1. Introduction
Sea surface temperature (SST) and its relationship with ocean currents has historically shaped the latitudinal distribution of marine species that respond by increasing in abundance and spatial distribution [
1]. Temperature is related to fundamental physiological processes from the cellular level to organ functioning. Marine organisms mostly live close to their thermal tolerance limits; thus, temperature increases negatively affect their performance and survival [
2]. Temperature is a key factor that determines the spatial distribution and physiological rates of marine macrophytes [
3]. Climate change is occurring at an unprecedented rate, with rates up to seven times higher in the ocean than on land [
4]. As the planet continues to warm up due to increasing atmospheric greenhouse gas concentrations, the ocean faces a variety of disturbances, including warming, acidification, decreasing oxygen concentrations, reduction of sea ice, and rising sea levels [
5].
There is evidence that the increase in SST affects different aspects of marine macroalgae, such as metabolism (especially at the level of photosynthesis or growth), cell damage, changes in stem morphology (reduction in size, branching patterns), thalli degradation, changes in phenology, latitudinal and vertical distribution, and community structure. The worst-case scenario is the extinction of several hundred macroalgal species [
6,
7,
8,
9,
10,
11,
12,
13].
Understanding how human stressors have affected biodiversity [
14] over time is crucial for understanding the biological impact [
15] they have had in the past, and for making predictions on how biodiversity will respond to future global environmental changes [
16]. Data from herbaria are traditionally used for taxonomic purposes, but they are also important as historical datasets for assessing recent changes in marine community assemblages [
1], providing unique information for studying long-term changes [
16]. Recently, the use of data from herbaria has gained relevance for the detection of morphological changes in organisms, which, on several occasions, have been related to the impact of climate change, particularly increased SST [
17]. These historical data reveal patterns that would not otherwise be observable from other data sources [
18,
19]. In the last decade or so, there has been an increasing recognition of the value of collections in the analysis of biodiversity, with interest in their potential applications, for example, for inferring threats to conservation and ecology associated with anthropogenic changes [
8,
19,
20,
21,
22].
Studies have been carried out using herbarium data to evaluate changes in the morphology and floristic composition of marine macroalgae, such as the study by Riera et al. [
1], who evaluated
Fucus limitaneus (formerly
Fucus guiryi) (Phaeophyceae) in the Canary Islands using long-term herbarium material. They recognized that the decrease in the size of the morphological characteristics, and even the decline in
F. limitaneus populations, were related to environmental variables such as temperature. Later, in the same area, Alfonso et al. [
23] evaluated the changes in the morphology and reproductive aspects of
Gelidium canariense (Rhodophyta) over time and found that the length of the thallus has been reduced by half over the last 40 years, coinciding with a significant increase in SST, air temperature above the sea surface, and ultraviolet radiation. Geppi and Riera [
3] studied the long-term variability (38-year period, 1978–2016) of the morphological characteristics of three fucoid species,
Gongolaria abies-marina,
Cystoseira compressa, and
C. humilis (Phaeophyceae), and recorded significant declines in thallus size, reproductive efficiency, and biomass, indicating the algae’s disappearance in some of the studied areas.
Mexico’s Pacific coast makes up 68% of the country’s coastline and comprises several different ecoregions [
24] at local, regional, and global levels [
25], thus covering a particularly wide range of habitats [
26]. This results in substantial coastal and marine diversity. The Mexican Pacific is among the most impacted sites due to various anthropogenic pressures that significantly affect biodiversity, such as habitat modification and loss, pollution, fisheries, coastal urbanization, tourism, overexploitation, species introduction, and climate change [
25,
27]. The Mexican Pacific is strongly influenced by large and mesoscale processes of the Pacific Ocean that closely interact with climate conditions [
24]. The warm and cold episodes of large-scale atmospheric circulation [
28] and the El Niño/Southern Oscillation (ENSO) [
29] are among these processes, and their variations associated with recent climate change can seriously affect the coastal zones of the Mexican Pacific [
25].
The genus
Ceratodictyon has 10 described species worldwide [
30], of which
C. variabile (J. Agardh) R.E. Norris and
C. tenue (Setchell & N.L. Gardner) J.N. Norris (Rhodophyta) have been recorded throughout the Mexican Pacific since 1950 [
31,
32,
33,
34]. Additionally, both species can be easily distinguished from each other by their morphological characteristics. As they are representative, frequent species and have clearly delimited morphological characteristics, they have been used in the present study to determine whether there was a relationship between morphology, SST, and the El Niño and La Niña events over time. Herbarium samples and recently collected material from the Mexican Pacific region were used in the analysis. This is the first study of its kind conducted in a tropical area, the aim of which is to begin to evaluate the possible existence and degree of influence, if any, of factors such as SST on macroalgae.
3. Results
A total of 107 specimens of
Ceratodictyon (53
C. tenue and 54
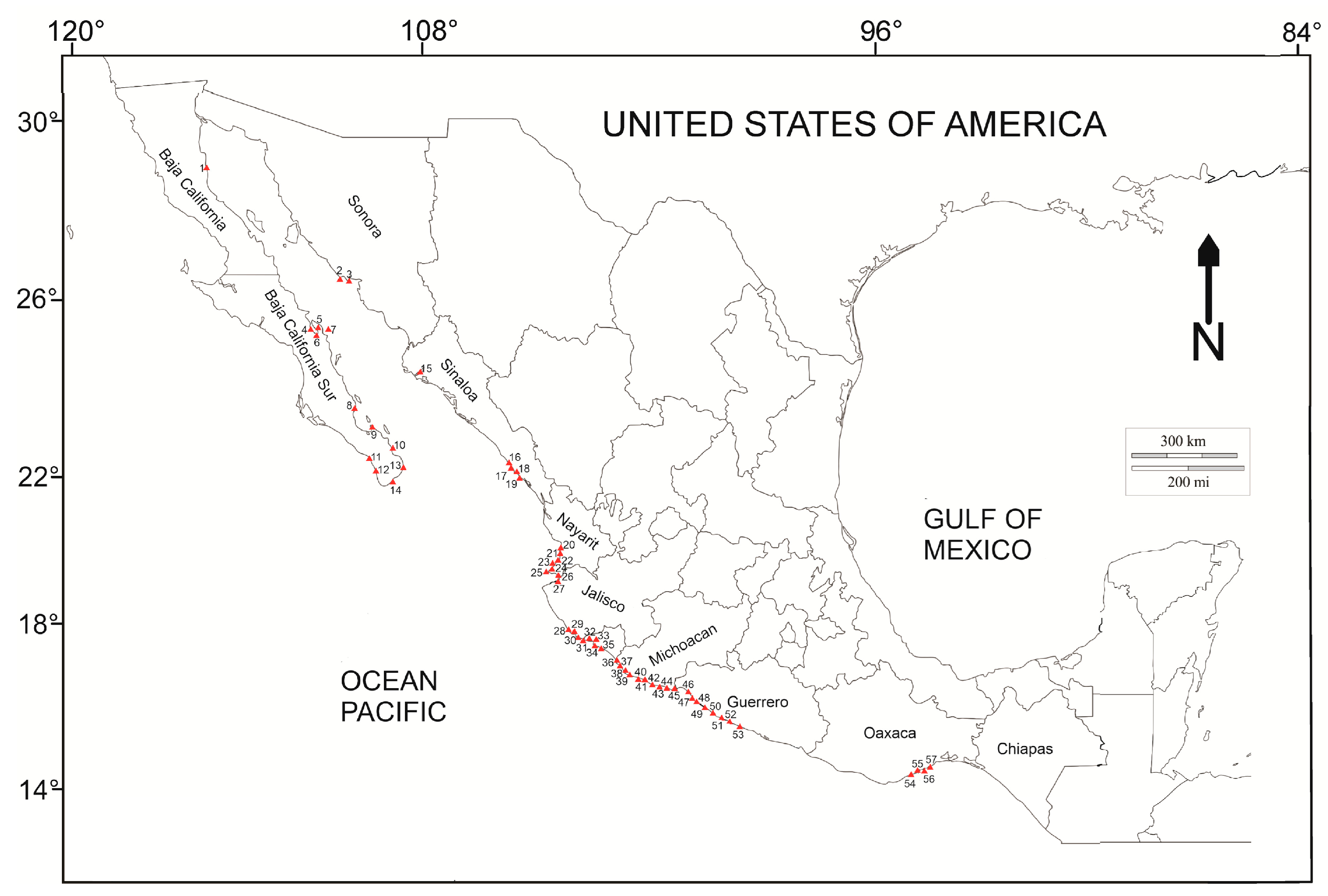
C. variabile) from the Mexican Pacific (Houston, TX, USA) were reviewed, of which 95 were deposited in the ENCB-IPN herbarium and 12 were obtained from recent collecting in the field. Samples from 57 localities were reviewed (
Figure 1) (
Supplementary File). Of the total number of characteristics evaluated, reproductive characteristics were not considered, since only two samples were in the tetrasporic phase, and the remainder were vegetative. Fourteen vegetative characteristics were considered to have significant variation, with thallus size and diameter and size of the medullary and cortical cells being the most strongly related to changes in SST (
Table 1,
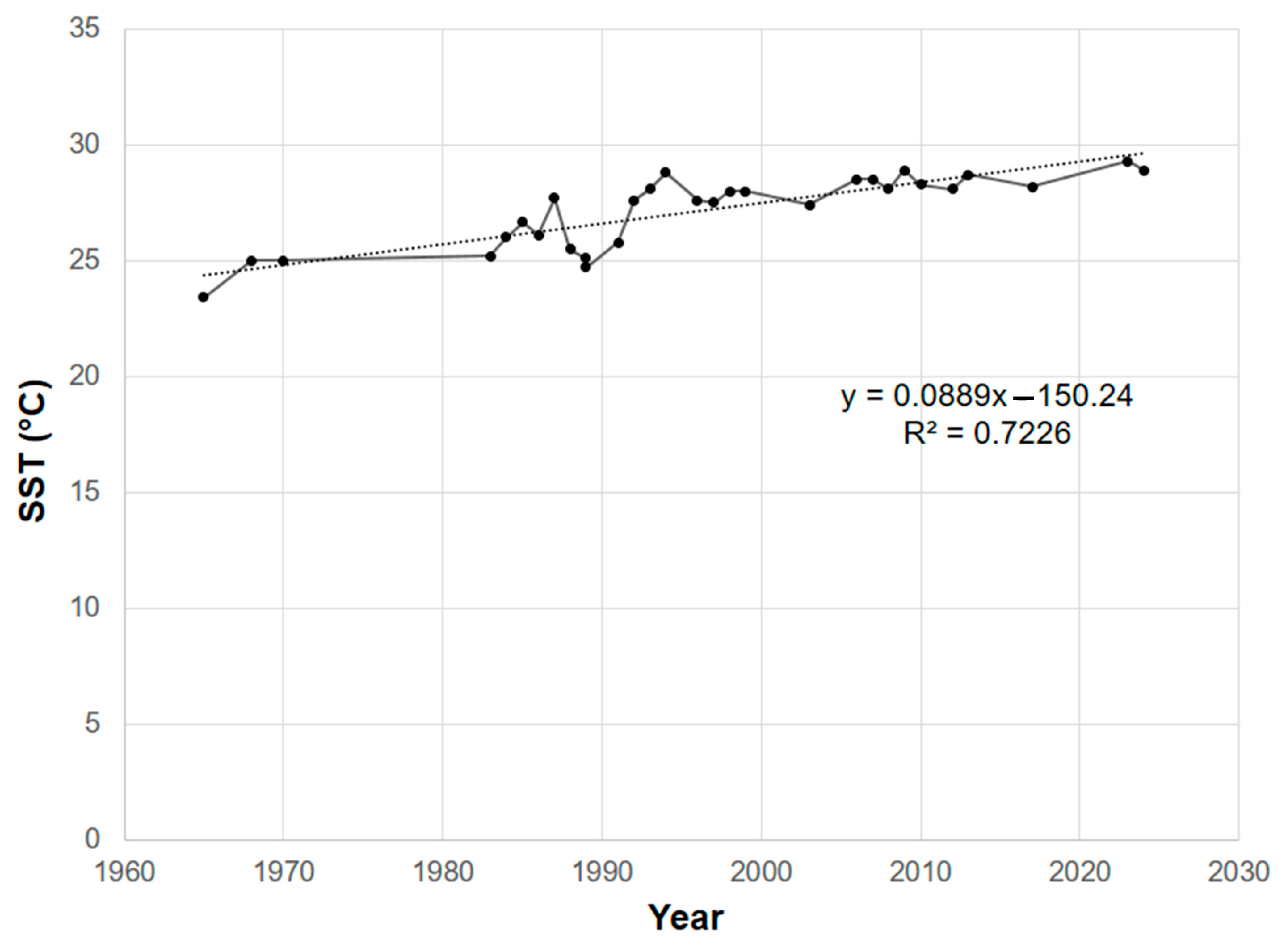
Figure 2). SST has increased throughout the Mexican Pacific since 1965 (
Figure 2), with an approximate increase of 0.5 °C per decade, and a positive correlation was found (R
2 = 0.7226) (
Figure 3).
3.1. Linear Regressions
For C. tenue, no significant differences were observed between SST and morphological characteristics except for thallus diameter. A slight tendency toward a decrease in thallus diameter with increasing temperature was observed. The linear regression of the average thallus diameter of C. tenue on the annual temperature was statistically significant (F = 5.04, df = 45, p = 0.02973), but showed a low correlation (R2 = 0.10).
The linear regression of the average medullar cell diameter of C. variabile on the annual temperature was statistically significant (F = 7.668, df = 41, p = 0.008402), but showed a low correlation (R2 = 0.15). Similarly, the correlation of the average cortical cell length with the year was statistically significant (F = 8.225, df = 41, p = 0.006498), but the correlation was weak (R2 = 0.16).
The correlations of the average cortical cells of C. variabile with latitude and longitude were statistically significant (F = 4.061, df = 40, p = 0.02479), but weak (R2 = 0.16). Moreover, the correlations of the average length of the thallus of C. variabile with latitude and annual temperature were also statistically significant (F = 5.246, DF = 40, p = 0.009479), but weak (R2 = 0.20). In the multiple linear regression, the average diameter was weakly correlated (R2 = 0.23) with annual temperature and the thallus length of C. variabile, but the regression was significant (F = 5.99, df = 40, p = 0.005301).
3.2. PERMANOVAS
A significant effect of the factor Mexican state (
F = 1.9523,
p = 0.0146) and the interaction between Mexican state and ENSO (
F = 1.2232,
p = 0.0041) were found for the average diameter of
C. tenue. The ENSO factor alone did not have a significant effect (
F = 0.3410,
p = 0.5829). Significant effects of the factors Mexican state (
F = 1.672,
p = 0.0233) and ENSO (
F = 4.3442,
p = 0.0038) on the average number of medullary cells were observed. A significant effect of the factor ENSO (
F = 3.1859,
p = 0.0174) on the average number of cortical cells was found (
Table 2).
One-way PERMANOVA was performed to evaluate the individual effects of the factors Mexican state and ENSO on some
C. tenue morphological variables. Significant effects were found for Mexican state (
F = 2.541,
p = 0.0212) and ENSO (
F = 6.225,
p = 0.0048) on average medullary cell count. A significant effect of ENSO (
F = 5.172,
p = 0.0107) on the average cortical cell count was observed. The effect of Mexican state on average thallus diameter was not significant (
F = 1.82,
p = 0.1006) (
Table 3).
In the paired PERMANOVA (post-hoc) for the effect of Mexican state on mean medullar cell count and the effect of ENSO on mean medullary and cortical cell counts, significant main effects were found in the one- and two-way PERMANOVAs (
Table 4).
Significant differences (p < 0.05) in the mean medullary cell count were found between the following pairs of Mexican states. Significant differences in mean medullary cell count were found between the following paired ENSO phases: La Niña–neutral (p = 0.0203) and La Niña–El Niño (p = 0.0022). A significant difference was also found in mean cortical cell count between the neutral ENSO phases and El Niño (p = 0.0020).
For
C. variabile, a significant effect of the factor Mexican state (
F = 5.7423,
p = 0.0002) on the average thallus length was found, as well as the effect of the factor ENSO (
F = 1.1874,
p = 0.1782). A significant effect of Mexican state (
F = 2.8235,
p = 0.0013) on the average thallus diameter was observed. A significant effect of Mexican state (
F = 5.5876,
p = 0.0001) on the average number of cortical cells was found (
Table 5). The one-way PERMANOVA showed significant effects of Mexican state on the average thallus length (
F = 8.099,
p = 0.0001), average thallus diameter (
F = 3.905,
p = 0.0017), and average cortical cell count (
F = 7.153,
p = 0.0001) (
Table 6).
The paired PERMANOVA (post-hoc) for the effects of Mexican state on the average thallus length and diameter and the average cortical cell were significantly different for the variables in which significant main effects were found in the one- and two-way PERMANOVAs. Significant differences (
p < 0.05) were found in the average thallus length and diameter and the average cortical cell count between pairs of states (
Table 7).
4. Discussion
The evaluation of
Ceratodictyon thalli over a long period of time reflects the relationships between some morphological characteristics and the average annual SST and El Niño and La Niña events. In several cases, the correlations were significant but low, which may indicate that these factors are not the only ones involved. Among the morphological characteristics that exhibited the greatest change were thallus size, diameter, and medullary and cortical cell diameters, with a slight tendency for these to decrease, which is related to the gradual increase in temperature. Changes in these characteristics in populations of brown algae have been noted by other authors, particularly Fucales and Laminariales [
42], such as in
Fucus guiryi [
43],
Gongolaria abies-marina,
Cystoseira compressa, and
C. humilis [
3], as well as in populations of red algae of the order Gelidiales, including
Gelidium amansii [
44],
G. canariense,
G. arbuscula,
Pterocladiella capillacea [
23,
45,
46], and
Gelidium robustum [
47]. Studies of macroalgae impacted by global warming are scarce, and those that do exist have been carried out in temperate zones. Therefore, studies in tropical zones are very important in order to recognize whether the impact is similar, lesser, or greater. Based on our observations and those of other authors, it appears that SST is not the predominant factor, as it very likely increases its effect when combined with other factors [
1,
43].
Morphological changes in response to environmental variables are very common among macroalgae, and SST has been identified as one of the main drivers of the changes in macroalgal development, abundance, and biomass [
3,
43,
47]. Previous studies have indicated a significant relationship between temperature increases and drastic reductions in the size and reproductive capacity of organisms [
23,
43,
48]. When under thermal stress, some macroalgal species undergo a process of metabolic acclimation, mainly with regard to photosynthesis and cellular respiration. However, this limits their growth, resulting in reduced thalli [
3]. Increased ocean temperature can have a stressful effect on species that modify their morphological structure due to physiological changes caused by changes in energy budgets [
11,
42].
Regarding the relationship with SST, significant differences were observed in the diameters of the medullary and cortical cells of
C. tenue during El Niño, La Niña, and neutral years. Larger diameters were observed in La Niña and neutral years compared to El Niño years. El Niño and La Niña events affect the morphology, abundance, and composition of macroalgal populations [
47,
49,
50]. Hernández-Guerrero et al. [
47] showed the impact of these meteorological events directly on the development and morphological characteristics of red algae. Important changes in the size, branching pattern, and reproduction of
Gelidium robustum occurred from 1980 to 1990, during which there were El Niño and La Niña events. The increase in temperature in an El Niño year had a greater effect on the thalli, reducing the size and branching patterns and altering the life cycle of this alga. During the La Niña event, larger thalli were present, which coincides with the results of our study, in which larger diameters occurred in the medullary and cortical cells during La Niña years, followed by neutral years, with the smallest sizes in El Niño years. This may be due to lower temperatures occurring during La Niña years, which favors the presence of nutrients and even upwellings in many places [
47,
51]. This type of thallus alteration caused by rising temperatures has been documented in various genera of macroalgae, mainly in temperate zones or for algae of commercial interest, leaving a significant information gap for tropical species and those that have no direct economic use [
47].
Furthermore, a significant relationship was observed between specimen distribution and morphology, which is linked to the mosaic of local climatic conditions, with important implications for the thermal sensitivity of organisms. Climatic variability can influence the range of thermal tolerance and is likely an important component of locally adapted responses to warming [
52]. This has been observed in other algae, such as
Fucus, in which significant changes in the morphology of nearby populations have been observed. The value of areas where environmental conditions represent a refuge for macroalgae is highlighted [
43], as this may occur in some sites in Mexico for
Ceratodictyon. Sites such as Michoacán have considerably larger thalli despite being exposed to high temperature conditions.
The low correlations observed may be related to the data used, since there are some biases regarding the number of specimens per location and per year, in addition to a significant period of time without information ranging from 2013 to 2017 and then from 2017 to 2023. Therefore, consecutive samplings should be carried out to determine significant changes in macroalgal populations, especially in tropical areas where climate change is not considered to have such a drastic effect, but is undocumented.
Although C. tenue and C. variabile are important components of rocky shorelines, much remains unknown regarding their physiology. However, our study presents evidence that distribution, El Niño and La Niña events, and SST have moderate to slight effects on these organisms. Long-term studies are needed to correlate these factors with other factors to detect the influence each may have on morphology.