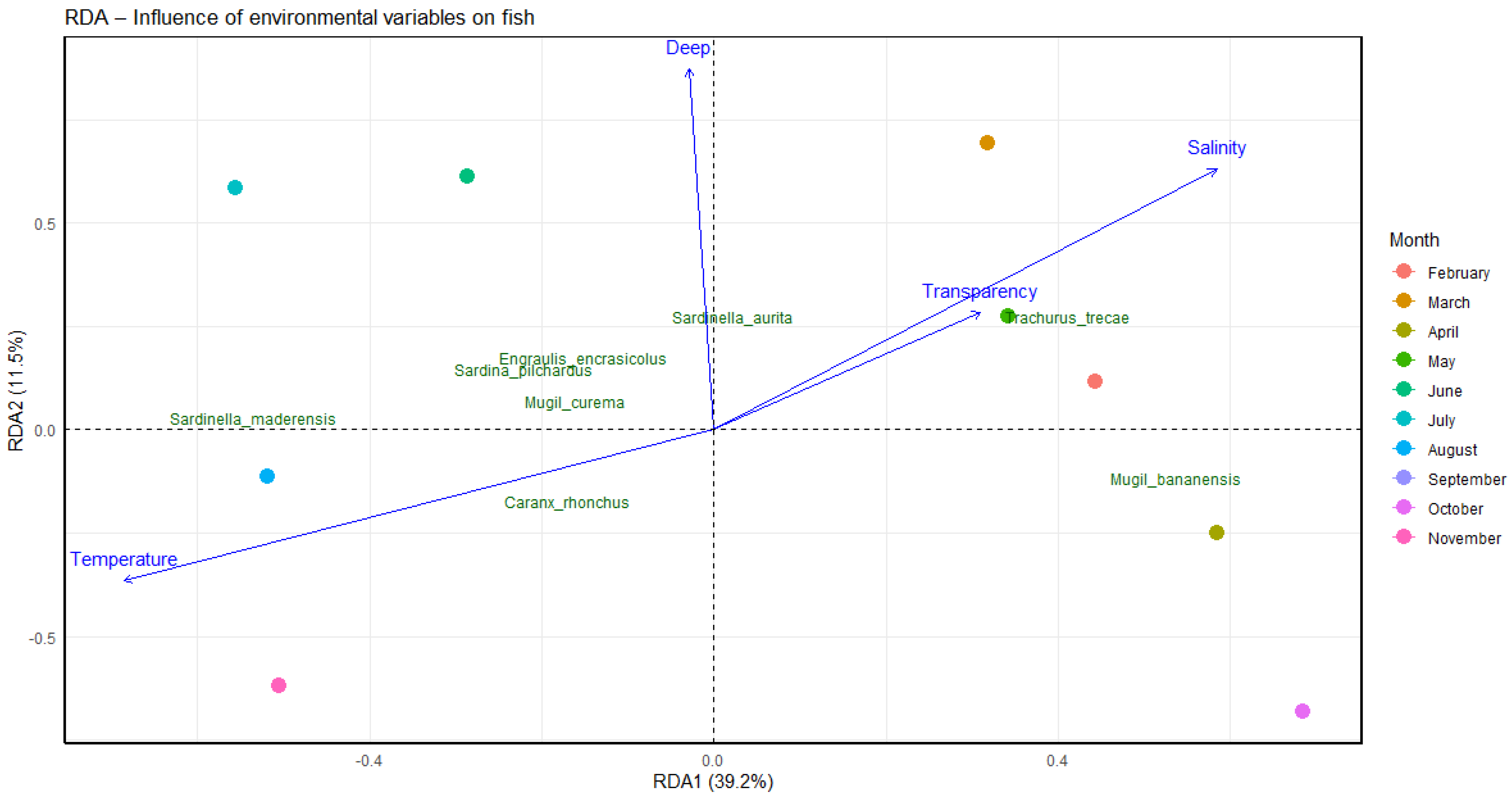
3.2. Environmental Drivers of Live Bait Fish Assemblages
3.3. Species Composition of Catches
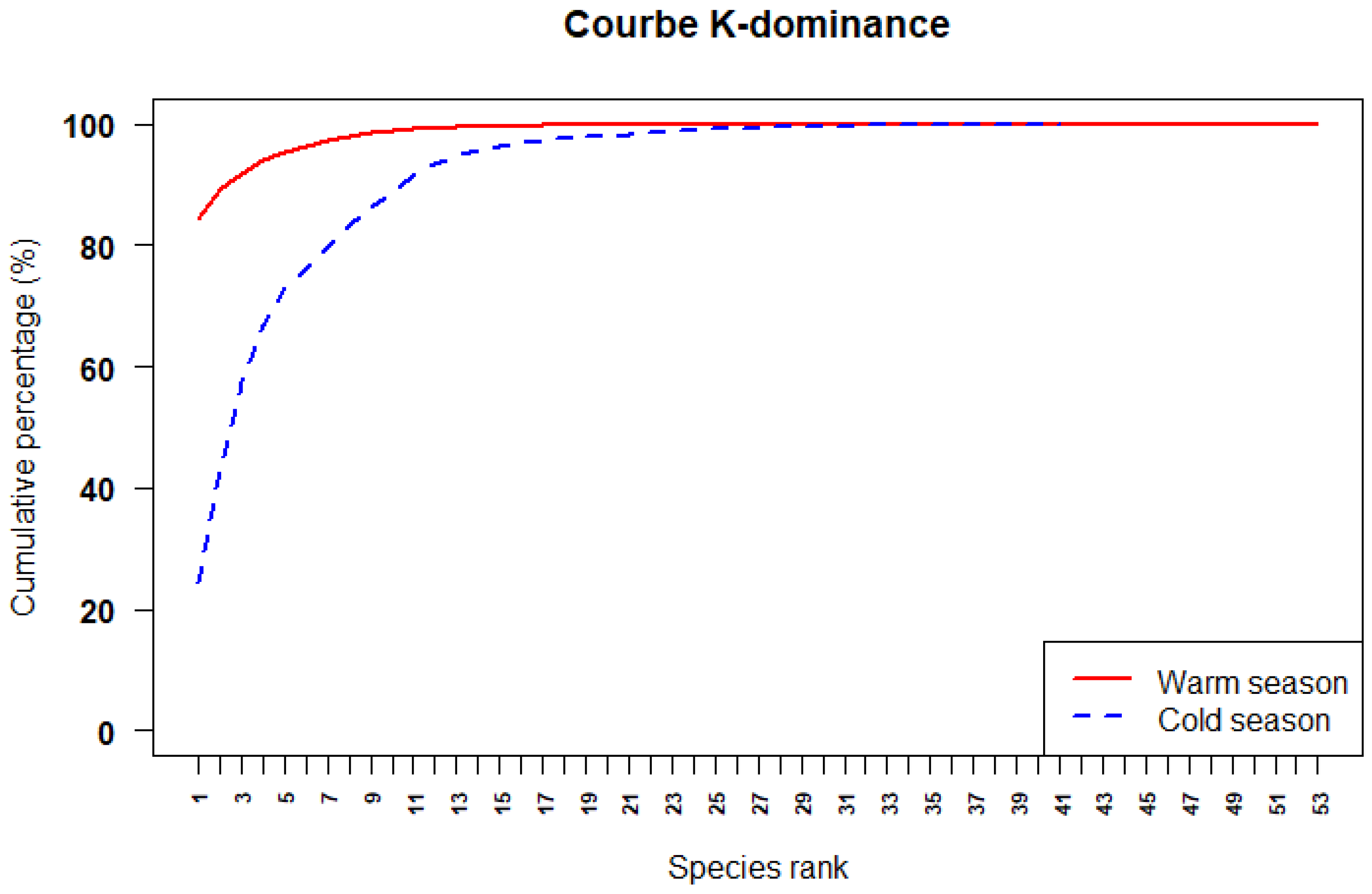
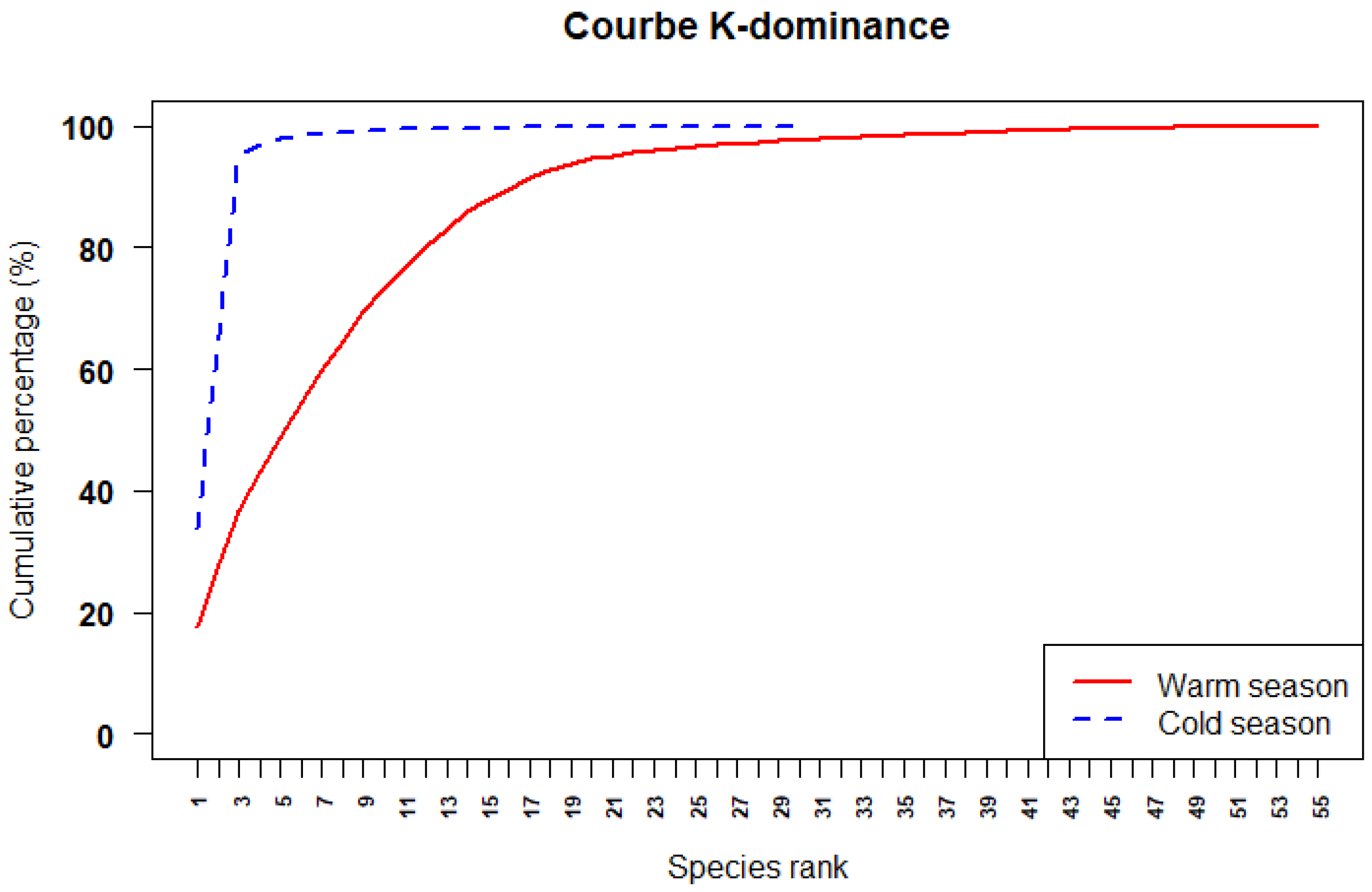
3.4. Seasonal Variation in Diversity Indices
3.5. Seasonal Variation in the Number and Weight of Live Baits
3.6. Proportion of Juvenile Live Baits in the Catches
4. Discussion
4.1. Aquatic Environment
4.2. Drivers of Community Structure in Live Bait Fish
4.3. Variation in Species Richness
4.4. Variation in Diversity Indices
4.5. Seasonal Variation in Species Dominance
4.6. Variation in Abundance and Biomass
4.7. Juvenile Exploitation and Implications for Sustainability
4.8. Impacts of Fishing on Biodiversity
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Appendix A
| Group | Family | Species | Beach Seine | Purse Seine |
|---|---|---|---|---|
| Live baits | Alosidae | Sardina pilchardus (Walbaum, 1792) | * | * |
| Carangidae | Caranx rhonchus (Geoffroy Saint-Hilaire, 1817) | * | * | |
| Trachurus trecae (Cadenat, 1950) | * | * | ||
| Dorosomatidae | Ethmalosa fimbriata (Bowdich, 1825) | * | ||
| Sardinella aurita (Valenciennes, 1847) | * | * | ||
| Sardinella maderensis (Lowe, 1838) | * | * | ||
| Engraulidae | Engraulis encrasicolus (Linnaeus, 1758) | * | * | |
| Mugilidae | Mugil bananensis (Pellegrin, 1927) | * | * | |
| Mugil cephalus (Linnaeus, 1758) | * | |||
| Mugil curema (Valenciennes, 1836) | * | * | ||
| Miscellaneous category | Ariidae | Carlarius parkii Cuvier, 1833 (Günther, 1864) | * | |
| Batrachoididae | Halobatrachus didactylus (Bloch & Schneider, 1801) | * | ||
| Belonidae | Ablennes hians (Valenciennes, 1846) | * | ||
| Tylosurus Acus Rafale (Collette & Parin, 1970) | * | * | ||
| Carangidae | Caranx crysos (Mitchill, 1815) | * | * | |
| Caranx hippos (Linnaeus, 1766) | * | |||
| Caranx senegallus (Cuvier, 1833) | * | * | ||
| Chloroscombrus chrysurus (Linnaeus, 1766) | * | * | ||
| Hemicaranx bicolor (Günther, 1860) | * | |||
| Lichia amia (Linnaeus, 1758) | * | |||
| Selene dorsalis (Gill, 1863) | * | * | ||
| Trachinotus ovatus (Linnaeus, 1758) | * | * | ||
| Centrolophidae | Schedophilus pemarco (Poll, 1959) | * | ||
| Cichlidae | Coptodon guineensis (Günther, 1862) | * | * | |
| Sarotherodon melanotheron (Rüppell, 1852) | * | * | ||
| Cyclopsettidae | Citharichthys stampflii (Steindachner, 1894) | * | ||
| Syacium guineensis (Bleeker, 1862) | * | * | ||
| Cynoglossidae | Cynoglossus senegalensis (Kaup, 1858) | * | * | |
| Dactylopteridae | Dactylopterus volitans (Linnaeus, 1758) | * | ||
| Diodontidae | Chilomycterus spinosus (Linnaeus, 1758) | * | * | |
| Drepaneidae | Drepane africana (Osório, 1892) | * | * | |
| Epinephelidae | Epinephelus aeneus (Geoffroy Saint-Hilaire, 1817) | * | * | |
| Exocoetidae | Exocoetus volitans (Linnaeus, 1758) | * | * | |
| Fodiator acutus (Valenciennes, 1847) | * | * | ||
| Fistulariidae | Fistularia petimba (Lacepède, 1803) | * | * | |
| Fistularia tabacaria (Linnaeus, 1758) | * | * | ||
| Gerreidae | Eucynostomus melanopterus (Bleeker, 1863) | * | * | |
| Gerres Nigri (Günther, 1859) | * | * | ||
| Haemulidae | Brachydeuterus auritus (Valenciennes, 1832) | * | * | |
| Pomadasys incisus (Bowdich, 1825) | * | * | ||
| Pomadasys jubelini (Cuvier, 1830) | * | |||
| Pomadasys peroteti (Cuvier, 1830) | * | * | ||
| Pomadasys rogeri (Cuvier, 1830) | * | |||
| Hemiramphidae | Hemiramphus brasiliensis (Linnaeus, 1758) | * | * | |
| Monacanthidae | Stephanolepis hispidus (Linnaeus, 1766) | * | * | |
| Mugilidae | Chelon dumerili (Steindachner, 1870) | * | ||
| Neochelon falcipinnis (Valenciennes, 1836) | * | * | ||
| Parachelon grandisquamis (Valenciennes, 1836) | * | |||
| Mullus barbatus (Linnaeus, 1758) | * | |||
| Pseudupeneus prayensis (Cuvier, 1829) | * | * | ||
| Polynemidae | Galeoides decadactylus (Bloch, 1795) | * | ||
| Priacanthidae | Priacanthus arenatus (Cuvier, 1829) | * | ||
| Scaridae | Scarus hoefleri (Steindachner, 1881) | * | ||
| Sciaenidae | Pseudotolithus senegallus (Cuvier, 1830) | * | * | |
| Umbrina canariensis (Valenciennes, 1843) | * | * | ||
| Scombridae | Auxis thazard (Lacepède, 1800) | * | ||
| Euthynnus alletteratus (Rafinesque, 1810) | * | * | ||
| Scomberomorus tritor (Cuvier, 1832) | * | |||
| Soleidae | Pegusa lascaris (Risso, 1810) | * | ||
| Solea senegalensis (Kaup, 1858) | * | |||
| Synaptura cadenati (Chabanaud, 1948) | * | |||
| Sparidae | Boops boops (Linnaeus, 1758) | * | ||
| Diplodus bellottii (Steindachner, 1882) | * | * | ||
| Diplodus cervinus (Lowe, 1838) | * | |||
| Diplodus sargus (Linnaeus, 1758) | * | |||
| Diplodus vulgaris (Geoffroy Saint-Hilaire, 1817) | * | * | ||
| Lithognathus mormyrus (Linnaeus, 1758) | * | * | ||
| Pagellus bellottii (Steindachner, 1882) | * | * | ||
| Pagrus auriga (Valenciennes, 1843) | * | |||
| Pagrus caeruleostictus (Valenciennes, 1830) | * | * | ||
| Spondyliosoma cantharus (Linnaeus, 1758) | * | * | ||
| Sphyraenidae | Sphyraena afra (Peters, 1844) | * | ||
| Sphyraena guachancho (Cuvier, 1829) | * | |||
| Synodontidae | Trachinocephalus myops (Forster, 1801) | * | ||
| Tetraodontidae | Ephippion guttifer (Bennett, 1831) | * | * | |
| Lagocephalus laevigatus (Linnaeus, 1766) | * | * | ||
| Sphoeroides marmoratus (Lowe, 1838) | * | * | ||
| Sphoeroides pachygaster (Müller & Troschel, 1848) | * | |||
| Torpedinidae | Torpedo mackayana (Metzelaar, 1919) | * | ||
| Torpedo marmorata (Risso, 1810) | * | |||
| Torpedo torpedo (Linnaeus, 1758) | * |
References
- Mbaye, A. Cadre Institutionnel et Réglementaire de la Pêche Artisanale Sénégalaise; Document Scientifique; CRODT/ISRA: Dakar, Senegal, 2014; p. 29. [Google Scholar]
- Gillett, R.E. Étude mondiale sur la gestion de la pêche d’appâts destinés aux thoniers canneurs. In Lettre d’Information sur les Pêches de la CPS n° 139—Septembre/Décembre 2012; International Seafood Sustainability Foundation (ISSF): Pittsburgh, PA, USA, 2012; pp. 31–34. [Google Scholar]
- Hallier, J.P.; Delgado De Molina, A. Baitboat as a tuna aggregating device. In Pêche Thonière et Dispositifs de Concentration de Poissons; Actes du Colloque IFREMER, 28, 553–578; IFREMER: Brest Cedex, France, 2000; p. 26. [Google Scholar]
- Ngom-Sow, F.; Thiam, N.; Ba, K. Appui à la Pêcherie Thonnière à la Canne au Sénégal; Rapport de consultance; FIP pêche à la canne et à la ligne; CRODT: Dakar, Senegal, 2020; p. 14. [Google Scholar]
- Barry, M.; Dème, M.; Diouf, T.; Fontana, A.; Samba, A.; Thiam, D. La pêche. In Bilan de la Recherche Agricole et Agro-Alimentaire au Sénégal; Document ISRA, ITA et CIRAD; Projet JOKO/FAC no 98004900; Ministère Français des Affaires Etrangères: Paris, France, 2005; pp. 345–367. [Google Scholar]
- Caillart, B.; Million, J.; Fonteneau, A.; Sculley, M. Étude de faisabilité du programme de marquage de thons tropicaux de l’océan Atlantique. Collect. Sci. Pap. ICCAT 2014, 71, 1–114. [Google Scholar]
- Dème, M.; Failler, P.; Dème, M. Contribution de la pêche artisanale migrante à l’approvisionnement des usines de farine de poisson au Sénégal, en Gambie et en Mauritanie. Front. Mar. Sci. 2022, 10, 871911. [Google Scholar] [CrossRef]
- Diarra, B.; Ndiaye, W.; Diouf, M. Characterization of Livebait Fishing by Pole-and-Line Tuna Vessels in Hann Bay, Senegal, West Africa. Am. J. Life Sci. 2022, 10, 88–94. [Google Scholar]
- Gueye, M.; Fall, M.; Diouf, M.; Baldé, B. Characterization of artisanal bait fishing using juveniles of round Sardinella aurita and flat Sardinella maderensis off Hann Bay (Dakar region, Senegal). Int. J. Fish. Aquat. Stud. 2020, 8, 164–171. [Google Scholar]
- Dème, E.B.; Dème, M. Mise en marché des petits pélagiques côtiers au Sénégal: Formes de valorisation et enjeux autour de la ressource. EchoGéo 2021, 58, 1–23. [Google Scholar] [CrossRef]
- Jacquemot, P. L’Afrique Face à L’épuisement de ses Ressources de la Pêche Maritime; Hal 04703863; Policy Center for the New South (PCNS): Rabat, Morocco, 2024. [Google Scholar]
- Ba, K.; Thiaw, M.; Lazar, N.; Sarr, A.; Brochier, T.; Ndiaye, I.; Faye, A.; Sadio, O.; Panfili, J.; Thiaw, O.T.; et al. Resilience of key biological parameters of the Senegalese flat sardinella to overfishing and climate change. PLoS ONE 2016, 11, e0156143. [Google Scholar] [CrossRef] [PubMed]
- Dème, M.; Inejih, C.; Baldé, B. Étude sur L’importance Économique, Sociale et Écologique des Petits Pélagiques au Sénégal, en Mauritanie et en Guinée-Bissau; Rapport Technique; PRCM: Dakar, Senegal, 2019; 62p. [Google Scholar]
- Legendre, P.; Legendre, L. Numerical Ecology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Borcard, D.; Gillet, F.; Legendre, P. Numerical Ecology with R, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Wagner, H. Vegan: Community Ecology Package. R Package Version 2.5-7. 2020. Available online: https://CRAN.R-project.org/package=vegan (accessed on 28 July 2025).
- Shannon, C.E. The mathematical theory of communication. Bell Syst. Tech. Jol. 1948, 27, 379–423, 623–656. [Google Scholar] [CrossRef]
- Pielou, E.C. Ecological Diversity; Wiley Interscience: New York, NY, USA, 1975; 165p. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing, version 4.4.2; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 28 July 2025).
- Froese, R.; Pauly, D. FishBase (Version 10/2024). 2024. Available online: www.fishbase.se (accessed on 28 July 2025).
- Ndour, I.; Ndoye, S.; Bâ, C.T.; Diadhiou, H.D.; Ndiaye, O. Size at first sexual maturity of anchovy, Engraulis encrasicolus, in Senegalese waters. AACL Bioflux 2021, 14, 424–429. [Google Scholar]
- Ndiaye, W.; Ndiaye, K.; Sarr, A.; Diédhiou, P. Effect of environmental parameters on the reproduction of the white mullet (Mugil curema, Valenciennes, 1836) in the Saloum Delta (Senegal). J. Biol. Life Sci. 2022, 13, 50–63. [Google Scholar] [CrossRef]
- Keznine, M.; Analla, M.; Aksissou, M.; El Meraoui, A. The reproduction and growth of the sardine Sardina pilchardus in West Mediterranean, Morocco. Egypt. J. Aquat. Biol. Fish. 2020, 24, 303–319. [Google Scholar] [CrossRef]
- Samba, O.; Diouf, K.; Fall, M.; Ndiaye, P.; Panfili, J. Long-Term Changes in Life History Traits and Catches of the Round Sardinella, Sardinella aurita, Along the Senegal Coast, West Africa; Elsevier: Amsterdam, The Netherlands, 2021; p. 26. [Google Scholar]
- Faye, A.; Sarr, A.; Thiaw, M.; Ndiaye, I.; Ba, K.; Fall, J.; Diouf, M.; Thiaw, O.T.; Lazard, N. Reproductive biology of Ethmalosa fimbriata (Bowdich) in Senegalese coastal waters. J. Biol. Life Sci. 2014, 5, 57–71. [Google Scholar] [CrossRef]
- Ndour, I.; Diadhiou, H.D.; Thiaw, O.T. Reproduction of yellow mullet Mugil cephalus on northern coast of Senegal, West Africa. AACL Bioflux 2013, 6, 439–445. [Google Scholar]
- Gobert, B. La Pêche D’appâts Vivants par les Thoniers au Sénégal; Archives, no 128; CRODT: Dakar, Senegal, 1983; 35p. [Google Scholar]
- UNESCO; ICES; SCOR; IAPSO. Background Papers and Supporting Data on the International Equation of State of Seawater 1980; Unesco Technical Papers in Marine Science, Nr. 38; UNESCO: Paris, France, 1981. [Google Scholar]
- Van Vlaardingen, P.L.A.; Verbruggen, E.M.J. Guidance for the Derivation of Environmental Risk Limits Within the Framework of the Project ‘International and National Environmental Quality Standards for Substances in the Netherlands’ (INS)—Revision 2007; Report no. 601782001; National Institute for Public Health and the Environment: Bilthoven, The Netherlands, 2007; p. 146. [Google Scholar]
- Rodier, J.; Legube, B.; Merlet, N. L’analyse de l ’eau; Dunod: Paris, France, 2009. [Google Scholar]
- Mbaye, M.; Hagemann, S.; Haensler, A.; Stacke, T.; Gaye, A.; Afouda, A. Assessment of climate change impact on water resources in the Upper Senegal Basin (West Africa). Am. J. Clim. Change 2015, 4, 77–93. [Google Scholar] [CrossRef]
- Simier, M.; Blanc, L.; Aliaume, C.; Diouf, P.S.; Albaret, J.J. Spatial and temporal structure of fish assemblages in an “inverse estuary” (Sine-Saloum, Senegal). Estuar. Coast. Shelf Sci. 2004, 59, 69–86. [Google Scholar] [CrossRef]
- Feuilloley, G. Analyse de la Variabilité Spatio-Temporelle du Zooplancton dans le Golfe du Lion et Conséquences sur les Populations de Petits Pélagiques. Ph.D. Thesis, Université de Montpellier, Montpellier, France, 2020. [Google Scholar]
- Ross, S.T. Resource partitioning in fish assemblages: A review of field studies. Copeia 1986, 1986, 352–388. [Google Scholar] [CrossRef]
- Diouf, P.S. Les Peuplements de Poissons des Milieux Estuariens de L’afrique de L’ouest: L’exemple de L’estuaire Hyperhalin du Sine-Saloum. Ph.D. Thesis, Université de Montpellier II, Montpellier, France, 1996. [Google Scholar]
- Tine, M.; Ndiaye, A.; Diouf, M.; Panfili, J. Impact of climate variability on estuarine fish communities in West Africa. Reg. Stud. Mar. Sci. 2019, 30, 100679. [Google Scholar]
- Perry, A.L.; Low, P.J.; Ellis, J.R.; Reynolds, J.D. Climate change and distribution shifts in marine fishes. Science 2005, 308, 1912–1915. [Google Scholar] [CrossRef]
- IPCC (Intergovernmental Panel on Climate Change). IPCC Special Report on the Ocean and Cryosphere in a Changing Climate; IPCC: Geneva, Switzerland, 2019. [Google Scholar]
- Blaber, E.B. Nonparametric estimation of fishery selectivity using generalized additive models. Fish. Res. 2000, 45, 153–164. [Google Scholar]
- Albaret, J.J.; Simier, M.; Darboe, F.S.; Ecoutin, J.M.; Raffray, J.; Tito de Morais, L. Fish diversity and distribution in the Gambia Estuary, West Africa, in relation to environmental variables. Estuar. Coast. Shelf Sci. 2004, 63, 421–435. [Google Scholar] [CrossRef]
- Laë, R. Does overfishing lead to a decrease in catches and yields? An example of two West African coastal lagoons. Fish. Manag. Ecol. 1997, 4, 149–164. [Google Scholar] [CrossRef]
- Simier, M.; Laurent, C.; Ecoutin, J.M.; Albaret, J.J. L’estuaire du fleuve Gambie: Une référence pour l’étude des assemblages de poissons estuariens en Afrique de l’Ouest. Estuar. Coast. Shelf Sci. 2006, 69, 615–628. [Google Scholar] [CrossRef]
- Binet, D. Effets des variations de l’harmattan et des upwellings sur les ressources pélagiques des côtes ouest-africaines. Oceanol. Acta. 1985, 8, 177–185. [Google Scholar]
- Cury, P.; Roy, C. Optimal environmental window and pelagic fish recruitment success in upwelling areas. Can. J. Fish. Aquat. Sci. 1989, 46, 670–680. [Google Scholar] [CrossRef]
- Longhurst, A.R.; Pauly, D. Ecology of Tropical Oceans; Academic Press: Cambridge, MA, USA, 1987. [Google Scholar]
- Sieben, C. Ecological Risk Assessment for the Impact of the Senegal-Based Tuna Pole-and-Line Fishery on Species Used as Live Bait; Rapport du Projet D’amélioration de la Pêche au thon à la Canne; Eastern Atlantic P&L Tuna FIP: Dakar, Senegal, 2020; p. 35. [Google Scholar]
- McClatchie, S.; Millar, R.B.; Webster, F.; Lester, P.J.; Hurst, R.; Bagley, N. Demersal fish community diversity off New Zealand: Is it related to depth, latitude and regional surface phytoplankton? Deep Sea Res. Part I Oceanogr. Res. Pap. 1997, 44, 647–667. [Google Scholar] [CrossRef]
- Bianchi, G.; Gislason, H.; Graham, K.; Hill, L.; Jin, X.; Koranteng, K.; Manickchand-Heileman, S.; Paya, I.; Sansbury, K.; Sanchez, F.; et al. Impact of fishing on size composition and diversity of demersal fish communities. ICES J. Mar. Sci. 2000, 57, 558–571. [Google Scholar] [CrossRef]
- Garibaldi, L.; Caddy, J.F. Biogeographic characterization of Mediterranean and Black Seas faunal provinces using GIS procedures. Ocean Coast. Manag. 1998, 39, 211–227. [Google Scholar] [CrossRef]
- Zhu, M.; Zhang, C.; Liu, Y.; Sun, J.; Gao, T.; Sun, Y. Spatiotemporal variation of fish species composition and its response to environmental variables in the East China Sea. Biology 2024, 13, 751. [Google Scholar]
- Zhang, W.; Ma, W.; Chen, L.; Huang, W. Effects of seasonal changes in environmental factors on fish community structure in a subtropical estuary. J. Mar. Sci. Eng. 2022, 10, 1679. [Google Scholar]
- Dalu, T.; Wasserman, R.J.; Froneman, P.W. Seasonal variability in the diet of selected fish species in a small temperate estuary. Environ. Biol. Fishes 2016, 99, 329–341. [Google Scholar]
- Anastasopoulou, A.; Mytilineou, C.; Smith, C.J.; Papadopoulou, K.N. Dominance and diversity patterns of demersal fish communities in a Mediterranean marine protected area. Mar. Biodivers. 2021, 51, 1–16. [Google Scholar]
- Binet, D.; Marchal, E.; Pezennec, O. Upwelling areas and coastal fisheries in West Africa. In African Oceanography; Crawford, R.J.M., Ed.; UNESCO: Paris, France, 1991; pp. 29–52. [Google Scholar]
- FAO. Field Identification Guide to the Living Marine Resources of Western Central Atlantic; FAO: Rome, Italy, 2021. [Google Scholar]
- Fréon, P.; Misund, O.A. Dynamics of Pelagic Fish Distribution and Behaviour: Effects on Fisheries and Stock Assessment; Blackwell Science: Oxford, UK, 1999. [Google Scholar]
- Belhabib, D.; Sumaila, U.R.; Pauly, D. Feeding the poor: Contribution of West African fisheries to employment and food security. Ocean Coast. Manag. 2016, 111, 72–81. [Google Scholar] [CrossRef]
- Diankha, O.; Thiaw, M.; Faye, A.; Diouf, P.S.; Panfili, J. Reproductive strategy and spawning pattern of Pseudotolithus senegalensis (Sciaenidae) in the Sine-Saloum estuary (Senegal, West Africa). Reg. Stud. Mar. Sci. 2018, 21, 3, 63–71. [Google Scholar]
- Chesson, P. Species competition and diversity: Coexistence, history, and the role of temporal environmental variation. BMC Biol. 2013, 11, 98. [Google Scholar]
- Aboua, B.R.D.; Konan, K.M.; Bamba, M. Seasonal variation in fish assemblage structure in a West African estuarine ecosystem (Ebrié Lagoon, Côte d’Ivoire). Aquat. Ecol. 2022, 56, 371–383. [Google Scholar]
- Yáñez-Arancibia, A.; Day, J.W. The Gulf of Mexico estuarine ecosystem: Ecological role and biological indicators. Estuaries Coasts 2004, 27, 1033–1046. [Google Scholar]
- Shimadzu, H.; Dornelas, M.; Henderson, P.A.; Magurran, A.E. Diversity is maintained by seasonal variation in species abundance. BMC Biol. 2013, 11, 98. [Google Scholar] [CrossRef]
- Gross, K.; Cardinale, B.J.; Fox, J.W.; Gonzalez, A.; Loreau, M.; Polley, H.W.; Reich, P.B.; Van Ruijven, J. Species richness and the temporal stability of biomass production: A new analysis of recent biodiversity experiments. Am. Nat. 2014, 183, 1–12. [Google Scholar] [CrossRef]
- Vilas, D.; Pennino, M.G.; Bellido, J.M.; Navarro, J.; Palomera, I.; Coll, M. Seasonality of spatial patterns of abundance, biomass, and biodiversity in a demersal community of the NW Mediterranean Sea. ICES J. Mar. Sci. 2020, 77, 567–580. [Google Scholar] [CrossRef]
- Beverton, R.J.H.; Holt, S.J. On the Dynamics of Exploited Fish Populations; Fishery Investigations Series II, Marine Fisheries; Springer: Dordrecht, The Netherlands, 1957; Volume 19, pp. 1–533. [Google Scholar]
- Bakun, A. Fronts and eddies as key structures in the habitat of marine fish larvae: Opportunity, adaptive response and competitive advantage. Sci. Mar. 2006, 70, 105–122. [Google Scholar] [CrossRef]
- Pikitch, E.K.; Rountos, K.J.; Essington, T.E.; Santora, C.; Pauly, D.; Watson, R.; Sumaila, U.R.; Boersma, P.D.; Boyd, I.L.; Conover, D.O.; et al. The global contribution of forage fish to marine fisheries and ecosystems. Fish Fish. 2014, 15, 43–64. [Google Scholar] [CrossRef]
- Chuenpagdee, R.; Morgan, L.E.; Maxwell, S.M.; Norse, E.A.; Pauly, D. Shifting gears: Assessing collateral impacts of fishing methods in US waters. Front. Ecol. Environ. 2003, 1, 517–524. [Google Scholar] [CrossRef]
- Kolding, J.; Van Zwieten, P.A.M. The tragedy of our legacy: How do global management discourses affect small-scale fisheries in the South? Forum Dev. Stud. 2011, 38, 267–297. [Google Scholar] [CrossRef]
- Tsikliras, A.C.; Dinouli, A.; Tsiros, V.Z.; Tsalkou, E. The Mediterranean and Black Sea fisheries at risk from overexploitation. PLoS ONE 2015, 10, e0121188. [Google Scholar] [CrossRef]
- Froese, R.; Zeller, D.; Kleisner, K.; Pauly, D. What catch data can tell us about the status of global fisheries. Mar. Biol. 2016, 163, 214. [Google Scholar] [CrossRef]
- Pauly, D.; Christensen, V.; Dalsgaard, J.; Froese, R.; Torres, F. Fishing down marine food webs. Science 1998, 279, 860–863. [Google Scholar] [CrossRef] [PubMed]
- Christensen, V.; Guénette, S.; Heymans, J.J.; Walters, C.J.; Watson, R.; Zeller, D.; Pauly, D. Hundred-year decline of North Atlantic predatory fishes. Fish Fish. 2003, 4, 1–24. [Google Scholar] [CrossRef]
- Cury, P.M.; Boyd, I.L.; Bonhommeau, S.; Anker-Nilssen, T.; Crawford, R.J.M.; Furness, R.W.; Mills, J.A.; Murphy, E.J.; Österblom, H.; Paleczny, M.; et al. Global seabird response to forage fish depletion—One-third for the birds. Science 2011, 334, 1703–1706. [Google Scholar] [CrossRef]


| Species | Beach Seine | Purse Seine | Beach Seine | Purse Seine | ||||
|---|---|---|---|---|---|---|---|---|
| Number of Individuals | % Number of Individuals | Number of Individuals | % Number of Individuals | Weight of Individuals (kg) | % Weight of Individuals | Weight of Individuals (kg) | % Weight of Individuals | |
| Caranx rhonchus | 1138 | 0.29 | 158 | 1.68 | 37.66 | 2.16 | 17.73 | 5.80 |
| Engraulis encrasicolus | 325,168 | 83.55 | 6 | 0.06 | 419.10 | 24.05 | 0.06 | 0.02 |
| Ethmalosa fimbriata | 481 | 0.12 | 4.89 | 0.28 | ||||
| Mugil bananensis | 35 | 0.01 | 14 | 0.15 | 2.25 | 0.13 | 2.32 | 0.76 |
| Mugil cephalus | 3 | 0.00 | 1.54 | 0.09 | ||||
| Mugil curema | 289 | 0.07 | 9 | 0.10 | 4.30 | 0.25 | 2.20 | 0.72 |
| Sardina pilchardus | 2214 | 0.57 | 109 | 1.16 | 11.35 | 0.65 | 2.53 | 0.83 |
| Sardinella aurita | 4201 | 1.08 | 113 | 1.20 | 55.26 | 3.17 | 1.37 | 0.45 |
| Sardinella maderensis | 5466 | 1.40 | 2651 | 28.18 | 66.35 | 3.81 | 87.36 | 28.57 |
| Trachurus trecae | 18,346 | 4.71 | 36 | 0.38 | 550.21 | 31.57 | 1.12 | 0.37 |
| Miscellaneous category | 31,830 | 8.18 | 6312 | 67.09 | 589.83 | 33.85 | 191.11 | 62.50 |
| Total | 389,171 | 9408 | 1743 | 306 | ||||
| Gear | Season | Individuals | Biomass (kg) | Seasonal Species Richness (S) | Shannon’s Index (H) | Pielou’s Evenness (J′) | ||
|---|---|---|---|---|---|---|---|---|
| H (0–ln(S)) | Mean ± SD | J′ | Mean ± SD | |||||
| Beach Seine | Warm | 385,301 | 1578 | 53 | 0.77 | 1.59 ± 1.15 | 0.19 | 0.42 ± 0.32 |
| (0–3.97) | ||||||||
| Cold | 3890 | 165 | 40 | 2.4 | 0.65 | |||
| (0–3.68) | ||||||||
| Purse Seine | Warm | 2347 | 132 | 53 | 2.96 | 2.15 ± 1.14 | 0.74 | 0.56 ± 0.34 |
| (0–3.97) | ||||||||
| Cold | 7061 | 174 | 30 | 1.34 | 0.39 | |||
| (0–3.40) | ||||||||
| Species | Gear | Numbers | Minimum (mm) | Maximum (mm) | L50 (mm) | Juvenile (%) |
|---|---|---|---|---|---|---|
| Caranx rhonchus | PS | 158 | 100 | 275 | 200 [20] | 32 |
| BS | 490 | 103 | 295 | 83 | ||
| Engraulis encrasicolus | PS | 7 | 75 | 130 | 96 [21] | 69 |
| BS | 496 | 55 | 127 | 82 | ||
| Mugil curema | PS | 2 | 211 | 224 | 220 [22] | 50 |
| BS | 18 | 205 | 247 | 22 | ||
| Sardina pilchardus | PS | 108 | 96 | 155 | 133 [23] | 79 |
| BS | 99 | 74 | 125 | 100 | ||
| Sardinella aurita | PS | 100 | 60 | 150 | 181 [24] | 100 |
| BS | 300 | 61 | 150 | 100 | ||
| Sardinella maderensis | PS | 987 | 80 | 322 | 166 [12] | 89 |
| BS | 427 | 35 | 210 | 99 | ||
| Trachurus trecae | PS | 36 | 102 | 210 | 168 [20] | 98 |
| BS | 345 | 40,4 | 222 | 99 | ||
| Ethmalosa fimbriata | BS | 435 | 54 | 287 | 172 [25] | 99 |
| Mugil bananensis | PS | 1 | 316 | 316 | 180 [20] | 0 |
| Mugil cephalus | BS | 72 | 45 | 230 | 390 [26] | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keita, M.; Thiam, N.; Ngom, F.; Kantoussan, J.; Ndour, I.; Sadio, O. Diversity and Seasonal Variation in Live Baits Caught in Hann Bay, Dakar, Senegal. Diversity 2025, 17, 608. https://doi.org/10.3390/d17090608
Keita M, Thiam N, Ngom F, Kantoussan J, Ndour I, Sadio O. Diversity and Seasonal Variation in Live Baits Caught in Hann Bay, Dakar, Senegal. Diversity. 2025; 17(9):608. https://doi.org/10.3390/d17090608
Chicago/Turabian StyleKeita, Maryam, Ndiaga Thiam, Fambaye Ngom, Justin Kantoussan, Ismaïla Ndour, and Oumar Sadio. 2025. "Diversity and Seasonal Variation in Live Baits Caught in Hann Bay, Dakar, Senegal" Diversity 17, no. 9: 608. https://doi.org/10.3390/d17090608
APA StyleKeita, M., Thiam, N., Ngom, F., Kantoussan, J., Ndour, I., & Sadio, O. (2025). Diversity and Seasonal Variation in Live Baits Caught in Hann Bay, Dakar, Senegal. Diversity, 17(9), 608. https://doi.org/10.3390/d17090608








