Activity Patterns of Bharal (Pseudois nayaur) from a Subtropical Forest Area Based on Camera Trap Data
Abstract
1. Introduction
2. Material and Methods
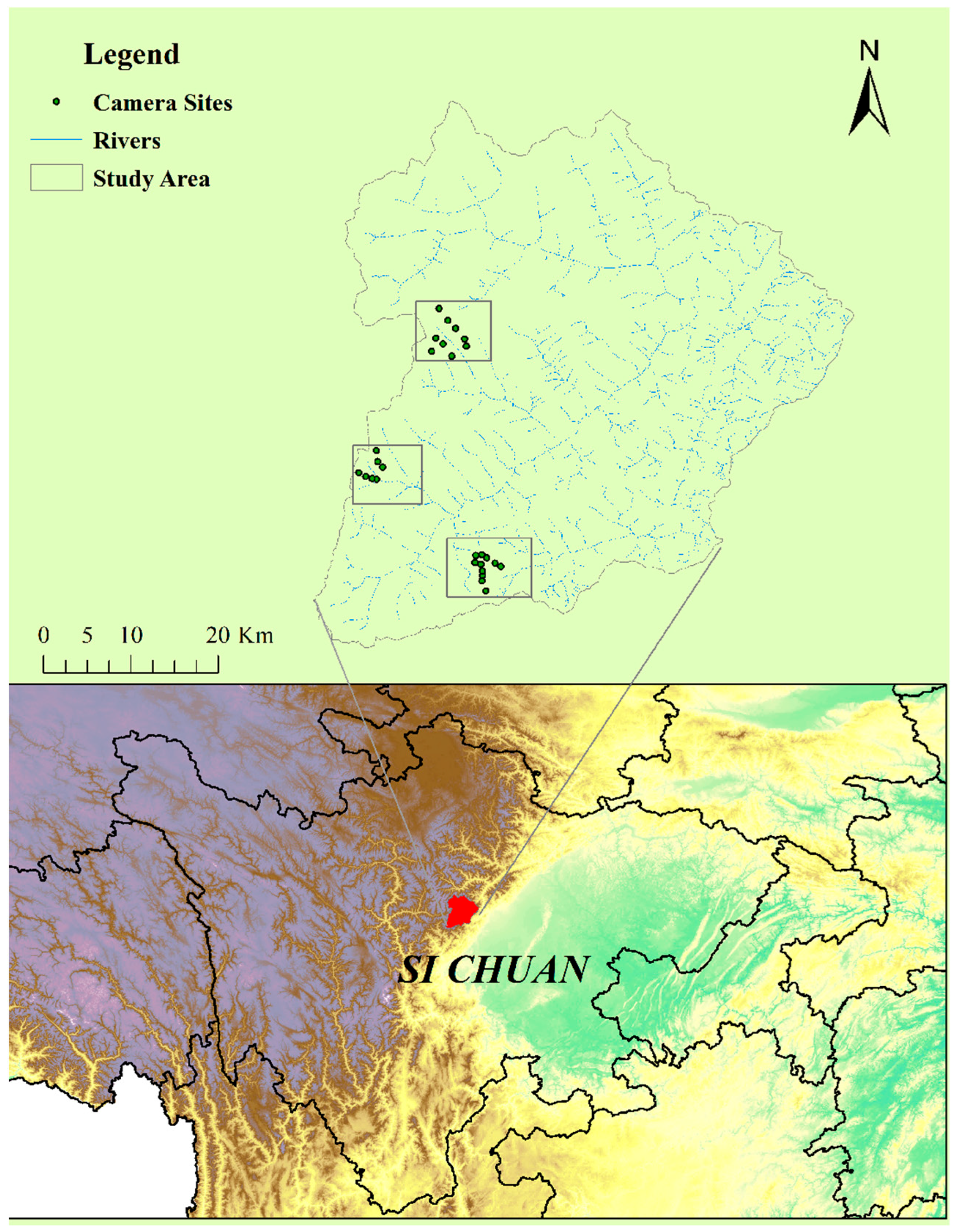
2.1. Study Site
2.2. Data Collection
2.3. Statistical Analysis
3. Results
3.1. Effective Captures
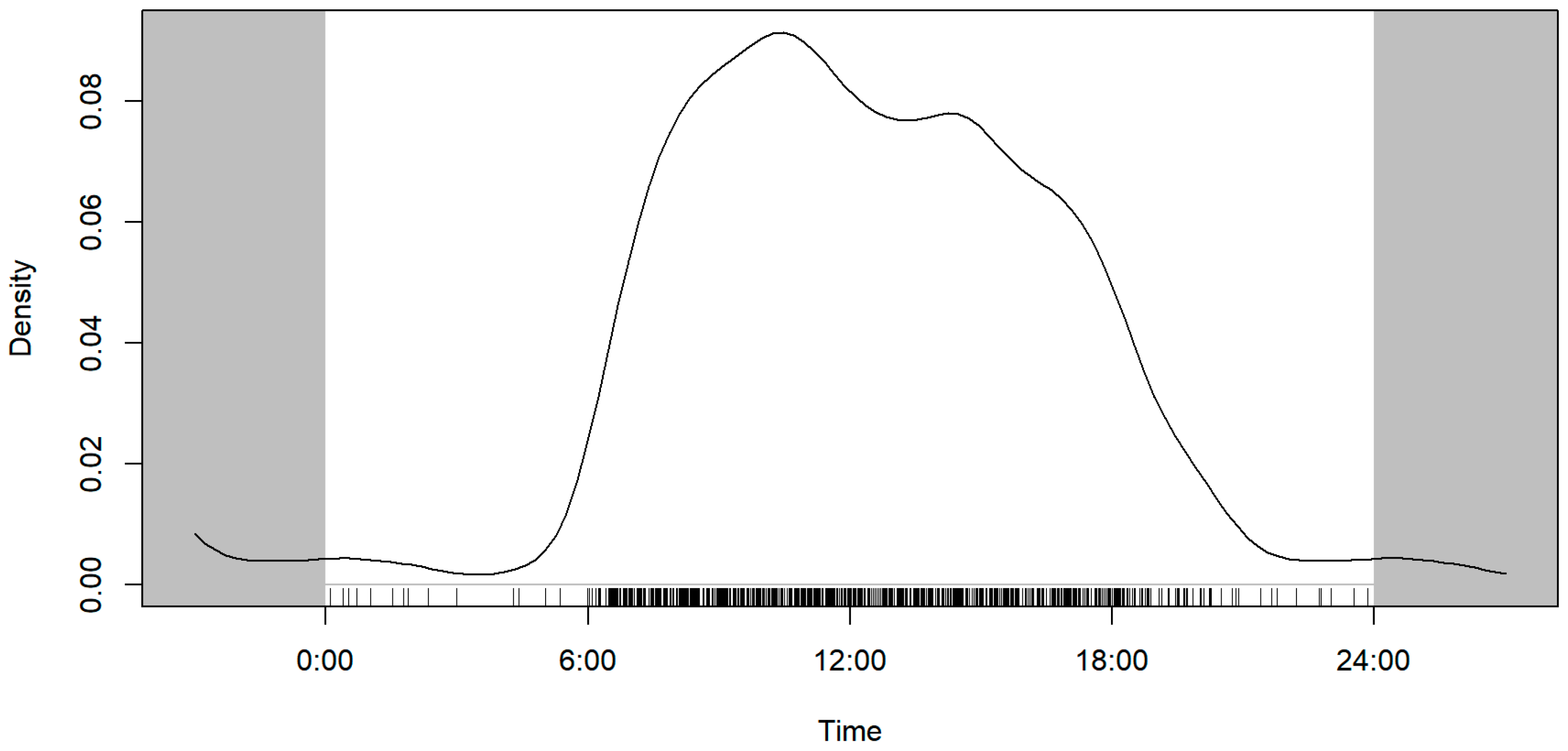
3.2. Diurnal Activity Patterns
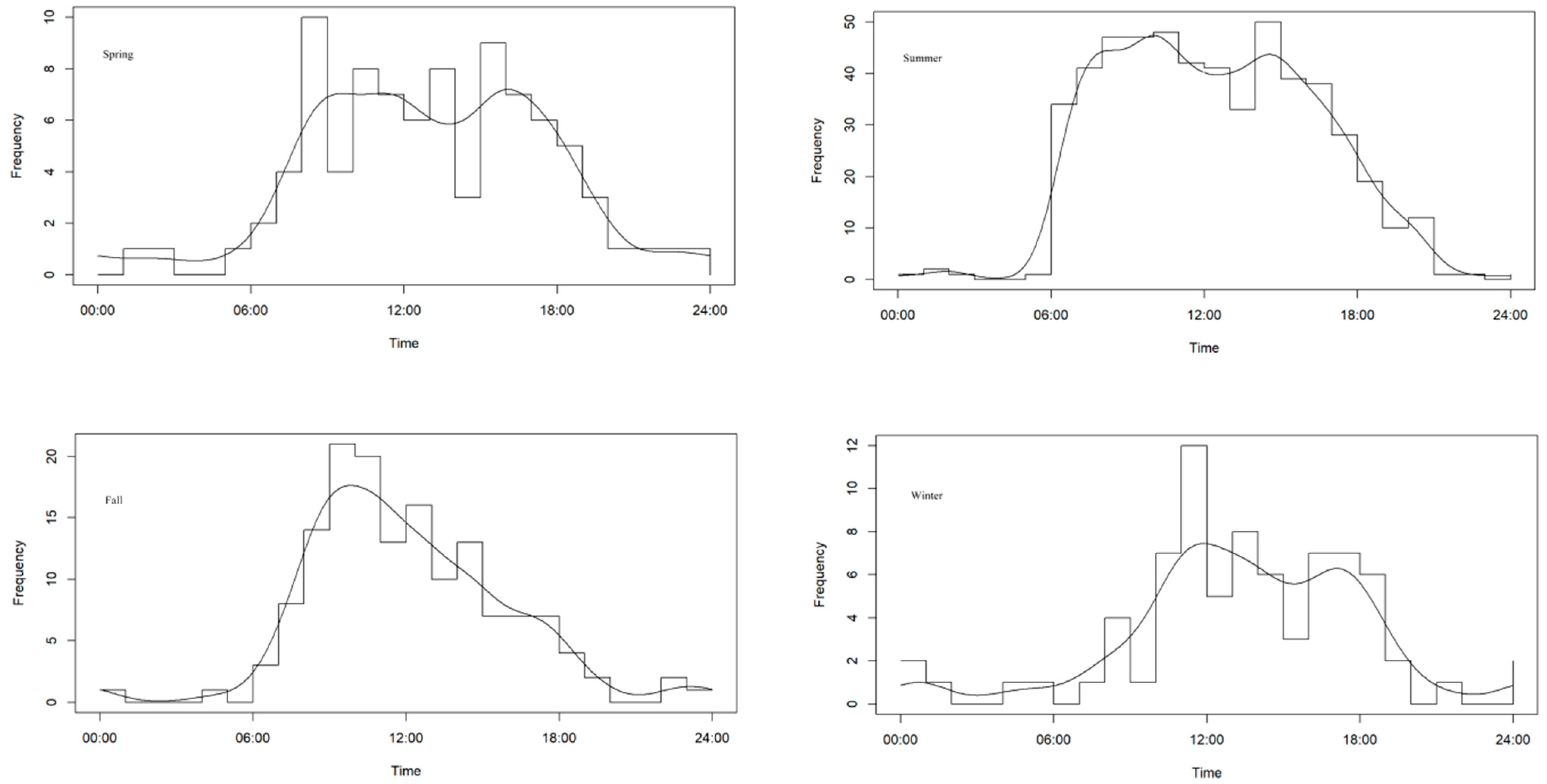
3.3. Seasonal Activity Patterns
3.4. Atmospheric Temperature and Activity Patterns
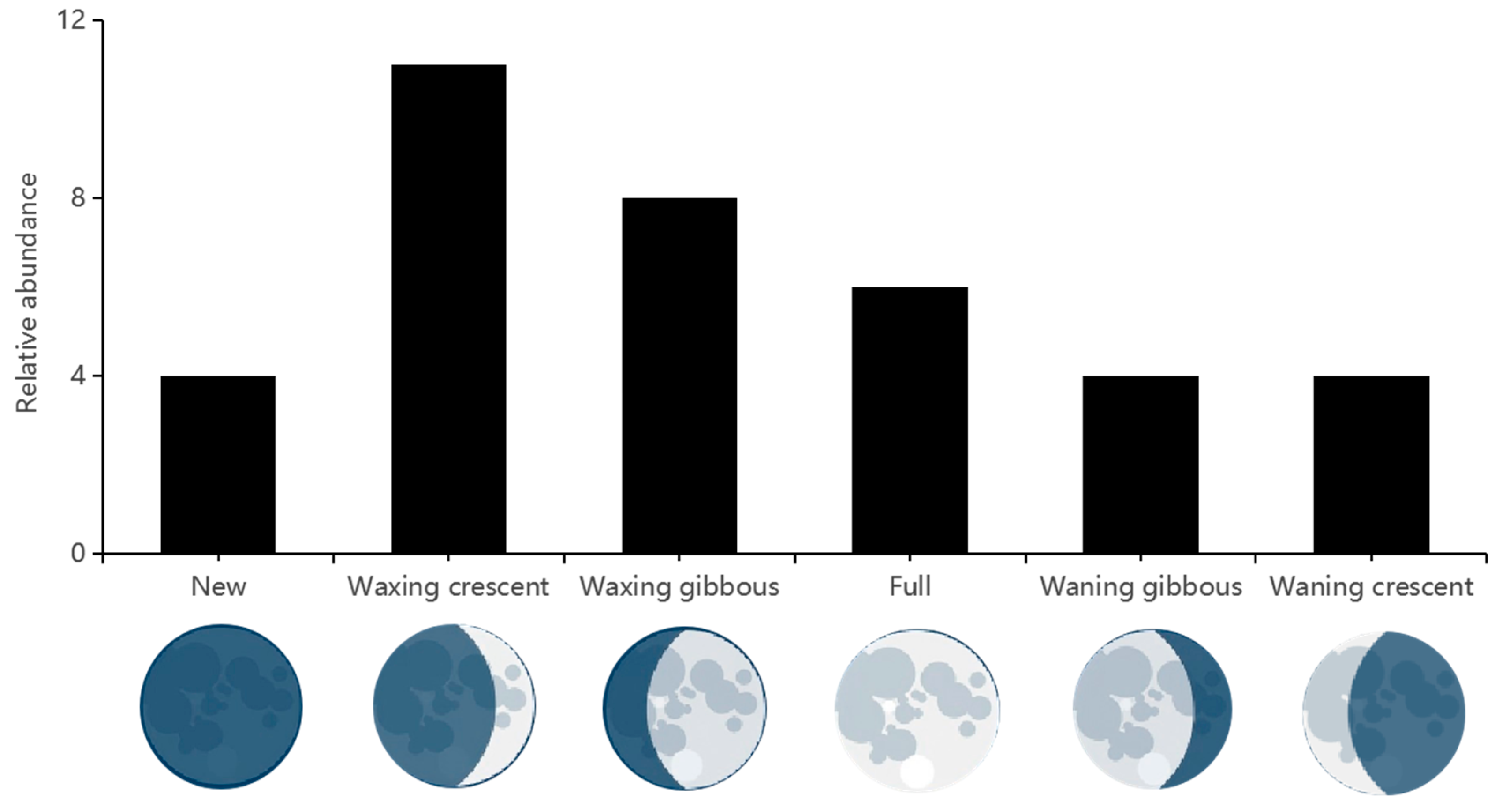
3.5. Moon Phase and Nocturnal Activity Pattern
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Aschoff, J. Tierische Periodik unter dem Einfluß von Zeitgebern. Z. Tierpsychol. 1958, 15, 1–30. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, C.J.; Wang, M.; Yang, W. Research progress on ecology and biology of blue sheep. Sichuan J. Zool. 2021, 40, 592–600. [Google Scholar]
- Wiskirchen, K.H.; Jacobsen, T.C.; Ditchkoff, S.S.; Demarais, S.; Gitzen, R.A. Behaviour of a large ungulate reflects temporal patterns of predation risk. Wildl. Res. 2022, 49, 500–512. [Google Scholar] [CrossRef]
- Kawecki, T. Adaptation to Marginal Habitats. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 321. [Google Scholar] [CrossRef]
- Xiang, D.; Meng, B.; Xie, B.; Huang, X.; Wang, C.; Ran, J.; Su, H.; Zhang, M. Daily activity rhythm of sympatric ungulate species in Fanjingshan Reserve, China. Glob. Ecol. Conserv. 2024, 56, e3271. [Google Scholar] [CrossRef]
- Berger, A. Activity patterns, chronobiology and the assessment of stress and welfare in zoo and wild animals. Int. Zoo Yearb. 2011, 45, 80–90. [Google Scholar] [CrossRef]
- Piccione, G.; Caola, G.; Refinetti, R. Circadian modulation of starvation-induced hypothermia in sheep and goats. Chronobiol. Int. 2002, 19, 531–541. [Google Scholar] [CrossRef]
- Silveira, L.; Jácomo, A.T.; Diniz-Filho, J.A.F. Camera trap, line transect census and track surveys: A comparative evaluation. Biol. Conserv. 2003, 114, 351–355. [Google Scholar] [CrossRef]
- Sheng, L.; Dajun, W.; Zhishu, X.; Xinhai, L.; Tianming, W.; Limin, F.; Yun, W. Camera-trapping in wildlife research and conservation in China: Review and outlook. Biodivers. Sci. 2014, 22, 685. [Google Scholar] [CrossRef]
- Harris, R.B. Pseudois nayaur. The IUCN Red List of Threatened Species, e.T61513537A64313015. 2014. Available online: https://www.iucnredlist.org/species/61513537/64313015 (accessed on 15 July 2025).
- Schaller, G.M. Mountain: Wild Sheep and Goats of Himalaya; University of Chicago Press: Chicago, IL, USA, 1977. [Google Scholar]
- Wang, Y. A Complete Checklist of Mammal Species and Subspecies in China: A Taxonomic and Geographic Reference; China Forestry Publishing House: Beijing, China, 2003; pp. 41–46. [Google Scholar]
- Wegge, P. Aspects of the population ecology of blue sheep in Nepal. J. Asian Ecol. 1979, 1, 10–20. [Google Scholar]
- Yu, Y.; Guo, S.; Bai, Q.; Li, Z.; Hu, T.; Lu, H. The seasonal change of blue sheep population structure in Helanshan Mountains. Acta Theriol. Sin. 2004, 24, 200–204. [Google Scholar]
- Wang, X.-m.; Liu, Z.-s.; Li, X.-q.; Li, Z.-g. Comparison of age structures of male blue sheep (Pseudois nayaur) on Helan Mountain in two periods, 1995 and 2004. Zool. Res. 2005, 26, 467–472. [Google Scholar]
- Harris, R.; Miller, D. Overlap in summer habitats and diets of Tibetan Plateau ungulates. Mammalia 1995, 59, 197–212. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, X.; Li, Z.; Cui, D. Comparison of seasonal feeding habitats by blue sheep (Pseudois nayaur) during winter and spring in Helan mountain, China. Dong Wu Xue Yan Jiu = Zool. Res. 2005, 26, 580–589. [Google Scholar]
- Liu, Z.S.; Wang, X.M.; Li, Z.G.; Cui, D.Y.; Li, X.Q. Diurnal activity rhythm of blue sheep (Pseudois nayaur) in Helan Mountain: Variation of season, age and sex. Zool. Res. 2005, 26, 350–357. [Google Scholar]
- Cao, L.; Liu, Z.; Wang, X.; Hu, T.; Li, T.; Zhai, H.; Hou, J. Preliminary study on group characteristics of blue sheep (Pseudois nayaur) in spring and early winter in Helan Mountains, China. Chin. J. Zool. 2005, 40, 28–33. [Google Scholar]
- Lirong, C.; Zhensheng, L.; Xiaoming, W.; Tianhua, H.; Hao, Z.; Jianhai, H. Winter group size and composition of blue sheep (Pseudois nayaur) in the Helan Mountains, China. Shou Lei Xue Bao = Acta Theriol. Sin. 2005, 25, 200–204. [Google Scholar]
- Khattak, R.H.; Hussain, A.; Rehman, E.U.; Nawaz, M.A. Population Structure of Blue Sheep (Pseudios nayaur) in Shimshal Valley Gilgit-Baltistan Pakistan. Pak. J. Zool. 2020, 52, 699. [Google Scholar] [CrossRef]
- Mingxing, L.; Xing, C.; Xingyu, H.; Yunxi, L.; Wenlong, J.; Kong, Y.; Sheng, L.; Tianpei, G. Group composition and seasonal changes of Bharal (Pseudois nayaur) in Wanglang National Nature Reserve, China. Acta Theriol. Sin. 2021, 41, 321. [Google Scholar]
- Wilson, P. Aspects of Reproductive Behaviour of Bharal (Pseudois nayaur) in Nepal; Die Gesellschaft: Berlin, Germany, 1984. [Google Scholar]
- Shrestha, R.; Wegge, P.; Koirala, R.A. Summer diets of wild and domestic ungulates in Nepal Himalaya. J. Zool. 2005, 266, 111–119. [Google Scholar] [CrossRef]
- Namgail, T.; Fox, J.L.; Bhatnagar, Y.V. Habitat segregation between sympatric Tibetan argali Ovis ammon hodgsoni and blue sheep Pseudois nayaur in the Indian Trans-Himalaya. J. Zool. 2004, 262, 57–63. [Google Scholar] [CrossRef]
- Mishra, C.; Van Wieren, S.E.; Ketner, P.; Heitkönig, I.M.; Prins, H.H. Competition between domestic livestock and wild bharal Pseudois nayaur in the Indian Trans-Himalaya. J. Appl. Ecol. 2004, 41, 344–354. [Google Scholar] [CrossRef]
- Parker, K.L.; Barboza, P.S.; Gillingham, M.P. Nutrition integrates environmental responses of ungulates. Funct. Ecol. 2009, 23, 57–69. [Google Scholar] [CrossRef]
- Antonovics, J.; McKane, A.; Newman, T. Spatiotemporal dynamics in marginal populations. Am. Nat. 2006, 167, 16–27. [Google Scholar] [CrossRef]
- Westrick, S.E.; Broder, E.D.; Reznick, D.N.; Ghalambor, C.K.; Angeloni, L. Rapid evolution and behavioral plasticity following introduction to an environment with reduced predation risk. Ethology 2019, 125, 232–240. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Z.; Li, J.; Teng, L.; Liu, Z. Diurnal Behaviors of Captive Blue Sheep in Summer. Chin. J. Wildl. 2018, 39, 796–800. [Google Scholar]
- Xing, C.; Lianjun, Z.; Qianqian, H.; Mingxing, L.; Chunping, L.; Shiwei, J.; Xiaodong, G.; Tianpei, G. The seasonal activity patterns of Bharal (Pseudois nayaur) in forest-meadow mosaic habitat. Angew. Chem. Int. Ed. Engl. 2020, 59, 9004–9010. [Google Scholar]
- Kays, R.W.; Slauson, K. Remote cameras. In Noninvasive Survey Methods for Carnivores; Island Press: Washington, DC, USA, 2008; pp. 110–140. [Google Scholar]
- Sollmann, R.; Tôrres, N.M.; Furtado, M.M.; de Almeida Jácomo, A.T.; Palomares, F.; Roques, S.; Silveira, L. Combining camera-trapping and noninvasive genetic data in a spatial capture–recapture framework improves density estimates for the jaguar. Biol. Conserv. 2013, 167, 242–247. [Google Scholar] [CrossRef]
- Bashir, T.; Bhattacharya, T.; Poudyal, K.; Sathyakumar, S.; Qureshi, Q. Integrating aspects of ecology and predictive modelling: Implications for the conservation of the leopard cat (Prionailurus bengalensis) in the Eastern Himalaya. Acta Theriol. 2014, 59, 35–47. [Google Scholar] [CrossRef]
- Wronski, T.; Apio, A.; Plath, M.; Ziege, M. Sex difference in the communicatory significance of localized defecation sites in Arabian gazelles (Gazella arabica). J. Ethol. 2013, 31, 129–140. [Google Scholar] [CrossRef]
- Samejima, H.; Ong, R.; Lagan, P.; Kitayama, K. Camera-trapping rates of mammals and birds in a Bornean tropical rainforest under sustainable forest management. For. Ecol. Manag. 2012, 270, 248–256. [Google Scholar] [CrossRef]
- Ohashi, H.; Saito, M.; Horie, R.; Tsunoda, H.; Noba, H.; Ishii, H.; Kuwabara, T.; Hiroshige, Y.; Koike, S.; Hoshino, Y. Differences in the activity pattern of the wild boar Sus scrofa related to human disturbance. Eur. J. Wildl. Res. 2013, 59, 167–177. [Google Scholar] [CrossRef]
- Edwards, S.; Noack, J.; Heyns, L.; Rodenwoldt, D. Are camera traps a reliable method for estimating activity patterns? A case study comparing technologies for estimating brown hyaena activity curves. Remote Sens. Ecol. Conserv. 2021, 7, 129–138. [Google Scholar] [CrossRef]
- O’Connell, A.F.; Nichols, J.D.; Karanth, K.U. Camera Traps in Animal Ecology: Methods and Analyses; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Karanth, K.U. Estimating tiger Panthera tigris populations from camera-trap data using capture—Recapture models. Biol. Conserv. 1995, 71, 333–338. [Google Scholar] [CrossRef]
- Ullas Karanth, K. Analysis of predator-prey balance in Bandipur Tiger Reserve with reference to census reports. J. Bombay Nat. Hist. Soc. 1988, 85, 1–8. [Google Scholar]
- Carbone, C.; Christie, S.; Conforti, K.; Coulson, T.; Franklin, N.; Ginsberg, J.; Griffiths, M.; Holden, J.; Kawanishi, K.; Kinnaird, M. The use of photographic rates to estimate densities of tigers and other cryptic mammals. In Animal Conservation Forum; Cambridge University Press: Cambridge, UK, 2001; Volume 4, pp. 75–79. [Google Scholar]
- Jackson, R.M.; Roe, J.D.; Wangchuk, R.; Hunter, D.O. Estimating snow leopard population abundance using photography and capture-recapture techniques. Wildl. Soc. Bull. 2006, 34, 772–781. [Google Scholar] [CrossRef]
- Liu, J.; Ouyang, Z.; Tan, Y.; Yang, J.; Zhang, H. Changes in human population structure: Implications for biodiversity conservation. Popul. Environ. 1999, 21, 45–58. [Google Scholar] [CrossRef]
- Li, Z.; Tang, Z.; Xu, Y.; Wang, Y.; Duan, Z.; Liu, X.; Wang, P.; Yang, J.; Chen, W.; Prins, H.H. Habitat use and activity patterns of mammals and birds in relation to temperature and vegetation cover in the alpine ecosystem of Southwestern China with camera-trapping monitoring. Animals 2021, 11, 3377. [Google Scholar] [CrossRef]
- Yasuda, M. Monitoring diversity and abundance of mammals with camera traps: A case study on Mount Tsukuba, central Japan. Mammal Study 2004, 29, 37–46. [Google Scholar] [CrossRef]
- Zhuo, T.; Jian, Y.; Xuehua, L.; Pengyan, W.; Zhouyuan, L. Research on snow leopards (Panthera uncia) using camera-trapping in Wolong National Nature Reserve, China. Biodivers. Sci. 2017, 25, 62. [Google Scholar] [CrossRef]
- Ridout, M.S.; Linkie, M. Estimating overlap of daily activity patterns from camera trap data. J. Agric. Biol. Environ. Stat. 2009, 14, 322–337. [Google Scholar] [CrossRef]
- Froy, O. Circadian rhythms, aging, and life span in mammals. Physiology 2011, 26, 225–235. [Google Scholar] [CrossRef]
- Vitaterna, M.H.; Takahashi, J.S.; Turek, F.W. Overview of circadian rhythms. Alcohol Res. Health 2001, 25, 85. [Google Scholar]
- Xiaoming, W.; Ming, L.; Shaoxiang, T.; Zhixiao, L. A preliminary study of some characters of blue sheep population ecology in spring. Shou Lei Xue Bao = Acta Theriol. Sin. 1998, 18, 27–33. [Google Scholar]
- Yan, Y.F.; Wang, L.C.; Zhu, J.; Ni, Z.Y.; Luo, T.W.; Liu, N.F. Preliminary study on the ecological behaviour of the Blue Sheep in Dongdashan Nature Reserve, China. Environ. Sci. Agric. Food Sci. 2006, 41, 53–59. [Google Scholar]
- Liu, Z.-s.; Wang, X.-m.; Li, Z.-g.; Cui, D.-y.; Li, X.-q. Seasonal variation of diurnal activity budgets by blue sheep (Pseudois nayaur) with different age-sex classes in Helan Mountain. Zool. Res. 2005, 26, 350–357. [Google Scholar]
- Madison, D. Activity rhythm and spacing. In Biology of New World Microtus; Tamarin, R., Ed.; The American Society of Mammalogists: Topeka, KS, USA, 1985; Volume 373–419. [Google Scholar]
- Kronfeld-Schor, N.; Bloch, G.; Schwartz, W.J. Animal Clocks: When Science Meets Nature; The Royal Society: London, UK, 2013; Volume 280, p. 20131354. [Google Scholar]
- Welbourne, D.J.; Claridge, A.W.; Paull, D.J.; Lambert, A. How do passive infrared triggered camera traps operate and why does it matter? Breaking down common misconceptions. Remote Sens. Ecol. Conserv. 2016, 2, 77–83. [Google Scholar] [CrossRef]
- Prugh, L.R.; Golden, C.D. Does moonlight increase predation risk? Meta-analysis reveals divergent responses of nocturnal mammals to lunar cycles. J. Anim. Ecol. 2014, 83, 504–514. [Google Scholar] [CrossRef]
- Taylor, P.; Swan, M.; Sitters, H.; Smith, A.; Di Stefano, J. Small mammals reduce activity during high moon illumination under risk of predation by introduced predators. Sci. Rep. 2023, 13, 10532. [Google Scholar] [CrossRef]
- Pratas-Santiago, L.P.; Gonçalves, A.L.; Nogueira, A.J.; Spironello, W.R. Dodging the moon: The moon effect on activity allocation of prey in the presence of predators. Ethology 2017, 123, 467–474. [Google Scholar] [CrossRef]
- Linley, G.; Pauligk, Y.; Marneweck, C.; Ritchie, E. Moon phase and nocturnal activity of native Australian mammals. Aust. Mammal. 2020, 43, 190–195. [Google Scholar] [CrossRef]



| df | F | p | |
|---|---|---|---|
| Age class | 3 | 5.164 | 0.002 |
| Season | 3 | 12.914 | <0.001 |
| Type of behavior | 6 | 12.931 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Z.; Chen, W.; Wang, S.; Li, Z.; Guan, T.; Yang, J. Activity Patterns of Bharal (Pseudois nayaur) from a Subtropical Forest Area Based on Camera Trap Data. Diversity 2025, 17, 525. https://doi.org/10.3390/d17080525
Tang Z, Chen W, Wang S, Li Z, Guan T, Yang J. Activity Patterns of Bharal (Pseudois nayaur) from a Subtropical Forest Area Based on Camera Trap Data. Diversity. 2025; 17(8):525. https://doi.org/10.3390/d17080525
Chicago/Turabian StyleTang, Zhuo, Wei Chen, Shufeng Wang, Zhouyuan Li, Tianpei Guan, and Jian Yang. 2025. "Activity Patterns of Bharal (Pseudois nayaur) from a Subtropical Forest Area Based on Camera Trap Data" Diversity 17, no. 8: 525. https://doi.org/10.3390/d17080525
APA StyleTang, Z., Chen, W., Wang, S., Li, Z., Guan, T., & Yang, J. (2025). Activity Patterns of Bharal (Pseudois nayaur) from a Subtropical Forest Area Based on Camera Trap Data. Diversity, 17(8), 525. https://doi.org/10.3390/d17080525






