Spread and Ecology of the Bumblebee Bombus haematurus (Hymenoptera: Apidae) in Northeastern Italy
Abstract
1. Introduction
2. Materials and Methods
3. Results
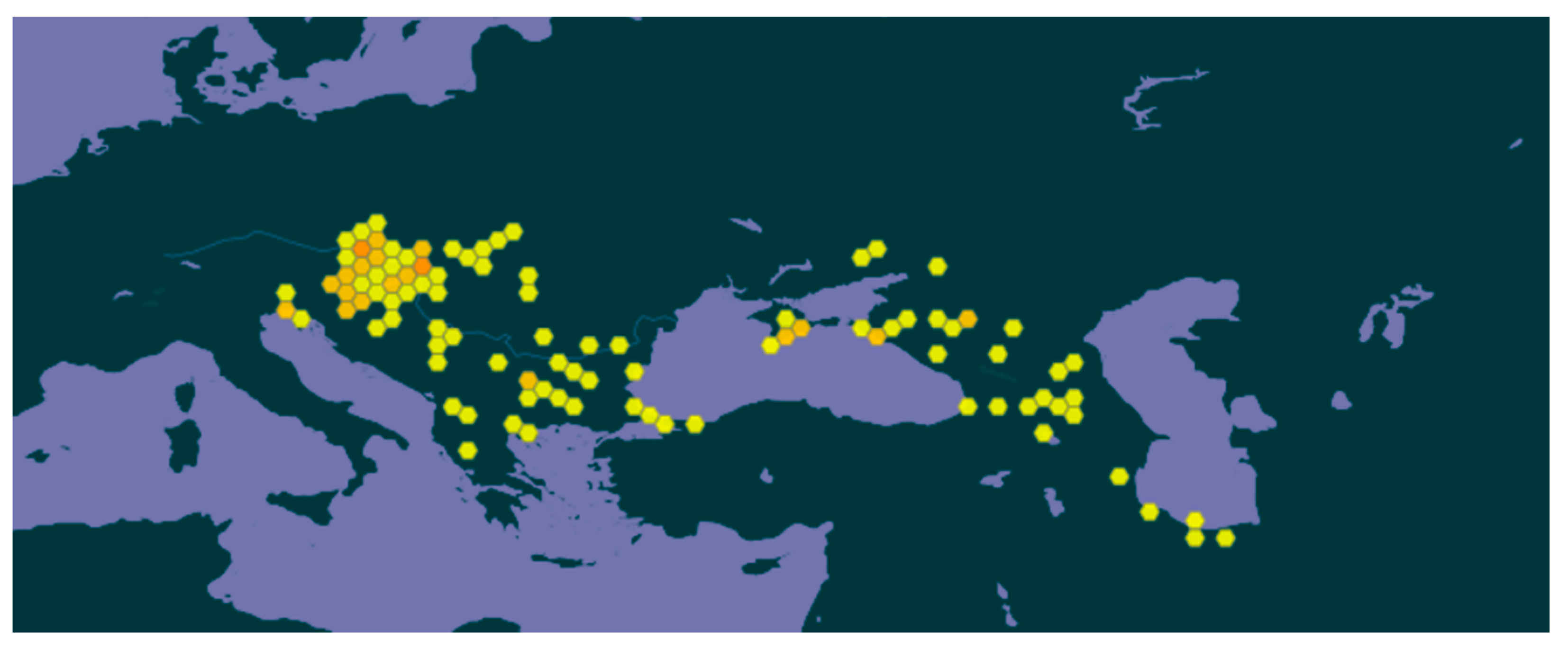
3.1. Specimens Recorded and Species Distribution
3.2. Habitats
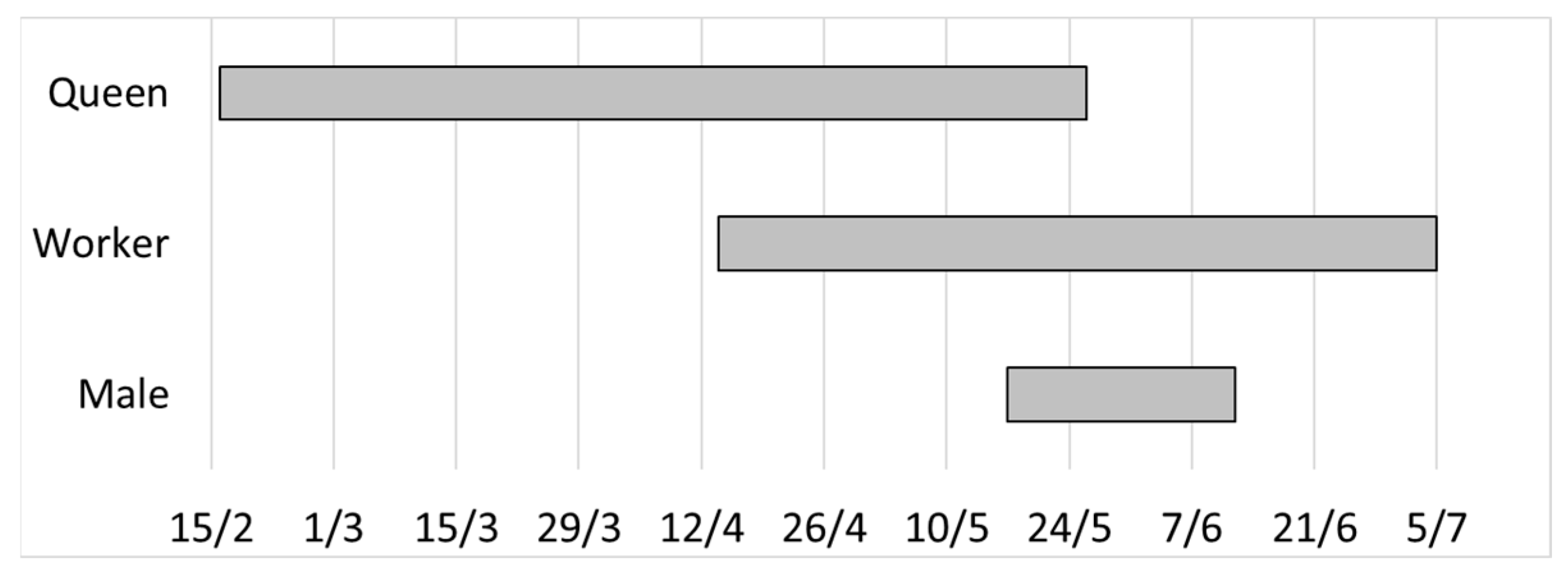
3.3. Preliminary Data on the Flying Season
3.4. Flowering Plants Visited
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neumayer, J. Erstfund von Bombus haematurus Kriechbaumer, 1870 (Hymenoptera, Apidae) in Österreich. Beitr. Entomofaunist. 2004, 5, 134–135. [Google Scholar]
- Šima, P.; Smetana, V. Current distribution of the bumble bee Bombus haematurus (Hymenoptera: Apidae, Bombini) in Slovakia. Klapalekiana 2009, 45, 209–212. [Google Scholar]
- Jenič, A.; Gogala, A.; Grad, J. Bombus haematurus (Hymenoptera: Apidae), new species in the Slovenian bumblebee fauna. Acta Entomol. Slov. 2010, 18, 168–170. [Google Scholar]
- Rasmont, P.; Franzén, M.; Lecocq, T.; Harpke, A.; Roberts, S.P.M.; Biesmeijer, J.C.; Castro, L.; Cederberg, B.; Dvorák, L.; Fitzpatrick, Ú.; et al. Climatic risk and distribution atlas of European bumblebees. BioRisk 2015, 10, 1–236. [Google Scholar] [CrossRef]
- Straka, J.; Bogusch, P.; Tyrner, P.; Říha, M.; Benda, D.; Čížek, O.; Halada, M.; Macháčková, L.; Marhoul, P.; Tropek, R. Faunistic records from the Czech Republic—380, Hymenoptera: Aculeata: Apoidea. Klapalekiana 2015, 51, 77–91. [Google Scholar]
- Biella, P.; Galimberti, A. The spread of Bombus haematurus in Italy and its first DNA barcode reference sequence (Hymenoptera: Apidae). Fragm. Entomol. 2020, 52, 67–70. [Google Scholar] [CrossRef]
- Czyżewski, S.; Kierat, J.; Zapotoczny, K. First record of Bombus haematurus Kriechbaumer, 1870 (Hymenoptera: Apidae) in Poland. Acta Zool. Cracoviensia 2023, 67, 7–10. [Google Scholar] [CrossRef]
- Biella, P.; Ćetković, A.; Gogala, A.; Neumayer, J.; Sárospataki, M.; Šima, P.; Smetana, V. Northwestward range expansion of the bumblebee Bombus haematurus into Central Europe is associated with warmer winters and niche conservatism. Insect Sci. 2021, 28, 861–872. [Google Scholar] [CrossRef]
- Ghisbain, G.; Gérard, M.; Wood, T.J.; Hines, M.H.; Michez, D. Expanding insect pollinators in the Anthropocene. Biol. Rev. 2021, 96, 2755–2770. [Google Scholar] [CrossRef] [PubMed]
- GBIF Occurrence Download. Available online: https://www.gbif.org/dataset/50c9509d-22c7-4a22-a47d-8c48425ef4a7 (accessed on 2 January 2025).
- Reinig, W.F. Ökologische Studien an mittel- und südosteuropäischen Hummeln (Bombus Latr., 1802; Hym., Apidae). Mitt. Münch. Entomol. Ges. 1972, 60, 1–56. [Google Scholar]
- Bossert, S.; Schneller, B. First records of Bombus haematurus Kriechbaumer, 1870 and Nomada moeschleri Alfken, 1913 (Hymenoptera: Apidae) for the state of Vienna (Austria). Beitr. Entomofaunist. 2014, 15, 95–100. [Google Scholar]
- Šima, P.; Smetana, V.; Riegler, M. Quo vadis Bombus haematurus? An outline of the ecology and biology of a species expanding in Slovakia. Acta Musei Tekovensis Levice 2018, 11, 41–65. [Google Scholar]
- Teppner, H. Flower visitation of Bombus haematurus Kriechbaumer 1870 (Hymenoptera, Apidae) in Graz, Styria. Linzer Biol. Beitr. 2010, 42, 89–94. [Google Scholar]
- Grad, J.; Gradišek, A. Bumblebee brood temperature and colony development: A field study. Acta Entomol. Slov. 2018, 26, 219–232. [Google Scholar]
- Nieto, A.; Roberts, S.P.M.; Kemp, J.; Rasmont, P.; Kuhlmann, M.; García Criado, M.; Biesmeijer, J.C.; Bogusch, P.; Dathe, H.H.; De la Rúa, P.; et al. European Red List of Bees; Publication Office of the European Union: Luxembourg, 2014; 84p. [Google Scholar] [CrossRef]
- Rasmont, P.; Devalez, J.; Pauly, A.; Michez, D.; Radchenko, V.G. Addition to the checklist of IUCN European wild bees (Hymenoptera: Apoidea). Ann. Soc. Entomol. Fr. 2017, 53, 17–32. [Google Scholar] [CrossRef]
- Reverté, S.; Miličić, M.; Ačanski, J.; Andrić, A.; Aracil, A.; Aubert, M.; Balzan, M.V.; Bartomeus, I.; Bogusch, P.; Bosch, J.; et al. National records of 3000 European bee and hoverfly species: A contribution to pollinator conservation. Insect Conserv. Diver. 2023, 16, 758–775. [Google Scholar] [CrossRef]
- Ghisbain, G.; Rosa, P.; Bogusch, P.; Flaminio, S.; Le Divelec, R.; Dorchin, A.; Kasparek, M.; Kuhlmann, M.; Litman, J.; Mignot, M.; et al. The new annotated checklist of the wild bees of Europe (Hymenoptera: Anthophila). Zootaxa 2023, 5327, 1–147. [Google Scholar] [CrossRef]
- Pagliano, G. Hymenoptera Apoidea. In Checklist delle Specie della Fauna Italiana, 106; Minelli, A., Ruffo, S., La Posta, S., Eds.; Calderini: Bologna, Italy, 1995; 25p. [Google Scholar]
- Quaranta, M.; Ambroselli, S.; Barro, P.; Bella, S.; Carini, A.; Celli, G.; Cogoi, P.; Comba, L.; Comoli, R.; Felicioli, A.; et al. Wild bees in agroecosystems and semi-natural landscapes. 1997–2000 collection period in Italy. Bull. Insectol. 2004, 57, 11–61. [Google Scholar]
- Barbattini, R.; Frilli, F.; Zandigiacomo, P.; Pagliano, G.; Quaranta, M. Apoidea del Friuli Venezia Giulia e di territori confinanti. II: Apidae. Gortania 2006, 28, 139–184. [Google Scholar]
- Quaranta, M.; Cornalba, M. Council for Agricultural Research and Economics, Research Centre for Agriculture and Environment—CREA, Bologna, Italy. 2025; manuscript in preparation. [Google Scholar]
- Pittioni, B. Die Hummeln und Schmarotzerhummeln der Balkan-Halbinsel. II. Spezieller Teil. Mitt. Königl. Nat.wiss. Inst. Sofia 1939, 12, 49–115. [Google Scholar]
- Gokcezade, J.F.; Gereben-Krenn, B.-A.; Neumayer, J.; Krenn, H.W. Feldbestimmungsschlüssel für die Hummeln Österreichs, Deutschlands und der Schweiz (Hymenoptera, Apidae). Linzer Boil. Beitr. 2015, 47, 5–42. [Google Scholar]
- Simonetti, G.; Frilli, F.; Barbattini, R.; Iob, M. Flora di interesse apistico. Uno studio di botanica applicata in Friuli-Venezia Giulia. Apicoltura 1989, 5, 1–377. [Google Scholar]
- Ricciardelli D’Albore, G.; Intoppa, F. Fiori e Api. La Flora Visitata dalle Api e Dagli Altri Apoidei in Europa; Calderini Edagricole: Bologna, Italy, 2000; 253p. [Google Scholar]
- Peruzzi, L. Crocus heuffelianus (Iridaceae), a new record for the Italian flora. Phytotaxa 2016, 261, 298–300. [Google Scholar] [CrossRef]
- Martini, F.; Bertani, G.; Boscutti, F.; Bruna, A.; Danelutto, A.; Pavan, R.; Peruzovich, C. Flora del Friuli Venezia Giulia. Repertorio Critico Diacronico e Atlante Corologico; Martini, F., Ed.; Forum: Udine, Italy, 2023; 1008p. [Google Scholar]
- Portal to the Flora of Italy. Available online: https://dryades.units.it/floritaly/ (accessed on 15 February 2025).
- Jakab, D.A.; Tóth, M.; Szarukán, I.; Szanyi, S.; Józan, Z.; Sárospataki, M.; Nagy, A. Long-term changes in the composition and distribution of the Hungarian bumble bee fauna (Hymenoptera, Apidae, Bombus). J. Hymenopt. Res. 2023, 96, 207–237. [Google Scholar] [CrossRef]
- Konovalova, I.B. The bumble bees of Ukraine: Species distribution and floral preferences. Psyche—J. Entomol. 2010, 2010, 819740. [Google Scholar] [CrossRef]
- Anagnostopoulos, I.T. The bumblebee fauna of Greece: An annotated species list including new records for Greece (Hymenoptera: Apidae, Bombini). Linzer Boil. Beitr. 2005, 37, 1013–1026. [Google Scholar]
- Özbek, H. On the bumblebee fauna of Turkey: II. The genus Pyrobombus (Hymenoptera, Apidae, Bombinae). Zool. Middle East 1998, 16, 89–106. [Google Scholar] [CrossRef]
- Baker, D.B. On a collection of bumble-bees from northern Iran. Beiträge Entomol. Beirt. Ent. 1996, 46, 109–132. [Google Scholar]
- Manino, A.; Patetta, A.; Porporato, M.; Quaranta, M.; Intoppa, F.; Piazza, M.G.; Frilli, F. Bumblebee (Bombus Latreille, 1802) distribution in high mountains and global warming. Redia 2007, 40, 125–128. [Google Scholar]
- Reinig, W.F. Zur Verbreitung einiger Hummelarten auf der Balkan-Halbinsel (Hym., Bombidae). Nachrichtenbl. Bayer. Entomol. 1974, 23, 11–13. [Google Scholar]
- Kozuharova, E.; Trifonov, T.; Stoycheva, C.; Zapryanova, N.; Sokolov, R.S. Plants for Wild Bees—Field Records in Bulgaria. Diversity 2025, 17, 214. [Google Scholar] [CrossRef]
- Teppner, H.; Ebmer, A.W.; Gusenleitner, F.; Schwarz, M. The bees (Apidae, Hymenoptera) of the Botanic Garden in Graz, an annotated list. Mitt. Naturwiss. Ver. Steiermark 2016, 146, 19–68. [Google Scholar]
- Michołap, P.; Sikora, A.; Pawlikowski, T.; Sikora, M. Dispersion of bumblebee Bombus semenoviellus Skorikov (Hymenoptera, Apidae) in Poland. J. Apic. Sci. 2020, 64, 47–54. [Google Scholar] [CrossRef]
- Biella, P.; Cornalba, M.; Rasmont, P.; Neumayer, J.; Mei, M.; Brambilla, M. Climate tracking by mountain bumblebees across a century: Distribution retreats, small refugia and elevational shifts. Glob. Ecol. Conserv. (GECCO) 2024, 54, e03163. [Google Scholar] [CrossRef]
- Haris, A.; Józan, Z.; Roller, L.; Šima, P.; Tóth, S. Changes in Population Densities and Species Richness of Pollinators in the Carpathian Basin during the Last 50 Years (Hymenoptera, Diptera, Lepidoptera). Diversity 2024, 16, 328. [Google Scholar] [CrossRef]
- Haris, A.; Józan, Z.; Schmidt, P.; Glemba, G.; Tomozii, B.; Csóka, G.; Hirka, A.; Šima, P.; Tóth, S. Climate Change Influences on Central European Insect Fauna over the Last 50 Years: Mediterranean Influx and Non-Native Species. Ecologies 2025, 6, 16. [Google Scholar] [CrossRef]
- Cornalba, M.; Quaranta, M.; Selis, M.; Flaminio, S.; Gamba, S.; Mei, M.; Bonifacino, M.; Cappellari, A.; Catania, R.; Niolu, P.; et al. Exploring the hidden riches: Recent remarkable faunistic records and range extensions in the bee fauna of Italy (Hymenoptera, Apoidea, Anthophila). Biodivers. Data J. 2024, 12, e116014. [Google Scholar] [CrossRef]
- Quaranta, M.; Sommaruga, A.; Balzarini, P.; Felicioli, A. A new species for the bee fauna of Italy: Megachile sculpturalis continues its colonization of Europe. Bull. Insectol. 2014, 67, 287–293. [Google Scholar]
- Bortolotti, L.; Luthi, F.; Flaminio, S.; Bogo, G.; Sgolastra, F. First record of the Asiatic bee Megachile disjunctiformis in Europe. Bull. Insectol. 2018, 71, 143–149. [Google Scholar]
- Flaminio, S.; Bortolotti, L.; Cilia, G. First report of Xylocopa aestuans in Italy: A new species for Europe? Bull. Insectol. 2023, 76, 161–166. [Google Scholar]


| District | Geographic Area | Site | m a.s.l. | N. of Visits | Bombus haematurus | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Q | W | M | Feb | Mar | Apr | May | June | July | |||||
| Gorizia | Eastern Friulian plains (Isonzo plain) | GO1 | 33 | 2 * | 2 | - | 2 | - | - | - | - | 1 | 1 | - |
| GO2 | 118 | 7; 3y | 26 | 6 | 18 | 2 | - | 6 (3) | 6 (2) | 14 (2) | - | - | ||
| GO3 | 116 | 1 | 1 | - | 1 | - | - | - | 1 | - | - | - | ||
| GO4 | 55 | 1 | 1 | - | 1 | - | - | - | - | - | 1 | - | ||
| GO5 | 33 | 3; 1y | 13 | 3 | 6 | 4 | - | - | 7 (2) | 6 | - | - | ||
| GO6 | 108 | 1 | 2 | 2 | - | - | - | 2 | - | - | - | - | ||
| Trieste | Coast | TS1 | 90 | 2; 2y | 2 | 2 | - | - | 1 | - | 1 | - | - | - |
| Karst plateau | TS2 | 197 | 1 | 1 | - | 1 | - | - | - | - | 1 | - | - | |
| TS3 | 128 | 1 | 1 | 1 | - | - | - | 1 | - | - | - | - | ||
| TS4 | 165 | 1 | 1 | 1 | - | - | - | 1 | - | - | - | - | ||
| Udine | Morainic hills | UD1 | 142 | 16, 3y | 147 | 40 | 80 | 27 | - | 29 (5) | 14 (5) | 103 (5) | 1 | - |
| UD2 | 161 | 2, 1y | 6 | 6 | - | - | - | 6 (2) | - | - | - | - | ||
| UD3 | 143 | 5, 1y | 6 | 5 | 1 | - | - | 2 (2) | 3 (2) | - | 1 | - | ||
| UD4 | 218 | 1 | 4 | 4 | - | - | - | 4 | - | - | - | - | ||
| UD5 | 147 | 1 | 1 | 1 | - | - | - | 1 | - | - | - | - | ||
| Julian Prealps | UD6 | 210 | 2, 2y | 2 | 2 | - | - | - | 1 | 1 | - | - | - | |
| UD7 | 364 | 1 | 5 | 5 | - | - | - | - | - | 5 | - | - | ||
| Central Friulian plains | UD8 | 64 | 2, 1y | 3 | - | 2 | 1 | - | - | - | 1 | 2 | - | |
| UD9 | 29 | 5, 1y | 10 | - | 10 | - | - | - | - | 4 | 5 (3) | 1 | ||
| UD10 | 10 | 1 | 1 | - | 1 | - | - | - | - | - | - | 1 | ||
| Pordenone | Carnic Prealps | PN1 | 135 | 1 | 1 | 1 | - | - | - | - | 1 | - | - | - |
| PN2 | 247 | 1 | 2 | - | 2 | - | - | - | - | - | 2 | - | ||
| Total | 57 | 238 | 79 | 125 | 34 | 1 | 53 | 34 | 135 | 13 | 2 | |||
| Family | Species | Plant Features | N. of Events | N. of Bombus haematurus | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Flowering Period | Nectar | Pollen | Feb | Mar | Apr | May | June | July | Tot. | |||
| Boraginaceae | Echium vulgare L. | IV–IX | +++ | ++ | 4, 1 site | - | - | - | 4 | 4 | - | 8 |
| Caprifoliaceae | Knautia drymeia Heuff. | V–VII | ++ (*) | ++ (*) | 2, 1 site | - | - | - | 1 | 1 | - | 2 |
| Lamiaceae | Lamium galeobdolon (L.) | IV–VIII | ++ | + | 11, 4 sites | - | - | 6 | 101 | 2 | - | 109 |
| Lamium maculatum L. | III–XII | + | ++ | 12, 5 sites | - | 3 | 13 | 13 | 1 | - | 30 | |
| Lamium orvala L. | III–VI | ++ | + | 11, 7 sites | - | 5 | 10 | 7 | 1 | - | 23 | |
| Lamium purpureum L. | III–X | + | ++ | 6, 5 sites | - | 13 | - | - | - | - | 13 | |
| Lavandula angustifolia Mill. | VI–IX | +++ | + | 3, 3 sites | - | - | - | - | 1 | 2 | 3 | |
| Salvia pratensis L. | V–III | ++ (*) | NR | 1 | - | - | - | 8 | - | - | 8 | |
| Salvia verticillata L. | VI–VII | ++ (*) | NR | 1 | - | - | - | - | 1 | - | 1 | |
| Teucrium fruticans L. | IV–VIII | ++ | + | 1 | - | - | 1 | - | - | - | 1 | |
| Fabaceae | Vicia cracca L. | V–VIII | + | + | 2, 2 sites | - | - | - | 1 | 1 | - | 2 |
| Orobanchaceae | Lathraea squamaria L. | III–VI | NR | NR | 3, 2 sites | - | 3 | 1 | - | - | - | 4 |
| Papaveraceae | Corydalis cava (L.) | III–V | + | ++ | 3, 1 site | - | 16 | - | - | - | - | 16 |
| Plantaginaceae | Veronica persica Poir. | I–XII | + | + | 1 | - | 1 | - | - | - | - | 1 |
| Rubiaceae | Asperula taurina L. | III–VI | + (*) | + (*) | 1 | - | - | 1 | - | - | - | 1 |
| Iridaceae | Crocus heuffelianus Herb. | II–IV | + | ++ | 1 | - | 1 | - | - | - | - | 1 |
| Liliaceae | Erythronium dens-canis L. | III–IV | NR | NR | 1 | - | 1 | - | - | - | - | 1 |
| Ranunculaceae | Helleborus odorus Waldst. & Kit. | II–IV | ++ | ++ | 2, 1 site | - | 2 | - | - | - | - | 1 |
| Asparagaceae | Scilla bifolia L. | III–IV | + | ++ | 1 | - | 1 | - | - | - | - | 1 |
| Aphid (Hemiptera: Aphidoidea) honeydew on leaves of apple trees | 1 | - | - | - | 1 | - | - | 1 | ||||
| Flying above ground | 6, 3 sites | 1 | 5 | 2 | - | - | - | 8 | ||||
| N. of Bombus haematurus | 1 | 53 | 34 | 136 | 12 | 2 | 238 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cargnus, E.; Quaranta, M.; Villani, A.; Zandigiacomo, P. Spread and Ecology of the Bumblebee Bombus haematurus (Hymenoptera: Apidae) in Northeastern Italy. Diversity 2025, 17, 534. https://doi.org/10.3390/d17080534
Cargnus E, Quaranta M, Villani A, Zandigiacomo P. Spread and Ecology of the Bumblebee Bombus haematurus (Hymenoptera: Apidae) in Northeastern Italy. Diversity. 2025; 17(8):534. https://doi.org/10.3390/d17080534
Chicago/Turabian StyleCargnus, Elena, Marino Quaranta, Alberto Villani, and Pietro Zandigiacomo. 2025. "Spread and Ecology of the Bumblebee Bombus haematurus (Hymenoptera: Apidae) in Northeastern Italy" Diversity 17, no. 8: 534. https://doi.org/10.3390/d17080534
APA StyleCargnus, E., Quaranta, M., Villani, A., & Zandigiacomo, P. (2025). Spread and Ecology of the Bumblebee Bombus haematurus (Hymenoptera: Apidae) in Northeastern Italy. Diversity, 17(8), 534. https://doi.org/10.3390/d17080534







