Mt. Fuji in the Ocean–Description of a Strange New Species of Sea Anemone, Discoactis tritentaculata fam., gen., and sp. nov. (Cnidaria; Anthozoa; Actiniaria; Actinostoloidea) from Japan, with the Foundation of a New Family and Genus †
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preservation
2.2. Preparation of Histological Sections
2.3. Observation with SEM
2.4. Cnidae Observation
2.5. Phylogenetic Analyses
| Marker | Primers | Sequences (5′-3′) | PCR Protocol | References | |
|---|---|---|---|---|---|
| 12S | 12S1a | TAAGTGCCAGCAGACGCGGT | (95 °C for 4 min) + 4 × [(94 °C for 30 s) → (50 °C for 1 min) → (72 °C for 2 min)] +30 × [(94 °C for 30 s) → (55 °C for 1 min) → (72 °C for 2 min)] + (72 °C for 4 min) | [22] | |
| 12S3r | ACGGGCAATTTGTACTAACA | ||||
| 16S | ANEM16SA | CACTGACCGTGATAATGTAGCGT | (95 °C for 4 min) + 30 × [(95 °C for 30 s) → (46 °C for 45 s) → (72 °C for 1 min)] + (72 °C for 5 min) | [23] | |
| ANEM16SB | CCCCATGGTAGCTTTTATTCG | ||||
| COXIII | COIIIF | CATTTAGTTGATCCTAGGCCTTGACC | (95 °C for 2 min) + 30 × [(95 °C for 30 s) → (46 °C for 45 s) → (72 °C for 1 min)] + (72 °C for 5 min) | [23] | |
| COIIIR | CAAACCACATCTACAAAATGCCAATATC | ||||
| 18S | PCR | 18SA | AACCTGGTTGATCCTGCCAGT | (94 °C for 4 min) + 35 × [(94 °C for 20 s) → (57 °C for 20 s) → (72 °C for 1 min 45 s)] + (72 °C for 7 min) | [24,25] |
| 18SB | TGATCCTTCCGCAGGTTCACCT | ||||
| Only Sequence | 18SL | CCAACTACGAGCTTTTTAACTG | |||
| 18SC | CGGTAATTCCAGCTCCAATAG | ||||
| 18SY | CAGACAAATCGCTCCACCAAC | ||||
| 18SO | AAGGGCACCACCAGGAGTGGAG | ||||
| 28S | PCR1 | F63sq | AATAAGCGGAGGAAAAGAAAC | (94 °C for 5 min) + 30 × [(94 °C for 30 s) → (45 °C for 1 min) → (72 °C for 3 min)] + (72 °C for 10 min) | [26] |
| R2077sq | GAGCCAATCCTTWTCCCGARGTT | ||||
| PCR2 | LSU5 | TAGGTCGACCCGCTGAAYTTAAGCA | (94 °C for 5 min) + 30 × [(94 °C for 30 s) → (45 °C for 1 min) → (72 °C for 3 min)] + (72 °C for 10 min) | [27,28,29] | |
| LSU1600R | AGCGCCATCCATTTTCAGG | ||||
| Only Sequence | LSU330F | CAAGTACCGTGAGGGAAAGTTG | |||
| LSU900F | CCGTCTTGAAACACGGACCAAG | ||||
| ECD2S | CTTGGTCCGTGTTTCAAGACGG | ||||
| Higher Taxa | Families | Genus | Species | (Remarks) | 12S | 16S | 18S | 28S | COXIII | |
|---|---|---|---|---|---|---|---|---|---|---|
| Actiniaria | ||||||||||
| Anenthemonae | ||||||||||
| Actinernoidea | Actinernidae | Actinernus | antarcticus | KJ482930 | KJ482966 | KJ483023 | KJ483126 | - | ||
| Isactinernus | quadrolobatus | KJ482932 | KJ482968 | KJ483024 | KJ483105 | KJ482998 | ||||
| Synhalcurias | laevis | KJ482942 | - | KJ483021 | KJ483120 | - | ||||
| Halcuriidae | Halcurias | pilatus | KJ482931 | KJ482967 | KJ483020 | KJ483109 | KJ482997 | |||
| Edwardsioidea | Edwardsiidae | Edwardsia | japonica | GU473274 | GU473288 | GU473304 | KJ483048 | GU473359 | ||
| Edwardsia | timida | GU473281 | - | GU473315 | KJ483088 | KJ482996 | ||||
| Edwardsianthus | gilbertensis | EU190728 | EU190772 | EU190859 | EU190817 | - | ||||
| Nematostella | vectensis | EU190750 | AY169370 | AF254382 | KJ483089 | FJ489501 | ||||
| Enthemonae | ||||||||||
| Actinostoloidea | Capneidae | Capnea | japonica | (CMNH-ZG 06547; off Misaki) | LC602145 | LC602146 | LC602147 | LC602148 | LC602149 | |
| Capnea | georgiana | - | KJ482951 | KJ483022 | KJ483050 | KJ482990 | ||||
| Discoactinidae | Discoactis | tritentaculata | (NSMT-Co 1908; off Misaki) | LC874807 | LC875472 | LC875474 | LC875477 | LC876766 | ||
| Discoactis | tritentaculata | (NSMT-Co 1909; off Iki Island) | LC874806 | LC875473 | LC875475 | - | LC876767 | |||
| Halcampulactinidae | Halcampulactis | solamar | MK279362 | MK279363 | MK279364 | MK279365 | MK279366 | |||
| Exocoelactinidae | Exocoelactis | actinostoloides | (CMNH-ZG 05926; off Misaki) | KP793003 | KP793004 | LC875476 | LC875478 | LC876768 | ||
| Actinostolidae | Actinostola | crassicornis | - | EU190753 | EU190843 | KJ483098 | GU473332 | |||
| Actinostola | chilensis | - | GU473285 | GU473302 | KJ483110 | GU473357 | ||||
| Actinostola | georgiana | KJ482928 | KJ482952 | KJ483032 | KJ483099 | KJ482991 | ||||
| Antholoba | achates | GU473269 | GU473284 | GU473301 | KJ483128 | GU473356 | ||||
| Stomphia | didemon | KJ482929 | EU190795 | EU190875 | KJ483127 | GU473348 | ||||
| Stomphia | selaginella | GU473280 | GU473298 | GU473314 | GU473331 | GU473349 | ||||
| Anthosactinidae | Anthosactis | janmayeni | KJ482938 | GU473292 | GU473308 | KJ483091 | GU473363 | |||
| Anthosactis | pearseae | EU190751 | EU190798 | EU190878 | EU190841 | GU473365 | ||||
| Hormosoma | scotti | EU190733 | EU190778 | EU190863 | KJ483090 | GU473366 | ||||
| Tealidium | konoplinorum | MZ569954 | MZ567264 | MZ569926 | MZ569979 | MZ576886 | ||||
| Sicyonidae | Sicyonis | denisovi | MZ569948 | MZ567258 | MZ569920 | MZ569973 | MZ576880 | |||
| Ophiodiscus | bukini | MZ569953 | MZ567263 | MZ569925 | MZ569978 | MZ576885 | ||||
| Actinoidea | Actiniidae | Actinia | fragacea | EU190714 | EU190756 | EU190845 | EU190845 | GU473334 | ||
| Anemonia | viridis | EU190718 | EU190760 | EU190849 | EU190849 | GU473335 | ||||
| Anthopleura | elegantissima | EU190713 | EU190755 | EU190844 | EU190844 | GU473333 | ||||
| Anthostella | stephensoni | JQ810719 | JQ810721 | JQ810723 | JQ810723 | JQ810726 | ||||
| Bolocera | kerguelensis | KJ482925 | KJ482965 | KJ483029 | KJ483029 | KJ482985 | ||||
| Bunodactis | verrucosa | EU190723 | EU190766 | EU190854 | EU190854 | - | ||||
| Bunodosoma | grandis | EU190722 | EU190765 | EU190853 | EU190853 | GU473336 | ||||
| Epiactis | lisbethae | EU190727 | EU190771 | EU190858 | EU190858 | GU473360 | ||||
| Glyphoperidium | bursa | KJ482923 | KJ482961 | KJ483033 | KJ483033 | KJ482982 | ||||
| Isotealia | antarctica | JQ810720 | JQ810722 | - | - | JQ810727 | ||||
| Isosicyonis | alba | - | KJ482959 | KJ483030 | KJ483030 | KJ482981 | ||||
| Isosicyonis | striata | EU190736 | EU190781 | EU190864 | EU190864 | FJ489493 | ||||
| Korsaranthus | natalinesis | KJ482920 | KJ482958 | KJ483017 | KJ483017 | KJ482987 | ||||
| Macrodactyla | doreenensis | EU190739 | EU190785 | EU190867 | EU190867 | GU473342 | ||||
| Urticina | coriacea | GU473282 | EU190797 | EU190877 | EU190877 | GU473351 | ||||
| Actinodendridae | Actinostephanus | haeckeli | KJ482936 | EU190762 | KJ483034 | KJ483034 | GU473353 | |||
| Haloclavidae | Haloclava | producta | EU190734 | EU190779 | AF254370 | AF254370 | JF833008 | |||
| Haloclava | sp. | KJ482924 | KJ482963 | KJ483031 | KJ483031 | KJ482989 | ||||
| Harenactis | argentina | KJ482926 | KJ482964 | KJ483026 | KJ483026 | KJ482984 | ||||
| Peachia | cylindrica | EU190743 | EU190789 | KJ483015 | KJ483015 | - | ||||
| Stephanthus | antarcticus | KJ482927 | KJ482960 | KJ483019 | KJ483019 | KJ482983 | ||||
| Liponematidae | Liponema | brevicornis | EU190738 | EU190784 | EU190866 | EU190866 | KJ483001 | |||
| Liponema | multiporum | KJ482922 | KJ482962 | - | - | - | ||||
| Phymanthidae | Phymanthus | loligo | EU190745 | EU190791 | EU190871 | EU190871 | GU473345 | |||
| Preactiidae | Dactylanthus | antarcticus | GU473272 | AY345877 | AF052896 | AF052896 | GU473358 | |||
| Preactis | milliardae | KJ482921 | KJ482957 | KJ483018 | KJ483018 | KJ482986 | ||||
| Stichodactidae | Heteractis | magnifica | EU190732 | EU190777 | EU190862 | EU190862 | KJ482988 | |||
| Metridioidea | Actinoscyphiidae | Actinoscyphia | plebeia | EU190712 | EU190754 | FJ489437 | KJ483067 | FJ489476 | ||
| Aiptasiidae | Aiptasia | mutabilis | JF832963 | FJ489418 | FJ489438 | KJ483115 | FJ489505 | |||
| Aiptasia | pallida | EU190715 | EU190757 | EU190846 | EU190803 | KJ482979 | ||||
| Bartholomea | annulata | EU190721 | EU190763 | EU190851 | KJ483068 | FJ489483 | ||||
| Neoaiptasia | morbilla | EU190742 | EU190788 | EU190869 | KJ483075 | JF833010 | ||||
| Aliciidae | Alicia | sansibarensis | KJ482933 | KJ482953 | KJ483016 | KJ483116 | KJ483000 | |||
| Triactis | producta | EU490525 | - | EU190876 | KJ483125 | GU473350 | ||||
| Amphianthidae | Amphianthus | sp. | FJ489413 | FJ489432 | FJ489450 | FJ489467 | FJ489502 | |||
| Peronanthus | sp. | KJ482917 | KJ482956 | KJ483014 | KJ483066 | KJ482976 | ||||
| Andvakiidae | Andvakia | boninensis | EU190717 | EU190759 | EU190848 | KJ483053 | FJ489479 | |||
| Andvakia | discipulorum | GU473273 | GU473287 | GU473316 | KJ483051 | - | ||||
| Telmatactis | sp. | JF832968 | JF832979 | KJ483013 | KJ483135 | - | ||||
| Antipodactinidae | Antipodactis | awii | GU473271 | GU473286 | GU473303 | KJ483074 | GU473337 | |||
| Bathyphelliidae | Bathyphellia | australis | FJ489402 | FJ489422 | EF589063 | EF589086 | FJ489482 | |||
| Boloceroididae | Boloceroides | mcmurrichi | GU473270 | - | EU190852 | KJ483103 | KJ483002 | |||
| Bunodeopsis | globulifera | KJ482940 | KJ482949 | KJ483025 | KJ483122 | KJ482992 | ||||
| Diadumenidae | Diadumene | cincta | EU190725 | EU190769 | EU190856 | KJ483106 | FJ489490 | |||
| Diadumene | leucolena | JF832957 | JF832977 | JF832986 | KJ483123 | JF833006 | ||||
| Diadumene | sp. | JF832960 | JF832976 | JF832980 | KJ483130 | JF833005 | ||||
| Galantheanthemidae | Galatheanthemum | sp. | KJ482918 | KJ482955 | KJ483012 | KJ483065 | KJ482977 | |||
| Galatheanthemum | profundus | KJ482919 | KJ482954 | KJ483011 | KJ483119 | KJ482978 | ||||
| Gonatiniidae | Gonactinia | prolifera | KJ482935 | - | KJ483008 | KJ483112 | KJ482994 | |||
| Protantea | simplex | KJ482939 | KJ482970 | KJ483010 | KJ483078 | KJ482993 | ||||
| Halcampidae | Cactosoma | sp. nov. | GU473279 | GU473297 | GU473313 | GU473329 | GU473346 | |||
| Halcampa | duodecimcirrata | JF832966 | EU190776 | AF254375 | EU190820 | - | ||||
| Halcampoides | purpurea | EU190735 | EU190780 | AF254380 | KJ483100 | - | ||||
| Haliplanellidae | Haliplanella | lineata | EU190730 | EU190774 | EU190860 | KJ483108 | FJ489506 | |||
| Hormathiidae | Actinauge | richardi | EU190719 | EU190761 | EU190850 | KJ483055 | FJ489480 | |||
| Adamsia | palliata | FJ489398 | FJ489419 | FJ489436 | KJ483101 | FJ489474 | ||||
| Allantactis | parasitica | FJ489399 | FJ489420 | FJ489439 | KJ483056 | FJ489478 | ||||
| Calliactis | japonica | FJ489403 | FJ489423 | FJ489441 | KJ483057 | FJ489486 | ||||
| Calliactis | parasitica | EU190711 | EU190752 | EU190842 | KJ483102 | FJ489475 | ||||
| Calliactis | polypus | FJ489407 | FJ489427 | FJ489445 | KJ483058 | FJ489485 | ||||
| Calliactis | tricolor | FJ489405 | FJ489425 | FJ489443 | KJ483059 | FJ489488 | ||||
| Chondrophellia | orangina | FJ489406 | FJ489426 | FJ489444 | KJ483060 | FJ489489 | ||||
| Hormathia | armata | EU190731 | EU190775 | EU190861 | KJ483062 | FJ489491 | ||||
| Hormathia | lacunifera | FJ489409 | FJ489428 | FJ489446 | KJ483063 | FJ489492 | ||||
| Hormathia | pectinata | FJ489415 | FJ489430 | FJ489448 | FJ489465 | FJ489497 | ||||
| Paracalliactis | japonica | FJ489411 | FJ489429 | FJ489447 | KJ483061 | FJ489496 | ||||
| Paraphelliactis | sp. | FJ489412 | FJ489431 | FJ489449 | FJ489466 | FJ489498 | ||||
| Isanthidae | Isanthus | capensis | JF832967 | GU473291 | GU473307 | KJ483096 | GU473362 | |||
| Kadosactinidae | Alvinactis | chessi | GU473278 | GU473296 | GU473312 | KJ483052 | GU473352 | |||
| Cyananthea | hourdezi | GU473275 | GU473293 | GU473309 | KJ483081 | GU473364 | ||||
| Kadosactis | antarctica | FJ489410 | EU190782 | EU190865 | KJ483080 | FJ489504 | ||||
| Metridium | senile | EU190740 | EU190786 | AF052889 | KJ483076 | FJ489494 | ||||
| Nemathidae | Nemanthus | nitidus | EU190741 | EU190787 | EU190868 | KJ483064 | FJ489495 | |||
| Phelliidae | Phellia | gausapata | EU190744 | EU190790 | EU190870 | KJ483054 | FJ489473 | |||
| Phellia | exlex | JF832958 | JF832978 | JF832984 | KJ483121 | JF833004 | ||||
| Sagartiidae | Actinothoe | sphyrodeta | FJ489401 | FJ489421 | FJ489440 | KJ483111 | FJ489481 | |||
| Anthothoe | chilensis | FJ489397 | FJ489416 | FJ489434 | FJ489453 | FJ489470 | ||||
| Cereus | pedunculatus | EU190724 | EU190767 | EU190855 | EU190813 | FJ489471 | ||||
| Cereus | herpetodes | JF832956 | JF832969 | JF832983 | JF832992 | - | ||||
| Sagartia | troglodytes | EU190746 | EU190792 | EU190872 | KJ483073 | FJ489499 | ||||
| Sagartia | ornata | JF832959 | JF832975 | JF832985 | KJ483069 | JF833011 | ||||
| Sagartiogeton | laceratus | EU190748 | EU190794 | EU190874 | KJ483071 | FJ489500 | ||||
| Sagartiogeton | undatus | FJ489400 | FJ489417 | FJ489435 | KJ483070 | FJ489472 | ||||
| Verrillactis | paguri | FJ489414 | FJ489433 | FJ489440 | KJ483046 | FJ489503 | ||||
| Zoanthidea | Epizoanthidae | Epizoanthus | illoricatus | AY995901 | EU591597 | KC218424 | KJ483036 | - | ||
| Parazoanthidae | Parazoanthus | axinellae | GQ464940 | EU828754 | KC218416 | KJ483044 | - | |||
| Savalia | savaglia | AY995905 | DQ825686 | HM044299 | HM044298 | - | ||||
| Undknown | Relicanthidae | Relicanthus | daphneae | KJ482934 | KJ482971 | KJ483028 | KJ483131 | KJ482999 | ||
3. Results and Discussions
3.1. Taxonomic Section
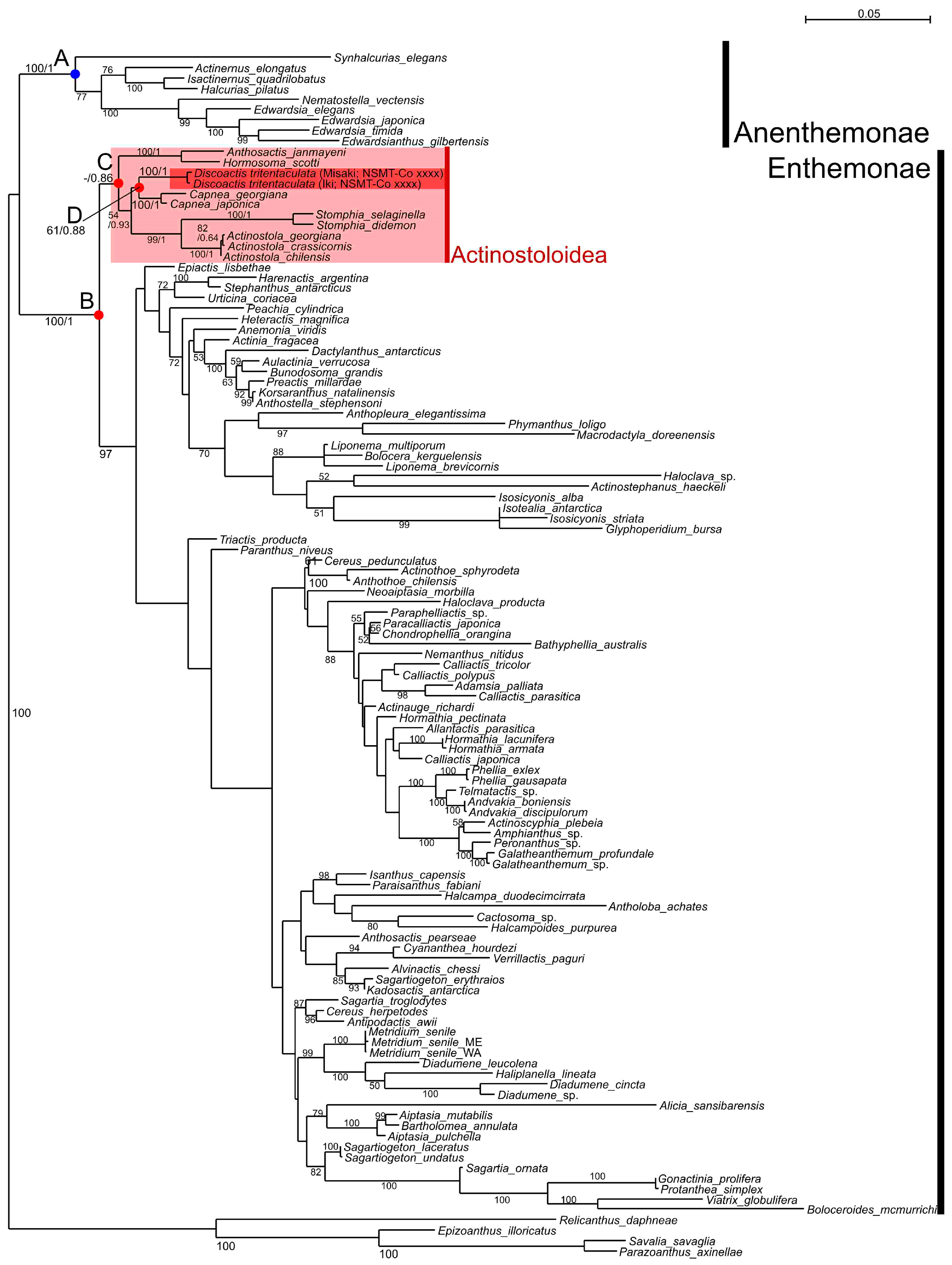
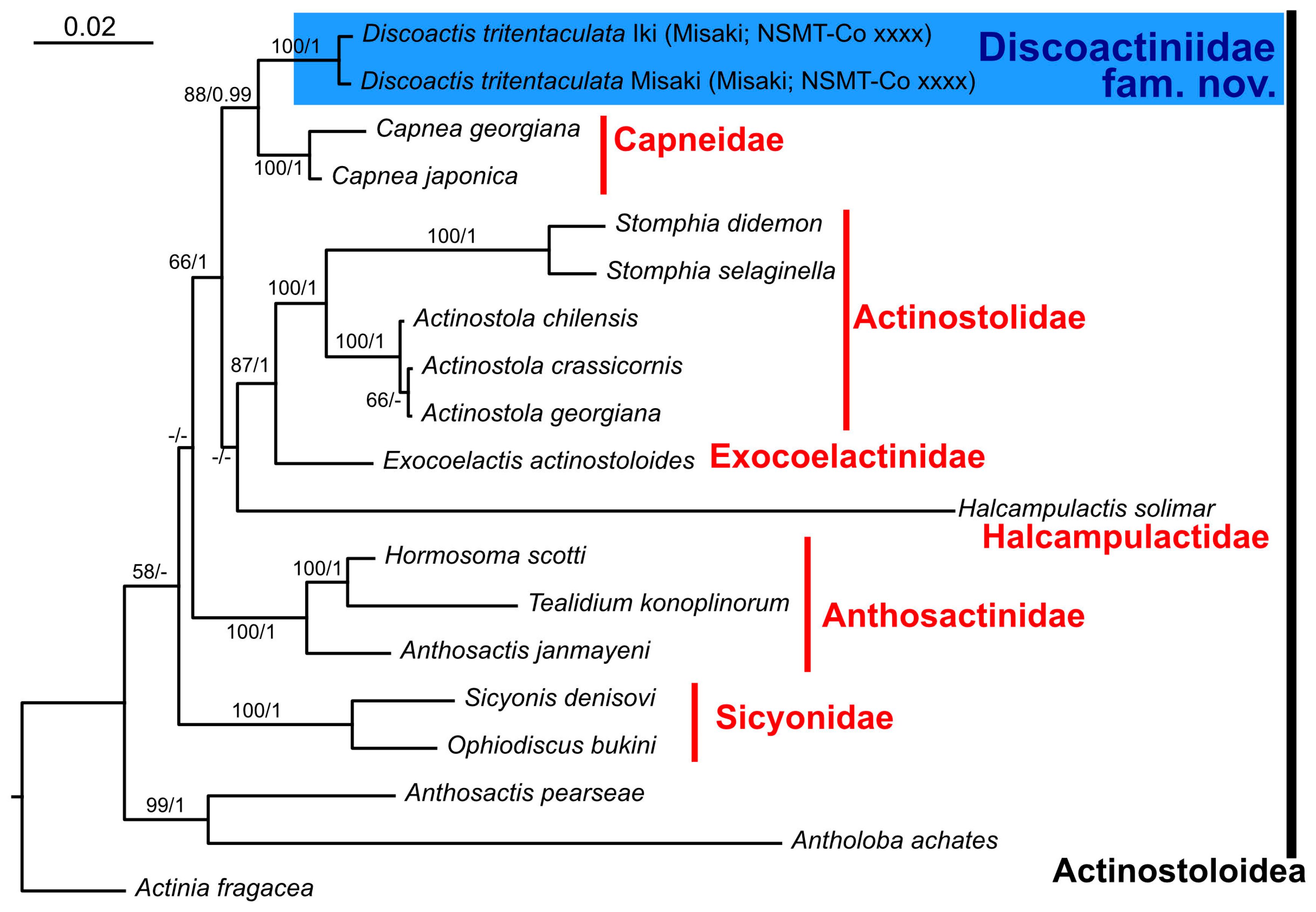
3.2. Molecular Phylogeny
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hertwig, R. Die Actinien der Challenger Expedition. Gustav Fisch 1882, 119, 14. [Google Scholar]
- Fautin, D.G. Catalog to families, genera, and species of orders Actiniaria and Corallimorpharia (Cnidaria: Anthozoa). Zootaxa 2016, 4145, 1–449. [Google Scholar] [CrossRef] [PubMed]
- Titus, B.M.; Benedict, C.; Laroche, R.; Gusmão, L.C.; Van Deusen, V.; Chiodo, T.; Meyer, C.P.; Berumen, M.L.; Bartholomew, A.; Yanagi, K.; et al. Phylogenetic relationships among the clownfish-hosting sea anemones. Molecular Phylogenetics and Evolution 2019, 139, 106526. [Google Scholar] [CrossRef]
- Yanagi, K. Chapter 4, Actiniaria. In How to Study Sessile Organisms: Revised; The Sessile Organisms Society of Japan, Ed.; Kouseisha Kouseikaku: Tokyo, Japan, 2017; pp. 53–87. (In Japanese) [Google Scholar]
- Carlgren, O. A Survey of the Ptychodactiaria, Corallimorpharia and Actiniaria; Kungliga Svenska Vetenskapsakademiens Handlingar: Stockholm, Sweden, 1949; Volume 1, pp. 1–121. [Google Scholar]
- Shick, J.M. A Functional Biology of Sea Anemones; Chapman & Hall: London, UK, 1991; 395p. [Google Scholar]
- Izumi, T.; Fujii, T.; Yanagi, K.; Fujita, T. Fluorescent Anemones in Japan—Comprehensive Revision of Japanese Actinernoidea (Cnidaria: Anthozoa: Actiniaria: Anenthemonae) with Rearrangements of the Classification. Diversity 2023, 15, 773. [Google Scholar] [CrossRef]
- Rodríguez, E.; Barbeitos, M.S.; Brugler, M.R.; Crowley, L.M.; Grajales, A.; Gusmão, L.; Häussermann, V.; Reft, A.; Daly, M. Hidden among sea anemones: The first comprehensive phylogenetic reconstruction of the Order Actiniaria (Cnidaria, Anthozoa, Hexacorallia) reveals a novel group of hexacorals. PLoS ONE 2014, 9, e96998. [Google Scholar] [CrossRef]
- Izumi, T.; Fujii, T.; Yanagi, K. Introduction to the latest classification system of Actiniaria and proposals for Japanese names of higher taxa. TAXA 2019, 46, 54–63. (In Japanese) [Google Scholar]
- Carlgren, O. Die Ceriantharien, Zoantharien und Actiniarien des arktischen Gebietes. In Eine Zusammenstellung der Arktischen Tierformen mit Besonderer Berücksichtigung des Spitzbergen-Gebietes auf Grund der Ergebnisse der Deutschen Expedition in das Nördliche Eismeer im Jahre 1898; Römer, F., Schaudinn, F., Brauer, A., Arndt, W., Eds.; Gustav Fischer: Jena, Germany, 1932; pp. 255–266. [Google Scholar]
- Carlgren, O. Zur Kenntnis der Hexacorallen. Zool. Anz. 1925, 65, 87–99. [Google Scholar]
- Gusmão, L.C.; Berniker, K.; Van Deusen, V.; Rodríguez, E. Halcampulactidae (Actiniaria, Actinostoloidea), a new family of burrowing sea anemones with external brooding from Antarctica. Polar Biol. 2019, 42, 1271–1286. [Google Scholar] [CrossRef]
- Andres, A. Prodromus neapolitanae actiniarum faunae addito generalis actiniarum bibliographiae catalogo. Mitth. Zool. Stn. Neapal 1881, 2, 305–371. [Google Scholar]
- Sanamyan, N.P.; Sanamyan, K.E.; Galkin, S.V.; Ivin, V.V.; Bocharova, E.S. Deep water Actiniaria (Cnidaria: Anthozoa) Sicyonis, Ophiodiscus and Tealidium: Re-evaluation of Actinostolidae and related families. Invertebr. Zool. 2021, 18, 385–449. [Google Scholar] [CrossRef]
- Forbes, E. Contributions to British actinology. I. On Kapnea, a new helianthoid polype. Ann. Mag. Nat. Hist. 1841, 7, 81–85. [Google Scholar] [CrossRef]
- Yanagi, K.; Izumi, T. Redescription of the sea anemone Capnea japonica (Cnidaria: Anthozoa: Actiniaria). Species Divers. 2021, 26, 153–163. [Google Scholar] [CrossRef]
- Momma, H.; Hotta, H. Deep Tow Dredge and Shovel Sampler. Jpn. Agency Mar.-Earth Sci. Technol. 1989, 21, 251–257. [Google Scholar]
- Carlgren, O. A contribution to the knowledge of the structure and distribution of the cnidae in the Anthozoa. Lunds Univ. Årsskrift 1940, 36, 1–62. [Google Scholar]
- Presnell, J.K.; Schreibman, M.P. Humason’s Animal Tissue Techniques, 5th ed.; Johns Hopkins University Press: Baltimore, MD, USA, 1997. [Google Scholar]
- Rasband, W.S. (1997–2012) ImageJ, U.S. National Institutes of Health: Bethesda, Maryland, USA. Available online: http://imagej.nih.gov/ij/ (accessed on 17 October 2020).
- Mariscal, R.N. Nematocysts. In Coelenterate Biology: Reviews and New Perspectives; Muscatine, L., Lenhoff, H.M., Eds.; Academic Press: New York, NY, USA, 1974; pp. 129–178. [Google Scholar]
- Sinniger, F.; Montoya-Burgos, J.I.; Chevaldonné, P.; Pawlowski, J. Phylogeny of the order Zoantharia (Anthozoa, Hexacorallia) based on the mitochondrial ribosomal genes. Mar. Biol. 2005, 147, 1121–1128. [Google Scholar] [CrossRef]
- Geller, J.B.; Walton, E.D. Breaking up and getting together: Evolution of symbiosis and cloning by fission in sea anemones (genus Anthopleura). Evolution 2001, 55, 1781–1794. [Google Scholar]
- Medlin, L.; Elwood, H.J.; Stickel, S.; Sogin, M.L. The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions. Gene 1988, 71, 491–499. [Google Scholar] [CrossRef]
- Apakupakul, K.; Siddall, M.E.; Burreson, E.M. Higher level relationships of leeches (Annelida: Clitellata: Euhirudinea) based on morphology and gene sequences. Mol. Phylogenet. Evol. 1999, 12, 350–359. [Google Scholar] [CrossRef]
- Medina, M.; Collins, A.G.; Silberman, J.D.; Sogin, M.L. Evaluating hypotheses of basal animal phylogeny using complete sequences of large and small subunit rRNA. Proc. Natl. Acad. Sci. USA 2001, 98, 9707–9712. [Google Scholar] [CrossRef]
- Littlewood, D.T.J.; Curini-Galletti, M.; Herniou, E.A. The interrelationships of Proseriata (Platyhelminthes: Seriata) tested with molecules and morphology. Mol. Phylogenet. Evol. 2000, 16, 449–466. [Google Scholar] [CrossRef]
- Williams, S.T.; Reid, D.G.; Littlewood, D.T.J. A molecular phylogeny of the Littorininae (Gastropoda: Littorinidae): Unequal evolutionary rates, morphological parallelism and biogeography of the Southern Ocean. Mol. Phylogenet. Evol. 2003, 28, 60–86. [Google Scholar] [CrossRef]
- Williams, S.T.; Ozawa, T. Molecular phylogeny suggests polyphyly of both the turban shells (family Turbinidae) and the superfamily Trochoidea (Mollusca: Vetigastropoda). Mol. Phylogenet. Evol. 2006, 39, 33–51. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Phylogenet. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Castresana, J. Gblocks Server. 2002. Available online: http://molevol.cmima.csic.es/castresana/Gblocks_server.html (accessed on 20 January 2022).
- Tanabe, A.S. Kakusan4 and Aminosan: Two programs for comparing nonpartitioned, proportional and separate models for combined molecular phylogenetic analyses of multilocus sequence data. Mol. Ecol. Resour. 2011, 11, 914–921. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 2006, 22, 2688–2690. [Google Scholar] [CrossRef]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef]
- Carlgren, O. Actiniaria Part, I. In Danish Ingolf-Expedition; Hagerup: Copenhagen, Denmark, 1921; Volume 5, pp. 1–241. [Google Scholar]
- Bourne, G.C. On some new Phelliinae from New Guinea. Q. J. Microsc. Sci. 1918, 63, 31–90. [Google Scholar] [CrossRef]
- Sanamyan, N.P.; Sanamyan, K.E. Tetracoelactis ioran, a new genus and species of deep-sea anemones of the family Exocoelactinidae (Cnidaria: Anthozoa: Actiniaria) from the Northwestern Pacific Ocean. Zoosystematica Ross. 2019, 28, 238–248. [Google Scholar] [CrossRef]
- Gosse, P.H. A History of the British Sea-Anemones and Corals; Van Voorst: London, UK, 1860; 362p. [Google Scholar]
- Rafinesque, C.S. Analyse de la Nature ou Tableau de l’Univers et des Corps Organisés; Rafinesque, C.S., Ed.; Gallia: Palerme, Italy, 1815; 224p. [Google Scholar]
- Carlgren, O. Studien über nordische Actinien. K. Sven. Vetenskapsakademiens Handl. 1893, 25, 1–148. [Google Scholar]
- Yanagi, K.; Fujii, T.; Hirose, M. Redescription of the sea anemone Exocoelactis actinostoloides (Cnidaria: Anthozoa: Actiniaria) based on a topotypic specimen collected from Tokyo Bay, Japan. Species Divers. 2015, 20, 199–209. [Google Scholar] [CrossRef]
- Gusmão, L.C.; Rodríguez, E. Two sea anemones (Cnidaria: Anthozoa: Actiniaria) from the Southern Ocean with evidence of a deep-sea, polar lineage of burrowing sea anemones. Zool. J. Linn. Soc. 2021, 193, 1–24. [Google Scholar] [CrossRef]
- Rodríguez, E.; Fautin, D.; Daly, M. World List of Actiniaria. Actinostolidae Carlgren, 1932. World Register of Marine Species. Available online: https://www.marinespecies.org/aphia.php?p=taxdetails&id=100655 (accessed on 7 January 2025).
- Verrill, A.E. Notice of recent additions to the marine fauna of the eastern coast of North America, No. 5. Am. J. Sci. Arts 1879, 17, 472–474. [Google Scholar] [CrossRef]

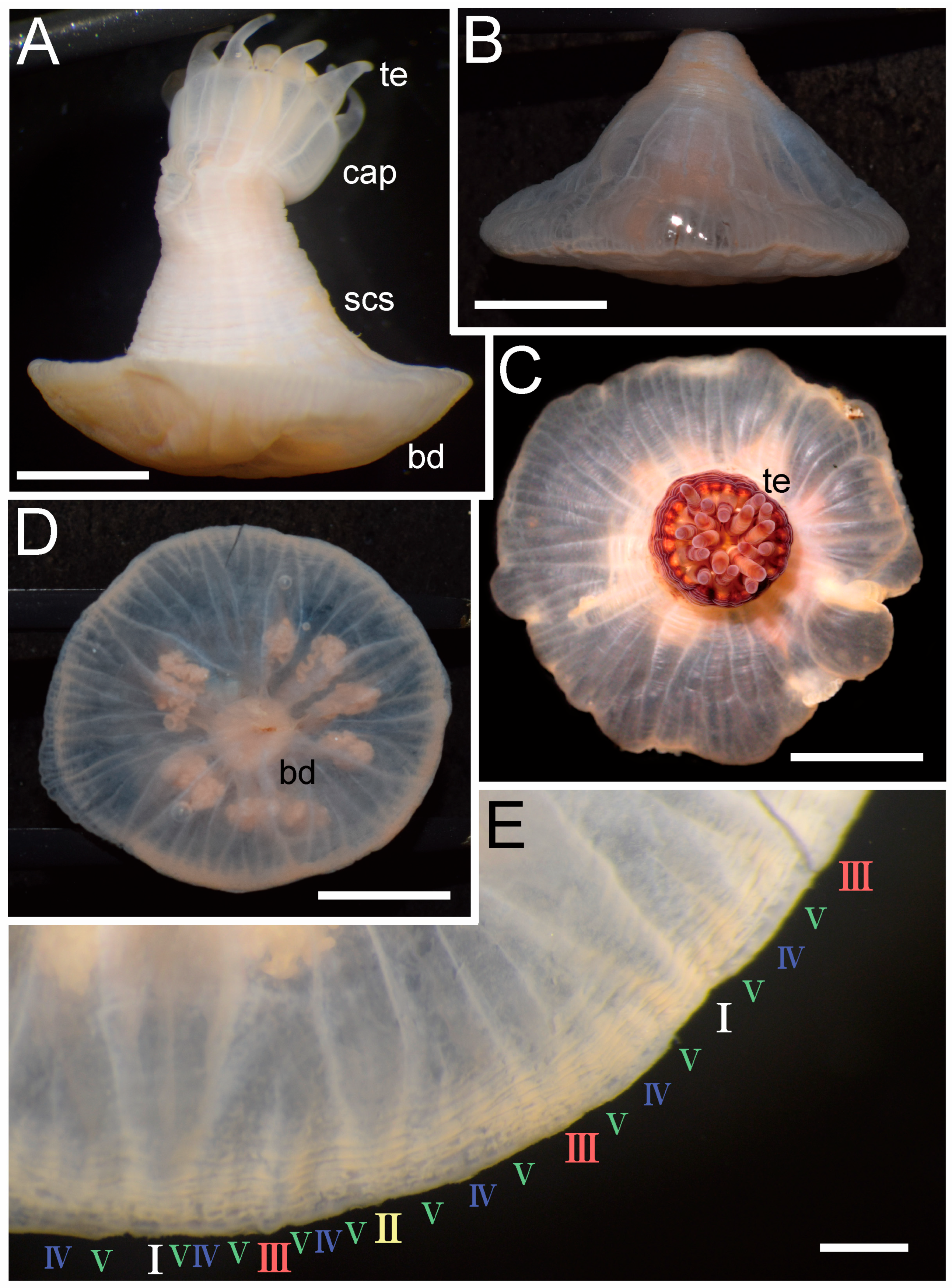
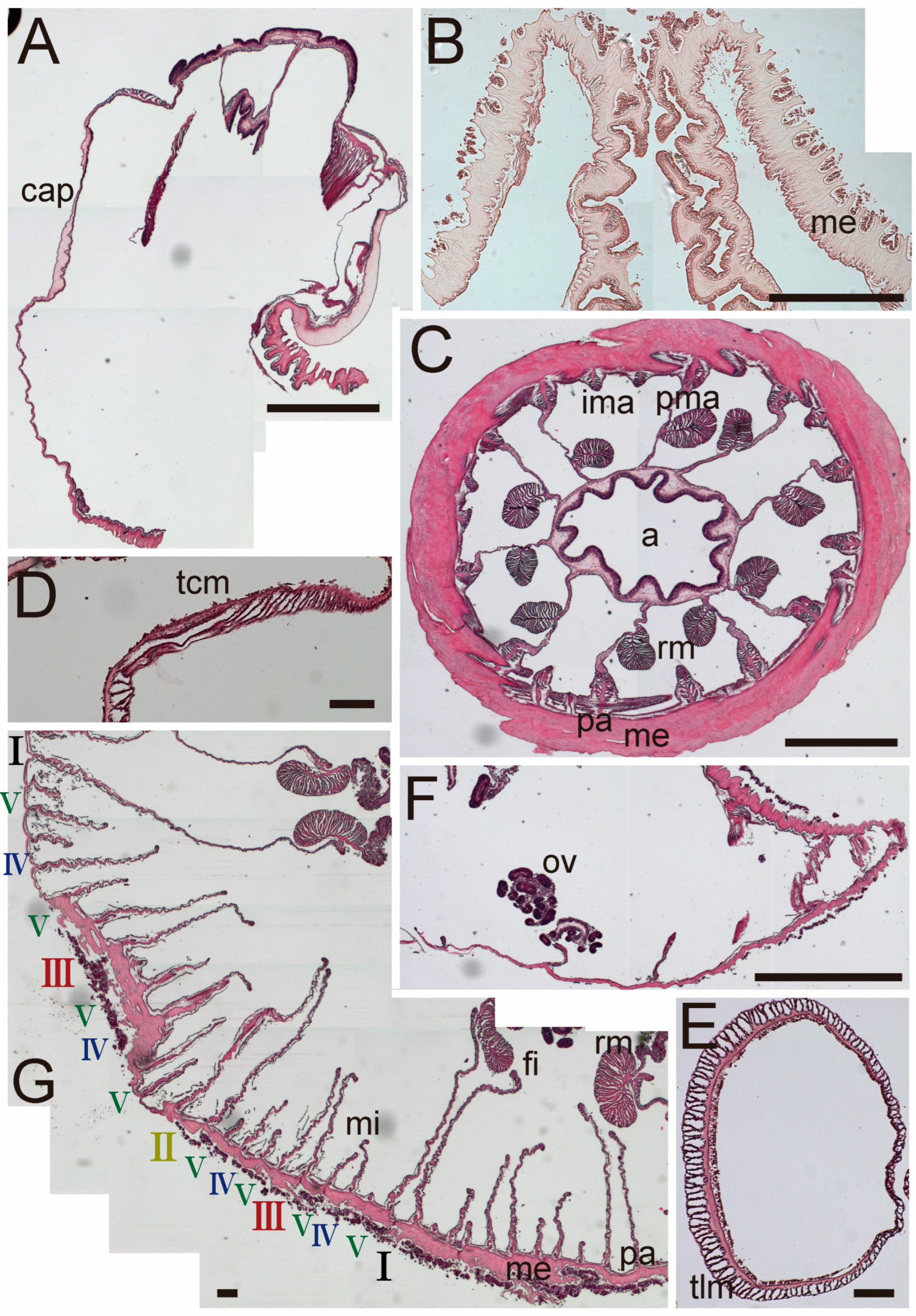
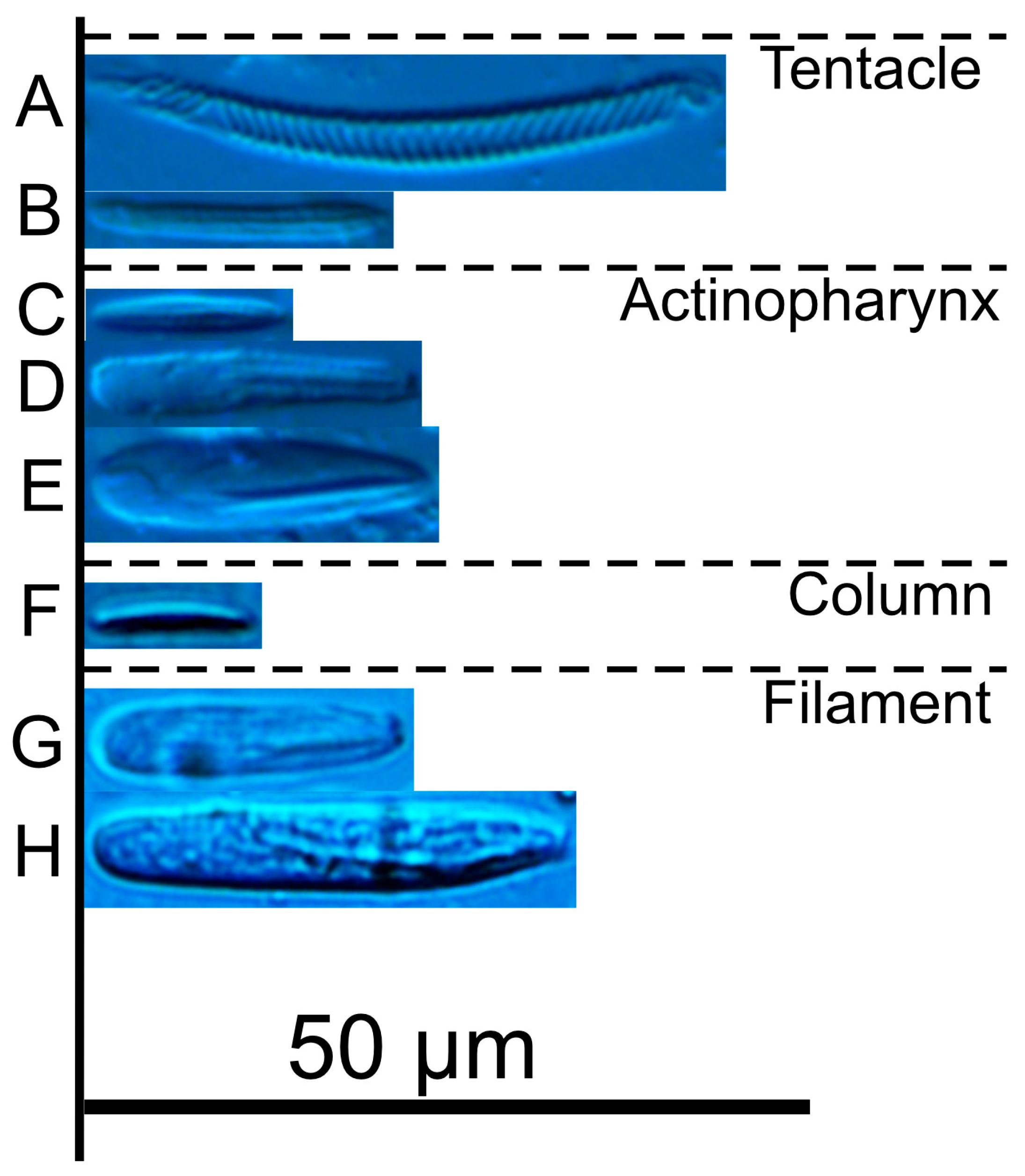
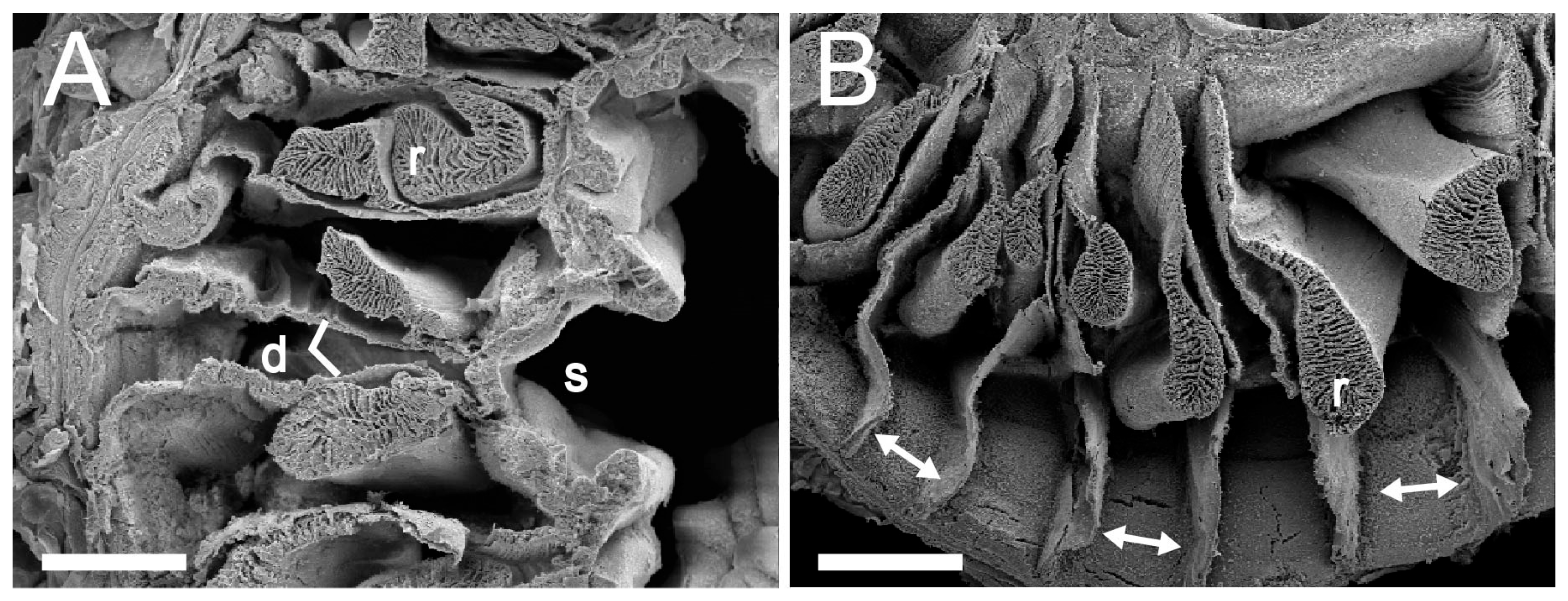
| Discoactis tritentaculata Izumi, Yanagi, and Kohtsuka, 202x | ||||||||||||
| CMNH-ZG 10795 (Suruga Bay) | NSMT-Co 1908 (Sagami Bay) | |||||||||||
| Length × Width (µm) | Mean | SD | N | Frequency | Length × Width (µm) | Mean | SD | N | Frequency | |||
| Tentacle | ||||||||||||
| basitrichs | 13.6–21.9 × 2.0–3.3 | 18.6 × 2.6 | 1.71 × 0.32 | 27 | numerous | 13.4–20.5 × 1.7–3.1 | 17.0 × 2.5 | 1.95 × 0.35 | 29 | numerous | ||
| spirocysts | 17.5–52.0 × 2.4–4.5 | 32.4 × 3.3 | 8.28 × 0.46 | 88 | numerous | 15.3–41.4 × 2.1–4.6 | 25.6 × 3.3 | 6.99 × 0.66 | 48 | numerous | ||
| Actinopharynx | ||||||||||||
| basitrichs | 11.7–15.4 × 1.9–2.9 | 13.3 × 2.4 | 1.07 × 0.28 | 13 | few | 11.8–14.1 × 1.8–2.6 | 13.4 × 2.1 | 0.82 × 0.28 | 5 | rare | ||
| microbasic p-mastigophores | gracile | 19.5–26.9 × 3.4–4.8 | 23.6 × 4.0 | 1.44 × 0.36 | 36 | numerous | 20.8–25.5 × 3.3–5.1 | 22.8 × 4.2 | 1.24 × 0.39 | 26 | numerous | |
| robust | 20.9–25.6 × 5.4–7.4 | 23.6 × 6.4 | 1.15 × 0.47 | 34 | numerous | 20.0–25.3 × 5.3–6.7 | 22.3 × 5.8 | 1.62 × 0.41 | 11 | few | ||
| Column | ||||||||||||
| basitrichs | 11.2–15.9 × 2.0–2.4 | 13.2 × 2.2 | 1.71 × 0.15 | 4 | rare | 10.0–16.2 × 1.7–2.8 | 13.1 × 2.3 | 1.88 × 0.30 | 4 | rare | ||
| Filament | ||||||||||||
| spirocysts | - | - | - | - | none | 21.3–26.6 × 2.5–3.0 | 24.6 × 2.8 | 2.39 × 0.25 | 3 | rare | ||
| holotrichs | - | - | - | - | none | 23.1–23.7 × 6.3–6.6 | 23.5 × 6.5 | 0.35 × 0.17 | 2 | rare | ||
| microbasic p-mastigophores | small | 18.8–21.7 × 4.1–6.2 | 20.6 × 4.9 | 0.82 × 0.51 | 10 | few | 16.2–20.8 × 3.3–5.1 | 18.2 × 4.2 | 1.50 × 0.50 | 9 | few | |
| large | 28.2–36.1 × 4.8–7.6 | 32.2 × 6.5 | 1.71 × 0.59 | 75 | numerous | 22.5–28.6 × 4.7–6.2 | 25.2 × 5.5 | 1.49 × 0.38 | 33 | numerous | ||
| Discoactis tritentaculata Izumi, Yanagi, and Kohtsuka, 202x | ||||||||||||
| CMNH-ZG 10797 (Otsuchi) | NSMT-Co 1908 (Sea of Japan) | |||||||||||
| Length × Width (µm) | Mean | SD | N | Frequency | Length × Width (µm) | Mean | SD | N | Frequency | |||
| Tentacle | ||||||||||||
| basitrichs | 14.2–18.9 × 2.0–3.7 | 16.5 × 2.5 | 0.99 × 0.25 | 29 | numerous | 13.9–18.3 × 2.1–2.9 | 16.3 × 2.5 | 1.20 × 0.22 | 15 | numerous | ||
| spirocysts | 15.2–30.1 × 2.1–3.8 | 22.6 × 3.2 | 4.57 × 0.46 | 20 | numerous | 14.2–42.4 × 2.1–4.1 | 24.8 × 2.9 | 6.77 × 0.51 | 49 | numerous | ||
| Actinopharynx | Damaged | |||||||||||
| basitrichs | 14.5–20.6 × 2.3–3.0 | 18.3 × 2.6 | 2.05 × 0.25 | 7 | few | |||||||
| microbasic p-mastigophores | 16.6–26.3 × 3.5–6.7 | 23.7 × 4.8 | 2.04 × 0.98 | 24 | numerous | |||||||
| Column | ||||||||||||
| basitrichs | 10.2–15.5 × 1.9–3.3 | 13.1 × 2.5 | 1.34 × 0.35 | 17 | numerous | 10.6–20.1 × 1.9–3.3 | 13.2 × 2.5 | 3.08 × 0.45 | 7 | few | ||
| Filament | No cnidae observed in the specimen | |||||||||||
| basitrichs | 17.2–21.1 × 1.7–3.0 | 19.4 × 2.5 | 1.17 × 0.28 | 22 | numerous | |||||||
| spirocysts | 27.1–31.2 × 2.5–3.0 | 29.1 × 2.7 | 2.02 × 0.25 | 2 | rare | |||||||
| microbasic p-mastigophores | small | 17.2–26.8 × 3.5–6.6 | 22.0 × 5.1 | 2.50 × 0.72 | 58 | numerous | ||||||
| large | ||||||||||||
| Discoactinidae fam. nov. | Capneidae Gosse, 1860 | Actinostolidae Carlgren, 1932 | Anthosactinidae Sanamyan, Sanamyan, Galkin, Ivin, and Bocharova, 2021 | ||
| Type genus | Discoactis gen. nov. | Capnea Forbes, 1841 | Actinostola Verrill, 1879 | Anthosactis Danielssen, 1890 | |
| The other genera | None | Actinoporus Duchassaing, 1850 | Antholoba Hertwig, 1882 Antiparactis Verrill, 1899 Bathydactylus Carlgren, 1928 Cnidanthus Carlgren, 1927 Glandulactis Riemann-Zürneck, 1978 Hadalanthus Carlgren, 1956 Ophiodiscus Hertwig, 1882 Paranthus Andres, 1883 Parasicyonis Carlgren, 1921 Pseudoparactis Stephenson, 1920 Pycnanthus McMurrich, 1893 Stomphia Gosse, 1859 Synsicyonis Carlgren, 1921 | Hormosoma Stephenson, 1918 Tealidium Hertwig, 1882 | |
| Characters | |||||
| Number of macrocnemes in the first cycle | 10 | 12 | 12 | 12 | |
| Marginal sphincter muscle | Present but weak | Present, strong | Present | Present | |
| (origin) | (mesogleal) | (endodermal) | (mesogleal) | (mesogleal) | |
| Location of unequal mesenterial pairs | Ventrolateral mesenteries in first cycle | None | Third cycle or fourth cycle | Fourth cycle | |
| Remarks on a mesenterial developing rule | Original development | Following the general rule of Actiniaria | Generally following the “Actinostola rule” | ||
| References | The present study | [5,15,16,38] | [4,5,10,14,43,44] | [14] | |
| Sicyonidae Hertwig, 1882 | Exocoelactinidae Carlgren, 1925 | Tetracoelactinidae Sanamyan, Sanamyan, Galkin, Ivin, and Bocharova, 2021 | Halcampulactidae Gusmão, Berniker, Deusen, Harris, and Rodriguez, 2019 | ||
| Type genus | Sicyonis Hertwig, 1882 | Exocoelactis Carlgren, 1925 | Tetracoelactis Sanamyan and Sanamyan, 2019 | Halcampulactis Gusmão, Berniker, Deusen, Harris, and Rodríguez, 2019 | |
| The other genera | None | None | None | None | |
| Characters | |||||
| Number of macrocnemes in the first cycle | 12 | 12 | 12 | 8 | |
| Marginal sphincter muscle | Absent | Present | Present | Absent | |
| (Origin) | - | (mesogleal) | (mesogleal) | - | |
| Location of unequal mesenterial pairs | Equal | After the third cycle | Second and third cycles | Lateral mesenteries in the first cycle | |
| Remarks of mesenterial developing rule | Following to “Exocoelactis rule” | (same as Edwardsiidae) | |||
| References | [14] | [4,41] | [14,37] | [12] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izumi, T.; Yanagi, K.; Kohtsuka, H. Mt. Fuji in the Ocean–Description of a Strange New Species of Sea Anemone, Discoactis tritentaculata fam., gen., and sp. nov. (Cnidaria; Anthozoa; Actiniaria; Actinostoloidea) from Japan, with the Foundation of a New Family and Genus. Diversity 2025, 17, 430. https://doi.org/10.3390/d17060430
Izumi T, Yanagi K, Kohtsuka H. Mt. Fuji in the Ocean–Description of a Strange New Species of Sea Anemone, Discoactis tritentaculata fam., gen., and sp. nov. (Cnidaria; Anthozoa; Actiniaria; Actinostoloidea) from Japan, with the Foundation of a New Family and Genus. Diversity. 2025; 17(6):430. https://doi.org/10.3390/d17060430
Chicago/Turabian StyleIzumi, Takato, Kensuke Yanagi, and Hisanori Kohtsuka. 2025. "Mt. Fuji in the Ocean–Description of a Strange New Species of Sea Anemone, Discoactis tritentaculata fam., gen., and sp. nov. (Cnidaria; Anthozoa; Actiniaria; Actinostoloidea) from Japan, with the Foundation of a New Family and Genus" Diversity 17, no. 6: 430. https://doi.org/10.3390/d17060430
APA StyleIzumi, T., Yanagi, K., & Kohtsuka, H. (2025). Mt. Fuji in the Ocean–Description of a Strange New Species of Sea Anemone, Discoactis tritentaculata fam., gen., and sp. nov. (Cnidaria; Anthozoa; Actiniaria; Actinostoloidea) from Japan, with the Foundation of a New Family and Genus. Diversity, 17(6), 430. https://doi.org/10.3390/d17060430









