Predicting Range Shifts in the Distribution of Arctic/Boreal Plant Species Under Climate Change Scenarios
Abstract
1. Introduction
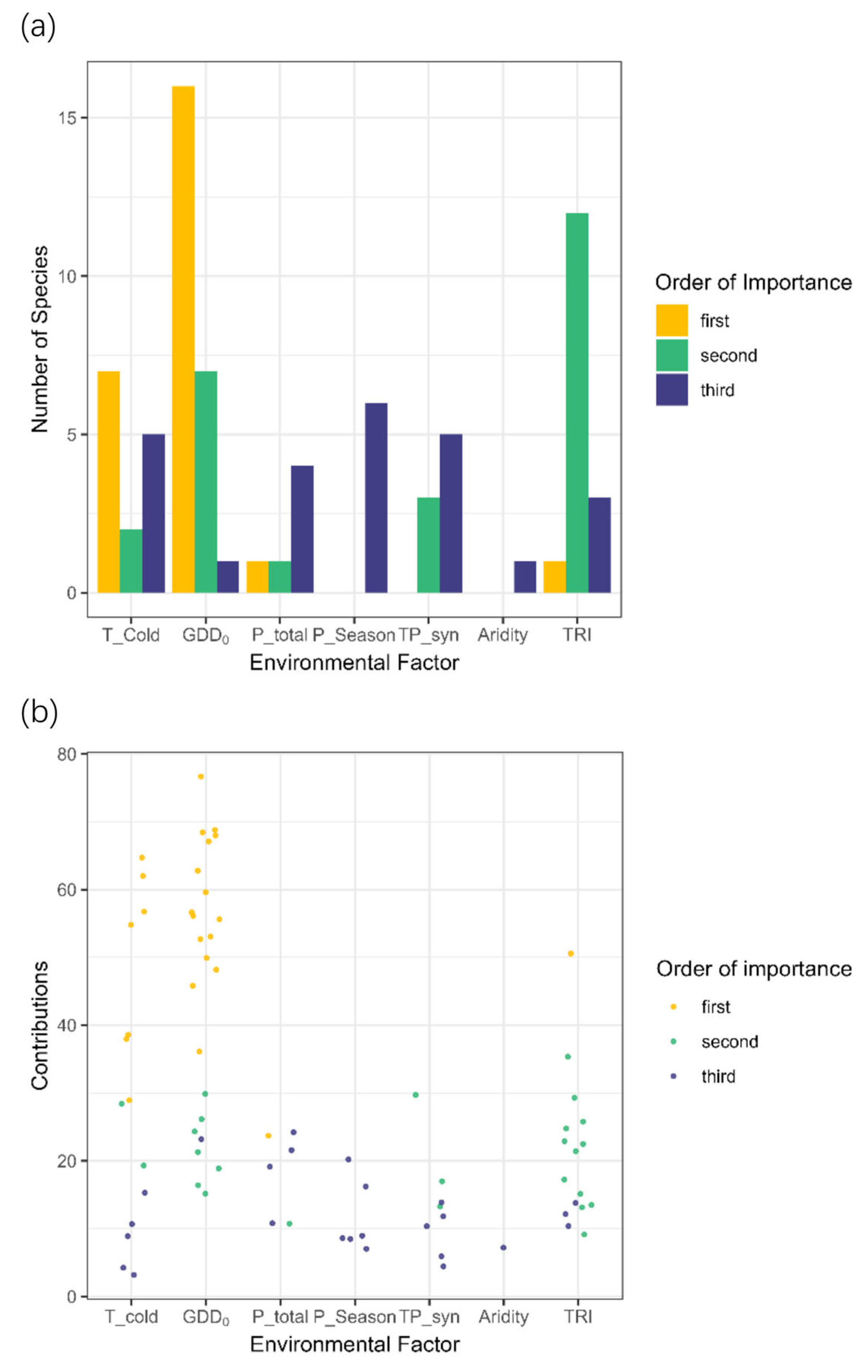
- What are the major environmental factors governing the distribution of focal plants, and thereby influencing their future range shifting responses?
- How do different SSPs play pivotal roles in determining the fates of these plants in future climate scenarios?
- Are Arctic plants (A_Spps) more vulnerable than Boreal plants (B_Spps) in general, as widely hypothesized, in changing climate scenarios?
- Are there general patterns in the timing of rapid range shift stages for Arctic/Boreal plants?
- Where are the possible climate refugia for species that are critically endangered as a result of climate change?
2. Methods
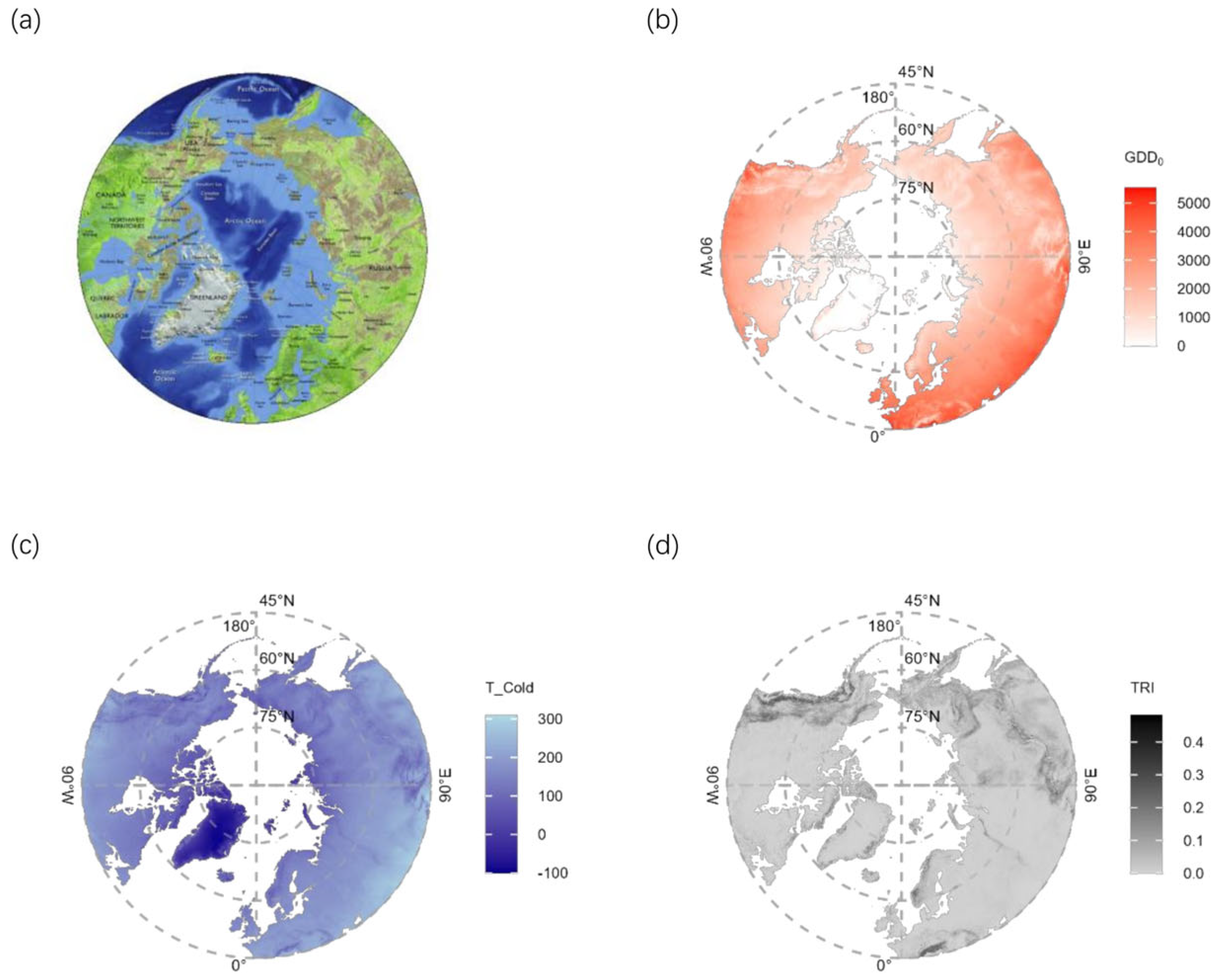
2.1. Research Area
2.2. Species Selection and Occurrence Data Preparation
2.3. BioPlantPolar Dataset Preparation
2.4. Model Selection
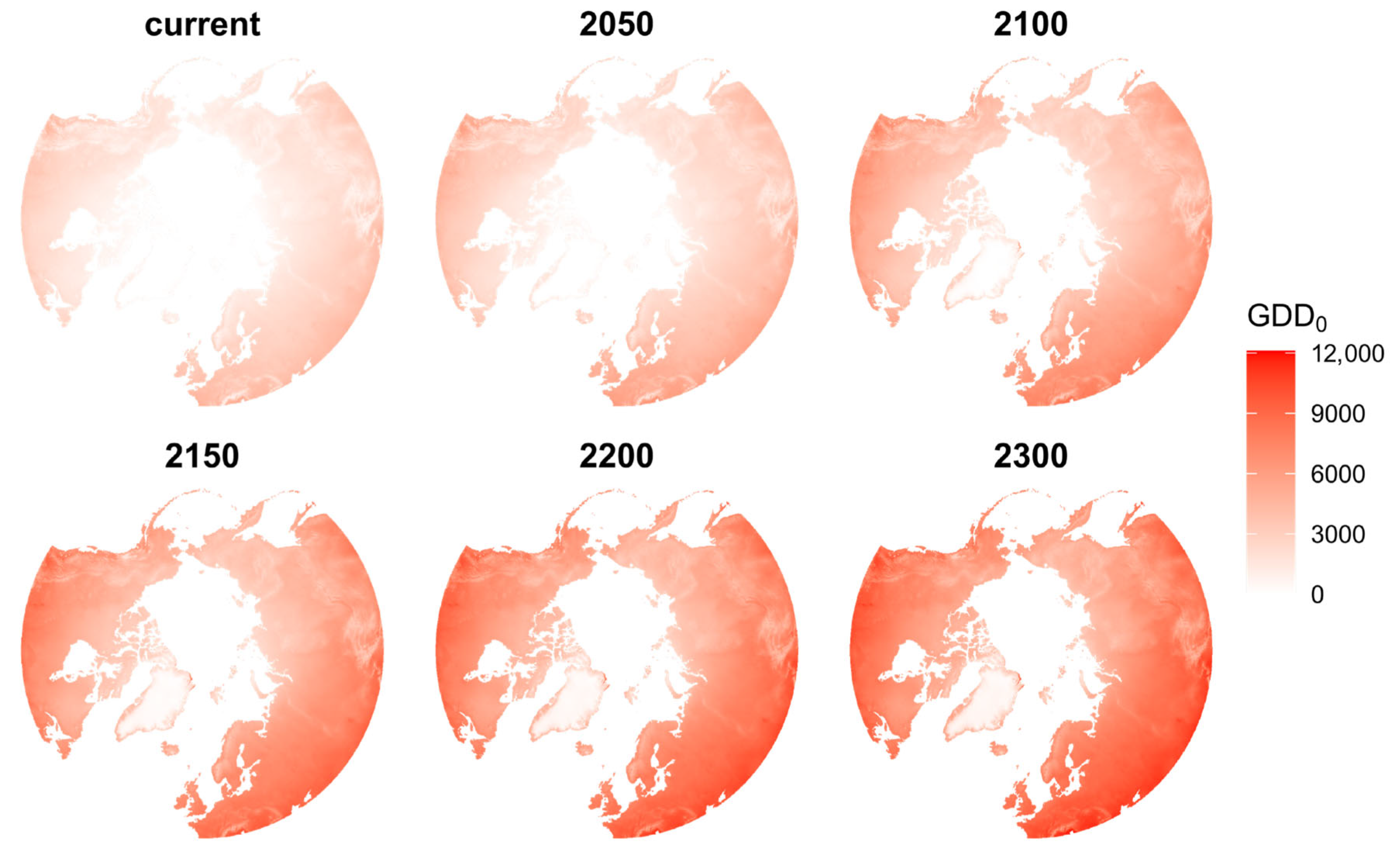
2.5. Species Suitability Projections
2.6. Range Shift Indices Calculation and Summarization
3. Results
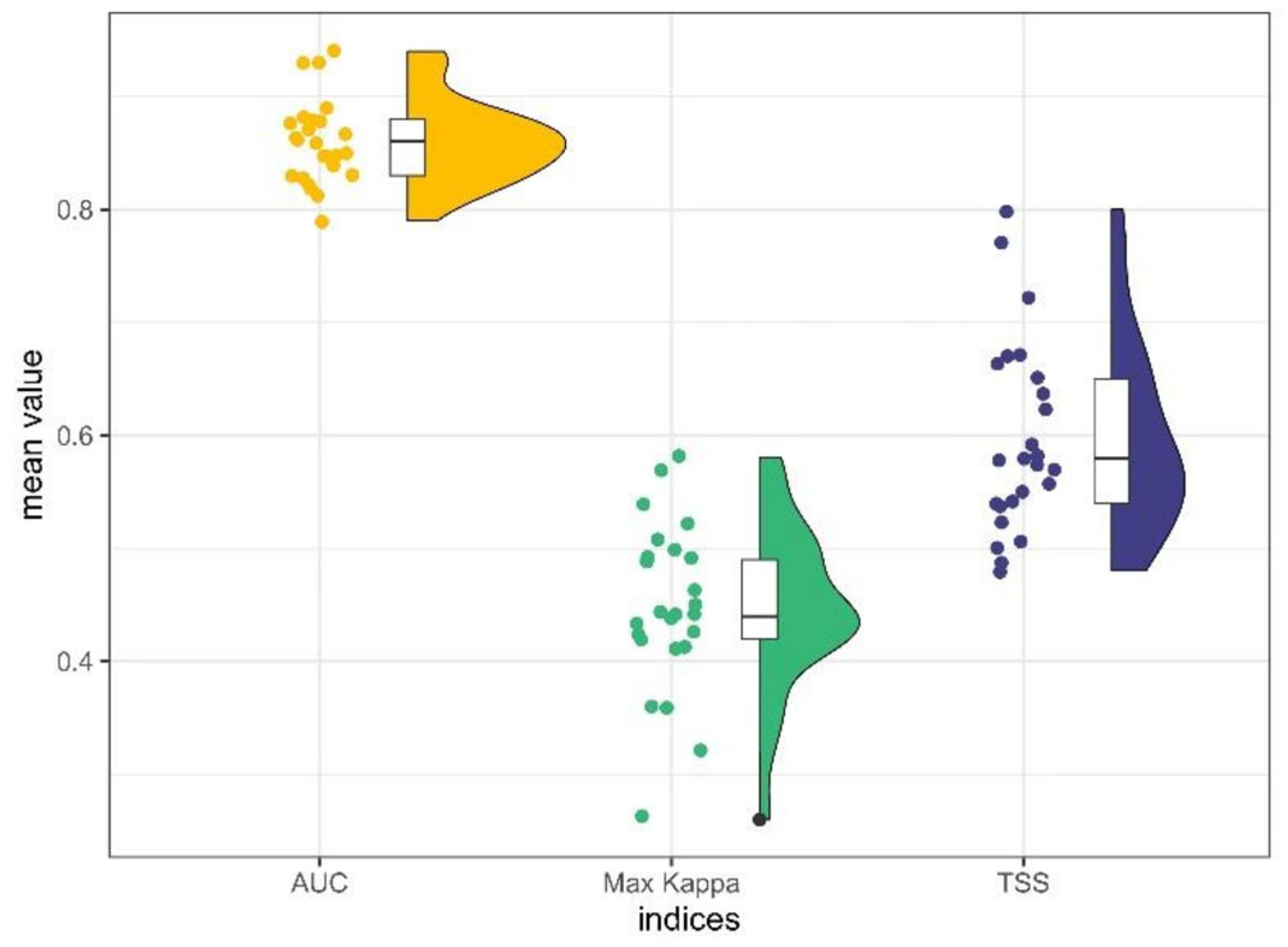
3.1. Model Selection and Performances
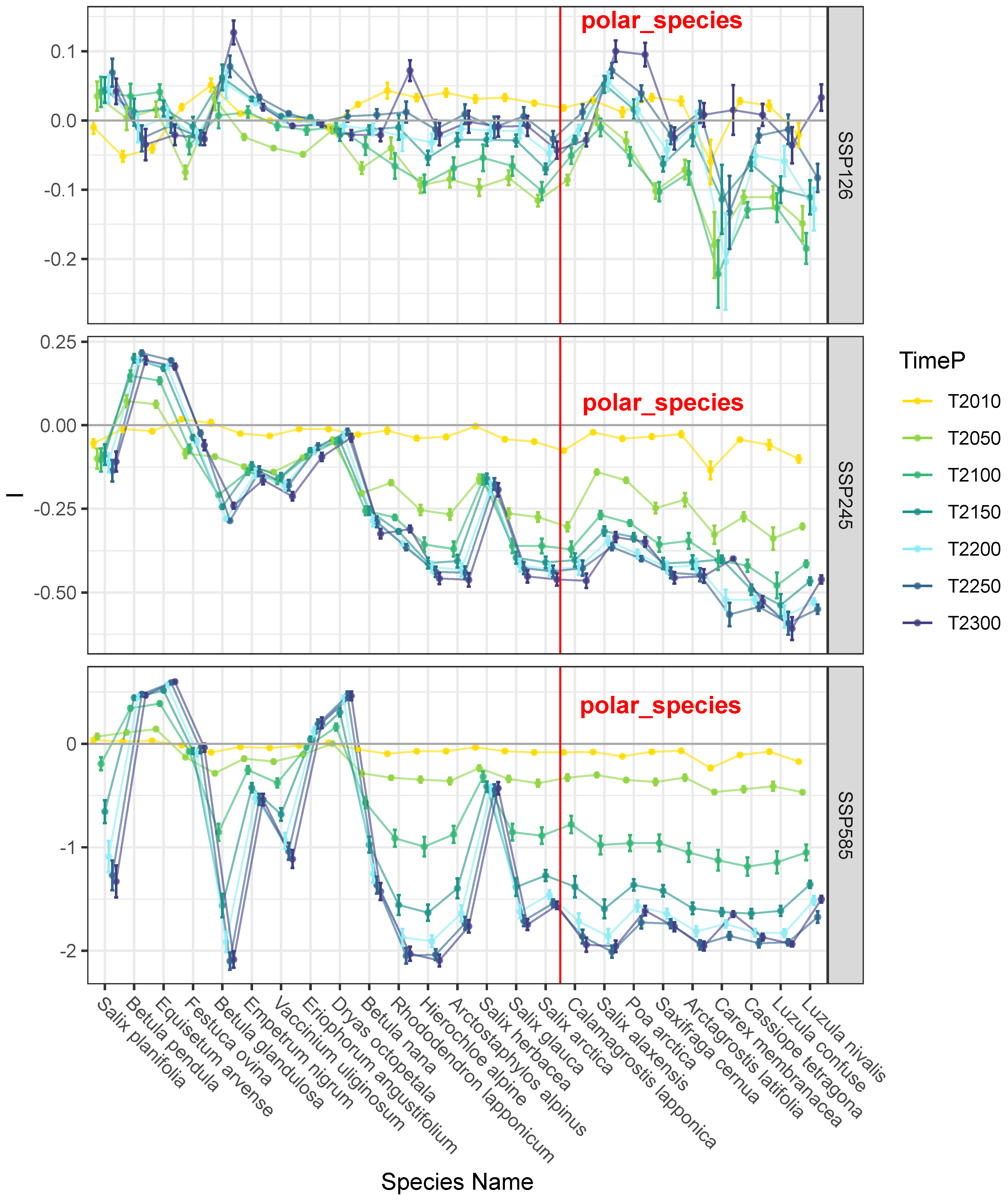
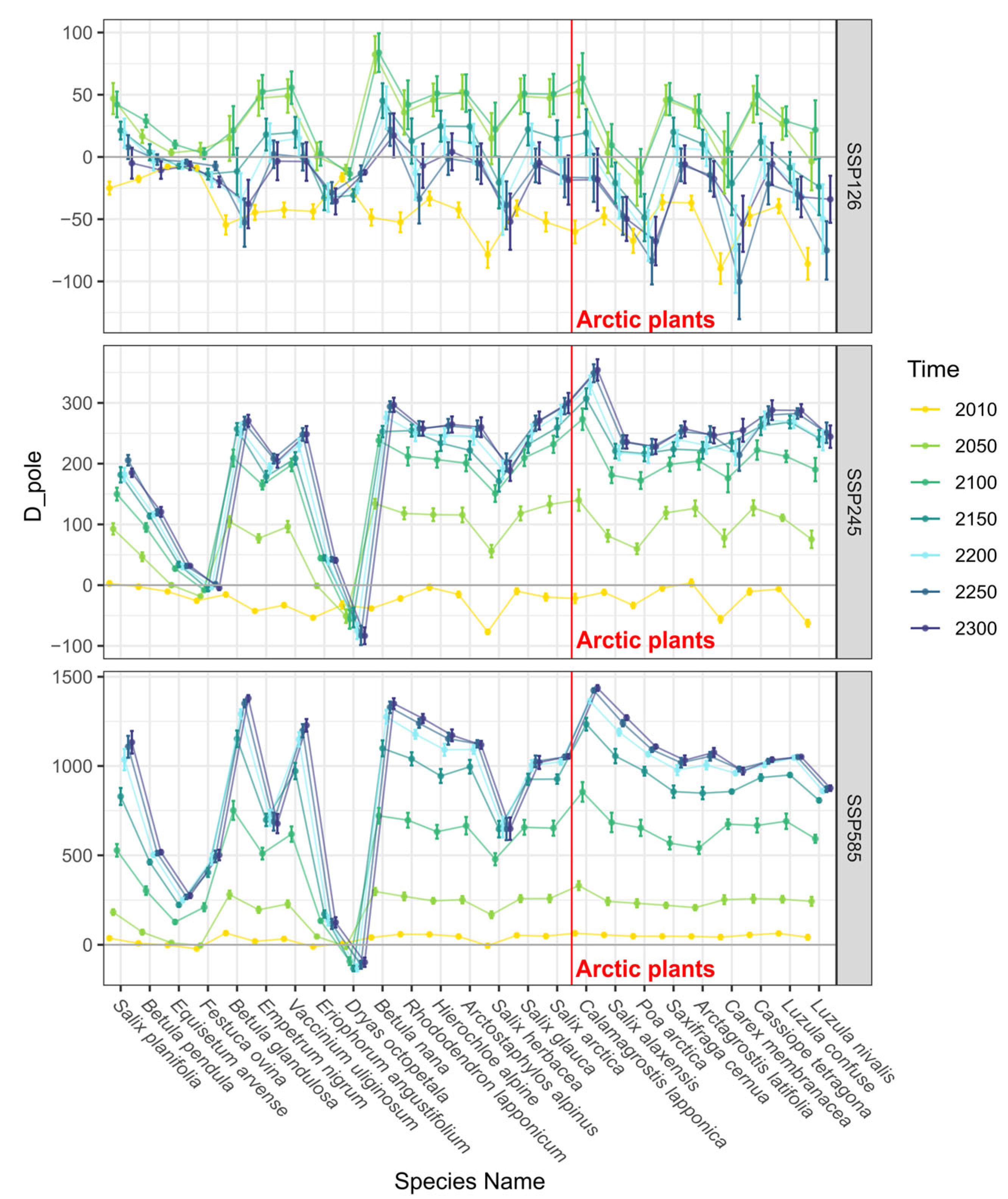
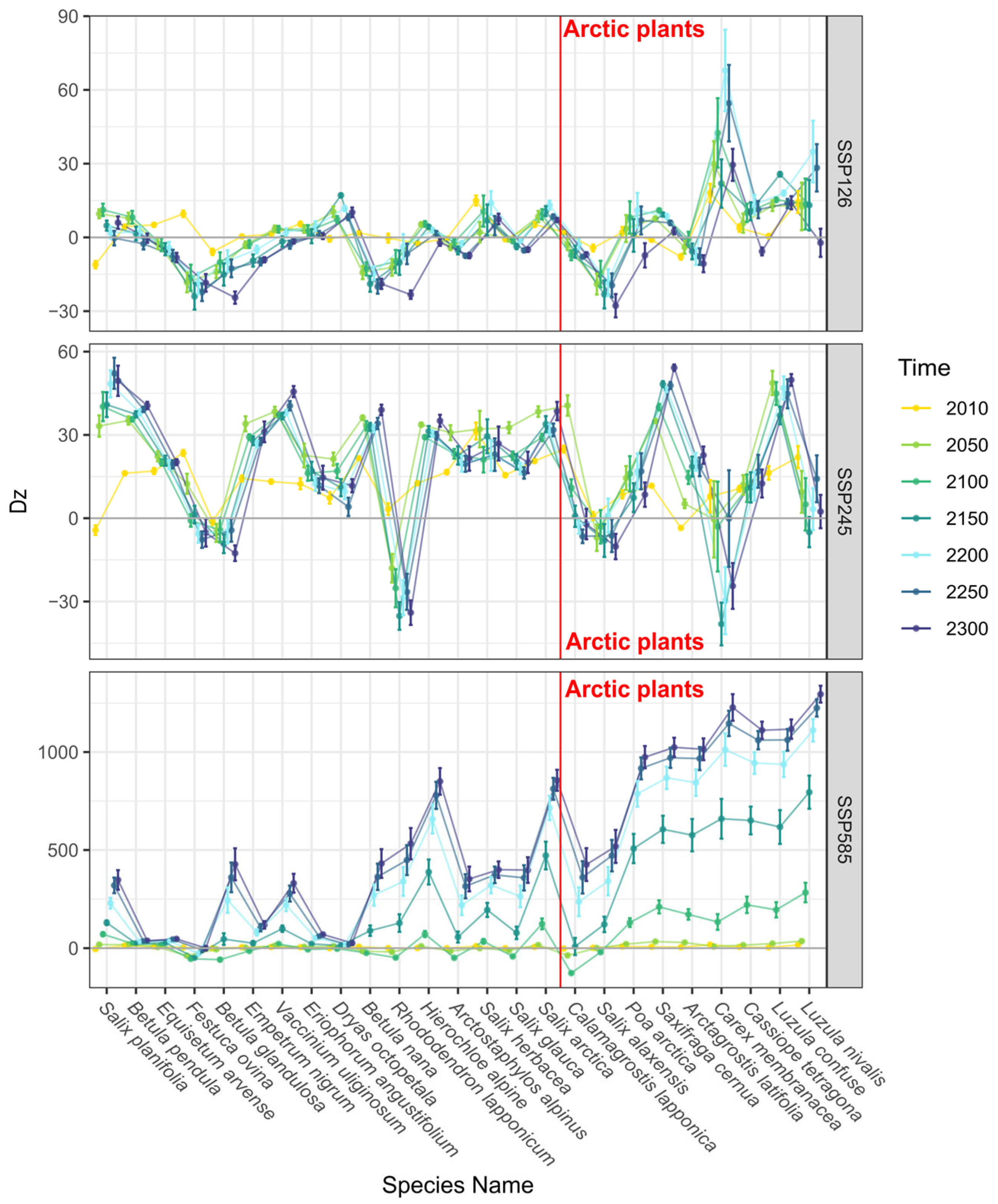
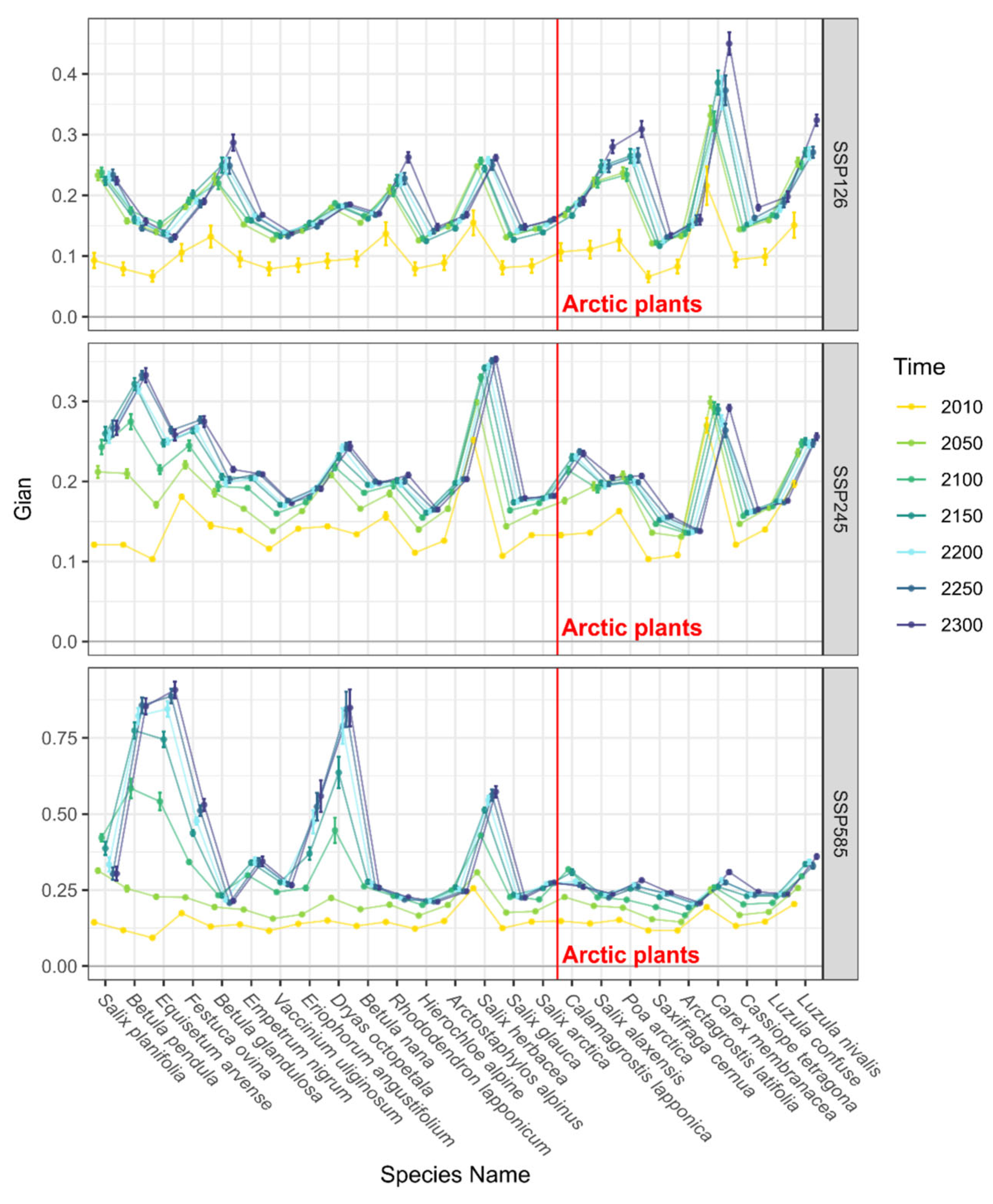
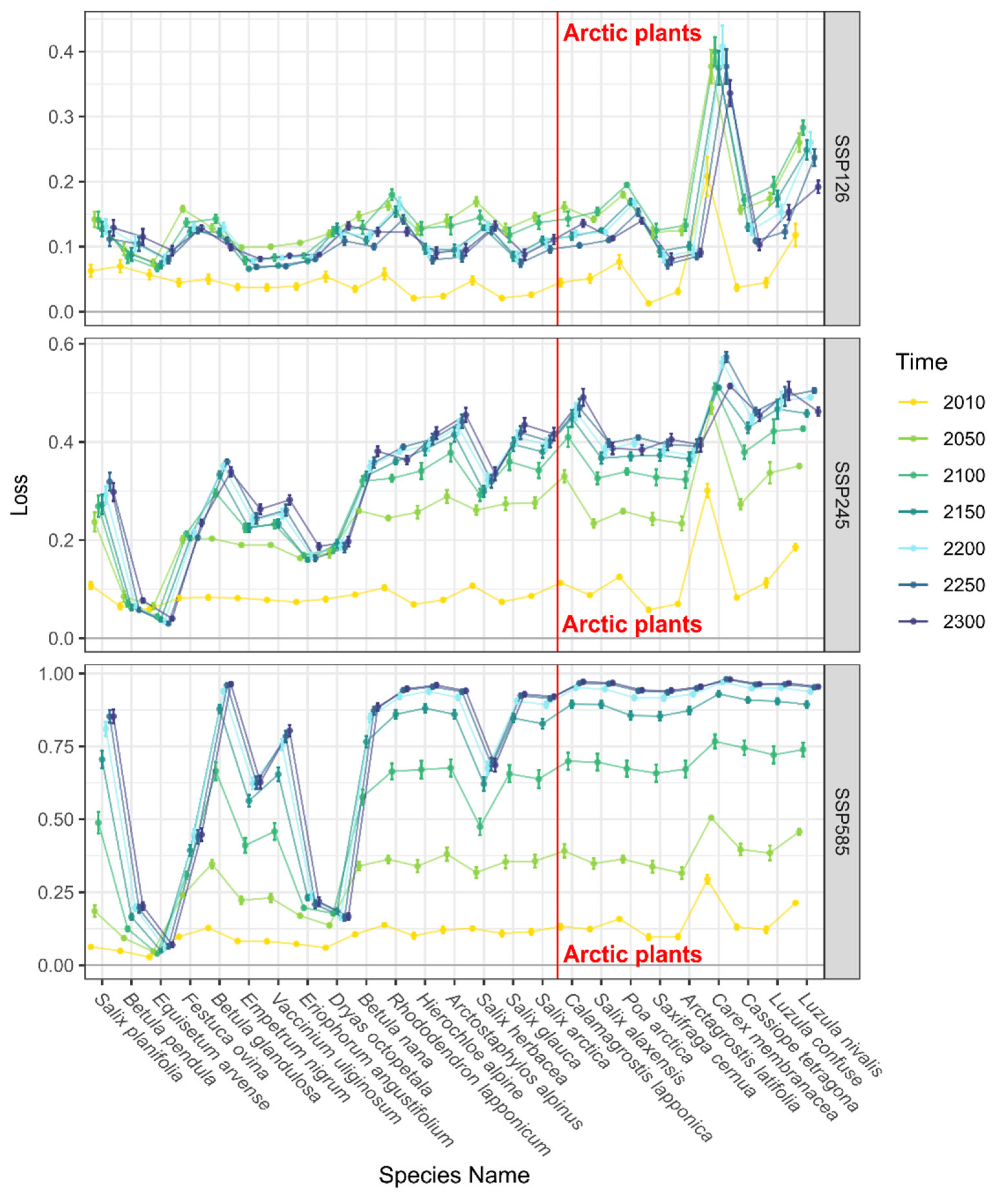
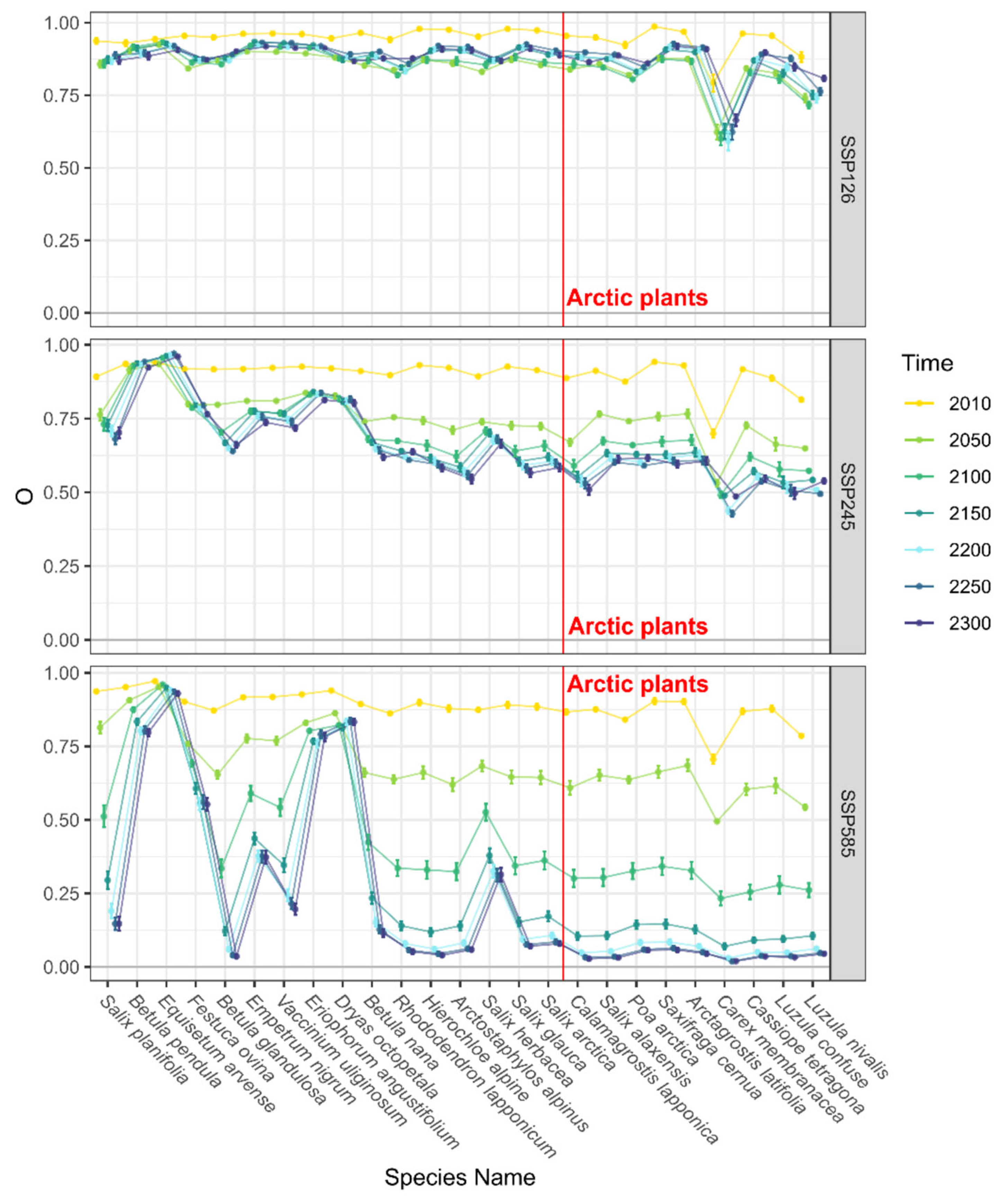
3.2. Trends in Range Shifts
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Thuiller, W.; Guéguen, M.; Renaud, J.; Karger, D.N.; Zimmermann, N.E. Uncertainty in ensembles of global biodiversity scenarios. Nat. Commun. 2019, 10, 1446. [Google Scholar] [CrossRef] [PubMed]
- Abrahms, B.; DiPietro, D.; Graffis, A.; Hollander, A. Managing biodiversity under climate change: Challenges, frameworks, and tools for adaptation. Biodivers. Conserv. 2017, 26, 2277–2293. [Google Scholar] [CrossRef]
- Bellard, C.; Bertelsmeier, C.; Leadley, P.; Thuiller, W.; Courchamp, F. Impacts of climate change on the future of biodiversity. Ecol. Lett. 2012, 15, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Garcia, R.A.; Cabeza, M.; Rahbek, C.; Araujo, M.B. Multiple Dimensions of Climate Change and Their Implications for Biodiversity. Science 2014, 344, 1247579. [Google Scholar] [CrossRef]
- Trew, B.T.; Maclean, I.M.D. Vulnerability of global biodiversity hotspots to climate change. Glob. Ecol. Biogeogr. 2021, 30, 768–783. [Google Scholar] [CrossRef]
- Kass, J.M.; Fukaya, K.; Thuiller, W.; Mori, A.S. Biodiversity modeling advances will improve predictions of nature’s contributions to people. Trends Ecol. Evol. 2024, 39, 338–348. [Google Scholar] [CrossRef]
- Poertner, H.O.; Scholes, R.J.; Arneth, A.; Barnes, D.K.A.; Burrows, M.T.; Diamond, S.E.; Duarte, C.M.; Kiessling, W.; Leadley, P.; Managi, S.; et al. Overcoming the coupled climate and biodiversity crises and their societal impacts. Science 2023, 380, 6642. [Google Scholar] [CrossRef] [PubMed]
- Antunes, A.C.; Berti, E.; Brose, U.; Hirt, M.R.; Karger, D.N.; O’Connor, L.M.J.; Pollock, L.J.; Thuiller, W.; Gauzens, B. Linking biodiversity, ecosystem function, and Nature’s contributions to people: A macroecological energy flux perspective. Trends Ecol. Evol. 2024, 39, 427–434. [Google Scholar] [CrossRef]
- Myrsky, E.; Mikola, J.; Kaarlejarvi, E.; Olofsson, J.; Sjogersten, S.; Tupek, B.; Mannisto, M.K.; Stark, S. Higher vascular plant abundance associated with decreased ecosystem respiration after 20 years of warming in the forest-tundra ecotone. Funct. Ecol. 2024, 38, 219–232. [Google Scholar] [CrossRef]
- Manlick, P.J.; Perryman, N.L.; Koltz, A.M.; Cook, J.A.; Newsome, S.D. Climate warming restructures food webs and carbon flow in high-latitude ecosystems. Nat. Clim. Change 2024, 14, 120–121. [Google Scholar] [CrossRef]
- Higgins, S.I.I.; Conradi, T.; Muhoko, E. Shifts in vegetation activity of terrestrial ecosystems attributable to climate trends. Nat. Geosci. 2023, 16, 147–153. [Google Scholar] [CrossRef]
- Schuur, E.A.G.; Abbott, B.W.; Commane, R.; Ernakovich, J.; Euskirchen, E.; Hugelius, G.; Grosse, G.; Jones, M.; Koven, C.; Leshyk, V.; et al. Permafrost and Climate Change: Carbon Cycle Feedbacks From the Warming Arctic. Annu. Rev. Environ. Resour. 2022, 47, 343–371. [Google Scholar] [CrossRef]
- Markley, P.T.; Gross, C.P.; Daru, B.H. The changing biodiversity of the Arctic flora in the Anthropocene. Am. J. Bot. 2025, 112, e16466. [Google Scholar] [CrossRef]
- Schaefer, J.A. Increases in graminoids after three decades of change in the High Arctic. Polar Res. 2023, 42. [Google Scholar] [CrossRef]
- Jonsdottir, I.S.; Halbritter, A.H.; Christiansen, C.T.; Althuizen, I.H.J.; Haugum, S.V.; Henn, J.J.; Bjornsdottir, K.; Maitner, B.S.; Malhi, Y.; Michaletz, S.T.; et al. Intraspecific trait variability is a key feature underlying high Arctic plant community resistance to climate warming. Ecol. Monogr. 2023, 93, e1555. [Google Scholar] [CrossRef]
- Mörsdorf, M.A.; Cooper, E.J. Habitat determines plant community responses to climate change in the High Arctic1. Arct. Sci. 2022, 8, 722–743. [Google Scholar] [CrossRef]
- Rantanen, M.; Karpechko, A.Y.; Lipponen, A.; Nordling, K.; Hyvärinen, O.; Ruosteenoja, K.; Vihma, T.; Laaksonen, A. The Arctic has warmed nearly four times faster than the globe since 1979. Commun. Earth Environ. 2022, 3, 168. [Google Scholar] [CrossRef]
- Chylek, P.; Folland, C.; Klett, J.D.; Wang, M.Y.; Hengartner, N.; Lesins, G.; Dubey, M.K. Annual Mean Arctic Amplification 1970–2020: Observed and Simulated by CMIP6 Climate Models. Geophys. Res. Lett. 2022, 49, e2022GL099371. [Google Scholar] [CrossRef]
- Niskanen, A.K.J.; Niittynen, P.; Aalto, J.; Väre, H.; Luoto, M. Lost at high latitudes: Arctic and endemic plants under threat as climate warms. Divers. Distrib. 2019, 25, 809–821. [Google Scholar] [CrossRef]
- Post, E.; Alley, R.B.; Christensen, T.R.; Macias-Fauria, M.; Forbes, B.C.; Gooseff, M.N.; Iler, A.; Kerby, J.T.; Laidre, K.L.; Mann, M.E.; et al. The polar regions in a 2 °C warmer world. Sci. Adv. 2019, 5, eaaw9883. [Google Scholar] [CrossRef] [PubMed]
- Gallois, E.C.; Myers-Smith, I.H.; Iversen, C.M.; Salmon, V.G.; Turner, L.L.; An, R.; Elmendorf, S.C.; Collins, C.G.; Anderson, M.J.R.; Young, A.; et al. Tundra Vegetation Community Type, Not Microclimate, Controls Asynchrony of Above- and Below-Ground Phenology. Glob. Change Biol. 2025, 31, e70153. [Google Scholar] [CrossRef]
- Finne, E.A.; Bjerke, J.W.; Stordal, F.; Tallaksen, L.M. Lichens are more tolerant against winter warming stress than vascular and non-vascular plants: Insights from an alpine field experiment. J. Ecol. 2025, 113, 518–530. [Google Scholar] [CrossRef]
- Quan, Q.; He, N.; Zhang, R.; Wang, J.; Luo, Y.; Ma, F.; Pan, J.; Wang, R.; Liu, C.; Zhang, J.; et al. Plant height as an indicator for alpine carbon sequestration and ecosystem response to warming. Nat. Plants 2024, 10, 890–900. [Google Scholar] [CrossRef] [PubMed]
- Berner, L.T.; Orndahl, K.M.; Rose, M.; Tamstorf, M.; Arndal, M.F.; Alexander, H.D.; Humphreys, E.R.; Loranty, M.M.; Ludwig, S.M.; Nyman, J.; et al. The Arctic Plant Aboveground Biomass Synthesis Dataset. Sci. Data 2024, 11, 305. [Google Scholar] [CrossRef]
- Labrecque-Foy, J.-P.; Gaspard, A.; Simard, M.; Boudreau, S. Radial growth of subarctic tree and shrub species: Relationships with climate and association with the greening of the forest-tundra ecotone of subarctic Quebec, Canada. Arct. Sci. 2024, 10, 201–214. [Google Scholar] [CrossRef]
- Johnson, S.A.; Ober, H.K.; Adams, D.C. Are keystone species effective umbrellas for habitat conservation? A spatially explicit approach. J. Nat. Conserv. 2017, 37, 47–55. [Google Scholar] [CrossRef]
- Marjakangas, E.-L.; Santangeli, A.; Kujala, H.; Mammola, S.; Lehikoinen, A. Identifying ‘climate keystone species’ as a tool for conserving ecological communities under climate change. Divers. Distrib. 2023, 29, 1341–1354. [Google Scholar] [CrossRef]
- Narango, D.L.; Tallamy, D.W.; Shropshire, K.J. Few keystone plant genera support the majority of Lepidoptera species. Nat. Commun. 2020, 11, 5751. [Google Scholar] [CrossRef]
- Patsopoulos, N.A.; Baranzini, S.E.; Santaniello, A.; Shoostari, P.; Cotsapas, C.; Wong, G.; Beecham, A.H.; James, T.; Replogle, J.; Vlachos, I.S.; et al. Multiple sclerosis genomic map implicates peripheral immune cells and microglia in susceptibility. Science 2019, 365, eaav7188. [Google Scholar] [CrossRef]
- Parmesan, C. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 637–669. [Google Scholar] [CrossRef]
- Warren, R.; Price, J.; Graham, E.; Forstenhaeusler, N.; VanDerWal, J. The projected effect on insects, vertebrates, and plants of limiting global warming to 1.5 °C rather than 2 °C. Science 2018, 360, 791–795. [Google Scholar] [CrossRef]
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef]
- Walther, G.R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.C.; Fromentin, J.M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological responses to recent climate change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef]
- Phoenix, G.K.; Bjerke, J.W.; Bjork, R.G.; Blok, D.; Bryn, A.; Callaghan, T.V.; Christiansen, C.T.; Cunliffe, A.M.; Davidson, S.J.; Epstein, H.E.; et al. Browning events in Arctic ecosystems: Diverse causes with common consequences. PLoS Clim. 2025, 4, e0000570. [Google Scholar] [CrossRef]
- Choler, P.; Bayle, A.; Fort, N.; Gascoin, S. Waning snowfields have transformed into hotspots of greening within the alpine zone. Nat. Clim. Change 2025, 15, 80–85. [Google Scholar] [CrossRef]
- Karlsen, S.R.; Elvebakk, A.; Stendardi, L.; Hogda, K.A.; Macias-Fauria, M. Greening of Svalbard. Sci. Total Environ. 2024, 945, 174130. [Google Scholar] [CrossRef]
- Phoenix, G.K.; Treharne, R. Arctic greening and browning: Challenges and a cascade of complexities. Glob. Change Biol. 2022, 28, 3481–3483. [Google Scholar] [CrossRef]
- Phoenix, G.K.; Bjerke, J.W. Arctic browning: Extreme events and trends reversing arctic greening. Glob. Change Biol. 2016, 22, 2960–2962. [Google Scholar] [CrossRef]
- Myers-Smith, I.H.; Elmendorf, S.C.; Beck, P.S.A.; Wilmking, M.; Hallinger, M.; Blok, D.; Tape, K.D.; Rayback, S.A.; Macias-Fauria, M.; Forbes, B.C.; et al. Climate sensitivity of shrub growth across the tundra biome. Nat. Clim. Change 2015, 5, 887–891. [Google Scholar] [CrossRef]
- Myers-Smith, I.H.; Forbes, B.C.; Wilmking, M.; Hallinger, M.; Lantz, T.; Blok, D.; Tape, K.D.; Macias-Fauria, M.; Sass-Klaassen, U.; Levesque, E.; et al. Shrub expansion in tundra ecosystems: Dynamics, impacts and research priorities. Environ. Res. Lett. 2011, 6, 045509. [Google Scholar] [CrossRef]
- Seider, J.H.; Lantz, T.C.; Bone, C. Tundra shrub expansion in a warming climate and the influence of data type on models of habitat suitability. Arct. Antarct. Alp. Res. 2022, 54, 488–506. [Google Scholar] [CrossRef]
- Mekonnen, Z.A.; Riley, W.J.; Berner, L.T.; Bouskill, N.J.; Torn, M.S.; Iwahana, G.; Breen, A.L.; Myers-Smith, I.H.; Criado, M.G.; Liu, Y.; et al. Arctic tundra shrubification: A review of mechanisms and impacts on ecosystem carbon balance. Environ. Res. Lett. 2021, 16, 053001. [Google Scholar] [CrossRef]
- Taylor, J.J.; Lawler, J.P.; Aronsson, M.; Barry, T.; Bjorkman, A.D.; Christensen, T.; Coulson, S.J.; Cuyler, C.; Ehrich, D.; Falk, K.; et al. Arctic terrestrial biodiversity status and trends: A synopsis of science supporting the CBMP State of Arctic Terrestrial Biodiversity Report. Ambio 2020, 49, 833–847. [Google Scholar] [CrossRef] [PubMed]
- van der Wal, R.; Stien, A. High-arctic plants like it hot: A long-term investigation of between-year variability in plant biomass. Ecology 2014, 95, 3414–3427. [Google Scholar] [CrossRef]
- Henry, G.H.R.; Hollister, R.D.; Klanderud, K.; Bjork, R.G.; Bjorkman, A.D.; Elphinstone, C.; Jónsdóttir, I.S.; Molau, U.; Petraglia, A.; Oberbauer, S.F.; et al. The International Tundra Experiment (ITEX): 30 years of research on tundra ecosystems1. Arct. Sci. 2022, 8, 550–571. [Google Scholar] [CrossRef]
- Bjorkman, A.D.; Criado, M.G.; Myers-Smith, I.H.; Ravolainen, V.; Jonsdottir, I.S.; Westergaard, K.B.; Lawler, J.P.; Aronsson, M.; Bennett, B.; Gardfjell, H.; et al. Status and trends in Arctic vegetation: Evidence from experimental warming and long-term monitoring. Ambio 2020, 49, 678–692. [Google Scholar] [CrossRef]
- Zong, S.; Lembrechts, J.J.; Du, H.; He, H.S.; Wu, Z.; Li, M.; Rixen, C. Upward range shift of a dominant alpine shrub related to 50 years of snow cover change. Remote Sens. Environ. 2022, 268, 112773. [Google Scholar] [CrossRef]
- Nill, L.; Grunberg, I.; Ullmann, T.; Gessner, M.; Boike, J.; Hostert, P. Arctic shrub expansion revealed by Landsat-derived multitemporal vegetation cover fractions in the Western Canadian Arctic. Remote Sens. Environ. 2022, 281, 113228. [Google Scholar] [CrossRef]
- Chardon, N.I.; Pironon, S.; Peterson, M.L.; Doak, D.F. Incorporating intraspecific variation into species distribution models improves distribution predictions, but cannot predict species traits for a wide-spread plant species. Ecography 2020, 43, 60–74. [Google Scholar] [CrossRef]
- Freeman, M.S.; Dick, J.T.; Reid, N. Dealing with non-equilibrium bias and survey effort in presence-only invasive Species Distribution Models (iSDM); Predicting the range of muntjac deer in Britain and Ireland. Ecol. Inform. 2022, 69, 101683. [Google Scholar] [CrossRef]
- Koldasbayeva, D.; Tregubova, P.; Gasanov, M.; Zaytsev, A.; Petrovskaia, A.; Burnaev, E. Challenges in data-driven geospatial modeling for environmental research and practice. Nat. Commun. 2024, 15, 10700. [Google Scholar] [CrossRef]
- Soley-Guardia, M.; Alvarado-Serrano, D.F.; Anderson, R.P. Top ten hazards to avoid when modeling species distributions: A didactic guide of assumptions, problems, and recommendations. Ecography 2024, 2024, e06852. [Google Scholar] [CrossRef]
- Zurell, D.; Franklin, J.; Koenig, C.; Bouchet, P.J.; Dormann, C.F.; Elith, J.; Fandos, G.; Feng, X.; Guillera-Arroita, G.; Guisan, A.; et al. A standard protocol for reporting species distribution models. Ecography 2020, 43, 1261–1277. [Google Scholar] [CrossRef]
- Riva, F.; Martin, C.J.; Acedo, C.G.; Bellon, E.N.; Keil, P.; Moran-Ordonez, A.; Fahrig, L.; Guisan, A. Incorporating effects of habitat patches into species distribution models. J. Ecol. 2024, 112, 2162–2182. [Google Scholar] [CrossRef]
- Santini, L.; Benitez-Lopez, A.; Maiorano, L.; Cengic, M.; Huijbregts, M.A.J. Assessing the reliability of species distribution projections in climate change research. Divers. Distrib. 2021, 27, 1035–1050. [Google Scholar] [CrossRef]
- Wang, F.; Sahana, M.; Pahlevanzadeh, B.; Pal, S.C.; Shit, P.K.; Piran, M.J.; Janizadeh, S.; Band, S.S.; Mosavi, A. Applying different resampling strategies in machine learning models to predict head-cut gully erosion susceptibility. Alex. Eng. J. 2021, 60, 5813–5829. [Google Scholar] [CrossRef]
- Tanaka, K.; Boucher, O.; Ciais, P.; Johansson, D.J.A.; Morfeldt, J. Cost-effective implementation of the Paris Agreement using flexible greenhouse gas metrics. Sci. Adv. 2021, 7, eabf9020. [Google Scholar] [CrossRef]
- Schoeman, D.S.; Sen Gupta, A.; Harrison, C.S.; Everett, J.D.; Brito-Morales, I.; Hannah, L.; Bopp, L.; Roehrdanz, P.R.; Richardson, A.J. Demystifying global climate models for use in the life sciences. Trends Ecol. Evol. 2023, 38, 843–858. [Google Scholar] [CrossRef]
- Pecl, G.T.; Araújo, M.B.; Bell, J.D.; Blanchard, J.; Bonebrake, T.C.; Chen, I.-C.; Clark, T.D.; Colwell, R.K.; Danielsen, F.; Evengård, B. Biodiversity redistribution under climate change: Impacts on ecosystems and human well-being. Science 2017, 355, eaai9214. [Google Scholar] [CrossRef]
- Sala, O.E.; Stuart Chapin, F.; Armesto, J.J.; Berlow, E.; Bloomfield, J.; Dirzo, R.; Huber-Sanwald, E.; Huenneke, L.F.; Jackson, R.B.; Kinzig, A. Global biodiversity scenarios for the year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef]
- Meinshausen, M.; Nicholls, Z.R.; Lewis, J.; Gidden, M.J.; Vogel, E.; Freund, M.; Beyerle, U.; Gessner, C.; Nauels, A.; Bauer, N. The shared socio-economic pathway (SSP) greenhouse gas concentrations and their extensions to 2500. Geosci. Model. Dev. 2020, 13, 3571–3605. [Google Scholar] [CrossRef]
- Patil, J.; Sharma, P.; Mhatre, K. Global warming induced stress and its impact on biodiversity. Sci. Technol. 2021, 6, 34–45. [Google Scholar]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudik, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Tórshavn, F.I. Conservation of Arctic Flora and Fauna (CAFF) Flora Group Workshop. Available online: https://www.geobotany.org/library/pubs/TalbotSS2008_caff_Report15.pdf (accessed on 23 June 2025).
- Nilsson, A.E. A changing Arctic climate: Science and policy in the Arctic Climate Impact Assessment. In Climate Governance in the Arctic; Springer: Berlin/Heidelberg, Germany, 2009; pp. 77–95. [Google Scholar]
- Lane, M.A.; Edwards, J.L. The global biodiversity information facility (GBIF). Syst. Assoc. Spec. Vol. 2007, 73, 1. [Google Scholar]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Steen, V.A.; Tingley, M.W.; Paton, P.W.; Elphick, C.S. Spatial thinning and class balancing: Key choices lead to variation in the performance of species distribution models with citizen science data. Methods Ecol. Evol. 2021, 12, 216–226. [Google Scholar] [CrossRef]
- Kou, X.; Li, Q.; Liu, S. High-resolution bioclimatic dataset derived from future climate projections for plant species distribution modeling. Ecol. Inform. 2011, 6, 196–204. [Google Scholar]
- Kou, X. Polar Plant Dataset; Figshare: London, UK, 2025. [Google Scholar]
- Yukimoto, S.; Koshiro, T.; Kawai, H.; Oshima, N.; Yoshida, K.; Urakawa, S.; Tsujino, H.; Deushi, M.; Tanaka, T.; Hosaka, M.; et al. MRI MRI-ESM2.0 Model Output Prepared for CMIP6 CMIP. World Data Center for Climate (WDCC) at DKRZ. 2023. Available online: https://www.wdc-climate.de/ui/entry?acronym=C6_5243961 (accessed on 23 June 2025).
- Boucher, O.; Denvil, S.; Levavasseur, G.; Cozic, A.; Caubel, A.; Foujols, M.-A.; Meurdesoif, Y.; Gastineau, G. IPSL IPSL-CM6A-LR Model Output Prepared for CMIP6 DAMIP. World Data Center for Climate (WDCC) at DKRZ. 2023. Available online: https://hdl.handle.net/21.14106/f8270bc2151936dffc83a10d69720a61f9de8eaa (accessed on 23 June 2025).
- Giorgetta, M.; Jungclaus, J.; Reick, C.; Legutke, S.; Brovkin, V.; Crueger, T.; Esch, M.; Fieg, K.; Glushak, K.; Gayler, V. CMIP5 Simulations of the Max Planck Institute for Meteorology (MPI-M) based on the MPI-ESM-LR model: The rcp85 Experiment, Served by ESGF. 2011. Available online: https://ipcc-browser.ipcc-data.org/browser/dataset/1260 (accessed on 23 June 2025).
- O’Neill, B.C.; Tebaldi, C.; Van Vuuren, D.P.; Eyring, V.; Friedlingstein, P.; Hurtt, G.; Knutti, R.; Kriegler, E.; Lamarque, J.-F.; Lowe, J. The scenario model intercomparison project (ScenarioMIP) for CMIP6. Geosci. Model. Dev. 2016, 9, 3461–3482. [Google Scholar] [CrossRef]
- Zhang, H.T.; Guo, W.Y.; Wang, W.T. The dimensionality reductions of environmental variables have a significant effect on the performance of species distribution models. Ecol. Evol. 2023, 13, e10747. [Google Scholar] [CrossRef] [PubMed]
- Kou, X.; Li, Q.; Beierkuhnlein, C.; Zhao, Y.; Liu, S. A new tool for exploring climate change induced range shifts of conifer species in China. PLoS ONE 2014, 9, e98643. [Google Scholar] [CrossRef]
- Oke, T.A.; Stralberg, D.; Reid, D.G.; Bennett, B.A.; Cannings, S.; Willier, C.; Fulkerson, J.R.; Cooke, H.A.; Mantyka-Pringle, C.S. Warming drives poleward range contractions of Beringian endemic plant species at high latitudes. Divers. Distrib. 2023, 29, 509–523. [Google Scholar] [CrossRef]
- Rew, L.J.; McDougall, K.L.; Alexander, J.M.; Daehler, C.C.; Essl, F.; Haider, S.; Kueffer, C.; Lenoir, J.; Milbau, A.; Nuñez, M.A.; et al. Moving up and over: Redistribution of plants in alpine, Arctic, and Antarctic ecosystems under global change. Arct. Antarct. Alp. Res. 2020, 52, 651–665. [Google Scholar] [CrossRef]
- Rastner, P.; Bolch, T.; Mölg, N.; Machguth, H.; Le Bris, R.; Paul, F. The first complete inventory of the local glaciers and ice caps on Greenland. Cryosphere 2012, 6, 1483–1495. [Google Scholar] [CrossRef]
- Bartsch, A.; Pointner, G.; Nitze, I.; Efimova, A.; Jakober, D.; Ley, S.; Högström, E.; Grosse, G.; Schweitzer, P. Expanding infrastructure and growing anthropogenic impacts along Arctic coasts. Environ. Res. Lett. 2021, 16, 115013. [Google Scholar] [CrossRef]
- Charles, K.M.; Stehlik, I. Assisted species migration and hybridization to conserve cold-adapted plants under climate change. Conserv. Biol. 2021, 35, 559–566. [Google Scholar] [CrossRef]
- Brun, P.; Thuiller, W.; Chauvier, Y.; Pellissier, L.; Wüest, R.O.; Wang, Z.; Zimmermann, N.E. Model complexity affects species distribution projections under climate change. J. Biogeogr. 2020, 47, 130–142. [Google Scholar] [CrossRef]
- Dilts, T.E.; Blum, M.E.; Shoemaker, K.T.; Weisberg, P.J.; Stewart, K.M. Topographic Ruggedness Indices in Ecology: Past, Present and Future. 2022. [CrossRef]
- Revell, L.J. Phylogenetic signal and linear regression on species data. Methods Ecol. Evol. 2010, 1, 319–329. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Li, S.; Su, Y.; Yang, B.; Kou, X. Predicting Range Shifts in the Distribution of Arctic/Boreal Plant Species Under Climate Change Scenarios. Diversity 2025, 17, 558. https://doi.org/10.3390/d17080558
Zhang Y, Li S, Su Y, Yang B, Kou X. Predicting Range Shifts in the Distribution of Arctic/Boreal Plant Species Under Climate Change Scenarios. Diversity. 2025; 17(8):558. https://doi.org/10.3390/d17080558
Chicago/Turabian StyleZhang, Yan, Shaomei Li, Yuanbo Su, Bingyu Yang, and Xiaojun Kou. 2025. "Predicting Range Shifts in the Distribution of Arctic/Boreal Plant Species Under Climate Change Scenarios" Diversity 17, no. 8: 558. https://doi.org/10.3390/d17080558
APA StyleZhang, Y., Li, S., Su, Y., Yang, B., & Kou, X. (2025). Predicting Range Shifts in the Distribution of Arctic/Boreal Plant Species Under Climate Change Scenarios. Diversity, 17(8), 558. https://doi.org/10.3390/d17080558





