Unravelling the Role of Predator Diversity in Shaping Plankton Dynamics: Evidence from a Mesocosm Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Setup
2.3. Physicochemical and Biological Sampling
2.4. Statistical Analyses
3. Results
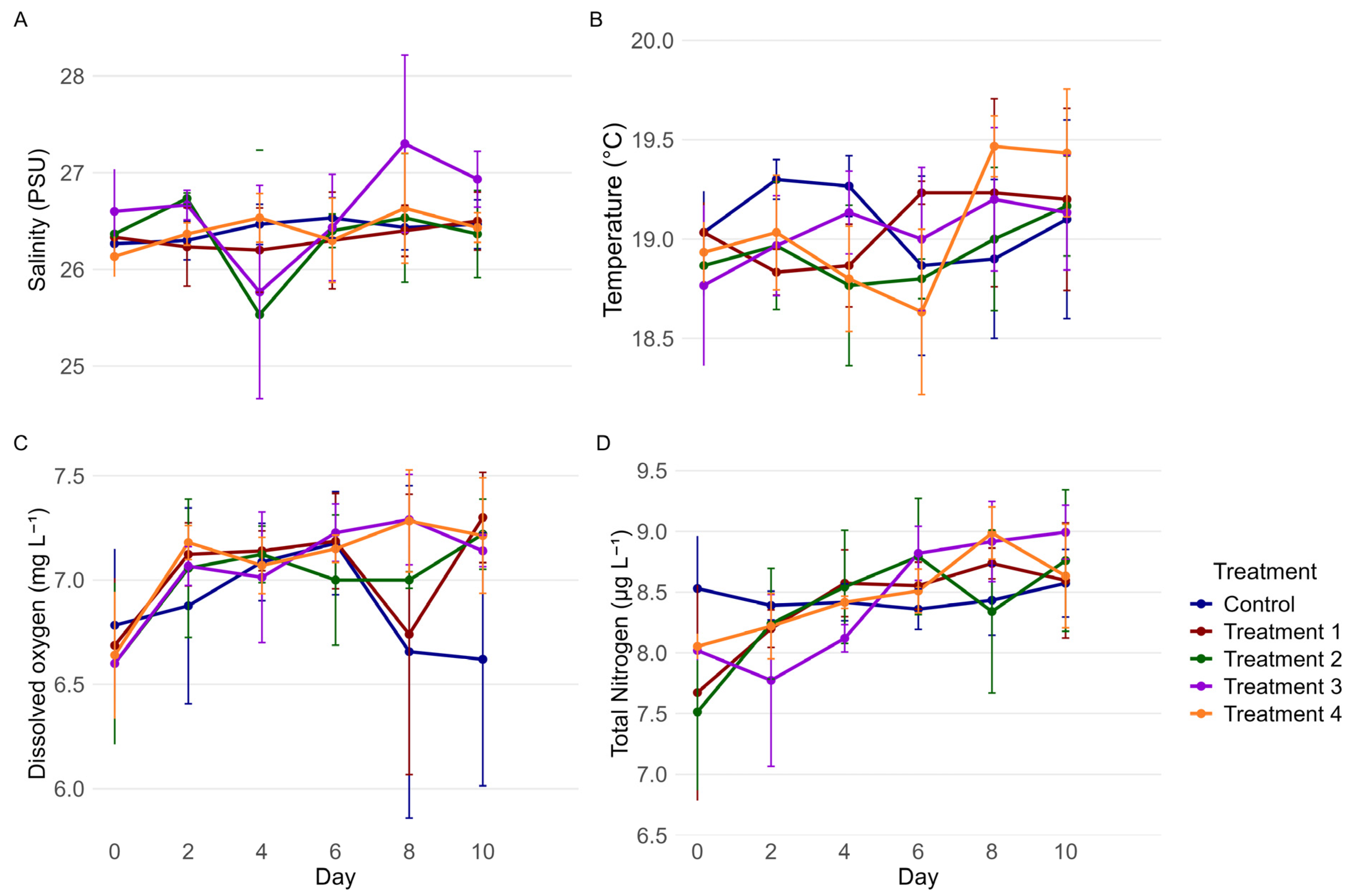
3.1. Physiochemical Results
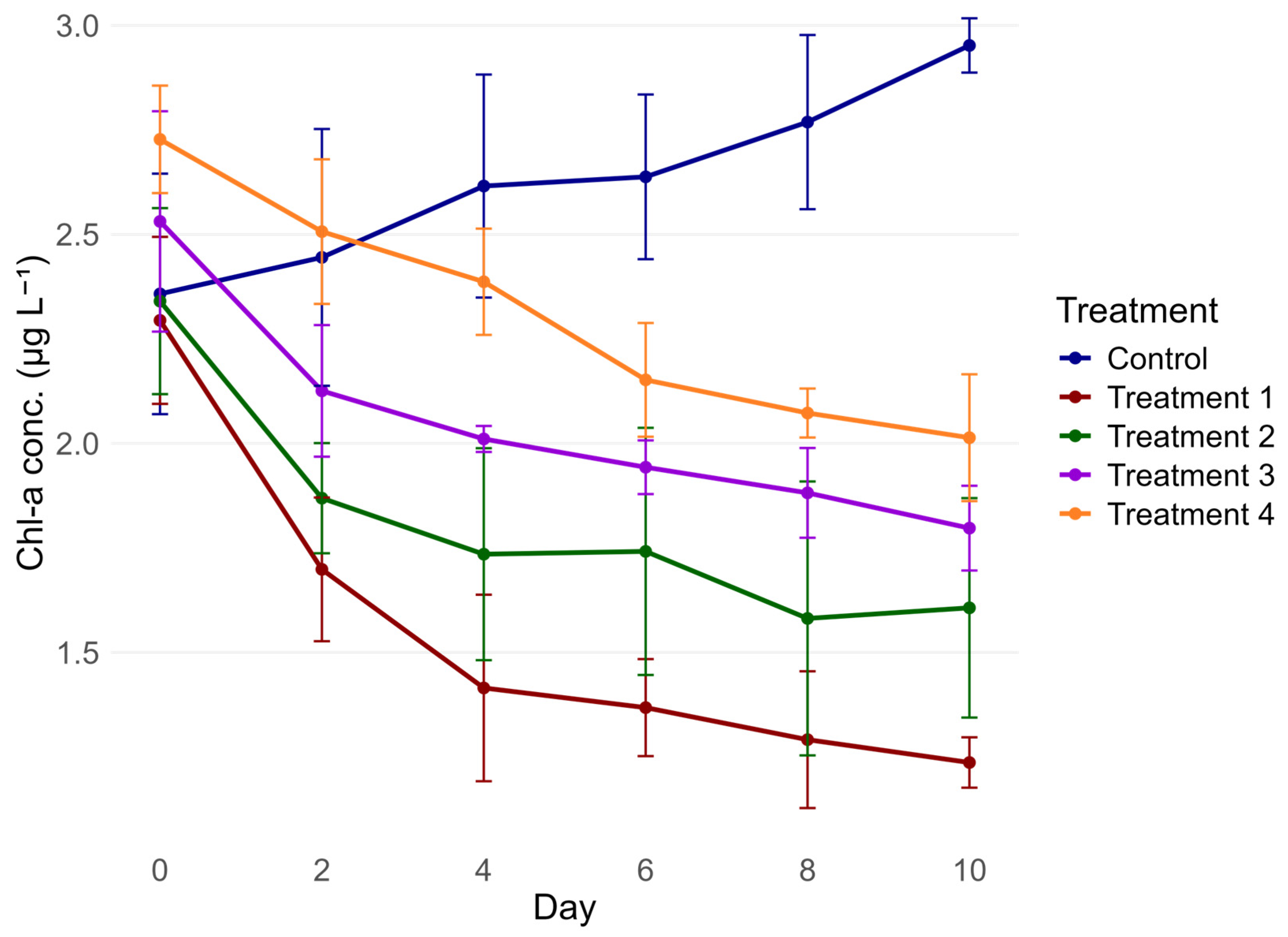
3.2. Chlorophyll-a Concentrations
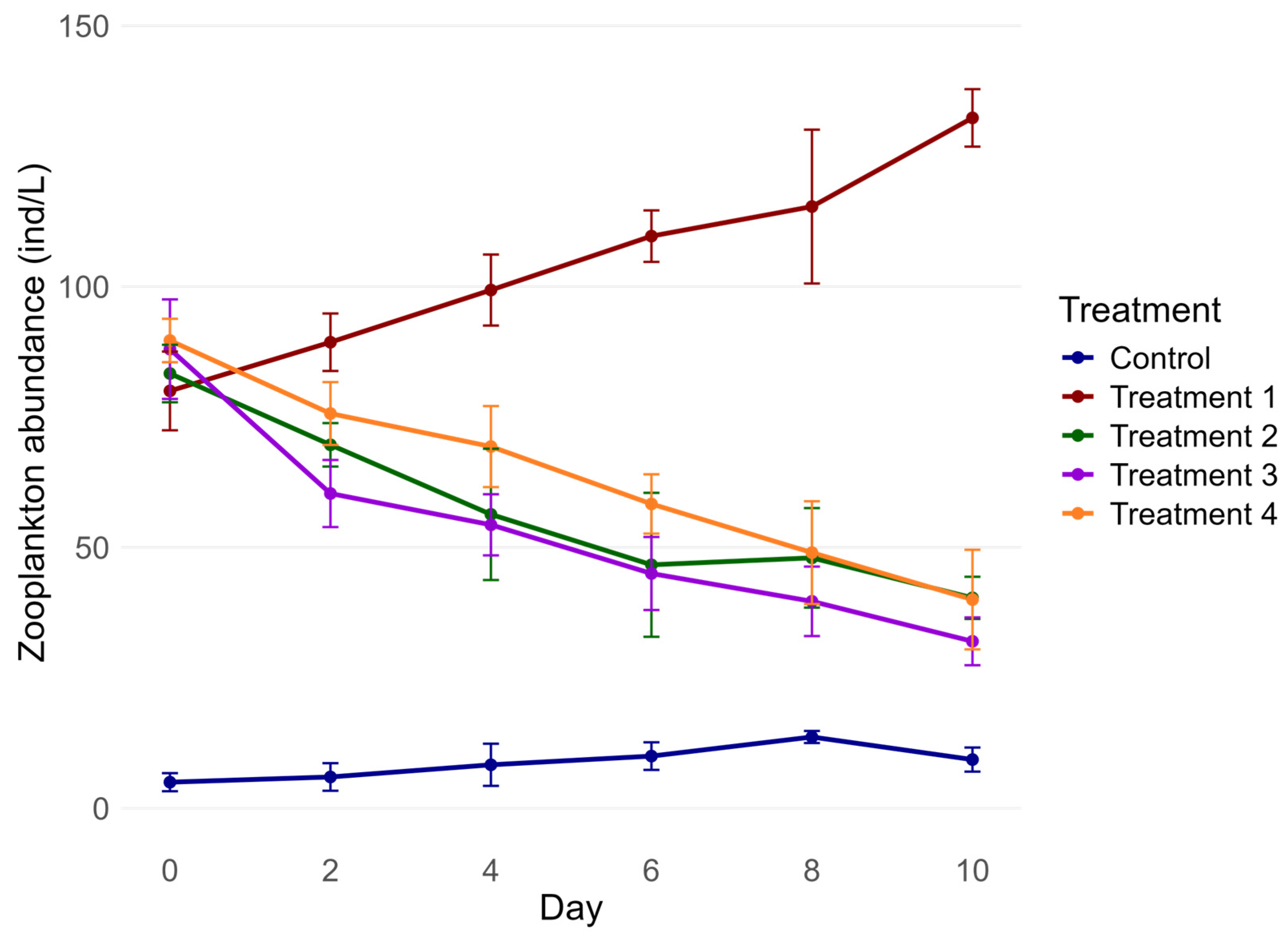
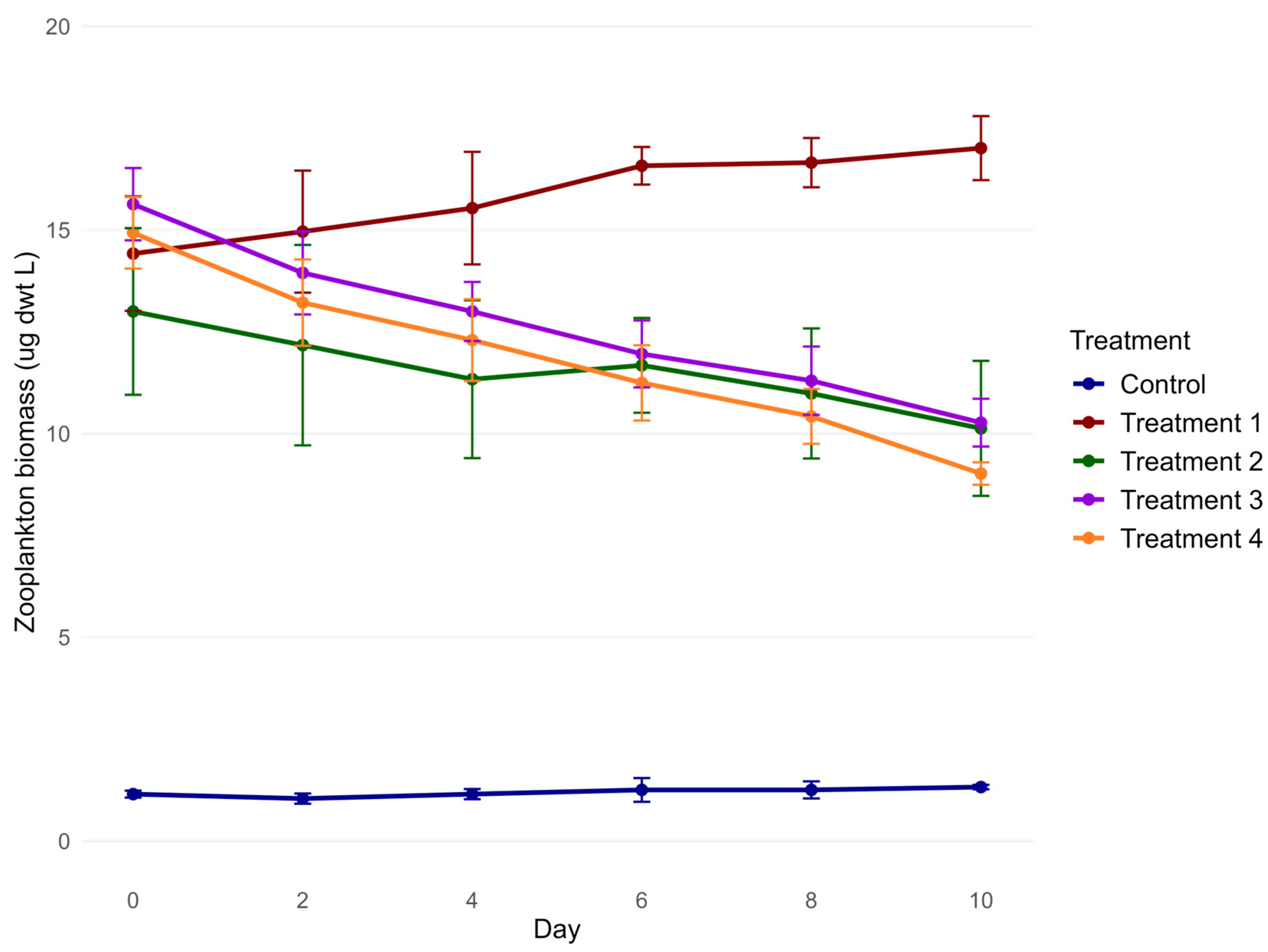
3.3. Zooplankton Abundance and Biomass
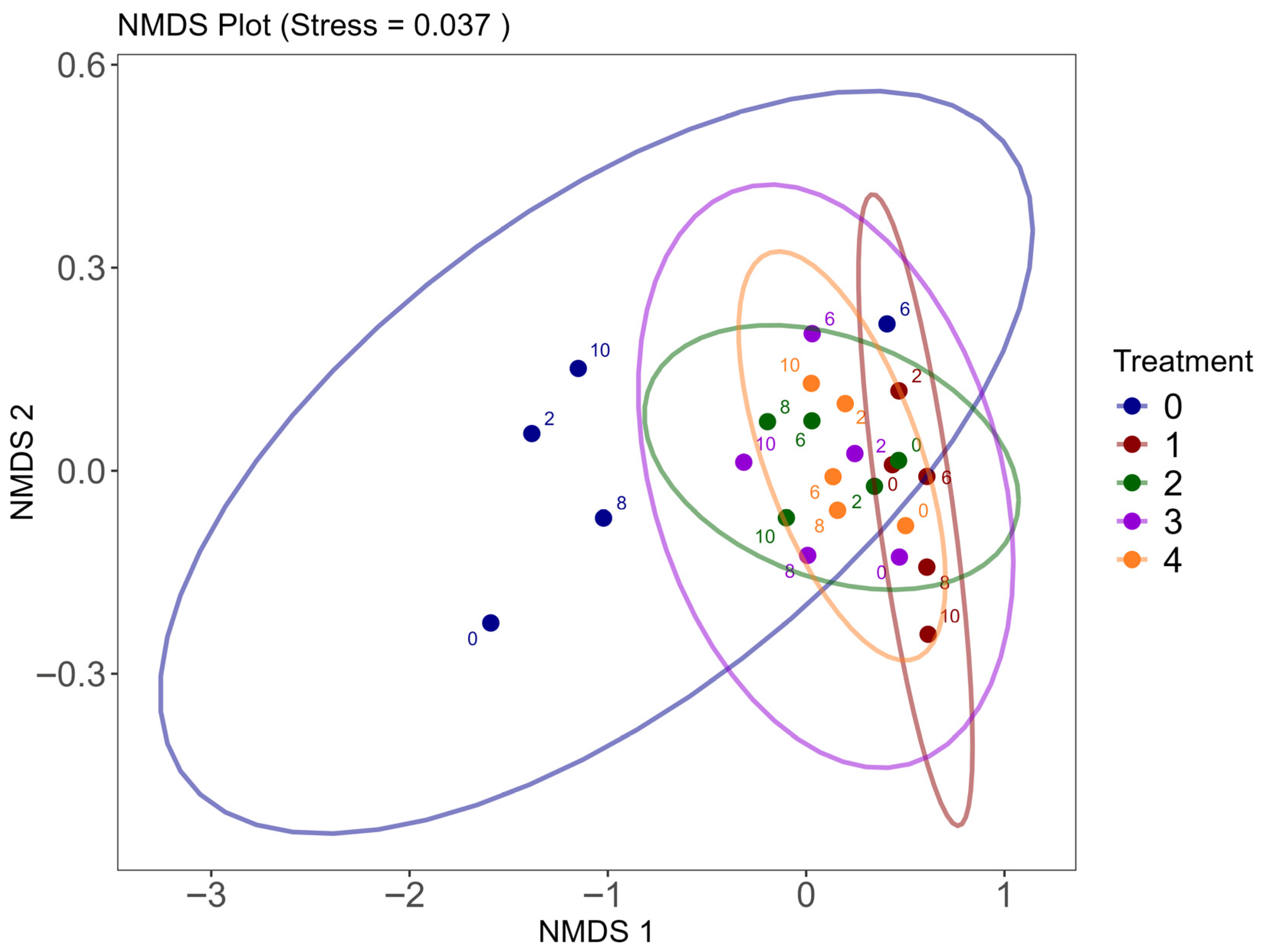
3.4. Community Effects
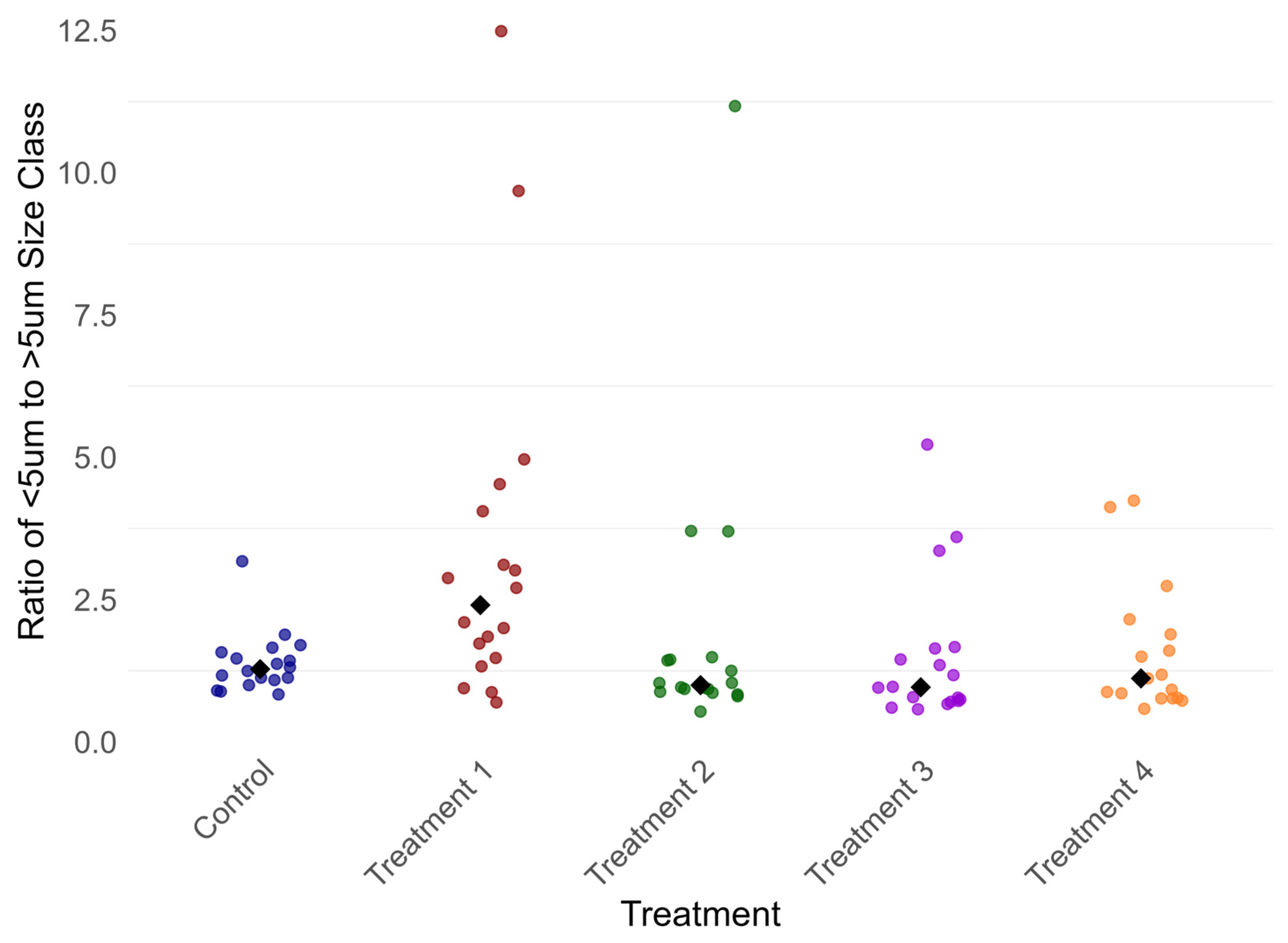
3.4.1. Phytoplankton
3.4.2. Zooplankton
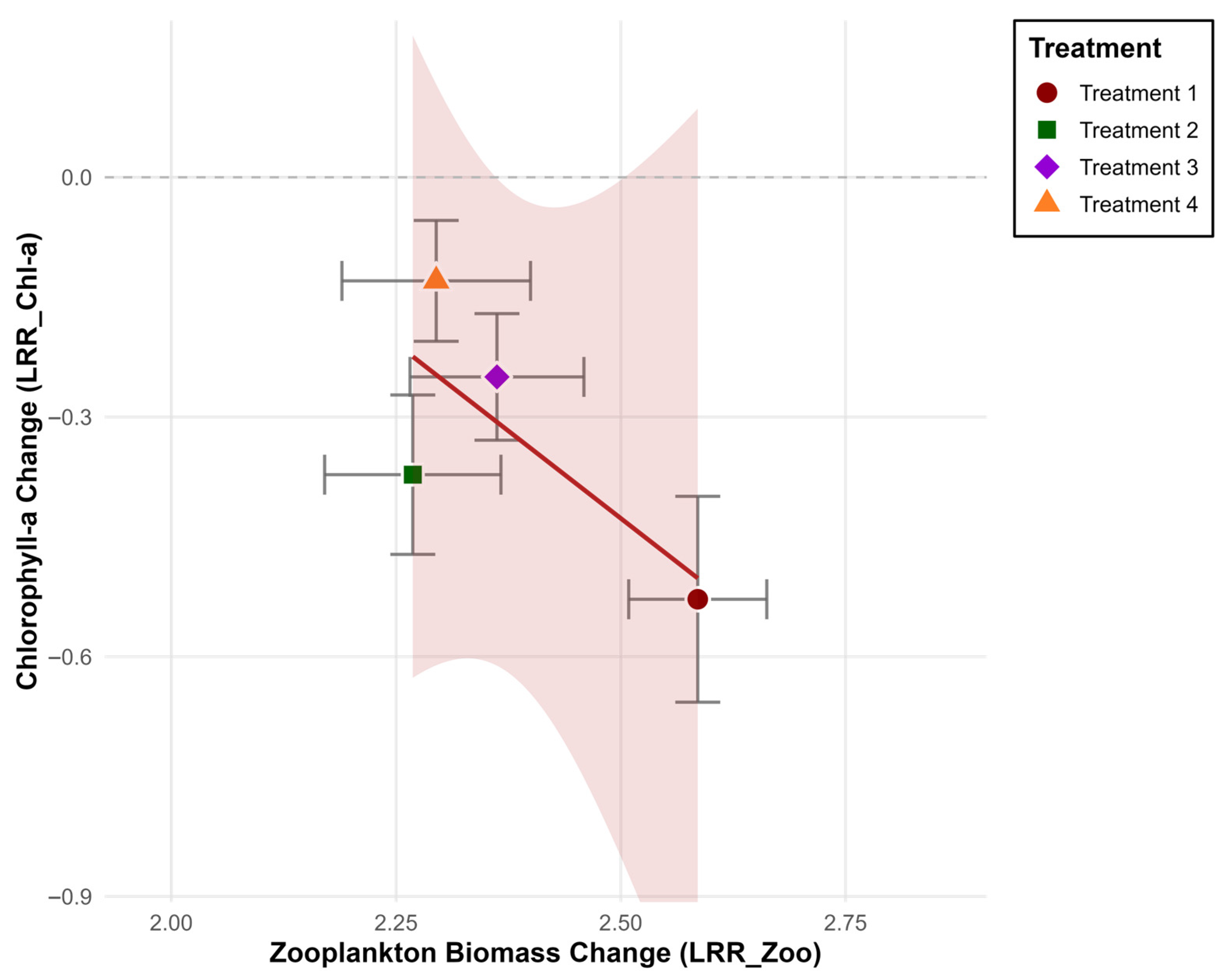
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Treatment | Manipulation |
|---|---|
| Control | Estuary water filtered through 80 μm filter |
| T1 | Estuary water filtered through 1000 μm filter |
| T2 | Estuary water filtered through 1000 μm filter 10 adult, Mesopodopsis wooldrigei |
| T3 | Estuary water filtered through 1000 μm filter 10 juvenile, Rhabdosargus holubi |
| T4 | Estuary water filtered through 1000 μm filter 5 Mesopodopsis wooldrigei 5 Rhabdosargus holubi |
| Comparisons | Z-Value | Unadjusted p-Value | Adjusted p-Value | Significance |
|---|---|---|---|---|
| Control vs. T1 | −2.041 | 0.0412 | 0.103 | Not significant |
| Control vs. T2 | 0.727 | 0.4671 | 0.778 | Not significant |
| T1 vs. T2 | 2.769 | 0.0056 | 0.028 | Significant |
| Control vs. T3 | 1.250 | 0.2111 | 0.422 | Not significant |
| T1 vs. T3 | 3.292 | 0.0010 | 0.001 | Significant |
| T2 vs. T3 | 0.523 | 0.6009 | 0.668 | Not significant |
| Control vs. T4 | 0.670 | 0.5029 | 0.718 | Not significant |
| T1 vs. T4 | 2.711 | 0.0067 | 0.022 | Significant |
| T2 vs. T4 | −0.057 | 0.9542 | 0.954 | Not significant |
| T3 vs. T4 | −0.581 | 0.5615 | 0.702 | Not significant |
| Treatment | LRR_Zoo | Direction | LRR Chl-a | Direction |
|---|---|---|---|---|
| Treatment 1 | 2.586 | Increase | −0.528 | Decrease |
| Treatment 2 | 2.269 | Increase | −0.372 | Decrease |
| Treatment 3 | 2.362 | Increase | −0.250 | Decrease |
| Treatment 4 | 2.295 | Increase | −0.130 | Decrease |
References
- Paine, R.T. Food webs: Linkage, interaction strength and community infrastructure. J. Anim. Ecol. 1980, 49, 667–685. [Google Scholar] [CrossRef]
- Hammerschlag, N.; Schmitz, O.J.; Flecker, A.S.; Lafferty, K.D.; Sih, A.; Atwood, T.B.; Gallagher, A.J.; Irschick, D.J.; Skubel, R.; Cooke, S.J. Ecosystem function and services of aquatic predators in the Anthropocene. Trends Ecol. Evol. 2019, 34, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, S.R.; Kitchell, J.F.; Hodgson, J.R. Cascading trophic interactions and lake productivity. BioScience 1985, 35, 634–639. [Google Scholar] [CrossRef]
- Schmitz, O.J.; Grabowski, J.H.; Peckarsky, B.L.; Preisser, E.L.; Trussell, G.C.; Vonesh, J.R. From individuals to ecosystem function: Toward an integration of evolutionary and ecosystem ecology. Ecology 2008, 89, 2436–2445. [Google Scholar] [CrossRef]
- Beauchamp, D.A.; Wahl, D.; Johnson, B.M. Predator–prey interactions. In Analysis and Interpretation of Freshwater Fisheries Data; American Fisheries Society: Bethesda, MD, USA, 2007; pp. 765–842. [Google Scholar]
- Miller, T.J.; Crowder, L.B.; Rice, J.A.; Marschall, E.A. Larval size and recruitment mechanisms in fishes: Toward a conceptual framework. Can. J. Fish. Aquat. Sci. 1988, 45, 1657–1670. [Google Scholar] [CrossRef]
- Dalton, C.M.; Tracy, K.E.; Hairston, N.G., Jr.; Flecker, A.S. Fasting or fear: Disentangling the roles of predation risk and food deprivation in the nitrogen metabolism of consumers. Ecology 2018, 99, 681–689. [Google Scholar] [CrossRef]
- Guariento, R.D.; Luttbeg, B.; Carneiro, L.S.; Caliman, A. Prey adaptive behaviour under predation risk modify stoichiometry predictions of predator-induced stress paradigms. Funct. Ecol. 2018, 32, 1631–1643. [Google Scholar] [CrossRef]
- McIntyre, P.B.; Jones, L.E.; Flecker, A.S.; Vanni, M.J. Fish extinctions alter nutrient recycling in tropical freshwaters. Proc. Natl. Acad. Sci. USA 2007, 104, 4461–4466. [Google Scholar] [CrossRef]
- Allgeier, J.E.; Valdivia, A.; Cox, C.; Layman, C.A. Fishing down nutrients on coral reefs. Nat. Commun. 2016, 7, 12461. [Google Scholar] [CrossRef]
- Hammerschlag, N.; Barley, S.C.; Irschick, D.J.; Meeuwig, J.J.; Nelson, E.R.; Meekan, M.G. Predator declines and morphological changes in prey: Evidence from coral reefs depleted of sharks. Mar. Ecol. Prog. Ser. 2018, 586, 127–139. [Google Scholar] [CrossRef]
- Griffen, B.D. Detecting emergent effects of multiple predator species. Oecologia 2006, 148, 702–709. [Google Scholar] [CrossRef]
- Sih, A.; Englund, G.; Wooster, D. Emergent impacts of multiple predators on prey. Trends Ecol. Evol. 1998, 13, 350–355. [Google Scholar] [CrossRef]
- Ives, A.R.; Cardinale, B.J.; Snyder, W.E. A synthesis of subdisciplines: Predator–prey interactions, and biodiversity and ecosystem functioning. Ecol. Lett. 2005, 8, 102–116. [Google Scholar] [CrossRef]
- Polis, G.A.; Holt, R.D. Intraguild predation: The dynamics of complex trophic interactions. Trends Ecol. Evol. 1992, 7, 151–154. [Google Scholar] [CrossRef]
- McCoy, M.W.; Stier, A.C.; Osenberg, C.W. Emergent effects of multiple predators on prey survival: The importance of depletion and the functional response. Ecol. Lett. 2012, 15, 1449–1456. [Google Scholar] [CrossRef] [PubMed]
- Vance-Chalcraft, H.D.; Soluk, D.A. Multiple predator effects result in risk reduction for prey across multiple prey densities. Oecologia 2005, 144, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, O.J. Behavior of predators and prey and links with population-level processes. In Ecology of Predator-Prey Interactions; Oxford Academic: New York, NY, USA, 2005; Volume 256. [Google Scholar]
- Power, M.E. Top-down and bottom-up forces in food webs: Do plants have primacy. Ecology 1992, 73, 733–746. [Google Scholar] [CrossRef]
- Kemp, J.; Froneman, P. Recruitment of ichthyoplankton and macrozooplankton during overtopping events into a temporarily open/closed southern African estuary. Estuar. Coast. Shelf Sci. 2004, 61, 529–537. [Google Scholar] [CrossRef]
- Strydom, N.A. Patterns in larval fish diversity, abundance, and distribution in temperate South African estuaries. Estuaries Coasts 2015, 38, 268–284. [Google Scholar] [CrossRef]
- James, N.C.; Leslie, T.D.; Potts, W.M.; Whitfield, A.K.; Rajkaran, A. The importance of different juvenile habitats as nursery areas for a ubiquitous estuarine-dependent marine fish species. Estuar. Coast. Shelf Sci. 2019, 226, 106270. [Google Scholar] [CrossRef]
- Wasserman, R.J.; Noyon, M.; Avery, T.S.; Froneman, P.W. Trophic level stability-inducing effects of predaceous early juvenile fish in an estuarine mesocosm study. PLoS ONE 2013, 8, e61019. [Google Scholar] [CrossRef]
- Morozov, A.; Arashkevich, E.; Nikishina, A.; Solovyev, K. Nutrient-rich plankton communities stabilized via predator—Prey interactions: Revisiting the role of vertical heterogeneity. Math. Med. Biol. A J. IMA 2011, 28, 185–215. [Google Scholar] [CrossRef]
- Johnson, J.B.; Belk, M.C. Predators as agents of selection and diversification. Diversity 2020, 12, 415. [Google Scholar] [CrossRef]
- Turner, A.M.; Mittelbach, G.G. Predator avoidance and community structure: Interactions among piscivores, planktivores, and plankton. Ecology 1990, 71, 2241–2254. [Google Scholar] [CrossRef]
- Froneman, P. Feeding ecology of the mysid, Mesopodopsis wooldridgei, in a temperate estuary along the eastern seaboard of South Africa. J. Plankton Res. 2001, 23, 999–1008. [Google Scholar] [CrossRef]
- Wasserman, R.J.; Strydom, N.A. The importance of estuary head waters as nursery areas for young estuary- and marine-spawned fishes in temperate South Africa. Estuar. Coast. Shelf Sci. 2011, 94, 56–67. [Google Scholar] [CrossRef]
- Blaber, S.J. The population structure and growth of juvenile Rhabdosargus holubi (Steindachner)(Teleostei: Sparidae) in a closed estuary. J. Fish Biol. 1974, 6, 455–460. [Google Scholar] [CrossRef]
- De Wet, P.; Marais, J. Stomach content analysis of juvenile Cape stumpnose Rhabdosargus holubi in the Swartkops Estuary, South Africa. S. Afr. J. Mar. Sci. 1990, 9, 127–133. [Google Scholar] [CrossRef]
- Froneman, P.W. Predator Diversity Does Not Contribute to Increased Prey Risk: Evidence from a Mesocosm Study. Diversity 2022, 14, 584. [Google Scholar] [CrossRef]
- Henninger, T.; Froneman, P.; Hodgson, A. The population dynamics of the estuarine isopod Exosphaeroma hylocoetes (Barnard, 1940) within three temporarily open/closed southern African estuaries. Afr. Zool. 2008, 43, 202–217. [Google Scholar] [CrossRef]
- Froneman, P. The importance of phytoplankton size in mediating trophic interactions within the plankton of a southern African estuary. Estuar. Coast. Shelf Sci. 2006, 70, 693–700. [Google Scholar] [CrossRef]
- Froneman, P. Seasonal changes in selected physico-chemical and biological variables in the temporarily open/closed Kasouga estuary, Eastern Cape, South Africa. Afr. J. Aquat. Sci. 2002, 27, 117–123. [Google Scholar] [CrossRef]
- Froneman, P.; Henninger, T. The influence of prolonged mouth closure on selected components of the hyperbenthos in the littoral zone of the temporarily open/closed Kasouga Estuary, South Africa. Estuar. Coast. Shelf Sci. 2009, 83, 326–332. [Google Scholar] [CrossRef]
- Wittmann, K.J. Morphogeographic variations in the genus Mesopodopsis Czerniavsky with descriptions of three new species (Crustacea, Mysidacea). Hydrobiologia 1992, 241, 71–89. [Google Scholar] [CrossRef]
- Steindachner, F. Ichthyologische Beiträge (X). In Sitzungsberichte der Kaiserlichen Akademie der Wissenschaften. Mathematisch-Naturwissenschaftliche Classe; Kaiserlichen Akademie der Wissenschaften: Wien, Austria, 1881. [Google Scholar]
- Wooldridge, T. Ecology of beach and surf-zone mysid shrimps in the Eastern Cape, South Africa. In Proceedings of the Sandy Beaches as Ecosystems: Based on the Proceedings of the First International Symposium on Sandy Beaches, Port Elizabeth, South Africa, 17–21 January 1983; pp. 449–460. [Google Scholar]
- Jerling, H.; Wooldridge, T. Plankton distribution and abundance in the Sundays River estuary, South Africa, with comments on potential feeding interactions. S. Afr. J. Mar. Sci. 1995, 15, 169–184. [Google Scholar] [CrossRef]
- Blaber, S. Population size and mortality of juveniles of the marine teleost Rhabdosargus holubi (Pisces: Sparidae) in a closed estuary. Mar. Biol. 1973, 21, 219–225. [Google Scholar] [CrossRef]
- Whitfield, A.; Paterson, A.; Bok, A.; Kok, H. A comparison of the ichthyofaunas in two permanently open eastern Cape estuaries. Afr. Zool. 1994, 29, 175–185. [Google Scholar] [CrossRef]
- Carassou, L.; Whitfield, A.; Bergamino, L.; Moyo, S.; Richoux, N. Trophic dynamics of the cape stumpnose (Rhabdosargus holubi, Sparidae) across three adjacent aquatic habitats. Estuaries Coasts 2016, 39, 1221–1233. [Google Scholar] [CrossRef]
- Blaber, S.J. Temperature and salinity tolerance of juvenile Rhabdosargus holubi [Steindachner (Teleostei: Sparidae)]. J. Fish Biol. 1973, 5, 593–598. [Google Scholar] [CrossRef]
- Spivak, A.C.; Vanni, M.J.; Mette, E.M. Moving on up: Can results from simple aquatic mesocosm experiments be applied across broad spatial scales? Freshw. Biol. 2011, 56, 279–291. [Google Scholar] [CrossRef]
- Holm-Hansen, O.; Riemann, B. Chlorophyll a determination: Improvements in methodology. Oikos 1978, 30, 438–447. [Google Scholar] [CrossRef]
- Broglio, E.; Saiz, E.; Calbet, A.; Trepat, I.; Alcaraz, M. Trophic impact and prey selection by crustacean zooplankton on the microbial communities of an oligotrophic coastal area (NW Mediterranean Sea). Aquat. Microb. Ecol. 2004, 35, 65–78. [Google Scholar] [CrossRef]
- Boltovskoy, D. South Atlantic Zooplankton; Backhuys Publishers: Leiden, The Netherlands, 1999. [Google Scholar]
- Girden, E.R. ANOVA: Repeated Measures; Sage Publications, Inc.: Thousand Oaks, CA, USA, 1992. [Google Scholar]
- Keselman, H.; Algina, J.; Kowalchuk, R.K. The analysis of repeated measures designs: A review. Br. J. Math. Stat. Psychol. 2001, 54, 1–20. [Google Scholar] [CrossRef]
- Singmann, H.; Bolker, B.; Westfall, J.; Aust, F.; Ben-Shachar, M.; Højsgaard, S.; Fox, J.; Lawrence, M.; Mertens, U.; Love, J. Package ‘afex’. 2015. Available online: https://github.Com/singmann/afex (accessed on 7 August 2025).
- Abdi, H. The greenhouse-geisser correction. Encycl. Res. Des. 2010, 1, 544–548. [Google Scholar]
- Lenth, R.V. emmeans: Estimated Marginal Means, aka Least-Squares Means. 2024. Available online: https://cran.r-project.org/web/packages/emmeans/emmeans.pdf (accessed on 7 August 2025).
- Lenth, R.; Lenth, M.R. Package ‘lsmeans’. Am. Stat. 2018, 34, 216–221. [Google Scholar]
- Kruskal, W.H.; Wallis, W.A. Use of ranks in one-criterion variance analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Dunn, O.J. Multiple comparisons using rank sums. Technometrics 1964, 6, 241–252. [Google Scholar] [CrossRef]
- Dinno, A. Nonparametric pairwise multiple comparisons in independent groups using Dunn’s test. Stata J. 2015, 15, 292–300. [Google Scholar] [CrossRef]
- Anderson, M. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; Primer-E Limited: Auckland, New Zealand, 2008. [Google Scholar]
- Hedges, L.V.; Gurevitch, J.; Curtis, P.S. The meta-analysis of response ratios in experimental ecology. Ecology 1999, 80, 1150–1156. [Google Scholar] [CrossRef]
- Viechtbauer, W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- Su, H.; Feng, Y.; Chen, J.; Chen, J.; Ma, S.; Fang, J.; Xie, P. Determinants of trophic cascade strength in freshwater ecosystems: A global analysis. Ecology 2021, 102, e03370. [Google Scholar] [CrossRef]
- Osenberg, C.W.; Sarnelle, O.; Cooper, S.D.; Holt, R.D. Resolving ecological questions through meta-analysis: Goals, metrics, and models. Ecology 1999, 80, 1105–1117. [Google Scholar] [CrossRef]
- Gurevitch, J.; Hedges, L.V. Statistical issues in ecological meta-analyses. Ecology 1999, 80, 1142–1149. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Wickham, H.; François, R.; Henry, L.; Müller, K.; Vaughan, D. dplyr: A Grammar of Data Manipulation, R package version 1.1.4; Springer: New York, NY, USA, 2023. [Google Scholar]
- Wickham, H.; Vaugh, D.; Girlich, M. tidyr: Tidy Messy Data, 1.3.1; CRAN; 2024. Available online: https://CRAN.R-project.org/package=tidyr (accessed on 7 August 2025).
- Fox, J.; Weisberg, S. An R Companion to Applied Regression; Sage Publications: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Ogle, D.H.; Doll, J.C.; Powell, W.A.; Dinno, A. FSA: Simple Fisheries Stock Assessment Methods, 0.9.5; CRAN; 2023. Available online: https://CRAN.R-project.org/package=FSA (accessed on 7 August 2025).
- Dinno, A. dunn.test: Dunn’s Test of Multiple Comparisons Using Rank Sums, 1.3.6; CRAN; 2024. Available online: https://CRAN.R-project.org/package=dunn.test (accessed on 7 August 2025).
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Henry, M.; Stevens, H.; et al. vegan: Community Ecology Package, 2.6-6.1; CRAN; 2024. Available online: https://CRAN.R-project.org/package=vegan (accessed on 7 August 2025).
- Slowikowski, K. ggrepel: Automatically Position Non-Overlapping Text Labels with ‘ggplot2’, 0.9.5; CRAN; 2024. Available online: https://www.bcgsc.ca/downloads/morinlab/UMontreal_MLLT/renv/library/R-4.1/x86_64-pc-linux-gnu/ggrepel/html/00Index.html (accessed on 7 August 2025).
- Duffy, J.E. Biodiversity and ecosystem function: The consumer connection. Oikos 2002, 99, 201–219. [Google Scholar] [CrossRef]
- Denno, R.F.; Lewis, D. Predator-prey interactions. In The Princeton Guide to Ecology; Princeton University Press: Princeton, NJ, USA, 2009; pp. 202–212. [Google Scholar]
- Finke, D.L.; Denno, R.F. Predator diversity and the functioning of ecosystems: The role of intraguild predation in dampening trophic cascades. Ecol. Lett. 2005, 8, 1299–1306. [Google Scholar] [CrossRef]
- Baum, J.K.; Worm, B. Cascading top-down effects of changing oceanic predator abundances. J. Anim. Ecol. 2009, 78, 699–714. [Google Scholar] [CrossRef]
- Benton, T.G.; Solan, M.; Travis, J.M.; Sait, S.M. Microcosm experiments can inform global ecological problems. Trends Ecol. Evol. 2007, 22, 516–521. [Google Scholar] [CrossRef]
- Stewart, R.I.; Dossena, M.; Bohan, D.A.; Jeppesen, E.; Kordas, R.L.; Ledger, M.E.; Meerhoff, M.; Moss, B.; Mulder, C.; Shurin, J.B. Mesocosm experiments as a tool for ecological climate-change research. Adv. Ecol. Res. 2013, 48, 71–181. [Google Scholar]
- Piehler, M.F.; Currin, C.A.; Hall, N.S. Estuarine intertidal sandflat benthic microalgal responses to in situ and mesocosm nitrogen additions. J. Exp. Mar. Biol. Ecol. 2010, 390, 99–105. [Google Scholar] [CrossRef]
- Carpenter, S.R. Microcosm experiments have limited relevance for community and ecosystem ecology. Ecology 1996, 77, 677–680. [Google Scholar] [CrossRef]
- Petersen, J.E.; Hastings, A. Dimensional approaches to scaling experimental ecosystems: Designing mousetraps to catch elephants. Am. Nat. 2001, 157, 324–333. [Google Scholar] [CrossRef]
- Petersen, J.E.; Englund, G. Dimensional approaches to designing better experimental ecosystems: A practitioners guide with examples. Oecologia 2005, 145, 215–223. [Google Scholar] [CrossRef]
- Auffan, M.; Tella, M.; Santaella, C.; Brousset, L.; Paillès, C.; Barakat, M.; Espinasse, B.; Artells, E.; Issartel, J.; Masion, A. An adaptable mesocosm platform for performing integrated assessments of nanomaterial risk in complex environmental systems. Sci. Rep. 2014, 4, 5608. [Google Scholar] [CrossRef]
- Siegfried, C.A.; Kopache, M.E. Feeding of Neomysis mercedis (Holmes). Biol. Bull. 1980, 159, 193–205. [Google Scholar] [CrossRef]
- Sanders, D.; Thébault, E.; Kehoe, R.; van Veen, F.F. Trophic redundancy reduces vulnerability to extinction cascades. Proc. Natl. Acad. Sci. USA 2018, 115, 2419–2424. [Google Scholar] [CrossRef] [PubMed]
- Eisaguirre, J.H.; Eisaguirre, J.M.; Davis, K.; Carlson, P.M.; Gaines, S.D.; Caselle, J.E. Trophic redundancy and predator size class structure drive differences in kelp forest ecosystem dynamics. Ecology 2020, 101, e02993. [Google Scholar] [CrossRef]
- Whitfield, A.K. Biology and Ecology of Fishes in Southern African Estuaries; J.L.B. Smith Institute of Ichthyology: Grahamstown, South Africa, 1998. [Google Scholar]
- Reynolds, P.L.; Bruno, J.F. Multiple predator species alter prey behavior, population growth, and a trophic cascade in a model estuarine food web. Ecol. Monogr. 2013, 83, 119–132. [Google Scholar] [CrossRef]
- Johnson, K.D.; Grabowski, J.H.; Smee, D.L. Omnivory dampens trophic cascades in estuarine communities. Mar. Ecol. Prog. Ser. 2014, 507, 197–206. [Google Scholar] [CrossRef]
- Tumolo, B.B.; Flinn, M.B. Top-down effects of an invasive omnivore: Detection in long-term monitoring of large-river reservoir chlorophyll-a. Oecologia 2017, 185, 293–303. [Google Scholar] [CrossRef]
- Sommer, U. Trophic Cascades in Marine and Freshwater Plankton. Int. Rev. Hydrobiol. 2008, 93, 506–516. [Google Scholar] [CrossRef]
- Borer, E.T.; Gruner, D.S. Top-down and bottom-up regulation of communities. In The Princeton Guide to Ecology; Princeton University Press: Princeton, NJ, USA, 2009; pp. 296–304. [Google Scholar]
- Jochum, M.; Schneider, F.D.; Crowe, T.P.; Brose, U.; O’Gorman, E.J. Climate-induced changes in bottom-up and top-down processes independently alter a marine ecosystem. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 2962–2970. [Google Scholar] [CrossRef]
- Otto, S.B.; Berlow, E.L.; Rank, N.E.; Smiley, J.; Brose, U. Predator diversity and identity drive interaction strength and trophic cascades in a food web. Ecology 2008, 89, 134–144. [Google Scholar] [CrossRef]
- Griffin, J.N.; Silliman, B.R. Predator size-structure and species identity determine cascading effects in a coastal ecosystem. Ecol. Evol. 2018, 8, 12435–12442. [Google Scholar] [CrossRef] [PubMed]
- Guidi, L.; Stemmann, L.; Jackson, G.A.; Ibanez, F.; Claustre, H.; Legendre, L.; Picheral, M.; Gorskya, G. Effects of phytoplankton community on production, size, and export of large aggregates: A world-ocean analysis. Limnol. Oceanogr. 2009, 54, 1951–1963. [Google Scholar] [CrossRef]
- Fuchs, H.L.; Franks, P.J. Plankton community properties determined by nutrients and size-selective feeding. Mar. Ecol. Prog. Ser. 2010, 413, 1–15. [Google Scholar] [CrossRef]
- Cottingham, K.L. Nutrients and zooplankton as multiple stressors of phytoplankton communities: Evidence from size structure. Limnol. Oceanogr. 1999, 44, 810–827. [Google Scholar] [CrossRef]
- Atkinson, C.L.; Capps, K.A.; Rugenski, A.T.; Vanni, M.J. Consumer-driven nutrient dynamics in freshwater ecosystems: From individuals to ecosystems. Biol. Rev. 2017, 92, 2003–2023. [Google Scholar] [CrossRef]
- Froneman, P. Seasonal changes in zooplankton biomass and grazing in a temperate estuary, South Africa. Estuar. Coast. Shelf Sci. 2001, 52, 543–553. [Google Scholar] [CrossRef]
- Froneman, P. Food web structure in three contrasting estuaries determined using stable isotope (∂ 13C) analysis. Afr. J. Aquat. Sci. 2002, 27, 107–115. [Google Scholar] [CrossRef]
- Froneman, P. Trophic cascading in an oligotrophic temperate estuary, South Africa. J. Plankton Res. 2002, 24, 807–816. [Google Scholar] [CrossRef]
- Finke, D.L.; Denno, R.F. Predator diversity dampens trophic cascades. Nature 2004, 429, 407–410. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fabian, R.S.; Froneman, W. Unravelling the Role of Predator Diversity in Shaping Plankton Dynamics: Evidence from a Mesocosm Study. Diversity 2025, 17, 591. https://doi.org/10.3390/d17090591
Fabian RS, Froneman W. Unravelling the Role of Predator Diversity in Shaping Plankton Dynamics: Evidence from a Mesocosm Study. Diversity. 2025; 17(9):591. https://doi.org/10.3390/d17090591
Chicago/Turabian StyleFabian, Robyn Shaylee, and William Froneman. 2025. "Unravelling the Role of Predator Diversity in Shaping Plankton Dynamics: Evidence from a Mesocosm Study" Diversity 17, no. 9: 591. https://doi.org/10.3390/d17090591
APA StyleFabian, R. S., & Froneman, W. (2025). Unravelling the Role of Predator Diversity in Shaping Plankton Dynamics: Evidence from a Mesocosm Study. Diversity, 17(9), 591. https://doi.org/10.3390/d17090591







