Ancient Lineages of the Western and Central Palearctic: Mapping Indicates High Endemism in Mediterranean and Arid Regions
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Lineage Coverage and Ages
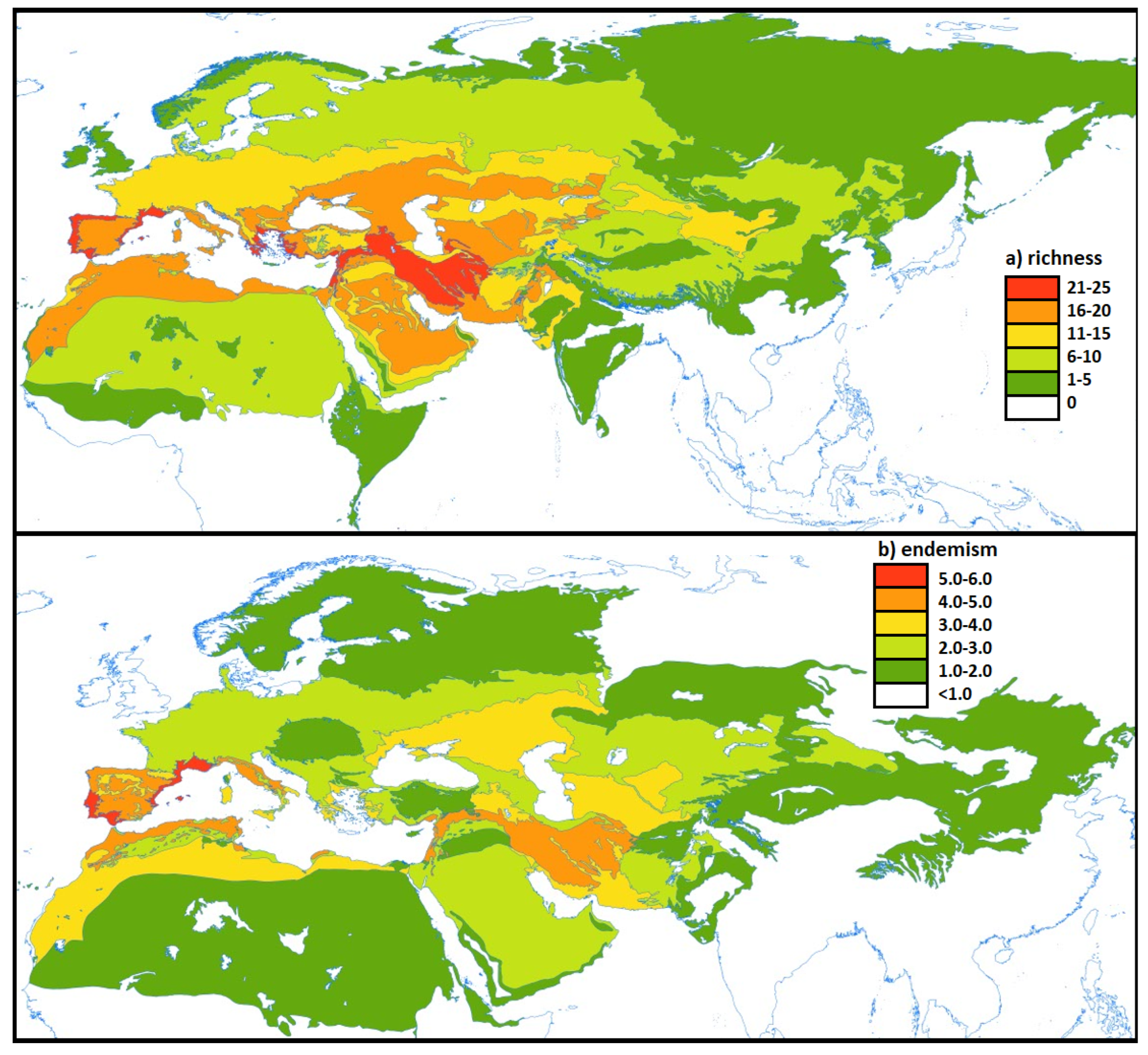
3.2. Lineage Richness
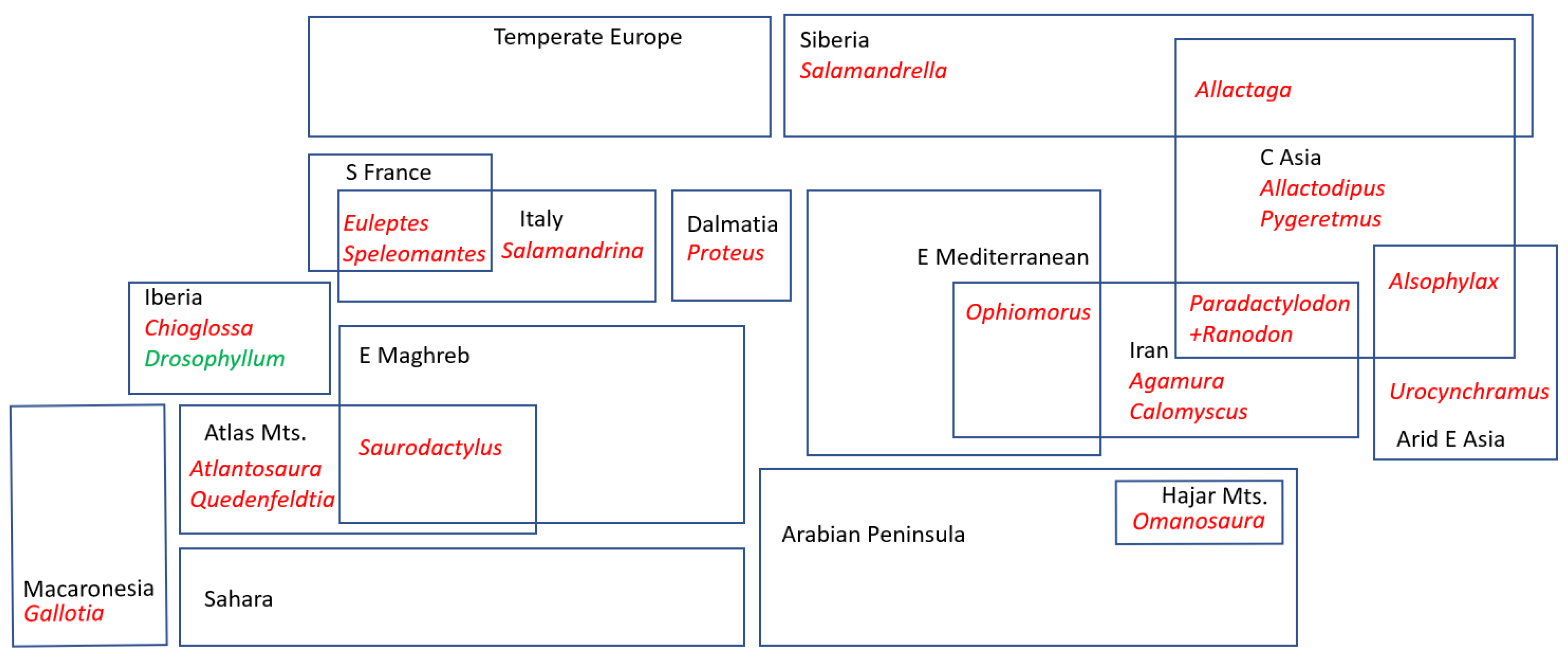
3.3. Lineage Endemism
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wallace, A.R. The Geographical Distribution of Animals; Harper & Brothers: New York, NY, USA, 1876; Volume 1, pp. 1–501. [Google Scholar]
- Kreft, H.; Jetz, W. A framework for delineating biogeographical regions based on species distributions. J. Biogeogr. 2010, 37, 2029–2053. [Google Scholar] [CrossRef]
- Procheş, Ş.; Ramdhani, S. The world’s zoogeographical regions confirmed by cross-taxon analyses. BioScience 2012, 62, 260–270. [Google Scholar] [CrossRef]
- Holt, B.G.; Lessard, J.-P.; Borregaard, M.K.; Fritz, S.A.; Araújo, M.B.; Dimitrov, D.; Fabre, P.-H.; Graham, C.H.; Graves, G.R.; Jønsson, K.A.; et al. An update of Wallace’s zoogeographic regions of the world. Science 2013, 339, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Procheş, Ş.; Ramdhani, S.; Perera, S.J.; Ali, J.R.; Gairola, S. Global hotspots in the present-day distribution of ancient animal and plant lineages. Sci. Rep. 2015, 5, 15457. [Google Scholar] [CrossRef] [PubMed]
- De Bruyn, M.; Stelbrink, B.; Morley, R.J.; Hall, R.; Carvalho, G.R.; Cannon, C.H.; Van Den Bergh, G.; Meijaard, E.; Metcalfe, I.; Boitani, L.; et al. Borneo and Indochina are major evolutionary hotspots for Southeast Asian biodiversity. Syst. Biol. 2014, 63, 879–901. [Google Scholar] [CrossRef] [PubMed]
- Barbolini, N.; Woutersen, A.; Dupont-Nivet, G.; Silvestro, D.; Tardif, D.; Coster, P.; Meijer, N.; Chang, C.; Zhang, H.-X.; Licht, A.; et al. Cenozoic evolution of the steppe-desert biome in Central Asia. Sci. Adv. 2020, 6, eabb8227. [Google Scholar] [CrossRef]
- Morrone, J.J.; Ebach, M.C. Toward a terrestrial biogeographical regionalisation of the world: Historical notes, characterisation and area nomenclature. Aust. Syst. Bot. 2022, 35, 187–224. [Google Scholar] [CrossRef]
- Roselaar, C.S.; Sluys, R.; Aliabadian, M.; Mekenkamp, P.G. Geographic patterns in the distribution of Palearctic songbirds. J. Ornithol. 2007, 148, 271–280. [Google Scholar] [CrossRef]
- Wan, T.; Gong, Y.; Liu, Z.; Zhou, Y.; Dai, C.; Wang, Q. Evolution of complex genome architecture in gymnosperms. GigaScience 2022, 11, giac078. [Google Scholar] [CrossRef]
- Ficetola, G.F.; Falaschi, M.; Bonardi, A.; Padoa-Schioppa, E.; Sindaco, R. Biogeographical structure and endemism pattern in reptiles of the Western Palearctic. Prog. Phys. Geogr. 2018, 42, 220–236. [Google Scholar] [CrossRef]
- Rosati, M.; Cantonati, M.; Primicerio, R.; Rossetti, G. Biogeography and relevant ecological drivers in spring habitats: A review on ostracods of the Western Palearctic. Int. Rev. Hydrobiol. 2014, 99, 409–424. [Google Scholar] [CrossRef]
- Padayachee, A.L.; Procheş, Ş. Patterns in the diversity and endemism of extant Eocene age lineages across southern Africa. Biol. J. Linn. Soc. 2016, 117, 482–491. [Google Scholar] [CrossRef]
- Rosindell, J.; Harmon, L.J. OneZoom: A fractal explorer for the tree of life. PLoS Biol. 2012, 10, e1001406. [Google Scholar]
- Roquet, C.; Lavergne, S.; Thuiller, W. One tree to link them all: A phylogenetic dataset for the European tetrapoda. PLoS Curr. Tree Life 2014, 6. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Porta, J.; Irisarri, I.; Kirchner, M.; Rodríguez, A.; Kirchhof, S.; Brown, J.L.; MacLeod, A.; Turner, A.P.; Ahmadzadeh, F.; Albaladejo, G.; et al. Environmental temperatures shape thermal physiology as well as diversification and genome-wide substitution rates in lizards. Nat. Comm. 2019, 10, 4077. [Google Scholar] [CrossRef] [PubMed]
- Olson, D.M.; Dinerstein, E.; Wikramanayake, E.D.; Burgess, N.D.; Powell, G.V.; Underwood, E.C.; D’amico, J.A.; Itoua, I.; Strand, H.E.; Morrison, J.C.; et al. Terrestrial ecoregions of the world: A new map of life on Earth. BioScience 2001, 51, 933–938. [Google Scholar] [CrossRef]
- World Wide Fund for Nature (WWF). WildFinder. Available online: www.worldwildlife.org/science/wildfinder (accessed on 8 December 2011).
- Angiosperm Phylogeny Website (APWeb). Available online: http://www.mobot.org/MOBOT/research/APweb (accessed on 18 June 2024).
- iNaturalist. Available online: https://www.inaturalist.org/observations (accessed on 10 July 2024).
- Global Biodiversity Information Facility (GBIF). Available online: https://www.gbif.org/ (accessed on 19 August 2024).
- Perera, S.J.; Herbert, D.G.; Procheş, Ş.; Ramdhani, S. Land snail biogeography and endemism in south-eastern Africa: Implications for the Maputaland-Pondoland-Albany biodiversity hotspot. PLoS ONE 2021, 16, e0248040. [Google Scholar] [CrossRef]
- Hurdu, B.-I.; Escalante, T.; Pușcaș, M.; Novikoff, A.; Bartha, L.; Zimmerman, N.E. Exploring the different facets of plant endemism in the South-Eastern Carpathians: A manifold approach for the determination of biotic elements, centres and areas of endemism. Biol. J. Linn. Soc. 2016, 119, 649–672. [Google Scholar] [CrossRef]
- Environmental Systems Research Institute (ERSI). ArcGIS; Pro 3.4; Environmental Systems Research Institute: Redlands, CA, USA, 2020. [Google Scholar]
- Rancilhac, L.; Irisarri, I.; Angelini, C.; Arntzen, J.W.; Babik, W.; Bossuyt, F.; Künzel, S.; Lüddecke, T.; Pasmans, F.; Sanchez, E.; et al. Phylotranscriptomic evidence for pervasive ancient hybridization among Old World salamanders. Mol. Phylogenet. Evol. 2021, 155, 106967. [Google Scholar] [CrossRef]
- Fritz, U.; Ayaz, D.; Hundsdörfer, A.K.; Kotenko, T.; Guicking, D.; Wink, M.; Tok, C.V.; Çiçek, K.; Buschbom, J. Mitochondrial diversity of European pond turtles (Emys orbicularis) in Anatolia and the Ponto-Caspian Region: Multiple old refuges, hotspot of extant diversification and critically endangered endemics. Org. Divers. Evol. 2009, 9, 100–114. [Google Scholar] [CrossRef]
- Zuntini, A.R.; Carruthers, T.; Maurin, O.; Bailey, P.C.; Leempoel, K.; Brewer, G.E.; Epitawalage, N.; Françoso, E.; Gallego-Paramo, B.; McGinnie, C.; et al. Phylogenomics and the rise of the angiosperms. Nature 2024, 629, 843–850. [Google Scholar] [CrossRef]
- Leslie, A.B.; Beaulieu, J.M.; Rai, H.S.; Crane, P.R.; Donoghue, M.J.; Mathews, S. Hemisphere-scale differences in conifer evolutionary dynamics. Proc. Natl. Acad. Sci. USA 2012, 109, 16217–16221. [Google Scholar] [CrossRef]
- Pérez-Gutiérrez, M.A.; Romero-García, A.T.; Fernández, M.C.; Blanca, G.; Salinas-Bonillo, M.J.; Suárez-Santiago, V.N. Evolutionary history of fumitories (subfamily Fumarioideae, Papaveraceae): An old story shaped by the main geological and climatic events in the Northern Hemisphere. Mol. Phylogenet. Evol. 2015, 88, 75–92. [Google Scholar] [CrossRef] [PubMed]
- Beilstein, M.A.; Nagalingum, N.S.; Clements, M.D.; Manchester, S.R.; Mathews, S. Dated molecular phylogenies indicate a Miocene origin for Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2010, 107, 18724–18728. [Google Scholar] [CrossRef]
- Appelhans, M.S.; Keßler, P.J.; Smets, E.; Razafimandimbison, S.G.; Janssens, S.B. Age and historical biogeography of the pantropically distributed Spathelioideae (Rutaceae, Sapindales). J. Biogeogr. 2012, 39, 1235–1250. [Google Scholar] [CrossRef]
- Hertweck, K.L.; Kinney, M.S.; Stuart, S.A.; Maurin, O.; Mathews, S.; Chase, M.W.; Gandolfo, M.A.; Pires, J.C. Phylogenetics, divergence times and diversification from three genomic partitions in monocots. Bot. J. Linn. Soc. 2015, 178, 375–393. [Google Scholar] [CrossRef]
- Valente, L.M.; Vargas, P. Contrasting evolutionary hypotheses between two mediterranean-climate floristic hotspots: The Cape of southern Africa and the Mediterranean Basin. J. Biogeogr. 2013, 40, 2032–2046. [Google Scholar] [CrossRef]
- Dufresnes, C. Patterns of amphibian diversity in the Western Palearctic. Herpetol. Bull. 2018, 145, 28–30. [Google Scholar]
- Barrón, E.; Rivas-Carballo, R.; Postigo-Mijarra, J.M.; Alcalde-Olivares, C.; Vieira, M.; Castro, L.; Pais, J.; Valle-Hernández, M. The Cenozoic vegetation of the Iberian Peninsula: A synthesis. Rev. Palaeobot. Palynol. 2010, 162, 382–402. [Google Scholar] [CrossRef]
- Irl, S.D.H.; Harter, D.E.; Steinbauer, M.J.; Gallego Puyol, D.; Fernández-Palacios, J.M.; Jentsch, A.; Beierkuhnlein, C. Climate vs. topography—Spatial patterns of plant species diversity and endemism on a high-elevation island. J. Ecol. 2015, 103, 1621–1633. [Google Scholar] [CrossRef]
- Badgley, C.; Smiley, T.M.; Terry, R.; Davis, E.B.; DeSantis, L.R.; Fox, D.L.; Hopkins, S.S.; Jezkova, T.; Matocq, M.D.; Matzke, N.; et al. Biodiversity and topographic complexity: Modern and geohistorical perspectives. Trends Ecol. Evol. 2017, 32, 211–226. [Google Scholar] [CrossRef] [PubMed]
- Kessler, M. The elevational gradient of Andean plant endemism: Varying influences of taxon-specific traits and topography at different taxonomic levels. J. Biogeogr. 2002, 29, 1159–1165. [Google Scholar] [CrossRef]
- Molina-Venegas, R.; Aparicio, A.; Lavergne, S.; Arroyo, J. Climatic and topographical correlates of plant palaeo- and neoendemism in a Mediterranean biodiversity hotspot. Ann. Bot. 2017, 119, 229–238. [Google Scholar] [CrossRef]
- Baquero, R.A.; Tellería, J.L. Species richness, rarity and endemicity of European mammals: A biogeographical approach. Biodivers. Conserv. 2001, 10, 29–44. [Google Scholar] [CrossRef]
- Médail, F.; Diadema, K. Glacial refugia influence plant diversity patterns in the Mediterranean Basin. J. Biogeogr. 2009, 36, 1333–1345. [Google Scholar] [CrossRef]
- Ricánková, V.P.; Robovský, J.; Riegert, J.; Zrzavý, J. Regional patterns of postglacial changes in the Palearctic mammalian diversity indicate retreat to Siberian steppes rather than extinction. Sci. Rep. 2015, 5, 12682. [Google Scholar] [CrossRef]
- Regnery, B.; Couvet, D.; Kubarek, L.; Julien, J.-F.; Kerbiriou, C. Tree microhabitats as indicators of bird and bat communities in Mediterranean forests. Ecol. Indic. 2013, 34, 221–230. [Google Scholar] [CrossRef]
- Martín, J.; Salvador, A. Microhabitat selection by the Iberian rock lizard Lacerta monticola: Effects on density and spatial distribution of individuals. Biol. Conserv. 1997, 79, 303–307. [Google Scholar] [CrossRef]
- Larrieu, L.; Paillet, Y.; Winter, S.; Bütler, R.; Kraus, D.; Krumm, F.; Lachat, T.; Michel, A.K.; Regnery, B.; Vandekerkhove, K. Tree related microhabitats in temperate and Mediterranean European forests: A hierarchical typology for inventory standardization. Ecol. Indic. 2018, 84, 194–207. [Google Scholar] [CrossRef]
- Gómez-Rodríguez, C.; Díaz-Paniagua, C.; Serrano, L.; Florencio, M.; Portheault, A. Mediterranean temporary ponds as amphibian breeding habitats: The importance of preserving pond networks. Aquatic Ecol. 2009, 43, 1179–1191. [Google Scholar] [CrossRef]
- Elverici, C.; Peterson, A.T.; Perktaş, U. Hotspots in transition: Mediterranean amphibian diversity under different climate scenarios. Biodivers. Conserv. 2025, 34, 589–604. [Google Scholar] [CrossRef]
- Ewers, R.M.; Didham, R.K. Confounding factors in the detection of species responses to habitat fragmentation. Biol. Rev. 2006, 81, 117–142. [Google Scholar] [CrossRef] [PubMed]
- Kuussaari, M.; Bommarco, R.; Heikkinen, R.K.; Helm, A.; Krauss, J.; Lindborg, R.; Öckinger, E.; Pärtel, M.; Pino, J.; Rodà, F.; et al. Extinction debt: A challenge for biodiversity conservation. Trends Ecol. Evol. 2009, 24, 564–571. [Google Scholar] [CrossRef]
- Guardiola, M.; Pino, J.; Rodà, F. Patch history and spatial scale modulate local plant extinction and extinction debt in habitat patches. Divers. Distrib. 2013, 19, 825–833. [Google Scholar] [CrossRef]
- Hooftman, D.A.; Edwards, B.; Bullock, J.M. Reductions in connectivity and habitat quality drive local extinctions in a plant diversity hotspot. Ecography 2016, 39, 583–592. [Google Scholar] [CrossRef]
- Esin, N.V.; Yanko-Hombach, V.V.; Esin, N.I. Evolutionary mechanisms of the Paratethys Sea and its separation into the Black Sea and Caspian Sea. Quat. Int. 2018, 465, 46–53. [Google Scholar] [CrossRef]
- Kvaček, Z.; Kováč, M.; Kovar-Eder, J.; Doláková, N.; Jechorek, H.; Parashiv, V.; Kováčová, M.; Sliva, L. Miocene evolution of landscape and vegetation in the Central Paratethys. Geol. Carpathica 2006, 57, 295–310. [Google Scholar]
- Jiménez-Moreno, G.; Pérez-Asensio, J.N.; Larrasoaña, J.C.; Aguirre, J.; Civis, J.; Rivas-Carballo, M.R.; Valle-Hernández, M.F.; González-Delgado, J.A. Vegetation, sea-level, and climate changes during the Messinian salinity crisis. GSA Bull. 2013, 125, 432–444. [Google Scholar] [CrossRef]
- Jiménez-Moreno, G.; Suc, J.-P.; Fauquette, S. Miocene to Pliocene vegetation reconstruction and climate estimates in the Iberian Peninsula from pollen data. Rev. Palaeobot. Palynol. 2010, 162, 403–415. [Google Scholar] [CrossRef]
- Noroozi, J.; Talebi, A.; Doostmohammadi, M.; Manafzadeh, S.; Asgarpour, Z.; Schneeweiss, G.M. Endemic diversity and distribution of the Iranian vascular flora across phytogeographical regions, biodiversity hotspots and areas of endemism. Sci. Rep. 2019, 9, 12991. [Google Scholar] [CrossRef]
- Jalilian, M.A.; Shayesteh, K.; Danehkar, A.; Salmanmahiny, A. A new ecosystem based land classification of Iran for conservation goals. Environ. Monit. Assess. 2020, 192, 182. [Google Scholar] [CrossRef] [PubMed]
- Ilanloo, S.S.; Ashrafi, S.; Shabani, A.A. Modeling habitat suitability of the red-backed shrike (Lanius collurio) in the Irano-Anatolian Biodiversity Hotspot. J. Zool. Res. 2021, 3. [Google Scholar] [CrossRef]
- Utzeri, V.J.; Cilli, E.; Fontani, F.; Zoboli, D.; Orsini, M.; Ribani, A.; Latorre, A.; Lissovsky, A.A.; Pillola, G.L.; Bovo, S.; et al. Ancient DNA re-opens the question of the phylogenetic position of the Sardinian pika Prolagus sardus (Wagner, 1829), an extinct lagomorph. Sci. Rep. 2023, 13, 13635. [Google Scholar] [CrossRef]
- Blondel, J. Origin, diversification, and biogeography of forest birds across temperate forest regions in the Northern Hemisphere. Front. Biogeogr. 2022, 14, e51998. [Google Scholar] [CrossRef]
- Covas, R.; Blondel, J. Biogeography and history of the Mediterranean bird fauna. Ibis 1998, 140, 395–407. [Google Scholar] [CrossRef]
- Poyarkov, N.A.; Kuzmin, S.L. Phylogeography of the Siberian newt Salamandrella keyserlingii by mitochondrial DNA sequence analysis. Russ. J. Genet. 2008, 44, 948–958. [Google Scholar] [CrossRef]
- Morrone, J.J. On the identification of areas of endemism. Syst. Biol. 1994, 43, 438–441. [Google Scholar] [CrossRef]
- Procheş, Ş.; Ramdhani, S. A global regionalisation based on the present-day distribution of broad plant lineages. Phytotaxa 2020, 442, 20–26. [Google Scholar] [CrossRef]
- Morrone, J.J. Biogeographical regionalisation of the world: A reappraisal. Aust. Syst. Bot. 2015, 28, 81–90. [Google Scholar] [CrossRef]
- Rundel, P.W.; Arroyo, M.T.; Cowling, R.M.; Keeley, J.E.; Lamont, B.B.; Vargas, P. Mediterranean biomes: Evolution of their vegetation, floras, and climate. Ann. Rev. Ecol. Evol. Syst. 2016, 47, 383–407. [Google Scholar] [CrossRef]
- Pullin, A.S.; Baldi, A.; Can, O.E.; Dieterich, M.; Kati, V.; Livoreil, B.; Lövei, G.; Mihok, B.; Nevin, O.; Selva, N.; et al. Conservation focus on Europe: Major conservation policy issues that need to be informed by conservation science. Conserv. Biol. 2009, 23, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.T.; Jolley-Rogers, G.; Mishler, B.D.; Thornhill, A.H. Phylogenetic diversity is a better measure of biodiversity than taxon counting. J. Syst. Evol. 2018, 56, 663–667. [Google Scholar] [CrossRef]
- Qian, J.; Zhuang, H.; Yang, W.; Chen, Y.; Chen, S.; Qu, Y.; Zhang, Y.; Yang, Y.; Wang, Y. Selecting flagship species to solve a biodiversity conservation conundrum. Plant Divers. 2020, 42, 488–491. [Google Scholar] [CrossRef] [PubMed]
- Isaac, N.J.; Turvey, S.T.; Collen, B.; Waterman, C.; Baillie, J.E. Mammals on the EDGE: Conservation priorities based on threat and phylogeny. PLoS ONE 2007, 2, e296. [Google Scholar] [CrossRef]
- Garamvölgyi, Á.; Hufnagel, L. Impacts of climate change on vegetation distribution No. 1: Climate change induced vegetation shifts in the Palearctic Region. Appl. Ecol. Environ. Res. 2013, 11, 79–122. [Google Scholar] [CrossRef]
| Genera Included | Family | Order | Age (Era/Period) | References |
|---|---|---|---|---|
| Salamandrella | Hynobiidae | Urodela | Cenozoic | [14,15] |
| Paradactylodon + Ranodon | Hynobiidae | Urodela | Cenozoic | [14,15] |
| Proteus | Proteidae | Urodela | Cretaceous | [14,15] |
| Chioglossa | Salamandridae | Urodela | Cenozoic | [14,15,25] |
| Salamandra + Lyciasalamandra | Salamandridae | Urodela | Cenozoic | [14,15,25] |
| Salamandrina | Salamandridae | Urodela | Cenozoic | [14,15,25] |
| Calotriton + Triturus + Lissotriton + Ommatotriton + Ichthyosaura + Neurergus | Salamandridae | Urodela | Cenozoic | [14,15,25] |
| Speleomantes | Pletodontidae | Urodela | Cenozoic | [14,15] |
| Alytes | Alytidae | Anura | Cretaceous | [14,15] |
| Discoglossus | Alytidae | Anura | Cretaceous | [14,15] |
| Pelobates | Pelobatidae | Anura | Cretaceous | [14,15] |
| Pelodytes | Pelodytidae | Anura | Jurassic | [14,15] |
| Desmana + Galemys | Talpidae | Eulipotyphla | Cenozoic | [14,15,26] |
| Muscardinus | Gliridae | Rodentia | Cenozoic | [14,15,26] |
| Selevinia + Myomimus | Gliridae | Rodentia | Cenozoic | [14,15,26] |
| Myoxus | Gliridae | Rodentia | Cenozoic | [14,15,26] |
| Allactaga | Dipodidae | Rodentia | Cenozoic | [14,15,26] |
| Allactodipus | Dipodidae | Rodentia | Cenozoic | [14,15,26] |
| Pygeretmus | Dipodidae | Rodentia | Cenozoic | [14,15,26] |
| Calomyscus | Calomyscidae | Rodentia | Cenozoic | [14,15,26] |
| Myospalax + Eospalax + Spalax + Nannospalax | Spalacidae | Rodentia | Cenozoic | [14,15,26] |
| Urocynchramus | Urocynchramidae | Passeriformes | Cenozoic | [14,15] |
| Natrix | Colubridae | Squamata | Cenozoic | [15] |
| Blanus | Blanidae | Squamata | Cenozoic | [15] |
| Euleptes | Sphaerodactylidae | Squamata | Cenozoic | [15] |
| Quedenfeldtia | Sphaerodactylidae | Squamata | Cenozoic | [15] |
| Saurodactylus | Sphaerodactylidae | Squamata | Cenozoic | [15] |
| Bunopus + Crossobamon | Gekkonidae | Squamata | Cenozoic | [15] |
| Tropiocolotes | Gekkonidae | Squamata | Cenozoic | [15] |
| Cyrtopodion + Carinatogecko | Gekkonidae | Squamata | Cenozoic | [15] |
| Agamura | Gekkonidae | Squamata | Cenozoic | [15] |
| Stenodactylus | Gekkonidae | Squamata | Cenozoic | [15] |
| Alsophylax | Gekkonidae | Squamata | Cenozoic | [15] |
| Ophiomorus | Scincidae | Squamata | Cretaceous | [15] |
| Ablepharus | Scincidae | Squamata | Cenozoic | [15] |
| Chalcides | Scincidae | Squamata | Cenozoic | [15] |
| Uromastyx + Saara | Agamidae | Squamata | Cenozoic | [15] |
| Phrynocephalus | Agamidae | Squamata | Cenozoic | [15] |
| Trapelus + Bufoniceps | Agamidae | Squamata | Cenozoic | [15] |
| Laudakia | Agamidae | Squamata | Cenozoic | [15] |
| Podarcis | Lacertidae | Squamata | Cenozoic | [15,16] |
| Zootoca + Archaeolacerta + Teira + Scelarcis | Lacertidae | Squamata | Cenozoic | [15,16] |
| Eremias | Lacertidae | Squamata | Cenozoic | [15,16] |
| Acanthodactylus | Lacertidae | Squamata | Cenozoic | [15,16] |
| Ophisops | Lacertidae | Squamata | Cenozoic | [15,16] |
| Mesalina | Lacertidae | Squamata | Cenozoic | [15,16] |
| Gallotia | Lacertidae | Squamata | Cenozoic | [15,16] |
| Psammodromus | Lacertidae | Squamata | Cenozoic | [15,16] |
| Atlantolacerta | Lacertidae | Squamata | Cenozoic | [15,16] |
| Omanosaura | Lacertidae | Squamata | Cenozoic | [15,16] |
| Cedrus | Pinaceae | Pinales | Jurassic–Cretaceous | [14,27,28] |
| Hypecoum | Papaveraceae | Ranunculales | Cretaceous | [14,27,29] |
| Leontice + Gymnospermium | Berberidaceae | Ranunculales | Cenozoic | [14,27] |
| Helleborus | Ranunculaceae | Ranunculales | Cenozoic | [14,27] |
| Nigella | Ranunculaceae | Ranunculales | Cenozoic | [14,27] |
| Aethionema + Moriera | Brassicaceae | Brassicales | Cenozoic–Cretaceous | [14,27,30] |
| Drosophyllum | Drosophyllaceae | Caryophyllales | Cenozoic | [14,27] |
| Cynomorium | Cynomoriaceae | Saxifragales | Cenozoic–Cretaceous | [19,27] |
| Biebersteinia | Biebersteiniaceae | Sapindales | Cretaceous | [14,27] |
| Tetradiclis | Nitrariaceae | Sapindales | Cenozoic | [14,27] |
| Cneorum | Rutaceae | Sapindales | Cenozoic | [14,27,31] |
| Ixolirion | Ixoliriaceae | Asparagales | Cenozoic–Cretaceous | [14,27,32] |
| Aphyllanthes | Asparagaceae | Asparagales | Cenozoic–Cretaceous | [14,27,32] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Procheş, Ş.; Ramdhani, S.; Kuppusamy, T. Ancient Lineages of the Western and Central Palearctic: Mapping Indicates High Endemism in Mediterranean and Arid Regions. Diversity 2025, 17, 444. https://doi.org/10.3390/d17070444
Procheş Ş, Ramdhani S, Kuppusamy T. Ancient Lineages of the Western and Central Palearctic: Mapping Indicates High Endemism in Mediterranean and Arid Regions. Diversity. 2025; 17(7):444. https://doi.org/10.3390/d17070444
Chicago/Turabian StyleProcheş, Şerban, Syd Ramdhani, and Tamilarasan Kuppusamy. 2025. "Ancient Lineages of the Western and Central Palearctic: Mapping Indicates High Endemism in Mediterranean and Arid Regions" Diversity 17, no. 7: 444. https://doi.org/10.3390/d17070444
APA StyleProcheş, Ş., Ramdhani, S., & Kuppusamy, T. (2025). Ancient Lineages of the Western and Central Palearctic: Mapping Indicates High Endemism in Mediterranean and Arid Regions. Diversity, 17(7), 444. https://doi.org/10.3390/d17070444






