Environmental Heterogeneity Drives Diversity Across Forest Strata in Hopea hainanensis Communities
Abstract
1. Introduction
2. Materials and Methods
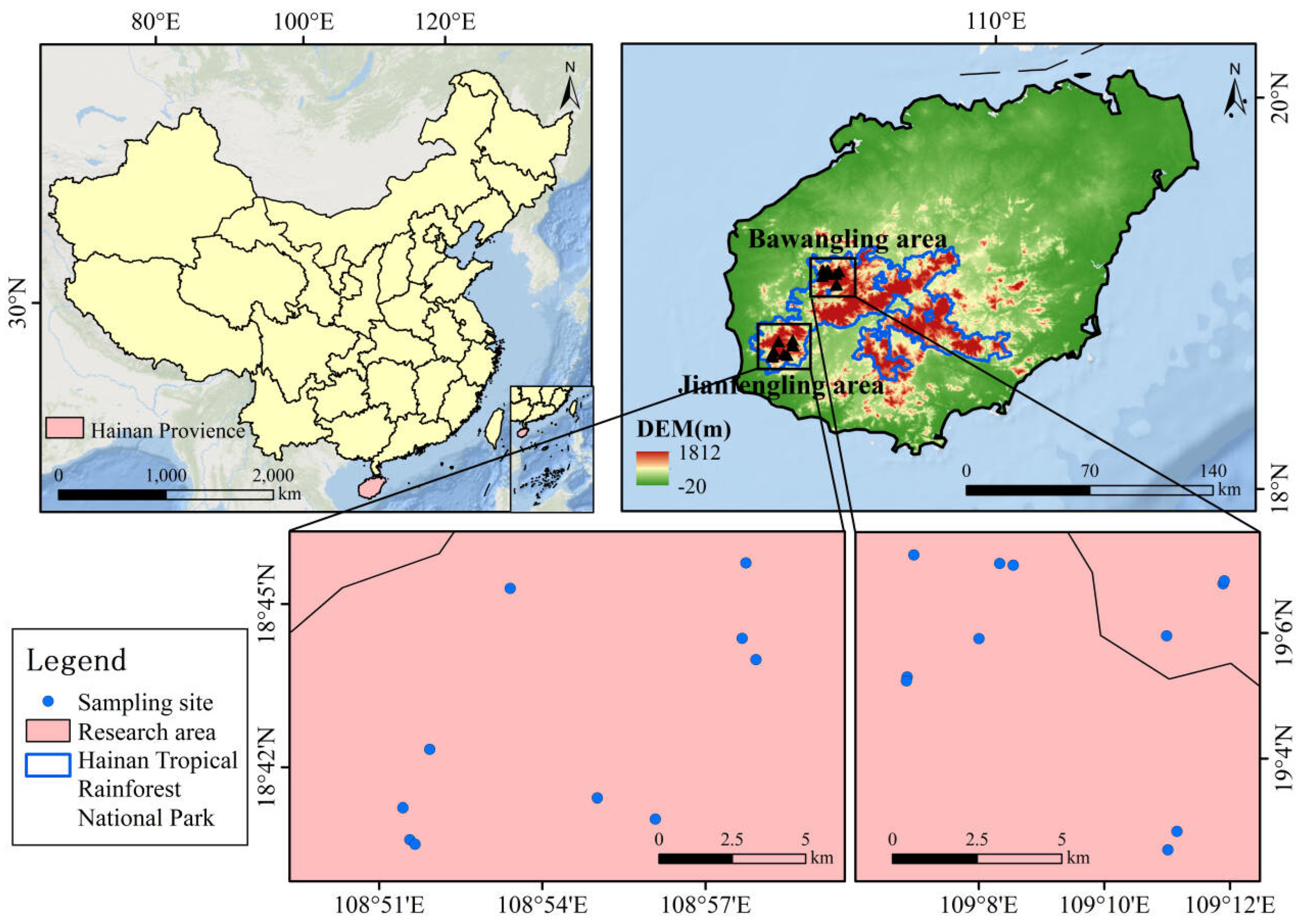
2.1. The Study Area
2.2. Data Processing and Analysis
2.2.1. Stratification of the Forest Layers and Calculation of the Importance Values and the Similarity Indices
2.2.2. Acquisition of Environmental Factors
2.2.3. Calculation of Species Diversity and Phylogenetic Diversity Indices
3. Results
3.1. Characteristics of the Community Where Hopea hainanensis Is Located
3.1.1. Species Composition of Hopea hainanensis Communities in Different Regions
3.1.2. The Importance Values of Species in Different Forest Layers of the Hopea hainanensis Community
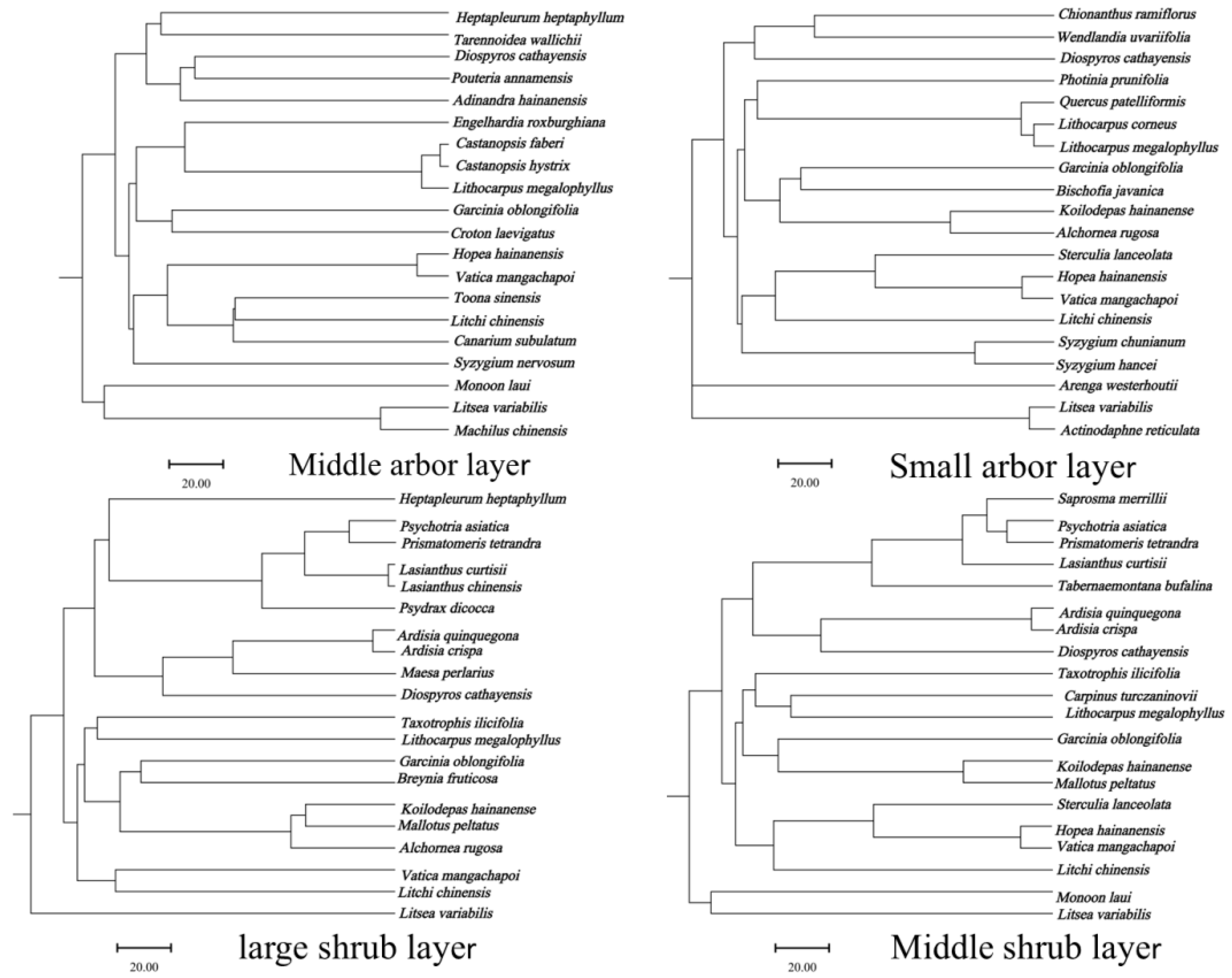
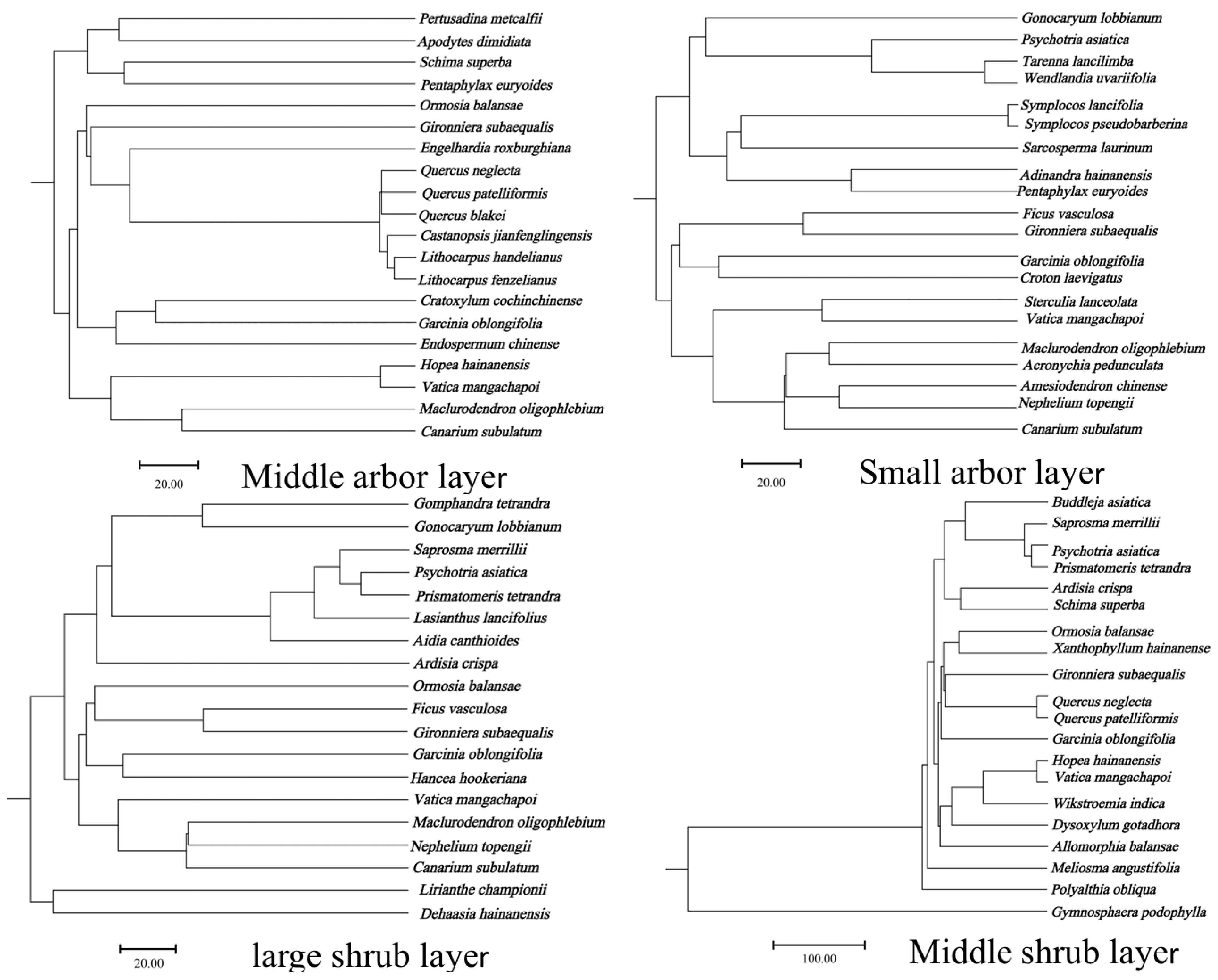
3.1.3. The Similarity of Different Forest Layers in the Community Where Hopea hainanensis Is Located
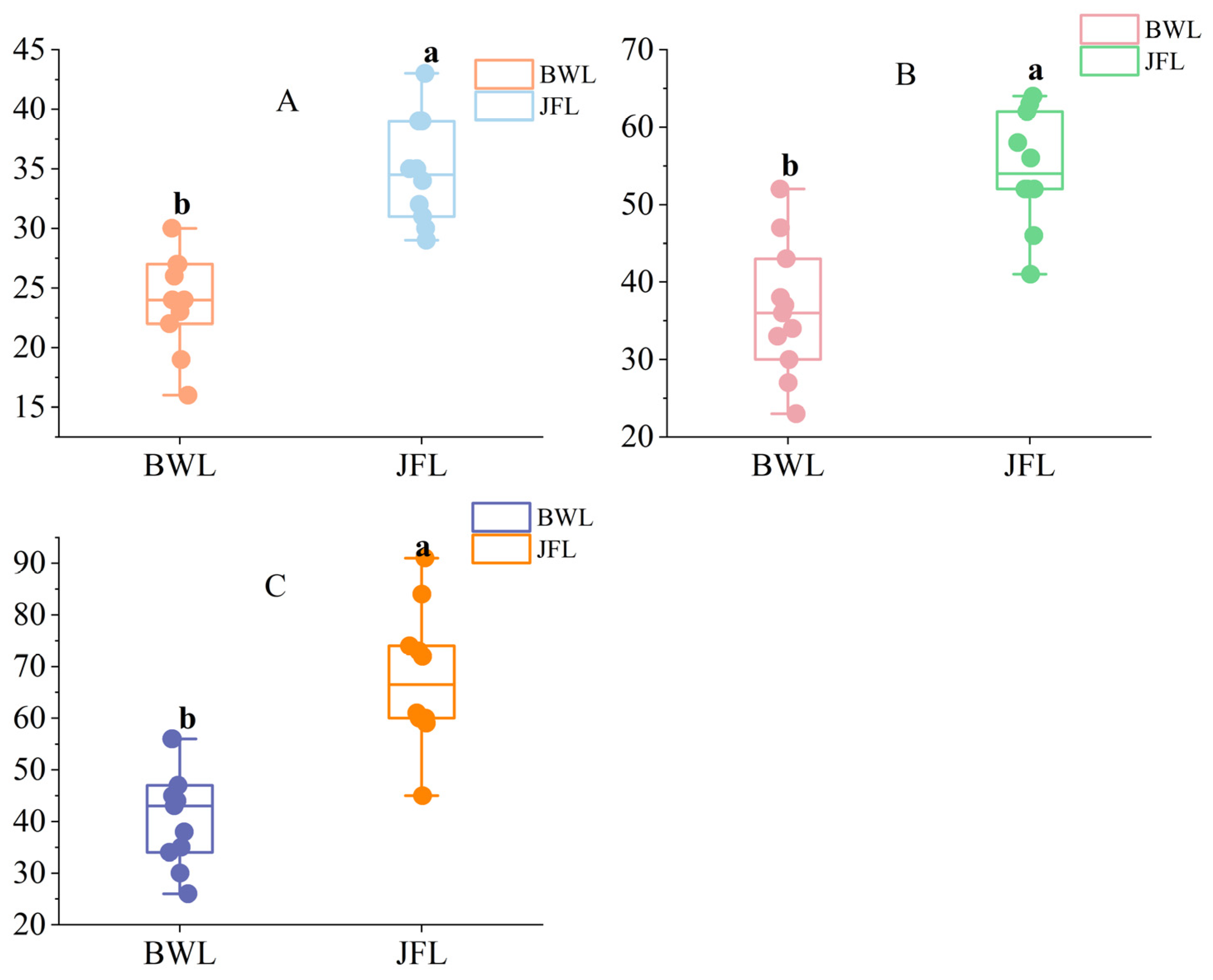
3.2. Diversity Indices of Communities Containing Hopea hainanensis in Different Regions
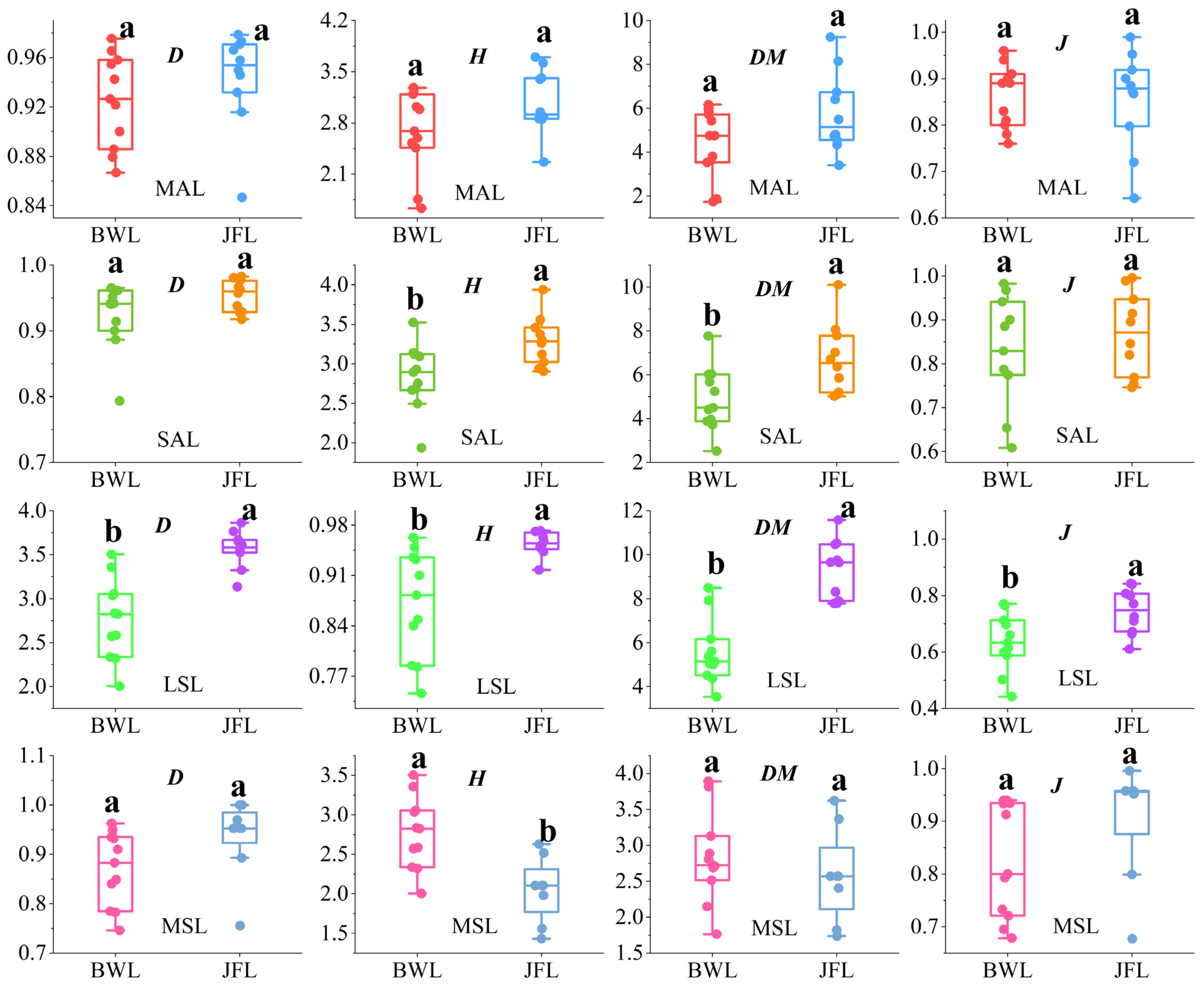
3.2.1. The Species Diversity Index
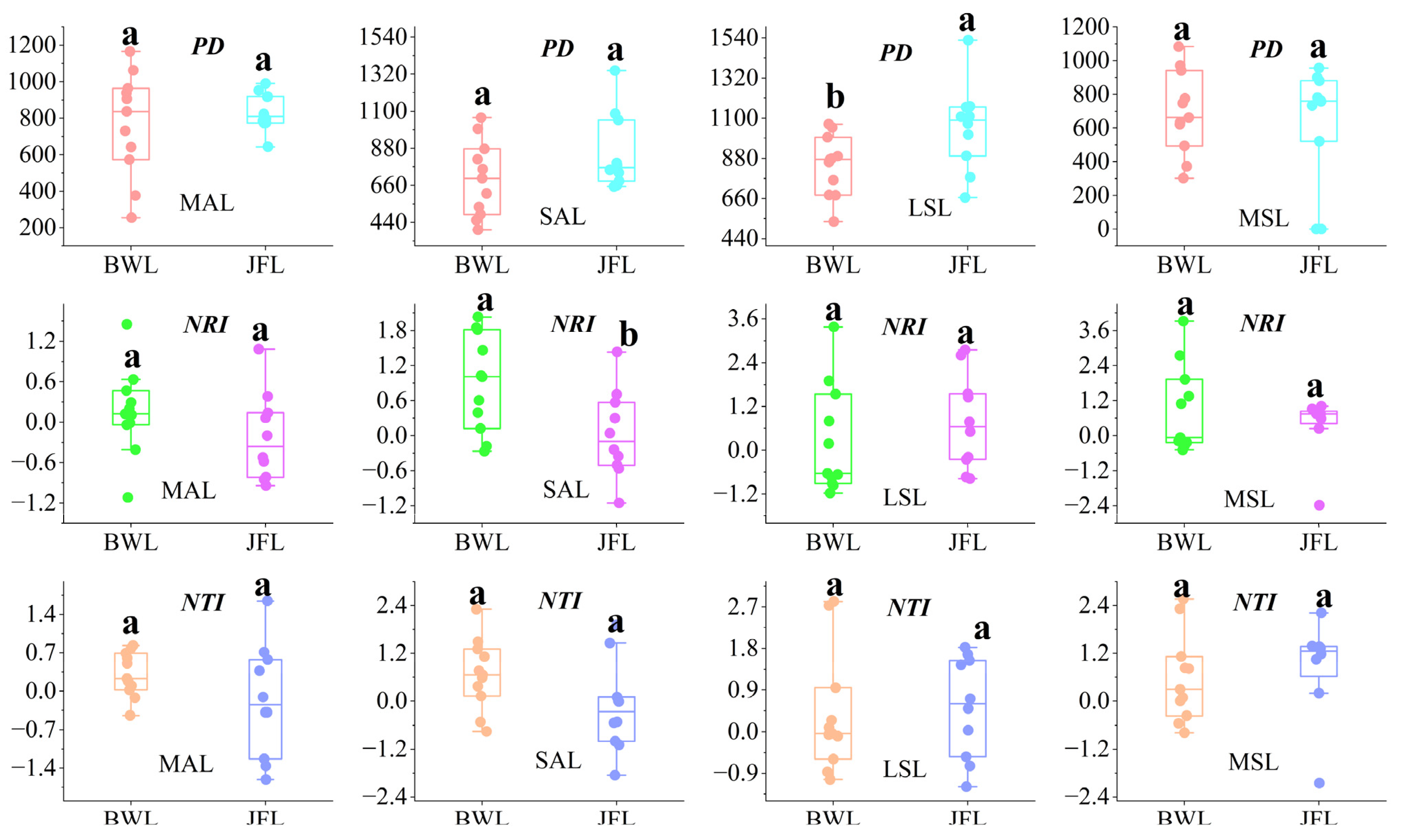
3.2.2. The Phylogenetic Diversity Index of Dominant Tree Species
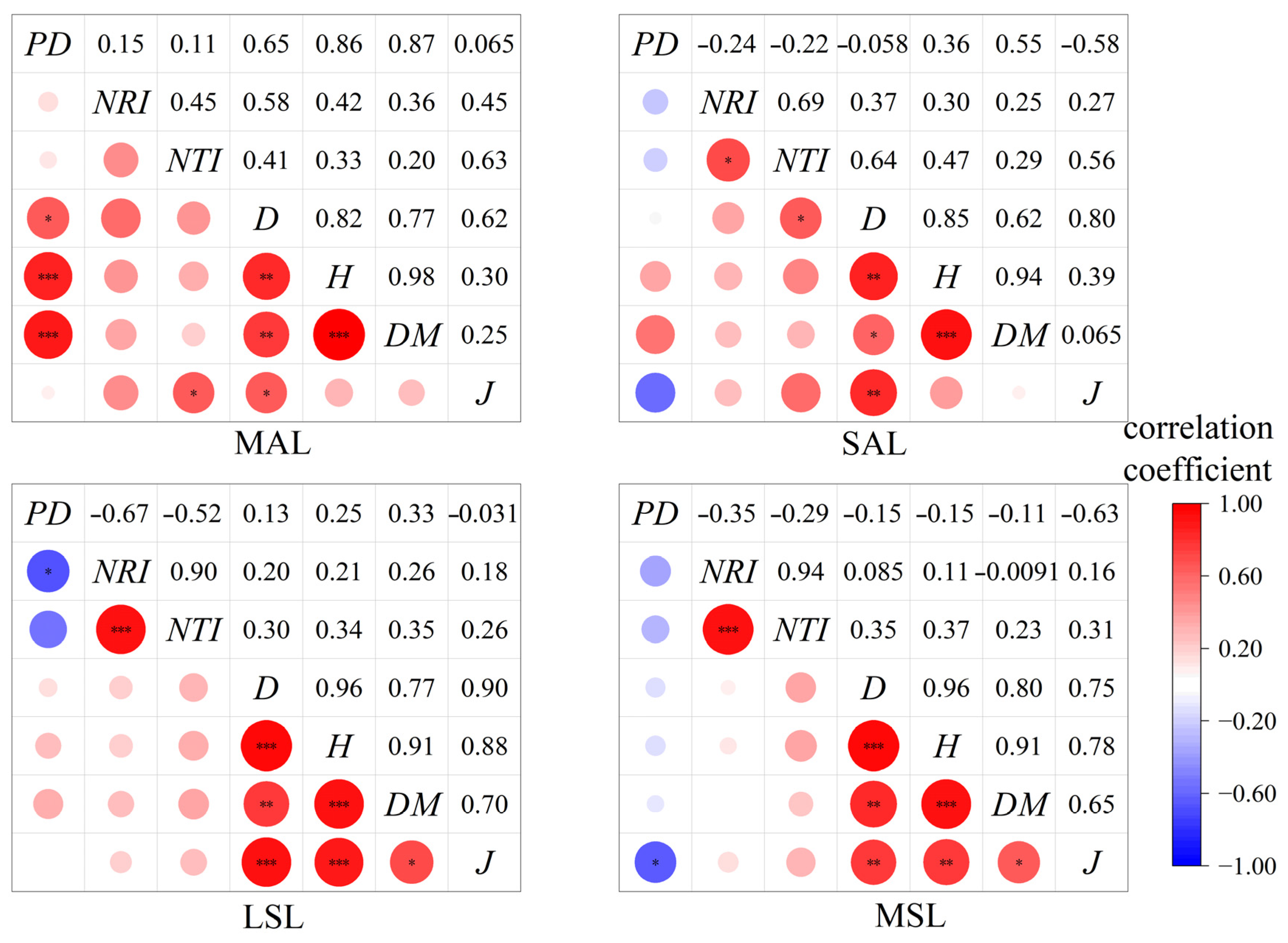
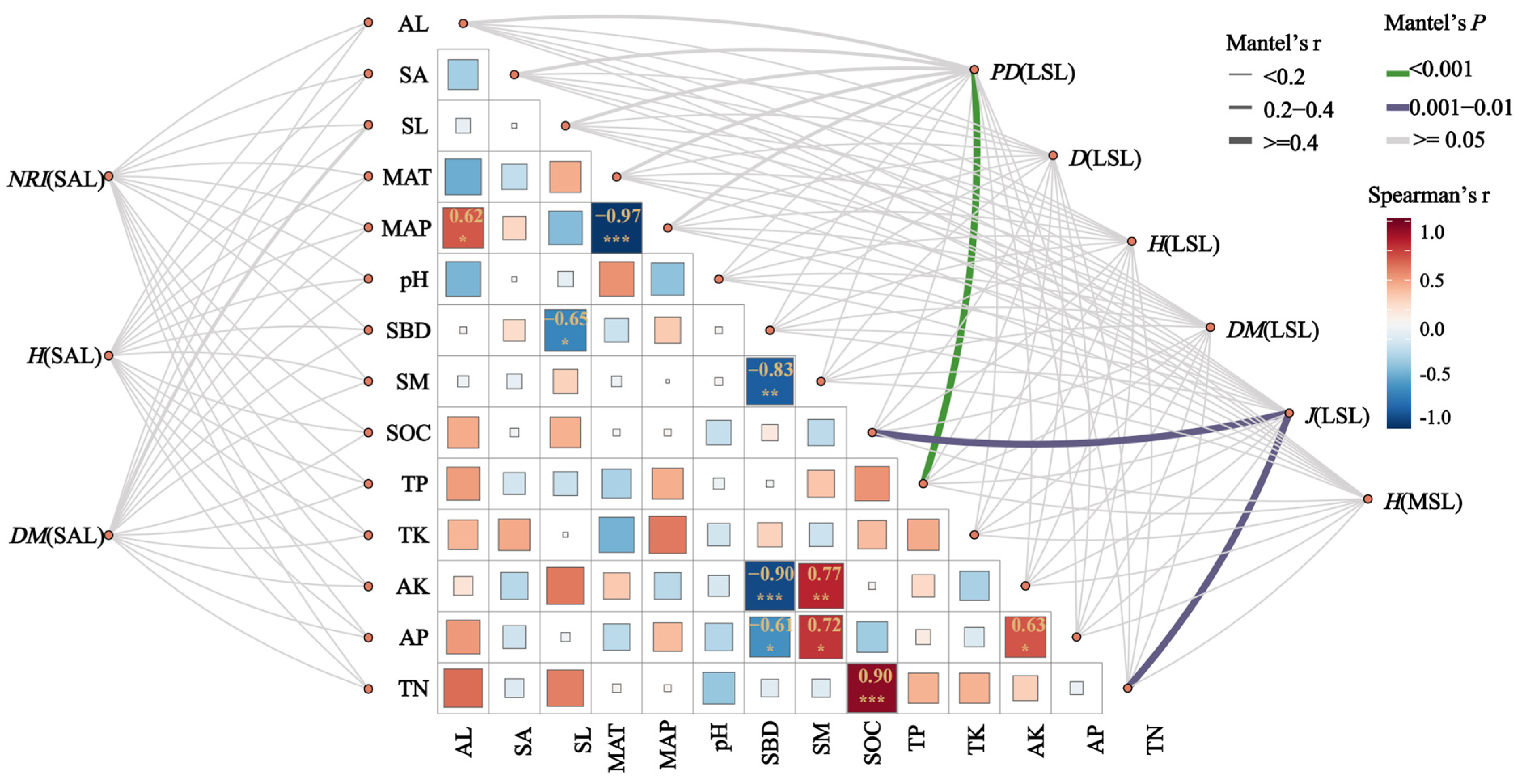
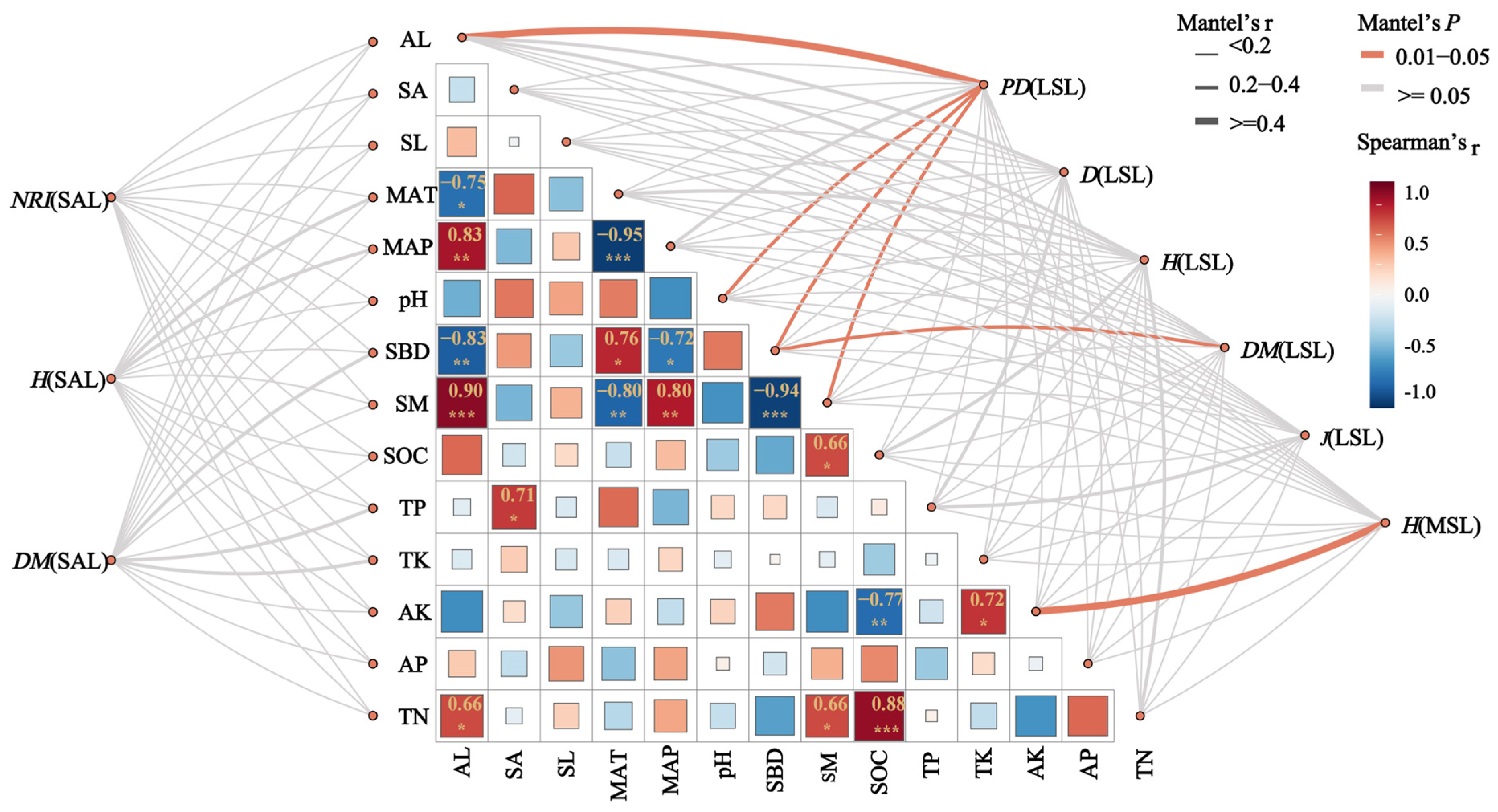
3.3. Correlations Among Diversity Indices in the Hopea hainanensis Community
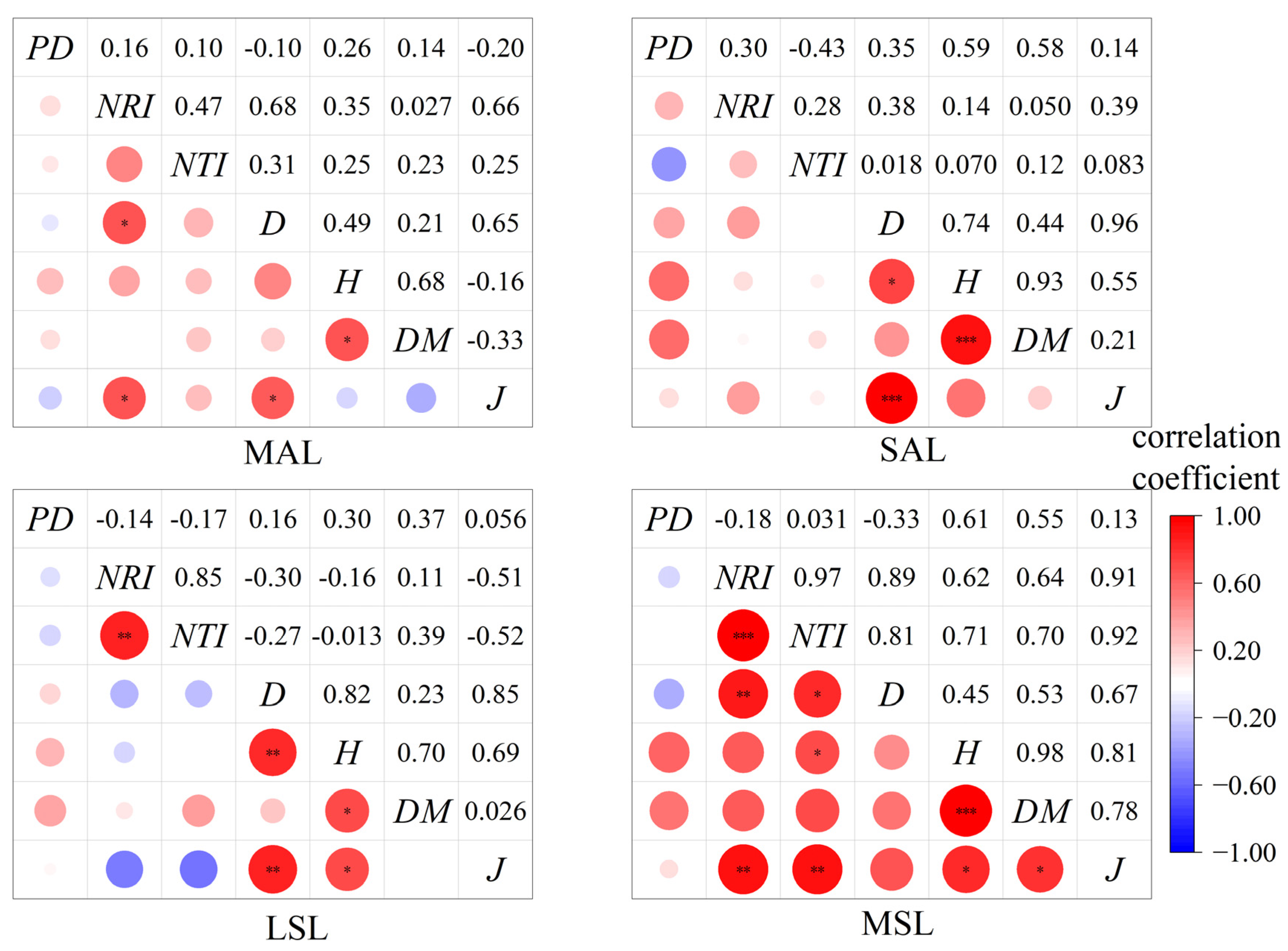
3.4. Factors Influencing the Diversity Index of Hopea chinensis Communities
4. Discussion
4.1. Community Diversity Indices of Different Forest Layers
4.2. The Correlation Between the Species Diversity Index and the Phylogenetic Diversity Index of Dominant Tree Species
4.3. The Correlation Between the Diversity Index and Environmental Factors
4.4. The Protection Strategy
4.5. Limitations and Prospects
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Tan, R.P.; Hou, K.; Wu, H.Q. The cost of biodiversity protection: National Key Ecological Functional Zone and labor demand in China. J. Asian. Econ. 2025, 97, 101895. [Google Scholar] [CrossRef]
- Pascual, U.; Adams, W.M.; Díaz, S.; Lele, S.; Mace, G.M.; Turnhout, E. Biodiversity and the challenge of pluralism. Nat. Sustain. 2021, 4, 567–572. [Google Scholar] [CrossRef]
- Srivathsa, A.; Vasudev, D.; Nair, T.; Chakrabarti, S.; Chanchani, P.; DeFries, R.; Deomurari, A.; Dutta, S.; Ghose, D.; Goswami, V.R.; et al. Prioritizing India’s landscapes for biodiversity, ecosystem services and human well-being. Nat. Sustain. 2023, 6, 568–577. [Google Scholar] [CrossRef]
- Wang, L.H.; Wei, F.L.; Tagesson, T.; Fang, Z.X.; Svenning, J.C. Transforming forest management through rewilding: Enhancing biodiversity, resilience, and biosphere sustainability under global change. One Earth 2025, 8, 101195. [Google Scholar] [CrossRef]
- Qu, Y.; Zeng, X.Y.; Luo, C.Y.; Zhang, H.Q.; Liu, Y.N.; Wang, J.F. Constructing wetland ecological corridor system based on hydrological connectivity with the goal of improving regional biodiversity. J. Environ. Manag. 2024, 368, 122074. [Google Scholar] [CrossRef]
- Swenson, N.G.; Erickson, D.L.; Mi, X.C.; Bourg, N.A.; Jimena, F.M.; Ge, X.J.; Robert, H.; Lake, J.K.; Liu, X.J.; Ma, K.P.; et al. Phylogenetic and functional alpha and beta diversity in temperate and tropical tree communities. Ecology 2012, 93, S112–S125. [Google Scholar] [CrossRef]
- McGill, B.J.; Etienne, R.S.; Gray, J.S.; Alonso, D.; Anderson, M.J.; Benecha, H.K.; Dornelas, M.; Enquist, B.J.; Green, J.L.; He, F.L.; et al. Species abundance distributions: Moving beyond single prediction theories to integration within an ecological framework. Ecol. Lett. 2007, 10, 995–1015. [Google Scholar] [CrossRef] [PubMed]
- Schweiger, O.; Klotz, S.; Durka, W.; Kühn, I. A comparative test of phylogenetic diversity indices. Oecologia 2008, 157, 485–495. [Google Scholar] [CrossRef]
- Che, Y.; Jin, G.Z. effects of species diversity and phylogenetic diversity on the productivity of broadleaved Korean pine forests. Chin. J. Appl. Ecol. 2019, 30, 2241–2248. [Google Scholar] [CrossRef]
- Merwin, L.; He, T.H.; Lamont, B.B. Phylogenetic and phenotypic structure among Banksia communities in south-western Australia. J. Biogeogr. 2012, 39, 397–407. [Google Scholar] [CrossRef]
- Lu, Y.X.; Ye, M.; Qian, J.R.; Chen, W.L.; Che, J.; Li, M.M.; Zeng, G.Y. Study on biodiversity and phylogenetic diversity of grasslands in the Habahe forest area of Xinjiang and analysis of influencing factors. ActaPratac.Sin. 2025, 34, 1–12. [Google Scholar]
- Yan, Y.; Yang, X.; Tang, Z. Patterns of species diversity and phylogenetic structure of vascular plants on the Q inghai-T ibetan P lateau. Ecol. Evol. 2010, 3, 4584–4595. [Google Scholar] [CrossRef]
- Gong, H.; Yu, T.; Zhang, X.; Zhang, P.; Han, J.; Gao, J. Effects of boundary constraints and climatic factors on plant diversity along an altitudinal gradient. Glob. Ecol. Conserv. 2019, 19, e00671. [Google Scholar] [CrossRef]
- Macheroum, A.; Kadik, L.; Neffar, S.; Chenchouni, H. Environmental drivers of taxonomic and phylogenetic diversity patterns of plant communities in semi-arid steppe rangelands of North Africa. Ecol. Indic. 2021, 132, 108279. [Google Scholar] [CrossRef]
- Wani, S.A.; Zargar, S.A.; Dar, F.A.; Khoja, A.A.; Malik, A.H.; Rashid, I.; Khuroo, A.A. Elevational patterns and drivers of taxonomic and phylogenetic diversity of pteridophytes: A case study from the Himalaya. Flora 2025, 323, 152654. [Google Scholar] [CrossRef]
- Zheng, J.; Arif, M.; He, X.; Ding, D.; Zhang, S.; Ni, X.; Li, C. Plant community assembly is jointly shaped by environmental and dispersal filtering along elevation gradients in a semiarid area, China. Front. Plant Sci. 2022, 13, 1041742. [Google Scholar] [CrossRef]
- Ndiribe, C.; Salamin, N.; Guisan, A. Understanding the concepts of community phylogenetics. Evol. Ecol. Res. 2013, 15, 853–868. [Google Scholar] [CrossRef]
- Editorial Committee of Flora of China; Chinese Academy of Sciences. Flora of China; Beijing Science Press: Beijing, China, 1990. [Google Scholar]
- Fu, L.G. China Red Data Book of Rare and Endangered Plants; Beijing Science Press: Beijing, China, 1991; Volume 1. [Google Scholar]
- Luo, W.; Xu, H.; Li, Y.P.; Xie, C.H.; Lu, C.Y.; Liang, C.S.; Su, H.H.; Li, Z.L. Population Structure and Quantitative Dynamics of a Wild Plantwith Extremely Small Populations Hopea hainanensis. For. Res. 2023, 36, 169–177. [Google Scholar] [CrossRef]
- Wu, E.H.; Nong, S.Q.; Yang, X.B.; Lu, G.; Li, D.H. Hopea hainanensis, a representative tree species of the Hainan tropical rainforest. For. Hum. 2021, 10, 42–45. [Google Scholar]
- Zhang, L.; Zhang, H.L.; Chen, Y.K.; Nizamani, M.M.; Zhou, Q.; Su, X. Analyses of community stability and inter-specific associations between a plant species with extremely small populations (Hopea hainanensis) and its associated species. Front. Ecol. Evol. 2022, 10, 922829. [Google Scholar] [CrossRef]
- Wang, C.; Ma, X.; Ren, M.; Tang, L. Genetic diversity and population structure in the endangered tree Hopea hainanensis (Dipterocarpaceae) on Hainan Island, China. PLoS ONE 2020, 15, e0241452. [Google Scholar] [CrossRef]
- Xiao, Y.X.; Yin, L.H.; Yu, W.B.; Tang, J.W. Study on population structure and seedling regeneration of Hopea hainanensis Merr. Chun underex situ conservation in XishuangbannaTropical Botanical Garden. Plant. Sci. J. 2023, 41, 604–612. [Google Scholar] [CrossRef]
- Zhang, D.; Qi, X.; Liu, S.; Lu, K.; Chen, Y.; Long, W. Seasonal home range utilization of Hainan gibbons (Nomascus hainanus) in a secondary tropical forest of Hainan Island, South China. Glob. Ecol. Conserv. 2024, 54, e03063. [Google Scholar] [CrossRef]
- Xie, C.; Chen, L.; Luo, W.; Jim, C.Y. Species diversity and distribution pattern of venerable trees in tropical Jianfengling National Forest Park (Hainan, China). J. Nat. Conserv. 2014, 77, 126542. [Google Scholar] [CrossRef]
- Wang, G.H.; Fang, J.Y.; Guo, K.; Xie, Z.Q.; Tang, Z.Y.; Shen, Z.H.; Wang, R.Q.; Wang, X.P.; Wang, D.L.; Qiang, S.; et al. Contents and protocols for the classification and description of Vegetation Formations, Alliances and Associations of vegetation of China. Chin. J. Plant. Ecol. 2020, 44, 128–178. [Google Scholar] [CrossRef]
- Zhao, Y.; Qi, R.; Li, B.; Liu, T.; Cao, J.H.; Li, Y. Niche of woody plant populations in Picea purpurea community in the upper Taohe River. Ecol. Indic. 2024, 166, 112557. [Google Scholar] [CrossRef]
- Li, L.; Wei, S.G.; Lian, J.Y.; Cao, H. Distributional regularity of species diversity in plant community at different latitudes in subtropics. Acta Ecol. Sin. 2020, 40, 1249–1257. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhang, Y.; Su, Q.; Gan, Q.; Zhou, Q.; Guo, Y.; Hassan, M.U. MaxEnt Modeling for Predicting the Potential Geographical Distribution of Camellia oleifera Abel Under Climate Change. Foresty 2025, 16, 1026. [Google Scholar] [CrossRef]
- Luo, X.; Yang, M.; Khan, M.S.; Huang, W.; Liu, S.; Liao, F.; Li, Y. Climate and topographic drivers of species and functional diversity in subtropical shrublands of Guangdong, China. Ecol. Indic. 2025, 176, 113641. [Google Scholar] [CrossRef]
- Yi, M. Soil Agro-Chemical Analysis; Agricultural Press: North Richmond, Australia, 1980. [Google Scholar]
- Su, Y.Q.; Zhang, Y.; Jia, X.R.; Xue, Y.G. Application of several diversity indexes in forest community analysis. Ecol. Sci. 2017, 36, 132–138. [Google Scholar] [CrossRef]
- Pommerening, A. Approaches to quantifying forest structures. Foresty 2002, 75, 305–324. [Google Scholar] [CrossRef]
- Gamito, S. Caution is needed when applying Margalef diversity index. Ecol. Indic. 2010, 10, 550–551. [Google Scholar] [CrossRef]
- Wu, H.P.; Liu, J.; Liu, L. Analysis of Plant Community Structure and Similarity at Different Altitudes in Yinggeling Tropical Montane Rainforest, Hainan. Bot. Res. 2024, 13, 1. [Google Scholar] [CrossRef]
- Zhong, Y.M.; Guo, X.S.; Yao, Z.Y. Community Composition and Species Diversity of Deciduous Oak Forest in South Subtropical Region of West Guangxi. Acta Bot. Bor-Occid. Sin. 2022, 42, 1945–1953. [Google Scholar] [CrossRef]
- Huang, R.X.; Xu, M.F.; Liu, T.; Wu, Z.L.; Su, Z.Y. Environmental Interpretation and Species Diversity of Understory Vegetation in 5 Subtropical Forest Types. J. Southwest For. Univ. (Nat. Sci. Ed.) 2020, 40, 53–62. [Google Scholar] [CrossRef]
- Wang, Q.; Li, J.B.; Yang, X.B.; Zeng, R.J.; Xia, D.; Wang, H.; Zhu, Z.C.; Jiang, Y.X.; Wang, C.Y.; Zhang, S.W.; et al. Community structure and species diversity of Pinuslatteri forests in Bawangling Ridge, Hainan. J. Trop. Biol. 2023, 14, 585–592. [Google Scholar] [CrossRef]
- Xu, H.; Li, Y.D.; Lin, M.X.; Wu, J.H.; Luo, T.S.; Zhou, Z.; Chen, D.X.; Yang, H.; Li, G.J.; Liu, S.R. Community characteristics of a 60 ha dynamics plot in the tropical montane rain forest in Jianfengling, Hainan Island. Biodivers. Sci. 2015, 23, 192–201. [Google Scholar] [CrossRef]
- Liu, H.D.; Chen, Q.; Xu, Z.Y.; Liu, Y.; Jiang, Y.; Chen, Y.F. Effects of topographical factors on species diversity across Dacrydium pectinatum natural community in Hainan Island. Chin. J. Ecol. 2020, 39, 394–403. [Google Scholar] [CrossRef]
- Wu, Y.P.; Xu, H.; Li, Y.D. Elevation Patterns of Tree and Shrub Species Diversity of Tropical Forests in Jianfengling, Hainan Island. Sci. Silv. Sin. 2013, 49, 16–23. [Google Scholar] [CrossRef]
- Xu, G.X.; Shi, Z.M.; Tang, J.C.; Xu, H.; Yang, H.; Liu, L.R.; Li, Y.D.; Li, M.X. Effects of species abundance and size classes on assessing community phylogenetic structure: A case study in Jianfengling tropical montane rainforest. Biodivers. Sci. 2016, 24, 617–628. [Google Scholar] [CrossRef]
- Sedio, B.E.; Parker, J.D.; McMahon, S.M.; Wright, S.J. Comparative metabolomics of forest communities: Species differences in foliar chemistry are greater in the tropics. bioRxiv 2018. [Google Scholar] [CrossRef]
- Asefa, M.; Cao, M.; Zhang, G.; Ci, X.; Li, J.; Yang, J. Environmental filtering structures tree functional traits combination and lineages across space in tropical tree assemblages. Sci. Rep. 2017, 7, 132. [Google Scholar] [CrossRef]
- Li, M.J.; He, Z.S.; Jiang, L.; Gu, X.G.; Ji, M.R.; Chen, B.; Liu, J.F. Distribution pattern and driving factors of species diversity and phylogenetic diversity along altitudinal gradient on the south slope of Daiyun Mountain. Acta. Ecol. Sin. 2021, 41, 1148–1157. [Google Scholar] [CrossRef]
- Khan, F.; Siddique, A.B.; Shabala, S. Phosphorus plays key roles in regulating plants’ physiological responses to abiotic stresses. Plants 2023, 12, 2861. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, X.; Su, J.; Bai, Y. Phosphorus availability and planting patterns regulate soil microbial effects on plant performance in a semiarid steppe. Ann. Bot. 2023, 131, 1081–1095. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Dai, L.; Lin, Z.B.; Wu, J.T.; Yan, W.; Wang, Z.J. Plant Diversity and Its Relationship with Soil Physicochemical Properties of Urban Forest Communities in Central Guizhou. J. Ecol. Environ. 2021, 30, 2130–2141. [Google Scholar] [CrossRef]
- Xiao, D.R.; Tian, K.; Zhang, L.Q. Relationship between plant diversity and soil fertility in Napahai wetland of Northwestern Yunnan Plateau. Acta Ecol. Sin. 2008, 28, 3116–3124. Available online: https://www.ecologica.cn/stxb/article/abstract/20080720?st=article_issue (accessed on 20 June 2025).
- Liang, J.; Zhang, J.; Wong, M.H. Effects of air-filled soil porosity and aeration on the initiation and growth of secondary roots of maize (Zea mays). Plant Soil 1996, 186, 245–254. [Google Scholar] [CrossRef]
- Qian, G.; Lu, G. Effects of soil factors on functional trait space of different vegetations in the Tianshan Mountains. Catena 2025, 252, 108852. [Google Scholar] [CrossRef]
- Xue, W.; Rillig, M.C.; Yu, M.F.; Hu, J.N.; Huang, L.; Yu, F.H. Soil nutrient heterogeneity alters productivity and diversity of experimental plant communities under multiple global change factors. Oikos 2023, 2023, e10189. [Google Scholar] [CrossRef]
| Similarity Index | SIJa | SISo | |
|---|---|---|---|
| Forest Layer | |||
| Middle arbor layer | 0.10 | 0.18 | |
| Small arbor layer | 0.12 | 0.21 | |
| Large shrub layer | 0.13 | 0.23 | |
| Middle shrub layer | 0.09 | 0.17 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, S.; Li, D.; Yang, X.; Qi, D.; Shang, N.; Liang, C.; Liu, R.; Du, C. Environmental Heterogeneity Drives Diversity Across Forest Strata in Hopea hainanensis Communities. Diversity 2025, 17, 556. https://doi.org/10.3390/d17080556
He S, Li D, Yang X, Qi D, Shang N, Liang C, Liu R, Du C. Environmental Heterogeneity Drives Diversity Across Forest Strata in Hopea hainanensis Communities. Diversity. 2025; 17(8):556. https://doi.org/10.3390/d17080556
Chicago/Turabian StyleHe, Shaocui, Donghai Li, Xiaobo Yang, Dongling Qi, Naiyan Shang, Caiqun Liang, Rentong Liu, and Chunyan Du. 2025. "Environmental Heterogeneity Drives Diversity Across Forest Strata in Hopea hainanensis Communities" Diversity 17, no. 8: 556. https://doi.org/10.3390/d17080556
APA StyleHe, S., Li, D., Yang, X., Qi, D., Shang, N., Liang, C., Liu, R., & Du, C. (2025). Environmental Heterogeneity Drives Diversity Across Forest Strata in Hopea hainanensis Communities. Diversity, 17(8), 556. https://doi.org/10.3390/d17080556





