Biodiversity of Scuttle Flies (Diptera: Phoridae) of Interfluves of the Moksha and Sura Rivers (European Russia)
Simple Summary
Abstract
1. Summary
2. Data Description
2.1. Dataset Description
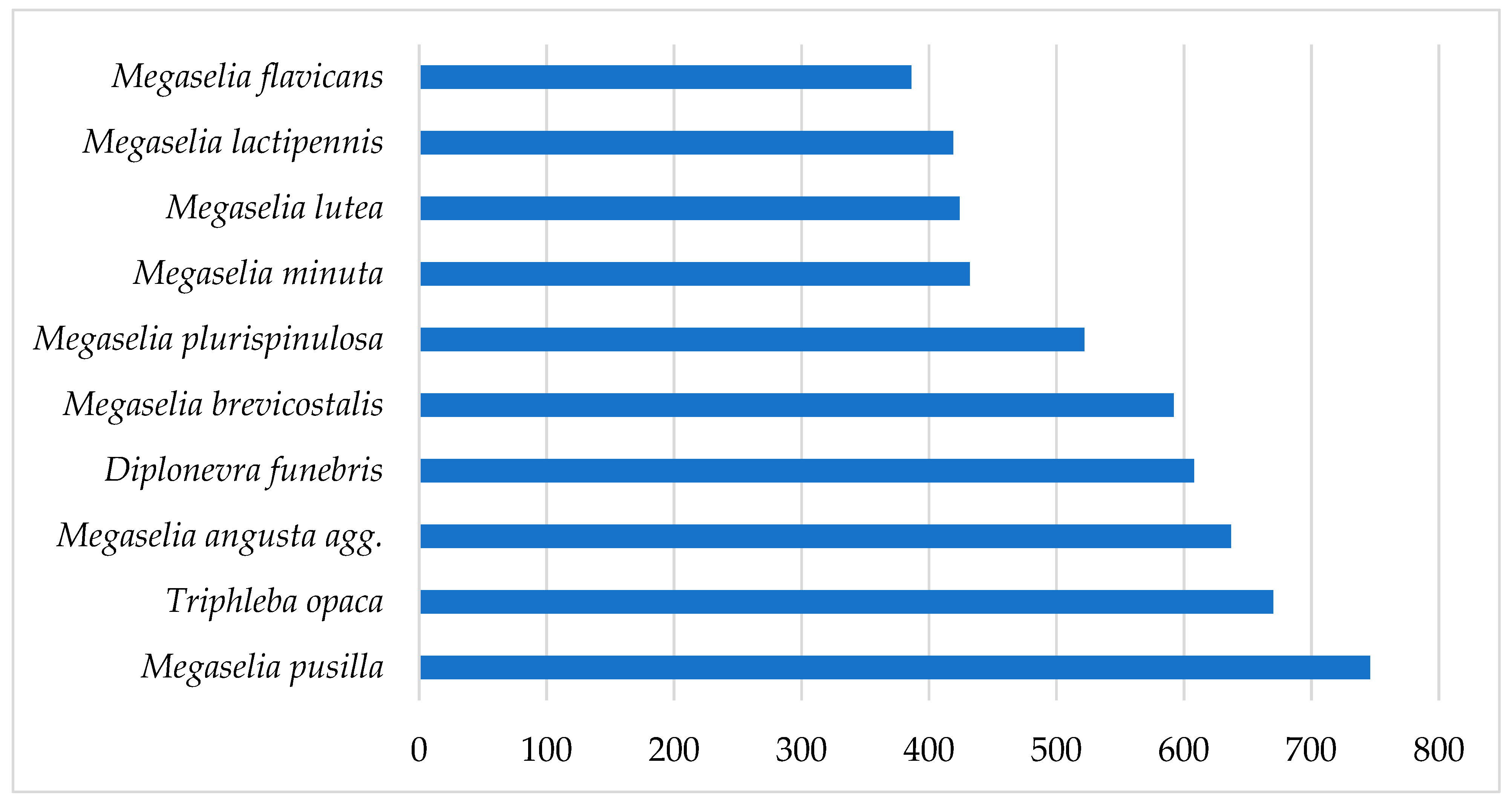
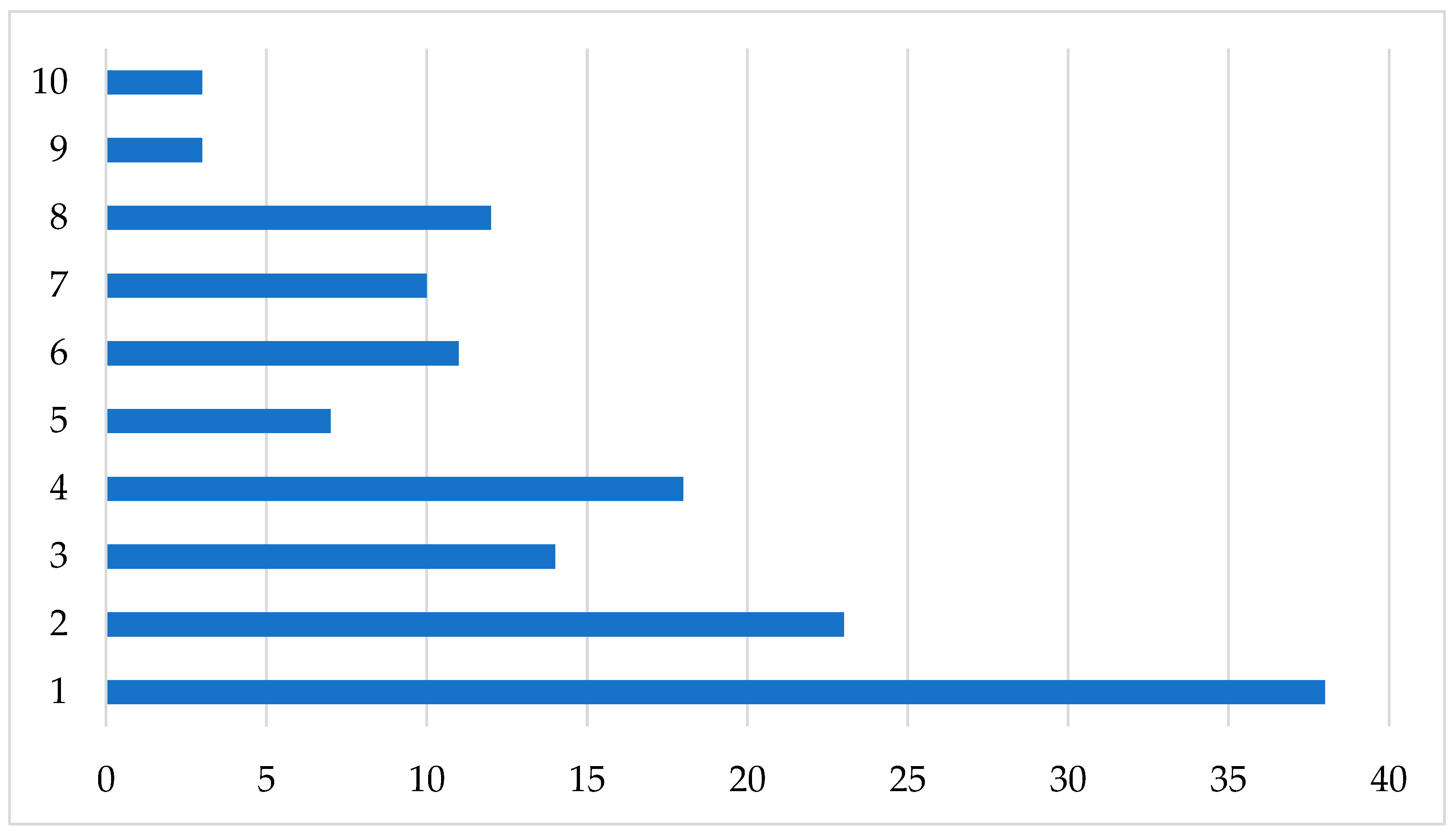
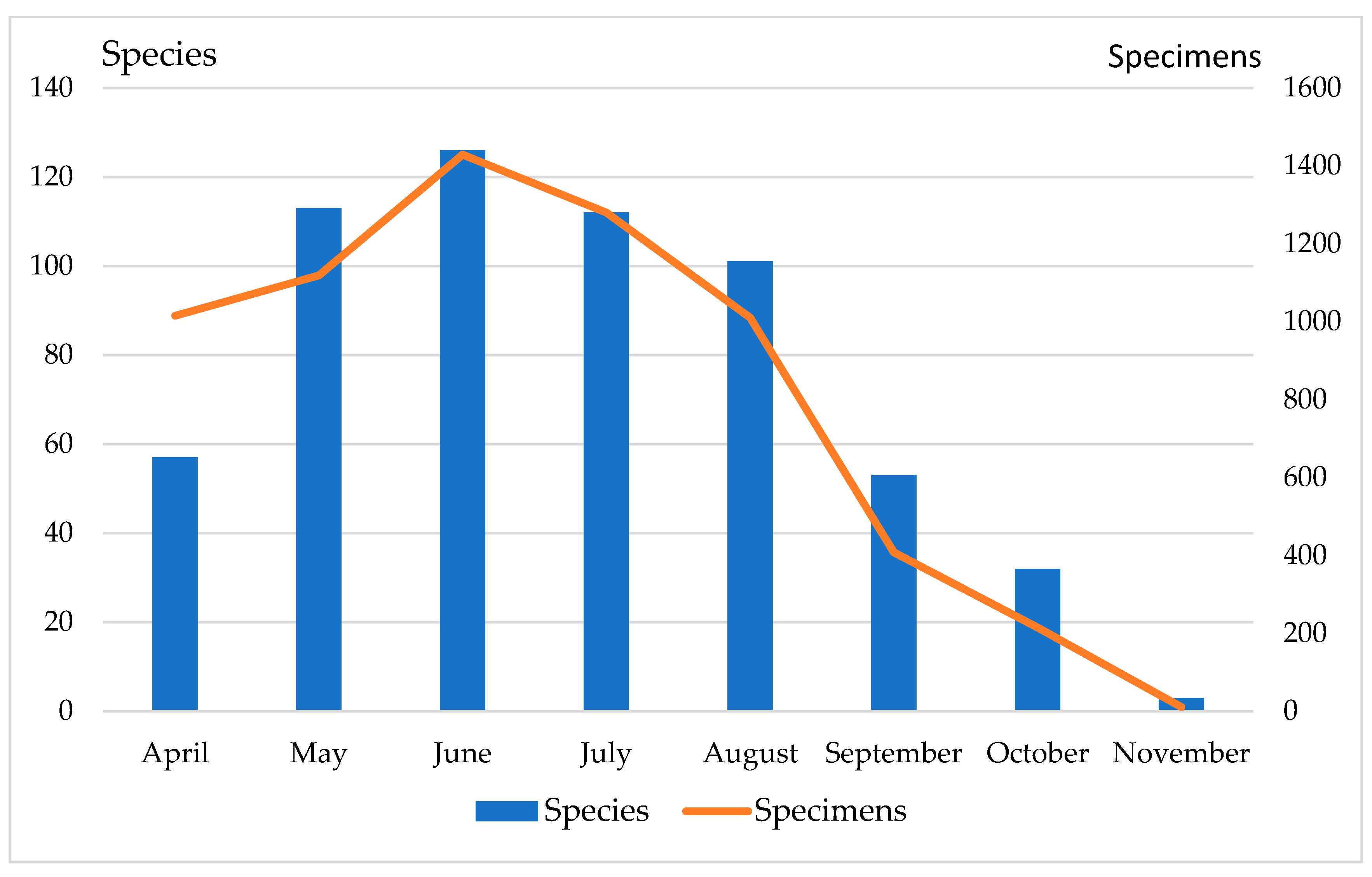
2.2. Biodiversity of Scuttle Flies
3. Methods
3.1. Study Area
3.2. Study Material
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Klink, R.; Sheard, J.K.; Høye, T.T.; Roslin, T.; Do Nascimento, L.A.; Bauer, S. Towards a toolkit for global insect biodiversity monitoring. Philos. Trans. R. Soc. B 2024, 379, 20230101. [Google Scholar] [CrossRef]
- Aleinikov, A.A.; Aleksutin, V.E.; Vozmitel, F.K.; Gunya, A.N. Long-term dynamics of forests with Pinus sibirica in the upper reaches of the River Kolva (Northern Pre-Urals, Russia) from 1938 to 2023. Nat. Conserv. Res. 2024, 9, 72–91. [Google Scholar] [CrossRef]
- Lopeztegui-Castillo, A. Long-term variations in nutritional condition of Panulirus argus (Decapoda: Palinuridae) in Cuba: Analytical and morphological approaches. Nat. Conserv. Res. 2023, 8, 48–60. [Google Scholar] [CrossRef]
- Korotyaev, B.A.; Markova, T.O.; Maslov, M.V. Species of the weevil genus Dorytomus Germar (Coleopera: Curculionidae) associated with poplars in the Ussuriysky Nature Reserve and adjacent area. Far East. Entomol. 2024, 505, 17–24. [Google Scholar] [CrossRef]
- Saroj, S.; Mandi, A.; Basak, N.; Mandal, P. The Buxa Tiger Reserve as a ‘hot spot’ of ant diversity in West Bengal state (Hymenoptera: Formicidae). Far East. Entomol. 2024, 503, 16–22. [Google Scholar] [CrossRef]
- Drago, M.C.; Vrcibradic, D. Will future extinctions occur at the same places where the past ones did? A review involving mammals and the IUCN Red List. Nat. Conserv. Res. 2023, 8, 1–9. [Google Scholar] [CrossRef]
- Saunders, M.E.; Lees, A.C.; Grames, E.M. Understanding and counteracting the denial of insect biodiversity loss. Curr. Opin. Insect Sci. 2025, 68, 101338. [Google Scholar] [CrossRef] [PubMed]
- Veen, G.F.; Vermaat, A.T.; Sitters, J.; Bakker, E.S. Vertebrate grazing can mitigate impacts of nutrient addition on plant diversity and insect abundance in a semi-natural grassland. Oikos 2024, 2024, e10422. [Google Scholar] [CrossRef]
- Trujillo-Arias, N.; Serrano-Cardozo, V.H.; Ramírez-Pinilla, M.P. Role of a Campesine Reserve Zone in the Magdalena Val-ley (Colombia) in the conservation of endangered tropical rainforests. Nat. Conserv. Res. 2023, 8, 49–63. [Google Scholar] [CrossRef]
- Svenningsen, C.S.; Schigel, D. Sharing insect data through GBIF: Novel monitoring methods, opportunities and standards. Phil. Trans. R. Soc. 2024, 379, 20230104. Available online: http://doi.org/10.1098/rstb.2023.0104 (accessed on 30 May 2025).
- Alfeus, M.; Irish, J.; Birkhofer, K. Recognition and completeness metrics from iNaturalist and GBIF can inform future citizen science and research projects: A case study on arthropods in Namibia. Biodivers Conserv. 2025, 34, 467–480. Available online: https://doi.org/10.1007/s10531-024-02981-z (accessed on 30 May 2025).
- Štípková, Z.; Tsiftsis, S.; Kindlmann, P. Is the GBIF appropriate for use as input in models of predicting species distributions? Study from the Czech Republic. Nat. Conserv. Res. 2024, 9, 84–95. Available online: https://dx.doi.org/10.24189/ncr.2024.008 (accessed on 30 May 2025). [CrossRef]
- Vasenkova, N.V.; Kuznetsova, N.A. A multiscale approach to evaluate the structure of diversity of Collembola in boreo-nemoral forests of the Russian Plain. Nat. Conserv. Res. 2022, 7 (Suppl. 1), 38–51. [Google Scholar] [CrossRef]
- van der Velden, J.A. Hoverflies (Diptera, Syrphidae) in a rural garden and their potential for citizen science. Contrib. Entomol. 2024, 74, 193–198. [Google Scholar] [CrossRef]
- Chursina, M.A.; Ruchin, A.B. A checklist of Syrphidae (Diptera) from Mordovia, Russia. Halteres 2018, 9, 57–73. [Google Scholar] [CrossRef]
- Blakeman, E.; Wilson, A.B.; Romer, S.; Olin, E.; Scott, C.; Popescu, V.; Brodie, B. Passively crowdsourcing images online for measuring broad-scale fly (Diptera) floral interactions and biodiversity. J. Pollinat. Ecol. 2023, 35, 180–193. [Google Scholar] [CrossRef]
- Rocha, L.C.; Faria, A.P.J.; Alvarado, S.T.; da Silva Carvalho-Filho, F.; Esposito, M.C.; Juen, L.; Sousa, J.R.P. Cerrado physiognomies in a protected area determine the distribution of necrophagous Diptera. J. Insect Conserv. 2024, 28, 749–761. [Google Scholar] [CrossRef]
- Yatsuk, A.A.; Matyukhin, A.V.; Anisimova, V.I.; Markovets, M.Y.; Nartshuk, E.P. A new Ornithophila (Diptera: Hippoboscidae) species from Baikal State Nature Reserve (Russia). Nat. Conserv. Res. 2024, 9, 100–104. [Google Scholar] [CrossRef]
- Disney, R.H.L. Scuttle Flies: The Phoridae; Chapman & Hall: London, UK, 1994; p. 467. [Google Scholar]
- Durska, E. Effects of disturbances on scuttle flies (Diptera: Phoridae) in Pine Forests. Biodivers. Conserv. 2013, 22, 1991–2021. [Google Scholar] [CrossRef]
- Grundmann, B.; Kappert, J. The Phoridae (Diptera) of NE-Westphalia: A field study over five years. Fragm. Faun. 2023, 66, 15–36. [Google Scholar] [CrossRef]
- Bragança, M.A.; Martins, H.C.; Oliveira, R.J.; Della Lucia, T.M. Parasitism of the leaf-cutting ant Atta bisphaerica by phorid flies: Biology and seasonal and intercolonial parasitism rates. Entomol. Exp. Et Appl. 2021, 169, 338–349. [Google Scholar] [CrossRef]
- Dogan, F.; Ozdemir, I.O.; Disney, R.H.L.; Karaborklu, S. The first record of the scuttle fly, Megaselia scalaris (Loew)(Diptera: Phoridae), parasitising the citrus longhorn beetle, Anoplophora chinensis Forster (Coleoptera: Cerambycidae), from Türkiye. Biocontrol Sci. Technol. 2024, 34, 776–781. [Google Scholar] [CrossRef]
- Shikano, I.; Woolcott, J.; Cloonan, K.; Andreadis, S.; Jenkins, N.E. Biology of Mushroom Phorid Flies, Megaselia halterata (Diptera: Phoridae): Effects of Temperature, Humidity, Crowding, and Compost Stage. Environ. Entomol. 2021, 50, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Grundmann, B.; Ruchin, A.; Esin, M.; Lobachev, E. Fauna of Scuttle Flies (Diptera: Phoridae) in Some Regions of European Russia; Joint Directorate of the Mordovia State Nature Reserve and National Park “Smolny”, Occurrence Dataset. GBIF.org. Available online: https://doi.org/10.15468/xr5s2j (accessed on 12 June 2025).
- Zaitsev, V.F. Phoridae—Scuttle Flies. In Keys to the Insects of the European Part of the USSR; Bey-Bienko, G.Y., Ed.; Schweizerbart and Borntraeger Science Publishers Position: Stuttgart, Germany, 1969; Volume 5, pp. 753–802. (In Russian) [Google Scholar]
- Nartshuk, E.P. Key to families of Diptera (Insecta) of the fauna of Russian and adjacent countries. Proc. Zool. Inst. Vol. 2003, 294, 1–252. [Google Scholar]
- Michailovskaya, M.V. Phoridae—Scuttle flies. In Keys to the Insects of the Russian Far East; Schweizerbart and Borntraeger Science Publishers Position: Stuttgart, Germany, 2004; Volume 3, pp. 9–39. (In Russian) [Google Scholar]
- Schmitz, H. Phoridae (1. Teilband). In Die Fliegen Der Paläarktischen Region; Lindner, E., Ed.; Schweizerbart and Borntraeger Science Publishers Position: Stuttgart, Germany, 1958; Volume 4, pp. 1–512. [Google Scholar]
- Durska, E. The phenology of Triphleba Rondani species (Diptera: Phoridae) in moist pine forests in the Bialowieza Forest. Entomol. Fenn. 2003, 14, 177–182. [Google Scholar] [CrossRef]
- Durska, E. The scuttle fly (Diptera: Phoridae) assemblages of pine plantations of the Biala Forest (Poland). Entomol. Fenn. 2009, 20, 170–178. [Google Scholar] [CrossRef]
- Golub, V.B.; Tsurikov, M.N.; Prokin, A.A. Insect Collections: Collection, Processing and Storage of Material; KMK Scientific Press Ltd.: Moscow, Russia, 2012; p. 339. [Google Scholar]
- Schmitz, H.; Beyer, E. Phoridae. In Die Fliegen Der Paläarktischen Region; Lindner, E., Ed.; Schweizerbart and Borntraeger Science Publishers Position: Stuttgart, Germany, 1965; Volume 4, pp. 513–608. [Google Scholar]
- Schmitz, H.; Delage, A. Phoridae. In Die Fliegen Der Paläarktischen Region; Lindner, E., Ed.; Schweizerbart and Borntraeger Science Publishers Position: Stuttgart, Germany, 1974; Volume 4, pp. 609–712. [Google Scholar]
- Buck, M.; Disney, R.H.L. Revision of the Megaselia giraudii and M. densior species complexes of Europe, including ecological notes (Diptera, Phoridae). Beiträge Zur Entomol. Contrib. Entomol. 2001, 51, 74–154. [Google Scholar] [CrossRef]
- Disney, R.H.L. Scuttle Flies. Diptera: Phoridae (Except Megaselia). In Handbooks for the Identification of British Insects; Royal Entomological Society of London: London, UK, 1983; Volume 10, pp. 1–81. [Google Scholar]
- Disney, R.H.L. Scuttle Flies. Diptera: Phoridae, Genus Megaselia. In Handbooks for the Identification of British Insects; Royal Entomological Society of London: London, UK, 1989; Volume 10, pp. 1–155. [Google Scholar]
- Disney, R.H.L. A troublesome sibling species complex of scuttle flies (Diptera, Phoridae) revisited. J. Nat. Hist. 1999, 33, 1159–1216. [Google Scholar] [CrossRef]


| Column Label | Column Description |
|---|---|
| occurrenceID | An identifier for the occurrence (as opposed to a particular digital record of the occurrence) |
| basisOfRecord | The specific nature of the data record: HumanObservation |
| eventDate | The date when material from the trap was collected or the range of dates during which the trap collected material |
| scientificNam | The full scientific name including the genus name and the lowest level of taxonomic rank with the authority |
| kingdom | The full scientific name of the kingdom in which the taxon is classified |
| decimalLatitude | The geographic latitude of location in decimal degrees |
| sex | The sex of the biological individual(s) represented in the occurrence |
| country | The name of the country in which the location occurs |
| countryCode | The standard code for the country in which the location occurs. |
| individualCount | The number of individuals represented present at the time of the occurrence |
| year | The integer year in which the event occurred |
| month | The ordinal month in which the event occurred |
| day | The integer day of the month on which the event occurred |
| recordedBy | A person, group, or organization responsible for recording the original occurrence |
| identifiedBy | A list of names of people who assigned the taxon to the subject |
| habitat | A category or description of the habitat in which the Event occurred |
| samplingProtocol | The names of the methods or protocols used during an event |
| locality_original | The specific description of the place. This term may contain information modified from the original to correct perceived errors or standardize the description |
| georeferenceSources | A list of maps, gazetteers, or other resources used to georeference the Location |
| coordinateUncertaintyInMeters | The maximum uncertainty distance in metres |
| geodeticDatum | The ellipsoid, geodetic datum, or spatial reference system (SRS) upon which the geographic coordinates given in decimalLatitude and decimalLongitude is based |
| Species | MSNR | NPS | Other District | Total |
|---|---|---|---|---|
| Aenigmatias brevifrons (Schmitz, 1955) * | 2 | 2 | ||
| Aenigmatias lubbocki (Verrall, 1877) | 7 | 28 | 13 | 48 |
| Anevrina curvinervis (Becker, 1901) | 116 | 6 | 1 | 123 |
| Anevrina thoracica (Meigen, 1804) | 77 | 3 | 14 | 94 |
| Anevrina unispinosa (Zetterstedt, 1860) | 31 | 13 | 38 | 82 |
| Anevrina urbana (Meigen, 1830 | 15 | 4 | 1 | 20 |
| Beckerina umbrimargo (Becker, 1901) * | 4 | 4 | ||
| Borophaga femorata (Meigen, 1830) | 118 | 10 | 2 | 130 |
| Borophaga subsultans (Linné, 1767) * | 4 | 2 | 1 | 7 |
| Chaetopleurophora bohemanni (Becker, 1901) | 1 | 4 | 5 | |
| Chaetopleurophora erythronota (Strobl, 1892) | 2 | 1 | 5 | 8 |
| Chaetopleurophora pygidialis Schmitz, 1941 | 1 | 1 | 2 | |
| Conicera dauci (Meigen, 1830) | 8 | 8 | ||
| Conicera floricola Schmitz, 1938 | 39 | 33 | 4 | 76 |
| Conicera similis (Haliday, 1833) | 208 | 51 | 19 | 278 |
| Conicera tarsalis Schmitz, 1920 | 1 | 1 | ||
| Conicera tibialis Schmitz, 1925 | 23 | 7 | 1 | 31 |
| Diplonevra abbreviata (von Roser, 1840) | 5 | 1 | 2 | 8 |
| Diplonevra florescens (Turton, 1801) | 3 | 3 | ||
| Diplonevra freyi (Schmitz, 1927) | 7 | 62 | 16 | 85 |
| Diplonevra funebris (Meigen, 1830) | 279 | 238 | 91 | 608 |
| Diplonevra glabra (Schmitz, 1927) * | 11 | 11 | ||
| Diplonevra nitidula (Meigen, 1830) * | 2 | 3 | 5 | |
| Diplonevra oldenbergi (Schmitz, 1920) * | 7 | 6 | 13 | |
| Diplonevra pilosella (Schmitz, 1927) * | 101 | 4 | 16 | 121 |
| Gymnophora arcuata (Meigen, 1830) | 18 | 22 | 40 | |
| Gymnophora healeyae Disney, 1980 * | 38 | 38 | ||
| Gymnophora integralis Schmitz, 1920 | 1 | 1 | ||
| Gymnoptera longicostalis Schmitz, 1933 | 1 | 1 | ||
| Hypocerides nearcticus Borgmeier, 1966 * | 1 | 1 | ||
| Megaselia aculeata (Schmitz, 1919) * | 1 | 14 | 7 | 22 |
| Megaselia aequalis (Wood, 1909) | 1 | 4 | 8 | 13 |
| Megaselia affinis (Wood, 1909) * | 2 | 2 | ||
| Megaselia albalucifrons Häggqvist, Ulefors & Ronquist, 2015 * | 1 | 1 | ||
| Megaselia albicaudata (Wood, 1910) * | 4 | 1 | 39 | 44 |
| Megaselia albiclava Schmitz, 1926 | 4 | 2 | 6 | |
| Megaselia albocingulata (Strobl, 1906) * | 46 | 54 | 26 | 126 |
| Megaselia altifrons (Wood, 1909) * | 98 | 143 | 7 | 248 |
| Megaselia analis (Lundbeck, 1920) * | 34 | 19 | 4 | 57 |
| Megaselia angularis (Schmitz, 1924) | 2 | 2 | ||
| Megaselia angusta (Wood, 1909) agg. | 202 | 74 | 361 | 637 |
| Megaselia annulipes (Schmitz, 1921) * | 4 | 1 | 2 | 7 |
| Megaselia armata (Wood, 1909) * | 2 | 2 | ||
| Megaselia barbulata (Wood, 1909) | 3 | 1 | 4 | |
| Megaselia basispinata (Lundbeck, 1920) | 2 | 3 | 3 | 8 |
| Megaselia berndseni (Schmitz, 1919) | 92 | 65 | 22 | 179 |
| Megaselia bifida Disney, 1983 * | 1 | 1 | ||
| Megaselia bifurcata Disney, 1983 * | 1 | 1 | ||
| Megaselia bovista (Gimmerthal, 1848) | 28 | 22 | 1 | 51 |
| Megaselia brevicostalis (Wood, 1910) | 343 | 74 | 175 | 592 |
| Megaselia brevior (Schmitz, 1924) | 134 | 47 | 109 | 290 |
| Megaselia brevissima (Schmitz, 1924) | 77 | 5 | 82 | |
| Megaselia breviterga (Lundbeck, 1920) | 1 | 1 | 10 | 12 |
| Megaselia campestris (Wood, 1908) * | 73 | 11 | 19 | 103 |
| Megaselia ciliata (Zetterstedt, 1848) | 1 | 1 | ||
| Megaselia cinerea Schmitz, 1938 * | 2 | 2 | 4 | |
| Megaselia cinereifrons (Strobl, 1910) * | 6 | 2 | 8 | |
| Megaselia citrinella Buck & Disney, 2001 * | 16 | 12 | 9 | 37 |
| Megaselia clemonsi Disney, 1984 * | 13 | 17 | 27 | 57 |
| Megaselia coccyx Schmitz, 1965 | 1 | 1 | ||
| Megaselia collini (Wood, 1909) * | 2 | 2 | ||
| Megaselia communiformis (Schmitz, 1918) * | 6 | 11 | 3 | 20 |
| Megaselia conformis (Wood, 1909) | 1 | 1 | ||
| Megaselia consetigera (Schmitz, 1925) * | 1 | 1 | ||
| Megaselia crassipes (Wood, 1909) * | 8 | 2 | 10 | |
| Megaselia curvicapilla Schmitz, 1947 * | 28 | 9 | 8 | 45 |
| Megaselia dahli (Becker, 1901) * | 1 | 1 | 2 | |
| Megaselia deltomera (Schmitz, 1924) * | 1 | 1 | ||
| Megaselia densior Schmitz, 1927 * | 38 | 13 | 16 | 67 |
| Megaselia depililobulus Disney & Durska, 2011 * | 3 | 3 | ||
| Megaselia devia Schmitz, 1936 * | 5 | 1 | 6 | |
| Megaselia differens Schmitz, 1948 | 1 | 3 | 4 | |
| Megaselia diversa (Wood, 1909) | 107 | 6 | 19 | 132 |
| Megaselia dubitalis (Wood, 1908) * | 4 | 4 | ||
| Megaselia eccoptomera Schmitz, 1927 | 1 | 1 | ||
| Megaselia eisfelderae Schmitzt, 1948 * | 1 | 3 | 4 | |
| Megaselia elongata (Wood, 1914) | 126 | 184 | 25 | 335 |
| Megaselia emarginata (Wood, 1908) | 174 | 129 | 10 | 313 |
| Megaselia erecta (Wood, 1910) * | 1 | 1 | ||
| Megaselia excorticata Disney, 2010 * | 6 | 1 | 7 | |
| Megaselia fenestralis (Schmitz, 1919) * | 58 | 58 | ||
| Megaselia feshiensis Disney, 1987 | 5 | 3 | 8 | |
| Megaselia flammula Schmitz, 1928 * | 63 | 19 | 39 | 121 |
| Megaselia flava (Fallén, 1823) | 13 | 4 | 108 | 125 |
| Megaselia flavescens (Wood, 1909) * | 7 | 4 | 2 | 13 |
| Megaselia flavicans Schmitz, 1935 * | 59 | 48 | 279 | 386 |
| Megaselia flavicoxa (Zetterstedt, 1848) * | 1 | 1 | ||
| Megaselia frameata Schmitz, 1927 * | 33 | 1 | 4 | 38 |
| Megaselia frontalis (Wood, 1909) * | 3 | 1 | 4 | |
| Megaselia fumata (Malloch, 1909) * | 2 | 2 | ||
| Megaselia funesta (Schmitz, 1935) * | 22 | 15 | 1 | 38 |
| Megaselia fusca (Wood, 1909) | 1 | 2 | 3 | |
| Megaselia fuscinervis (Wood, 1908) * | 1 | 1 | ||
| Megaselia fuscipalpis (Lundbeck, 1920) * | 1 | 1 | 4 | 6 |
| Megaselia fuscovariana Schmitz, 1933 | 1 | 1 | 2 | |
| Megaselia giraudii (Egger, 1862) | 28 | 6 | 8 | 42 |
| Megaselia glabrifrons (Wood, 1909) * | 6 | 7 | 1 | 14 |
| Megaselia gregaria (Wood, 1910) * | 26 | 3 | 2 | 31 |
| Megaselia hendersoni Disney, 1979 * | 3 | 1 | 4 | |
| Megaselia hibernans Schmitz, 1934 * | 30 | 33 | 7 | 70 |
| Megaselia hilaris Schmitz, 1927 * | 8 | 51 | 48 | 107 |
| Megaselia hirsuta (Wood, 1910) * | 1 | 1 | ||
| Megaselia hirticaudata (Wood, 1910) * | 1 | 1 | 4 | 6 |
| Megaselia hirticrus (Schmitz, 1918) | 132 | 6 | 26 | 164 |
| Megaselia hirtiventris (Wood, 1909) | 7 | 1 | 1 | 9 |
| Megaselia hobroensis Disney & Boggild, 2019 * | 32 | 8 | 40 | |
| Megaselia horsfieldi Disney, 1986 * | 19 | 12 | 31 | |
| Megaselia humeralis (Zetterstedt, 1838) | 1 | 2 | 3 | |
| Megaselia hyalipennis (Wood, 1912) | 21 | 21 | ||
| Megaselia ignobilis (Schmitz, 1919) * | 5 | 6 | 11 | |
| Megaselia immodensior Disney, 2001 | 1 | 1 | ||
| Megaselia incrassata (Schmitz, 1920) * | 2 | 6 | 2 | 10 |
| Megaselia indifferens (Lundbeck, 1920) * | 1 | 1 | ||
| Megaselia infraposita (Wood, 1909) * | 3 | 8 | 11 | |
| Megaselia insons (Lundbeck, 1920) * | 26 | 14 | 40 | |
| Megaselia intonsa Schmitz, 1948 | 1 | 1 | ||
| Megaselia involuta (Wood, 1910) * | 13 | 28 | 4 | 45 |
| Megaselia jani Disney, 2012 * | 1 | 1 | ||
| Megaselia joannae Disney, 1998 * | 2 | 2 | 4 | |
| Megaselia lactipennis (Lundbeck, 1920) * | 115 | 302 | 2 | 419 |
| Megaselia largifrontalis Schmitz, 1939 * | 2 | 2 | 4 | |
| Megaselia lata (Wood, 1910) * | 15 | 12 | 25 | 52 |
| Megaselia latifemorata (Becker, 1901) * | 5 | 1 | 1 | 7 |
| Megaselia latior Schmitz, 1936 * | 2 | 2 | ||
| Megaselia ledburiensis Brues, 1915 * | 23 | 51 | 8 | 82 |
| Megaselia leucozona Schmitz, 1930 | 92 | 106 | 40 | 238 |
| Megaselia limburgensis (Schmitz, 1918) | 2 | 1 | 3 | |
| Megaselia limpachensis Disney & Prescher, 2015 * | 118 | 17 | 6 | 141 |
| Megaselia lobatafurcae Disney, 2009 * | 1 | 1 | ||
| Megaselia longicostalis (Wood, 1912) | 9 | 14 | 133 | 156 |
| Megaselia longifurca Lundbeck, 1921 | 4 | 2 | 2 | 8 |
| Megaselia longipalpis (Wood, 1910) * | 69 | 7 | 5 | 81 |
| Megaselia longiseta (Wood, 1909) | 3 | 3 | 6 | |
| Megaselia lucifrons (Schmitz, 1918) * | 102 | 87 | 26 | 215 |
| Megaselia lutea (Meigen, 1830) | 88 | 17 | 319 | 424 |
| Megaselia luteipes (Schmitz, 1918) * | 1 | 2 | 3 | |
| Megaselia major (Wood, 1912) * | 21 | 27 | 1 | 49 |
| Megaselia malhamensis Disney, 1986 | 1 | 1 | ||
| Megaselia mallochi (Wood, 1909) * | 2 | 1 | 3 | |
| Megaselia manicata (Wood, 1910) | 36 | 13 | 22 | 71 |
| Megaselia manualis Schmitz, 1919 | 3 | 1 | 4 | |
| Megaselia marekdurskii Disney, 1998 * | 4 | 1 | 7 | 12 |
| Megaselia maura (Wood, 1910) * | 33 | 7 | 1 | 41 |
| Megaselia meigeni (Becker, 1901) | 14 | 14 | ||
| Megaselia melanostola Schmitz, 1942 * | 6 | 6 | ||
| Megaselia mimodensior Buck, 2001 * | 123 | 20 | 17 | 160 |
| Megaselia minor (Zetterstedt, 1848) * | 4 | 6 | 4 | 14 |
| Megaselia minuta (Aldrich, 1892) * | 12 | 1 | 419 | 432 |
| Megaselia mixta (Schmitz, 1918) * | 2 | 2 | ||
| Megaselia nasoni (Malloch, 1914) | 9 | 5 | 9 | 23 |
| Megaselia nigra (Meigen, 1830) | 2 | 2 | 4 | |
| Megaselia nigrescens (Wood, 1910) * | 2 | 2 | ||
| Megaselia nigriceps (Loew, 1866) | 22 | 2 | 19 | 43 |
| Megaselia nudiventris (Wood, 1909) | 2 | 2 | ||
| Megaselia obscuripennis (Wood, 1909) * | 2 | 1 | 3 | |
| Megaselia palmeni (Becker, 1901) | 1 | 1 | ||
| Megaselia parnassia Disney, 1986 | 22 | 22 | ||
| Megaselia parva (Wood, 1909) * | 3 | 1 | 4 | 8 |
| Megaselia parvula Schmitz, 1930 | 47 | 12 | 6 | 65 |
| Megaselia pectoralis (Wood, 1910) | 16 | 15 | 31 | |
| Megaselia pectorella Schmitz, 1929 * | 1 | 1 | ||
| Megaselia pedatella (Schmitz, 1926) * | 2 | 1 | 3 | |
| Megaselia perdistans (Schmitz, 1924) | 4 | 91 | 4 | 99 |
| Megaselia picta (Lehmann, 1822) | 10 | 15 | 25 | |
| Megaselia pleuralis (Wood, 1909) | 76 | 17 | 68 | 161 |
| Megaselia plurispinulosa (Zetterstedt, 1860) | 301 | 158 | 63 | 522 |
| Megaselia producta (Schmitz, 1921) | 3 | 1 | 4 | |
| Megaselia propinqua (Wood, 1909) * | 57 | 11 | 27 | 95 |
| Megaselia pseudogiraudii (Schmitz, 1920) * | 2 | 2 | ||
| Megaselia pulicaria (Fallén, 1823) | 8 | 1 | 10 | 19 |
| Megaselia pumila (Meigen, 1830) | 188 | 4 | 3 | 195 |
| Megaselia pusilla (Meigen, 1830) * | 388 | 309 | 49 | 746 |
| Megaselia pygmaea (Zetterstedt, 1848) | 54 | 54 | 126 | 234 |
| Megaselia quadriseta (Schmitz, 1918) * | 20 | 2 | 11 | 33 |
| Megaselia qurigolensis Disney et al., 2019 * | 2 | 2 | ||
| Megaselia raruvesiculae Disney, 2001 * | 20 | 18 | 2 | 40 |
| Megaselia rubella (Schmitz, 1920) | 3 | 5 | 55 | 63 |
| Megaselia rubescens (Wood, 1912) | 40 | 25 | 2 | 67 |
| Megaselia rufa (Wood, 1908) * | 9 | 1 | 1 | 11 |
| Megaselia ruficornis (Meigen, 1830) | 3 | 8 | 17 | 28 |
| Megaselia russellsmithi Disney, 2015 * | 14 | 12 | 26 | |
| Megaselia scutellaris (Wood, 1909) | 5 | 1 | 6 | |
| Megaselia sepulchralis (Lundbeck, 1920) | 1 | 1 | ||
| Megaselia serrata (Wood, 1910) * | 6 | 6 | ||
| Megaselia setulipalpis Schmitz, 1938 * | 2 | 2 | ||
| Megaselia simplex (Wood, 1910) * | 31 | 42 | 1 | 74 |
| Megaselia simulans (Wood, 1912) | 5 | 21 | 26 | |
| Megaselia sordida (Zetterstedt, 1838) | 3 | 3 | ||
| Megaselia specularis Schmitz, 1935 * | 2 | 2 | ||
| Megaselia speiseri Schmitz, 1929 | 3 | 2 | 2 | 7 |
| Megaselia spinata (Wood, 1910) * | 1 | 7 | 8 | |
| Megaselia spinicincta (Wood, 1910) | 22 | 1 | 23 | |
| Megaselia spinigera (Wood, 1908) | 4 | 4 | ||
| Megaselia stichata (Lundbeck, 1920) * | 5 | 2 | 7 | |
| Megaselia stigmatica (Schmitz, 1920) | 3 | 3 | ||
| Megaselia striolata Schmitz, 1940 * | 101 | 76 | 35 | 212 |
| Megaselia styloprocta (Schmitz, 1921) | 12 | 17 | 29 | |
| Megaselia subcarpalis (Lundbeck, 1920) | 6 | 6 | ||
| Megaselia subconvexa (Lundbeck, 1920) * | 1 | 1 | ||
| Megaselia subfraudulenta Schmitz, 1933 * | 1 | 1 | ||
| Megaselia subnitida (Lundbeck, 1920) * | 4 | 1 | 1 | 6 |
| Megaselia subnudipennis (Schmitz, 1919) * | 45 | 23 | 132 | 200 |
| Megaselia subpalpalis (Lundbeck, 1920) * | 8 | 8 | ||
| Megaselia subpleuralis (Wood, 1909) * | 1 | 1 | ||
| Megaselia subtumida (Wood, 1909) | 7 | 3 | 15 | 25 |
| Megaselia sulphuripes (Meigen, 1830) | 2 | 2 | 4 | |
| Megaselia superciliata (Wood, 1910) * | 7 | 7 | 13 | 27 |
| Megaselia surdifrons (Wood, 1909) * | 4 | 6 | 10 | |
| Megaselia sylvatica (Wood, 1910) | 1 | 1 | ||
| Megaselia tama (Schmitz, 1926) * | 32 | 21 | 1 | 54 |
| Megaselia tarsalis (Wood, 1910) * | 24 | 15 | 17 | 56 |
| Megaselia tarsella (Lundbeck, 1921) | 4 | 4 | ||
| Megaselia thomseni Disney & Bøggild, 2021 * | 3 | 1 | 1 | 5 |
| Megaselia tonyirwini Disney, 1988 * | 5 | 5 | ||
| Megaselia trichorrhoea Schmitz, 1921 | 29 | 29 | ||
| Megaselia triquetra Schmitz, 1927 * | 2 | 2 | 4 | |
| Megaselia tubularia Disney, 2016 | 1 | 1 | ||
| Megaselia uliginosa (Wood, 1909) * | 2 | 2 | ||
| Megaselia unguicularis (Wood, 1909) * | 1 | 1 | ||
| Megaselia unicolor (Schmitz, 1919) * | 13 | 8 | 21 | |
| Megaselia variana Schmitz, 1926 | 3 | 2 | 5 | |
| Megaselia verna Schmitz, 1932 * | 1 | 1 | ||
| Megaselia verralli (Wood, 1910) | 20 | 160 | 12 | 192 |
| Megaselia vestita (Wood, 1914) | 28 | 28 | ||
| Megaselia viklundi Disney & Prescher, 2018 * | 8 | 1 | 9 | |
| Megaselia villicauda Schmitz, 1927 * | 1 | 1 | ||
| Megaselia virilis (Schmitz, 1919) * | 1 | 1 | 2 | |
| Megaselia woodi (Lundbeck, 1922) * | 161 | 1 | 2 | 164 |
| Megaselia xanthozona (Strobl, 1892) * | 112 | 1 | 2 | 115 |
| Menozziola obscuripes (Schmitz, 1927) | 1 | 4 | 5 | |
| Metopina braueri (Strobl, 1880) * | 2 | 1 | 3 | |
| Metopina galeata Haliday, 1833 * | 10 | 2 | 12 | |
| Metopina oligoneura (Mik, 1867) * | 42 | 27 | 107 | 176 |
| Metopina perpusilla (Six, 1878) * | 5 | 4 | 14 | 23 |
| Metopina pileata Schmitz, 1936 * | 1 | 4 | 8 | 13 |
| Microselia southwoodi Disney, 1988 * | 1 | 7 | 8 | |
| Peromitra agilis (Meigen, 1830) | 1 | 1 | 5 | 7 |
| Peromitra carinifrons (Zetterstedt, 1848) | 32 | 29 | 68 | 129 |
| Peromitra incrassata (Meigen, 1830) * | 138 | 60 | 53 | 251 |
| Phalacrotophora beuki Disney, 1997 * | 6 | 6 | ||
| Phalacrotophora delageae Disney, 1979 * | 2 | 2 | ||
| Phalacrotophora fasciata (Fallén, 1823) | 3 | 3 | 2 | 8 |
| Phalacrotophora flavidus Khameneh & Disney, 2021 * | 1 | 1 | 1 | 3 |
| Phora atra (Meigen, 1804) | 4 | 69 | 12 | 85 |
| Phora bullata Schmitz, 1927 | 3 | 4 | 7 | |
| Phora dubia (Zetterstedt, 1848) | 5 | 5 | ||
| Phora edentata Schmitz, 1920 | 7 | 3 | 1 | 11 |
| Phora hamata Schmitz, 1927 | 1 | 1 | ||
| Phora holosericea Schmitz, 1920 | 54 | 16 | 8 | 78 |
| Phora tincta Schmitz, 1920 | 12 | 27 | 39 | |
| Plectanocnema nudipes (Becker, 1901) | 2 | 2 | ||
| Pseudacteon brevicauda Schmitz, 1925 * | 76 | 123 | 4 | 203 |
| Pseudacteon fennicus Schmitz, 1927 * | 12 | 1 | 3 | 16 |
| Pseudacteon formicarum (Verrall, 1877) * | 38 | 32 | 1 | 71 |
| Pseudacteon lundbecki Schmitz, 1924 * | 117 | 63 | 14 | 194 |
| Spiniphora bergenstammi (Mik, 1864) | 4 | 3 | 7 | |
| Spiniphora excisa (Becker, 1901) | 2 | 1 | 3 | |
| Stichillus coronatus (Becker, 1901) * | 2 | 2 | ||
| Triphleba aequalis (Schmitz, 1919) * | 9 | 9 | ||
| Triphleba disparinervis (Schmitz, 1947) * | 4 | 4 | ||
| Triphleba distinguenda (Strobl, 1892) | 1 | 1 | 2 | |
| Triphleba dudai (Schmitz, 1918) * | 1 | 1 | ||
| Triphleba excisa (Lundbeck, 1921) * | 1 | 1 | ||
| Triphleba intermedia (Malloch, 1908) * | 15 | 15 | ||
| Triphleba lugubris (Meigen, 1830) * | 14 | 7 | 21 | |
| Triphleba minuta (Fabricius, 1787) | 7 | 7 | ||
| Triphleba nudipalpis (Becker, 1901) | 7 | 30 | 4 | 41 |
| Triphleba opaca (Meigen, 1830) | 642 | 1 | 27 | 670 |
| Triphleba papillata (Wingate, 1906) * | 22 | 2 | 24 | |
| Triphleba trinervis (Becker, 1901) | 17 | 17 | ||
| Xenotriphleba dentistylata Buck, 1997 | 1 | 1 | ||
| Shannon Index | 4.34 | 4.07 | 3.85 | |
| Simpson Index | 0.97 | 0.97 | 0.96 | |
| Margalef Index | 25.1 | 21.2 | 21.0 | |
| Pielou Index | 0.80 | 0.79 | 0.75 | |
| Berger-Parker Index | 0.08 | 0.08 | 0.11 | |
| Totalspecimens | 7678 | 4048 | 3975 | 15,701 |
| Total species | 226 | 177 | 175 | 271 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grundmann, B.; Ruchin, A.B.; Esin, M.N.; Lobachev, E.A. Biodiversity of Scuttle Flies (Diptera: Phoridae) of Interfluves of the Moksha and Sura Rivers (European Russia). Diversity 2025, 17, 502. https://doi.org/10.3390/d17080502
Grundmann B, Ruchin AB, Esin MN, Lobachev EA. Biodiversity of Scuttle Flies (Diptera: Phoridae) of Interfluves of the Moksha and Sura Rivers (European Russia). Diversity. 2025; 17(8):502. https://doi.org/10.3390/d17080502
Chicago/Turabian StyleGrundmann, Bernd, Alexander B. Ruchin, Mikhail N. Esin, and Evgeniy A. Lobachev. 2025. "Biodiversity of Scuttle Flies (Diptera: Phoridae) of Interfluves of the Moksha and Sura Rivers (European Russia)" Diversity 17, no. 8: 502. https://doi.org/10.3390/d17080502
APA StyleGrundmann, B., Ruchin, A. B., Esin, M. N., & Lobachev, E. A. (2025). Biodiversity of Scuttle Flies (Diptera: Phoridae) of Interfluves of the Moksha and Sura Rivers (European Russia). Diversity, 17(8), 502. https://doi.org/10.3390/d17080502







