Climate in Europe and Africa Sequentially Shapes the Spring Passage of Long-Distance Migrants at the Baltic Coast in Europe
Abstract
1. Introduction
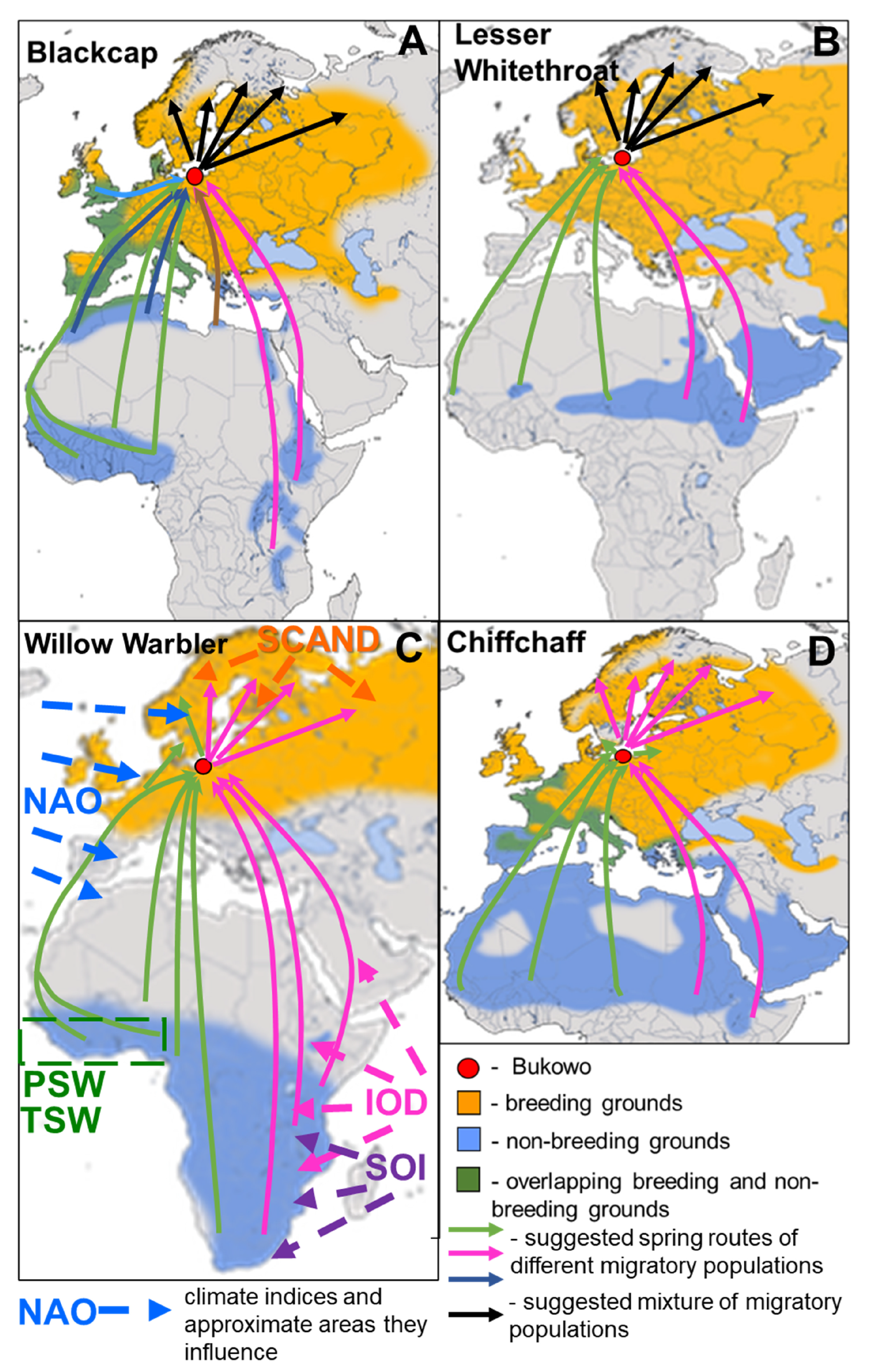
2. Materials and Methods
2.1. Study Species and Their Spring Migration Routes
2.1.1. Blackcap
2.1.2. Lesser Whitethroat
2.1.3. Willow Warbler
2.1.4. Chiffchaff
2.2. Study Site and Methods of Fieldwork
2.3. Methods of Analysing Data on Migrant Birds
2.4. Climate Indices Selected for the Study
2.5. Methods of Statistical Analysis
3. Results
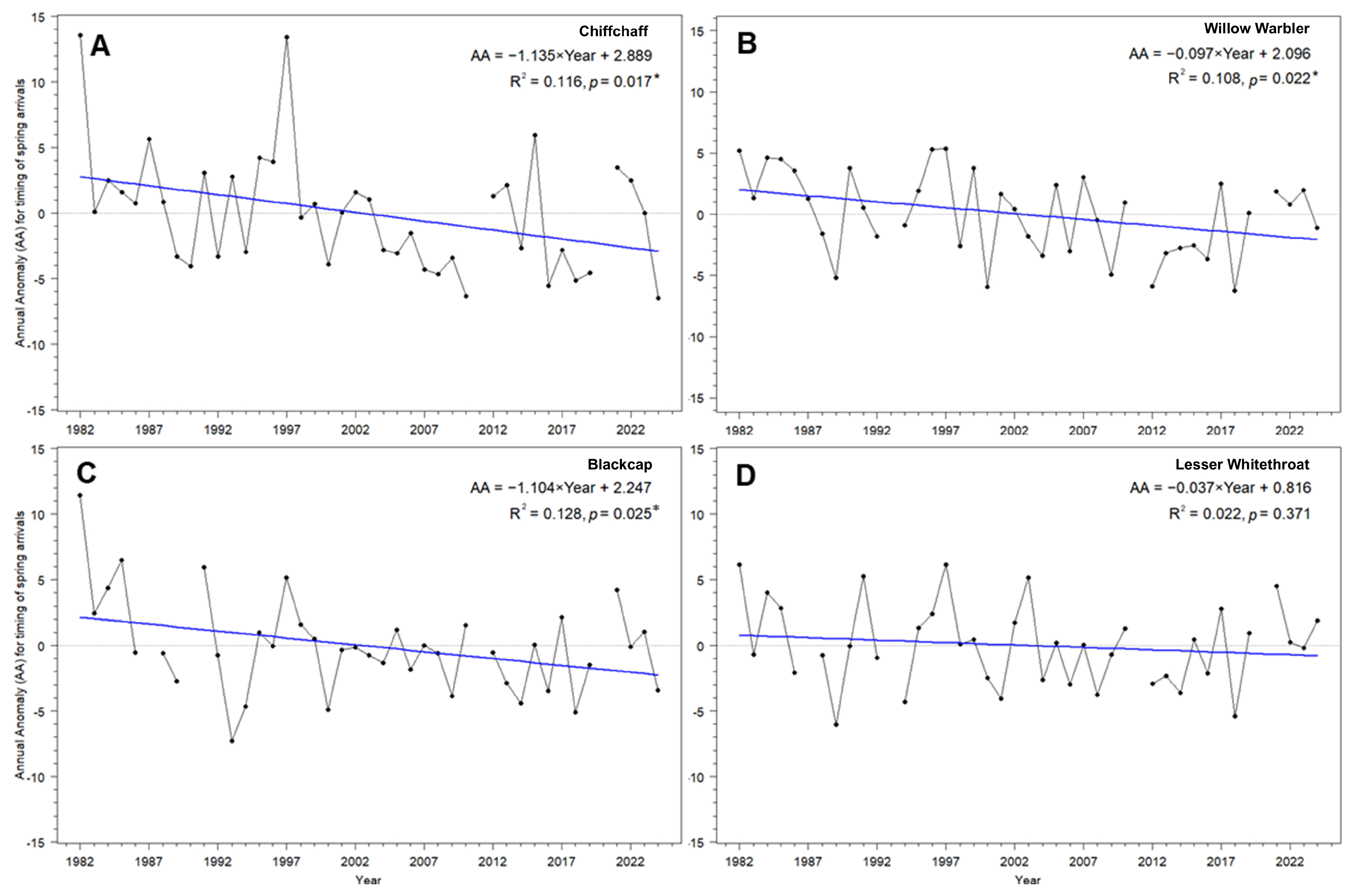
3.1. Multi-Year Trends in the Spring Passage Timing at the Baltic Sea Coast for the Four Study Species
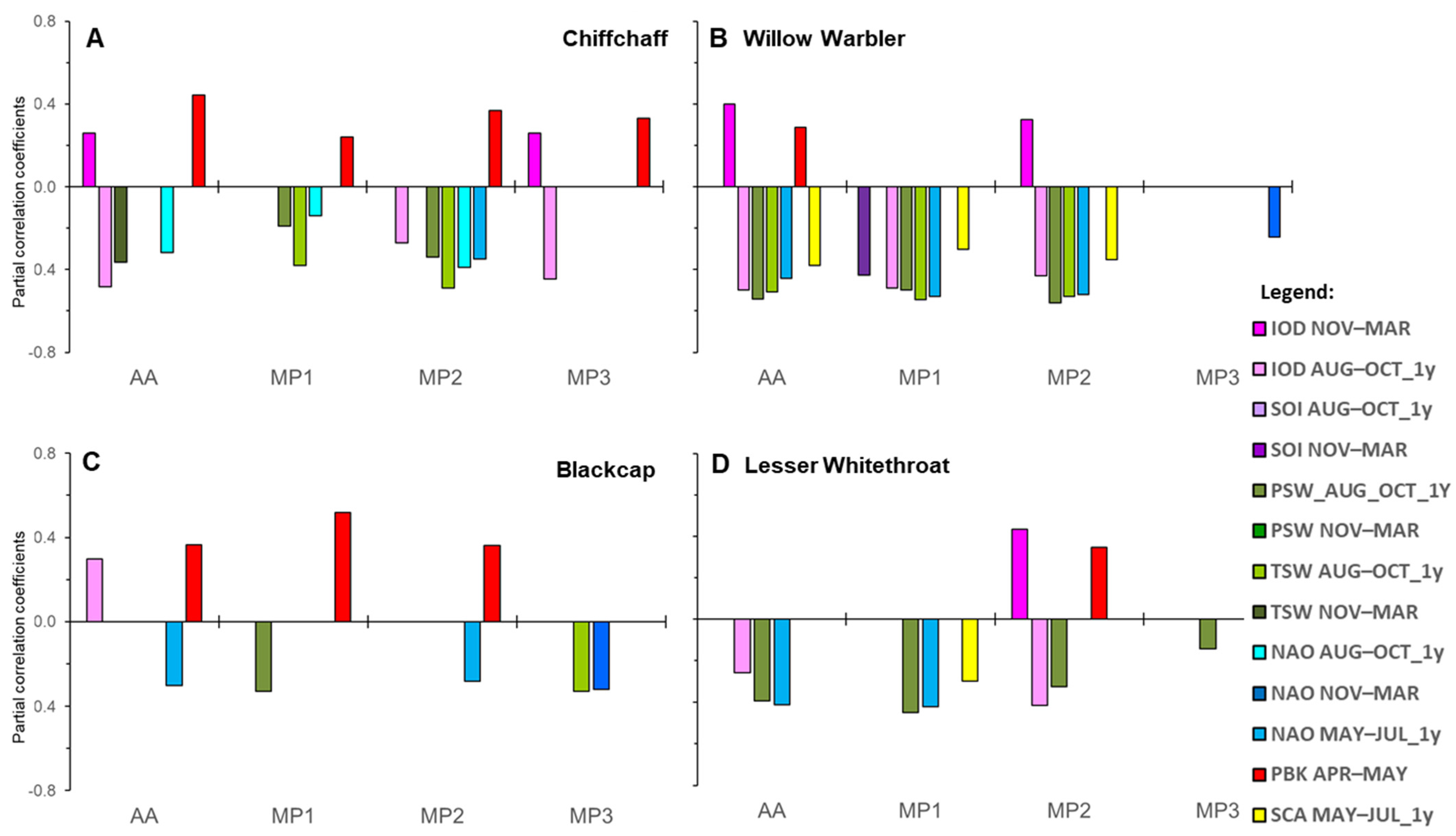
3.2. Effects of Climate Factors on the Entire Spring Passage (AA) and Its Three Main Periods (MP1–MP3) in the Four Studied Species
3.3. Effects of Climate Factors on Migrants in Nine Overlapping Sub-Periods of Passage (SP1–SP9)
4. Discussion
4.1. Long-Term Trends in the Overall Spring Migration Timing in the Four Species
4.2. Sequential Effects of Climate Factors on Spring Passage on the Southern Baltic Coast in the Context of Migration Patterns of the Four Long-Distance Migrants
4.2.1. Carry-Over Effect of Conditions During the Previous Autumn and Winter on Spring Passage of the Long-Distance Migrants on the Southern Baltic Coast
4.2.2. The Climatic Influence of Conditions in West and East Africa on the Four Species as an Evidence of the Crossroads of Western and Eastern Flyways at the Baltic Coast
4.2.3. The Variety of Climate Factors That Influence Each Species in the Context of Its Geographical Range
4.2.4. The Temporal Changes in the Influence of Climate Factors as a Reflection of the Sequence of Passage of Different Migratory Populations
Comparison of the Climatic Effects in the Two Leaf Warblers
Comparison of the Climatic Effects in the Two Larger Warblers
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Year | Location | N Chiffchaff | N Willow Warbler | N Lesser Whitethroat | N Blackcap | N Nets |
|---|---|---|---|---|---|---|
| 1982 | Kopań | 90 | 1016 | 123 | 88 | 57 |
| 1983 | Kopań | 100 | 498 | 186 | 80 | 57 |
| 1984 | Kopań | 52 | 180 | 48 | 22 | 42 |
| 1985 | Kopań | 39 | 189 | 42 | 13 | 51 |
| 1986 | Kopań | 29 | 62 | 12 | 24 | 50 |
| 1987 | Kopań | 42 | 76 | – | – | 45 |
| 1988 | Kopań | 28 | 64 | 25 | 35 | 45 |
| 1989 | Kopań | 48 | 84 | 40 | 11 | 45 |
| 1990 | Kopań | 31 | 34 | 20 | – | 45 |
| 1991 | Kopań | 77 | 52 | 50 | 28 | 45 |
| 1992 | Kopań | 18 | 31 | 31 | 16 | 37 |
| 1993 | Kopań | 73 | – | – | – | – |
| 1994 | Kopań | 58 | 71 | 85 | 60 | 47 |
| 1995 | Kopań | 59 | 79 | 99 | 35 | 47 |
| 1996 | Kopań | 51 | 108 | 88 | 64 | 47 |
| 1997 | Kopań | 63 | 161 | 80 | 110 | 47 |
| 1998 | Kopań | 92 | 80 | 112 | 117 | 47 |
| 1999 | Kopań | 70 | 133 | 80 | 133 | 47 |
| 2000 | Kopań | 85 | 72 | 89 | 120 | 47 |
| 2001 | Kopań | 91 | 95 | 147 | 180 | 57 |
| 2002 | Kopań | 61 | 92 | 99 | 136 | 47 |
| 2003 | Kopań | 91 | 58 | 67 | 175 | 46 |
| 2004 | Kopań | 92 | 99 | 108 | 163 | 47 |
| 2005 | Kopań | 132 | 177 | 228 | 280 | 47 |
| 2006 | Kopań | 108 | 107 | 126 | 297 | 47 |
| 2007 | Kopań | 87 | 147 | 183 | 212 | 54 |
| 2008 | Kopań | 129 | 115 | 182 | 150 | 50 |
| 2009 | Kopań | 131 | 107 | 155 | 257 | 46 |
| 2010 | Kopań | 148 | 85 | 163 | 288 | 35 |
| 2011 | – | – | – | – | – | – |
| 2012 | Bukowo | 89 | 138 | 63 | 334 | 56 |
| 2013 | Bukowo | 88 | 85 | 115 | 391 | 51 |
| 2014 | Bukowo | 120 | 71 | 103 | 224 | 48 |
| 2015 | Bukowo | 75 | 42 | 62 | 198 | 51 |
| 2016 | Bukowo | 87 | 72 | 54 | 374 | 52 |
| 2017 | Bukowo | 47 | 78 | 71 | 186 | 52 |
| 2018 | Bukowo | 110 | 59 | 89 | 279 | 52 |
| 2019 | Bukowo | 138 | 73 | 88 | 295 | 52 |
| 2020 | – | – | – | – | – | – |
| 2021 | Bukowo | 79 | 68 | 87 | 370 | 46 |
| 2022 | Bukowo | 154 | 118 | 130 | 326 | 57 |
| 2023 | Bukowo | 192 | 88 | 85 | 335 | 47 |
| 2024 | Bukowo | 337 | 58 | 19 | 434 | 47 |
| N years | 41 | 40 | 39 | 38 |
| IOD NOV_ MAR | NAO APR_MAY | NAO AUG_ OCT_1Y | NAO NOV_ MAR | PBK APR_ MAY | PSW AUG_ OCT_1Y | PSW NOV_ MAR | SCA MAY_ JUL_1Y | SOI NOV_ MAR | TBK APR_ MAY | TSW AUG_ OCT_1Y | TSW NOV_ MAR | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IOD_AUG_OCT_1Y | 0.60 | −0.19 | −0.20 | 0.25 | −0.04 | 0.15 | −0.15 | −0.03 | −0.39 | 0.11 | 0.14 | 0.03 |
| IOD_NOV_MAR | −0.25 | −0.17 | −0.03 | −0.32 | 0.18 | −0.29 | 0.22 | −0.10 | 0.19 | 0.14 | 0.22 | |
| NAO_APR_MAY | −0.16 | 0.06 | −0.20 | 0.07 | 0.10 | −0.01 | 0.07 | −0.07 | −0.13 | −0.19 | ||
| NAO_AUG_OCT_1Y | −0.09 | 0.24 | −0.09 | 0.23 | 0.16 | −0.15 | 0.00 | −0.27 | −0.21 | |||
| NAO_NOV_MAR | −0.23 | 0.15 | −0.20 | −0.06 | −0.15 | 0.53 | −0.17 | −0.43 | ||||
| PBK_APR_MAY | −0.18 | 0.04 | −0.11 | −0.29 | −0.40 | −0.10 | −0.25 | |||||
| PSW_AUG_OCT_1Y | −0.16 | 0.01 | 0.26 | 0.26 | −0.43 | −0.02 | ||||||
| PSW_NOV_MAR | −0.14 | −0.23 | −0.18 | −0.04 | 0.06 | |||||||
| SCA_MAY_JUL_1Y | 0.15 | 0.12 | −0.23 | −0.29 | ||||||||
| SOI_NOV_MAR | 0.06 | 0.00 | 0.13 | |||||||||
| TBK_APR_MAY | 0.10 | 0.05 | ||||||||||
| TSW_AUG_OCT_1Y | 0.67 |
| Species | ß Slope | SE | R2 | t | p | 43 Years × ß (Days) |
|---|---|---|---|---|---|---|
| Chiffchaff Phylloscopus collybita | −0.14 | 0.05 | −2.49 | 0.0170 | 0.14 | −5.8 |
| Willow Warbler Phylloscopus trochilus | −0.10 | 0.04 | −2.39 | 0.0221 | 0.13 | −4.2 |
| Blackcap Sylvia atricapilla | −0.10 | 0.04 | −2.33 | 0.0253 | 0.13 | −4.5 |
| Lesser Whitethroat Curruca curruca | −0.04 | 0.04 | −0.91 | 0.3710 | 0.02 | −1.6 |
| Response Variable /Climate Variable | Estimate | SE | t | p | VIF | pR |
|---|---|---|---|---|---|---|
| AA Full model: F13,28 = 1.99, AdjR2 = 24.0% | ||||||
| TBK_APR_MAY | −0.12 | 0.13 | −0.91 | 0.3683 | 1.05 | 0.03 |
| PBK_APR_MAY | 1.71 | 0.90 | 1.90 | 0.0681 | 2.15 | 0.11 |
| NAO_APR_MAY | −0.73 | 0.73 | −1.00 | 0.3277 | 1.35 | 0.03 |
| NAO_NOV_MAR | 0.22 | 0.97 | 0.23 | 0.8219 | 2.33 | 0.00 |
| IOD_NOV_MAR | 1.60 | 1.06 | 1.51 | 0.1425 | 2.80 | 0.08 |
| SOI_NOV_MAR | 0.23 | 0.84 | 0.27 | 0.7858 | 1.69 | 0.00 |
| NAO_AUG_OCT_1Y | −1.42 | 0.72 | −1.97 | 0.0588 | 1.32 | 0.12 |
| IOD_AUG_OCT_1Y | −2.51 | 1.00 | −2.52 | 0.0179 | 2.14 | 0.18 |
| SCA_MAY_JUL_1Y | −0.43 | 0.76 | −0.57 | 0.5758 | 1.56 | 0.01 |
| PSW_NOV_MAR | 0.28 | 0.73 | 0.39 | 0.7023 | 1.44 | 0.01 |
| PSW_AUG_OCT_1Y | −1.32 | 0.95 | −1.39 | 0.1765 | 2.15 | 0.06 |
| TSW_NOV_MAR | −1.33 | 1.29 | −1.03 | 0.3119 | 4.13 | 0.04 |
| TSW_AUG_OCT_1Y | −1.13 | 1.16 | −0.97 | 0.3384 | 3.63 | 0.03 |
| TBK_APR_MAY | −0.12 | 0.13 | −0.91 | 0.3683 | 1.05 | 0.03 |
| MP1 Full model: F13,28 = 1.45, AdjR2 = 12.0% | ||||||
| TBK_APR_MAY | −0.04 | 0.06 | −0.77 | 0.4471 | 1.05 | 0.02 |
| PBK_APR_MAY | 0.62 | 0.38 | 1.66 | 0.1090 | 2.15 | 0.09 |
| NAO_APR_MAY | 0.07 | 0.31 | 0.23 | 0.8233 | 1.35 | 0.00 |
| NAO_NOV_MAR | 0.22 | 0.41 | 0.54 | 0.5964 | 2.33 | 0.01 |
| IOD_NOV_MAR | 0.28 | 0.44 | 0.63 | 0.5337 | 2.80 | 0.01 |
| SOI_NOV_MAR | 0.19 | 0.35 | 0.54 | 0.5908 | 1.69 | 0.01 |
| NAO_AUG_OCT_1Y | −0.60 | 0.30 | −2.01 | 0.0540 | 1.32 | 0.13 |
| IOD_AUG_OCT_1Y | −0.44 | 0.42 | −1.05 | 0.3021 | 2.14 | 0.04 |
| SCA_MAY_JUL_1Y | −0.04 | 0.32 | −0.11 | 0.9104 | 1.56 | 0.00 |
| PSW_NOV_MAR | 0.34 | 0.31 | 1.13 | 0.2695 | 1.44 | 0.04 |
| PSW_AUG_OCT_1Y | −0.44 | 0.40 | −1.11 | 0.2757 | 2.15 | 0.04 |
| TSW_NOV_MAR | −0.23 | 0.54 | −0.43 | 0.6677 | 4.13 | 0.01 |
| TSW_AUG_OCT_1Y | −0.71 | 0.48 | −1.46 | 0.1558 | 3.63 | 0.07 |
| MP2 Full model: F13,28 = 2.33, AdjR2 = 29.7% | ||||||
| TBK_APR_MAY | −0.05 | 0.05 | −1.00 | 0.3280 | 1.05 | 0.03 |
| TBK_APR_MAY | 0.65 | 0.32 | 2.05 | 0.0499 | 2.15 | 0.13 |
| PBK_APR_MAY | −0.48 | 0.26 | −1.86 | 0.0727 | 1.35 | 0.11 |
| NAO_APR_MAY | 0.17 | 0.34 | 0.50 | 0.6196 | 2.33 | 0.01 |
| NAO_NOV_MAR | 0.55 | 0.37 | 1.48 | 0.1504 | 2.80 | 0.07 |
| IOD_NOV_MAR | −0.08 | 0.30 | −0.26 | 0.7941 | 1.69 | 0.00 |
| SOI_NOV_MAR | −0.58 | 0.25 | −2.27 | 0.0313 | 1.32 | 0.16 |
| NAO_AUG_OCT_1Y | −0.79 | 0.35 | −2.25 | 0.0324 | 2.14 | 0.15 |
| IOD_AUG_OCT_1Y | 0.02 | 0.27 | 0.07 | 0.9472 | 1.56 | 0.00 |
| SCA_MAY_JUL_1Y | 0.16 | 0.26 | 0.62 | 0.5403 | 1.44 | 0.01 |
| PSW_NOV_MAR | −0.54 | 0.34 | −1.62 | 0.1166 | 2.15 | 0.09 |
| PSW_AUG_OCT_1Y | −0.27 | 0.46 | −0.60 | 0.5556 | 4.13 | 0.01 |
| TSW_NOV_MAR | −0.65 | 0.41 | −1.59 | 0.1228 | 3.63 | 0.08 |
| TSW_AUG_OCT_1Y | −0.05 | 0.05 | −1.00 | 0.3280 | 1.05 | 0.03 |
| MP3 Full model: F13,28 = 1.31, AdjR2 = 8.9% | ||||||
| TBK_APR_MAY | −0.03 | 0.06 | −0.50 | 0.6212 | 1.05 | 0.01 |
| TBK_APR_MAY | 0.44 | 0.44 | 1.00 | 0.3249 | 2.15 | 0.03 |
| TBK_APR_MAY | −0.32 | 0.35 | −0.90 | 0.3779 | 1.35 | 0.03 |
| PBK_APR_MAY | −0.17 | 0.47 | −0.36 | 0.7224 | 2.33 | 0.00 |
| NAO_APR_MAY | 0.77 | 0.51 | 1.50 | 0.1449 | 2.80 | 0.07 |
| NAO_NOV_MAR | 0.12 | 0.41 | 0.29 | 0.7739 | 1.69 | 0.00 |
| IOD_NOV_MAR | −0.24 | 0.35 | −0.68 | 0.4990 | 1.32 | 0.02 |
| SOI_NOV_MAR | −1.28 | 0.48 | −2.65 | 0.0130 | 2.14 | 0.20 |
| NAO_AUG_OCT_1Y | −0.41 | 0.37 | −1.12 | 0.2713 | 1.56 | 0.04 |
| IOD_AUG_OCT_1Y | −0.22 | 0.35 | −0.63 | 0.5360 | 1.44 | 0.01 |
| SCA_MAY_JUL_1Y | −0.34 | 0.46 | −0.73 | 0.4729 | 2.15 | 0.02 |
| PSW_NOV_MAR | −0.83 | 0.62 | −1.32 | 0.1972 | 4.13 | 0.06 |
| PSW_AUG_OCT_1Y | 0.23 | 0.56 | 0.41 | 0.6884 | 3.63 | 0.01 |
| TSW_NOV_MAR | −0.03 | 0.06 | −0.50 | 0.6212 | 1.05 | 0.01 |
| TSW_AUG_OCT_1Y | 0.44 | 0.44 | 1.00 | 0.3249 | 2.15 | 0.03 |
| TBK_APR_MAY | −0.32 | 0.35 | −0.90 | 0.3779 | 1.35 | 0.03 |



| Response Variable /Climate Variable | Estimate | SE | t | p | VIF | pR |
|---|---|---|---|---|---|---|
| AA Full model: F13,28 = 3.88, AdjR2 = 48.4% | ||||||
| TBK_APR_MAY | −0.03 | 0.08 | −0.33 | 0.7409 | 1.05 | 0.00 |
| PBK_APR_MAY | 0.37 | 0.60 | 0.62 | 0.5416 | 2.38 | 0.01 |
| NAO_APR_MAY | −1.51 | 0.46 | −3.30 | 0.0027 | 1.42 | 0.29 |
| NAO_NOV_MAR | −0.59 | 0.59 | −1.00 | 0.3284 | 2.29 | 0.04 |
| IOD_NOV_MAR | 1.28 | 0.65 | 1.98 | 0.0585 | 2.80 | 0.13 |
| SOI_NOV_MAR | −0.52 | 0.54 | −0.97 | 0.3394 | 1.84 | 0.03 |
| NAO_AUG_OCT_1Y | −0.64 | 0.45 | −1.42 | 0.1665 | 1.39 | 0.07 |
| IOD_AUG_OCT_1Y | −2.02 | 0.62 | −3.27 | 0.0029 | 2.11 | 0.28 |
| SCA_MAY_JUL_1Y | −0.95 | 0.46 | −2.06 | 0.0493 | 1.55 | 0.14 |
| PSW_NOV_MAR | 0.32 | 0.45 | 0.72 | 0.4795 | 1.46 | 0.02 |
| PSW_AUG_OCT_1Y | −1.66 | 0.63 | −2.65 | 0.0133 | 2.39 | 0.21 |
| TSW_NOV_MAR | −0.88 | 0.79 | −1.12 | 0.2731 | 4.07 | 0.04 |
| TSW_AUG_OCT_1Y | −1.39 | 0.77 | −1.82 | 0.0803 | 4.05 | 0.11 |
| MP1 Full model: F13,28 = 4.32, AdjR2 = 51.2% | ||||||
| TBK_APR_MAY | −0.02 | 0.04 | −0.59 | 0.5574 | 1.05 | 0.01 |
| PBK_APR_MAY | 0.27 | 0.28 | 0.98 | 0.3337 | 2.38 | 0.03 |
| NAO_APR_MAY | −0.58 | 0.21 | −2.78 | 0.0097 | 1.42 | 0.22 |
| NAO_NOV_MAR | −0.08 | 0.27 | −0.30 | 0.7645 | 2.29 | 0.00 |
| IOD_NOV_MAR | 0.48 | 0.30 | 1.61 | 0.1190 | 2.80 | 0.09 |
| SOI_NOV_MAR | −0.49 | 0.25 | −1.99 | 0.0574 | 1.84 | 0.13 |
| NAO_AUG_OCT_1Y | −0.18 | 0.21 | −0.85 | 0.4027 | 1.39 | 0.03 |
| IOD_AUG_OCT_1Y | −0.96 | 0.28 | −3.37 | 0.0023 | 2.11 | 0.30 |
| SCA_MAY_JUL_1Y | −0.41 | 0.21 | −1.92 | 0.0658 | 1.55 | 0.12 |
| PSW_NOV_MAR | 0.19 | 0.21 | 0.94 | 0.3535 | 1.46 | 0.03 |
| PSW_AUG_OCT_1Y | −0.77 | 0.29 | −2.67 | 0.0126 | 2.39 | 0.21 |
| TSW_NOV_MAR | −0.17 | 0.36 | −0.47 | 0.6403 | 4.07 | 0.01 |
| TSW_AUG_OCT_1Y | −0.78 | 0.35 | −2.22 | 0.0349 | 4.05 | 0.15 |
| MP2 Full model: F13,28 = 3.18, AdjR2 = 41.6% | ||||||
| TBK_APR_MAY | −0.01 | 0.04 | −0.27 | 0.7865 | 1.05 | 0.00 |
| TBK_APR_MAY | 0.05 | 0.29 | 0.18 | 0.8549 | 2.38 | 0.00 |
| PBK_APR_MAY | −0.74 | 0.22 | −3.40 | 0.0021 | 1.42 | 0.30 |
| NAO_APR_MAY | −0.31 | 0.28 | −1.11 | 0.2750 | 2.29 | 0.04 |
| NAO_NOV_MAR | 0.54 | 0.31 | 1.75 | 0.0908 | 2.80 | 0.10 |
| IOD_NOV_MAR | −0.09 | 0.26 | −0.35 | 0.7256 | 1.84 | 0.00 |
| SOI_NOV_MAR | −0.30 | 0.21 | −1.40 | 0.1725 | 1.39 | 0.07 |
| NAO_AUG_OCT_1Y | −0.81 | 0.29 | −2.74 | 0.0107 | 2.11 | 0.22 |
| IOD_AUG_OCT_1Y | −0.46 | 0.22 | −2.09 | 0.0466 | 1.55 | 0.14 |
| SCA_MAY_JUL_1Y | 0.06 | 0.21 | 0.28 | 0.7807 | 1.46 | 0.00 |
| PSW_NOV_MAR | −0.76 | 0.30 | −2.54 | 0.0170 | 2.39 | 0.19 |
| PSW_AUG_OCT_1Y | −0.53 | 0.38 | −1.40 | 0.1717 | 4.07 | 0.07 |
| TSW_NOV_MAR | −0.55 | 0.36 | −1.51 | 0.1434 | 4.05 | 0.08 |
| TSW_AUG_OCT_1Y | −0.01 | 0.04 | −0.27 | 0.7865 | 1.05 | 0.00 |
| MP3 Full model: F13,28 = 1.03, AdjR2 = 0.9% | ||||||
| TBK_APR_MAY | 0.01 | 0.02 | 0.25 | 0.8060 | 1.05 | 0.00 |
| TBK_APR_MAY | 0.05 | 0.17 | 0.28 | 0.7780 | 2.38 | 0.00 |
| TBK_APR_MAY | −0.19 | 0.13 | −1.47 | 0.1524 | 1.42 | 0.07 |
| PBK_APR_MAY | −0.19 | 0.16 | −1.18 | 0.2468 | 2.29 | 0.05 |
| NAO_APR_MAY | 0.26 | 0.18 | 1.46 | 0.1559 | 2.80 | 0.07 |
| NAO_NOV_MAR | 0.06 | 0.15 | 0.39 | 0.7004 | 1.84 | 0.01 |
| IOD_NOV_MAR | −0.17 | 0.12 | −1.32 | 0.1965 | 1.39 | 0.06 |
| SOI_NOV_MAR | −0.26 | 0.17 | −1.51 | 0.1433 | 2.11 | 0.08 |
| NAO_AUG_OCT_1Y | −0.09 | 0.13 | −0.68 | 0.5030 | 1.55 | 0.02 |
| IOD_AUG_OCT_1Y | 0.07 | 0.12 | 0.54 | 0.5918 | 1.46 | 0.01 |
| SCA_MAY_JUL_1Y | −0.13 | 0.17 | −0.78 | 0.4439 | 2.39 | 0.02 |
| PSW_NOV_MAR | −0.19 | 0.22 | −0.85 | 0.4027 | 4.07 | 0.03 |
| PSW_AUG_OCT_1Y | −0.06 | 0.21 | −0.29 | 0.7705 | 4.05 | 0.00 |
| TSW_NOV_MAR | 0.01 | 0.02 | 0.25 | 0.8060 | 1.05 | 0.00 |
| TSW_AUG_OCT_1Y | 0.05 | 0.17 | 0.28 | 0.7780 | 2.38 | 0.00 |
| Response Variable /Climate Variable | Estimate | SE | t | p | VIF | pR |
|---|---|---|---|---|---|---|
| AA Full model: F13,28 = 1.58, AdjR2 = 16.1% | ||||||
| TBK_APR_MAY | −0.07 | 0.11 | −0.60 | 0.5554 | 1.06 | 0.01 |
| PBK_APR_MAY | 0.77 | 0.76 | 1.02 | 0.3182 | 2.14 | 0.04 |
| NAO_APR_MAY | −1.11 | 0.65 | −1.70 | 0.1010 | 1.40 | 0.10 |
| NAO_NOV_MAR | −0.87 | 0.82 | −1.07 | 0.2961 | 2.36 | 0.04 |
| IOD_NOV_MAR | 0.99 | 0.89 | 1.11 | 0.2751 | 2.82 | 0.05 |
| SOI_NOV_MAR | −0.13 | 0.72 | −0.18 | 0.8584 | 1.68 | 0.00 |
| NAO_AUG_OCT_1Y | 0.05 | 0.61 | 0.09 | 0.9325 | 1.29 | 0.00 |
| IOD_AUG_OCT_1Y | −1.43 | 0.85 | −1.68 | 0.1049 | 2.13 | 0.10 |
| SCA_MAY_JUL_1Y | −0.74 | 0.64 | −1.17 | 0.2534 | 1.56 | 0.05 |
| PSW_NOV_MAR | −0.23 | 0.62 | −0.38 | 0.7094 | 1.46 | 0.01 |
| PSW_AUG_OCT_1Y | −0.80 | 0.80 | −1.01 | 0.3226 | 2.13 | 0.04 |
| TSW_NOV_MAR | −1.48 | 1.09 | −1.36 | 0.1865 | 4.15 | 0.07 |
| TSW_AUG_OCT_1Y | 0.20 | 0.98 | 0.21 | 0.8384 | 3.57 | 0.00 |
| MP1 Full model: F13,28 = 1.99, AdjR2 = 24.8% | ||||||
| TBK_APR_MAY | −0.03 | 0.05 | −0.59 | 0.5616 | 1.06 | 0.01 |
| PBK_APR_MAY | 0.65 | 0.30 | 2.17 | 0.0394 | 2.14 | 0.15 |
| NAO_APR_MAY | −0.29 | 0.26 | −1.11 | 0.2779 | 1.40 | 0.05 |
| NAO_NOV_MAR | −0.07 | 0.32 | −0.22 | 0.8250 | 2.36 | 0.00 |
| IOD_NOV_MAR | 0.40 | 0.35 | 1.15 | 0.2600 | 2.82 | 0.05 |
| SOI_NOV_MAR | −0.13 | 0.28 | −0.44 | 0.6604 | 1.68 | 0.01 |
| NAO_AUG_OCT_1Y | 0.02 | 0.24 | 0.08 | 0.9344 | 1.29 | 0.00 |
| IOD_AUG_OCT_1Y | −0.52 | 0.34 | −1.55 | 0.1326 | 2.13 | 0.08 |
| SCA_MAY_JUL_1Y | −0.26 | 0.25 | −1.03 | 0.3124 | 1.56 | 0.04 |
| PSW_NOV_MAR | −0.01 | 0.24 | −0.03 | 0.9765 | 1.46 | 0.00 |
| PSW_AUG_OCT_1Y | −0.54 | 0.31 | −1.72 | 0.0971 | 2.13 | 0.10 |
| TSW_NOV_MAR | 0.03 | 0.43 | 0.07 | 0.9446 | 4.15 | 0.00 |
| TSW_AUG_OCT_1Y | −0.29 | 0.39 | −0.73 | 0.4692 | 3.57 | 0.02 |
| MP2 Full model: F13,28 = 1.43, AdjR2 = 21.1% | ||||||
| TBK_APR_MAY | −0.03 | 0.04 | −0.61 | 0.5499 | 1.06 | 0.01 |
| PBK_APR_MAY | 0.28 | 0.28 | 1.00 | 0.3254 | 2.14 | 0.04 |
| NAO_APR_MAY | −0.44 | 0.24 | −1.83 | 0.0783 | 1.40 | 0.11 |
| NAO_NOV_MAR | −0.31 | 0.30 | −1.02 | 0.3148 | 2.36 | 0.04 |
| IOD_NOV_MAR | 0.33 | 0.33 | 1.00 | 0.3242 | 2.82 | 0.04 |
| SOI_NOV_MAR | 0.10 | 0.26 | 0.36 | 0.7192 | 1.68 | 0.01 |
| NAO_AUG_OCT_1Y | 0.05 | 0.22 | 0.23 | 0.8188 | 1.29 | 0.00 |
| IOD_AUG_OCT_1Y | −0.38 | 0.31 | −1.21 | 0.2372 | 2.13 | 0.05 |
| SCA_MAY_JUL_1Y | −0.38 | 0.23 | −1.60 | 0.1207 | 1.56 | 0.09 |
| PSW_NOV_MAR | −0.06 | 0.23 | −0.27 | 0.7887 | 1.46 | 0.00 |
| PSW_AUG_OCT_1Y | −0.19 | 0.29 | −0.65 | 0.5200 | 2.13 | 0.02 |
| TSW_NOV_MAR | −0.64 | 0.40 | −1.59 | 0.1245 | 4.15 | 0.09 |
| TSW_AUG_OCT_1Y | 0.26 | 0.36 | 0.71 | 0.4868 | 3.57 | 0.02 |
| MP3 Full model: F13,28 = 0.82, AdjR2 = 6.5% | ||||||
| TBK_APR_MAY | −0.02 | 0.05 | −0.33 | 0.7460 | 1.06 | 0.00 |
| PBK_APR_MAY | −0.16 | 0.33 | −0.48 | 0.6372 | 2.14 | 0.01 |
| NAO_APR_MAY | −0.38 | 0.28 | −1.35 | 0.1874 | 1.40 | 0.07 |
| NAO_NOV_MAR | −0.49 | 0.36 | −1.38 | 0.1784 | 2.36 | 0.07 |
| IOD_NOV_MAR | 0.26 | 0.39 | 0.67 | 0.5098 | 2.82 | 0.02 |
| SOI_NOV_MAR | −0.10 | 0.31 | −0.32 | 0.7525 | 1.68 | 0.00 |
| NAO_AUG_OCT_1Y | −0.02 | 0.27 | −0.07 | 0.9409 | 1.29 | 0.00 |
| IOD_AUG_OCT_1Y | −0.53 | 0.37 | −1.43 | 0.1642 | 2.13 | 0.07 |
| SCA_MAY_JUL_1Y | −0.11 | 0.28 | −0.39 | 0.6974 | 1.56 | 0.01 |
| PSW_NOV_MAR | −0.16 | 0.27 | −0.61 | 0.5465 | 1.46 | 0.01 |
| PSW_AUG_OCT_1Y | −0.07 | 0.35 | −0.20 | 0.8399 | 2.13 | 0.00 |
| TSW_NOV_MAR | −0.87 | 0.47 | −1.84 | 0.0769 | 4.15 | 0.12 |
| TSW_AUG_OCT_1Y | 0.23 | 0.43 | 0.54 | 0.5910 | 3.57 | 0.01 |

| Response Variable /Climate Variable | Estimate | SE | t | p | VIF | pR |
|---|---|---|---|---|---|---|
| AA Full model: F13,28 = 1.54, AdjR2 = 15.4% | ||||||
| TBK_APR_MAY | −0.03 | 0.10 | −0.33 | 0.7469 | 1.06 | 0.00 |
| PBK_APR_MAY | 0.23 | 0.72 | 0.32 | 0.7545 | 2.36 | 0.00 |
| NAO_APR_MAY | −1.08 | 0.58 | −1.88 | 0.0717 | 1.45 | 0.12 |
| NAO_NOV_MAR | −0.20 | 0.71 | −0.29 | 0.7776 | 2.30 | 0.00 |
| IOD_NOV_MAR | 1.20 | 0.77 | 1.56 | 0.1319 | 2.81 | 0.09 |
| SOI_NOV_MAR | −0.68 | 0.65 | −1.05 | 0.3019 | 1.79 | 0.04 |
| NAO_AUG_OCT_1Y | −0.20 | 0.54 | −0.38 | 0.7070 | 1.39 | 0.01 |
| IOD_AUG_OCT_1Y | −1.67 | 0.74 | −2.27 | 0.0316 | 2.09 | 0.17 |
| SCA_MAY_JUL_1Y | −0.57 | 0.55 | −1.03 | 0.3144 | 1.55 | 0.04 |
| PSW_NOV_MAR | 0.02 | 0.54 | 0.03 | 0.9725 | 1.49 | 0.00 |
| PSW_AUG_OCT_1Y | −1.22 | 0.74 | −1.65 | 0.1120 | 2.38 | 0.09 |
| TSW_NOV_MAR | −0.29 | 0.94 | −0.31 | 0.7590 | 3.98 | 0.00 |
| TSW_AUG_OCT_1Y | −0.22 | 0.92 | −0.24 | 0.8134 | 3.97 | 0.00 |
| TBK_APR_MAY | −0.03 | 0.10 | −0.33 | 0.7469 | 1.06 | 0.00 |
| MP1 Full model: F13,28 = 1.76, AdjR2 = 20.2% | ||||||
| TBK_APR_MAY | −0.02 | 0.05 | −0.46 | 0.6463 | 1.06 | 0.01 |
| PBK_APR_MAY | 0.08 | 0.34 | 0.23 | 0.8169 | 2.36 | 0.00 |
| NAO_APR_MAY | −0.56 | 0.27 | −2.07 | 0.0483 | 1.45 | 0.14 |
| NAO_NOV_MAR | 0.00 | 0.33 | −0.01 | 0.9923 | 2.30 | 0.00 |
| IOD_NOV_MAR | 0.45 | 0.36 | 1.26 | 0.2195 | 2.81 | 0.06 |
| SOI_NOV_MAR | −0.23 | 0.30 | −0.76 | 0.4541 | 1.79 | 0.02 |
| NAO_AUG_OCT_1Y | −0.05 | 0.25 | −0.19 | 0.8493 | 1.39 | 0.00 |
| IOD_AUG_OCT_1Y | −0.57 | 0.34 | −1.65 | 0.1112 | 2.09 | 0.09 |
| SCA_MAY_JUL_1Y | −0.40 | 0.26 | −1.54 | 0.1348 | 1.55 | 0.08 |
| PSW_NOV_MAR | 0.20 | 0.25 | 0.78 | 0.4399 | 1.49 | 0.02 |
| PSW_AUG_OCT_1Y | −0.61 | 0.35 | −1.75 | 0.0918 | 2.38 | 0.11 |
| TSW_NOV_MAR | 0.03 | 0.44 | 0.08 | 0.9404 | 3.98 | 0.00 |
| TSW_AUG_OCT_1Y | −0.07 | 0.43 | −0.17 | 0.8626 | 3.97 | 0.00 |
| MP2 Full model: F13,28 = 1.40, AdjR2 = 11.6% | ||||||
| TBK_APR_MAY | −0.01 | 0.05 | −0.25 | 0.8049 | 1.06 | 0.00 |
| PBK_APR_MAY | 0.10 | 0.34 | 0.30 | 0.7678 | 2.36 | 0.00 |
| NAO_APR_MAY | −0.39 | 0.27 | −1.47 | 0.1548 | 1.45 | 0.08 |
| NAO_NOV_MAR | −0.15 | 0.33 | −0.45 | 0.6535 | 2.30 | 0.01 |
| IOD_NOV_MAR | 0.63 | 0.36 | 1.73 | 0.0951 | 2.81 | 0.10 |
| SOI_NOV_MAR | −0.32 | 0.30 | −1.07 | 0.2951 | 1.79 | 0.04 |
| NAO_AUG_OCT_1Y | 0.00 | 0.25 | −0.01 | 0.9956 | 1.39 | 0.00 |
| IOD_AUG_OCT_1Y | −0.83 | 0.34 | −2.41 | 0.0232 | 2.09 | 0.18 |
| SCA_MAY_JUL_1Y | −0.17 | 0.26 | −0.66 | 0.5171 | 1.55 | 0.02 |
| PSW_NOV_MAR | −0.16 | 0.25 | −0.62 | 0.5425 | 1.49 | 0.01 |
| PSW_AUG_OCT_1Y | −0.51 | 0.35 | −1.48 | 0.1520 | 2.38 | 0.08 |
| TSW_NOV_MAR | −0.21 | 0.44 | −0.48 | 0.6331 | 3.98 | 0.01 |
| TSW_AUG_OCT_1Y | −0.15 | 0.43 | −0.36 | 0.7205 | 3.97 | 0.01 |
| TBK_APR_MAY | −0.01 | 0.05 | −0.25 | 0.8049 | 1.06 | 0.00 |
| MP3 Full model: F13,28 = 0.74, AdjR2 = 9.4% | ||||||
| TBK_APR_MAY | 0.00 | 0.02 | 0.03 | 0.9746 | 1.06 | 0.00 |
| PBK_APR_MAY | 0.05 | 0.14 | 0.34 | 0.7361 | 2.36 | 0.00 |
| NAO_APR_MAY | −0.13 | 0.12 | −1.15 | 0.2616 | 1.45 | 0.05 |
| NAO_NOV_MAR | −0.05 | 0.14 | −0.35 | 0.7321 | 2.30 | 0.00 |
| IOD_NOV_MAR | 0.13 | 0.15 | 0.81 | 0.4240 | 2.81 | 0.02 |
| SOI_NOV_MAR | −0.13 | 0.13 | −1.01 | 0.3231 | 1.79 | 0.04 |
| NAO_AUG_OCT_1Y | −0.15 | 0.11 | −1.44 | 0.1611 | 1.39 | 0.07 |
| IOD_AUG_OCT_1Y | −0.28 | 0.15 | −1.90 | 0.0691 | 2.09 | 0.12 |
| SCA_MAY_JUL_1Y | 0.00 | 0.11 | −0.01 | 0.9957 | 1.55 | 0.00 |
| PSW_NOV_MAR | −0.02 | 0.11 | −0.21 | 0.8327 | 1.49 | 0.00 |
| PSW_AUG_OCT_1Y | −0.11 | 0.15 | −0.71 | 0.4843 | 2.38 | 0.02 |
| TSW_NOV_MAR | −0.11 | 0.19 | −0.60 | 0.5532 | 3.98 | 0.01 |
| TSW_AUG_OCT_1Y | 0.01 | 0.18 | 0.06 | 0.9546 | 3.97 | 0.00 |
References
- Miller-Rushing, A.J.; Lloyd-Evans, T.L.; Primack, R.B.; Satzinger, P. Bird Migration Times, Climate Change, and Changing Population Sizes. Glob. Change Biol. 2008, 14, 1959–1972. [Google Scholar] [CrossRef]
- Usui, T.; Butchart, S.H.M.; Phillimore, A.B. Temporal Shifts and Temperature Sensitivity of Avian Spring Migratory Phenology: A Phylogenetic Meta-Analysis. J. Anim. Ecol. 2017, 86, 250–261. [Google Scholar] [CrossRef]
- Gordo, O.; Sanz, J.J. Climate Change and Bird Phenology: A Long-Term Study in the Iberian Peninsula. Glob. Change Biol. 2006, 12, 1993–2004. [Google Scholar] [CrossRef]
- Saino, N.; Ambrosini, R.; Rubolini, D.; Von Hardenberg, J.; Provenzale, A.; Hüppop, K.; Hüppop, O.; Lehikoinen, A.; Lehikoinen, E.; Rainio, K.; et al. Climate warming, ecological mismatch at arrival and population decline in migratory birds. Proc. R. Soc. B Biol. Sci. R. Soc. 2011, 278, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Gordo, O.; Brotons, L.; Ferrer, X.; Comas, P. Do Changes in Climate Patterns in Wintering Areas Affect the Timing of the Spring Arrival of Trans-Saharan Migrant Birds? Glob. Change Biol. 2005, 11, 12–21. [Google Scholar] [CrossRef]
- Saino, N.; Ambrosini, R. Climatic Connectivity between Africa and Europe May Serve as a Basis for Phenotypic Adjustment of Migration Schedules of Trans-Saharan Migratory Birds. Glob. Change Biol. 2008, 14, 250–263. [Google Scholar] [CrossRef]
- Remisiewicz, M.; Underhill, L.G. Climatic Variation in Africa and Europe Has Combined Effects on Timing of Spring Migration in a Long-Distance Migrant Willow Warbler Phylloscopus trochilus. PeerJ 2020, 8, e8770. [Google Scholar] [CrossRef] [PubMed]
- Remisiewicz, M.; Underhill, L.G. Large-Scale Climatic Patterns Have Stronger Carry-Over Effects than Local Temperatures on Spring Phenology of Long-Distance Passerine Migrants between Europe and Africa. Animals 2022, 12, 1732. [Google Scholar] [CrossRef]
- Remisiewicz, M.; Underhill, L.G. Climate in Africa Sequentially Shapes Spring Passage of Willow Warbler Phylloscopus trochilus across the Baltic Coast. PeerJ 2022, 10, e12964. [Google Scholar] [CrossRef]
- Spina, F.; Baillie, S.R.; Bairlein, F.; Fiedler, W.; Thorup, K. (Eds.) The Eurasian African Bird Migration Atlas. 2022. Available online: https://migrationatlas.org/ (accessed on 23 July 2025).
- Valkama, J.; Saurola, P.; Lehikoinen, A.; Lehikoinen, E.; Piha, M.; Sola, P.; Velmala, W. Suomen Rengastysatlas. [The Finnish Bird Ringing Atlas]; Finnish Museum of Natural History and Ministry of Environment: Helsinki, Finland, 2014; Volume II. [Google Scholar]
- Fransson, T.; Hall-Karlsson, S. Svensk Ringmärkningsatlas; Naturhistoriska riksmuseet & Sveriges Ornitologiska Forening: Stockholm, Sweden, 2008; Volume 3. [Google Scholar]
- Zwarts, L.; Bijlsma, R.G.; van der Kamp, J.; Wymenga, E. Living on the Edge: Wetlands and Birds in a Changing Sahel; KNNV Publishing: Zeist, The Netherlands, 2009. [Google Scholar]
- Cramp, S.; Brooks, D.J. The Birds of the Western Palearctic. In Handbook of the Birds of Europe, the Middle East and North Africa; Oxford University Press: Oxford, UK, 1992; Volume VI: Warblers. [Google Scholar]
- Hušek, J.; Adamík, P.; Cepák, J.; Tryjanowski, P. The Influence of Climate and Population Size on the Distribution of Breeding Dates in the Red-Backed Shrike (Lanius collurio). Ann. Zool. Fennici 2009, 46, 439–450. [Google Scholar] [CrossRef]
- Tryjanowski, P.; Stenseth, N.C.; Matysioková, B. The Indian Ocean Dipole as an Indicator of Climatic Conditions Affecting European Birds. Clim. Res. 2013, 57, 45–49. [Google Scholar] [CrossRef]
- Tøttrup, A.P.; Klaassen, R.H.G.; Kristensen, M.W.; Strandberg, R.; Vardanis, Y.; Lindström, Å.; Rahbek, C.; Alerstam, T.; Thorup, K. Drought in Africa Caused Delayed Arrival of European Songbirds. Science 2012, 338, 1307. [Google Scholar] [CrossRef] [PubMed]
- Tobolka, M.; Dylewski, L.; Wozna, J.T.; Zolnierowicz, K.M. How Weather Conditions in Non-Breeding and Breeding Grounds Affect the Phenology and Breeding Abilities of White Storks. Sci. Total Environ. 2018, 636, 512–518. [Google Scholar] [CrossRef]
- Askeyev, O.V.; Sparks, T.H.; Askeyev, I.V.; Tryjanowski, P. Spring Migration Timing of Sylvia Warblers in Tatarstan (Russia) 1957–2008. Cent. Eur. J. Biol. 2009, 4, 595–602. [Google Scholar] [CrossRef]
- Askeyev, O.V.; Sparks, T.H.; Askeyev, I.V.; Tryjanowski, P. Is Earlier Spring Migration of Tatarstan Warblers Expected under Climate Warming? Int. J. Biometeorol. 2007, 51, 459–463. [Google Scholar] [CrossRef]
- Cotton, P.A. Avian Migration Phenology and Global Climate Change. Proc. Natl. Acad. Sci. USA 2003, 100, 12219–12222. [Google Scholar] [CrossRef]
- Saino, N.; Rubolini, D.; Jonzén, N.; Ergon, T.; Montemaggiori, A.; Stenseth, N.C.; Spina, F. Temperature and Rainfall Anomalies in Africa Predict Timing of Spring Migration in Trans-Saharan Migratory Birds. Clim. Res. 2007, 35, 123–134. [Google Scholar] [CrossRef]
- Haest, B.; Hüppop, O.; Bairlein, F. Weather at the Winter and Stopover Areas Determines Spring Migration Onset, Progress, and Advancements in Afro-Palearctic Migrant Birds. Proc. Natl. Acad. Sci. USA 2020, 117, 17056–17062. [Google Scholar] [CrossRef] [PubMed]
- Tomotani, B.M.; van der Jeugd, H.; Gienapp, P.; de la Hera, I.; Pilzecker, J.; Teichmann, C.; Visser, M.E. Climate Change Leads to Differential Shifts in the Timing of Annual Cycle Stages in a Migratory Bird. Glob. Change Biol. 2018, 24, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Redlisiak, M.; Remisiewicz, M.; Nowakowski, J.K. Long-Term Changes in Migration Timing of Song Thrush Turdus Philomelos at the Southern Baltic Coast in Response to Temperatures on Route and at Breeding Grounds. Int. J. Biometeorol. 2018, 62, 1595–1605. [Google Scholar] [CrossRef]
- Ouwehand, J.; Both, C. African Departure Rather than Migration Speed Determines Variation in Spring Arrival in Pied Flycatchers. J. Anim. Ecol. 2017, 86, 88–97. [Google Scholar] [CrossRef]
- Remisiewicz, M.; Bernitz, Z.; Bernitz, H.; Burman, M.S.; Raijmakers, J.M.H.; Raijmakers, J.H.F.A.; Underhill, L.G.; Rostkowska, A.; Barshep, Y.; Soloviev, S.A.; et al. Contrasting Strategies for Wing-Moult and Pre-Migratory Fuelling in Western and Eastern Populations of Common Whitethroat Sylvia Communis. Ibis 2019, 161, 824–838. [Google Scholar] [CrossRef]
- Alerstam, T. Bird Migration; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar]
- Gill, F.B. Ornithology, 3rd ed.; W. H. Freeman and Company: New York, NY, USA, 2006. [Google Scholar]
- O’Connor, R.S.; Love, O.P.; Régimbald, L.; Le Pogam, A.; Gerson, A.R.; Elliott, K.H.; Hargreaves, A.L.; Vézina, F. An Arctic Breeding Songbird Overheats during Intense Activity Even at Low Air Temperatures. Sci. Rep. 2024, 14, 15193. [Google Scholar] [CrossRef] [PubMed]
- Zwarts, L.; Bijlsma, R.G.; Van Der Kamp, J. The Fortunes of Migratory Birds from Eurasia: Being on a Tightrope in the Sahel. Ardea 2023, 111, 397–437. [Google Scholar] [CrossRef]
- Allan, D.G.; Harrison, J.A.; Herremans, M.; Navarro, R.; Underhill, L.G. Southern African Geography: Its Relevance to Birds. In The Atlas of Southern African Birds Volume 1: Non-Passerines; Harrison, J.A., Allan, D.G., Underhill, L.G., Herremans, M., Tree, A.J., Parker, V., Brown, C.J., Eds.; BirdLife South Africa: Johannesburg, South Africa, 1997. [Google Scholar]
- Katti, M.; Price, T. Annual Variation in Fat Storage by a Migrant Warbler Overwintering in the Indian Tropics. J. Anim. Ecol. 1999, 68, 815–823. [Google Scholar] [CrossRef]
- Hüppop, O.; Hüppop, K. North Atlantic Oscillation and Timing of Spring Migration in Birds. Proc. R. Soc. B Biol. Sci. 2003, 270, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Stenseth, N.C.; Ottersen, G.; Hurrell, J.W.; Mysterud, A.; Lima, M.; Chan, K.S.; Yoccoz, N.G.; Ådlandsvik, B. Studying Climate Effects on Ecology through the Use of Climate Indices: The North Atlantic Oscillation, El Niño Southern Oscillation and Beyond. Proc. R. Soc. B Biol. Sci. 2003, 270, 2087–2096. [Google Scholar] [CrossRef]
- Haest, B.; Hüppop, O.; Bairlein, F. Challenging a 15-Year-Old Claim: The North Atlantic Oscillation Index as a Predictor of Spring Migration Phenology of Birds. Glob. Change Biol. 2018, 24, 1523–1537. [Google Scholar] [CrossRef]
- Trepte, A. Avi-Fauna—Vögel in Deutschland. Available online: https://www.avi-fauna.info/ (accessed on 23 July 2025).
- Kopiec, K.; Ożarowska, A. The Origin of Blackcaps Sylvia atricapilla Wintering on the British Isles. Ornis Fenn. 2012, 4, 254–263. [Google Scholar] [CrossRef]
- Delmore, K.E.; Van Doren, B.M.; Conway, G.J.; Curk, T.; Garrido-Garduño, T.; Germain, R.R.; Hasselmann, T.; Hiemer, D.; van der Jeugd, H.P.; Justen, H.; et al. Individual Variability and Versatility in an Eco-Evolutionary Model of Avian Migration. Proc. R. Soc. B Biol. Sci. 2020, 287, 20201339. [Google Scholar] [CrossRef]
- Ożarowska, A.; Yosef, R.; Busse, P. Orientation of Chiffchaff (Phylloscopus collybita), Blackcap (Sylvia atricapilla) and Lesser Whitethroat (S. curruca) on Spring Migration at Eilat, Israel. Avian Ecol. Behav. 2004, 12, 1–10. [Google Scholar]
- Maciąg, T.; Remisiewicz, M.; Nowakowski, J.K.; Redlisiak, M.; Rosińska, K.; Stępniewski, K.; Stępniewska, K.; Szulc, J. Maps of Ringing Recoveries. Website of the Bird Migration Research Station. Available online: https://en-sbwp.ug.edu.pl/badania/monitoringwyniki/maps-of-ringing-recoveries/ (accessed on 23 July 2025).
- Lerche-Jørgensen, M.; Willemoes, M.; Tøttrup, A.P.; Snell, K.R.S.; Thorup, K. No Apparent Gain from Continuing Migration for More than 3000 Kilometres: Willow Warblers Breeding in Denmark Winter across the Entire Northern Savannah as Revealed by Geolocators. Mov. Ecol. 2017, 5, 17. [Google Scholar] [CrossRef]
- Hansson, M.C.; Bensch, S.; Brännström, O. Range Expansion and the Possibility of an Emerging Contact Zone between Two Subspecies of Chiffchaff Phylloscopus collybita ssp. J. Avian Biol. 2000, 31, 548–558. [Google Scholar] [CrossRef]
- Bakken, V.; Runde, O.J.; Tjørve, E.; Koblik, E.A. Norsk Ringmerkingsatlas; Stavanger Museum: Stavanger, Norway, 2003; Volume 1. [Google Scholar]
- Ciach, M. Leaf Warblers (Phylloscopus spp.) As a Model Group in Migration Ecology Studies. Ring 2009, 31, 3–13. [Google Scholar] [CrossRef]
- Berthold, P.; Terrill, S.B. Migratory Behaviour and Population Growth of Blackcaps Wintering in Britain and Ireland: Some Hypotheses. Ringing Migr. 1988, 9, 153–159. [Google Scholar] [CrossRef]
- Ożarowska, A.; Meissner, W. Increasing Body Condition of Autumn Migrating Eurasian Blackcaps Sylvia Atricapilla over Four Decades. Eur. Zool. J. 2024, 91, 151–161. [Google Scholar] [CrossRef]
- Ożarowska, A. Contrasting fattening strategies in related migratory species: The blackcap, garden warbler, common whitethroat and lesser whitethroat. Ann. Zool. Fenn. 2015, 52, 115–127. [Google Scholar] [CrossRef]
- Hedenström, A.; Pettersson, J. Migration Routes and Wintering Areas of Willow Warblers Phylloscopus trochilus (L.) Ringed in Fennoscandia. Ornis Fenn. 1987, 64, 137–143. [Google Scholar]
- Zhao, T.; Ilieva, M.; Larson, K.; Lundberg, M.; Neto, J.M.; Sokolovskis, K.; Åkesson, S.; Bensch, S. Autumn Migration Direction of Juvenile Willow Warblers (Phylloscopus t. trochilus and P. t. acredula) and Their Hybrids Assessed by QPCR SNP Genotyping. Mov. Ecol. 2020, 8, 22. [Google Scholar] [CrossRef]
- Bensch, S.; Andersson, T.; Akesson, S. Morphological And Molerecular Variaton Across a Migratory Divide in Willow Warblers, Phylloscopus trochilus. Evolution 1999, 53, 1925–1935. [Google Scholar] [CrossRef] [PubMed]
- Tomiałojć, L.; Stawarczyk, T. Awifauna Polski: Rozmieszczenie, Liczebność i Zmiany; PTPP “pro Natura”: Wrocław, Poland, 2003. [Google Scholar]
- Bensch, S.; Bengtsson, G.; Åkesson, S. Patterns of Stable Isotope Signatures in Willow Warbler Phylloscopus trochilus Feathers Collected in Africa. J. Avian Biol. 2006, 37, 323–330. [Google Scholar] [CrossRef]
- Bensch, S.; Grahn, M.; MÜller, N.; Gay, L.; Åkesson, S. Genetic, Morphological, and Feather Isotope Variation of Migratory Willow Warblers Show Gradual Divergence in a Ring. Mol. Ecol. 2009, 18, 3087–3096. [Google Scholar] [CrossRef]
- Lindström, Å.; Svensson, S.; Green, M.; Ottval, R. Distribution and Population Changes of Two Subspecies of Chiffchaff Phylloscopus collybita in Sweden. Ornis Svec. 2007, 17, 137–147. [Google Scholar] [CrossRef]
- Ellergren, H.; Pettersson, J. Gransångarens Phylloscopus colybita Vårforekomst Vid Ottenby. Calidris 1985, 14, 3–12. [Google Scholar]
- Busse, P.; Meissner, W. Bird Ringing Station Manual; De Gruyter Open Ltd.: Warsaw, Poland; Berlin, Germany, 2015. [Google Scholar]
- Demongin, L. Identification Guide to Birds in the Hand; Laurent Demongin: Beauregard-Vendon, France, 2016. [Google Scholar]
- Svensson, L. Identification Guide to European Passerines; British Trust for Ornithology: Thetford, UK, 1992. [Google Scholar]
- Pinszke, A.; Remisiewicz, M. Long-Term Changes in Autumn Migration Timing of Garden Warblers Sylvia borin at the Southern Baltic Coast in Response to Spring, Summer and Autumn Temperatures. Eur. Zool. J. 2023, 90, 283–295. [Google Scholar] [CrossRef]
- Gołębiewski, I.; Remisiewicz, M. Carry-Over Effects of Climate Variability at Breeding and Non-Breeding Grounds on Spring Migration in the European Wren Troglodytes troglodytes at the Baltic Coast. Animals 2023, 13, 2015. [Google Scholar] [CrossRef]
- Barstone, A.G.; Livezey, R.E. Classification, Seasonality and Persistence of Low–Frequency Atmospheric Circulation Patterns. Mon. Weather Rev. 1987, 115, 1083–1126. [Google Scholar]
- Bueh, C.; Nakamura, H. Scandinavian Pattern and Its Climatic Impact. Q. J. R. Meteorol. Soc. 2007, 133, 2117–2131. [Google Scholar] [CrossRef]
- Markus Meier, H.E.; Kniebusch, M.; Dieterich, C.; Gröger, M.; Zorita, E.; Elmgren, R.; Myrberg, K.; Ahola, M.P.; Bartosova, A.; Bonsdorff, E.; et al. Climate Change in the Baltic Sea Region: A Summary. Earth Syst. Dyn. 2022, 13, 457–593. [Google Scholar] [CrossRef]
- Hurrell, J.W. Decadal Trends in the North Atlantic Oscillation: Regional Temperatures and Precipitation. Science 1995, 269, 676–679. [Google Scholar] [CrossRef]
- Osborn, T.J. Recent Variations in the Winter North Atlantic Oscillation. Weather 2006, 61, 353–355. [Google Scholar] [CrossRef]
- Wang, L.; Ting, M. Stratosphere-Troposphere Coupling Leading to Extended Seasonal Predictability of Summer North Atlantic Oscillation and Boreal Climate. Geophys. Res. Lett. 2022, 49. [Google Scholar] [CrossRef]
- Simpson, I.; Hanna, E.; Baker, L.; Sun, Y.; Wei, H. North Atlantic Atmospheric Circulation Indices: Links with Summer and Winter Temperature and Precipitation in North-west Europe, Including Persistence and Variability. Int. J. Climatol. 2024, 44, 902–922. [Google Scholar] [CrossRef]
- Wang, S.Y.; Gillies, R.R. Observed Change in Sahel Rainfall, Circulations, African Easterly Waves, and Atlantic Hurricanes since 1979. Int. J. Geophys. 2011, 2011, 1–14. [Google Scholar] [CrossRef]
- Biasutti, M. Rainfall Trends in the African Sahel: Characteristics, Processes, and Causes. Wiley Interdiscip. Rev. Clim. Change 2019, 10, e591. [Google Scholar] [CrossRef] [PubMed]
- Saji, N.H.; Goswami, B.N.; Vinayachandran, P.N.; Yamagata, T. A dipole mode in the tropical Indian Ocean. Nature 1999, 401, 360–363. [Google Scholar] [CrossRef] [PubMed]
- Marchant, R.; Mumbi, C.; Behera, S.; Yamagata, T. The Indian Ocean Dipole-The Unsung Driver of Climatic Variability in East Africa. Afr. J. Ecol. 2007, 45, 4–16. [Google Scholar] [CrossRef]
- Stige, L.C.; Stave, J.; Chan, K.-S.; Ciannelli, L.; Pettorelli, N.; Glantz, M.; Herren, H.R.; Stenseth, N.C. The Effect of Climate Variation on Agro-Pastoral Production in Africa. Proc. Natl. Acad. Sci. USA 2006, 103, 3049–3053. [Google Scholar] [CrossRef]
- Swapna, P.; Sandeep, N.; Alsumaina, K.N.; Krishnan, R.; Ajinkya, A.; Shamal, M.; Sreejith, O.P. Contrasting tropical precipitation and ecosystem response to Indian Ocean Dipole in a warming climate. Sci. Total Environ. 2025, 971, 179081. [Google Scholar] [CrossRef]
- McPhaden, M.J.; Santoso, A.; Cai, W. El Niño Southern Oscillation in a Changing Climate; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2021; ISBN 9781119548126. [Google Scholar]
- NOAA National Oceanic and Atmospheric Administration US Department of Commerce National Weather Service. Climate Prediction Center. Climate & Weather Linkage. Available online: https://www.cpc.ncep.noaa.gov/ (accessed on 31 May 2025).
- Maciag, T.; Remisiewicz, M. Climate Change Impact on the Populations of Goldcrest Regulus regulus and Firecrest Regulus ignicapilla Migrating Through the Southern Baltic Coast. Sustainability 2025, 17, 1243. [Google Scholar] [CrossRef]
- Gould, L.A.; Manning, A.D.; McGinness, H.M.; Hansen, B.D. A Review of Electronic Devices for Tracking Small and Medium Migratory Shorebirds. Anim. Biotelemetry 2024, 12, 11. [Google Scholar] [CrossRef]
- Finch, T.; Pearce-Higgins, J.W.; Leech, D.I.; Evans, K.L. Carry-over Effects from Passage Regions Are More Important than Breeding Climate in Determining the Breeding Phenology and Performance of Three Avian Migrants of Conservation Concern. Biodivers. Conserv. 2014, 23, 2427–2444. [Google Scholar] [CrossRef]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A Review of Methods to Deal with It and a Simulation Study Evaluating Their Performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Bartoń, K. MuMIn: Multi-Model Inference. R Package Version 1.48.11: 2025. Available online: https://CRAN.R-project.org/package=MuMIn (accessed on 24 July 2025).
- Frost, J. Regression Analysis. An Intuitive Guide for Using and Interpreting Linear Models, 1st ed. 2019. Available online: https://www.amazon.com/dp/1735431184?asin=1735431184&revisionId=&format=4&depth=1 (accessed on 24 July 2025).
- Crawley, M.J. The R Book; John Wiley & Sons: Chichester, UK, 2013. [Google Scholar]
- Pareto, A. R Script. Available online: https://rpubs.com/ratherbit/102428 (accessed on 30 June 2025).
- Tukey, J. We Need Both Exploratory and Confirmatory. Am. Stat. 1980, 34, 352–354. [Google Scholar] [CrossRef]
- Sparks, T.H.; Tryjanowski, P. The Detection of Climate Impacts: Some Methodological Considerations. Int. J. Climatol. 2005, 25, 271–277. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2025. [Google Scholar]
- Sokolov, L.V.; Markovets, M.Y.; Shapoval, A.P.; Morozov, Y.G. Long-Term Trends in the Timing of Spring Migration of Passerines on the Courish Spit of the Baltic Sea. Avian Ecol. Behav. 1998, 1, 1–21. [Google Scholar]
- Tøttrup, A.P.; Thorup, K.; Rahbek, C. Patterns of change in timing of spring migration in North European songbird populations. J. Avian Biol. 2006, 37, 84–92. [Google Scholar] [CrossRef]
- Miles, W.T.S.; Bolton, M.; Davis, P.; Dennis, R.; Broad, R.; Robertson, I.; Riddiford, N.J.; Harvey, P.V.; Riddington, R.; Shaw, D.N.; et al. Quantifying Full Phenological Event Distributions Reveals Simultaneous Advances, Temporal Stability and Delays in Spring and Autumn Migration Timing in Long-Distance Migratory Birds. Glob. Change Biol. 2017, 23, 1400–1414. [Google Scholar] [CrossRef]
- Ockendon, N.; Leech, D.; Pearce-Higgins, J.W. Climatic Effects on Breeding Grounds Are More Important Drivers of Breeding Phenology in Migrant Birds than Carryover Effects from Wintering Grounds. Biol. Lett. 2013, 9, 0669. [Google Scholar] [CrossRef] [PubMed]
- Hirons, L.; Turner, A. The Impact of Indian Ocean Mean-State Biases in Climate Models on the Representation of the East African Short Rains. J. Clim. 2018, 31, 6611–6631. [Google Scholar] [CrossRef]
- ECMWF. European Centre for Medium-Range Weather Forecasts. Copernicus Services Copernicus: Global Sea Ice Cover at a Record Low and Third-Warmest February Globally. Available online: https://climate.copernicus.eu/copernicus-global-sea-ice-cover-record-low-and-third-warmest-february-globally (accessed on 9 June 2025).
- Tøttrup, A.P.; Rainio, K.; Coppack, T.; Lehikoinen, E.; Rahbek, C.; Thorup, K. Local Temperature Fine-Tunes the Timing of Spring Migration in Birds. Proc. Integr. Comp. Biol. 2010, 50, 293–304. [Google Scholar] [CrossRef]
- Peach, W.; Baillie, S.; Underhill, L.G. Survival of British Sedge Warblers Acrocephalus schoenobaenus in Relation to West African Rainfall. Ibis 1991, 133, 300–305. [Google Scholar] [CrossRef]
- Maselli, F. Monitoring Forest Conditions in a Protected Mediterranean Coastal Area by the Analysis of Multiyear NDVI Data. Remote Sens. Environ. 2004, 89, 423–433. [Google Scholar] [CrossRef]
- Bartsch, S.; Stegehuis, A.I.; Boissard, C.; Lathière, J.; Peterschmitt, J.-Y.; Reiter, I.M.; Gauquelin, T.; Baldy, V.; Genesio, L.; Matteucci, G.; et al. Impact of Precipitation, Air Temperature and Abiotic Emissions on Gross Primary Production in Mediterranean Ecosystems in Europe. Eur. J. For. Res. 2020, 139, 111–126. [Google Scholar] [CrossRef]
- Peñuelas, J. Phenology Feedbacks on Climate Change. Science 2009, 324, 887–888. [Google Scholar] [CrossRef]
- Berthold, P. Control of Bird Migration; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1996. [Google Scholar]
- Briedis, M.; Bauer, S.; Adamík, P.; Alves, J.A.; Costa, J.S.; Emmenegger, T.; Gustafsson, L.; Koleček, J.; Krist, M.; Liechti, F.; et al. Broad-Scale Patterns of the Afro-Palaearctic Landbird Migration. Glob. Ecol. Biogeogr. 2020, 29, 722–735. [Google Scholar] [CrossRef]
- Briedis, M.; Hahn, S.; Adamík, P. Cold Spell En Route Delays Spring Arrival and Decreases Apparent Survival in a Long-Distance Migratory Songbird. BMC Ecol. 2017, 17, 11. [Google Scholar] [CrossRef] [PubMed]
- Catry, P.; Bearhop, S.; Lecoq, M. Sex Differences in Settlement Behaviour and Condition of Chiffchaffs Phylloscopus Collybita at a Wintering Site in Portugal. Are Females Doing Better? J. Ornithol. 2007, 148, 241–249. [Google Scholar] [CrossRef]
- Herremans, M. Willow Warbler Phylloscopus trochilus. In The Atlas of Southern African Birds; Harrison, J.A., Allan, D.G., Underhill, L.G., Herremans, M., Tree, A.J., Parker, V., Brown, C.J., Eds.; BirdLife: Johannesburg, South Africa, 1999; Volume 2: Passerines, pp. 254–267. [Google Scholar]
- Alerstam, T. Bird Migration Speed. In Avian Migration; Springer: Berlin/Heidelberg, Germany, 2003; pp. 253–267. [Google Scholar]
- Pulido, F.; Berthold, P. Microevolutionary Response to Climatic Change. Adv. Ecol. Res. 2004, 35, 151–183. [Google Scholar] [CrossRef]
- Ożarowska, A.; Zaniewicz, G.; Meissner, W. Sex and Age Specific Differences in Wing Pointedness and Wing Length in Blackcaps Sylvia atricapilla Migrating through the Southern Baltic Coast. Curr. Zool. 2020, 67, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Elkins, N. Weather and Bird Behaviour, 2nd ed.; Poyser: London, UK, 2018. [Google Scholar]
- Nowakowski, J. Ringing Data from the Bird Migration Research Station, University of Gdańsk. University of Gdańsk. 2017. (GBIF.org). Occurrence Dataset. Available online: https://doi.org/10.15468/q5o88l (accessed on 9 June 2025).
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
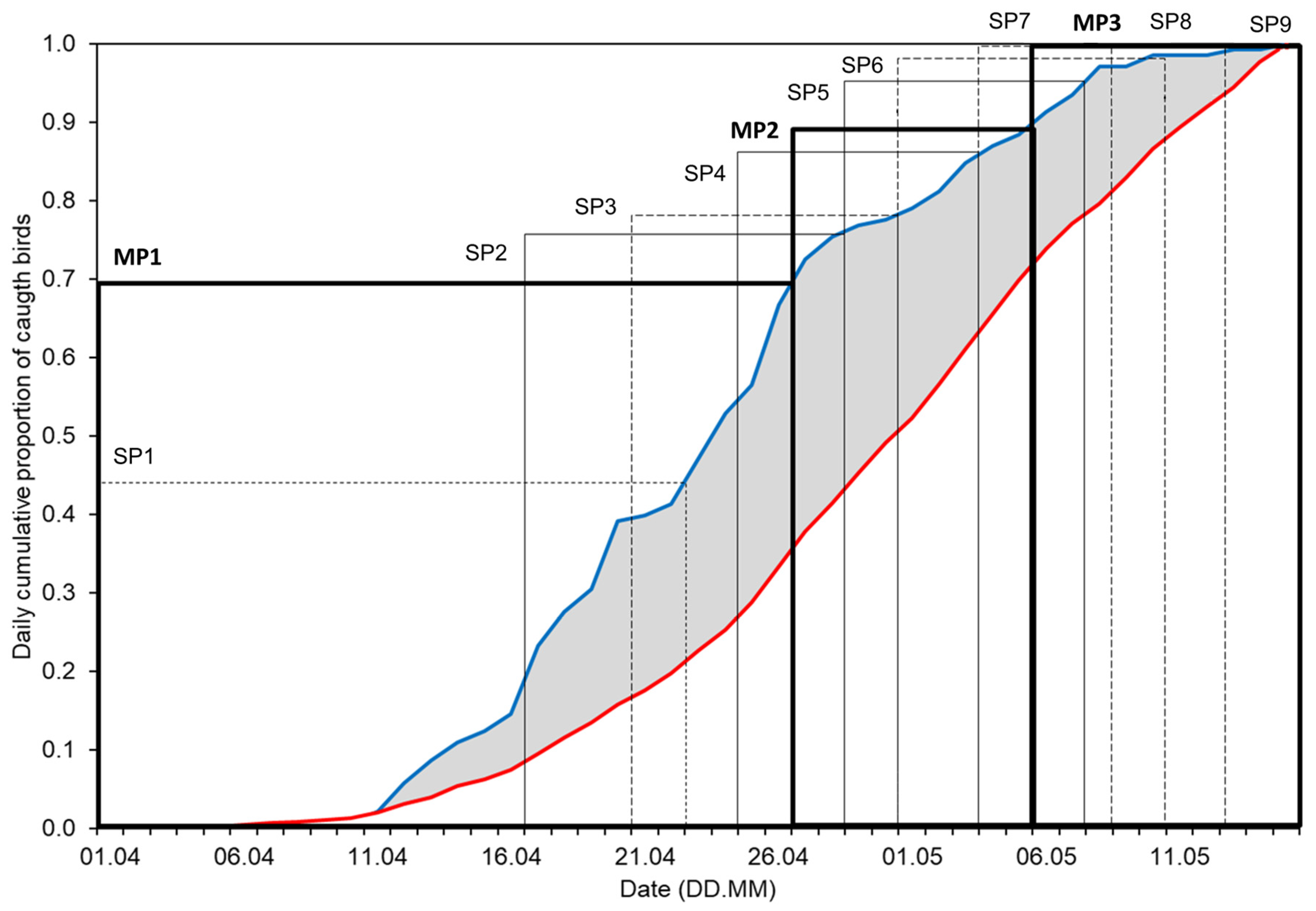
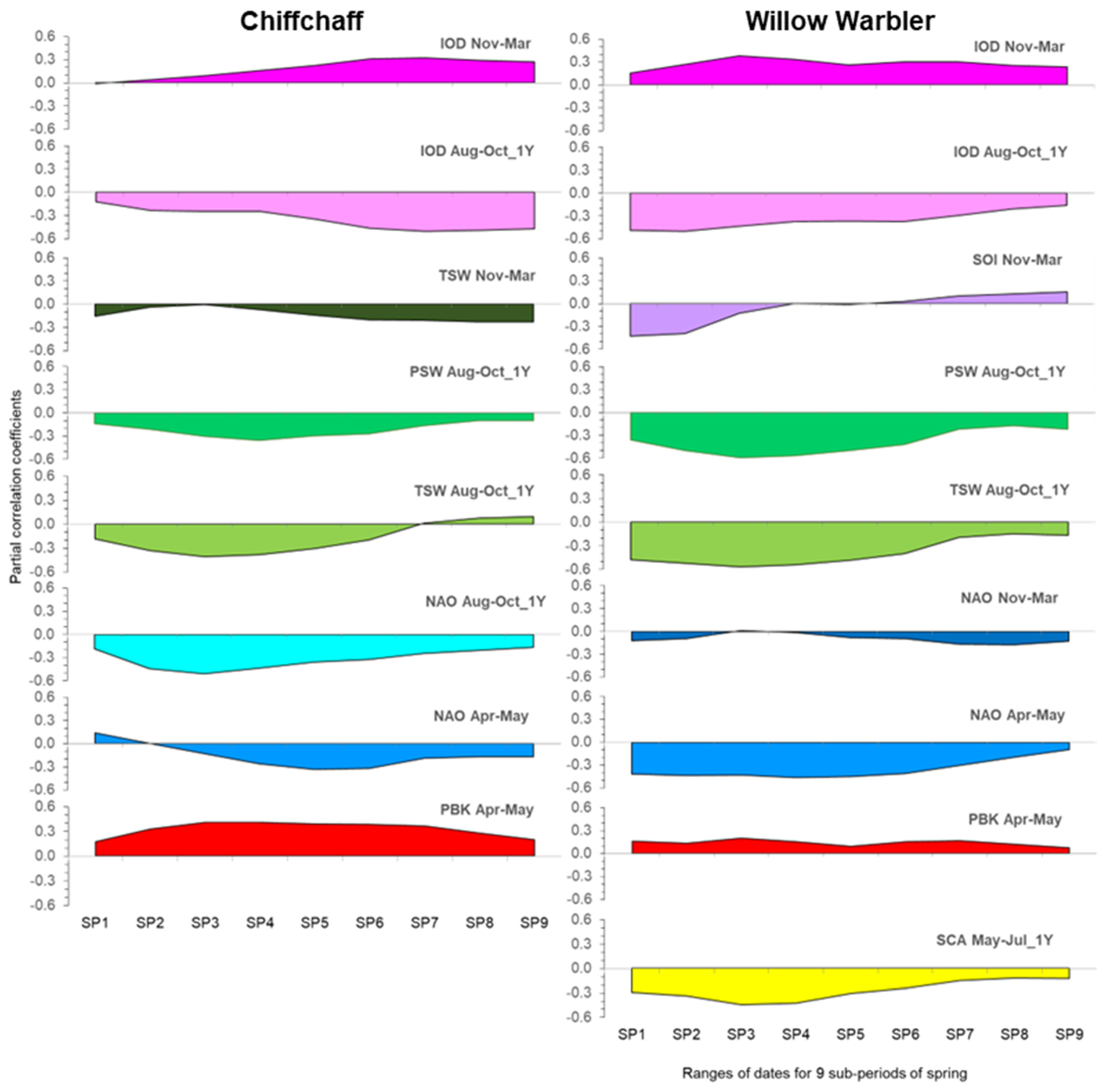
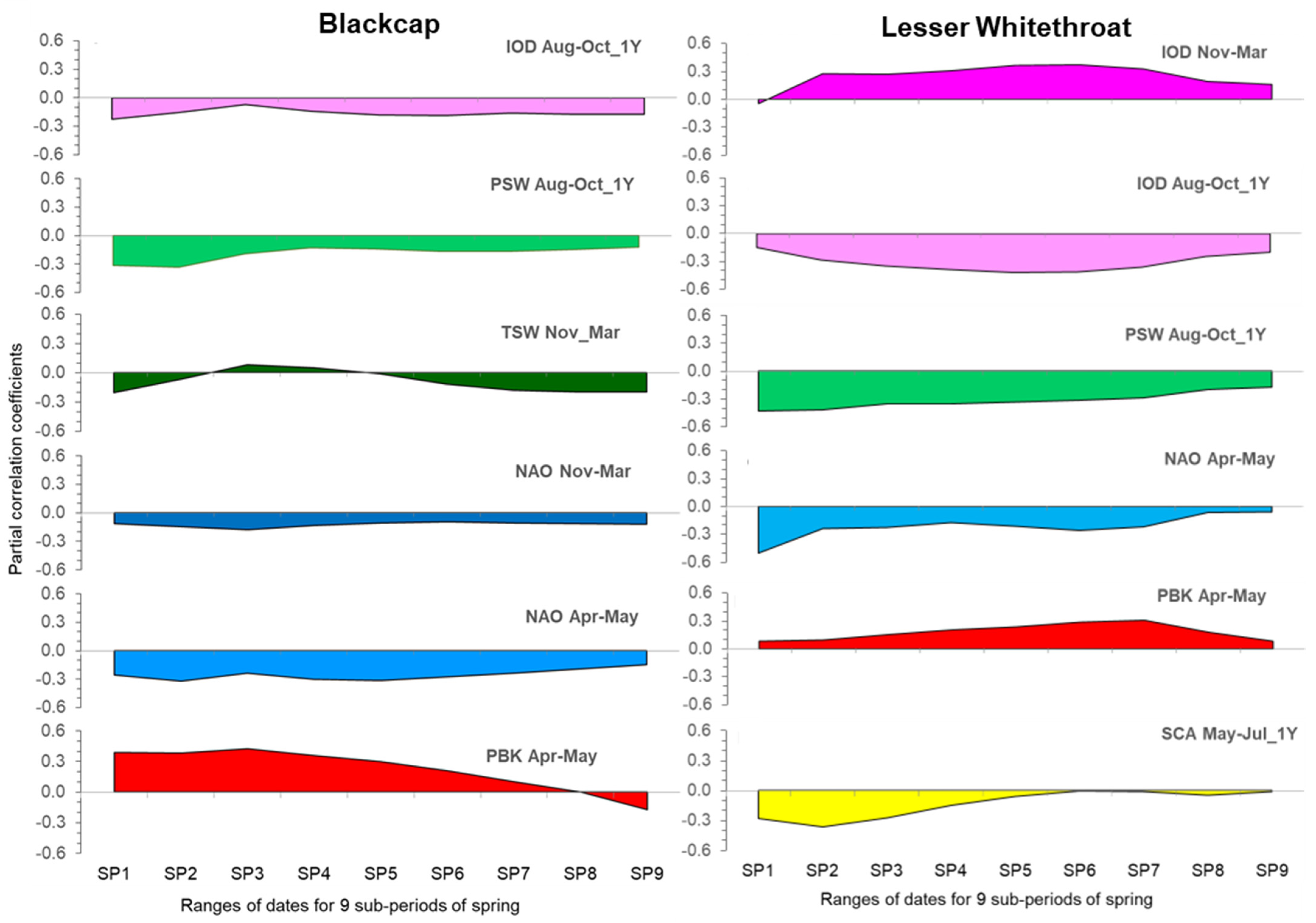
| Symbol | Percentiles | Dates for Each Period by Species | |||
|---|---|---|---|---|---|
| Chiffchaff | Willow Warbler | Blackcap | Lesser Whitethroat | ||
| AA | 0–100% | 26 March–15 May 1 | |||
| MP1 | 0–33% | 26 Mar–12 Apr | 1–26 Apr | 1–30 Apr | 1–30 Apr |
| MP2 | 34–66% | 13–23 Apr | 27 Apr–5 May | 27 Apr–5 May | 1–8 May |
| MP3 | 67–100% | 24 Apr–15 May | 6–15 May | 6–15 May | 9–15 May |
| SP1 | 0–20% | 26 Mar–7 Apr | 1–22 Apr | 1–23 Apr | 1–24 Apr |
| SP2 | 11–30% | 4–11 Apr | 18–25 Apr | 20–26 Apr | 25–30 Apr |
| SP3 | 21–40% | 8–14 Apr | 24–28 Apr | 24–28 Apr | 28 Apr–2 May |
| SP4 | 31–50% | 12–17 Apr | 26 Apr–1 May | 27–30 Apr | 1–4 May |
| SP5 | 41–60% | 15–20 Apr | 29 Apr–3 May | 29 Apr–2 May | 3–6 May |
| SP6 | 51–70% | 18–24 Apr | 2–5 May | 1–6 May | 5–9 May |
| SP7 | 61–80% | 21–28 Apr | 4–7 May | 3–8 May | 7–10 May |
| SP8 | 71–90% | 25 Apr–4 May | 6–11 May | 7–10 May | 10–13 May |
| SP9 | 81–100% | 29 Apr–15 May | 9–15 May | 11–15 May | 11–15 May |
| No | Symbol of Variable 1 | Climate Index | Region Under Climatic Influence | Key References | Source |
|---|---|---|---|---|---|
| 1 | SCA APR–MAY | Scandinavian Pattern Index | Scandinavia, western and central Russia | [62,63] | ftp://ftp.cpc.ncep.noaa.gov/wd52dg/data/indices/scand_index.tim (accessed on 23 June 2025) |
| 2 | SCA MAY–JUL_1Y | ||||
| 3 | TBK APR–MAY | Mean local temperature | Baltic coast near Bukowo (54–55° N, 16° E–18° E) | [64] | http://climexp.knmi.nl/select.cgi?era5_t2m_daily (accessed on 23 June 2025) |
| 4 | PBK APR–MAY | Mean local precipitation | https://climexp.knmi.nl/select.cgi?gpcc (accessed on 23 June 2025) | ||
| 5 | NAO APR–MAY | North Atlantic Oscillation Index | Northwestern and Central Europe, Western Mediterranean area, Northwestern Africa | [35,65,66,67,68] | https://www.cpc.ncep.noaa.gov/products/precip/CWlink/pna/norm.nao.monthly.b5001.current.ascii.table (accessed on 23 June 2025) |
| 6 | NAO AUG–OCT_1Y | ||||
| 7 | NAO NOV–MAR | ||||
| 8 | TSW AUG–OCT_1Y | Temperature in the Western Sahel | Western Sahel (15–20° N, 20° W–10° E) | [69,70] | http://climexp.knmi.nl/select.cgi?era5_t2m_daily (accessed on 23 June 2025) |
| 9 | TSW NOV–MAR | ||||
| 10 | PSW AUG–OCT_1Y | Precipitation for the Western Sahel | https://climexp.knmi.nl/select.cgi?gpcc (accessed on 23 June 2025) | ||
| 11 | PSW NOV–MAR | ||||
| 12 | IOD AUG–OCT_1Y | Indian Ocean Dipole | East Africa, Middle East | [71,72,73,74] | http://climexp.knmi.nl/getindices.cgi?WMO=UKMOData/hadisst1_dmi&STATION=DMI_HadISST1&TYPE=i&id=someone@somewhere (accessed on 23 June 2025) |
| 13 | IOD NOV–MAR | ||||
| 14 | SOI NOV–MAR | Southern Oscillation Index | East Africa, southeastern Africa | [75] | http://climexp.knmi.nl/getindices.cgi?WMO=CRUData/soi&STATION=SOI&TYPE=i&id=someone@somewhere (accessed on 23 June 2025) |
| Response Variable /Climate Variable | Estimate | SE | t | p | VIF | pR |
|---|---|---|---|---|---|---|
| AA Best model: F5,36 = 4.79, AdjR2 = 31.6%, AdjR2/R2 = 0.79, AdjR2 − predR2 = 0.15 | ||||||
| IOD_AUG_OCT_1Y | –2.66 | 0.81 | –3.29 | 0.0023 | 1.57 | –0.48 |
| IOD_NOV_MAR | 1.28 | 0.80 | 1.61 | 0.1163 | 1.76 | 0.26 |
| NAO_AUG_OCT_1Y | –1.26 | 0.62 | –2.02 | 0.0508 | 1.10 | –0.32 |
| PBK_APR_MAY | 1.91 | 0.64 | 2.97 | 0.0052 | 1.22 | 0.45 |
| TSW_NOV_MAR | –1.48 | 0.64 | –2.33 | 0.0256 | 1.12 | –0.36 |
| MP1 Best model: F4,37 = 4.44, AdjR2 = 25.1%, AdjR2/R2 = 0.78, AdjR2 − predR2 = 0.08 | ||||||
| NAO_AUG_OCT_1Y | –0.57 | 0.26 | –2.23 | 0.0316 | 1.13 | –0.14 |
| PBK_APR_MAY | 0.46 | 0.25 | 1.89 | 0.0671 | 1.08 | 0.24 |
| PSW_AUG_OCT_1Y | –0.53 | 0.30 | –1.78 | 0.0841 | 1.40 | –0.19 |
| TSW_AUG_OCT_1Y | –0.97 | 0.29 | –3.40 | 0.0016 | 1.48 | –0.38 |
| MP2 Best model: F6,35 = 4.86, AdjR2 = 36.1%, AdjR2/R2 = 0.79, AdjR2 − predR2 = 0.16 | ||||||
| IOD_AUG_OCT_1Y | –0.40 | 0.24 | –1.64 | 0.1101 | 1.12 | –0.27 |
| NAO_APR_MAY | –0.49 | 0.22 | –2.21 | 0.0341 | 1.12 | –0.35 |
| NAO_AUG_OCT_1Y | –0.57 | 0.23 | –2.48 | 0.0179 | 1.20 | –0.39 |
| PBK_APR_MAY | 0.51 | 0.22 | 2.34 | 0.0252 | 1.12 | 0.37 |
| PSW_AUG_OCT_1Y | –0.57 | 0.27 | –2.14 | 0.0397 | 1.50 | –0.34 |
| TSW_AUG_OCT_1Y | –0.86 | 0.26 | –3.33 | 0.0021 | 1.61 | –0.49 |
| MP3 Best model: F3,38 = 4.12, AdjR2 = 18.6%, AdjR2/R2 = 0.76, AdjR2 − predR2 = 0.10 | ||||||
| IOD_AUG_OCT_1Y | –1.16 | 0.38 | –3.05 | 0.0041 | 1.49 | –0.44 |
| IOD_NOV_MAR | 0.61 | 0.37 | 1.64 | 0.1084 | 1.67 | 0.26 |
| PBK_APR_MAY | 0.65 | 0.30 | 2.15 | 0.0377 | 1.16 | 0.33 |
| Response Variable /Climate Variable | Estimate | SE | t | p | VIF | pR |
|---|---|---|---|---|---|---|
| AA Best model: F7,33 = 6.80, AdjR2 = 50.4%, AdjR2/R2 = 0.85, AdjR2 − predR2 = 0.09 | ||||||
| IOD_AUG_OCT_1Y | –1.67 | 0.51 | –3.27 | 0.0025 | 1.49 | –0.50 |
| IOD_NOV_MAR | 1.34 | 0.55 | 2.47 | 0.0190 | 2.05 | 0.40 |
| NAO_APR_MAY | –1.16 | 0.41 | –2.83 | 0.0079 | 1.19 | –0.44 |
| PBK_APR_MAY | 0.78 | 0.46 | 1.68 | 0.1030 | 1.48 | 0.29 |
| PSW_AUG_OCT_1Y | –2.02 | 0.55 | –3.67 | 0.0009 | 1.93 | –0.54 |
| SCA_MAY_JUL_1Y | –0.96 | 0.41 | –2.33 | 0.0259 | 1.28 | –0.38 |
| TSW_AUG_OCT_1Y | –1.82 | 0.54 | –3.37 | 0.0019 | 2.08 | –0.51 |
| MP1 Best model: F6,34 = 8.78, AdjR2 = 53.9%, AdjR2/R2 = 0.89, AdjR2 − predR2 = 0.12 | ||||||
| IOD_AUG_OCT_1Y | –0.73 | 0.22 | –3.28 | 0.0024 | 1.34 | –0.49 |
| NAO_APR_MAY | –0.64 | 0.18 | –3.62 | 0.0009 | 1.04 | –0.53 |
| PSW_AUG_OCT_1Y | –0.80 | 0.24 | –3.33 | 0.0021 | 1.76 | –0.50 |
| SCA_MAY_JUL_1Y | –0.33 | 0.18 | –1.84 | 0.0741 | 1.14 | –0.30 |
| SOI_NOV_MAR | –0.57 | 0.21 | –2.73 | 0.0099 | 1.34 | –0.43 |
| TSW_AUG_OCT_1Y | –0.86 | 0.23 | –3.77 | 0.0006 | 1.77 | –0.54 |
| MP2 Best model: F6,34 = 6.30, AdjR2 = 44.3%, AdjR2/R2 = 0.84, AdjR2 − predR2 = 0.09 | ||||||
| IOD_AUG_OCT_1Y | –0.66 | 0.24 | –2.77 | 0.0089 | –0.43 | 1.45 |
| IOD_NOV_MAR | 0.49 | 0.24 | 2.00 | 0.0534 | 0.33 | 1.82 |
| NAO_APR_MAY | –0.65 | 0.18 | –3.54 | 0.0012 | –0.52 | 1.06 |
| PSW_AUG_OCT_1Y | –0.98 | 0.25 | –3.95 | 0.0004 | –0.56 | 1.76 |
| SCA_MAY_JUL_1Y | –0.43 | 0.19 | –2.18 | 0.0360 | –0.35 | 1.27 |
| TSW_AUG_OCT_1Y | –0.88 | 0.24 | –3.63 | 0.0009 | –0.53 | 1.87 |
| MP3 Best model: F1,39 = 5.55, AdjR2 = 10.2%, AdjR2/R2 = 0.82, AdjR2 − predR2 = 0.01 | ||||||
| NAO_NOV_MAR | –0.24 | 0.10 | –2.36 | 0.0236 | – | –0.24 |
| Response Variable /Climate Variable | Estimate | SE | t | p | VIF | pR |
|---|---|---|---|---|---|---|
| AA Best model: F3,36 = 5.07, AdjR2 = 23.9%, AdjR2/R2 = 0.80, AdjR2 − predR2 = 0.04 | ||||||
| IOD_AUG_OCT_1Y | –1.04 | 0.56 | –1.86 | 0.1189 | 1.02 | 0.30 |
| NAO_APR_MAY | –1.03 | 0.55 | –1.87 | 0.0113 | 1.09 | –0.30 |
| PBK_APR_MAY | 1.20 | 0.51 | 2.35 | 0.0160 | 1.08 | 0.36 |
| MP1 Best model: F2,37 = 10.17, AdjR2 = 32.0%, AdjR2/R2 = 0.90, AdjR2 − predR2 = 0.04 | ||||||
| PBK_APR_MAY | 0.72 | 0.20 | 3.68 | 0.0007 | 1.02 | 0.52 |
| PSW_AUG_OCT_1Y | –0.43 | 0.21 | –2.08 | 0.0448 | 1.02 | –0.33 |
| MP2 Best model: F2,37 = 5.81, AdjR2 = 19.8%, AdjR2/R2 = 0.83, AdjR2 − predR2 = 0.02 | ||||||
| NAO_APR_MAY | –0.36 | 0.20 | –1.78 | 0.0829 | 1.07 | –0.28 |
| PBK_APR_MAY | 0.45 | 0.19 | 2.36 | 0.0234 | 1.07 | 0.36 |
| MP3 Best model: F2,37 = 2.99, AdjR2 = 9.2%, AdjR2/R2 = 0.67, AdjR2 − predR2 = 0.07 | ||||||
| NAO_NOV_MAR | –0.49 | 0.24 | –2.04 | 0.0487 | 1.25 | –0.32 |
| TSW_NOV_MAR | –0.51 | 0.24 | –2.11 | 0.0413 | 1.25 | –0.33 |
| Response Variable /Climate Variable | Estimate | SE | t | p | VIF | pR |
|---|---|---|---|---|---|---|
| AA Best model: F3,36 = 5.48, AdjR2 = 25.6%, AdjR2/R2 = 0.85, AdjR2 − predR2 = 0.07 | ||||||
| IOD_AUG_OCT_1Y | –0.77 | 0.48 | –1.60 | 0.1189 | 1.03 | –0.26 |
| NAO_APR_MAY | –1.21 | 0.45 | –2.67 | 0.0113 | 1.02 | –0.41 |
| PSW_AUG_OCT_1Y | –1.15 | 0.46 | –2.53 | 0.0160 | 1.01 | –0.39 |
| MP1 Best model: F3,36 = 6.84, AdjR2 = 30.1%, AdjR2/R2 = 0.89, AdjR2 − predR2 = 0.09 | ||||||
| NAO_APR_MAY | –0.58 | 0.21 | –2.78 | 0.0087 | 1.00 | –0.42 |
| PSW_AUG_OCT_1Y | –0.63 | 0.21 | –3.01 | 0.0047 | 1.00 | –0.45 |
| SCA_MAY_JUL_1Y | –0.36 | 0.19 | –1.88 | 0.0681 | 1.00 | –0.30 |
| MP2 Best model: F4,35 = 4.15, AdjR2 = 24.4%, AdjR2/R2 = 0.84, AdjR2 − predR2 = 0.07 | ||||||
| IOD_AUG_OCT_1Y | –0.71 | 0.26 | –2.67 | 0.1189 | 1.44 | –0.41 |
| IOD_NOV_MAR | 0.73 | 0.26 | 2.83 | 0.0113 | 1.68 | 0.43 |
| PBK_APR_MAY | 0.48 | 0.22 | 2.16 | 0.0160 | 1.22 | 0.35 |
| PSW_AUG_OCT_1Y | –0.43 | 0.21 | –2.01 | 0.1189 | 1.06 | –0.32 |
| MP3 Best model: F1,38 = 2.51, AdjR2 = 3.7%, AdjR2/R2 = 0.82, AdjR2 − predR2 = 0.01 | ||||||
| PSW_AUG_OCT_1Y | –0.14 | 0.09 | –1.58 | 0.1217 | – | –0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Remisiewicz, M.; Underhill, L.G. Climate in Europe and Africa Sequentially Shapes the Spring Passage of Long-Distance Migrants at the Baltic Coast in Europe. Diversity 2025, 17, 528. https://doi.org/10.3390/d17080528
Remisiewicz M, Underhill LG. Climate in Europe and Africa Sequentially Shapes the Spring Passage of Long-Distance Migrants at the Baltic Coast in Europe. Diversity. 2025; 17(8):528. https://doi.org/10.3390/d17080528
Chicago/Turabian StyleRemisiewicz, Magdalena, and Les G. Underhill. 2025. "Climate in Europe and Africa Sequentially Shapes the Spring Passage of Long-Distance Migrants at the Baltic Coast in Europe" Diversity 17, no. 8: 528. https://doi.org/10.3390/d17080528
APA StyleRemisiewicz, M., & Underhill, L. G. (2025). Climate in Europe and Africa Sequentially Shapes the Spring Passage of Long-Distance Migrants at the Baltic Coast in Europe. Diversity, 17(8), 528. https://doi.org/10.3390/d17080528






