Spatial Forecasting and Social Acceptance of Human-Wildlife Conflicts Involving Semi-Aquatic Species in Romania
Abstract
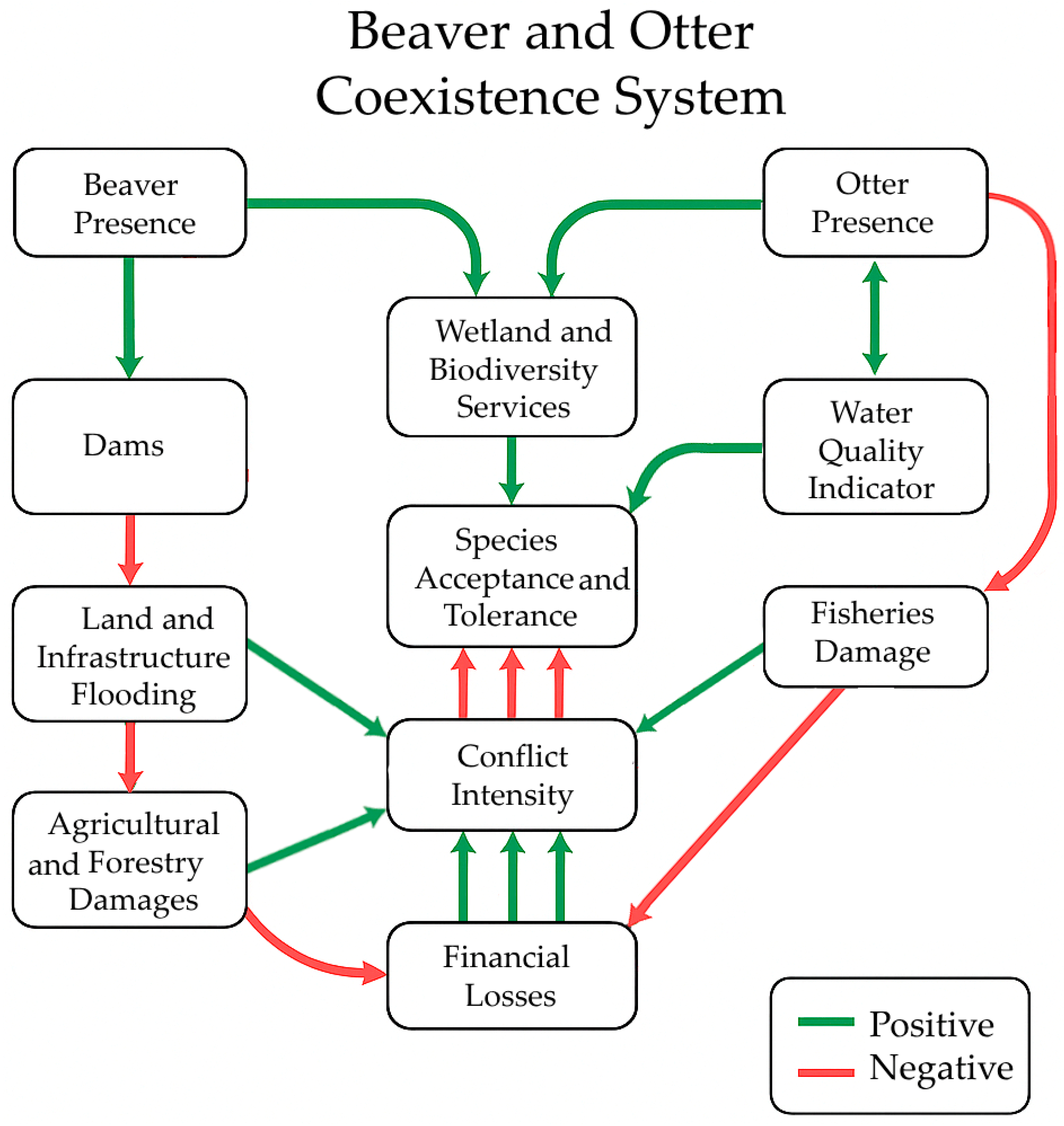
1. Introduction
2. Materials and Methods
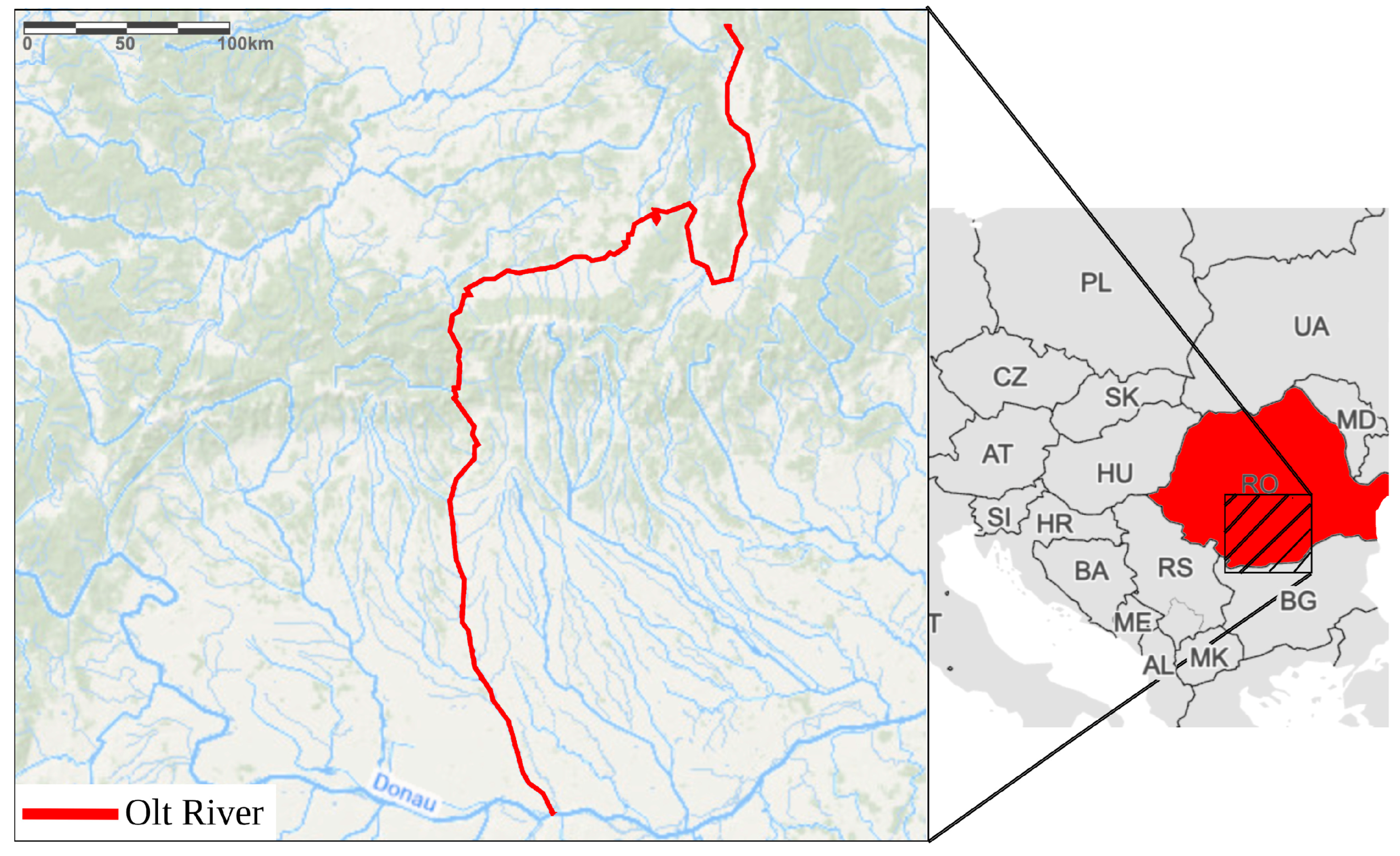
2.1. Study Area
2.2. Data Collection
2.3. Forecasting of HWC Areas and Statistical Analysis
3. Results
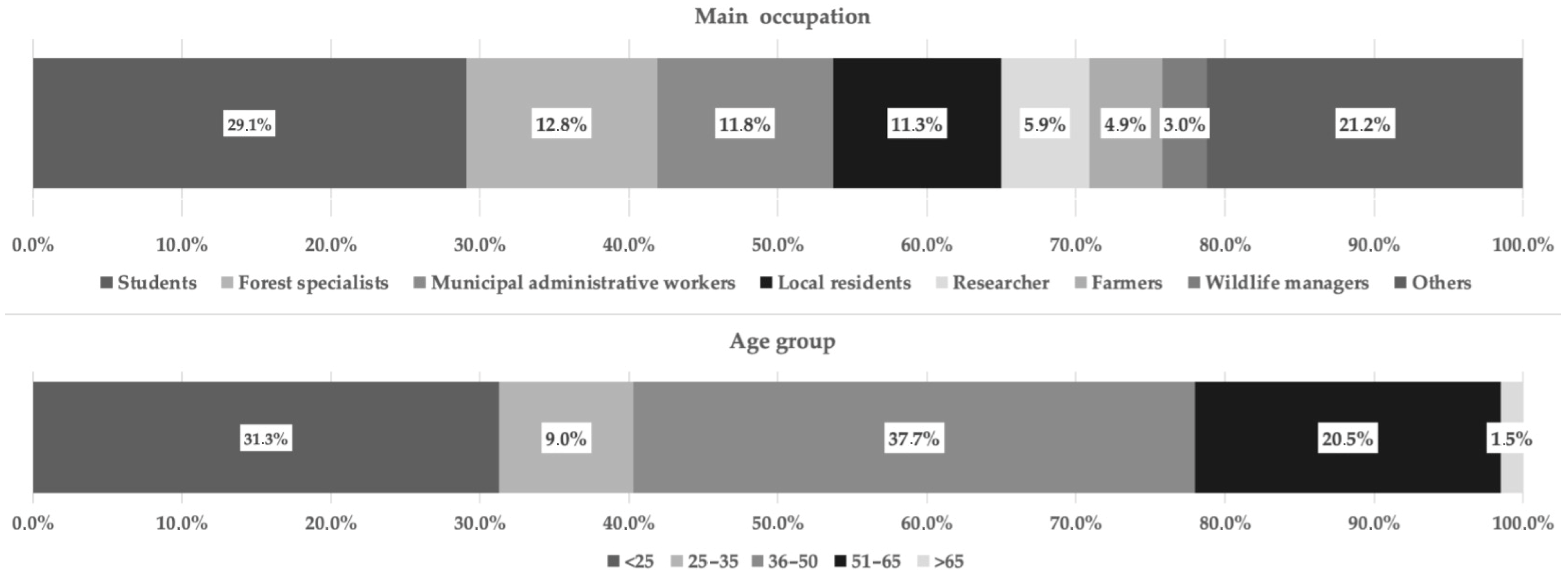
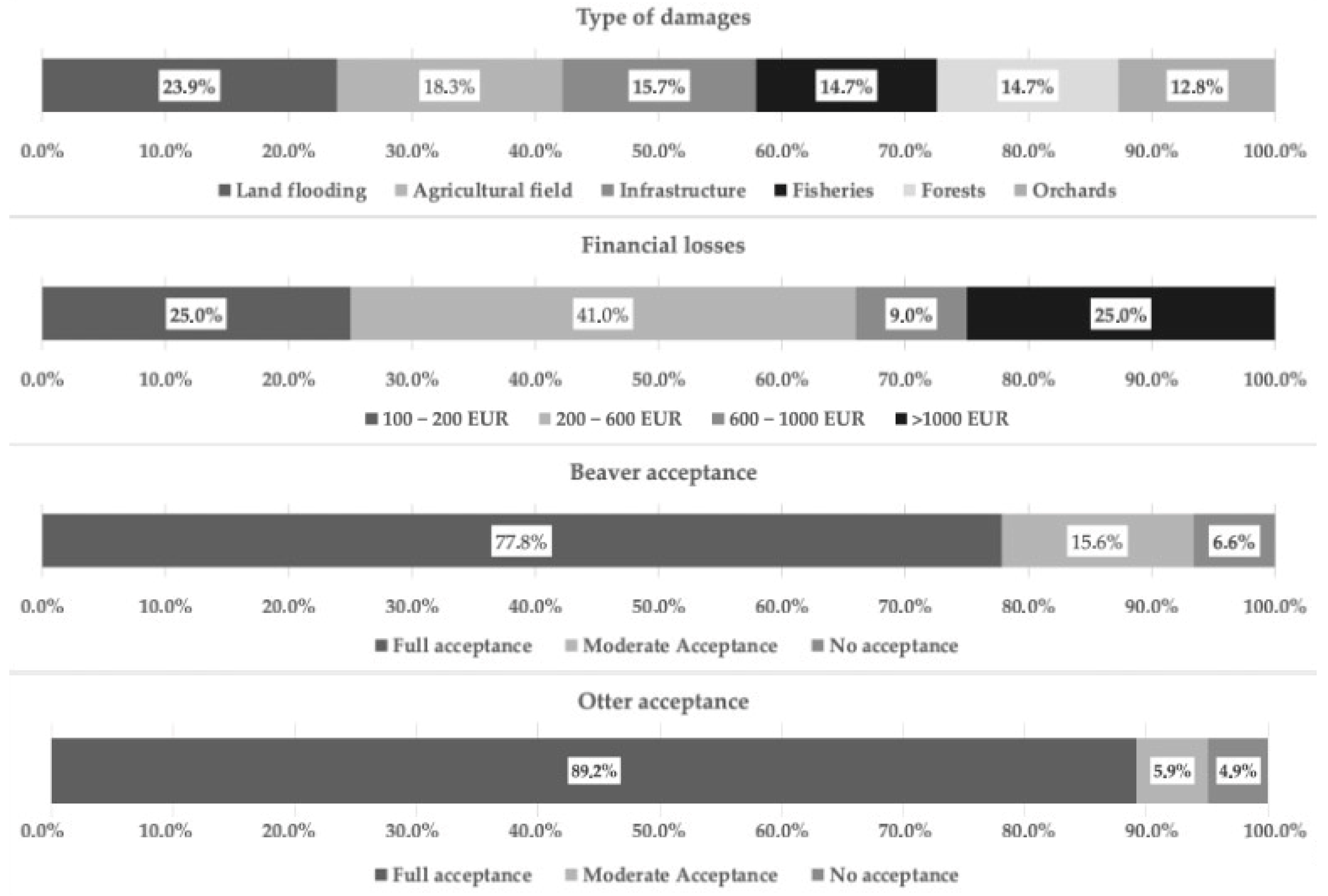
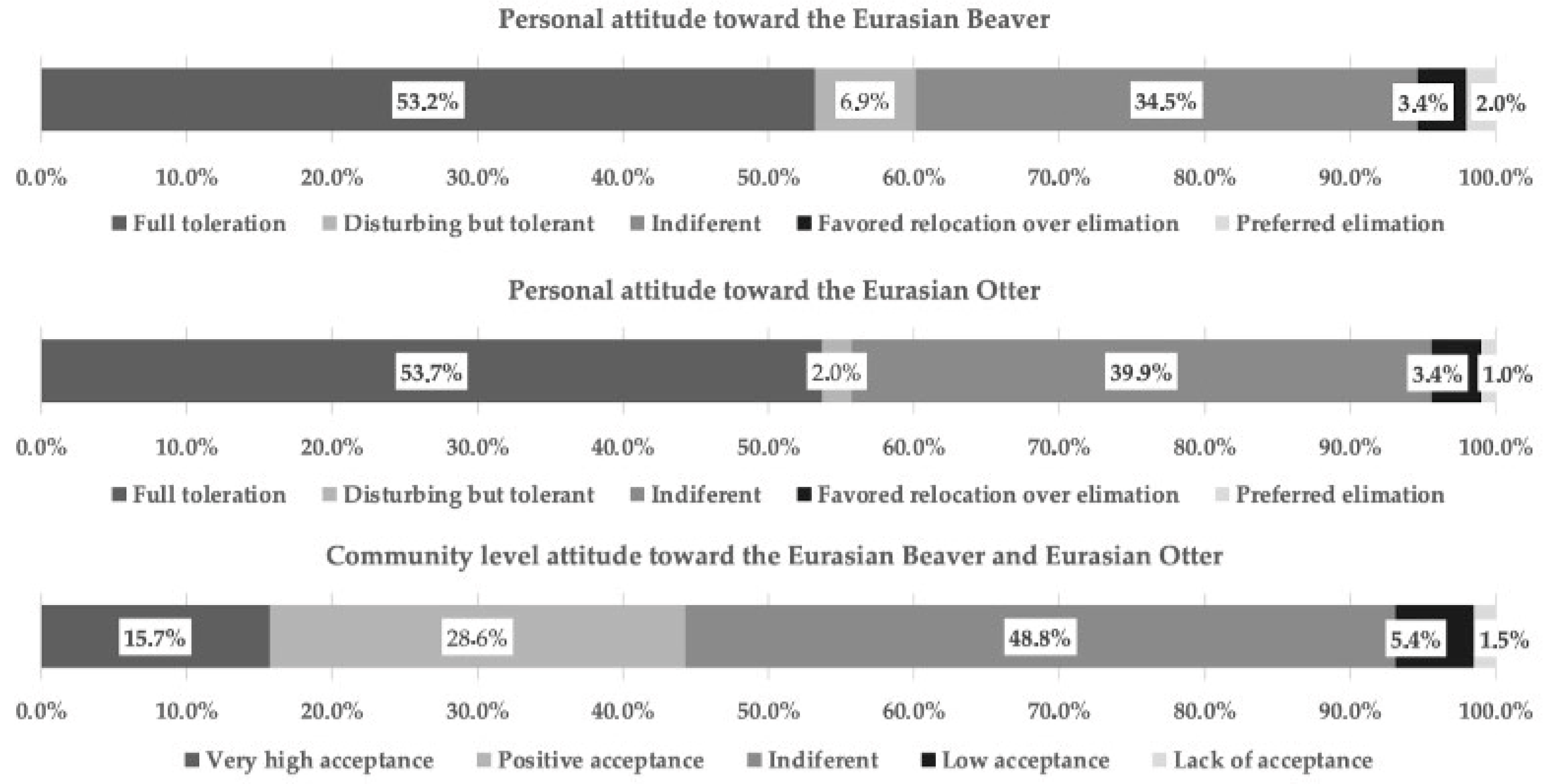
3.1. Stakeholder Group Survey
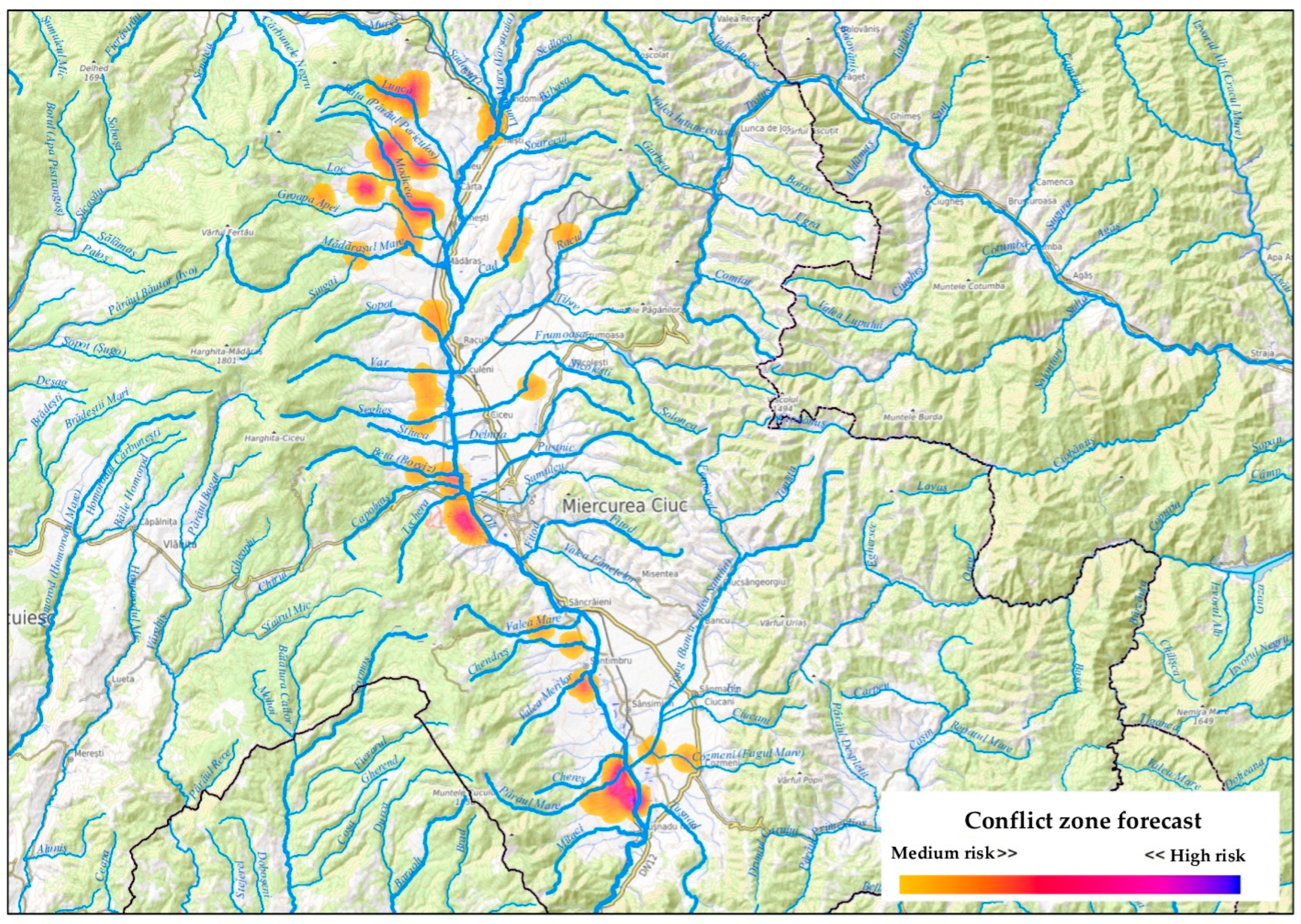
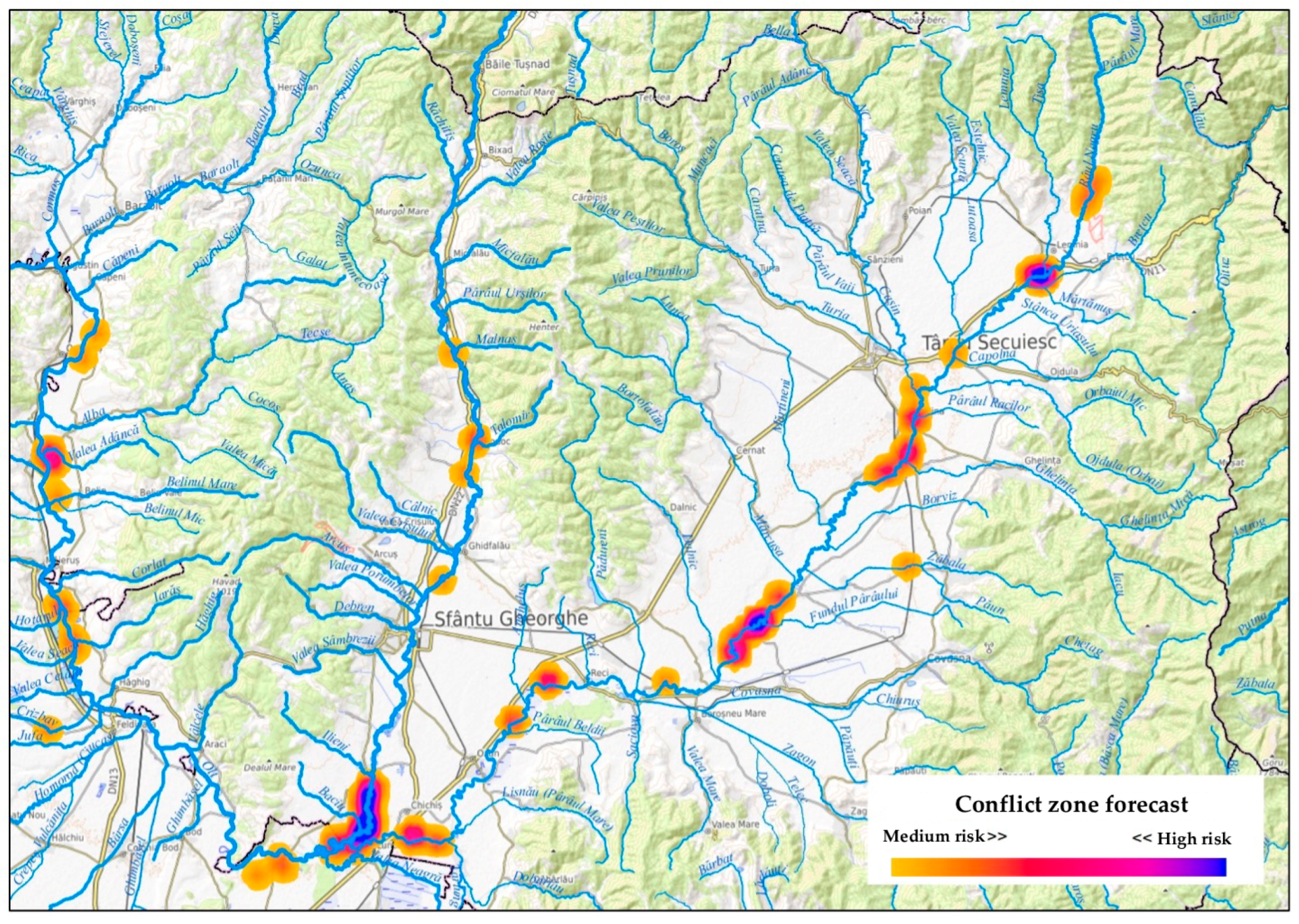
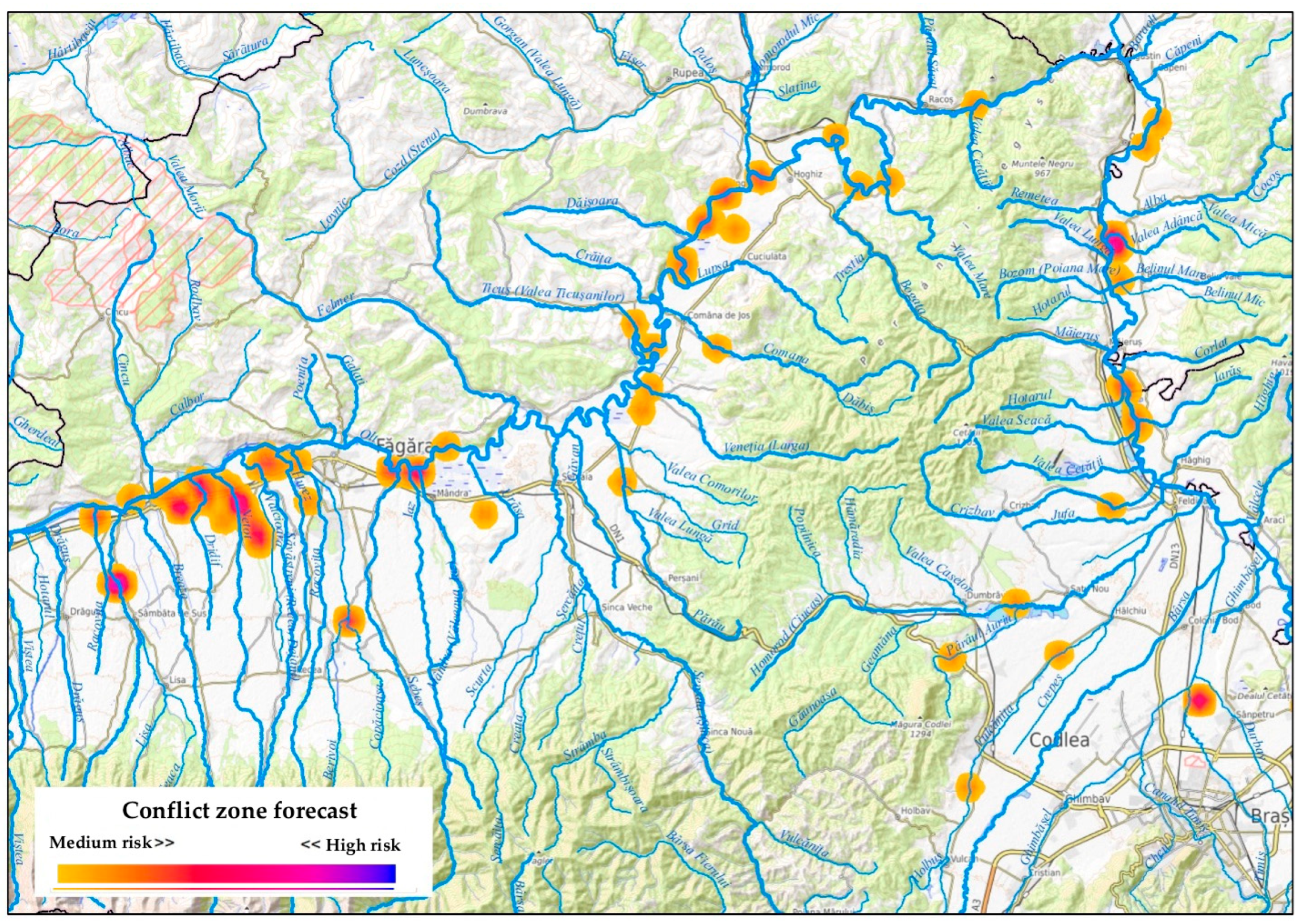
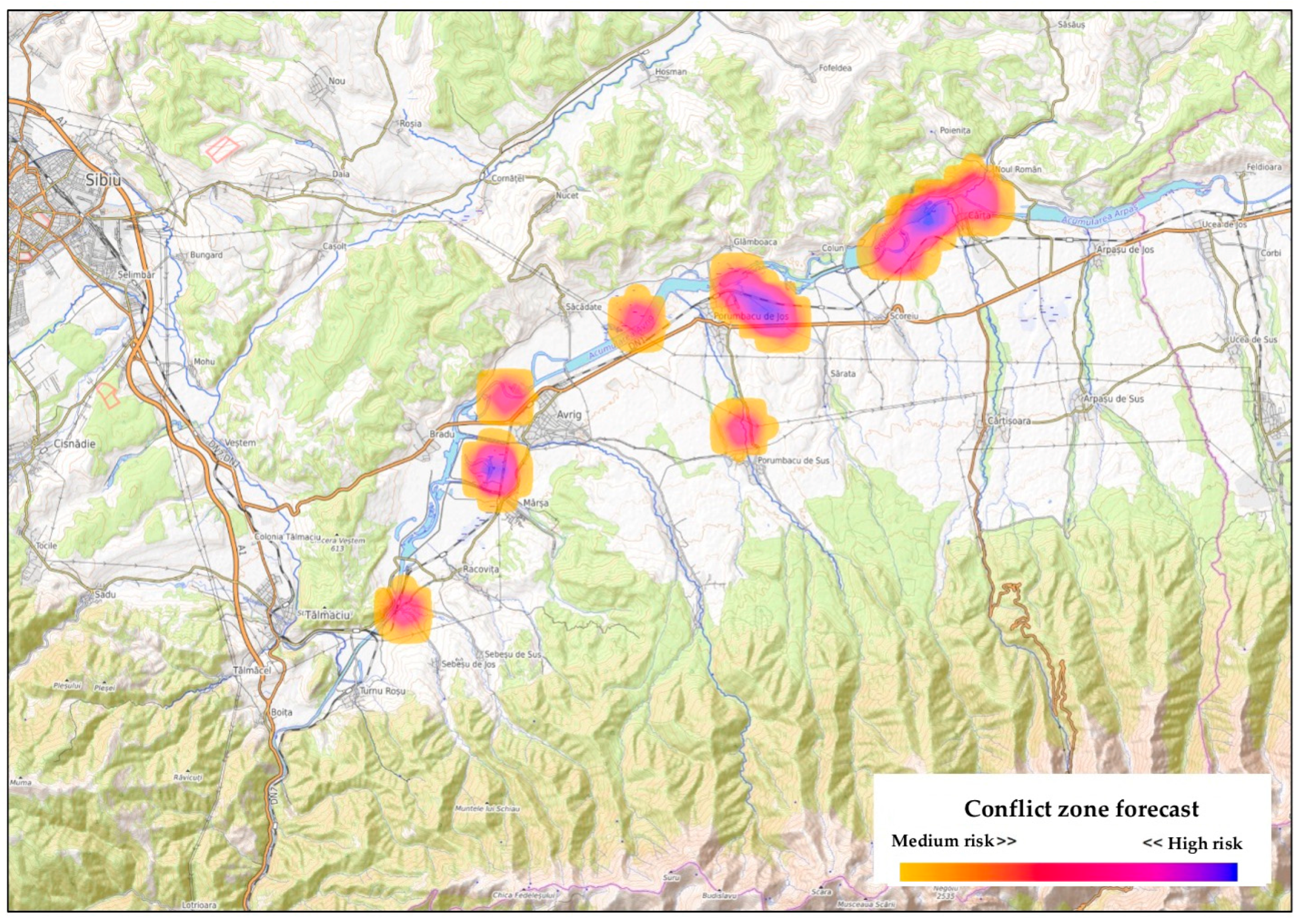
3.2. Institutional Reporting and Forecasted HWC Hotspots
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Izmailova, A.V.; Korneenkova, N.Y. Water Resources of Natural and Artificial Water Bodies in Europe. Water Resour. 2022, 49, 1–9. [Google Scholar] [CrossRef]
- Irfan, S.; Alatawi, A.M.M.; Irfan, S.; Alatawi, A.M.M. Aquatic Ecosystem and Biodiversity: A Review. Open J. Ecol. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Sánchez-Montoya, M.M.; Moleón, M.; Sánchez-Zapata, J.A.; Escoriza, D. The Biota of Intermittent and Ephemeral Rivers: Amphibians, Reptiles, Birds, and Mammals. In Intermittent Rivers and Ephemeral Streams: Ecology and Management; Academic Press: Cambridge, MA, USA, 2017; pp. 299–322. [Google Scholar]
- Glynnis, H. Semi-Aquatic Mammals: Ecology and Biology; Johns Hopkins University Press: Baltimore, MD, USA, 2020. [Google Scholar]
- Mortensen, R.M.; Reinhardt, S.; Hjønnevåg, M.E.; Wilson, R.P.; Rosell, F. Aquatic habitat use in a semi-aquatic mammal: The Eurasian beaver. Anim. Biotelemetry 2021, 9, 35. [Google Scholar] [CrossRef]
- Macdonald, S.M.; Mason, C.F. Some factors influencing the distribution of otters (Lutra lutra). Mamm. Rev. 1983, 13, 1–10. [Google Scholar] [CrossRef]
- Lister, A.M. Speciation and evolutionary trends in Quaternary vertebrates. In Reference Module in Earth Systems and Environmental Sciences; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Uehlinger, U.; Arndt, H.; Wantzen, K.M.; Leuven, R.S.E.W. The Rhine River Basin. In Rivers of Europe; Elsevier: Amsterdam, The Netherlands, 2009; pp. 199–245. [Google Scholar]
- Gethöffer, F.; Siebert, U. Current knowledge of the Neozoa, Nutria and Muskrat in Europe and their environmental impacts. J. Wildl. Biodivers. 2020, 4, 1–12. [Google Scholar]
- Irving, L. Aquatic Mammals. In Comparative Physiology of Thermoregulation; Whittow, G.C., Ed.; Academic Press: Cambridge, MA, USA, 1973; pp. 47–96. [Google Scholar]
- Council of the European Communities. Council Directive 92/43/EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora. Off. J. Eur. Community 1992, 206, 7–50. [Google Scholar]
- Nolet, B.A.; Rosell, F. Comeback of the beaver Castor fiber: An overview of old and new conservation problems. Biol. Conserv. 1998, 83, 165–173. [Google Scholar] [CrossRef]
- Burdock, G.A. Safety assessment of castoreum extract as a food ingredient. Int. J. Toxicol. 2007, 26, 51–55. [Google Scholar] [CrossRef]
- Treves, A.; Comino, E. A bibliometric literature review in beaver management: When does the beaver become a resource? Mamm. Rev. 2024, 54, 213–228. [Google Scholar] [CrossRef]
- Paderewski, J.; Sienkiewicz-Paderewska, D. Long-term dynamics, distribution, and protection of the Eurasian beaver Castor fiber in Poland. J. Water Land Dev. 2025, 155–162. [Google Scholar] [CrossRef]
- Nolet, B. Management of the Beaver (Castor Fiber): Towards Restoration of Its Former Distribution and Ecological Function in Europe; Council of Europe: Strasbourg, France, 1997. [Google Scholar]
- Campbell-Palmer, R.; Gow, D.; Campbell, R.; Dickinson, H.; Girling, S.; Gurnell, J.; Halley, D.; Jones, S.; Lisle, S.; Parker, H.; et al. The Eurasian Beaver Handbook; Pelagic Publishing: London, UK, 2016. [Google Scholar]
- Dijkstra, V.A.A. Reintroduction of the Beaver, Castor Fiber, in The Netherlands. In Beaver Protection, Management, and Utilization in Europe and North America; Springer: New York, NY, USA, 1999; pp. 15–16. [Google Scholar]
- Halley, D.J.; Jones, A.L.; Chesworth, S.; Hall, C.; Gow, D.; Jones-Parry, R.; Walsh, J. The Reintroduction of the Eurasian Beaver Castor Fiber to Wales: An Ecological Feasibility Study; NINA Report 457; Norsk Institutt for Naturforskning: Trondheim, Norway, 2015; 66p. [Google Scholar]
- Gaywood, M.J. Reintroducing the Eurasian beaver Castor fiber to Scotland. Mamm. Rev. 2018, 48, 48–61. [Google Scholar] [CrossRef]
- Kitchener, A.C.; Conroy, J.W.H. The history of the Eurasian Beaver Castor fiber in Scotland. Mamm. Rev. 1997, 27, 95–108. [Google Scholar] [CrossRef]
- Ionescu, G.; Ionescu, O.; Pasca, C.; Visan, D. Castorul in Romania; Editura Silvica: Bucharest, Romania, 2010. [Google Scholar]
- Ionescu, G.; Troidl, C. Beaver project Romania—A reintroduction with special focus on antropic factors. In Proceedings of the European Beaver Symposium, Bratislava, Slovakia, 15–19 September 1997. [Google Scholar]
- Thompson, S.; Vehkaoja, M.; Pellikka, J.; Nummi, P. Ecosystem services provided by beavers Castor spp. Mamm. Rev. 2021, 51, 25–39. [Google Scholar]
- Law, A.; Mclean, F.; Willby, N.J. Habitat engineering by beaver benefits aquatic biodiversity and ecosystem processes in agricultural streams. Freshw. Biol. 2016, 61, 486–499. [Google Scholar] [CrossRef]
- Lorimer, J. Worlding and weirding with beaver: A more-than-human political ecology of ecosystem engineering. Trans. Inst. Br. Geogr. 2025, 50, e12698. [Google Scholar] [CrossRef]
- Auster, R.E.; Barr, S.W.; Brazier, R.E. Beavers and flood alleviation: Human perspectives from downstream communities. J. Flood Risk Manag. 2022, 15, e12789. [Google Scholar] [CrossRef]
- Gridan, A.; Ionescu, O.; Ionescu, G.; Fedorca, A.; Ciocirlan, E.; Pașca, C.; Hardalau, D. The Ecological Impacts and Modeling of the Beaver Dam Distribution: A Study on Habitat Characteristics and Environmental Factors in Romania. Ecologies 2025, 6, 34. [Google Scholar] [CrossRef]
- Parker, J.D.; Caudill, C.C.; Hay, M.E. Beaver herbivory on aquatic plants. Oecologia 2007, 151, 616–625. [Google Scholar] [CrossRef] [PubMed]
- García-Silva, O.; Gallo-Reynoso, J.P.; Bucio-Pacheco, M.; Medrano-López, J.M.; Meza-Inostroza, P.M.; Grave-Partida, R.A.; García-Silva, O.; Gallo-Reynoso, J.P.; Bucio-Pacheco, M.; Medrano-López, J.M.; et al. Neotropical otter diet variation between a lentic and a lotic systems. Therya 2021, 12, 93–103. [Google Scholar] [CrossRef]
- Almeida, D.; Copp, G.H.; Masson, L.; Miranda, R.; Murai, M.; Sayer, C.D. Changes in the diet of a recovering Eurasian otter population between the 1970s and 2010. Aquat. Conserv. 2012, 22, 26–35. [Google Scholar] [CrossRef]
- Conroy, J.W.H.; Yoxon, P.; Gutleb, A.C. The status of the Eurasian otter (Lutra lutra) in Europe—A review. In Proceedings of the First Otter Toxicology Conference No 1, Skye, Scotland, 21–25 September 2000. [Google Scholar]
- Rehbein, M. From Historical Archives to Algorithms: Reconstructing Biodiversity Patterns in 19th Century Bavaria. Diversity 2025, 17, 315. [Google Scholar] [CrossRef]
- Manzoor, M.; Bhat, K.A.; Khurshid, N.; Yatoo, A.M.; Zaheen, Z.; Ali, S.; Ali, M.N.; Amin, I.; Mir, M.U.R.; Rashid, S.M.; et al. Bio-Indicator Species and Their Role in Monitoring Water Pollution. In Freshwater Pollution and Aquatic Ecosystems; Apple Academic Press: New York, NY, USA, 2021; pp. 321–347. [Google Scholar]
- Lee, C.; Luan, X. A Review on Eurasian Otters in Urban Areas: Principles for the Enhancement of Biodiversity. Diversity 2025, 17, 356. [Google Scholar] [CrossRef]
- Yoxon, P.; Yoxon, B. Eurasian otter (Lutra lutra): A Review of the current world status. J. Int. Otter Surviv. Fund 2019, 5, 53–73. [Google Scholar]
- Roos, A.; Loy, A.; de Silva, P.; Hajkova, P.; Zemanova, B. Lutra lutra. The IUCN Red List of Threatened Species. June 2015. Available online: https://www.iucnredlist.org/species/12419/21935287 (accessed on 5 June 2019).
- Bifolchi, A.; Lodé, T. Efficiency of conservation shortcuts: An investigation with otters as umbrella species. Biol. Conserv. 2005, 126, 523–527. [Google Scholar] [CrossRef]
- Koelewijn, H.P.; Pérez-Haro, M.; Jansman, H.A.H.; Boerwinkel, M.C.; Bovenschen, J.; Lammertsma, D.R.; Niewold, F.J.J.; Kuiters, A.T. The reintroduction of the Eurasian otter (Lutra lutra) into the Netherlands: Hidden life revealed by noninvasive genetic monitoring. Conserv. Genet. 2010, 11, 601–614. [Google Scholar] [CrossRef]
- Saavedra Bendito, D. Reintroduction of the Eurasian otter (Lutra lutra) in Muga and Fluvià basins (north-eastern Spain): Viability, development, monitoring and trends of the new population. Ph.D. Thesis, University of Girona, Girona, Spain, June 2003; p. 218. [Google Scholar]
- Santos-Reis, M.; Santos, R.; Antunes, P.; Sales-Luís, T.; Gomes, J.; Freitas, D.; Madruga, L. Reconciliation of the Conflict Between Otters and Fish Farmers. In Environmental Science and Engineering; Springer: Berlin/Heidelberg, Germany, 2013; pp. 49–79. [Google Scholar]
- Vaclavikova, M.; Vaclavik, T.; Kostkan, V. Otters vs. fishermen: Stakeholders’ perceptions of otter predation and damage compensation in the Czech Republic. J. Nat. Conserv. 2011, 19, 95–102. [Google Scholar] [CrossRef]
- Kloskowski, J. Otter Lutra lutra damage at farmed fisheries in southeastern Poland, I: An interview survey. Wildl. Biol. 2005, 11, 201–206. [Google Scholar] [CrossRef]
- Hohm, M.; Moesch, S.S.; Bahm, J.; Haase, D.; Jeschke, J.M.; Balkenhol, N. Reintroduced, but not accepted: Stakeholder perceptions of beavers in Germany. People Nat. 2024, 6, 1681–1695. [Google Scholar] [CrossRef]
- Auster, R.E.; Barr, S.W.; Brazier, R.E. Improving engagement in managing reintroduction conflicts: Learning from beaver reintroduction. J. Environ. Plan. Manag. 2020, 64, 1713–1734. [Google Scholar] [CrossRef]
- Viviano, A.; Auster, R.E.; Mazza, G.; Lagrotteria, A.; Pucci, C.; Senserini, D.; Campbell-Palmer, R.; Needham, R.; Curci, D.; Mori, E. Eurasian beavers in Central Italy: Perceptions in the local community. Naturwissenschaften 2023, 110, 30. [Google Scholar] [CrossRef] [PubMed]
- Holmes, G.; Rowland, G.; Fox, K. Eager about beavers? Understanding opposition to species reintroduction, and its implications for conservation. People Nat. 2024, 6, 1524–1537. [Google Scholar] [CrossRef]
- Freitas, D.; Gomes, J.; Luis, T.S.; Madruga, L.; Marques, C.; Baptista, G.; Rosalino, L.M.; Antunes, P.; Santos, R.; Santos-Reis, M. Otters and fish farms in the Sado estuary: Ecological and socio-economic basis of a conflict. Hydrobiologia 2007, 587, 51–62. [Google Scholar] [CrossRef]
- Špur, N.; Gomboc, K.Ž.; Šorgo, A. Public acceptability of measures to prevent from predation on commercial fish by the endangered Eurasian otter (Lutra lutra) in Natura 2000. J. Nat. Conserv. 2018, 44, 21–32. [Google Scholar] [CrossRef]
- Morzillo, A.T.; Needham, M.D. Landowner Incentives and Normative Tolerances for Managing Beaver Impacts. Hum. Dimens. Wildl. 2015, 20, 514–530. [Google Scholar] [CrossRef]
- Taylor, J.D.; Singleton, R.D. The evolution of flow devices used to reduce flooding by beavers: A review. Wildl. Soc. Bull. 2014, 38, 127–133. [Google Scholar] [CrossRef]
- Ulicsni, V.; Babai, D.; Juhász, E.; Molnár, Z.; Biró, M. Local knowledge about a newly reintroduced, rapidly spreading species (Eurasian beaver) and perception of its impact on ecosystem services. PLoS ONE 2020, 15, e0233506. [Google Scholar] [CrossRef]
- Janiszewski, P.; Hermanowska, Z. Damage Caused by the European Beaver (Castor Fiber L.) in Agricul-tural and Forest Farms in View of Selected Atmospheric Factors and Animal Behavior. Appl. Ecol. Environ. Res. 2019, 17, 15633–15642. [Google Scholar] [CrossRef]
- Graham, H.A.; Puttock, A.; Macfarlane, W.W.; Wheaton, J.M.; Gilbert, J.T.; Campbell-Palmer, R.; Elliott, M.; Gaywood, M.J.; Anderson, K.; Brazier, R.E. Modelling Eurasian beaver foraging habitat and dam suitability, for predicting the location and number of dams throughout catchments in Great Britain. Eur. J. Wildl. Res. 2020, 66, 42. [Google Scholar] [CrossRef]
- Maringer, A.; Slotta-Bachmayr, L. A GIS-based habitat-suitability model as a tool for the management of beavers Castor fiber. Acta Theriol. 2006, 51, 373–382. [Google Scholar] [CrossRef]
- Hardalau, D.; Codrean, C.; Iordache, D.; Fedorca, M.; Ionescu, O. The Expanding Thread of Ungulate Browsing—A Review of Forest Ecosystem Effects and Management Approaches in Europe. Forests 2024, 15, 1311. [Google Scholar] [CrossRef]
- Hardalau, D.; Fedorca, M.; Popovici, D.-C.; Ionescu, G.; Fedorca, A.; Mirea, I.; Daniel, I.; Ionescu, O. Insights in Managing Ungulates Population and Forest Sustainability in Romania. Diversity 2025, 17, 194. [Google Scholar] [CrossRef]
- Anthony, B.; Moldovan, D. Poised for engagement? Local communities and Măcin Mountains National Park, Romania. Int. J. Biodivers. Sci. Manag. 2008, 4, 230241. [Google Scholar] [CrossRef]
- Popovici, D.C.; Ionescu, O.; Ionescu, G.; Hardalau, D. Two Decades of Expansion: Population Dynamics and Spatial Distribution of the Golden Jackal in Romania between 2004–2025. Bull. Transilv. Univ. Brasov. Ser. II For. Wood Ind. Agric. Food Eng. 2025, 18, 41–54. [Google Scholar] [CrossRef]
- Stăncioiu, P.T.; Dutcă, I.; Bălăcescu, M.C.; Ungurean, Ş.V. Coexistence with Bears in Romania: A Local Community Perspective. Sustainability 2019, 11, 7167. [Google Scholar] [CrossRef]
- Cimpoca, A.; Voiculescu, M. Patterns of Human–Brown Bear Conflict in the Urban Area of Brașov, Romania. Sustainability 2022, 14, 7833. [Google Scholar] [CrossRef]
- Salvatori, V.; Mertends, A. Damage prevention methods in Europe: Experiences from LIFE nature projects. Assoc. Teriologica Ital. 2012, 23, 73–79. [Google Scholar]
- Geacu, S. Damage incurred by the big carnivorous mammals on Romania territory over 1940–1950. Revista de Silvicultură și Cinegetică 2017, 22, 98–102. [Google Scholar]
- Anthony, B.P.; Szabo, A. Protected Areas: Conservation Cornerstones or Paradoxes? Insights from Human-Wildlife Conflicts in Africa and Southeastern Europe. In The Importance of Biological Interactions in the Study of Biodiversity; IntechOpen: London, UK, 2011. [Google Scholar]
- Treves, A.; Wallace, R.B.; Naughton-Treves, L.; Morales, A. Co-Managing Human-Wildlife Conflicts: A Review. Hum. Dimens. Wildl. 2006, 11, 383–396. [Google Scholar] [CrossRef]
- Nyhus, P.J. Human-Wildlife Conflict and Coexistence. Annu. Rev. Environ. Resour. 2016, 41, 143–171. [Google Scholar] [CrossRef]
- König, H.J.; Kiffner, C.; Kramer-Schadt, S.; Fürst, C.; Keuling, O.; Ford, A.T. Human-Wildlife coexistence in a changing world. Conserv. Biol. 2020, 34, 786–794. [Google Scholar] [CrossRef]
- Patterson, M.E.; Montag, J.M.; Williams, D.R. The urbanization of wildlife management: Social science, conflict, and decision making. Urban For. Urban Green. 2003, 1, 171–183. [Google Scholar] [CrossRef]
- Liordos, V.; Kontsiotis, V.J.; Georgari, M.; Baltzi, K.; Baltzi, I. Public acceptance of management methods under different Human-Wildlife conflict scenarios. Sci. Total Environ. 2017, 579, 685–693. [Google Scholar] [CrossRef]
- Oliveira, S.; Buckley, P.; Consorte-McCrea, A. A glimpse of the long view: Human attitudes to an established population of Eurasian beaver (castor fiber) in the lowlands of south-east England. Front. Conserv. Sci. 2022, 3, 925594. [Google Scholar] [CrossRef]
- Li, M.; Jiang, W.; Li, B.; Butt, N. Social and cultural aspects of Human-Wildlife conflicts: Understanding people’s attitudes to crop-raiding animals and other wildlife in agricultural systems of the Tibetan Plateau. Integr. Conserv. 2023, 2, 214–225. [Google Scholar] [CrossRef]
- Seddon, P.J.; van Heezik, Y. Reintroductions to ‘Ratchet Up’ Public Perceptions of Biodiversity. In Ignoring Nature No More; University of Chicago Press: Chicago, IL, USA, 2015; pp. 137–152. [Google Scholar]
- Hermann, N.; Menzel, S. Threat Perception and Attitudes of Adolescents Towards Re-Introduced Wild Animals: A qualitative study of young learners from affected regions in Germany. Int. J. Sci. Educ. 2013, 35, 3062–3094. [Google Scholar] [CrossRef]
- Ravenelle, J.; Nyhus, P.J. Global patterns and trends in Human-Wildlife conflict compensation. Conserv. Biol. 2017, 31, 1247–1256. [Google Scholar] [CrossRef]
- Dickman, A.J.; Macdonald, E.A.; Macdonald, D.W. A review of financial instruments to pay for predator conservation and encourage human-carnivore coexistence. Proc. Natl. Acad. Sci. USA 2011, 108, 13937–13944. [Google Scholar] [CrossRef] [PubMed]
- Mikulka, O.; Homolka, M.; Drimaj, J.; Kamler, J. European beaver (Castor fiber) in open agricultural landscapes: Crop grazing and the potential for economic damage. Eur. J. Wildl. Res. 2020, 66, 101. [Google Scholar] [CrossRef]
- Lodberg-Holm, H.K.; Garvik, E.S.; Fountain, M.S.; Reinhardt, S.; Rosell, F. Crop circles revealed spatio-temporal patterns of beaver foraging on cereal fields. Agric. Ecosyst. Environ. 2022, 337, 108066. [Google Scholar] [CrossRef]
- Hood, G.A.; Manaloor, V.; Dzioba, B. Mitigating infrastructure loss from beaver flooding: A cost–benefit analysis. Hum. Dimens. Wildl. 2018, 23, 146–159. [Google Scholar] [CrossRef]
- Liordos, V.; Kontsiotis, V.J.; Nevolianis, C.; Nikolopoulou, C.E. Stakeholder preferences and consensus associated with managing an endangered aquatic predator: The Eurasian otter (Lutra lutra). Hum. Dimens. Wildl. 2019, 24, 446–462. [Google Scholar] [CrossRef]
- Digun-Aweto, O.; Van Der Merwe, P.; Saayman, M. Tolerance factors in human-wildlife conflicts in protected areas: The case of Cross River National Park, Cross River State Nigeria. GeoJournal 2022, 87, 349–361. [Google Scholar] [CrossRef]
- Liordos, V.; Kontsiotis, V.J.; Anastasiadou, M.; Karavasias, E. Effects of attitudes and demography on public support for endangered species conservation. Sci. Total Environ. 2017, 595, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Newsom, A.; Lozano, J.; Martín-López, B. Social perceptions of carnivores across the globe—A literature review. Hum. Dimens. Wildl. 2025. [Google Scholar] [CrossRef]
- Kleiven, J.; Bjerke, T.; Kaltenborn, B.P. Factors influencing the social acceptability of large carnivore behaviours. Biodivers. Conserv. 2004, 13, 1647–1658. [Google Scholar] [CrossRef]
- Kansky, R.; Kidd, M.; Knight, A.T. A wildlife tolerance model and case study for understanding human wildlife conflicts. Biol. Conserv. 2016, 201, 137–145. [Google Scholar] [CrossRef]
- Kosko, B. Fuzzy cognitive maps. Int. J. Man. Mach. Stud. 1986, 24, 65–75. [Google Scholar] [CrossRef]
- Larsen, A.; Larsen, J.R.; Lane, S.N. Dam builders and their works: Beaver influences on the structure and function of river corridor hydrology, geomorphology, biogeochemistry and ecosystems. Earth. Sci. Rev. 2021, 218, 103623. [Google Scholar] [CrossRef]
- Serenari, C. Beyond Tolerance: Mitigating Human-Wildlife Conflict with Hospitality. Animals 2024, 14, 1185. [Google Scholar] [CrossRef]
- Zhu, D.; Yu, S. Building Ecosystems: The Transformative Role of Beavers. Biol. Evid. 2024, 14, 172–183. [Google Scholar] [CrossRef]
- Narváez, M.; Cabezas, S.; Blanco-Garrido, F.; Baos, R.; Clavero, M.; Delibes, M. Eurasian otter (Lutra lutra) diet as an early indicator of recovery in defaunated river communities. Ecol. Indic. 2020, 117, 106547. [Google Scholar] [CrossRef]
- Bedford, S.J. The effects of riparian habitat quality and biological water quality on the European Otter (Lutra lutra) in Devon. Biosci. Horiz. Int. J. Stud. Res. 2009, 2, 125–133. [Google Scholar] [CrossRef]
- Fletcher, R.; Massarella, K.; Ferraz, K.M.P.M.B.; Kiwango, W.A.; Komi, S.; Mabele, M.B.; Marchini, S.; Nygren, A.; Sandroni, L.T.; Alagona, P.S.; et al. The production-protection nexus: How political-economic processes influence prospects for transformative change in human-wildlife interactions. Glob. Environ. Change 2023, 82, 102723. [Google Scholar] [CrossRef]
- Bruggeman, D.J.; Jones, M.L.; Lupi, F.; Scribner, K.T. Landscape Equivalency Analysis: Methodology for estimating spatially explicit biodiversity credits. Environ. Manag. 2005, 36, 518–534. [Google Scholar] [CrossRef]
- Laramie, H.A. A Device for Control of Problem Beavers. J. Wildl. Manag. 1963, 27, 471. [Google Scholar] [CrossRef]
- Simon, L.J.; Org, E. Solving Beaver Flooding Problems through the Use of Water Flow Control Devices. Proc. Vertebr. Pest Conf. 2006, 22, 22. [Google Scholar] [CrossRef]
- Sales-Luís, T.; Freitas, D.; Santos-Reis, M. Key landscape factors for Eurasian otter Lutra lutra visiting rates and fish loss in estuarine fish farms. Eur. J. Wildl. Res. 2009, 55, 345–355. [Google Scholar] [CrossRef]
- Miller, J.R.B. Mapping attack hotspots to mitigate human–carnivore conflict: Approaches and applications of spatial predation risk modeling. Biodivers. Conserv. 2015, 24, 2887–2911. [Google Scholar] [CrossRef]
- Spencer, R.D.; Beausoleil, R.A.; Martorello, D.A. How Agencies Respond to Human–black Bear Conflicts: A Survey of Wildlife Agencies in North America. Ursus 2007, 18, 217–229. [Google Scholar] [CrossRef]
- Madden, F.; McQuinn, B. Conservation’s blind spot: The case for conflict transformation in wildlife conservation. Biol. Conserv. 2014, 178, 97–106. [Google Scholar] [CrossRef]
- Quansah, J.E.; Engel, B.; Rochon, G.L. Early Warning Systems: A Review. J. Terr. Obs. 2010, 2, 2. [Google Scholar]
- Trouwborst, A. Conserving European Biodiversity in a Changing Climate: The Bern Convention, the European Union Birds and Habitats Directives and the Adaptation of Nature to Climate Change. Rev. Eur. Community Int. Environ. Law 2011, 20, 62–77. [Google Scholar] [CrossRef]
- Pasca, C.; Ionescu, G.; Fedorca, A.; Jurj, R.; Sirbu, G.; Popa, M.; Gridan, A.; Ionescu, O. Beaver management in Romania between challenges and perspectives. The National Action Plan for the Conservation of the Eurasian beaver population (Castor fiber L.)—Synthesis. Rev. Silv. Cinegetică 2022, 50, 109–118. [Google Scholar]
- Hofmann, D.D.; Behr, D.M.; McNutt, J.W.; Ozgul, A.; Cozzi, G. Bound within boundaries: Do protected areas cover movement corridors of their most mobile, protected species? J. Appl. Ecol. 2021, 58, 1133–1144. [Google Scholar] [CrossRef]
- Wikar, Z.; Ciechanowski, M. Beaver Dams and Fallen Trees as Ecological Corridors Allowing Movements of Mammals across Water Barriers—A Case Study with the Application of Novel Substrate for Tracking Tunnels. Animals 2023, 13, 1302. [Google Scholar] [CrossRef]
- Vaske, J.J.; Carlos, A.W.D.; Manfredo, M.J.; Teel, T.L. Evaluating alternative survey methodologies in human dimensions of wildlife research. Hum. Dimens. Wildl. 2023, 28, 320–334. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gridan, A.; Pașca, C.; Ionescu, G.; Sîrbu, G.; Spătaru, C.; Ionescu, O.; Hardalau, D. Spatial Forecasting and Social Acceptance of Human-Wildlife Conflicts Involving Semi-Aquatic Species in Romania. Diversity 2025, 17, 559. https://doi.org/10.3390/d17080559
Gridan A, Pașca C, Ionescu G, Sîrbu G, Spătaru C, Ionescu O, Hardalau D. Spatial Forecasting and Social Acceptance of Human-Wildlife Conflicts Involving Semi-Aquatic Species in Romania. Diversity. 2025; 17(8):559. https://doi.org/10.3390/d17080559
Chicago/Turabian StyleGridan, Alexandru, Claudiu Pașca, Georgeta Ionescu, George Sîrbu, Cezar Spătaru, Ovidiu Ionescu, and Darius Hardalau. 2025. "Spatial Forecasting and Social Acceptance of Human-Wildlife Conflicts Involving Semi-Aquatic Species in Romania" Diversity 17, no. 8: 559. https://doi.org/10.3390/d17080559
APA StyleGridan, A., Pașca, C., Ionescu, G., Sîrbu, G., Spătaru, C., Ionescu, O., & Hardalau, D. (2025). Spatial Forecasting and Social Acceptance of Human-Wildlife Conflicts Involving Semi-Aquatic Species in Romania. Diversity, 17(8), 559. https://doi.org/10.3390/d17080559








