Diversity of the Soil Bacterial Community of Abandoned Jujube Land in the Loess Area of Northern Shaanxi in Different Years
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area Overview and Formation Mechanisms of Abandoned Land
2.2. Sample Collection
2.3. Experimental Method
2.4. Soil Bacterial Community Structure Analysis
2.5. Statistical Analysis and Data Analysis
3. Results and Analysis
3.1. Characteristics of Soil Physical and Chemical Properties of Jujube Forests at Different Abandonment Years
3.2. Soil Bacterial Community Composition Characteristics of Jujube Forest at Different Abandoned Years
3.3. Diversity Analysis of Soil Bacterial Community
3.4. Similarity Analysis of Soil Bacterial Community
3.5. Relationship Between Soil Microbial Community and Soil Physicochemical Properties
4. Discussions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, L.; Gao, J.; Huang, T.; Kendall, J.R.A.; Shen, Q.; Zhang, R. Parental material and cultivation determine soil bacterial community structure and fertility. FEMS Microbiol. Ecol. 2015, 91, 1–10. [Google Scholar] [CrossRef]
- Beckers, B.; Op De Beeck, M.; Weyens, N.; Boerjan, W.; Vangronsveld, J. Structural variability and niche differentiation in the rhizosphere and endosphere bacterial microbiome of field-grown poplar trees. Microbiome 2017, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Blagodatskaya, E.; Kuzyakov, Y. Active microorganisms in soil: Critical review of estimation criteria and approaches. Soil Biol. Biochem. 2013, 67, 192–211. [Google Scholar] [CrossRef]
- Wilpiszeski, R.L.; Aufrecht, J.A.; Retterer, S.T.; Sullivan, M.B.; Graham, D.E.; Pierce, E.M.; Zablocki, O.D.; Palumbo, A.V.; Elias, D.A. Soil aggregate microbial communities: Towards understanding microbiome interactions at biologically relevant scales. Appl. Environ. Microbiol. 2019, 85, e00324-19. [Google Scholar] [CrossRef] [PubMed]
- Ciric, V.; Manojlovic, M.; Nesic, L.; Belic, M. Soil dry aggregate size distribution: Effects of soil type and land use. J. Soil Sci. Plant Nutr. 2012, 12, 689–703. [Google Scholar] [CrossRef]
- Graham, E.B.; Knelman, J.E.; Schindlbacher, A.; Siciliano, S.; Breulmann, M.; Yannarell, A.; Beman, J.; Abell, G.; Philippot, L.; Prosser, J. Microbes as engines of ecosystem function: When does community structure enhance predictions of ecosystem processes? Front. Microbiol. 2016, 7, 214. [Google Scholar] [CrossRef]
- Huang, X.-F.; Chaparro, J.M.; Reardon, K.F.; Zhang, R.; Shen, Q.; Vivanco, J.M. Rhizosphere interactions: Root exudates, microbes, and microbial communities. Botany 2014, 92, 267–275. [Google Scholar] [CrossRef]
- Carvalhais, L.C.; Dennis, P.G.; Badri, D.V.; Kidd, B.N.; Vivanco, J.M.; Schenk, P.M. Linking jasmonic acid signaling, root exudates, and rhizosphere microbiomes. Mol. Plant-Microbe Interact. 2015, 28, 1049–1058. [Google Scholar] [CrossRef]
- Tao, S.; Veen, G.F.; Zhang, N.; Yu, T.; Qu, L. Tree and shrub richness modifies subtropical tree productivity by regulating the diversity and community composition of soil bacteria and archaea. Microbiome 2023, 11, 261. [Google Scholar] [CrossRef]
- Zhelezova, A.; Chernov, T.; Nikitin, D.; Tkhakakhova, A.; Ksenofontova, N.; Zverev, A.; Kutovaya, O.; Semenov, M. Seasonal Dynamics of Soil Bacterial Community under Long-Term Abandoned Cropland in Boreal Climate. Agronomy 2022, 12, 519. [Google Scholar] [CrossRef]
- Fierer, N.; Lauber, C.L.; Ramirez, K.S.; Zaneveld, J.; Bradford, M.A.; Knight, R. Comparative metagenomic, phylogenetic and physiological analyses of soil microbial communities across nitrogen gradients. ISME J. 2012, 6, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zeng, D.; Huang, Y.; Zhong, L.; Liao, J.; Shi, Y.; Jiang, H.; Luo, Y.; Liang, Y.; Chai, S. The structure and diversity of bacteria and fungi in the roots and rhizosphere soil of three different species of Geodorum. BMC Genom. 2024, 25, 222. [Google Scholar] [CrossRef]
- Lai, L.; Wu, C.; Zhang, H.; Zhu, Z.; Yang, J.; Kuzyakov, Y.; Chen, J.; Ge, T. Microbial diversity loss and plant genotype modulates rhizosphere microbial β-diversity to constrain soil functioning. Soil Ecol. Lett. 2025, 7, 250308. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Pieterse, C.M.; Bakker, P.A. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef]
- Fierer, N. Embracing the unknown: Disentangling the complexities of the soil microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef]
- Yu, F.; Liang, J.; Shi, J.; Wang, S.; Lu, J. Effects of canopy damage on soil CO2 fixation bacterial community structure in Xiaokeng forest farm. Microbiol. China 2017, 44, 2297–2306. [Google Scholar]
- Wang, L.; Zhou, G.; Zhu, X.; Gao, B.; Xu, H. Effects of litter on soil organic carbon and microbial functional diversity. Acta Ecol. Sin. 2021, 41, 2709–2718. [Google Scholar]
- Sun, H.; Li, X.; Jin, L.; Li, C.; Zhang, J. Change over time in soil microbial diversity of artificial grassland in the Yellow River source zone. Acta Prataculturae Sin. 2021, 30, 46–58. [Google Scholar]
- Cheng, Z.; Yang, L.; Sui, X.; Zhang, T.; Wang, W.; Yin, W.; Li, G.; Song, F. Soil Microbial Functional Diversity Responses to Different evegetation Types in Heilongjiang Zhongyangzhan Black-Billed Capercaillie Nature Reserv. Res. Environ. Sci. 2021, 34, 1177–1186. [Google Scholar]
- Ye, H.; Hong, M.; Xu, X.; Liang, Z.; Jiang, N.; Tu, N.; Wu, Z. Responses of plant diversity and soil microorganism diversity to nitrogen addition in the desert steppe, China. J. Arid. Land 2024, 16, 447–459. [Google Scholar] [CrossRef]
- Bian, Y.; Zhang, Z.; Fu, Z.; Liu, W. Distribution characteristics of soil Microbial communities of different vegetation restoration Modelsand their correlation with soil physical and chemical properties in desert steppe. Acta Agrestia Sin. 2021, 29, 655–663. [Google Scholar]
- Yang, Y.; Xu, M.; Zou, X.; Chen, J.; Zhang, J.; Zhang, J. Effects of Different Vegetation Types on the Characteristics of Soil Bacterial Communities in the Hilly Area of Central Guizhou. J. Ecol. Rural. Environ. 2021, 37, 518–525. [Google Scholar]
- Wu, L.; Ren, C.; Jiang, H.; Zhang, W.; Chen, N.; Zhao, X.; Wei, G.; Shu, D. Land abandonment transforms soil microbiome stability and functional profiles in apple orchards of the Chinese Losses Plateau. Sci. Total Environ. 2024, 906, 167556. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Zhu, Z.; Yuan, H.; Ge, T.; Wang, J.; Chen, M.; Wu, J. Effects of Different Plantation Type on the Abundance and Diversity of Soil Microbes in Subtropical Red Soil. Environ. Sci. 2015, 36, 3839–3844. [Google Scholar]
- Tang, H.; Xiao, X.; Li, C.; Tang, W.; Guo, L.; Wang, K.; Sun, Y.; Cheng, K.; Sun, G.; Pan, X. Effects of recycling straw of different winter covering crops on rhizospheric microbial community functional diversity in a double-cropped paddy field. Acta Ecol. Sin. 2018, 38, 6559–6569. [Google Scholar]
- Liu, Z.; Huang, F.; Li, J.; Zhang, P.; Yang, B.; Ding, R.; Nie, J.; Jia, Z. Effects of farmland mulching patterns on soil microbial diversity and community structure in dryland. Acta Ecol. Sin. 2021, 41, 2750–2760. [Google Scholar]
- Wei, X.; Fu, T.; He, G.; Zhong, Z.; Yang, M.; Lou, F.; He, T. Types of vegetables shape composition, diversity, and co-occurrence networks of soil bacteria and fungi in karst areas of southwest China. BMC Microbiol. 2023, 23, 194. [Google Scholar] [CrossRef]
- Cui, F.; Li, Q.; Shang, S.; Hou, X.; Miao, H.; Chen, X. Effects of cotton peanut rotation on crop yield soil nutrients and microbial diversity. Sci. Rep. 2024, 14, 28072. [Google Scholar] [CrossRef]
- Jia, P.; Feng, H.; Li, M. Soil microbial diversity of black soil under different land use patterns in northeast China. Trans. Chin. Soc. Agric. Eng. 2020, 36, 171–178. [Google Scholar]
- Zhu, S.; Xia, B.; Hao, W.; Xu, M. Functional diversity of soil microbial community on eroded slope in the Loess Plateau Region. China Environ. Sci. 2020, 40, 4099–4105. [Google Scholar]
- Wen, J.; Li, X. Research on Exploitation and Utilization of Rural Abandoned Land. Mod. Agric. Sci. Technol. 2016, 297–299. [Google Scholar]
- Xiang, T.; Gao, R.; Qiang, F.; Yang, N.; Liu, G.; Liu, C.; Ai, N. Coupling Relationship between Soil Organic Carbon Storage and Soil Water Storage in Abandoned Economic Forests in the Loess Hilly Areas. Forests 2023, 14, 221. [Google Scholar] [CrossRef]
- Ren, Q.; Qiang, F.; Liu, G.; Liu, C.; Ai, N. Response of soil quality to ecosystems after revegetation in a coal mine reclamation area. Catena 2025, 257, 109038. [Google Scholar] [CrossRef]
- Institute of Soil Science, Chinese Academy of Sciences. Soil Physical Properties Determination Method; Science Press: Beijing, China, 1978. [Google Scholar]
- Bao, S. Soil Agrochemical Analysis; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Xu, G.; Zheng, H. Study Way of Soil Enzymes; Agriculture Press: Beijing, China, 1986. [Google Scholar]
- Zhou, Y.; Liu, C.; Ai, N.; Qin, J.; Shi, J.; Nan, Z. Soil fauna community characteristics and driving factors of Pinus tabuliformis in the loess region of northern Shaanxi. Catena 2023, 229, 107196. [Google Scholar] [CrossRef]
- Xu, M.; Xi, J.; Liu, Y.; Li, S. Adaptation strategies of soil microorganisms in resource changes and stoichiometric imbalances induced by secondary succession on the loess plateau. J. Environ. Manag. 2024, 370, 122668. [Google Scholar] [CrossRef]
- Park, H.; Kim, K.; Walitang, D.I.; Sayyed, R.; Sa, T. Shifts in Soil Bacterial Community Composition of Jujube Orchard Influenced by Organic Fertilizer Amendment. J. Microbiol. Biotechnol. 2024, 34, 2539–2546. [Google Scholar] [CrossRef]
- Eisenlord, S.D.; Zak, D.R. Simulated atmospheric nitrogen deposition alters actinobacterial community composition in forest soils. Soil Sci. Soc. Am. J. 2010, 74, 1157–1166. [Google Scholar] [CrossRef]
- Hill, P.; Krištůfek, V.; Dijkhuizen, L.; Boddy, C.; Kroetsch, D.; van Elsas, J.D. Land use intensity controls actinobacterial community structure. Microb. Ecol. 2011, 61, 286–302. [Google Scholar] [CrossRef]
- Xia, Y.; He, X.; Feng, Z.; Zhang, Q.; Yang, H. A comprehensive analysis of the microbial diversity in natural and engineered ecosystems based on high-throughput sequencing of 16S rRNA gene. Int. Biodeterior. Biodegrad. 2019, 140, 160–168. [Google Scholar] [CrossRef]
- Lauber, C.L.; Strickland, M.S.; Bradford, M.A.; Fierer, N. The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol. Biochem. 2008, 40, 2407–2415. [Google Scholar] [CrossRef]
- Yang, F.; Wu, J.; Zhang, D.; Chen, Q.; Zhang, Q.; Cheng, X. Soil bacterial community composition and diversity in relation to edaphic properties and plant traits in grasslands of southern China. Appl. Soil Ecol. 2018, 128, 43–53. [Google Scholar] [CrossRef]
- Harrop-Archibald, H.; Didham, R.K.; Standish, R.J.; Tibbett, M.; Hobbs, R.J. Mechanisms linking fungal conditioning of leaf litter to detritivore feeding activity. Soil Biol. Biochem. 2016, 93, 119–130. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, Y.; Ni, Y.; Zhong, Q. Effects of artificial forest and grass on soil fungal community at southern Ningxia mountain. China Environ. Sci. 2018, 38, 1449–1458. [Google Scholar]
- Balasooriya, W.; Denef, K.; Peters, J.; Verhoest, N.; Boeckx, P. Vegetation composition and soil microbial community structural changes along a wetland hydrological gradient. Hydrol. Earth Syst. Sci. 2008, 12, 277–291. [Google Scholar] [CrossRef]
- Tian, J.; Shu, C.; Chen, H.; Qiao, Y.; Yang, G.; Xiong, W.; Wang, L.; Sun, J.; Liu, X. Response of archaeal communities to water regimes under simulated warming and drought conditions in Tibetan Plateau wetlands. J. Soils Sediments 2015, 15, 179–188. [Google Scholar] [CrossRef]
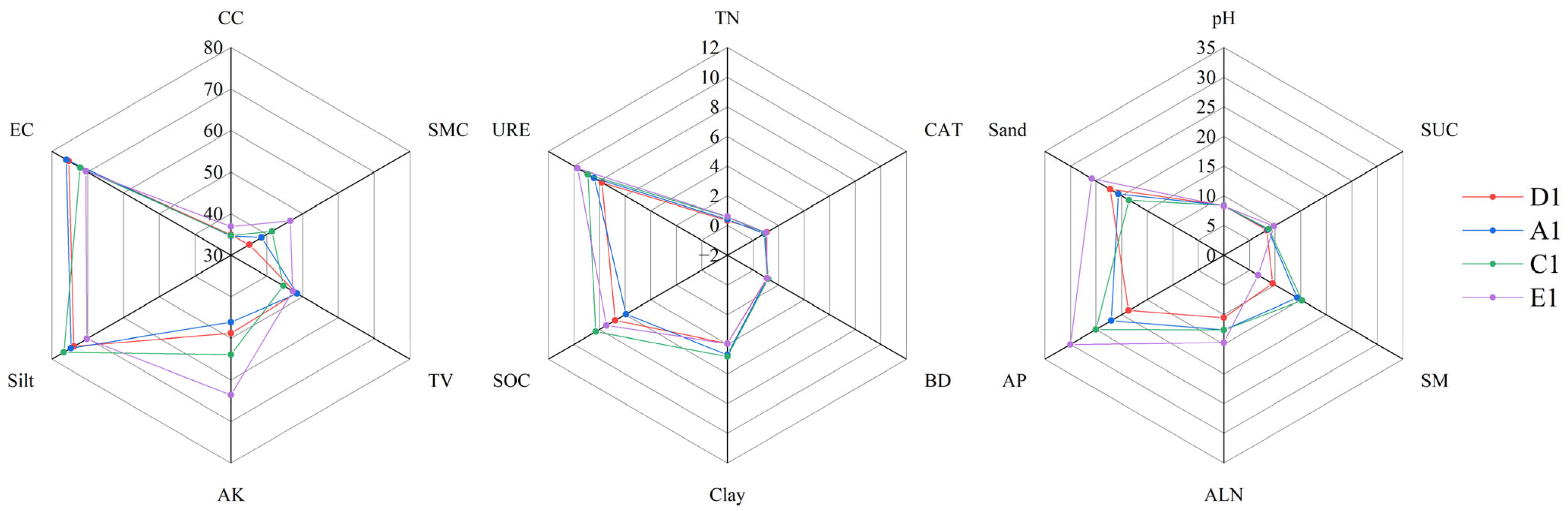
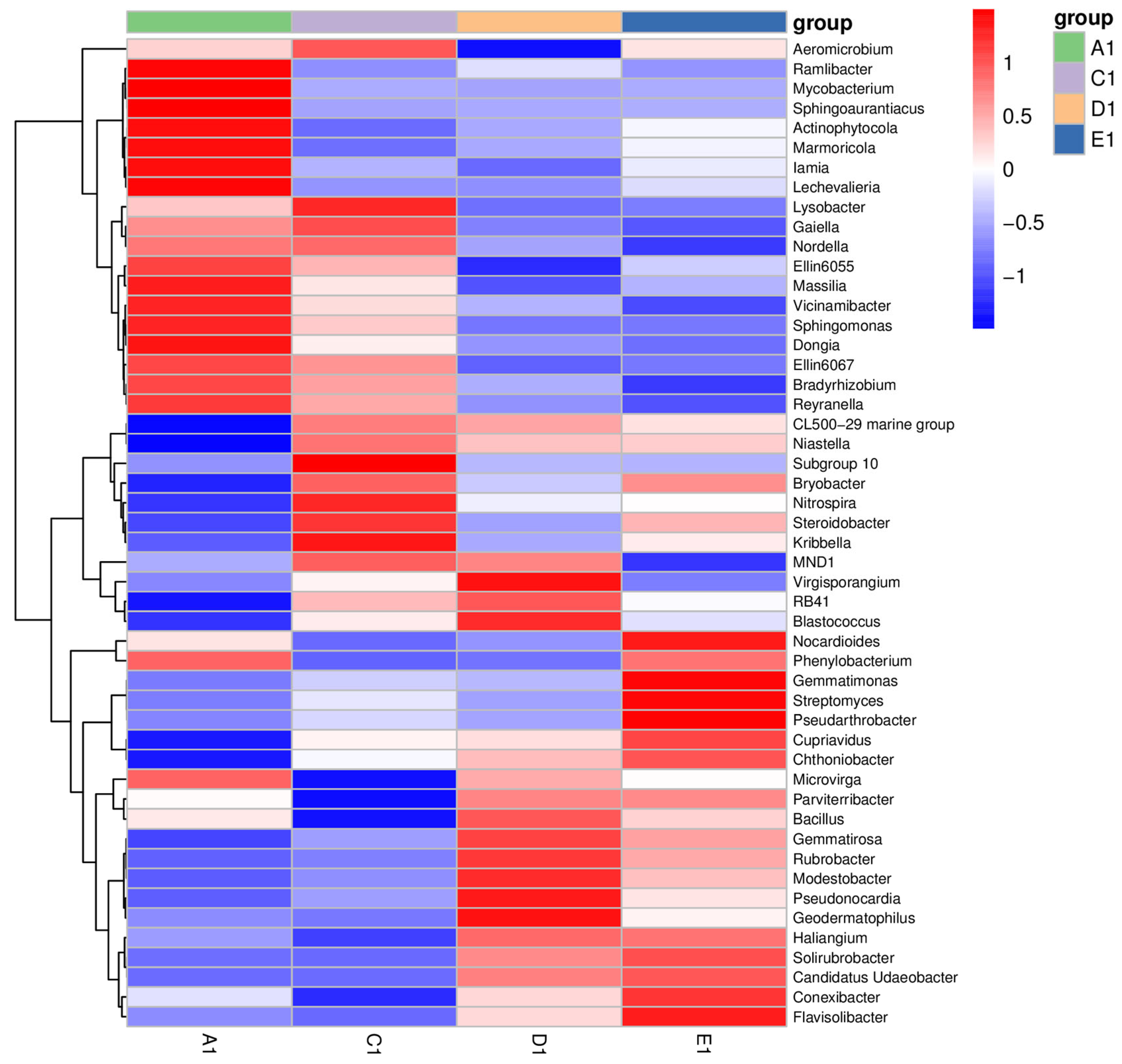
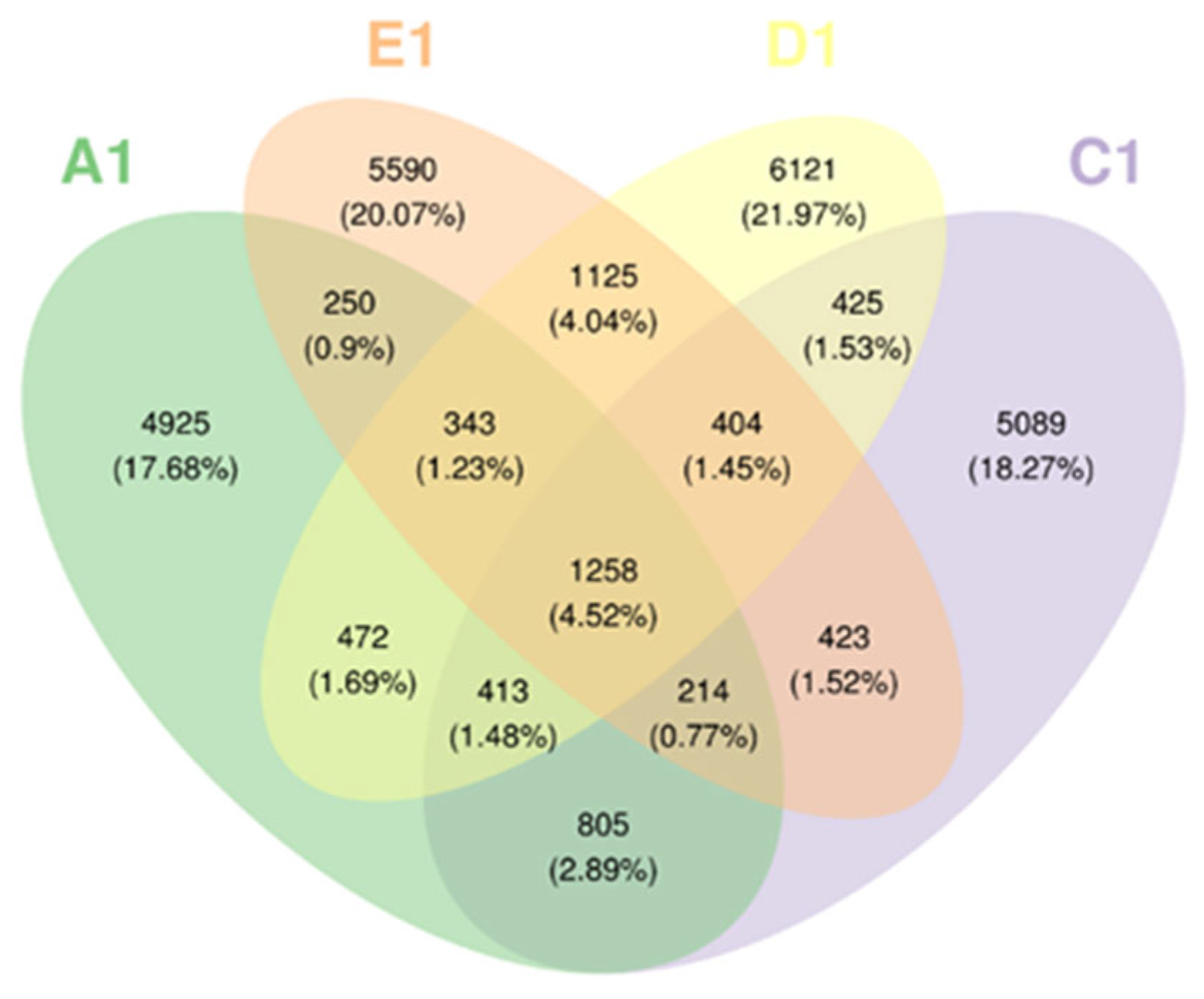
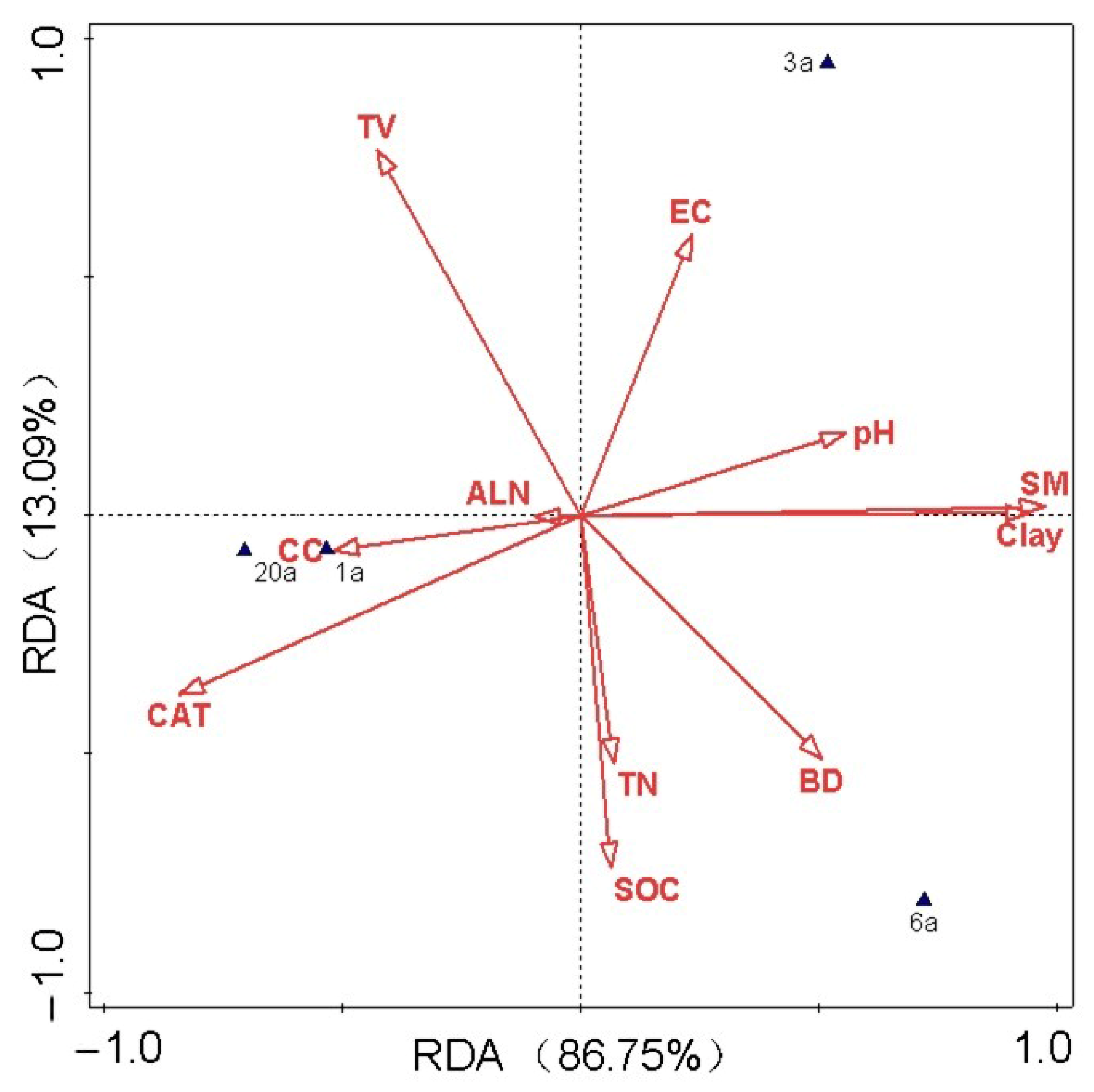
| Sample Plots | Elevation/m | Slopes Position | Abandoned Years (a) | Land Preparation Method |
|---|---|---|---|---|
| Jujube forest | 880 | Downslope segment | 1 | None |
| Jujube forest | 867 | Downslope segment | 3 | None |
| Jujube forest | 836 | Downslope segment | 6 | None |
| Jujube forest | 880 | Downslope segment | 20 | None |
| Group Distribution | Relative Abundance of Bacteria | |||
|---|---|---|---|---|
| 1 a | 3 a | 6 a | 20 a | |
| Alphaproteobacteria | 0.10 | 0.16 | 0.14 | 0.10 |
| Gammaproteobacteria | 0.10 | 0.15 | 0.13 | 0.09 |
| Phylum Actinomycetota | 0.12 | 0.12 | 0.10 | 0.12 |
| Thermoleophilia | 0.13 | 0.08 | 0.09 | 0.14 |
| Acidimicrobiia | 0.07 | 0.08 | 0.05 | 0.07 |
| Subgroup 6 | 0.05 | 0.06 | 0.08 | 0.05 |
| Gemmatimonadetes | 0.06 | 0.05 | 0.07 | 0.06 |
| Deltaproteobacteria | 0.05 | 0.03 | 0.04 | 0.05 |
| Blastocatellia | 0.04 | 0.03 | 0.04 | 0.03 |
| Bacteroidia | 0.02 | 0.03 | 0.03 | 0.03 |
| Miscellaneous | 0.26 | 0.21 | 0.23 | 0.26 |
| Total | 1.00 | 1.00 | 1.00 | 1.00 |
| Sample Area | Shannon Index | Simpson Index | Chao 1 Index | Observed Species Index | Goods Coverage Index |
|---|---|---|---|---|---|
| 1 a | 12.06 | 0.9993 | 11,888.50 | 10,838.7 | 0.9815 |
| 3 a | 11.54 | 0.9990 | 9296.42 | 8828.5 | 0.9884 |
| 6 a | 11.69 | 0.9990 | 9777.03 | 9199.2 | 0.9874 |
| 20 a | 11.96 | 0.9992 | 9867.50 | 9711.7 | 0.9925 |
| Gate Level Classification | Actinob Acteria | Proteoba Cteria | Acidobac Teria | Gemmatimo Nadetes | Chloro Flexi | Bacte Roidetes | Plancto Mycetes | Verruco Microbia | Rokub Acteria | Patesci Bacteria |
|---|---|---|---|---|---|---|---|---|---|---|
| Clay | −0.984 * | 0.713 | 0.946 | −0.065 | 0.388 | 0.784 | 0.738 | −0.601 | 0.553 | 0.087 |
| Silt | −0.971 * | 0.746 | 0.926 | −0.108 | 0.21 | 0.533 | 0.768 | −0.77 | 0.752 | 0.196 |
| Sand | 0.981 * | −0.746 | −0.937 | 0.101 | −0.242 | −0.58 | −0.77 | 0.748 | −0.725 | −0.179 |
| SM | −0.958 * | 0.908 | 0.789 | −0.398 | −0.049 | 0.45 | 0.922 | −0.898 | 0.869 | 0.471 |
| BD | −0.849 | 0.424 | 0.941 | 0.251 | 0.694 | 0.91 | 0.455 | −0.268 | 0.219 | −0.263 |
| SMC | 0.061 | −0.164 | −0.04 | 0.127 | 0.48 | 0.66 | −0.16 | 0.558 | −0.639 | −0.325 |
| CC | 0.944 | −0.843 | −0.823 | 0.295 | 0.006 | −0.394 | −0.859 | 0.89 | −0.875 | −0.394 |
| TV | 0.662 | −0.106 | −0.899 | −0.578 | −0.868 | −0.794 | −0.142 | 0.039 | −0.017 | 0.564 |
| EC | −0.021 | 0.36 | −0.169 | −0.513 | −0.795 | −0.719 | 0.343 | −0.667 | 0.726 | 0.67 |
| pH | −0.525 | 0.604 | 0.404 | −0.341 | −0.417 | −0.268 | 0.606 | −0.88 | 0.923 | 0.52 |
| SOC | −0.524 | −0.07 | 0.832 | 0.728 | 0.875 | 0.612 | −0.033 | 0.039 | −0.034 | −0.679 |
| AP | 0.091 | −0.252 | −0.02 | 0.242 | 0.564 | 0.66 | −0.246 | 0.625 | −0.7 | −0.434 |
| TN | −0.234 | −0.113 | 0.373 | 0.395 | 0.794 | 0.875 | −0.093 | 0.448 | −0.52 | −0.544 |
| ALN | 0.087 | −0.093 | −0.13 | −0.023 | 0.335 | 0.596 | −0.095 | 0.499 | −0.585 | −0.182 |
| AK | 0.382 | −0.64 | −0.162 | 0.57 | 0.668 | 0.418 | −0.631 | 0.888 | −0.925 | −0.727 |
| CAT | 0.732 | −0.941 | −0.395 | 0.754 | 0.276 | −0.494 | −0.938 | 0.716 | −0.642 | −0.708 |
| SUC | 0.392 | −0.478 | −0.302 | 0.277 | 0.445 | 0.4 | −0.478 | 0.8 | −0.857 | −0.468 |
| URE | 0.076 | −0.23 | −0.014 | 0.221 | 0.554 | 0.668 | −0.223 | 0.608 | −0.684 | −0.414 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ai, N.; Zou, M.; Yu, X.; Gao, J. Diversity of the Soil Bacterial Community of Abandoned Jujube Land in the Loess Area of Northern Shaanxi in Different Years. Diversity 2025, 17, 462. https://doi.org/10.3390/d17070462
Ai N, Zou M, Yu X, Gao J. Diversity of the Soil Bacterial Community of Abandoned Jujube Land in the Loess Area of Northern Shaanxi in Different Years. Diversity. 2025; 17(7):462. https://doi.org/10.3390/d17070462
Chicago/Turabian StyleAi, Ning, Menghuan Zou, Xuejiao Yu, and Jie Gao. 2025. "Diversity of the Soil Bacterial Community of Abandoned Jujube Land in the Loess Area of Northern Shaanxi in Different Years" Diversity 17, no. 7: 462. https://doi.org/10.3390/d17070462
APA StyleAi, N., Zou, M., Yu, X., & Gao, J. (2025). Diversity of the Soil Bacterial Community of Abandoned Jujube Land in the Loess Area of Northern Shaanxi in Different Years. Diversity, 17(7), 462. https://doi.org/10.3390/d17070462






