The Biological Diversity of Fruit Flies (Diptera: Drosophilidae) in Russia: A Description of a Set of Own and Published Data and a Complete List of Species
Abstract
1. Summary
2. Data Description
2.1. Dataset Description
2.2. Biodiversity of Fruit Flies
3. Methods
3.1. Study Area
3.2. Study Material
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Steganinae |
|---|
| Acletoxenus formosus (Loew, 1864) |
| Amiota aquilotaurusata Takada, Beppu & Toda, 1979 |
| Amiota clavata Okada, 1960 |
| Amiota delta Takada, Beppu & Toda, 1979 |
| Amiota dentata Okada, 1971 |
| Amiota eos Sidorenko, 1989 |
| Amiota elongata Okada, 1960 |
| Amiota falcilis Takada, Beppu & Toda, 1979 |
| Amiota palpifera Okada, 1971 |
| Amiota promissa Okada, 1960 |
| Amiota subfurcata Okada, 1971 |
| Amiota stylopyga Wakahama & Okada, 1958 |
| Amiota taurusata Takada, Beppu & Toda, 1979 |
| Amiota todai Sidorenko, 1989 |
| Amiota trifurcata Okada, 1968 |
| Cacoxenus indagator Loew, 1858 |
| Cacoxenus kaszabi (Okada, 1973) |
| Cacoxenus romankovae (Sidorenko, 1991) |
| Leucophenga maculata (Dufour, 1839) |
| Leucophenga todai Sidorenko, 1991 |
| Phortica okadai (Máca, 1977) |
| Stegana ctenaria Nishiharu, 1979 |
| Stegana nigrithorax Strobl, 1898 |
| Stegana sidorenkoi Hu & Toda, 1994 |
| Stegana singularis Sidorenko, 1990 |
| Stegana taba Okada, 1971 |
| Stegana xuei Hu & Toda, 1994 |
| Drosophilinae |
| Drosophila auraria Peng, 1937 |
| Drosophila biauraria Bock & Wheeler, 1972 |
| Drosophila bondarenkoi Sidorenko, 1993 |
| Drosophila brachynephros Okada, 1956 |
| Drosophila calidata Takada, Beppu & Toda, 1979 |
| Drosophila curvispina Watabe & Toda, 1984 |
| Drosophila imaii Moriwaki & Okada, 1967 |
| Drosophila kanekoi Watabe & Higuchi, 1979 |
| Drosophila lacertosa Okada, 1956 |
| Drosophila limbata Roser, 1840 |
| Drosophila moriwakii Okada & Kurokawa, 1957 |
| Drosophila multispina Okada, 1956 |
| Drosophila neokadai Kaneko & Takada, 1966 |
| Drosophila okadai Takada, 1959 |
| Drosophila orientacea Grimaldi, James & Jaenike, 1992 |
| Drosophila parakuntzei Okada, 1973 |
| Drosophila rellima Wheeler, 1960 |
| Drosophila sordidula Kikkawa & Peng, 1938 |
| Drosophila subauraria Kimura, 1983 |
| Drosophila tsigana Burla & Gloor, 1952 |
| Drosophila unispina Okada, 1956 |
| Hirtodrosophila alboralis (Momma & Takada, 1954) |
| Hirtodrosophila kangi (Okada & Lee, 1961) |
| Hirtodrosophila macromaculata (Kang & Lee, 1961) |
| Hirtodrosophila makinoi (Okada, 1956) |
| Hirtodrosophila nokogiri (Okada, 1956) |
| Hirtodrosophila pseudonokogiri (Kang, Lee & Bahng, 1965) |
| Hirtodrosophila shaitanensis (Sidorenko, 1995) |
| Hirtodrosophila quadrivittata (Okada, 1956) |
| Hirtodrosophila trilineata (Chung, 1960) |
| Hirtodrosophila ussurica (Duda, 1935) |
| Lordiphosa clarofinis (Lee, 1959) |
| Lordiphosa collinella (Okada, 1968) |
| Lordiphosa hexasticha (Papp, 1971) |
| Lordiphosa magnipectinata Okada, 1956 |
| Lordiphosa pseudotenuicauda (Toda, 1983) |
| Lordiphosa stackelbergi (Duda, 1935) |
| Lordiphosa pseudotenuicauda (Okada, 1956) |
| Microdrosophila cristata Okada, 1960 |
| Mycodrosophila celesta Sidorenko, 1992 |
| Mycodrosophila japonica Okada, 1956 |
| Mycodrosophila nigropteropleura Kang, Lee & Bahng, 1965 |
| Mycodrosophila planipalpis Kang, Lee & Bahng, 1966 |
| Mycodrosophila shikokuana Okada, 1956 |
| Mycodrosophila takachihonis Okada, 1956 |
| Nesiodrosophila okadai Nishiharu, 1981 |
| Scaptodrosophila puncticeps (Okada, 1956) |
| Scaptodrosophila throckmortoni (Okada, 1973) |
| Scaptomyza baechlii Sidorenko, 1993 |
| Scaptomyza montana Wheeler, 1949 |
| Scaptomyza polygonia Okada, 1956 |
| Scaptomyza subsplendens (Duda, 1935) |
| Scaptomyza trochanterata Collin, 1953 |
References
- Trujillo-Arias, N.; Serrano-Cardozo, V.H.; Ramírez-Pinilla, M.P. Role of a Campesine Reserve Zone in the Magdalena Valley (Colombia) in the conservation of endangered tropical rainforests. Nat. Conserv. Res. 2023, 8, 49–63. [Google Scholar] [CrossRef]
- Duco, R.A.J.; Bejar, S.G.F.; Bañez, J.G.; Fidelino, J.S.; Duya, M.V.; Ong, P.S.; Duya, M.R.M. Forest fragments matter: A look into the species richness and diversity patterns of birds in a small, isolated, Protected Landscape in Mindanao Island, Philippines. Nat. Conserv. Res. 2024, 9, 47–60. [Google Scholar] [CrossRef]
- Jain, A.; Cunha, F.; Bunsen, M.J.; Cañas, J.S.; Pasi, L.; Pinoy, N.; Helsing, F.; Russo, J.; Botham, M.; Sabourin, M.; et al. Insect Identification in the Wild: The AMI Dataset. In Computer Vision—ECCV 2024. ECCV 2024. Lecture Notes in Computer Science; Leonardis, A., Ricci, E., Roth, S., Russakovsky, O., Sattler, T., Varol, G., Eds.; Springer: Cham, Switzerland, 2025; Volume 15095. [Google Scholar] [CrossRef]
- Drago, M.C.; Vrcibradic, D. Will future extinctions occur at the same places where the past ones did? A review involving mammals and the IUCN Red List. Nat. Conserv. Res. 2023, 8, 1–9. [Google Scholar] [CrossRef]
- Alfeus, M.; Irish, J.; Birkhofer, K. Recognition and completeness metrics from iNaturalist and GBIF can inform future citizen science and research projects: A case study on arthropods in Namibia. Biodivers. Conserv. 2025, 34, 467–480. [Google Scholar] [CrossRef]
- Štípková, Z.; Tsiftsis, S.; Kindlmann, P. Is the GBIF appropriate for use as input in models of predicting species distributions? Study from the Czech Republic. Nat. Conserv. Res. 2024, 9, 84–95. [Google Scholar] [CrossRef]
- Ostrauskas, H.; Pakalniškis, S.; Taluntytė, L. The species composition of plant mining dipterous (Insecta: Diptera) of greenhouse surroundings in Lithuania. Ekologija 2003, 3, 3–11. [Google Scholar]
- Krivosheina, N.P. Macromycete fruit bodies as a habitat for dipterans (Insecta, Diptera). Entomol. Rev. 2008, 88, 778–792. [Google Scholar] [CrossRef]
- Oliveira-Christe, R.; Marrelli, M.T. Taxonomic history, biology and ecology of Culex (Microculex) (Diptera: Culicidae). Acta Trop. 2024, 259, 107387. [Google Scholar] [CrossRef] [PubMed]
- Izwan-Anas, N.; Halim, M.R.A.; Low, V.L.; Adler, P.H.; Ya’cob, Z. Wild-caught adult black flies (Diptera: Simuliidae) from various ecological landscapes in Malaysia. Acta Trop. 2024, 259, 107374. [Google Scholar] [CrossRef] [PubMed]
- Bächli, G. TaxoDros: The Database on Taxonomy of Drosophilidae. 2024. Available online: https://www.taxodros.uzh.ch/ (accessed on 10 November 2024).
- Gottschalk, M.; Hofmann, P.; Valente, V. Diptera, Drosophilidae: Historical occurrence in Brazil. Check List 2008, 4, 485–518. [Google Scholar] [CrossRef]
- Prigent, S.R.; Lang, M.; Nagy, O.; Acurio, A.; Matamoro-Vidal, A.; Courtier-Orgogozo, V.; David, J.R. Field collections reveal that São Tomé is the Afrotropical island with the highest diversity of drosophilid flies (Diptera: Drosophilidae). Ann. Société Entomol. Fr. (N.S.) 2020, 56, 1–14. [Google Scholar] [CrossRef]
- Hubenov, Z. Species Composition and Distribution of the Dipterans (Insecta: Diptera) in Bulgaria; Pensoft & National Museum of Natural History: Sofia, Bulgaria, 2021; 276p. [Google Scholar]
- Savage, J.; Borkent, A.; Brodo, F.; Cumming, J.M.; Curler, G.; Currie, D.C.; deWaard, J.R.; Gibson, J.F.; Hauser, M.; Laplante, L.; et al. Diptera of Canada. In The Biota of Canada—A Biodiversity Assessment; Langor, D.W., Sheffield, C.S., Eds.; Part 1: The Terrestrial Arthropods; ZooKeys: Sofia, Bulgaria, 2019; Volume 819, pp. 397–450. [Google Scholar] [CrossRef]
- Kahanpää, J. Checklist of the Diptera (Insecta) of Finland: An introduction and a summary of results. Zookeys 2014, 19, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Gammelmo, Ø.; Søli, G. Notes on new and interesting Diptera from Norway. Nor. J. Entomol. 2011, 58, 189–195. [Google Scholar]
- Nartshuk, E.P. Key to families of Diptera (Insecta) of the fauna of Russian and adjacent countries. In Proceedings of the Zoological Institute; Zoological Institute of the Russian Academy of Sciences: Saint Petersburg, Russia, 2003; Volume 294, pp. 1–252. [Google Scholar]
- Sidorenko, V.S. Drosophilidae/Key to the Insects of Russian Far East. Vol. VI. Diptera and Siphonaptera. Pt. 2; Dalnauka: Vladivostok, Russia, 2001; pp. 211–242. [Google Scholar]
- Mitsui, H.; Van Achterberg, K.; Nordlander, G.; Kimura, M.T. Geographical distributions and host associations of larval parasitoids of frugivorous Drosophilidae in Japan. J. Nat. Hist. 2007, 41, 1731–1738. [Google Scholar] [CrossRef]
- Batista, M.R.; Uno, F.; Chaves, R.D.; Tidon, R.; Rosa, C.A.; Klaczko, L.B. Differential attraction of drosophilids to banana baits inoculated with Saccharomyces cerevisiae and Hanseniaspora uvarum within a Neotropical forest remnant. PeerJ 2017, 5, e3063. [Google Scholar] [CrossRef] [PubMed]
- Hochmüller, C.J.C.; Lopes-da-Silva, M.; Valente, V.L.S.; Schmitz, H.J. The drosophilid fauna (Diptera, Drosophilidae) of the transition between the Pampa and Atlantic Forest Biomes in the state of Rio Grande do Sul, southern Brazil: First records. Papéis Avulsos Zool. 2010, 50, 285–295. [Google Scholar] [CrossRef]
- Gottschalk, M.S.; De Toni, D.C.; Valente, V.L.S.; Hofmann, P.R.P. Changes in Brazilian Drosophilidae (Diptera) assemblages across an urbanisation gradient. Neotrop. Entomol. 2007, 36, 848–862. [Google Scholar] [CrossRef] [PubMed]
- Duarte, L.B.; Valer, F.B.; Vizentin-Bugoni, J.; Bernardi, E.; Valente, V.L.D.S.; Gottschalk, M.S. From visit to emergence: Interactions between mycophagous Drosophilidae (Insecta, Diptera) and macroscopic fungi (Basidiomycota) and their patterns in ecological networks. Rev. Bras. Entomol. 2024, 68, e20230097. [Google Scholar] [CrossRef]
- Gornostaev, N.G.; Ruchin, A.B.; Esin, M.N.; Lazebny, O.E.; Kulikov, A.M. Vertical distribution of fruit flies (Diptera: Drosophilidae) in deciduous forests in the center of European Russia. Insects 2023, 14, 822. [Google Scholar] [CrossRef] [PubMed]
- Ruchin, A.B.; Egorov, L.V.; MacGowan, I.; Makarkin, V.N.; Antropov, A.V.; Gornostaev, N.G.; Khapugin, A.A.; Dvorak, L.; Esin, M.N. Post-fire insect fauna explored by crown fermental traps in forests of the European Russia. Sci. Rep. 2021, 11, 21334. [Google Scholar] [CrossRef] [PubMed]
- Vrdoljak, J.; Padró, J.; De Panis, D.; Soto, I.M.; Carreira, V.P. Protein–alkaloid interaction in larval diet affects fitness in cactophilic Drosophila (Diptera: Drosophilidae). Biol. J. Linn. Soc. 2019, 127, 44–55. [Google Scholar] [CrossRef]
- Yoshimoto, J.; Nishida, T. Plant-mediated indirect effects of carpenterworms on the insect communities attracted to fermented tree sap. Popul. Ecol. 2008, 50, 25–34. [Google Scholar] [CrossRef]
- Schmitz, H.J.; da Silva Valente, V.L. The flower flies and the unknown diversity of Drosophilidae (Diptera): A biodiversity inventory in the Brazilian fauna. Papéis Avulsos Zool. 2019, 59, e20195945. [Google Scholar] [CrossRef]
- Gornostaev, N.; Esin, M.; Ruchin, A.; Esina, I. Biodiversity of Fruit Flies (Diptera: Drosophilidae) of Russia. Joint Directorate of the Mordovia State Nature Reserve and National Park “Smolny”. 2024. GBIF Occurrence Download. Available online: https://doi.org/10.15468/dyqr7q (accessed on 28 October 2024).
- Shtakelberg, A.A. Drosophilidae. In Key of Insects of the European Part of the USSR; Israel Program for Scientific Translations: Jerusalem, Israel, 1970; Volume 5, Part 2; pp. 390–399. [Google Scholar]
- Sidorenko, V.S. Drosophilid Flies of the Subfamily Steganinae (Diptera: Drosophilidae) of the Fauna of Russia and Neighbouring Counties; Dalnauka: Vladivostok, Russia, 2008; 231p. [Google Scholar]
- Máca, J.; Roháček, J.; Vilela, C.R.; Březíková, M. New and interesting records of Drosophilidae (Diptera) from the Czech Republic and Slovakia. Acta Mus. Siles. Sci. Natur. 2015, 64, 101–106. [Google Scholar]
- Gornostaevl, N.G.; Ruchinl, A.B.; Esin, M.N. An annotated list of fruit flies (Diptera: Drosophilidae) of the Republic of Mordovia. Far East. Entomol. 2024, 503, 7–15. [Google Scholar] [CrossRef]
- Gornostaev, N.G.; Ruchin, A.B.; Esin, M.N.; Kulikov, A.M. Seasonal dynamics of fruit flies (Diptera: Drosophilidae) in forests of the European Russia. Insects 2022, 13, 751. [Google Scholar] [CrossRef] [PubMed]
- Sidorenko, V.S.; Storozhenko, S.Y. Complexes of the Drosophilid flies (Diptera, Drosophilidae)—Inhabitants of xylotrophic fungi from the south of Primorye Region. Izv. St. Peterbg. Lesoteh. Akad. 2009, 187, 310–317. [Google Scholar]
- Tsacas, L.; de Chenon, R.D. Taxinomie et Biogéographie des «Genres» Cacoxenus—Paracacoxenus—Gitonides—Gitona [Dipt., Drosophilidae] et Biologie d’Une Nouvelle Espèce Africaine Commensale d’Apoidea [Hymenoptera] . Ann. Société Entomol. Fr. (N.S.) 1976, 12, 491–507. [Google Scholar] [CrossRef]
- Cini, A.; Loriatti, C.; Anfora, G. A review of the invasion of Drosophila suzukii in Europe and a draft research agenda for integrated pest management. Bull Insectology 2012, 65, 149–160. [Google Scholar]
- dos Santos, L.A.; Mendes, M.F.; Krüger, A.P.; Blauth, M.L.; Gottschalk, M.S.; Garcia, F.R.M. Global potential distribution of Drosophila suzukii (Diptera, Drosophilidae). PLoS ONE 2017, 12, e0174318. [Google Scholar] [CrossRef] [PubMed]
- Bieńkowski, A.O.; Orlova-Bienkowskaja, M.J. Invasive agricultural pest Drosophila suzukii (Diptera, Drosophilidae) appeared in the Russian Caucasus. Insects 2020, 11, 826. [Google Scholar] [CrossRef] [PubMed]
- Sidorenko, V.S. New and unrecorded species of Drosophilidae from Soviet Far East (Diptera, Brachycera). Spixiana 1992, 15, 93–95. [Google Scholar]
- Rastegaeva, V.M.; Kuzina, N.P.; Shirokova, O.A. Field testing of different doses of synthetic attractant in combination with colored “Plastina” sticky traps for early detection and monitoring of Drosophila suzukii. Plant Health Quar. 2024, 12, 26–33. [Google Scholar] [CrossRef]
- Martin, N.A. History of an invader, Scaptomyza flava (Fallen, 1823) (Diptera: Drosophilidae). N. Zealand J. Zool. 2004, 31, 27–32. [Google Scholar] [CrossRef]
- Stalker, H.D. On the biology and genetics of Scaptomyza graminum Fallen (Diptera, Drosophilidae). Genetics 1945, 30, 266–279. [Google Scholar] [CrossRef] [PubMed]
- Szwejda, J.H. Diptera pests occurring on vegetable crops in Poland. J. Hortic. Res. 2023, 31, 169–188. [Google Scholar] [CrossRef]
- Gross, H.; Christian, E. Drosophilid communities along an urban gradient across Vienna. Z. Für Okol. Naturschutz 1994, 3, 81–86. [Google Scholar]
- Poppe, J.L.; Valente, V.L.D.S.; Schmitz, H.J. Structure of Drosophilidae assemblage (Insecta, Diptera) in Pampa Biome (São Luiz Gonzaga, RS). Papéis Avulsos Zool. 2012, 52, 185–195. [Google Scholar] [CrossRef]
- Ruchin, A.B.; Egorov, L.V.; Khapugin, A.A.; Vikhrev, N.E.; Esin, M.N. The use of simple crown traps for the insects collection. Nat. Conserv. Res. 2020, 5, 87–108. [Google Scholar] [CrossRef]
- Gornostaev, N.G. A key to the Drosophilid flies (Diptera, Drosophilidae) from European Russia and neighbouring countries. Entomol. Obozr. 2001, 80, 908–915. [Google Scholar]
- Grimaldi, D.A. A phylogenetic revised classificationof genera in the Drosophilidae (Diptera). Bull. Am. Mus. Nat. Hist. 1990, 197, 139p. [Google Scholar]
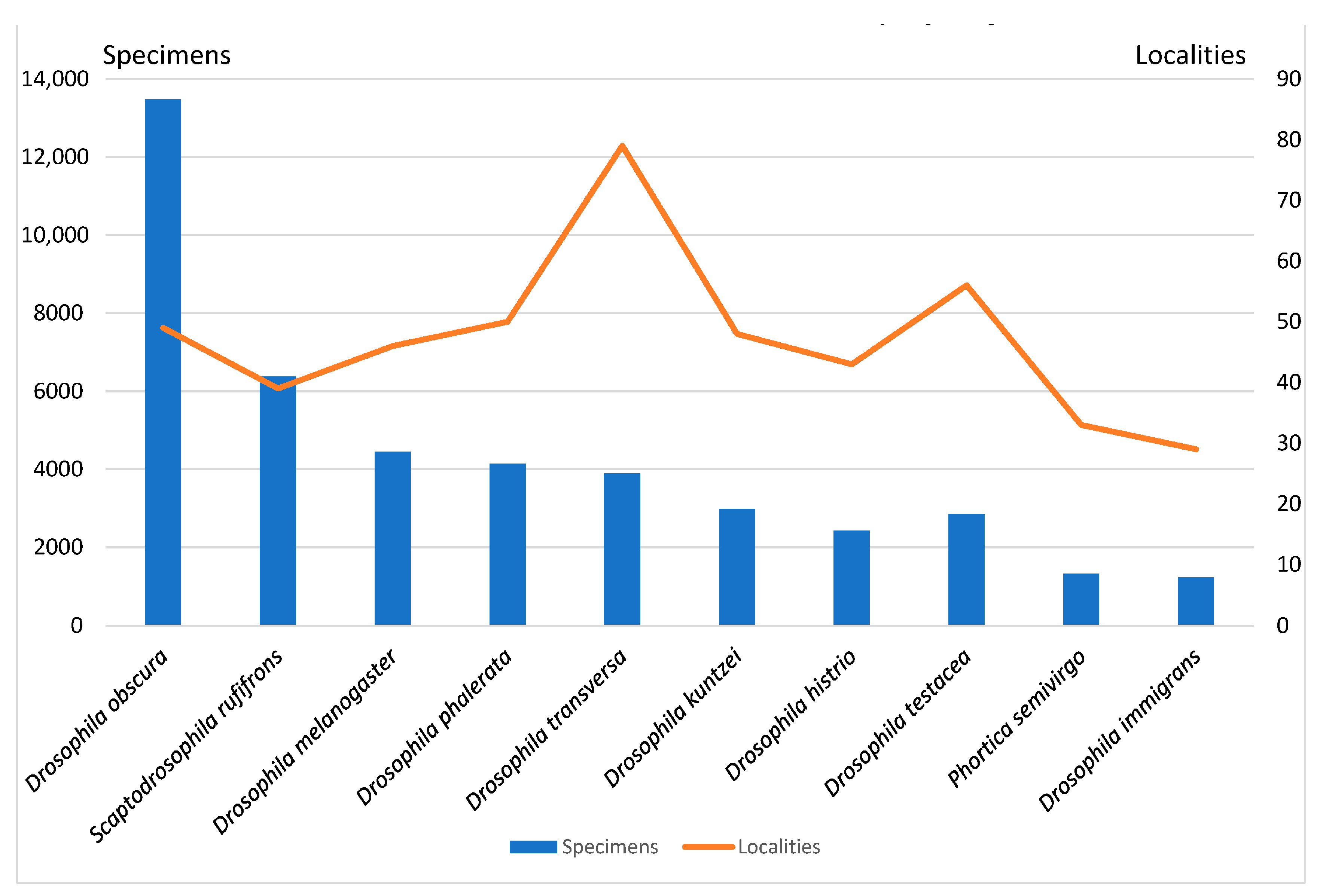
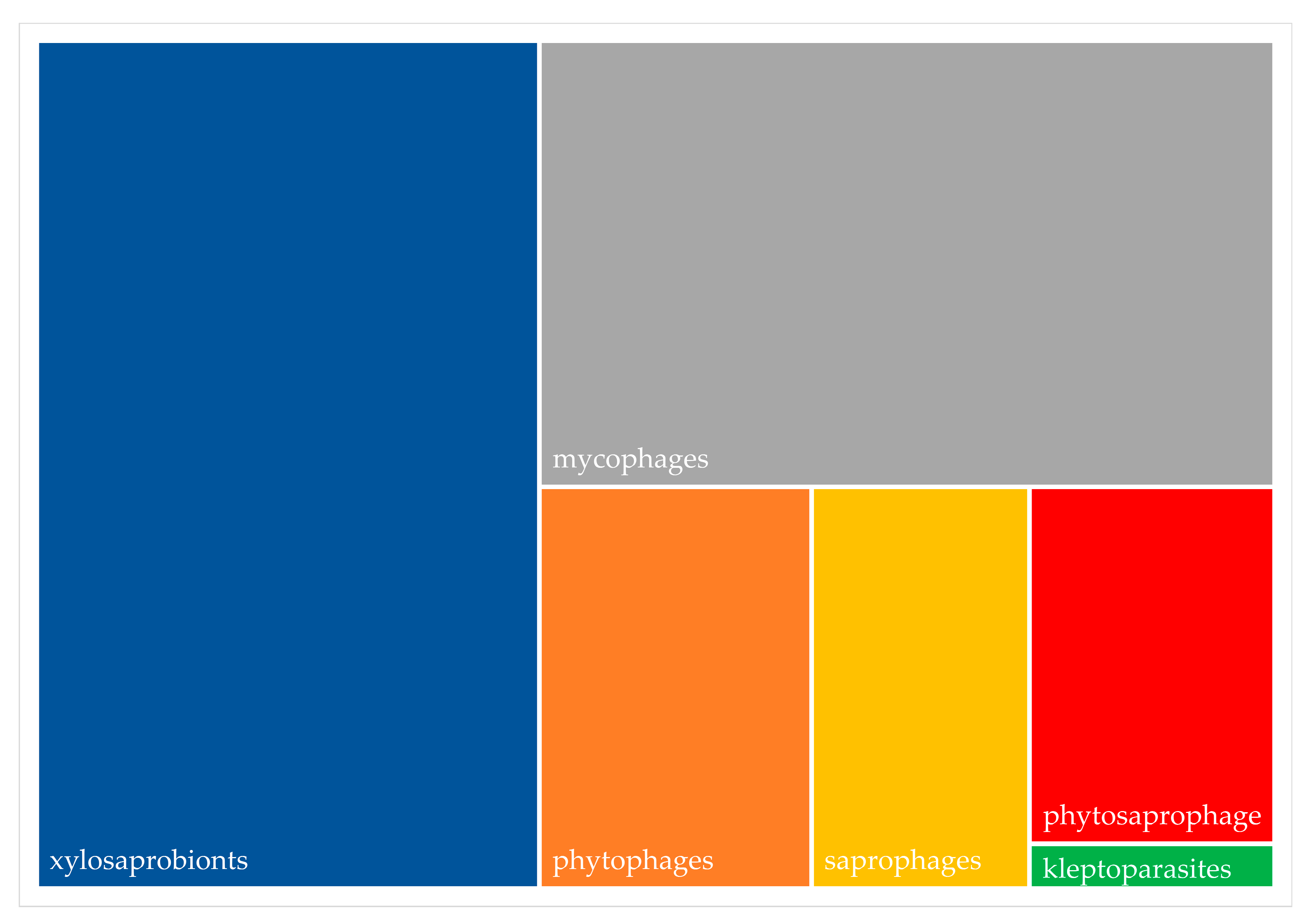
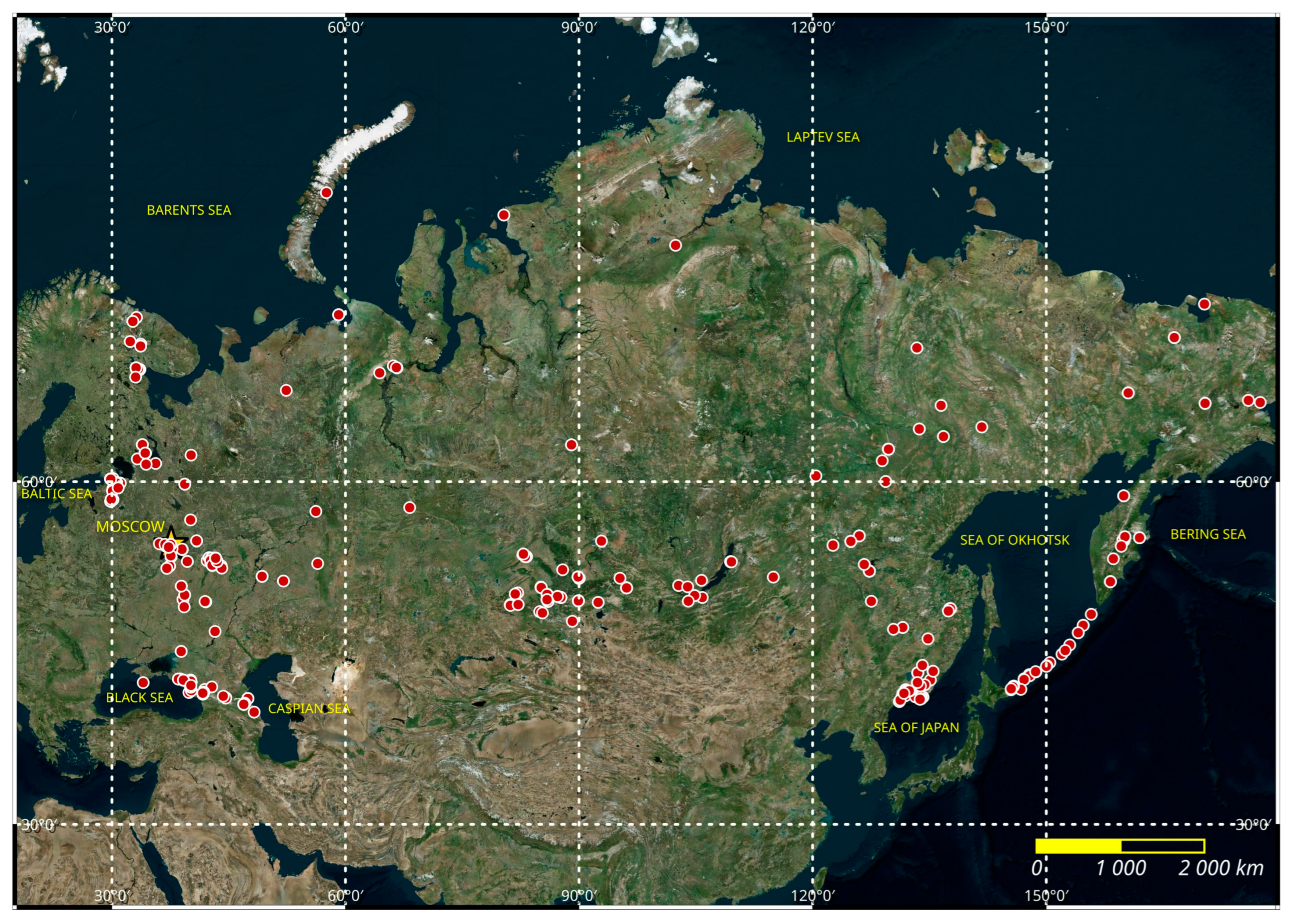
| Column Label | Column Description |
|---|---|
| occurrenceID | An identifier for the occurrence (as opposed to a particular digital record of the occurrence) |
| basisOfRecord | The specific nature of the data record: HumanObservation |
| scientificNam | The full scientific name including the genus name and the lowest level of taxonomic rank with the authority |
| Kingdom | The full scientific name of the kingdom in which the taxon is classified |
| decimalLatitude | The geographic latitude of the location in decimal degrees |
| geodeticDatum | The ellipsoid, geodetic datum, or spatial reference system (SRS) upon which the geographic coordinates given in decimalLatitude and decimalLongitude is based |
| Country | The name of the country in which the location occurs |
| countryCode | The standard code for the country in which the location occurs |
| individualCount | The number of individuals present at the time of the occurrence |
| eventDate | The date when material from the trap was collected or the range of dates during which the trap collected material |
| Year | The integer year in which the event occurred |
| Month | The ordinal month in which the event occurred |
| Day | The integer day of the month on which the event occurred |
| recordedBy | A person, group, or organization responsible for recording the original occurrence |
| identifiedBy | A list of names of people who assigned the taxon to the subject |
| bibliographicCitation | A bibliographic reference for the resource |
| Species | Specimens | Locality |
|---|---|---|
| Steganinae | ||
| Amiota albilabris (Roth, 1860) | 88 | 26 |
| Amiota alboguttata (Wahlberg, 1839) | 89 | 23 |
| Amiota flagellata Okada, 1971 | 4 | 1 |
| Amiota rufescens (Oldenberg, 1914) | 41 | 16 |
| Amiota subtusradiata (Duda, 1934) | 54 | 20 |
| Cacoxenus vlasovi (Duda, 1934) | 1 | 1 |
| Gitona distigma Meigen, 1830 | 144 | 36 |
| Leucophenga maculata (Dufour, 1839) | 53 | 14 |
| Leucophenga quadripunctata (Meijere, 1908) | 2 | 1 |
| Leucophenga quinquemaculata Strobl, 1893 | 717 | 40 |
| Leucophenga quinquemaculipennis Okada, 1956 | 9 | 2 |
| Leucophenga sorii Kang, Lee & Bhang 1965 | 3 | 3 |
| Leucophenga stackelbergi Duda, 1934 | 10 | 4 |
| Phortica chi (Toda & Sidorenko, 1996) | 17 | 6 |
| Phortica conifera (Okada, 1977) | 60 | 5 |
| Phortica iota (Toda & Sidorenko, 1996) | 141 | 10 |
| Phortica semivirgo (Máca, 1977) | 1327 | 33 |
| Phortica variegata (Fallén, 1823) | 9 | 6 |
| Stegana baechlii Laštovka & Máca, 1982 | 1 | 1 |
| Stegana coleoptrata (Scopoli, 1763) | 11 | 10 |
| Stegana furta (Linnaeus, 1767) | 16 | 7 |
| Stegana hypoleuca Meigen, 1830 | 10 | 5 |
| Stegana longifibula Takada, 1968 | 7 | 4 |
| Stegana masanoritodai Okada & Sidorenko, 1992 | 1 | 1 |
| Stegana mehadiae Duda, 1924 | 3 | 2 |
| Stegana sibirica (Duda, 1934) | 14 | 1 |
| Stegana similis Laštovka & Máca, 1982 | 2 | 2 |
| Drosophilinae | ||
| Chymomyza amoena (Loew, 1862) | 519 | 21 |
| Chymomyza caudatula Oldenberg, 1914 | 40 | 19 |
| Chymomyza costata (Zetterstedt, 1838) | 167 | 23 |
| Chymomyza distincta (Egger, 1862) | 33 | 6 |
| Chymomyza fuscimana (Zetterstedt, 1838) | 89 | 21 |
| Drosophila alpina Burla, 1948 | 188 | 12 |
| Drosophila altukhovi Imasheva, Lazebny, Cariou, David & Tsacas, 1994 | 1 | 1 |
| Drosophila ambigua Pomini, 1940 | 1 | 1 |
| Drosophila angularis Okada, 1956 | 17 | 4 |
| Drosophila bifasciata Pomini, 1940 | 355 | 26 |
| Drosophila busckii Coquillett, 1901 | 211 | 17 |
| Drosophila ezoana Takada & Okada, 1957 | 133 | 7 |
| Drosophila funebris (Fabricius, 1787) | 956 | 29 |
| Drosophila helvetica Burla, 1948 | 38 | 7 |
| Drosophila histrio Meigen, 1830 | 2426 | 43 |
| Drosophila hydei Sturtevant, 1921 | 147 | 18 |
| Drosophila imaii Moriwaki & Okada, 1967 | 10 | 1 |
| Drosophila immigrans Sturtevant, 1921 | 1226 | 29 |
| Drosophila ingrica Hackman, 1957 | 4 | 3 |
| Drosophila kuntzei Duda, 1924 | 2978 | 48 |
| Drosophila limbata Roser, 1840 | 24 | 10 |
| Drosophila littoralis Meigen, 1830 | 6 | 6 |
| Drosophila lummei Hackman, 1972 | 1 | 1 |
| Drosophila melanogaster Meigen, 1830 | 4445 | 46 |
| Drosophila mercatorum Patterson & Wheeler, 1942 | 35 | 2 |
| Drosophila montana Stone, Griffen & Patterson, 1941 | 42 | 5 |
| Drosophila nigromaculata Kikkawa et Peng, 1938 | 22 | 7 |
| Drosophila nigrosparsa Strobl, 1898 | 11 | 1 |
| Drosophila obscura Fallén, 1823 | 13,480 | 49 |
| Drosophila parakuntzei Okada, 1973 | 2 | 2 |
| Drosophila phalerata Meigen, 1830 | 4132 | 50 |
| Drosophila picta Zetterstedt, 1847 | 2 | 2 |
| Drosophila rellima Wheeler, 1960 | 18 | 2 |
| Drosophila repleta Wollaston, 1858 | 41 | 7 |
| Drosophila simulans Sturtevant, 1919 | 6 | 1 |
| Drosophila subobscura Collin, 1936 | 311 | 23 |
| Drosophila subsilvestris Hardy & Kaneshiro, 1968 | 362 | 21 |
| Drosophila suzukii (Matsumura, 1931) | 12 | 5 |
| Drosophila testacea Roser, 1840 | 2839 | 56 |
| Drosophila transversa Fallén, 1823 | 3891 | 79 |
| Drosophila tristis Fallén, 1823 | 16 | 6 |
| Drosophila unispina Okada, 1956 | 20 | 3 |
| Drosophila virilis Sturtevant, 1916 | 6 | 3 |
| Hirtodrosophila baikalensis Watabe, Toda & Sidorenko, 1996 | 7 | 3 |
| Hirtodrosophila cameraria (Haliday, 1833) | 13 | 6 |
| Hirtodrosophila confusa (Staeger, 1844) | 165 | 30 |
| Hirtodrosophila histrioides (Okada & Kurokawa, 1957) | 31 | 3 |
| Hirtodrosophila lundstroemi (Duda, 1935) | 1 | 1 |
| Hirtodrosophila oldenbergi (Duda, 1924) | 4 | 2 |
| Hirtodrosophila sexvittata (Okada, 1956) | 56 | 2 |
| Hirtodrosophila shaitanensis Sidorenko, 1996 | 1 | 1 |
| Hirtodrosophila subarctica (Hackman, 1969) | 317 | 22 |
| Hirtodrosophila toyohiokadai (Sidorenko, 1990) | 54 | 10 |
| Hirtodrosophila trivittata (Strobl, 1893) | 59 | 20 |
| Lordiphosa acuminata (Collin, 1952) | 3 | 2 |
| Lordiphosa andalusiaca (Strobl, 1906) | 1 | 1 |
| Lordiphosa fenestrarum (Fallén, 1823) | 17 | 11 |
| Lordiphosa miki (Duda, 1924) | 3 | 1 |
| Lordiphosa mommai (Takada & Okada, 1960) | 560 | 1 |
| Lordiphosa nigricolor (Strobl, 1898) | 11 | 6 |
| Microdrosophila congesta (Zetterstedt, 1847) | 2 | 2 |
| Microdrosophila cristata Okada, 1960 | 1 | 1 |
| Mycodrosophila celesta Sidorenko, 1992 | 7 | 2 |
| Mycodrosophila poecilogastra (Loew, 1874) | 59 | 7 |
| Mycodrosophila shikokuana Okada, 1956 | 8 | 1 |
| Mycodrosophila takachihonis Okada, 1956 | 1 | 1 |
| Nesiodrosophila magnidentata (Lee, 1964) | 1 | 1 |
| Scaptodrosophila coracina (Kikkawa & Peng, 1938) | 54 | 2 |
| Scaptodrosophila deflexa (Duda, 1924) | 92 | 2 |
| Scaptodrosophila rufifrons (Loew, 1873) | 6374 | 39 |
| Scaptomyza carinata Okada, 1973 | 216 | 6 |
| Scaptomyza consimilis Hackman, 1955 | 27 | 11 |
| Scaptomyza flava (Fallén, 1823) | 50 | 20 |
| Scaptomyza graminum (Fallén, 1823) | 59 | 22 |
| Scaptomyza okadai Hackman, 1959 | 13 | 7 |
| Scaptomyza pallida (Zetterstedt, 1847) | 538 | 43 |
| Scaptomyza taigensis Sidorenko & Toda, 1996 | 8 | 2 |
| Scaptomyza teinoptera Hackman, 1955 | 2 | 1 |
| Scaptomyza unipunctum (Zetterstedt, 1847) | 13 | 9 |
| Scaptomyza yakutica Sidorenko & Toda, 1996 | 80 | 2 |
| Zaprionus flavofasciatus (Takada, Beppu & Toda, 1979) | 1 | 1 |
| Total | 51,006 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gornostaev, N.G.; Ruchin, A.B.; Esin, M.N.; Lobachev, E.A.; Esina, I.G. The Biological Diversity of Fruit Flies (Diptera: Drosophilidae) in Russia: A Description of a Set of Own and Published Data and a Complete List of Species. Diversity 2025, 17, 490. https://doi.org/10.3390/d17070490
Gornostaev NG, Ruchin AB, Esin MN, Lobachev EA, Esina IG. The Biological Diversity of Fruit Flies (Diptera: Drosophilidae) in Russia: A Description of a Set of Own and Published Data and a Complete List of Species. Diversity. 2025; 17(7):490. https://doi.org/10.3390/d17070490
Chicago/Turabian StyleGornostaev, Nikolai G., Alexander B. Ruchin, Mikhail N. Esin, Evgeniy A. Lobachev, and Irina G. Esina. 2025. "The Biological Diversity of Fruit Flies (Diptera: Drosophilidae) in Russia: A Description of a Set of Own and Published Data and a Complete List of Species" Diversity 17, no. 7: 490. https://doi.org/10.3390/d17070490
APA StyleGornostaev, N. G., Ruchin, A. B., Esin, M. N., Lobachev, E. A., & Esina, I. G. (2025). The Biological Diversity of Fruit Flies (Diptera: Drosophilidae) in Russia: A Description of a Set of Own and Published Data and a Complete List of Species. Diversity, 17(7), 490. https://doi.org/10.3390/d17070490







