Study of the Geographical Distribution, Ecological–Biological Characteristics, and Economic Value of Rosa acicularis Lindl., Rosa laxa Retz., and Rosa spinosissima L. (Rosaceae) in Kazakhstan’s Part of the Altai Mountains
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Rio Declaration on Environment and Development. Available online: https://www.un.org/ru/documents/decl_conv/declarations/riodecl.shtml (accessed on 19 May 2025).
- International Treaty on Plant Genetic Resources for Food and Agriculture. Available online: https://www.fao.org/plant-treaty/en/ (accessed on 19 May 2025).
- Guo, Y.; Zhao, W.; He, Y.; Li, A.; Feng, Q.; Tian, L. Research on the pharmacognostic characteristics, physicochemical properties and in vitro antioxidant potency of Rosa laxa Retz. flos. Microsc. Res. Tech. 2024, 87, 2487–2503. [Google Scholar] [CrossRef] [PubMed]
- Bingjing, Z.; Li, D. Study on plant landscape of Beijing urban linear parks based on bird diversity improvement. Landsc. Archit. 2019, 26, 53–57. [Google Scholar]
- Tumbas, V.T.; Canadanovic-Brunet, J.M.; Cetojevic-Simin, D.D.; Cetkovic, G.S.; Ethilas, S.M.; Gille, L. Effect of rosehip (Rosa canina L.) phytochemicals on stable free radicals and human cancer cells. J. Sci. Food Agric. 2012, 92, 1273–1281. [Google Scholar] [CrossRef]
- Nagatomo, A.; Nishida, N.; Matsuura, Y.; Shibata, N. Rosehip extract inhibits lipid accumulation in white adipose tissue by suppressing the expression of peroxisome proliferator-activated receptor gamma. Prev. Nutr. Food Sci. 2013, 18, 85–91. [Google Scholar] [CrossRef]
- Andersson, S.C.; Olsson, M.E.; Gustavsson, K.E.; Johansson, E.; Rumpunen, K. Tocopherols in rose hips (Rosa spp.) during ripening. J. Sci. Food Agric. 2012, 92, 2116–2121. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, M.; Luo, L.; Pan, N.; Zhang, Q.; Yu, C. Metabolic profiles, bioactive compounds and antioxidant activity of rosehips from Xinjiang, China. LWT 2023, 174, 114451. [Google Scholar] [CrossRef]
- Rovná, K.; Ivanišová, E.; Žiarovská, J.; Ferus, P.; Terentjeva, M.; Kowalczewski, P.L.; Kačániová, M. Characterization of Rosa canina fruits collected in urban areas of Slovakia. Genome Size, iPBS profiles and antioxidant and antimicrobial activities. Molecules 2020, 25, 1888. [Google Scholar] [CrossRef]
- Muranets, A.; Yessimseitova, A.; Dyussembekova, D.; Nurtaza, A.; Kalybayev, K.; Kozhanov, K.; Kakimzhanova, A. Study of biodiversity of wild plants of the genus Rosa L. in Kazakhstan and their molecular genetic identification. Bull. L.N. Gumilyov Eurasian Natl. Univ. Biosci. Ser. 2022, 139, 44–60. (In Russian) [Google Scholar] [CrossRef]
- Guney, M. Determination of fatty acid profile and antioxidant activity of rosehip seeds from Turkey. Int. J. Agric. Environ. Food Sci. 2020, 4, 114–118. [Google Scholar] [CrossRef]
- Baitenov, M.B. Flora of Kazakhstan; Pavlov, N.V., Ed.; Publishing House of the Academy of Sciences of the Kazakh SSR: Alma-Ata, Kazakhstan, 1961; Volume 4, pp. 485–503. (In Russian) [Google Scholar]
- Koblanova, S.; Mukhtubayeva, S.; Kakimzhanova, A.; Orazov, A.; Dyussembekova, D.; Abileva, G. Diversity of Birch and Alder Forests in the Kostanay Region of Kazakhstan. Forests 2024, 15, 1680. [Google Scholar] [CrossRef]
- Dzhangaliev, A.D.; Salova, T.N.; Turekhanova, P.M. The Wild Fruit and Nut Plants of Kazakhstan; Janick, J., Ed.; Springer Science + Business Media: New York, NY, USA, 2003; Volume 29, pp. 305–372. [Google Scholar]
- Sitpayeva, G.T.; Kudabayeva, G.M.; Dimeyeva, L.A.; Gemejiyeva, N.G.; Vesselova, P.V. Crop wild relatives of Kazakhstani Tien Shan: Flora, vegetation, resources. Plant Divers. 2020, 42, 19–32. [Google Scholar] [CrossRef]
- Sumbembayev, A.; Tergenbaeva, Z.; Kudabayeva, G.; Tashmetova, R.; Genievskaya, Y.; Szlachetko, D. Assessment of state of Dactylorhiza fuchsii (Orchidaceae) populations from the Altai mountains of Kazakhstan. Biodiversitas 2022, 23, 4385–4399. [Google Scholar] [CrossRef]
- Kubentayev, S.A.; Khapilina, O.N.; Ishmuratova, M.Y.; Sarkytbayeva, A.K.; Turzhanova, A.S.; Imanbayeva, A.A.; Zhumagul, M.Z. Current state of natural populations of Paeonia anomala (Paeoniaceae) in East Kazakhstan. Diversity 2023, 15, 1127. [Google Scholar] [CrossRef]
- Sabitov, A.; Gaweł-Bęben, K.; Sakipova, Z.; Strzępek-Gomółka, M.; Hoian, U.; Satbayeva, E.; Głowniak, K.; Ludwiczuk, A. Rosa platyacantha Schrenk from Kazakhstan—Natural Source of Bioactive Compounds with Cosmetic Significance. Molecules 2021, 26, 2578. [Google Scholar] [CrossRef] [PubMed]
- Orazov, A.; Yermagambetova, M.; Myrzagaliyeva, A.; Mukhitdinov, N.; Tustubayeva, S.; Turuspekov, Y.; Almerekova, S. Plant height variation and genetic diversity between Prunus ledebouriana (Schlecht.) Y.Y. Yao and Prunus tenella Batsch based on using SSR markers in East Kazakhstan. PeerJ 2024, 12, e16735. [Google Scholar] [CrossRef]
- Zhang, S.; Isermann, M.; Gan, W.; Breed, M. Invasive Rosa rugosa populations outperform native populations, but some populations have greater invasive potential than others. Sci. Rep. 2018, 8, 5735. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, X.; Zhang, L.; Zhao, Y. Effects of deproteinization on rheological properties of polysaccharides from Rosa acicularis ‘Lu He’ and Rosa acicularis Lindl fruits. J. Food Meas. Charact. 2021, 15, 2500–2515. [Google Scholar] [CrossRef]
- Razgonova, M.P.; Bazhenova, B.A.; Zabalueva, Y.Y.; Burkhanova, A.G.; Zakharenko, A.M.; Kupriyanov, A.N.; Golokhvast, K.S. Rosa davurica Pall., Rosa rugosa Thumb., and Rosa acicularis Lindl. originating from Far Eastern Russia: Screening of 146 chemical constituents in three species of the genus Rosa. Appl. Sci. 2022, 12, 9401. (In Russian) [Google Scholar] [CrossRef]
- Osada, A.; Horikawa, K.; Wakita, Y.; Nakamura, H.; Ukai, M.; Shimura, H.; Suzuki, T. Rosa davurica Pall., a useful Rosa species for functional rose hip production with high content of antioxidants and multiple antioxidant activities in hydrophilic extract. Sci. Hortic. 2022, 291, 110528. [Google Scholar] [CrossRef]
- Choi, J.W.; Choi, H.J.; Jeong, J.B. Rosa acicularis leaves exert anti-obesity activity through AMPK-dependent lipolysis and thermogenesis in mouse adipocytes, 3T3-L1 cells. Korean J. Plant Resour. 2024, 37, 247–255. [Google Scholar]
- Karychev, R.K.; Salnikov, Y.; Nurtazin, M.T.; Miller, D.D. Tree fruit growing in Kazakhstan. Chron. Horticult. 2005, 45, 21–23. [Google Scholar]
- Imanbayeva, A.; Duisenova, N.; Orazov, A.; Sagyndykova, M.; Belozerov, I.; Tuyakova, A. Study of the floristic, morphological, and genetic (atpF–atpH, ITS, matK, psbK–psbI, rbcL, and trnH–psbA) differences in Crataegus ambigua populations in Mangistau (Kazakhstan). Plants 2024, 13, 1591. [Google Scholar] [CrossRef] [PubMed]
- Vdovina, T.A.; Isakova, E.A.; Lagus, O.A.; Sumbembayev, A.A. Selection assessment of promising forms of natural Hippophae rhamnoides (Elaeagnaceae) populations and their offspring in the Kazakhstan Altai Mountains. Biodiversitas 2024, 25, 1809–1822. [Google Scholar] [CrossRef]
- Shcherbakov, A.V.; Mayorov, A.V. Field Study of Flora and Herbarization of Plants; Moscow State University Press: Moscow, Russia, 2006; p. 84. [Google Scholar]
- Herbarium Fund of Altai botanical Garden (ABG). [CrossRef]
- Rumyantsev, D.E.; Lipatkin, V.A.; Zagreeva, A.B. Fundamentals of Geobotany; NPO “Professional Science”: Nizhny Novgorod, Russia, 2023; p. 67. (In Russian) [Google Scholar]
- Plants of the World Online (POWO 2024). Available online: https://powo.science.kew.org/results?q=31 (accessed on 19 May 2025).
- Gemedzhieva, N.G. Alkaloid-Bearing Plants of Kazakhstan and Prospects for Their Use (on the Example of the Dzungar-Northern Tien Shan Province); Institute of Botany and Phytointroduction: Almaty, Kazakhstan, 2012; p. 312. [Google Scholar]
- Aimenova, Z.E.; Matchanov, A.D.; Esanov, R.S.; Sumbembayev, A.A.; Duissebayev, S.E.; Dzhumanov, S.D.; Smailov, B.M. Phytochemical profile of Eranthis longistipitata Regel from three study sites in the Kazakhstan part of the Western Tien Shan. Biodiversitas J. Biol. Divers. 2023, 24, 6031–6038. [Google Scholar] [CrossRef]
- Preston, F.W. The Commonness, and Rarity, of Species. Ecology 1948, 29, 254–283. [Google Scholar] [CrossRef]
- Morris, P. Methods of Environmental Assessment; University College London Press: London, UK, 1995; p. 236. [Google Scholar]
- MacAdam, D.L. Uniform color scales. J. Opt. Soc. Am. JOSA 1974, 64, 1691–1702. [Google Scholar] [CrossRef]
- Semenyutina, A.V.; Kostyukov, S.M.; Kashchenko, E.V. Methods for identifying adaptation mechanisms of tree species in connection with their introduction into arid regions. Adv. Mod. Nat. Sci. 2016, 2, 103–109. [Google Scholar]
- Vdovina, T.A.; Isakova, E.A.; Lagus, O.A. Physiological bases of adaptation processes of tree species in the Altai Botanical Garden. Probl. Bot. South. Sib. Mong. 2023, 22, 72–79. (In Russian) [Google Scholar] [CrossRef]
- Dimeyeva, L.; Sitpayeva, G.; Sultanova, B.; Ussen, K.; Islamgulova, A. High-Altitude Flora and Vegetation of Kazakhstan and Climate Change Impacts. In Climate Change Impacts on High-Altitude Ecosystems; Öztürk, M., Hakeem, K., Faridah-Hanum, I., Efe, R., Eds.; Springer: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Ouerghemmi, S.; Rhimi, A.; Achour, H.; Dhaouadi, K.; Khebour Allouche, F.; Chaar, H.; Sebei, H. Ecological distribution, phytochemistry and biological properties of Rosa species in Tunisia. In Agriculture Productivity in Tunisia Under Stressed Environment; Springer: Cham, Switzerland, 2021; pp. 73–136. [Google Scholar]
- Singh, K.; Sharma, Y.P.; Gairola, S. Distribution status and ethnomedicinal importance of genus Rosa L. (Rosaceae) in India. Ethnobot. Res. Appl. 2023, 25, 1–22. [Google Scholar]
- Arslan, E.S.; Akyol, A.; Örücü, Ö.K.; Sarıkaya, A.G. Distribution of rose hip (Rosa canina L.) under current and future climate conditions. Reg. Environ. Change 2020, 20, 107. [Google Scholar] [CrossRef]
- Tishkina, E.A.; Chermnykh, A.I. Ecological and phytocenotic characteristics of Rosa acicularis L. in Ekaterinburg forest park zone. RUDN J. Agron. Anim. Ind. 2019, 14, 49–56. (In Russian) [Google Scholar] [CrossRef]
- Ullah, F.; Gao, Y.; Sari, İ.; Jiao, R.-F.; Saqib, S.; Gao, X.-F. Macro-morphological and ecological variation in Rosa sericea complex. Agronomy 2022, 12, 1078. [Google Scholar] [CrossRef]
- Gitonga, V.W.; Koning-Boucoiran, C.F.; Verlinden, K.; Dolstra, O.; Visser, R.G.; Maliepaard, C.; Krens, F.A. Genetic variation, heritability and genotype by environment interaction of morphological traits in a tetraploid rose population. BMC Genet. 2014, 15, 146. [Google Scholar] [CrossRef] [PubMed]
- Koczka, N.; Stefanovits-Bányai, É.; Ombódi, A. Total polyphenol content and antioxidant capacity of rosehips of some Rosa species. Medicines 2018, 5, 84. [Google Scholar] [CrossRef]
- Szołtysik, M.; Kucharska, A.Z.; Sokół-Łętowska, A.; Dąbrowska, A.; Bobak, Ł.; Chrzanowska, J. The effect of Rosa spinosissima fruits extract on lactic acid bacteria growth and other yoghurt parameters. Foods 2020, 9, 1167. [Google Scholar] [CrossRef]
- Türkben, C.; Barat, E.; Çopur, Ö.U.; Durgut, E.; Himelrick, D.G. Evaluation of rose hips (Rosa spp.) selections. Int. J. Fruit Sci. 2005, 5, 113–121. [Google Scholar] [CrossRef]
- Guan, H.; Huang, B.; Yan, X.; Zhao, J.; Yang, S.; Wu, Q.; Bao, M.; Bendahmane, M.; Fu, X. Identification of distinct roses suitable for future breeding by phenotypic and genotypic evaluations of 192 rose germplasms. Hort. Adv. 2024, 2, 5. [Google Scholar] [CrossRef]
- Kunc, N.; Hudina, M.; Bavcon, J.; Vreš, B.; Luthar, Z.; Gostinčar, K.; Ravnjak, B. Characterization of the Slovene autochthonous rose hybrid Rosa pendulina × spinosissima (Rosa reversa Waldst. and Kit) using biochemical patterns of the plant blossoms. Plants 2023, 12, 505. [Google Scholar] [CrossRef]
- Fascella, G.; Mammano, M.M.; D’Angiolillo, F. Leaf methanolic extracts from four Sicilian rose species: Bioactive compounds content and antioxidant activity. Acta Hortic. 2019, 1232, 81–88. [Google Scholar] [CrossRef]
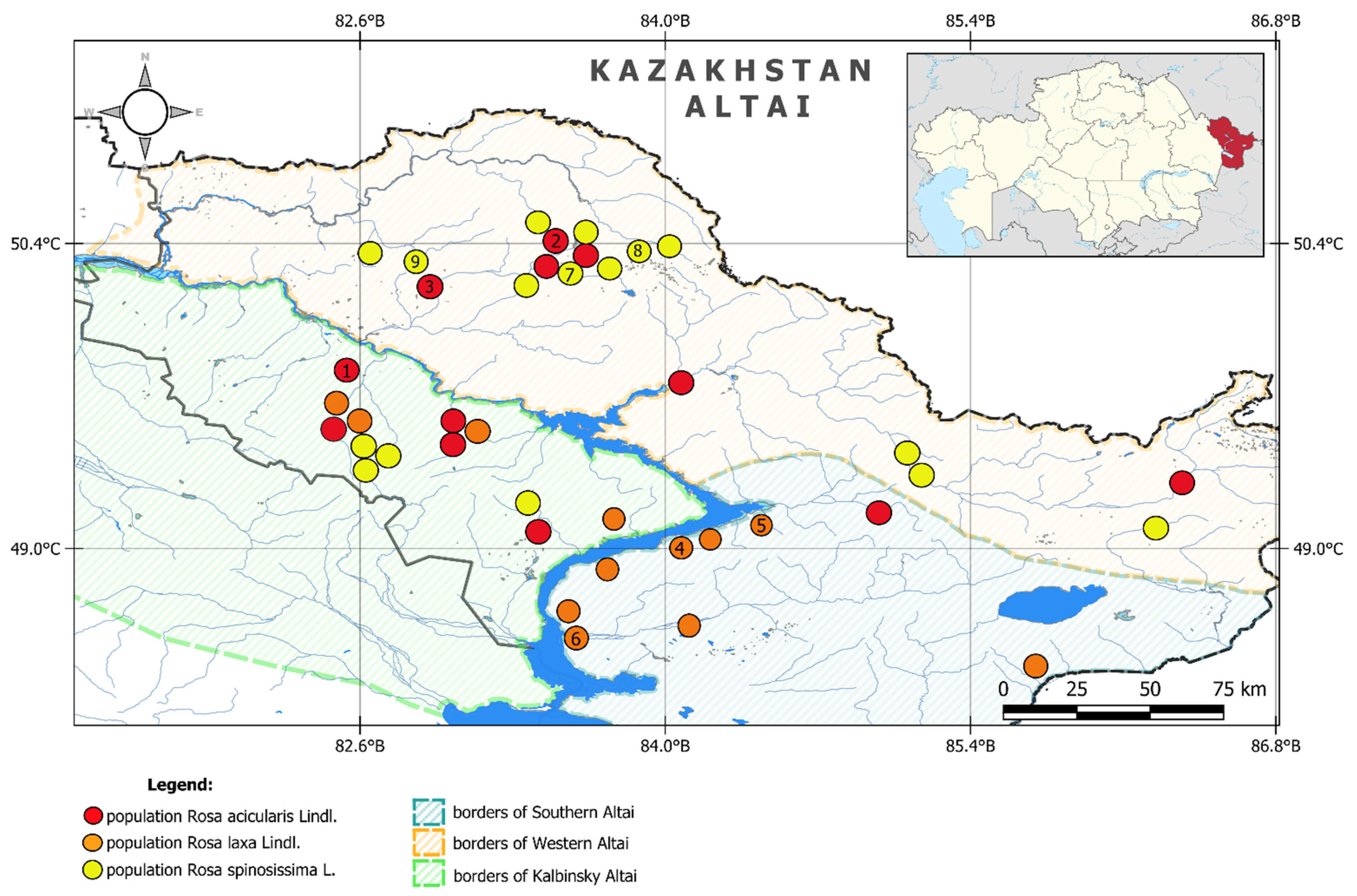

| Species Name | Population | Population Location | Latitude | Longitude | Altitude Above Sea Level | Area, ha | Plant Community |
|---|---|---|---|---|---|---|---|
| Rosa acicularis | Pop1 | Kalbinsky Altai, Eastern Kalba ridge, northwestern slope, valley of the Baichi river | 49.8269 | 82.5269 | 620.0 | 23.5 | Forb–shrub moderately moist meadow |
| Pop2 | Southwestern Altai, Ubinsky ridge, southwestern foothills, Zhuravlikha river valley, close to Krolchatnik village | 50.4217 | 83.4978 | 913.0 | 63.0 | Forb–grass shrubby meadows | |
| Pop3 | Southwestern Altai, Ubinsky ridge, southwestern foothills, Ubinka river valley, close to Zimovye village | 50.3069 | 82.8588 | 448.0 | 35.0 | Mixed moderately humid shrubby forest | |
| Rosa laxa | Pop4 | Narymsky Ridge, southwestern slope, Kanai tract, Kanasai River valley | 49.0486 | 84.0650 | 408.0 | 12.5 | Steppe shrub–forb meadows |
| Pop5 | Narymsky Ridge, northwestern slope, near the village Kokterek, Kudassai tract, Kudas River | 49.1086 | 84.4389 | 578.0 | 5.0 | Steppe shrub–forb meadows | |
| Pop6 | Kurchumsky Ridge, southwestern foothills of the Kurchum River valley | 48.5896 | 83.5764 | 578.0 | 40.0 | Shrub thickets in steppe areas of the meadow | |
| Rosa spinosissima | Pop7 | Southwestern Altai, Ivanovsky ridge, northwestern foothills, Shirokiy Log tract | 50.2900 | 83.5556 | 976.0 | 35.0 | Forb–cereal moderately moist shrubby meadow |
| Pop8 | Spurs of the Ivanovsky ridge, Mount Gavrina, northeastern slope, Seroluzhanskaya village | 50.36806 | 83.9042 | 1216.0 | 23.0 | Sparse aspen–birch bush forest | |
| Pop9 | Southwestern Altai, Ubinsky ridge, southwestern foothills, Ubinka river valley, near the Zimovye village | 50.3069 | 82.8588 | 448.0 | 12.5 | Forb–cereal moderately moist shrubby meadow |
| Population | Area, ha | Number of Plants, Count./m2 | Productivity, kg/Plant | Yield, kg/ha | Operational Reserve, Tonn | Volume of Possible Raw Material Collection, Tonn | |
|---|---|---|---|---|---|---|---|
| Total | Occupied by the Species | ||||||
| R. acicularis | |||||||
| Pop1 | 23.5 | 15.3 | 0.12 | 0.39 | 468.00 | 7.160 | 5.728 |
| Pop2 | 63.0 | 28.0 | 0.22 | 0.32 | 704.00 | 19.712 | 15.770 |
| Pop3 | 35.0 | 8.3 | 0.05 | 0.15 | 150.00 | 12.450 | 4.814 |
| R. laxa | |||||||
| Pop4 | 12.5 | 5.6 | 0.20 | 0.41 | 820.00 | 4.592 | 3.684 |
| Pop5 | 5.0 | 2.3 | 0.15 | 0.32 | 495.65 | 1.104 | 0.883 |
| Pop6 | 40.0 | 26.8 | 0.38 | 0.47 | 1786.01 | 47.865 | 38.292 |
| R. spinosissima | |||||||
| Pop7 | 35.0 | 17.5 | 0.28 | 0.52 | 1456.00 | 25.480 | 20.384 |
| Pop8 | 23.0 | 11.5 | 0.31 | 0.45 | 1395.04 | 16.043 | 12.834 |
| Pop9 | 12.5 | 8.75 | 0.09 | 0.28 | 252.00 | 2.205 | 1.764 |
| Parameter | Rosa acicularis | Rosa laxa | Rosa spinosissima | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Pop1 | Pop2 | Pop3 | Pop4 | Pop5 | Pop6 | Pop7 | Pop8 | Pop9 | |
| Qualitative characteristics | |||||||||
| Bush shape | Erect | Spreading | Spreading | Spreading | Spreading | Spreading | Oval | Spreading | Spreading |
| Amount of thorns | High | Moderate | High | High | High | Moderate | High | Moderate | High |
| Coloring of the leaf surface | Green | Green and light green | Green | Green and light green | Green | Green | Green and light green | Green | Green and light green |
| Fruit shape | Ovoid or elliptical, narrowed at the base | Elliptical, tapering at the base | Ovoid or elliptical, narrowed at the base | Oval or elliptical, tapering at the base | Oval, tapering at the base | Oval, tapering at the base | Rounded-flattened | Rounded-flattened | Rounded-flattened |
| Hypanthium coloring | Dark cherry | Orange-red | Bright orange | Red | Dark cherry | Raspberry | Black- brown | Black | Black-brown |
| Flavor profile | Sweet with a hint of sourness | Sweet with a slight sourness | Bland-sweet | Sweet-mealy | Bland-sweet | Sweet-mealy | Sweet-mealy | Sweet, slightly astringent | Sweet, slightly astringent |
| Quantitative characteristics | |||||||||
| Bush height, cm | 142.30 ± 5.45 | 115.7 ± 5.21 | 155.80 ± 3.12 | 167.10 ± 4.15 | 166.40 ± 4.87 | 155.20 ± 5.12 | 218.20 ± 5.15 | 151.50 ± 5.45 | 203.40 ± 4.29 |
| Bush diameter, cm | 103.30 ± 3.60 | 100.5 ± 3.79 | 124.20 ± 4.02 | 141.70 ± 4.67 | 151.70 ± 4.67 | 137.40 ± 9.80 | 196.70 ± 3.62 | 144.60 ± 10.30 | 185.90 ± 5.45 |
| Leaf length, cm | 5.71 ± 0.58 | 3.41 ± 0.30 | 3.72 ± 0.15 | 3.75 ± 0.21 | 4.73 ± 0.15 | 3.48 ± 0.12 | 3.50 ± 1.94 | 3.47 ± 2.03 | 3.06 ± 1.54 |
| Leaf width, cm | 1.54 ± 0.08 | 1.63 ± 0.11 | 1.72 ± 0.12 | 1.56 ± 0.0.08 | 2.67 ± 0.21 | 2.16 ± 0.12 | 1.90 ± 1.87 | 1.27 ± 1.96 | 1.21 ± 1.07 |
| Leaf area, cm2 | 39.09 ± 1.25 | 38.91 ± 4.65 | 36.90 ± 6.65 | 53.15 ± 7.65 | 50.62 ± 7.64 | 47.87 ± 6.46 | 20.17 ± 2.46 | 19.51 ± 3.68 | 18.71 ± 2.97 |
| Species | Population | Statistics | Fruit Size, cm | Mean Fruit Weight, g | Number of Seeds Per Fruit, Count. | |
|---|---|---|---|---|---|---|
| Length | Width | |||||
| Rosa acicularis | Pop1 | M ± m | 2.60 ± 0.09 | 1.80 ± 0.05 | 2.40 ± 0.24 | 34.71 ± 2.94 |
| C% | 11.41 | 5.65 | 17.43 | 12.82 | ||
| P% | 0.28 | 0.11 | 0.57 | 4.05 | ||
| Pop2 | M ± m | 2.20 ± 0.12 | 1.70 ± 0.08 | 2.47 ± 0.22 | 33.00 ± 3.03 | |
| C% | 11.41 | 9.38 | 18.70 | 13.92 | ||
| P% | 0.28 | 0.18 | 0.52 | 4.40 | ||
| Pop3 | M ± m | 2.60 ± 0.08 | 1.00 ± 0.04 | 2.23 ± 0.11 | 29.80 ± 2.33 | |
| C% | 6.42 | 8.47 | 19.78 | 11.82 | ||
| P% | 0.18 | 0.10 | 0.27 | 3.74 | ||
| Rosa laxa | Pop4 | M ± m | 2.20 ± 0.11 | 1.70 ± 0.08 | 2.28 ± 0.21 | 20.20 ± 2.11 |
| C% | 9.05 | 10.49 | 20.19 | 21.16 | ||
| P% | 0.26 | 0.20 | 0.52 | 4.67 | ||
| Pop5 | M ± m | 1.90 ± 0.11 | 1.70 ± 0.06 | 2.37 ± 0.16 | 17.90 ± 2.69 | |
| C% | 8.45 | 6.20 | 20.33 | 22.72 | ||
| P% | 2.26 | 1.66 | 5.43 | 5.19 | ||
| Pop6 | M ± m | 2.30 ± 0.20 | 1.20 ± 0.09 | 2.68 ± 0.26 | 17.50 ± 2.12 | |
| C% | 15.20 | 13.44 | 17.56 | 18.32 | ||
| P% | 4.06 | 3.59 | 4.69 | 5.19 | ||
| Rosa spinosissima | Pop7 | M ± m | 1.90 ± 0.08 | 2.10 ± 0.13 | 2.17 ± 0.40 | 17.20 ± 2.80 |
| C% | 7.29 | 13.62 | 32.97 | 24.64 | ||
| P% | 1.95 | 3.64 | 4.81 | 4.79 | ||
| Pop8 | M ± m | 2.40 ± 0.07 | 2.70 ± 0.09 | 2.55 ± 0.20 | 15.90 ± 2.21 | |
| C% | 3.72 | 9.02 | 19.63 | 21.06 | ||
| P% | 1.18 | 2.85 | 5.21 | 4.66 | ||
| Pop9 | M ± m | 1.30 ± 0.08 | 1.62 ± 0.08 | 2.26 ± 0.32 | 13.40 ± 2.77 | |
| C% | 9.46 | 9.46 | 21.45 | 31.31 | ||
| P% | 2.99 | 2.99 | 3.78 | 4.90 | ||
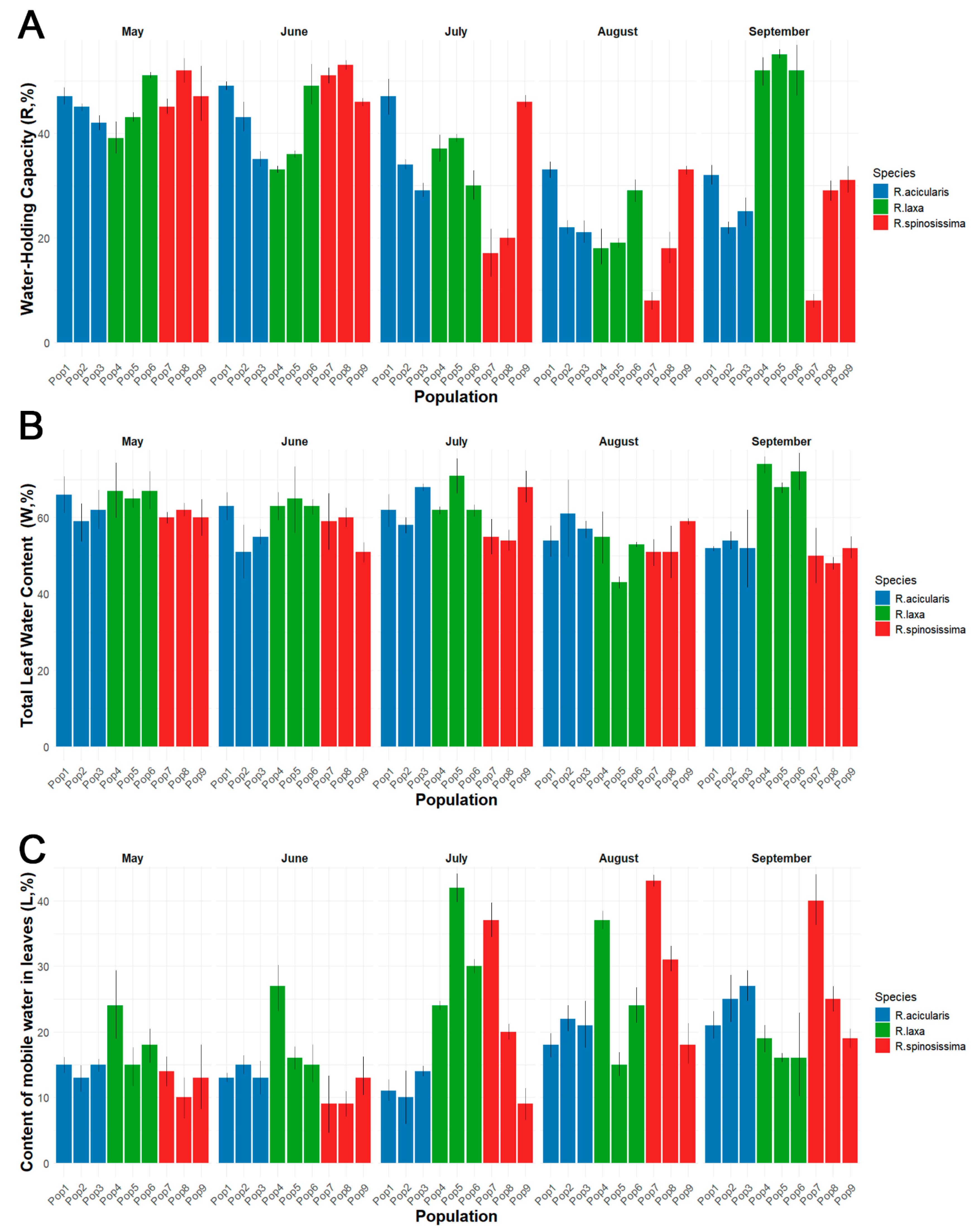
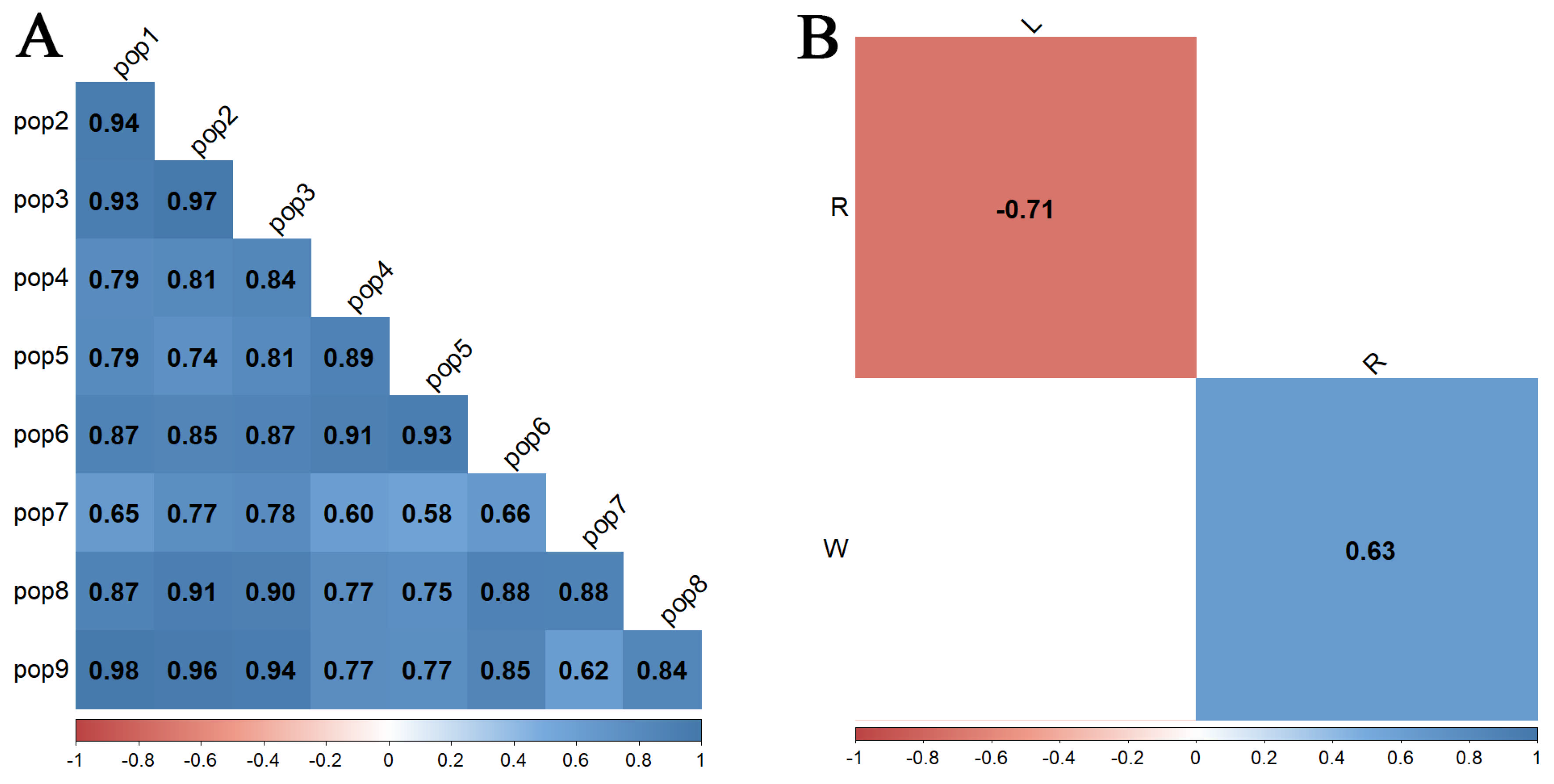
| Water-Holding Capacity (R, %) | df | SS | MS | F-Value | p-Value |
|---|---|---|---|---|---|
| Species | 2 | 208 | 104.2 | 1.632 | 0.21241000 |
| Month | 4 | 3203 | 800.8 | 12.540 | 0.00000411 |
| Population × Month | 8 | 1832 | 229.0 | 3.586 | 0.00495000 |
| Residuals | 30 | 1916 | 63.9 | ||
| Total leaf water content (W, %) | df | SS | MS | F-value | p-value |
| Species | 2 | 422.9 | 211.47 | 11.29 | 0.000221 |
| Month | 4 | 498.3 | 124.58 | 6.65 | 0.005890 |
| Population × Month | 8 | 670 | 83.74 | 4.47 | 0.001188 |
| Residuals | 30 | 562 | 18.73 | ||
| “Mobile” water content in leaves (L, %) | df | SS | MS | F-value | p-value |
| Species | 2 | 247.7 | 123.84 | 2.386 | 0.1092 |
| Month | 4 | 867.4 | 216.84 | 4.178 | 0.0083 |
| Population × Month | 8 | 905.4 | 113.17 | 2.181 | 0.0584 |
| Residuals | 30 | 1556.8 | 51.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danilova, A.N.; Vdovina, T.A.; Kotukhov, Y.A.; Anufriyeva, O.A.; Vinokurov, A.A.; Isakova, E.A.; Lagus, O.A.; Sumbembayev, A.A. Study of the Geographical Distribution, Ecological–Biological Characteristics, and Economic Value of Rosa acicularis Lindl., Rosa laxa Retz., and Rosa spinosissima L. (Rosaceae) in Kazakhstan’s Part of the Altai Mountains. Diversity 2025, 17, 441. https://doi.org/10.3390/d17070441
Danilova AN, Vdovina TA, Kotukhov YA, Anufriyeva OA, Vinokurov AA, Isakova EA, Lagus OA, Sumbembayev AA. Study of the Geographical Distribution, Ecological–Biological Characteristics, and Economic Value of Rosa acicularis Lindl., Rosa laxa Retz., and Rosa spinosissima L. (Rosaceae) in Kazakhstan’s Part of the Altai Mountains. Diversity. 2025; 17(7):441. https://doi.org/10.3390/d17070441
Chicago/Turabian StyleDanilova, Alevtina N., Tatyana A. Vdovina, Yuriy A. Kotukhov, Olga A. Anufriyeva, Andrey A. Vinokurov, Elena A. Isakova, Olga A. Lagus, and Aidar A. Sumbembayev. 2025. "Study of the Geographical Distribution, Ecological–Biological Characteristics, and Economic Value of Rosa acicularis Lindl., Rosa laxa Retz., and Rosa spinosissima L. (Rosaceae) in Kazakhstan’s Part of the Altai Mountains" Diversity 17, no. 7: 441. https://doi.org/10.3390/d17070441
APA StyleDanilova, A. N., Vdovina, T. A., Kotukhov, Y. A., Anufriyeva, O. A., Vinokurov, A. A., Isakova, E. A., Lagus, O. A., & Sumbembayev, A. A. (2025). Study of the Geographical Distribution, Ecological–Biological Characteristics, and Economic Value of Rosa acicularis Lindl., Rosa laxa Retz., and Rosa spinosissima L. (Rosaceae) in Kazakhstan’s Part of the Altai Mountains. Diversity, 17(7), 441. https://doi.org/10.3390/d17070441






