Growth Patterns of Reef-Building Porites Species in the Remote Clipperton Atoll Reef
Abstract
1. Introduction
2. Materials and Methods
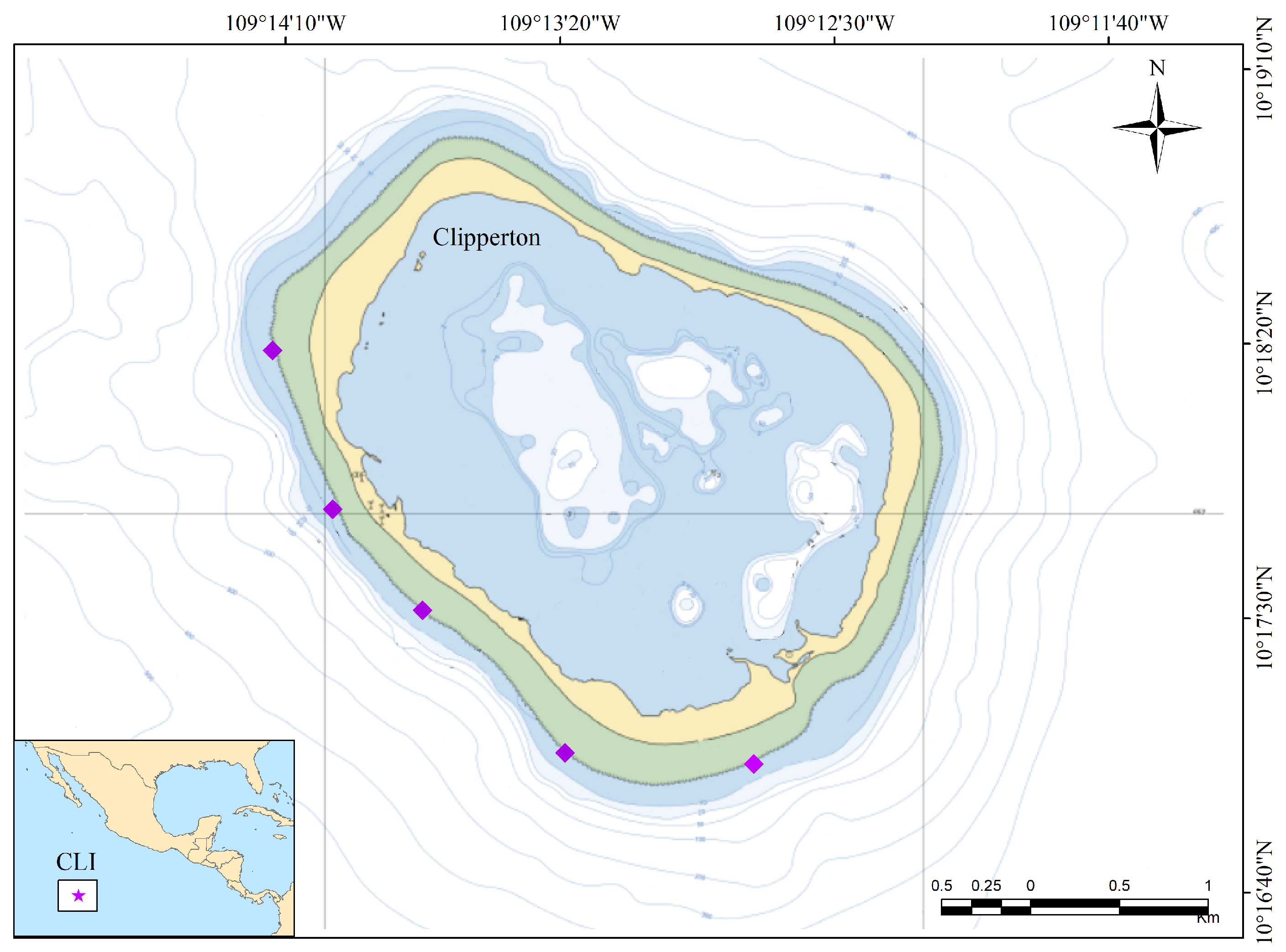
2.1. Study Area
2.2. Sample Collection
2.3. Data Analysis
3. Results
4. Discussion
Supplementary Materials
 Porites lutea,
Porites lutea,  Porites lobata,
Porites lobata,  Porites arnaudi,
Porites arnaudi,  Porites australiensis. Figure S3. Linear regressions between growth parameters (skeletal density, extension rate, calcification rate) from top to bottom: Porites lutea, P. lobata, P. arnaudi, P. australiensis.
Porites australiensis. Figure S3. Linear regressions between growth parameters (skeletal density, extension rate, calcification rate) from top to bottom: Porites lutea, P. lobata, P. arnaudi, P. australiensis.Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spalding, M.; Ravilious, C.; Green, E.P. World Atlas of Coral Reefs; University of California Press: Berkeley, CA, USA, 2001. [Google Scholar]
- Lough, J.M.; Cantin, N.E. Perspectives on massive coral growth rates in a changing ocean. Biol. Bull. 2014, 226, 187–202. [Google Scholar] [CrossRef] [PubMed]
- Manzello, D.P. Coral growth with thermal stress and ocean acidification: Lessons from the eastern tropical Pacific. Coral Reefs 2010, 29, 749–758. [Google Scholar] [CrossRef]
- Link, H.F. Beschreibung der Naturalien-Sammlung der Universität zu Rostock; Adlers Erben: Rostock, Germany, 1807; Volume 4, pp. 1–30. [Google Scholar]
- Veron, J.E.N.; Stafford-Smith, M.G.; Turak, E.; DeVantier, L.M. Corals of the World, Version 0.01 Beta. 2016. Available online: http://www.coralsoftheworld.org (accessed on 11 April 2017).
- Veron, J.E.N. Conservation of biodiversity: A critical time for the hermatypic corals of Japan. Coral Reefs 1992, 11, 13–21. [Google Scholar] [CrossRef]
- Pacherres, C.O.; Schmidt, G.M.; Richter, C. Autotrophic and heterotrophic responses of the coral Porites lutea to large amplitude internal waves. J. Exp. Biol. 2013, 216, 4365–4374. [Google Scholar]
- Sagawa, N.; Nakamori, T.; Iryu, Y. Pleistocene reef development in the southwest Ryukyu Islands, Japan. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2001, 175, 303–323. [Google Scholar] [CrossRef]
- Lough, J.M.; Barnes, D.J. Environmental controls on growth of the massive coral Porites. J. Exp. Mar. Biol. Ecol. 2000, 245, 225–243. [Google Scholar] [CrossRef]
- Lough, J.M. Coral calcification from skeletal records revisited. Mar. Ecol. Prog. Ser. 2008, 373, 257–264. [Google Scholar] [CrossRef]
- Veron, J.E.N.; Pichon, M. Scleractinia of Eastern Australia: Part. IV. Family Poritidae. Monogr. Ser. Aust. Inst. Mar Sci. 1982, 5, 1–159. [Google Scholar]
- Veron, J.E.N. Corals of Australia and the Indo-Pacific; Angus and Robertson: North Ryde, NSW, Australia, 1986. [Google Scholar]
- Forsman, Z.H.; Barshis, D.J.; Hunter, C.L.; Toonen, R.J. Shape-shifting corals: Molecular markers show morphology is evolutionarily plastic in Porites. BMC Evol. Biol. 2009, 9, 1–9. [Google Scholar] [CrossRef]
- Freeman, L.A.; Kleypas, J.A.; Miller, A.J. Coral reef habitat response to climate change scenarios. PLoS ONE 2013, 8, 12. [Google Scholar] [CrossRef]
- Cortés, J. Coastal morphology and coral reefs. In Central America: Geology, Resources and Hazards; Bundschuc, J., Alvarado, G., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 185–200. [Google Scholar]
- Glynn, P.W.; Veron, J.E.N.; Wellington, G.M. Clipperton Atoll (eastern Pacific): Oceanography, geomorphology, reef-building coral ecology and biogeography. Coral Reefs 1996, 15, 71–99. [Google Scholar] [CrossRef]
- Tortolero-Langarica, J.A.; Clua, E.; Rodríguez-Zaragoza, F.A.; Caselle, J.E.; Rodríguez-Troncoso, A.P.; Adjeroud, M.; Brown, E.K. Spatial and temporal patterns in the coral assemblage at Clipperton Atoll: A sentinel reef in the Eastern Tropical Pacific. Coral Reefs 2022, 41, 1405–1415. [Google Scholar] [CrossRef]
- Allen, G.R. Conservation hotspots of biodiversity and endemism for Indo-Pacific coral reef fishes. Aquat. Conserv. Mar. Freshw. Ecosyst. 2008, 18, 541–556. [Google Scholar] [CrossRef]
- Robertson, D.R.; Cramer, K.L. Shore fishes and biogeographic subdivisions of the Tropical Eastern Pacific. Mar Ecol Prog Ser. 2009, 380, 1–17. [Google Scholar] [CrossRef]
- Perry, C.T.; Alvarez-Filip, L.; Graham, N.A.; Mumby, P.J.; Wilson, S.K.; Kench, P.S.; Macdonald, C. Loss of coral reef growth capacity to track future increases in sea level. Nature 2018, 558, 396–400. [Google Scholar] [CrossRef]
- Wang, C.; Fiedler, P.C. ENSO variability and the eastern tropical Pacific: A review. Prog. Oceanogr. 2006, 69, 239–266. [Google Scholar] [CrossRef]
- Wu, H.C.; Moreau, M.; Linsley, B.K.; Schrag, D.P.; Corrège, T. Investigation of sea surface temperature changes from replicated coral Sr/Ca variations in the eastern equatorial Pacific (Clipperton Atoll) since 1874. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2014, 412, 208–222. [Google Scholar] [CrossRef]
- Zhao, H.; Raga, G.B. On the distinct interannual variability of tropical cyclone activity over the eastern North Pacific. Atmósfera 2015, 28, 161–178. [Google Scholar] [CrossRef]
- Carricart-Ganivet, J.P.; Reyes-Bonilla, H. New and Previous Records of Scleractinian Corals from Clipperton Atoll, Eastern Pacific. Pac. Sci. 1999, 53, 370–375. [Google Scholar]
- Vaughan, T.W. Some Shallow-Water Corals from Murray Island (Australia), Cocos-Keeling Island, and Fanning Island; Department of Marine Biology of the Carnegie Institution of Washington: Washington, DC, USA, 1918; Volume 9, pp. 49–234. [Google Scholar]
- Reyes-Bonilla, H.; Carricart-Ganivet, J.P. Porites arnaudi, a new species of stony coral (Anthozoa: Scleractinia: Poritidae) from oceanic islands of the eastern Pacific Ocean. Proc. Biol. Soc. Wash. 2000, 113, 561–571. [Google Scholar]
- Milne Edwards, H.; Haime, J. Recherches sur les polypiers. Mémoire 7. Monographie des Poritides. Ann. Sci. Nat. Zool. 1951, 16, 21–70. [Google Scholar]
- Dana, J.D. Zoophytes: United States Exploring Expedition During the Years 1838–1842; Lea and Blanchard: Philadelphia, PA, USA, 1846; pp. 1–120, 709–720. [Google Scholar]
- Carricart-Ganivet, J.P.; Barnes, D.J. Densitometry from digitized images of X radiographs: Methodology for measurement of coral skeletal density. J. Exp. Mar. Biol. Ecol. 2007, 344, 67–72. [Google Scholar] [CrossRef]
- Duprey, N.; Bouscher, H.; Jiménez, C. Digital correction of computed X radiographs for coral densitometry. J. Exp. Mar. Bio Ecol. 2012, 438, 84–92. [Google Scholar] [CrossRef]
- Rico-Esenaro, S.D.; Sanchez-Cabeza, J.A.; Carricart-Ganivet, J.P.; Montagna, P.; Ruiz-Fernández, A.C. Uncertainty and variability of extension rate, density and calcification rate of a hermatypic coral (Orbicella faveolata). Sci. Total Environ. 2019, 650, 1576–1581. [Google Scholar] [CrossRef] [PubMed]
- Behbehani, M.; Uddin, S.; Dupont, S.; Sajid, S.; Al-Musalam, L.; Al-Ghadban, A. Response of corals Acropora pharaonis and Porites lutea to changes in pH and temperature in the Gulf. Sustainability 2019, 11, 3156. [Google Scholar] [CrossRef]
- Cole, C.; Finch, A.A.; Hintz, C. Effects of seawater pCO2 and temperature on calcification and productivity in the coral genus Porites spp.: An exploration of potential interaction mechanisms. Coral Reefs 2018, 37, 471–481. [Google Scholar] [CrossRef]
- Kavousi, J.; Keppel, G. Clarifying the concept of climate change refugia for coral reefs. ICES J. Mar. Sci. 2018, 75, 43–49. [Google Scholar] [CrossRef]
- Alvarez-Filip, L.; Carricart-Ganivet, J.P.; Horta-Puga, G.; Iglesias-Prieto, R. Shifts in coral-assemblage composition do not ensure persistence of reef functionality. Sci. Rep. 2013, 3, 3486. [Google Scholar] [CrossRef]
- Lesser, M.P.; Slattery, M.; Leichter, J.J. Ecology of mesophotic coral reefs. J. Exp. Mar. Biol. Ecol. 2009, 375, 1–8. [Google Scholar] [CrossRef]
- Bessat, F.; Buigues, D. Two centuries of variation in coral growth in a massive Porites colony from Moorea (French Polynesia): A response of ocean-atmosphere variability from south central Pacific. Palaeo3 2001, 175, 381–392. [Google Scholar] [CrossRef]
- Glynn, P.W.; Wellington, G.M. Corals and Coral Reefs of the Galápagos Islands; University of California Press: Berkeley, CA, USA, 1983. [Google Scholar]
- Risk, M.J.; Sammarco, P.W. Cross-shelf trends in skeletal density of the massive coral Porites lobata from the Great Barrier Reef. Mar. Ecol. Prog. Ser. 1991, 69, 195–200. [Google Scholar] [CrossRef]
- Barnes, D.J.; Lough, J.M. Systematic variations in the depth of skeleton occupied by coral tissue in massive colonies of Porites from the Great Barrier Reef. J. Exp. Mar. Biol. Ecol. 1992, 159, 113–128. [Google Scholar] [CrossRef]
- Tortolero-Langarica, J.J.A.; Cupul-Magana, A.L.; Carricart-Ganivet, J.P.; Mayfield, A.B.; Rodriguez-Troncoso, A.P. Differences in growth and calcification rates in the reef-building coral Porites lobata: The implications of morphotype and gender on coral growth. Front. Mar. Sci. 2016, 3, 179. [Google Scholar] [CrossRef]
- Medellín-Maldonado, F.; Cabral-Tena, R.A.; López-Pérez, A.; Calderón-Aguilera, L.E.; Norzagaray-López, C.; Chapa-Balcorta, C.; Zepeta-Vilchis, R.C. Calcification of the main reef-building coral species on the Pacific coast of southern Mexico. Cien Mar. 2016, 42, 209–225. [Google Scholar] [CrossRef]
- Tortolero-Langarica, J.J.A.; Rodríguez-Troncoso, A.P.; Carricart-Ganivet, J.P.; Cupul-Magaña, A.L. Skeletal extension, density and calcification rates of massive free-living coral Porites lobata Dana, 1846. J. Exp. Mar. Biol. Ecol. 2016, 478, 68–76. [Google Scholar] [CrossRef]
- Knutson, D.W.; Buddemeier, R.W.; Smith, S.V. Coral chronometers: Seasonal growth bands in reef corals. Science 1972, 177, 270–272. [Google Scholar] [CrossRef] [PubMed]
- Buddemeier, R.W.; Maragos, J.E.; Knutson, D.W. Radiographic studies of reef coral exoskeletons: Rates and patterns of coral growth. J. Exp. Mar. Bio Ecol. 1974, 14, 179–199. [Google Scholar] [CrossRef]
- Highsmith, R.C. Coral growth rates and environmental control of density banding. J. Exp. Mar. Bio Ecol. 1979, 37, 105–125. [Google Scholar] [CrossRef]
- Scoffin, T.P.; Tudhope, A.W.; Brown, B.E.; Chansang, H.; Cheeney, R.F. Patterns and possible environmental controls of skeletogenesis of Porites lutea, South Thailand. Coral Reefs 1992, 11, 1–11. [Google Scholar] [CrossRef]
- Flora, C.J.; Ely, P.S. Surface growth rings of Porites lutea microatolls accurately track their annual growth. Northwest Sci. 2003, 77, 237–245. [Google Scholar]
- Zamani, N.P.; Arman, A. The growth rate of coral Porites lutea relating to the El Niño phenomena at Tunda Island, Banten Bay, Indonesia. Procedia Environ. Sci. 2016, 33, 505–511. [Google Scholar] [CrossRef]
- Ong, C.K.; Lee, J.N.; Tanzil, J.T.I. Skeletal Growth Rates in Porites lutea Corals from Pulau Tinggi, Malaysia. Water 2021, 14, 38. [Google Scholar] [CrossRef]
- Smith, L.W.; Barshis, D.J.; Birkeland, C. Phenotypic plasticity for skeletal growth, density and calcification of Porites lobata in response to habitat type. Coral Reefs 2007, 26, 559–567. [Google Scholar] [CrossRef]
- Tortolero-Langarica, J.J.A.; Carricart-Ganivet, J.P.; Cupul-Magaña, A.L.; Rodríguez-Troncoso, A.P. Historical insights on growth rates of the reef-building corals Pavona gigantea and Porites panamensis from the Northeastern tropical Pacific. Mar. Environ. Res. 2017, 132, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Lough, J.M.; Cooper, T.F. New insights from coral growth band studies in an era of rapid environmental change. Earth Sci. Rev. 2011, 108, 170–184. [Google Scholar] [CrossRef]
- Fabricius, K.; Langdon, C.; Uthicke, S.; Humphrey, C.; Noonan, S.; De’ath, D.; Okazaki, R.; Muehllehner, N.; Glas, M.; Lough, J.M. Losers and winners in coral reefs acclimatized to elevated carbon dioxide concentrations. Nat. Clim. Change 2011, 1, 165–169. [Google Scholar] [CrossRef]
- Eakin, C.M.; Sweatman, H.P.; Brainard, R.E. The 2014–2017 global-scale coral bleaching event: Insights and impacts. Coral Reefs 2019, 38, 539–545. [Google Scholar] [CrossRef]
- Lough, J.M.; Barnes, D.J.; Devereux, M.J.; Tobin, B.J.; Tobin, S. Variability in Growth Characteristics of Massive Porites on the Great Barrier Reef. Technical Report 28; CRC Reef Research Centre: Townsville, QLD, Australia, 1999; 95p. [Google Scholar]
- Lough, J.M. A strategy to improve the contribution of coral data to high-resolution paleoclimatology. Palaeogeogr. Paleoclimatol. Palaeoecol. 2004, 204, 115–143. [Google Scholar] [CrossRef]
- Glynn, P.W.; Manzello, D.P. Bioerosion and Coral Reef Growth: A Dynamic Balance. In Coral Reefs in the Anthropocene; Birkeland, C., Ed.; Springer: Dordrecht, The Netherlands, 2015; pp. 67–97. [Google Scholar]
- Robertson, D.R.; Allen, G.R. Zoogeography of the shore fish fauna of Clipperton Atoll. Coral Reefs 1996, 15, 121–131. [Google Scholar] [CrossRef]
- Fang, L.S.; Chen, Y.W.J.; Chen, C.S. Why does the white tip of stony coral grow so fast without zooxanthellae? Mar. Biol. 1989, 103, 359–363. [Google Scholar] [CrossRef]
- Carricart-Ganivet, J.P.; Merino, M. Growth responses of the reef-building coral Montastraea annularis along a gradient of continental influence in the southern Gulf of Mexico. Bull. Mar. Sci. 2001, 68, 133–146. [Google Scholar]
- Cabral-Tena, R.A.; Reyes-Bonilla, H.; Lluch-Cota, S.; Paz-García, D.A.; Calderón-Aguilera, L.E.; Norzagaray-López, O.; Balart, E.F. Different calcification rates in males and females of the coral Porites panamensis in the Gulf of California. Mar. Ecol. Prog. Ser. 2013, 476, 1–8. [Google Scholar] [CrossRef]
- Munk, W.H.; Sargent, M.C. Bikini and nearby atolls, Marshall Islands. US Geol. Surv. Prof. Pap. 1954, 260, 275–280. [Google Scholar][Green Version]
- National Oceanic and Atmospheric Administration, National Centers for Environmental Information. 2019 Eastern Pacific Hurricane Season. 2019. Available online: https://www.nhc.noaa.gov/data/tcr/index.php?season=2019&basin=epac (accessed on 29 November 2024).
- Muko, S.; Kawasaki, K.; Sakai, K.; Takasu, F.; Shigesada, N. Morphological plasticity in the coral Porites sillimaniani and its adaptive significance. Bull. Mar. Sci. 2000, 66, 225–239. [Google Scholar][Green Version]
- Todd, P.A. Morphological plasticity in Scleractinia corals. Biol. Rev. 2008, 83, 315–337. [Google Scholar] [CrossRef]
- Kitano, Y.F.; Benzoni, F.; Arrigoni, R.; Shirayama, Y.; Wallace, C.C.; Fukami, H. A Phylogeny of the Family Poritidae (Cnidaria, Scleractinia) Based on Molecular and Morphological Analyses. PLoS ONE 2014, 9, e98406. [Google Scholar] [CrossRef]
- Edmunds, P.J.; Brown, D.; Moriarty, V. Interactive effects of ocean acidification and temperature on two Scleractinia corals from Moorea, French Polynesia. Glob. Change Biol. 2012, 18, 2173–2183. [Google Scholar] [CrossRef]
- Crook, E.D.; Cohen, A.L.; Rebolledo-Vieyra, M.; Hernandez, L.; Paytan, A. Reduced calcification and lack of acclimatization by coral colonies growing in areas of persistent natural acidification. Proc. Natl. Acad. Sci. USA 2013, 110, 11044–11049. [Google Scholar] [CrossRef] [PubMed]
- Cooper, T.F.; O’Leary, R.A.; Lough, J.M. Growth of Western Australian corals in the Anthropocene. Science 2012, 335, 593–596. [Google Scholar] [CrossRef] [PubMed]
- Todd, P.A.; Ladle, R.J.; Lewin-Koh, N.; Chou, L.M. Genotype x environment interactions in transplanted clones of the massive corals Favia speciosa and Diploastrea heliopora. Mar. Ecol. Prog. Ser. 2004, 271, 167–182. [Google Scholar] [CrossRef]

 Porites lutea,
Porites lutea,  Porites lobata,
Porites lobata,  Porites arnaudi,
Porites arnaudi,  Porites australiensis.
Porites australiensis.
 Porites lutea,
Porites lutea,  Porites lobata,
Porites lobata,  Porites arnaudi,
Porites arnaudi,  Porites australiensis.
Porites australiensis.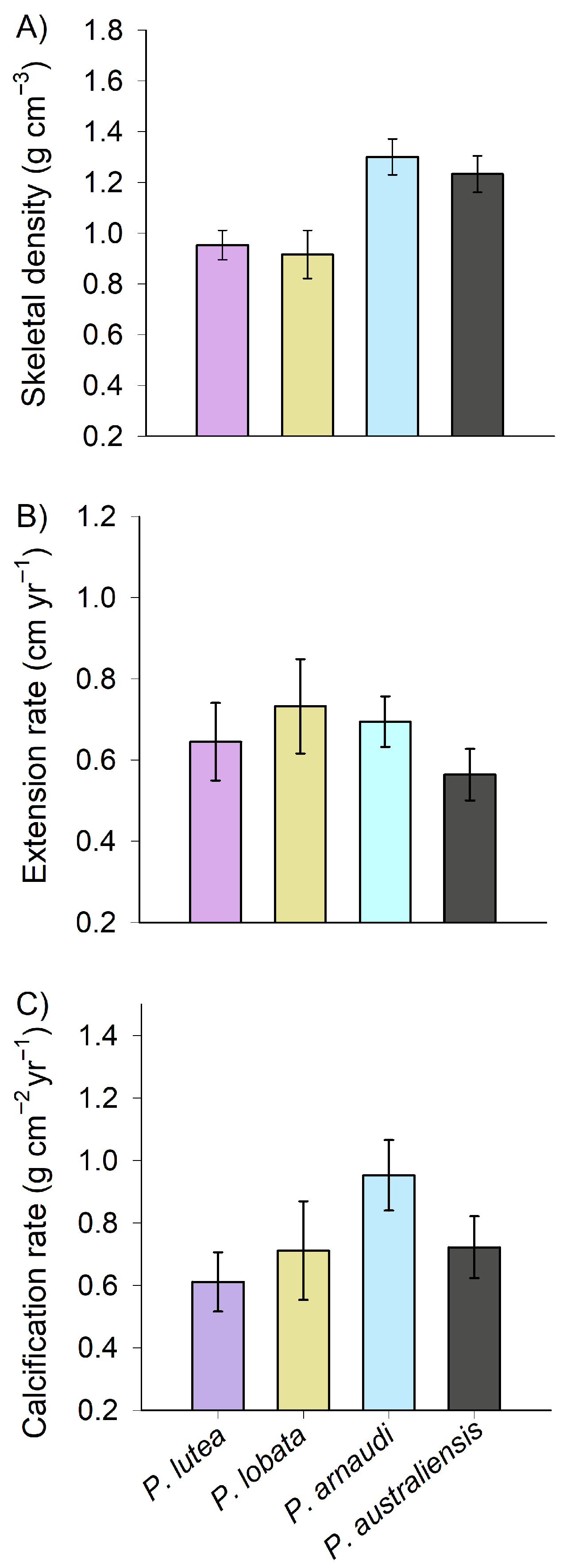

| Specie | No. Colonies | No. Tracks | Growth Parameters | ||
|---|---|---|---|---|---|
| Density (g cm−3) | Extension (cm yr−1) | Calcification (g cm−2 yr−1) | |||
| P. lutea | 6 | 10 | 0.96 ± 0.19 | 0.65 ± 0.33 | 0.62 ± 0.33 |
| P. lobata | 5 | 9 | 0.91 ± 0.27 | 0.73 ± 0.33 | 0.71 ± 0.45 |
| P. arnaudi | 8 | 16 | 1.30 ± 0.31 | 0.69 ± 0.28 | 0.95 ± 0.50 |
| P. australiensis | 6 | 10 | 1.22 ± 0.27 | 0.55 ± 0.23 | 0.70 ± 0.37 |
| Total number | 25 | 45 | |||
| PERMANOVA | PERMDISP | Pairwise Comparison | ||||
|---|---|---|---|---|---|---|
| Source | Pseudo-F | p | CV% | p | Groups | p (perm) |
| Temperature | 0.8 | 0.3962 | 2.07 | P. lut, P.aus | 0.0001 | |
| Species | 10.6 | 0.0001 | 33.2 | 0.184 | P. lut, P. lob | 0.3186 |
| Year | 1.4 | 0.2029 | 8.02 | 0.1091 | P. lut, P. arn | 0.0001 |
| Species x Year | 0.4 | 0.9876 | 18.4 | 0.3 | P. lob, P. arn | 0.0001 |
| P. lob, P. aus | 0.0004 | |||||
| P. arn, P. aus | 0.0091 | |||||
| Specie | Year (Yr) | Density (g cm−3) | Extension (cm yr−1) | Calcification (g cm−2 yr−1) |
|---|---|---|---|---|
| P. lutea | 2019 | 0.96 ± 0.25 | 0.44 ± 0.10 | 0.42 ± 0.14 |
| 2018 | 0.99 ± 0.24 | 0.68 ± 0.30 | 0.68 ± 0.33 | |
| 2017 | 0.92 ± 0.17 | 0.58 ± 0.20 | 0.54 ± 0.19 | |
| 2016 | 0.91 ± 0.11 | 0.77 ± 0.51 | 0.70 ± 0.46 | |
| 2015 | 0.96 ± 0.17 | 0.75 ± 0.37 | 0.72 ± 0.39 | |
| P. lobata | 2019 | 1.00 ± 0.19 | 0.54 ± 0.14 | 0.55 ± 0.20 |
| 2018 | 0.97 ± 0.26 | 0.77 ± 0.46 | 0.80 ± 0.58 | |
| 2017 | 0.94 ± 0.26 | 0.82 ± 0.44 | 0.81 ± 0.55 | |
| 2016 | 0.84 ± 0.32 | 0.80 ± 0.29 | 0.74 ± 0.49 | |
| 2015 | 0.80 ± 0.32 | 0.70 ± 0.25 | 0.62 ± 0.43 | |
| P. arnaudi | 2019 | 1.36 ± 0.33 | 0.62 ± 0.18 | 0.89 ± 0.42 |
| 2018 | 1.33 ± 0.26 | 0.71 ± 0.33 | 1.01 ± 0.63 | |
| 2017 | 1.28 ± 0.33 | 0.69 ± 0.27 | 0.95 ± 0.49 | |
| 2016 | 1.28 ± 0.32 | 0.65 ± 0.31 | 0.90 ± 0.51 | |
| 2015 | 1.22 ± 0.35 | 0.77 ± 0.27 | 0.98 ± 0.49 | |
| P. autraliensis | 2019 | 1.28 ± 0.25 | 0.55 ± 0.20 | 0.72 ± 0.33 |
| 2018 | 1.23 ± 0.31 | 0.52 ± 0.17 | 0.67 ± 0.32 | |
| 2017 | 1.23 ± 0.31 | 0.55 ± 0.24 | 0.71 ± 0.40 | |
| 2016 | 1.22 ± 0.29 | 0.57 ± 0.30 | 0.75 ± 0.47 | |
| 2015 | 1.17 ± 0.24 | 0.61 ± 0.29 | 0.74 ± 0.40 |
| Specie | Location | Density (g cm−3) | Extension (cm yr−1) | Calcification (g cm−2 yr−1) | Reference |
|---|---|---|---|---|---|
| Porites | Great Barrier Reef, Australia | 1.28 ± 0.16 | 1.3 ± 0.34 | 1.63 ± 0.38 | [6] |
| Porites | Moorea (French Polynesia) | 1.15 | 1.86 | 1.25 | [37] |
| P. lobata | Galapagos Islands | - | 0.81 | - | [38] |
| P. lobata | Great Barrier Reef, Australia | 1.1 | - | - | [39] |
| P. lobata | Australia | 1.41 ± 0.13 | 1.23 ± 0.24 | 1.71 ± 0.25 | [40] |
| P. lobata | Isla Isabel, Mexico | 1.08 ± 0.14 | 0.47 ± 0.23 | 0.51 ± 0.26 | [41] |
| P. lobata | Zacatoso, Mexico | 1.20 ± 0.07 | 0.60 ± 0.16 | 0.72 ± 0.22 | [42] |
| P. lobata | Isla Isabel, Mexico | 1.17 ± 0.02 | 0.57 ± 0.03 | 0.65 ± 0.03 | [43] |
| P. lutea | Enewetak Atoll | - | 1.35 | - | [44] |
| P. lutea | Enewetak Atoll | 1–1.40 | 0.4–0.5 1.35 | - | [45] |
| P. lutea | Enewetak Atoll | - | 0.76 | - | [46] |
| P. lutea | South Thailand | - | 2.25 | - | [47] |
| P. lutea | Abaiang Atoll, Republic of Kiribat | - | 2.12 ± 0.10 | - | [48] |
| P. lutea | Tunda Island, Indonesia | - | 0.5–2.2 | - | [49] |
| P. lutea | Pulau Tinggi, Malaysia | 1.23 ± 0.04 | 1.88 ± 0.15 | 2.01 ± 0.08 | [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ochoa-Serena, A.; Tortolero-Langarica, J.J.A.; Rodríguez-Zaragoza, F.A.; Carricart-Ganivet, J.P.; Clua, E.; Rodríguez-Troncoso, A.P. Growth Patterns of Reef-Building Porites Species in the Remote Clipperton Atoll Reef. Diversity 2025, 17, 492. https://doi.org/10.3390/d17070492
Ochoa-Serena A, Tortolero-Langarica JJA, Rodríguez-Zaragoza FA, Carricart-Ganivet JP, Clua E, Rodríguez-Troncoso AP. Growth Patterns of Reef-Building Porites Species in the Remote Clipperton Atoll Reef. Diversity. 2025; 17(7):492. https://doi.org/10.3390/d17070492
Chicago/Turabian StyleOchoa-Serena, Ania, J. J. Adolfo Tortolero-Langarica, Fabián A. Rodríguez-Zaragoza, Juan P. Carricart-Ganivet, Eric Clua, and Alma P. Rodríguez-Troncoso. 2025. "Growth Patterns of Reef-Building Porites Species in the Remote Clipperton Atoll Reef" Diversity 17, no. 7: 492. https://doi.org/10.3390/d17070492
APA StyleOchoa-Serena, A., Tortolero-Langarica, J. J. A., Rodríguez-Zaragoza, F. A., Carricart-Ganivet, J. P., Clua, E., & Rodríguez-Troncoso, A. P. (2025). Growth Patterns of Reef-Building Porites Species in the Remote Clipperton Atoll Reef. Diversity, 17(7), 492. https://doi.org/10.3390/d17070492











