Scaling Plant Functional Strategies from Species to Communities in Regenerating Amazonian Forests: Insights for Restoration in Deforested Landscapes
Abstract
1. Introduction
2. Materials and Methods
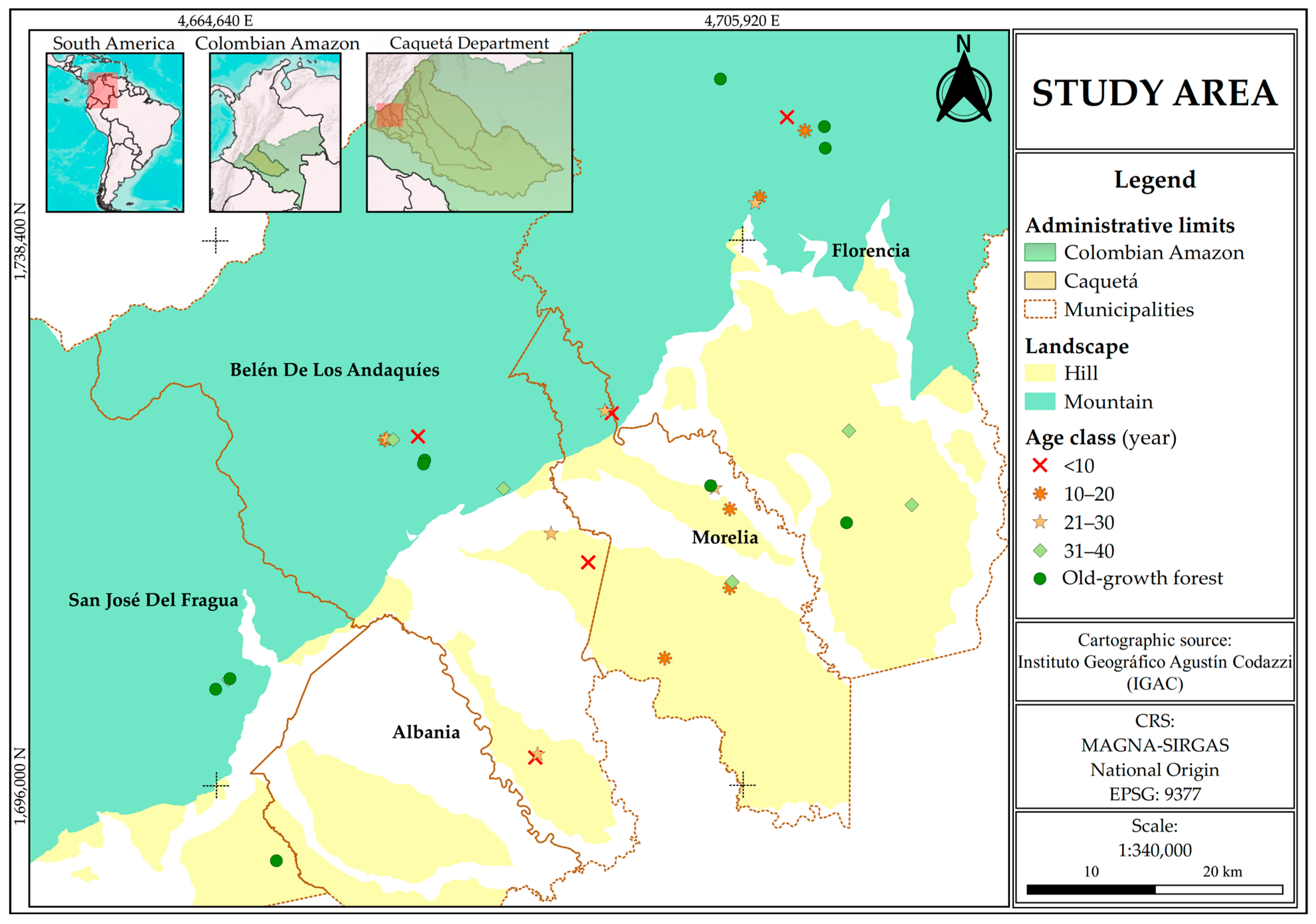
2.1. Study Area and Sampling Design
2.2. Floristic Data Collection
2.3. Trait Sampling and Life-History Strategies
2.4. Environmental Parameters
2.5. Data Analysis
3. Results
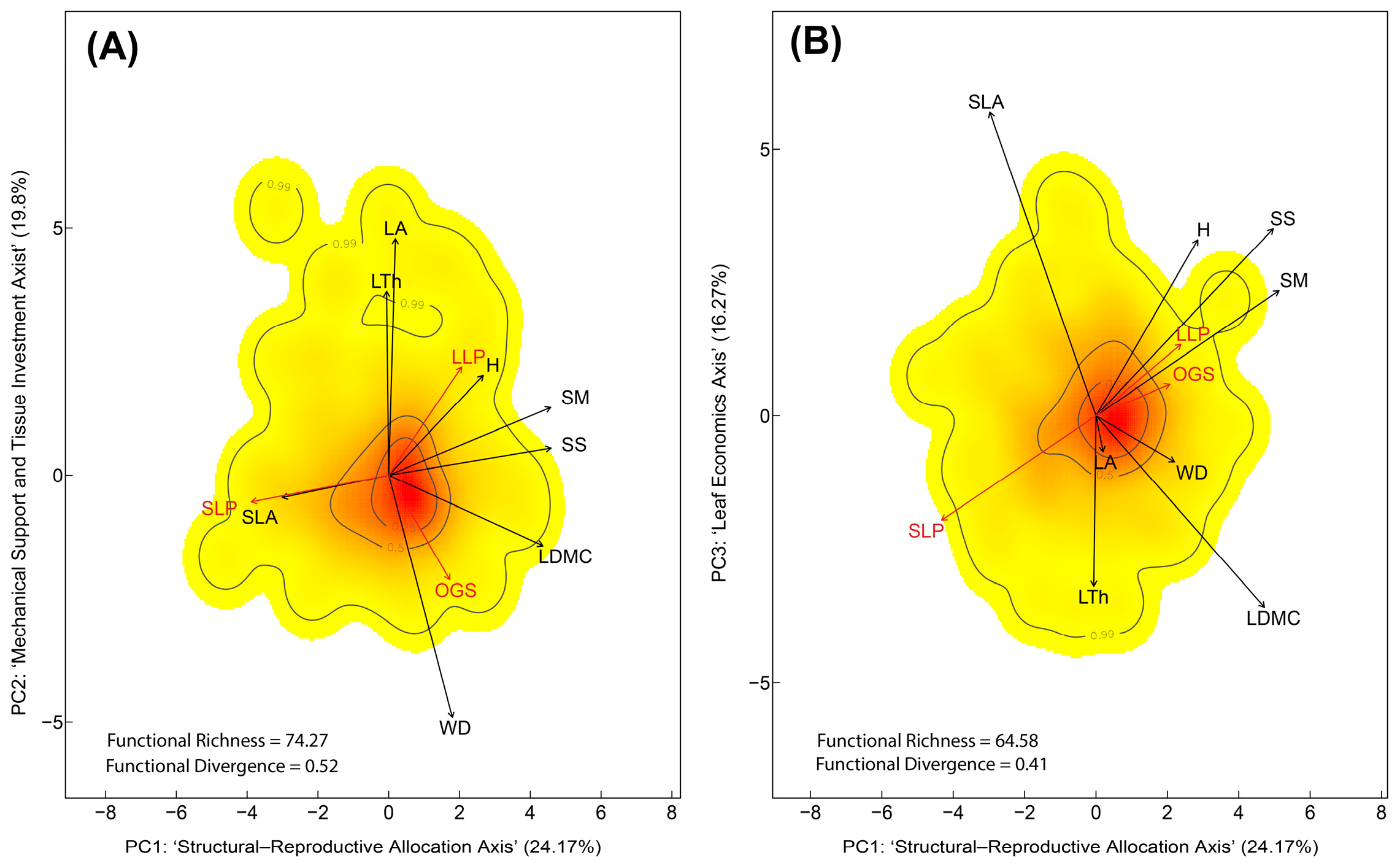
3.1. Plant Functional Trait Space at Species Level
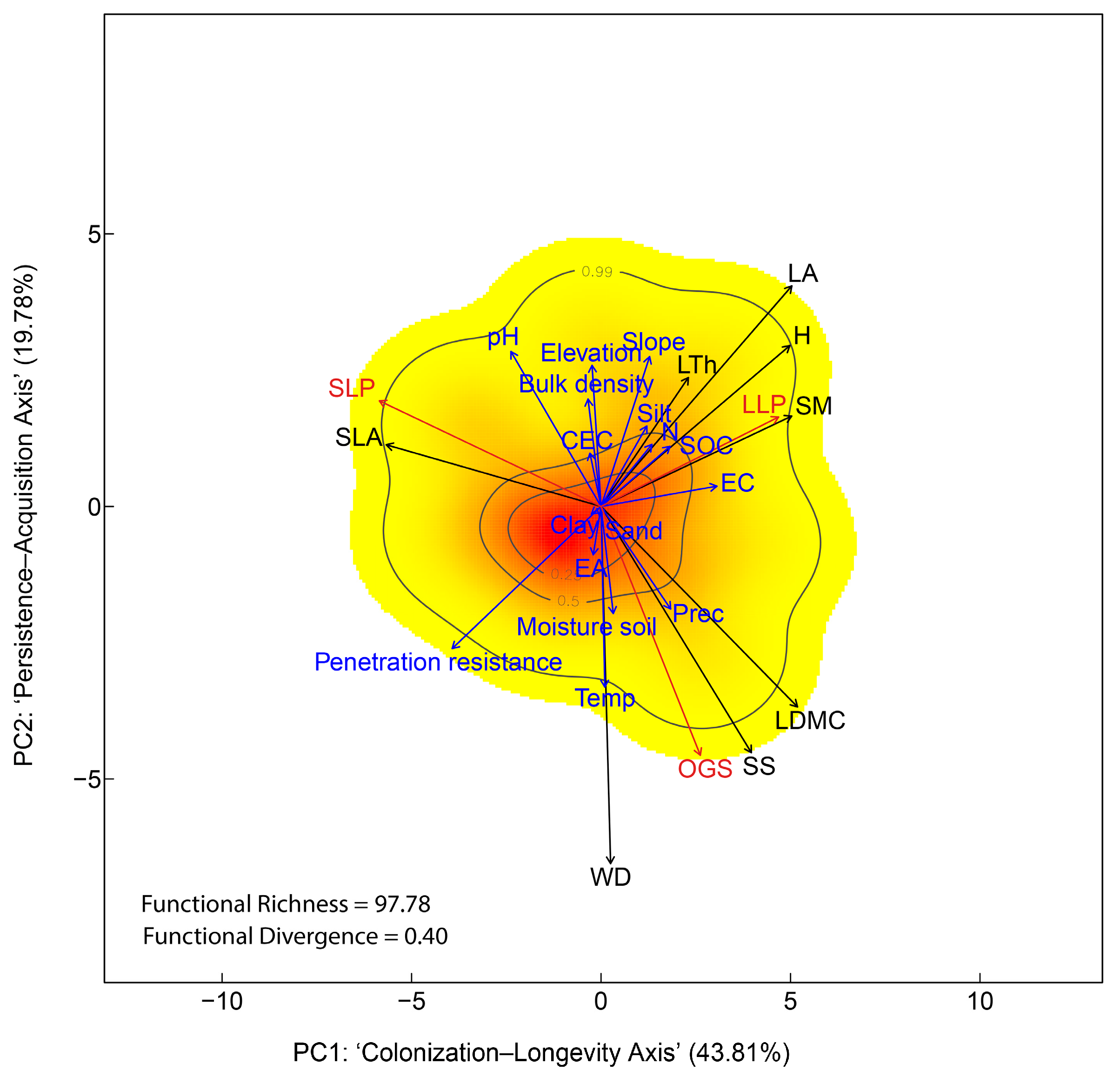
3.2. Plant Functional Trait Space at Community-Level
3.3. Correspondence Betweeen Plant Functional Trait Space at Species and Community Levels
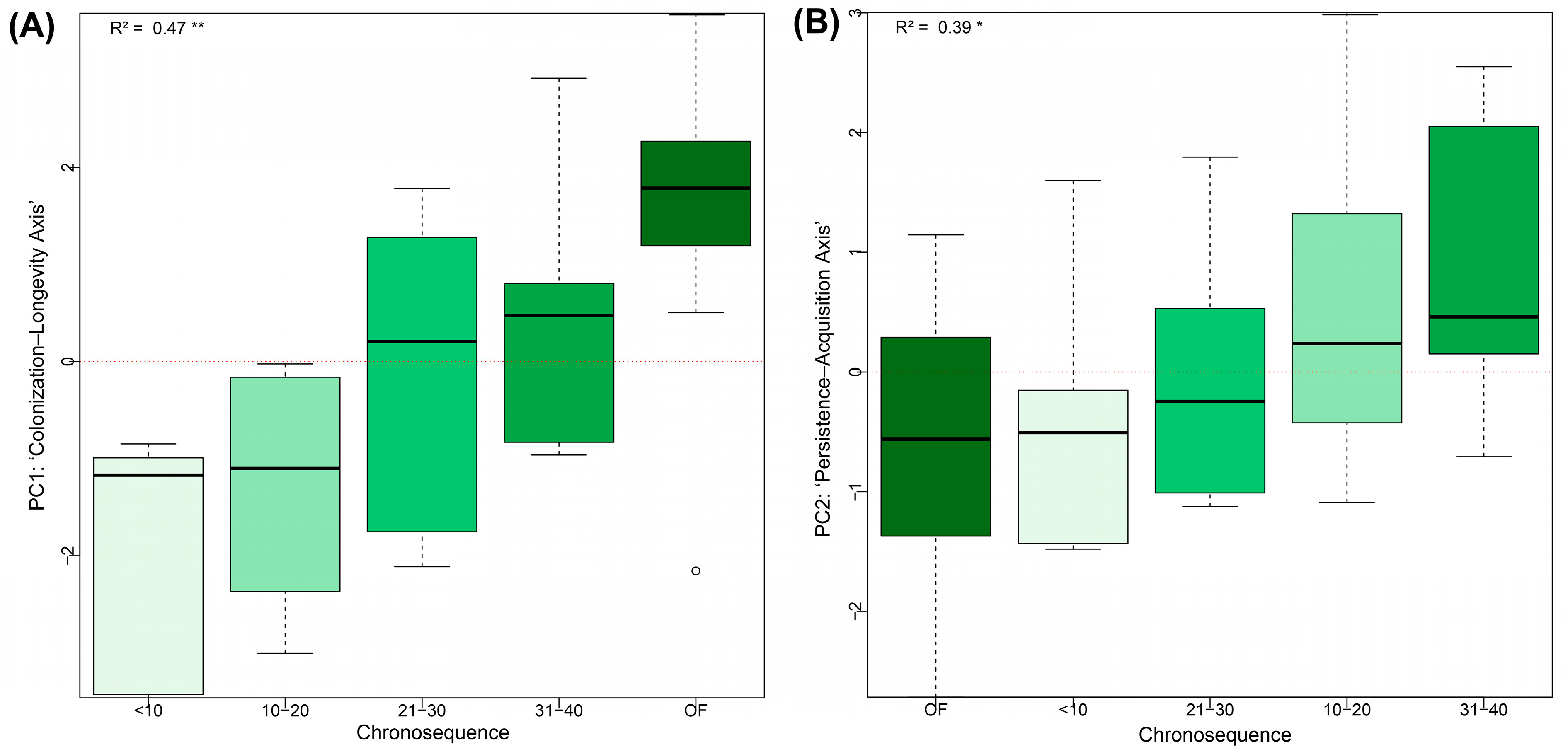
3.4. Relationships Between Environmental Factors, Forest Age, Richness, and Functional Trait Space
4. Discussion
4.1. Species-Level Functional Trait Space Reflect Life-History Trade-Offs
4.2. Community-Level Functional Trait Space Mirrors Species-Level Patterns
4.3. Community Functional Trait Space Driven by Environmental Factors, Forest Age, and Species Richness
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flores, B.M.; Montoya, E.; Sakschewski, B.; Nascimento, N.; Staal, A.; Betts, R.A.; Levis, C.; Lapola, D.M.; Esquível-Muelbert, A.; Jakovac, C.; et al. Critical Transitions in the Amazon Forest System. Nature 2024, 626, 555–564. [Google Scholar] [CrossRef]
- Williams, C.A.; de Almeida, C.T.; Silva Junior, C.H.L. Unraveling the Drivers and Feedbacks of Deforestation across the Amazon Basin. Nat. Sustain. 2024, 7, 110–120. [Google Scholar]
- Baragwanath, K.; Goldblatt, D.; Orellana, A. Infrastructure, Access, and Deforestation in the Amazon. Proc. Natl. Acad. Sci. USA 2021, 118. [Google Scholar]
- Batistella, M.; Brondízio, E.S.; Moran, E.; Brondizio, E.S.; Moran, E.F. Comparative Analysis of Landscape Fragmentation in Ron-dônia, Brazilian Amazon. Int. Arch. Photogramm. Remote Sens. 2000, 33 Pt 7, 148–155. [Google Scholar]
- Sierra, R. Dynamics and Patterns of Deforestation in the Western Amazon: The Napo Deforestation Front, 1986–1996. Appl. Geogr. 2000, 20, 1–16. [Google Scholar] [CrossRef]
- Armenteras, D.; Rudas, G.; Rodriguez, N.; Sua, S.; Romero, M. Patterns and Causes of Deforestation in the Colombian Amazon. Ecol. Indic. 2006, 6, 353–368. [Google Scholar] [CrossRef]
- Armenteras, D.; Murcia, U.; González, T.M.; Barón, O.J.; Arias, J.E. Scenarios of Land Use and Land Cover Change for NW Amazonia: Impact on Forest Intactness. Glob. Ecol. Conserv. 2019, 17, e00567. [Google Scholar] [CrossRef]
- Jiménez, J.G.; Mantilla, L.M.; Barrera, J.A. Enfoque Agroambiental: Una Mirada Distinta a las Intervenciones Productivas en la Amazonia. Caquetá y Guaviare; Instituto SINCHI: Bogotá, DC, Colombia, 2019. [Google Scholar]
- González-González, A.; Villegas, J.C.; Clerici, N.; Salazar, J.F. Spatial-Temporal Dynamics of Deforestation and Its Drivers Indicate Need for Locally-Adapted Environmental Governance in Colombia. Ecol. Indic. 2021, 126, 107695. [Google Scholar] [CrossRef]
- Poorter, L.; Craven, D.; Jakovac, C.C.; van der Sande, M.T.; Amissah, L.; Bongers, F.; Chazdon, R.L.; Farrior, C.E.; Kambach, S.; Meave, J.A.; et al. Multidimensional Tropical Forest Recovery. Science 2021, 374, 1370–1376. [Google Scholar] [CrossRef] [PubMed]
- Chazdon, R.L.; Broadbent, E.N.; Rozendaal, D.M.A.; Bongers, F.; Zambrano, A.M.A.; Aide, T.M.; Balvanera, P.; Becknell, J.M.; Boukili, V.; Brancalion, P.H.S.; et al. Carbon Sequestration Potential of Second-Growth Forest Regeneration in the Latin American Tropics. Sci. Adv. 2016, 2, e1501639. [Google Scholar] [CrossRef]
- Rozendaal, D.M.A.; Bongers, F.; Aide, T.M.; Alvarez-Dávila, E.; Ascarrunz, N.; Balvanera, P.; Becknell, J.M.; Bentos, T.V.; Brancalion, P.H.S.; Cabral, G.A.L.; et al. Biodiversity Recovery of Neotropical Secondary Forests. Sci. Adv. 2019, 5, eaau3114. [Google Scholar] [CrossRef]
- Elias, F.; Ferreira, J.; Resende, A.F.; Berenguer, E.; França, F.; Smith, C.C.; Schwartz, G.; Nascimento, R.O.; Guedes, M.; Rossi, L.C.; et al. Comparing Contemporary and Lifetime Rates of Carbon Accumulation from Secondary Forests in the Eastern Amazon. For. Ecol. Manag. 2022, 508, 120053. [Google Scholar] [CrossRef]
- Arroyo-Rodríguez, V.; Melo, F.P.L.; Martínez-Ramos, M.; Bongers, F.; Chazdon, R.L.; Meave, J.A.; Norden, N.; Santos, B.A.; Leal, I.R.; Tabarelli, M. Multiple Successional Pathways in Human-Modified Tropical Landscapes: New Insights from Forest Succession, Forest Fragmentation and Landscape Ecology Research. Biol. Rev. 2017, 92, 326–340. [Google Scholar] [CrossRef] [PubMed]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The Worldwide Leaf Economics Spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Díaz, S.; Kattge, J.; Cornelissen, J.H.C.; Wright, I.J.; Lavorel, S.; Dray, S.; Reu, B.; Kleyer, M.; Wirth, C.; Prentice, I.C.; et al. The Global Spectrum of Plant Form and Function. Nature 2016, 529, 167–171. [Google Scholar] [CrossRef]
- Violle, C.; Navas, M.-L.; Vile, D.; Kazakou, E.; Fortunel, C.; Hummel, I.; Garnier, E. Let the Concept of Trait Be Functional! Oikos 2007, 116, 882–892. [Google Scholar] [CrossRef]
- Reich, P.B. The World-Wide ‘fast–Slow’ Plant Economics Spectrum: A Traits Manifesto. J. Ecol. 2014, 102, 275–301. [Google Scholar] [CrossRef]
- Maracahipes, L.; Carlucci, M.B.; Lenza, E.; Marimon, B.S.; Marimon, B.H.; Guimarães, F.A.; Cianciaruso, M.V. How to Live in Contrasting Habitats? Acquisitive and Conservative Strategies Emerge at Inter- and Intraspecific Levels in Savanna and Forest Woody Plants. Perspect. Plant Ecol. Evol. Syst. 2018, 34, 17–25. [Google Scholar] [CrossRef]
- Maynard, D.S.; Bialic-Murphy, L.; Zohner, C.M.; Averill, C.; van den Hoogen, J.; Ma, H.; Mo, L.; Smith, G.R.; Acosta, A.T.R.; Aubin, I.; et al. Global Relationships in Tree Functional Traits. Nat. Commun. 2022, 13, 3185. [Google Scholar] [CrossRef]
- Carmona, C.P.; de Bello, F.; Mason, N.W.H.; Lepš, J. Traits Without Borders: Integrating Functional Diversity Across Scales. Trends Ecol. Evol. 2016, 31, 382–394. [Google Scholar] [CrossRef]
- Mason, N.W.H.; Mouillot, D.; Lee, W.G.; Wilson, J.B. Functional Richness, Functional Evenness and Functional Divergence: The Primary Components of Functional Diversity. Oikos 2005, 111, 112–118. [Google Scholar] [CrossRef]
- Westoby, M.; Falster, D.S.; Moles, A.T.; Vesk, P.A.; Wright, I.J. Plant Ecological Strategies: Some Leading Dimensions of Vari-ation between Species. Annu. Rev. Ecol. Evol. Syst. 2002, 33, 125–159. [Google Scholar] [CrossRef]
- Carmona, C.P.; De Bello, F.; Mason, N.W.H.; Leps, J. Trait Probability Density (TPD): Measuring Functional Diversity across Scales Based on TPD with R. Ecology 2019, 100, e02876. [Google Scholar] [CrossRef] [PubMed]
- ter Steege, H.; Poorter, L.; Aguirre-Gutiérrez, J.; Fortunel, C.; Magnusson, W.E.; Phillips, O.L.; Pos, E.; Luize, B.G.; Baraloto, C.; Guevara, J.E.; et al. Functional Composition of the Amazonian Tree Flora and Forests. Commun. Biol. 2025, 8, 355. [Google Scholar] [CrossRef]
- Cadotte, M.W.; Carscadden, K.; Mirotchnick, N. Beyond Species: Functional Diversity and the Maintenance of Ecological Processes and Services. J. Appl. Ecol. 2011, 48, 1079–1087. [Google Scholar] [CrossRef]
- Sakschewski, B.; Von Bloh, W.; Boit, A.; Poorter, L.; Peña-Claros, M.; Heinke, J.; Joshi, J.; Thonicke, K. Resilience of Amazon Forests Emerges from Plant Trait Diversity. Nat. Clim. Change 2016, 6, 1032–1036. [Google Scholar] [CrossRef]
- Schmitt, S.; Maréchaux, I.; Chave, J.; Fischer, F.J.; Piponiot, C.; Traissac, S.; Hérault, B. Functional Diversity Improves Tropical Forest Resilience: Insights from a Long-Term Virtual Experiment. J. Ecol. 2020, 108, 831–843. [Google Scholar] [CrossRef]
- Fauset, S.; Johnson, M.O.; Gloor, M.; Baker, T.R.; Monteagudo, A.M.; Brienen, R.J.; Feldpausch, T.R.; Lopez-Gonzalez, G.; Malhi, Y.; Ter Steege, H.; et al. Hyperdominance in Amazonian Forest Carbon Cycling. Nat. Commun. 2015, 6, 6857. [Google Scholar] [CrossRef]
- Lohbeck, M.; Bongers, F.; Martinez-Ramos, M.; Poorter, L. The Importance of Biodiversity and Dominance for Multiple Ecosystem Functions in a Human-Modified Tropical Landscape. Ecology 2016, 97, 2772–2779. [Google Scholar] [CrossRef]
- Ali, A.; Yan, E.-R.; Chang, S.X.; Cheng, J.-Y.; Liu, X.-Y. Community-Weighted Mean of Leaf Traits and Divergence of Wood Traits Predict Aboveground Biomass in Secondary Subtropical Forests. Sci. Total Environ. 2017, 574, 654–662. [Google Scholar] [CrossRef]
- Tilman, D.; Knops, J.; Wedin, D.; Reich, P.; Ritchie, M.; Siemann, E. The Influence of Functional Diversity and Composition on Ecosystem Processes. Science 1997, 277, 1300–1302. [Google Scholar] [CrossRef]
- Yuan, Z.; Wang, S.; Gazol, A.; Mellard, J.; Lin, F.; Ye, J.; Hao, Z.; Wang, X.; Loreau, M. Multiple Metrics of Diversity Have Different Effects on Temperate Forest Functioning over Succession. Oecologia 2016, 182, 1175–1185. [Google Scholar] [CrossRef]
- Yuan, Z.; Wang, S.; Ali, A.; Gazol, A.; Ruiz-Benito, P.; Wang, X.; Lin, F.; Ye, J.; Hao, Z.; Loreau, M. Aboveground Carbon Storage Is Driven by Functional Trait Composition and Stand Structural Attributes Rather than Biodiversity in Temperate Mixed Forests Recovering from Disturbances. Ann. For. Sci. 2018, 75, 67. [Google Scholar] [CrossRef]
- Prado-Junior, J.A.; Schiavini, I.; Vale, V.S.; Raymundo, D.; Lopes, S.F.; Poorter, L. Functional Traits Shape Size-Dependent Growth and Mortality Rates of Dry Forest Tree Species. J. Plant Ecol. 2016, 10, 895–906. [Google Scholar] [CrossRef]
- Pothasin, P.; Paradis, E.; Brockelman, W.Y.; Nathalang, A.; Khemrugka, T.; Lomwong, N.; Thripob, P.; Saenprasert, R.; Chanthorn, W. Seed Size Variation of Trees and Lianas in a Tropical Forest of Southeast Asia: Allometry, Phylogeny, and Seed Trait-Plant Functional Trait Relationships. Front. Plant Sci. 2022, 13, 852167. [Google Scholar] [CrossRef]
- Grime, J.P. (Ed.) Plant Strategies, Vegetation Processes and Ecosystem Properties; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2001; ISBN 0471496014. [Google Scholar]
- Wang, W.; Zhao, J.; Zhang, B.; Deng, G.; Maimaiti, A.; Guo, Z. Patterns and Drivers of Tree Species Diversity in a Coniferous Forest of Northwest China. Front. For. Glob. Change 2024, 7, 1333232. [Google Scholar] [CrossRef]
- Laughlin, D.C. The Intrinsic Dimensionality of Plant Traits and Its Relevance to Community Assembly. J. Ecol. 2013, 102, 186–193. [Google Scholar] [CrossRef]
- IGAC. Caquetá, Características Geográficas; Imprenta nacional de Colombia: Bogotá, DC, Colombia, 2010.
- Rodríguez-León, C.H.; Peña-Venegas, C.P.; Sterling, A.; Castro, D.; Mahecha-Virguez, L.K.; Virguez-Díaz, Y.R.; Silva-Olaya, A.M. Soil Quality Restoration during the Natural Succession of Abandoned Cattle Pastures in Deforested Landscapes in the Colombian Amazon. Agronomy 2021, 11, 2484. [Google Scholar] [CrossRef]
- Rodríguez-León, C.H.; Sterling, A.; Trujillo-Briñez, A.; Suárez-Córdoba, Y.D.; Roa-Fuentes, L.L. Forest Attribute Dynamics in Secondary Forests: Insights for Advancing Ecological Restoration and Transformative Territorial Management in the Amazon. Diversity 2025, 17, 39. [Google Scholar] [CrossRef]
- Chisholm, R.A.; Muller-Landau, H.C.; Rahman, K.A.; Bebber, D.P.; Bin, Y.; Bohlman, S.A.; Bourg, N.A.; Brinks, J.; Bunyavejchewin, S.; Butt, N.; et al. Scale-Dependent Relationships between Tree Species Richness and Ecosystem Function in Forests. J. Ecol. 2013, 101, 1214–1224. [Google Scholar] [CrossRef]
- Baraloto, C.; Paine, C.E.T.; Poorter, L.; Beauchene, J.; Bonal, D.; Domenach, A.; Hérault, B.; Patiño, S.; Roggy, J.; Chave, J. Decoupled Leaf and Stem Economics in Rain Forest Trees. Ecol. Lett. 2010, 13, 1338–1347. [Google Scholar] [CrossRef] [PubMed]
- Lohbeck, M.; Lebrija-Trejos, E.; Martínez-Ramos, M.; Meave, J.A.; Poorter, L.; Bongers, F. Functional Trait Strategies of Trees in Dry and Wet Tropical Forests Are Similar but Differ in Their Consequences for Succession. PLoS ONE 2015, 10, e0123741. [Google Scholar] [CrossRef] [PubMed]
- Condit, R.; Ashton, P.S.; Baker, P.; Bunyavejchewin, S.; Gunatilleke, S.; Gunatilleke, N.; Hubbell, S.P.; Foster, R.B.; Itoh, A.; LaFrankie, J.V.; et al. Spatial Patterns in the Distribution of Tropical Tree Species. Science 2000, 288, 1414–1418. [Google Scholar] [CrossRef] [PubMed]
- Buzzard, V.; Hulshof, C.M.; Birt, T.; Violle, C.; Enquist, B.J. Re-Growing a Tropical Dry Forest: Functional Plant Trait Composition and Community Assembly during Succession. Funct. Ecol. 2016, 30, 1006–1013. [Google Scholar] [CrossRef]
- The Angiosperm Phylogeny Group; Chase, M.W.; Christenhusz, M.J.M.; Fay, M.F.; Byng, J.W.; Judd, W.S.; Soltis, D.E.; Mabberley, D.J.; Sennikov, A.N.; Soltis, P.S.; et al. An Update of the Angiosperm Phylogeny Group Classification for the Orders and Families of Flowering Plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar] [CrossRef]
- Montoya, E.C.; Rangel, J.O.; Peña, A.C. Functional Diversity Guides Enrichment Planting Strategies in Andean Secondary Forests. Restor. Ecol. 2022, 30. [Google Scholar]
- Lohbeck, M.; Poorter, L.; Martínez-Ramos, M.; Rodriguez-Velázquez, J.; van Breugel, M.; Bongers, F. Changing Drivers of Species Dominance during Tropical Forest Succession. Funct. Ecol. 2014, 28, 1052–1058. [Google Scholar] [CrossRef]
- Baraloto, C.; Paine, C.E.T.; Patiño, S.; Bonal, D.; Hérault, B.; Chave, J. Functional Trait Variation and Sampling Strategies in Species-Rich Plant Communities. Funct. Ecol. 2010, 24, 208–216. [Google Scholar] [CrossRef]
- Pérez-Harguindeguy, N.; Díaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. Corrigendum to: New Handbook for Standardised Measurement of Plant Functional Traits Worldwide. Aust. J. Bot. 2016, 64, 715–716. [Google Scholar] [CrossRef]
- Malhi, Y.; Girardin, C.; Metcalfe, D.B.; Doughty, C.E.; Aragão, L.E.; Rifai, S.W.; Oliveras, I.; Shenkin, A.; Aguirre-Gutiérrez, J.; Dahlsjö, C.A.; et al. The Global Ecosystems Monitoring Network: Monitoring Ecosystem Productivity and Carbon Cycling across the Tropics. Biol. Conserv. 2021, 253, 108889. [Google Scholar] [CrossRef]
- ForestPlots.net; Blundo, C.; Carilla, J.; Grau, R.; Malizia, A.; Malizia, L.; Osinaga-Acosta, O.; Bird, M.; Bradford, M.; Catchpole, D.; et al. Taking the Pulse of Earth’s Tropical Forests Using Networks of Highly Distributed Plots. Biol. Conserv. 2021, 260, 108849. [Google Scholar] [CrossRef]
- Maitner, B.S.; Boyle, B.; Casler, N.; Condit, R.; Donoghue, J., II; Durán, S.M.; Guaderrama, D.; Hinchliff, C.E.; Jørgensen, P.M.; Kraft, N.J.; et al. The Bien r Package: A Tool to Access the Botanical Information and Ecology Network (BIEN) Database. Methods Ecol. Evol. 2018, 9, 373–379. [Google Scholar] [CrossRef]
- Kattge, J.; Díaz, S.; Lavorel, S.; Prentice, I.C.; Leadley, P.; Bönisch, G.; Garnier, E.; Westoby, M.; Reich, P.B.; Wright, I.J.; et al. TRY—A Global Database of Plant Traits. Glob. Change Biol. 2011, 17, 2905–2935. [Google Scholar] [CrossRef]
- Kattge, J.; Bönisch, G.; Díaz, S.; Lavorel, S.; Prentice, I.C.; Leadley, P.; Tautenhahn, S.; Werner, G.D.A.; Aakala, T.; Abedi, M.; et al. TRY Plant Trait Database—Enhanced Coverage and Open Access. Glob. Change Biol. 2020, 26, 119–188. [Google Scholar] [CrossRef]
- Weigelt, P.; König, C.; Kreft, H. GIFT—A Global Inventory of Floras and Traits for Macroecology and Biogeography. J. Biogeogr. 2020, 47, 16–43. [Google Scholar] [CrossRef]
- Díaz, S.; Kattge, J.; Cornelissen, J.H.C.; Wright, I.J.; Lavorel, S.; Dray, S.; Reu, B.; Kleyer, M.; Wirth, C.; Prentice, I.C.; et al. The Global Spectrum of Plant Form and Function: Enhanced Species-Level Trait Dataset. Sci. Data 2022, 9, 755. [Google Scholar] [CrossRef] [PubMed]
- Green, P.T.; Juniper, P.A. Seed–Seedling Allometry in Tropical Rain Forest Trees: Seed Mass-Related Patterns of Resource Allocation and the ‘Reserve Effect’. J. Ecol. 2004, 92, 397–408. [Google Scholar] [CrossRef]
- Ley-López, J.M.; Wawrzyniak, M.K.; Chacón-Madrigal, E.; Chmielarz, P. Seed Traits and Tropical Arboreal Species Conservation: A Case Study of a Highly Diverse Tropical Humid Forest Region in Southern Costa Rica. Biodivers. Conserv. 2023, 32, 1573–1590. [Google Scholar] [CrossRef]
- Ter Steege, H.; Welch, I.; Zagt, R. Long-Term Effect of Timber Harvesting in the Bartica Triangle, Central Guyana. For. Ecol. Manag. 2002, 170, 127–144. [Google Scholar] [CrossRef]
- Finegan, B. Pattern and Process in Neotropical Secondary Rain Forests: The First 100 Years of Succession. Trends Ecol. Evol. 1996, 11, 119–124. [Google Scholar] [CrossRef]
- Rodríguez-León, C.H.; Roa-Fuentes, L.L.; Sterling, A.; Suárez, J.C. Plant Biodiversity Homogenization across the Chronosequence in Highly Fragmented Landscapes in the Colombian Andean–Amazonian Transition. Forests 2022, 13, 1422. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-Km Spatial Resolution Climate Surfaces for Global Land Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Natural Resources Conservation Service. Soil Survey Staff Kellogg Soil Survey Laboratory Methods Manual. In Soil Survey Investigations Report No. 42; Version 6.0; USA Department of Agriculture: Lincoln, NE, USA, 2022. [Google Scholar]
- Zamudio, A.M.; Carrascal Carrascal, M.L.; Pulido Roa, C.E.; Gallardo, J.F.; Gómez Guzmán, I.D. Métodos Analíticos del Laboratorio de Suelos; Instituto Geografico Agustin Codazzi (IGAC): Bogotá, DC, Colombia, 2006; Volume 6, ISBN 9789589067987/9589067980. [Google Scholar]
- R Core Team and Contributors Worldwide. The R Stats Package, Version 4.3.3; R Core Team: Vienna, Austria, 2024. [Google Scholar]
- Carmona, C.P.; Pavanetto, N.; Puglielli, G. Funspace: Creating and Representing Functional Trait Spaces. R Package Version 0.2.2. CRAN: Contributed Packages. 2024. Available online: https://CRAN.R-project.org/package=funspace (accessed on 9 April 2025).
- Carmona, C.P.; Pavanetto, N.; Puglielli, G. Funspace: An R Package to Build, Analyse and Plot Functional Trait Spaces. Divers. Distrib. 2024, 30, e13820. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2024. [Google Scholar]
- Laliberté, E.; Legendre, P.; Shipley, B. Measuring Functional Diversity (FD) from Multiple Traits, and Other Tools for Functional Ecology. 2023. Available online: https://cran.r-project.org/web/packages/FD/FD.pdf (accessed on 10 May 2025).
- Dinno, A. Paran: Horn’s Test of Principal Components/Factors. R Package Version 1.5.4. CRAN: Contributed Packages. 2025. Available online: https://CRAN.R-project.org/package=paran (accessed on 9 May 2025).
- Gross, N.; Maestre, F.T.; Liancourt, P.; Berdugo, M.; Martin, R.; Gozalo, B.; Ochoa, V.; Delgado-Baquerizo, M.; Maire, V.; Saiz, H.; et al. Unforeseen Plant Phenotypic Diversity in a Dry and Grazed World. Nature 2024, 632, 808–814. [Google Scholar] [CrossRef]
- Dixon, P. VEGAN, a Package of R Functions for Community Ecology. J. Veg. Sci. 2009, 14, 927–930. [Google Scholar] [CrossRef]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D. Nlme: Linear and Nonlinear Mixed Effects Models. R Package Version 3.1-131.1. 2018. Available online: https://CRAN.R-project.org/package=nlme (accessed on 30 April 2025).
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. InfoStat Versión 2020. 2020. Available online: https://www.infostat.com.ar/ (accessed on 30 April 2025).
- Huang, H. LinkET: Everything Is Linkable. R Package Version 0.0.3. CRAN Contributed. 2021. Available online: https://github.com/Hy4m/linkET (accessed on 20 May 2025).
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S.; Bojesen, R.H.; Singmann, H.; Dai, B.; Scheipl, F.; Grothendieck, G.; Green, P.; et al. Package Lme4: Linear Mixed-Effects Models Using “Eigen” and S4 Package Version: 1.1-27. 2021. Available online: https://CRAN.R-project.org/package=lme4 (accessed on 10 May 2025).
- Kuznetsova, A.; Bruun Brockhoff, P.; Haubo Bojesen Christensen, R.; Pødenphant Jensen, S. LmerTest: Tests in Linear Mixed Effects Models. R Package Version 3.1-3. CRAN: Contributed Packages. 2020. Available online: https://CRAN.R-project.org/package=lmerTest (accessed on 17 May 2025).
- Fox, J.; Weisberg, S.; Price, B. R-Core Car: Companion to Applied Regression. R Package Version 3.1-3. CRAN: Contributed Packages. 2024. Available online: https://CRAN.R-project.org/package=car (accessed on 25 May 2025).
- Bartoń, K. MuMIn: Multi-Model Inference. R Package Version 1.43. 2020. Available online: https://CRAN.R-project.org/package=MuMIn (accessed on 19 May 2025).
- Rosseel, Y. Lavaan: An R Package for Structural Equation Modeling. J. Stat. Softw. 2012, 48, 1–36. [Google Scholar] [CrossRef]
- Epskamp, S.; Stuber, S.; Nak, J.; Veenman, M.; Jorgensen, T.D. SemPlot: Path Diagrams and Visual Analysis of Various SEM Packages’. Output. R Package Version 1.1.6. CRAN: Contributed Packages. 2022. Available online: https://CRAN.R-project.org/package=semPlot (accessed on 11 May 2025).
- RStudio. RStudio Version 2025.05.0. 2025. Available online: https://posit.co/blog/rstudio-2025-05-0-whats-new/ (accessed on 10 May 2025).
- Cope, O.L.; Lindroth, R.L.; Helm, A.; Keefover-Ring, K.; Kruger, E.L. Trait Plasticity and Trade-Offs Shape Intra-Specific Variation in Competitive Response in a Foundation Tree Species. New Phytol. 2021, 230, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ramos, M.; Barragán, F.; Mora, F.; Maza-Villalobos, S.; Arreola-Villa, L.F.; Bhaskar, R.; Bongers, F.; Lemus-Herrera, C.; Paz, H.; Martínez-Yrizar, A.; et al. Differential Ecological Filtering across Life Cycle Stages Drive Old-Field Succession in a Neotropical Dry Forest. For. Ecol. Manag. 2021, 482, 118810. [Google Scholar] [CrossRef]
- Poorter, L.; Bongers, F.; Aide, T.M.; Almeyda Zambrano, A.M.; Balvanera, P.; Becknell, J.M.; Boukili, V.; Brancalion, P.H.S.; Broadbent, E.N.; Chazdon, R.L.; et al. Biomass Resilience of Neotropical Secondary Forests. Nature 2016, 530, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Oliver, T.H.; Heard, M.S.; Isaac, N.J.B.; Roy, D.B.; Procter, D.; Eigenbrod, F.; Freckleton, R.P.; Hector, A.L.; Orme, C.D.L.; Petchey, O.; et al. Biodiversity and Resilience of Ecosystem Functions. Trends Ecol. Evol. 2015, 30, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Garnier, E.; Cortez, J.; Billès, G.; Navas, M.-L.; Roumet, C.; Debussche, M.; Laurent, G.; Blanchard, A.; Aubry, D.; Bellmann, A.; et al. Plant Functional Markers Capture Ecosystem Properties during Secondary Succession. Ecology 2004, 85, 2630–2637. [Google Scholar] [CrossRef]
- Loreau, M.; de Mazancourt, C. Biodiversity and Ecosystem Stability: A Synthesis of Underlying Mechanisms. Ecol. Lett. 2013, 16, 106–115. [Google Scholar] [CrossRef]
- Holl, K.D.; Reid, J.L.; Cole, R.J.; Oviedo-Brenes, F.; Rosales, J.A.; Zahawi, R.A. Applied Nucleation Facilitates Tropical Forest Recovery: Lessons Learned from a 15-Year Study. J. Appl. Ecol. 2020, 57, 2316–2328. [Google Scholar] [CrossRef]
- Palma, A.C.; Laurance, S.G. A Review of the Use of Direct Seeding and Seedling Plantings in Restoration: What Do We Know and Where Should We Go? Appl. Veg. Sci. 2015, 18, 561–568. [Google Scholar] [CrossRef]
- Rodrigues, R.R.; Brancalion, P.H.S.; Isernhagen, I. Ecological Restoration: Theory, Principles and Practice. Braz. J. Biol. 2021, 81, 12. [Google Scholar]
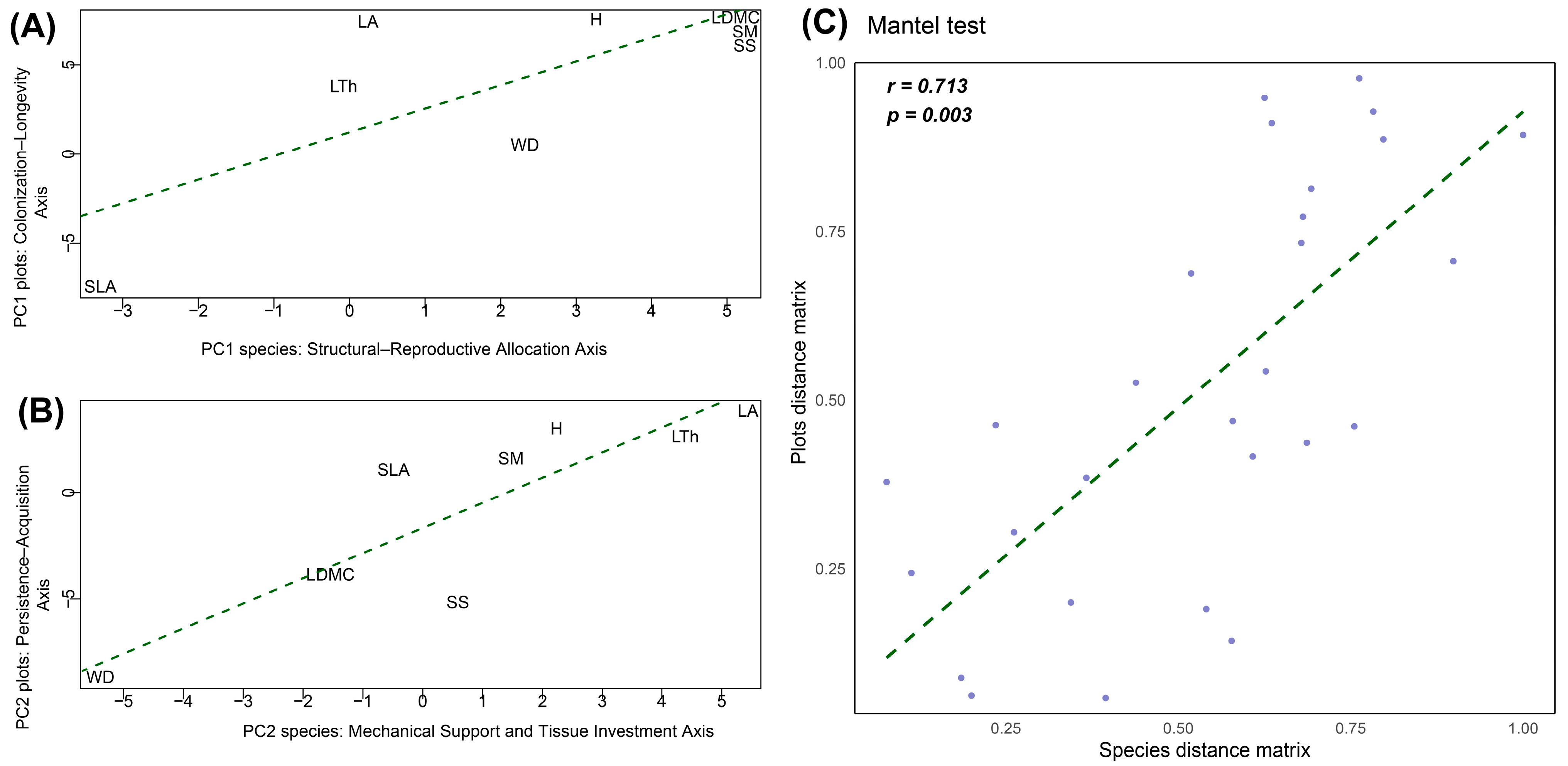
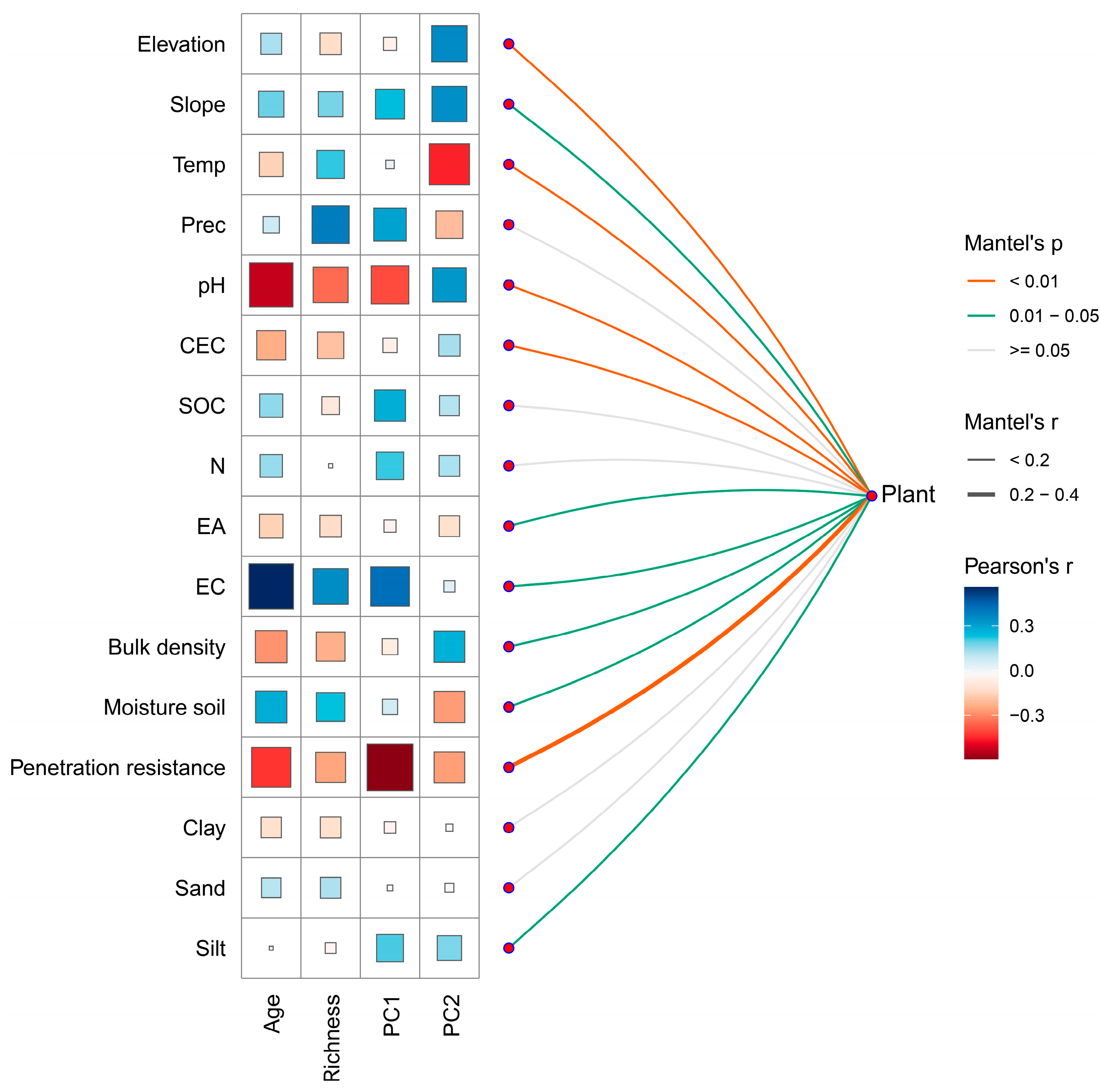


| Trait | Trait Variation PC1–PC2 = 43.97% | Trait Variation PC1–PC3 = 40.44% | Axis | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PC1 | PC2 | R2 | p-Value | PC1 | PC3 | R2 | p-Value | ||
| WD | 0.091 | 0.551 | 0.641 | 0.001 | 0.091 | 0.009 | 0.100 | 0.001 | MSTIA |
| LA | 0.001 | 0.538 | 0.539 | 0.001 | 0.001 | 0.008 | 0.009 | 0.368 | MSTIA |
| SM | 0.500 | 0.037 | 0.536 | 0.001 | 0.500 | 0.071 | 0.570 | 0.001 | SRAA |
| SS | 0.496 | 0.006 | 0.501 | 0.001 | 0.496 | 0.167 | 0.662 | 0.001 | SRAA |
| LDMC | 0.454 | 0.040 | 0.494 | 0.001 | 0.454 | 0.176 | 0.630 | 0.001 | LEA |
| LTh | 0.000 | 0.325 | 0.325 | 0.001 | 0.000 | 0.183 | 0.183 | 0.001 | MSTIA |
| H | 0.179 | 0.084 | 0.263 | 0.001 | 0.179 | 0.162 | 0.341 | 0.001 | SRAA |
| SLA | 0.213 | 0.004 | 0.217 | 0.001 | 0.213 | 0.526 | 0.739 | 0.001 | LEA |
| Trait | PC1 | PC2 | R2 | p-Value | Axis |
|---|---|---|---|---|---|
| WD | 0.003 | 0.816 | 0.819 | 0.001 | PAA |
| LA | 0.615 | 0.179 | 0.794 | 0.001 | CLA |
| LDMC | 0.191 | 0.578 | 0.769 | 0.001 | PAA |
| SS | 0.067 | 0.621 | 0.688 | 0.001 | PAA |
| H | 0.551 | 0.087 | 0.639 | 0.001 | CLA |
| SLA | 0.625 | 0.011 | 0.636 | 0.001 | CLA |
| SM | 0.509 | 0.025 | 0.534 | 0.001 | CLA |
| LTh | 0.141 | 0.067 | 0.208 | 0.027 | CLA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-León, C.H.; Sterling, A.; Daza-Giraldo, D.D.; Suárez-Córdoba, Y.D.; Roa-Fuentes, L.L. Scaling Plant Functional Strategies from Species to Communities in Regenerating Amazonian Forests: Insights for Restoration in Deforested Landscapes. Diversity 2025, 17, 570. https://doi.org/10.3390/d17080570
Rodríguez-León CH, Sterling A, Daza-Giraldo DD, Suárez-Córdoba YD, Roa-Fuentes LL. Scaling Plant Functional Strategies from Species to Communities in Regenerating Amazonian Forests: Insights for Restoration in Deforested Landscapes. Diversity. 2025; 17(8):570. https://doi.org/10.3390/d17080570
Chicago/Turabian StyleRodríguez-León, Carlos H., Armando Sterling, Dorman D. Daza-Giraldo, Yerson D. Suárez-Córdoba, and Lilia L. Roa-Fuentes. 2025. "Scaling Plant Functional Strategies from Species to Communities in Regenerating Amazonian Forests: Insights for Restoration in Deforested Landscapes" Diversity 17, no. 8: 570. https://doi.org/10.3390/d17080570
APA StyleRodríguez-León, C. H., Sterling, A., Daza-Giraldo, D. D., Suárez-Córdoba, Y. D., & Roa-Fuentes, L. L. (2025). Scaling Plant Functional Strategies from Species to Communities in Regenerating Amazonian Forests: Insights for Restoration in Deforested Landscapes. Diversity, 17(8), 570. https://doi.org/10.3390/d17080570







