A Review of Bat Fleas (Siphonaptera: Ischnopsyllidae) from Russia
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Medvedev, S.G. Features of the evolution of bat fleas. Parazitologiya 1990, 24, 457–465. (In Russian) [Google Scholar]
- Krichbaum, K.; Perkins, S.; Gannon, M.R. Host-parasite interactions of tropical bats in Puerto Rico. Acta Chiropterologica 2009, 11, 157–162. [Google Scholar] [CrossRef]
- Wagner, J. Aphanipterologische Studien. III. Proc. Russ. Entomol. Soc. 1898, 31, 555–594. [Google Scholar]
- Wagner, J. Notes on Aphaniptera of the Crimean Peninsula. Proc. Mus. Tauride Prov. Zemstvo 1914—Simferopol 1916, 1–6. [Google Scholar]
- Wagner, J. Vierter Nachtrag zum Kataloge der Palaearktischen Aphanipteren, 1st ed.; Konowia: Vienna, Austria, 1930; 55p. (In German) [Google Scholar]
- Dampf, A. Eine neue Aphanipteren-Art (Ischnopsyllus dolosus sp. n.) aus dem Kaukasus. Rev. Russe d’Entomol. 1912, 12, 41–59. (In German) [Google Scholar]
- Ioff, I.G. New instances of Formation of Species in Fleas when changing the Host. Dokl. Akad. Nauk SSSR 1953, 89, 189–192. [Google Scholar]
- Ioff, I.G.; Skalon, O.I. Key to flea of Eastern Siberia, Far East and Adjacent Regions; Medgiz: Moscow, Russia, 1954; 275p. (In Russian) [Google Scholar]
- Ioff, I.G.; Tiflov, V.E. Key to Aphaniptera (Suctoria–Aphaniptera) of the South–East of the USSR; Stavropol Book Publisher: Stavropol, Russia, 1954; 201p. [Google Scholar]
- Ioff, I.G.; Bondar, E.P. The fleas of Turkmenistan. Tr. Nauch.-Issled. protivochumn. Inst. Kavk. Zakavk. 1956, 1, 29–119. (In Russian) [Google Scholar]
- Ioff, I.G.; Ivanova, M.A. Aphaniptera Armenii. Zool. Sb. 1956, 9, 21–31. (In Russian) [Google Scholar]
- Skalon, O.I. Materials on the fauna of fleas (Aphaniptera) of Siberia and the Far Eastern region. News Irkutsk. State Res. Anti-Plague Inst. Sib. Far East 1936, 6, 46–57. [Google Scholar]
- Scalon, O.I. New species bat fleas Ischnopsyllus (Hexactenopsylla) transcaucasicus spfn. (Siphonaptera, Ischnopsyllidae). Entomol. Rev. 1979, 58, 901–903. [Google Scholar]
- Zhovtiy, I.F.; Zarubina, V.N.; Prokopiev, V.N.; Shvedko, L.P. About bat ectoparasites of Southeast Transbaikalia and adjacent areas of the Mongolian People’s Republic. Proc. Irkutsk. State Sci. AntiPlague Inst. Sib. Far East 1962, 24, 338–343. (In Russian) [Google Scholar]
- Labunets, N.F.; Degtyareva, L.V. About bat fleas in Northern Caucasus. Parazitologiya 1985, 19, 177–180. (In Russian) [Google Scholar]
- Kucheruk, V.V. The Successes of Modern Theriology. Mammals Are Carriers of Diseases Dangerous to Humans; Kucheruk, V.V., Ed.; Nauka: Moscow, Russia, 1977; pp. 75–92. (In Russian) [Google Scholar]
- Zhdanov, V.M.; Lvov, D.K. Evolution of Pathogens of Infectious Diseases; Medicine: Moscow, Russia, 1984; 300p. (In Russian) [Google Scholar]
- Kucheruk, V.V. (Ed.) Medical Theriology, 1st ed.; Nauka: Moscow, Russia, 1989; 269p. [Google Scholar]
- Medvedev, S.G. The structure of the aedeagus of Ischnopsyllidae (Siphonaptera). Entomol. Rev. 1984, 63, 236–249. (In Russian) [Google Scholar]
- Medvedev, S.G. Revision of the family Ischnopsyllidae (Siphonaptera). Parazitologiya 1985, 19, 14–26. (In Russian) [Google Scholar]
- Medvedev, S.G. Fleas of Family Ischnopsyllidae (Siphonaptera) USSR. Abstract of Ph.D. Thesis, Zoological institute of Russian Academy of Sciences, Saint Petersburg, Russia, 1984; 24p. [Google Scholar]
- Medvedev, S.G. Ecological peculiarities and distribution of fleas of fam. Ischnopsyllidae (Siphonaptera). Parasitol. Sb. 1989, 36, 21–43. (In Russian) [Google Scholar]
- Medvedev, S.G. Ecology of fleas of the family Ischnopsyllidae (Siphonaptera) of the fauna of the USSR. Parasitol. Dig. 1992, 37, 17–40. [Google Scholar]
- Medvedev, S.G.; Stanyukovich, M.K.; Tiunov, M.P.; Farafonova, G.V. Ectoparasites of bats of the Russian Far East. Parazitologiya 1991, 25, 27–37. (In Russian) [Google Scholar]
- Medvedev, S.G.; Mazing, M.V. Fleas of fam. Ischnopsyllidae (Siphonaptera) of Baltic States. Parazitologiya 1987, 21, 459–466. (In Russian) [Google Scholar]
- Medvedev, S.G.; Chistyakov, D.V.; Stanyukovich, M.K.; Paskhina, M.V. Bat ectoparasites fauna of Sebezh National Park. Nat. Pskov Reg. 2000, 11, 22–25. (In Russian) [Google Scholar]
- Medvedev, S.G.; Khabilov, T.K.; Rybin, S.N. On the biology of bat fleas (Ischnopsyllidae; Siphonaptera) Central Asia and Southern Kazakhstan. Parazitologiya 1984, 18, 140–149. (In Russian) [Google Scholar]
- Medvedev, S.G.; Polkanov, A.Y. Contribution to the flea fauna of fam. Ischnopsyllidae (Siphonaptera) of Middle Asia and Kazakhstan. Parazitologiya 1997, 31, 13–22. (In Russian) [Google Scholar]
- Medvedev, S.G. Host-Parasite Relations in Fleas (Siphonaptera). II. Entomol. Rev. 1997, 77, 511–521. [Google Scholar]
- Medvedev, S.G. Structure of the Thorax of Fleas (Siphonaptera). III. Entomol. Rev. 1991, 70, 774–784. [Google Scholar]
- Medvedev, S.G. Structure of the Aedeagus of Fleas (Siphonaptera). II. Entomol. Rev. 1993, 72, 519–536. [Google Scholar]
- Medvedev, S.G. Structure of the Modified Abdominal Segments in Fleas (Siphonaptera). Entomol. Rev. 1993, 72, 747–763. [Google Scholar]
- Orlova, M.V. Ectoparasite associations of bats from the Urals (Russia). Hystrix Ital. J. Mammal. 2011, 22, 105–110. [Google Scholar] [CrossRef]
- Orlova, M.V.; Zhigalin, A.V.; Orlov, O.L. New records of ectoparasites of the eastern water bat Myotis petax Hollister, 1912 (Vespertilionidae, Chiroptera) and the revision of the material previously collected from Myotis daubentonii s. lato in the Eastern Palaearctic. Entomol. Rev. 2014, 94, 1306–1312. [Google Scholar] [CrossRef]
- Malyavina, M.S.; Smirnov, D.G. Structure of ectoparasite communities of Nyctalus species (Chiroptera: Vespertilionidae) in the Zhiguli State Nature Reserve and Samarskaya Luka National Park (European Russia). Nat. Conserv. Res. 2024, 9, 1–24. [Google Scholar] [CrossRef]
- Orlova, M.V.; Zhigalin, A.V.; Orlov, O.L.; Kruskop, S.V.; Bogdanov, I.I. Contribution to the ectoparasite fauna of rare and poor studied bat species of Southern Siberia. Biol. Bull. 2015, 42, 254–259. [Google Scholar] [CrossRef]
- Orlova, M.V.; Zhigalin, A.V.; Khritankov, A.M. New findings of bat ectoparasites (Chiroptera: Vespertilionidae) in Southern Siberia. Entomol. Rev. 2015, 95, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Orlova, M.V.; Kazakov, D.V.; Gashev, S.N. New data on ectoparasites (Acarina; Insecta) of bats (Chiroptera: Vespertilionidae) of Baikal Siberia. Bull. Mosc. Soc. Nat. 2016, 121, 20–25. [Google Scholar] [CrossRef]
- Medvedev, S.G. Morphological basis of the classification of fleas (Siphonaptera). Entomol. Rev. 1995, 73, 30–51. [Google Scholar]
- Baryshnikov, G.F.; Garutt, V.E.; Gromov, I.M.; Gureev, A.A.; Kuz’mina, I.E.; Sokolov, A.S.; Strelkov, P.P.; Godina, A.V.; Zhegallo, V.I. Catalog of Mammals of the USSR (Pliocene–Modernity); Gromov, I.M., Baranova, G.I., Eds.; Nauka: Saint Petersburg, Russia, 1981; Volume 2, pp. 31–53. (In Russian) [Google Scholar]
- Dietz, C.; Von Helversen, O.; Nill, D. Bats of Britain, Europe and Northwest Africa; Black Publishers Ltd.: London, UK, 2009; 400p, ISBN 1408105314/9781408105313. [Google Scholar]
- Simmons, N.B. Order Chiroptera. In Mammal species of the World, 3rd ed.; A taxonomic and geographic reference; Wilson, D.E., Reeder, D.M., Eds.; John Hopkins University Press, College Park: Baltimore, MD, USA, 2005; Volume 1, pp. 312–529. ISBN 0801882214/9780801882210. [Google Scholar]
- Matveev, V.A.; Kruskop, S.V.; Kramerov, D.A. Revalidation of Myotis petax Hollister, 1912 and its new status in connection with Myotis daubentonii (Kuhl, 1817) (Vespertilionidae, Chiroptera). Acta Chiropterologica 2005, 7, 23–37. [Google Scholar] [CrossRef]
- Kruskop, S.V. Order Chiroptera. In Mammals of Russia: A Systematic and Geographical Reference Book; Pavlinov, I.Y., Lisovsky, A.A., Eds.; KMK Scientific Publications: Moscow, Russia, 2012; pp. 73–126. 604p. [Google Scholar]
- Benda, P.; Faizolâhi, K.; Andreas, M.; Obuch, J.; Reiter, A.; Ševčík, M.; Uhrin, M.; Vallo, P.; Ashrafi, S. Bats (Mammalia: Chiroptera) of the Eastern Mediterranean and Middle East. Part 10. Bat fauna of Iran. Acta Soc. Zool. Bohemicae 2012, 76, 163–582. [Google Scholar]
- Kruskop, S.V.; Borisenko, A.V.; Ivanova, N.V.; Lim, B.K.; Iger, J.L. The use of DNA barcodes for identifying phylogeographic breaks among bats of Eastern Palaearctic. In Molekulyarno-Geneticheskie Osnovy Sokhraneniya Bioraznoobraziya Mlekopitayushchikh Golarktiki: Materialy Mezhdunarodnoi Konkerentsii Molecular and Genetic Bases of biodiversity of Mammals of Holarctic: Proceedings of International Conference; KMK Ltd.: Moscow, Russia, 2007; pp. 115–121. [Google Scholar]
- Kruskop, S.V.; Borisenko, A.V.; Ivanova, N.V.; Lim, B.K.; Iger, J.L. Genetic diversity of northeastern Palaearctic bats as revealed by DNA barcodes. Acta Chiropterologica 2012, 14, 1–14. [Google Scholar] [CrossRef]
- Horaček, I.; Hanak, V. Comments on the systematics and phylogeny of Myotis nattereri (Kuhl, 1818). Myotis 1984, 21, 20–29. [Google Scholar]
- Yoshiyuki, M. A Systematic Study of the Japanese Chiroptera; National Science Museum: Tokyo, Japan, 1989; ISSN 13429574.
- Kawai, K.; Nikaido, M.; Harada, M.; Matsumura, S.; Lin, L.K.; Wu, Y.; Hasegawa, M.; Okada, N. The status of the Japanese and East Asian bats of the genus Myotis (Vespertilionidae) based on mitochondrial sequences. Mol. Phylogenetics Evol. 2003, 28, 297–307. [Google Scholar] [CrossRef]
- Appleton, B.R.; McKenzie, J.A.; Christidis, L. Molecular systematics and biogeography of the bentwing bat complex Miniopterus schreibersii (Kuhl, 1817) (Chiroptera: Vespertilionidae). Mol. Phylogenetics Evol. 2004, 31, 431–439. [Google Scholar] [CrossRef]
- Tian, L.; Liang, B.; Maeda, K.; Metzner, W.; Zhang, S. Molecular studies on the classification of Miniopterus schreibersii (Chiroptera: Vespertilionidae) inferred from mitochondrial cytochrome b sequences. Folia Zool. 2004, 53, 303–311. [Google Scholar]
- Whitaker, J.O., Jr. Collecting and preserving ectoparasites for ecological study. In Ecological and Behavioral Methods for the Study of Bats; Kunz, T.H., Ed.; Smithsonian Institution Press: Washington, DC, USA, 1988; pp. 459–474. [Google Scholar]
- Hopkins, G.H.E.; Rothschild, M. An Illustrated Catalogue of the Rothschild Collection of Fleas (Siphonaptera) in the British Museum (Natural History). Vol. II: Coptopsyllidae, Vermipsyllidae, Stephanocircidae, Ischnopsyllidae, Hypsopthalmidae and Xiphiopsyllidae; Oxford University Press: London, UK, 1956. [Google Scholar]
- Medvedev, S.G. Fleas of fam. Ischnopsyllidae (Siphonaptera) in fauna of Russia and adjacent countries. Entomol. Rev. 1996, 75, 438–454. [Google Scholar]
- Dormann, C.F.; Gruber, B.; Fründ, J. Introducing the bipartite package: Analysing ecological networks. Interaction 2008, 1, 8–11. [Google Scholar]
- Smit, F.G.A.M. A catalogue of the Siphonaptera of Finland with distribution maps of all Fennoscandian species. Ann. Zool. Fenn. 1969, 6, 47–86. Available online: https://www.jstor.org/stable/23731537 (accessed on 29 May 2025).
- Markova, L.I. Influence of hibernation on bat parasite fauna. Zool. Zhurnal 1938, 17, 133–145. (In Russian) [Google Scholar]
- Vysotskaja, S.O.; Sazonova, O.N. About fleas of the fauna of Leningrad district. Parazitol. Sb. Zool. Instituta Akad. Nauch SSSR 1953, 15, 386–409. (In Russian) [Google Scholar]
- Vashchenok, V.S. Species composition of fleas (Siphonaptera) of North-West of Russia. Parazitologiya 1996, 30, 410–424. (In Russian) [Google Scholar]
- Tiflov, V.E.; Skalon, O.I.; Rostigaev, B.A. Key to Fleas of Caucasus; Stavropol’skoe Knizhnoe Izdatel’stvo: Stavropol, Russia, 1977. (In Russian) [Google Scholar]
- Aleksanov, V.V.; Alekseev, S.K.; Bolshakov, L.V.; Vasilyeva, O.L.; Galchenkov, Y.D.; Garkunov, M.I.; Karpushin, S.E.; Korzikov, V.A.; Perov, V.V.; Khvaletsky, D.V. Fauna of the Kaluga City Forest; Series “Cadastral and Monitoring Studies of Biological Diversity in the Kaluga Region”; Eidos: Kaluga, Russia, 2022. [Google Scholar]
- Ioff, I.G.; Tiflov, V.E. On the Fauna and Ecology of Forest-Steppe Fleas; Parasitological Collection: Saint Petersburg, Russia, 1930; Volume 3, pp. 193–227. [Google Scholar]
- Orlova, M.V.; Kapitonov, V.I.; Griroriev, A.K.; Orlov, O.L. Bat ectoparasites of Udmurtia Republic. Vestn. Udmurt. Univ. Ser. Biol. Nauk. Zemle 2011, 2, 134–138. (In Russian) [Google Scholar]
- Orlova, M.V.; Thong, V.D.; Smirnov, D.G.; Zabashta, A.V.; Orlov, O.L. New geographical and host records of bat fleas (Siphonaptera: Ischnopsyllidae) in Russia. Ann. Parasitol. 2022, 68, 121–128. (In Russian) [Google Scholar]
- Bernikov, K.A.; Starikov, V.P. Fauna and ecological-biological peculiarity of bats (Chiroptera) of Khanty-Mansi Autonomous Region. Vestn. Orenb. Univ. 2009, 2, 117–123. (In Russian) [Google Scholar]
- Orlova, M.V.; Tomishina, A.A. New findings of bat ectoparasites (Acari, Insecta) in “Malaya Sos’va” Nature Reserve, with the revision of previous findings on the Territory of the Khanty-Mansi Autonomous Area. Plecotus 2019, 22, 49–58. (In Russian) [Google Scholar]
- Zakharov, E.S.; Troeva, I.S.; Orlova, M.V.; Koryakina, L.P.; Pavlova, A.I. On the Fauna and Ecology of Bats in Central Yakutia. Nat. Resour. Arct. Subarct. 2016, 4, 134–140. (In Russian) [Google Scholar]
- Darskaya, N.F.; Suvorova, L.G. The flea fauna of the tick-borne encephalitis focal region in the eastern part of the Russian Plain. Folia Parasitol. 1984, 31, 69–77. [Google Scholar] [PubMed]
- Nazarova, I.V. Fleas of Volga-Kama Territory; Nauka: Moscow, Russia, 1981; 168p. (In Russian) [Google Scholar]
- Jaunbauere, G.; Salmane, I.; Spungis, V. Occurrence of bat ectoparasites in Latvia. Latv. Entomol. 2008, 45, 38–42. [Google Scholar]
- Vinarskaya, N.; Orlova, M. Ectoparasites of the pond bats (Myotis dasycneme Boie, 1825) and parti-coloured bats (Vespertilio murinus Linnaeus, 1758 non Schreber, 1775) in the Middle Urals. J. Sci. Dialogue 2012, 2, 23–30. (In Russian) [Google Scholar]
- Strelkov, P.P.; Ilyin, V.Y. Bats (Chiroptera, Vespertilionidae) of the southern Middle and Lower Volga region. Proc. Zool. Inst. USSR Acad. Sci. 1990, 225, 42–167. (In Russian) [Google Scholar]
- Orlova, M.V.; Kazakov, D.V.; Kravchenko, L.B.; Zhigalin, A.V. Ectoparasite fauna of the Siberian bat Myotis sibiricus (Chiroptera: Vespertilionidae) with a revision of previous data on ectoparasites from Brandt’s bat Myotis brandtii s. l. and the whiskered bat M. mystacinus s. l. of the Eastern Palaearctic. Entomol. Rev. 2017, 97, 1166–1173. [Google Scholar] [CrossRef]
- Takahashi, M.; Misumi, H.; Kawai, K.; Sato, M. The first finding of a bat flea Myodopsylla trisellis (Siphonaptera: Ischnopsyllidae) on Myotis gracilis (Chiroptera: Vespertilionidae) in Japan. Med. Entomol. Zool. 2016, 67, 29–33. [Google Scholar] [CrossRef]
- Komarov, Y.E.; Labunets, N.F. Fleas Inhabiting Bird Nests in the Mountain Part of North Ossetia. In Parasitological Studies in Nature Reserves, Ivanov, I.A., Ed.; TSNIL Glavokhot: Moscow, Russia, 1983; pp. 94–97. (In Russian) [Google Scholar]
- Kotti, B.K. Fleas (Siphonaptera) of mammals and birds in the Great Caucasus. Entomol. Rev. 2015, 95, 728–738. [Google Scholar] [CrossRef]
- Albayrak, I. The bats of the Eastern Black Sea region in Turkey (Mammalia: Chiroptera). Turk. J. Zool. 2003, 27, 269–273. [Google Scholar]
- Benda, P.; Gazaryan, S.; Vallo, P. On the distribution and taxonomy of bats of the Myotis mystacinus morphogroup from the Caucasus region (Chiroptera: Vespertilionidae). Turk. J. Zool. 2016, 40, 842–863. [Google Scholar] [CrossRef]
- Orlova, M.V.; Orlov, O.L.; Kazakov, D.V.; Zhigalin, A.V. Approaches to the identification of ectoparasite complexes of bats (Chiroptera: Vespertilionidae, Miniopteridae, Rhinolophidae, Molossidae) in Palaearctic. Entomol. Rev. 2017, 97, 684–701. [Google Scholar] [CrossRef]
- Hůrka, K. Bat fleas (Aphaniptera, Ischnopsyllidae) of Czechoslovakia. Contribution to the distribution, morphology, bionomy, ecology and systematics. Pt, I. Subgenus Ischnopsyllus Westw. Acta Faun. Entomol. Musei Natl. Pragae 1963, 9, 57–120. [Google Scholar]
- George, R.S. Provisional Atlas of the Insects of the British Isles. Part 4. Siphonaptera; Natural Environment Research Council: Swindon, UK, 1974; ISBN 0900848707. [Google Scholar]
- Sleeman, D.P.; Smiddy, P. Bat fleas in Ireland: A review. Ir. Nat. J. 1994, 24, 444–448. Available online: http://www.jstor.org/stable/25535847 (accessed on 29 May 2025).
- Walter, G.; Kock, D. Distribution and host species of German bat fleas (Insecta: Siphonaptera: Ischnopsyllidae). Senckenberg. Biol. 1994, 74, 103–125. [Google Scholar]
- Brinck-Lindroth, G.; Smit, F.G. The fleas (Siphonaptera) of Fennoscandia and Denmark; Brill: Leiden, The Netherlands, 2007; Volume 41, ISBN 978-90-04-15151-2. [Google Scholar]
- Sakaguti, K.E.; Jameson, W., Jr. The Siphonaptera of Japan; Bishop Museum: Honolulu, HI, USA, 1962; 169p. [Google Scholar]
- Li, G.; Wang, D.; Xie, B. Ischnopsyllidae. In Fauna Sinica, Insecta, Siphonaptera; Liu, Z., Ed.; Science Press: Beijing, China, 1986; pp. 621–661. ISBN 9787030157409. (In Chinese) [Google Scholar]
- Ioff, I.G. Fleas (Aphaniptera) of Belovezhskaya Pushcha (with Notes about Geography of Fleas of Forest Zone of Northern Europe); Ectoparasites: Moscow, Russia, 1956; Volume 3, pp. 127–148. [Google Scholar]
- Porshakov, A.M.; Lamikhov, A.M.; Ozerova, R.A. Parasitocenosis of bats in the Saratov region. Entomol. Parasitolog. Stud. Volga Region 2007, 139–140. (In Russian) [Google Scholar]
- Shtutser, M.I.; Ioff, I.G. (Eds.) Digest of works of the anti-plague organization of the North Caucasus region for 1928. Proc. State Microbiolog. Inst. Rostov-on-Don 1929, 9. [Google Scholar]
- Zabashta, M.; Orlova, M.; Pichurina, N.; Khametova, A.; Romanova, L.; Borodina, T.; Zabashta, A. Participation of Bats (Chiroptera, Mammalia) and Their Ectoparasites in Circulation of Pathogens of Natural Focal Infections in the South of Russia. Entomol. Rev. 2019, 99, 1–9. [Google Scholar] [CrossRef]
- Medvedev, S.G. Fleas of Family Ischnopsyllidae (Siphonaptera) USSR. Ph.D. Thesis, Zoological Institute Russian Academy of Sciences, Saint Petersburg, Russia, 1985; 299p. (In Russian). [Google Scholar]
- Quetglas, J.; Nogueras, J.; Ibáñez, C.; Beaucournu, J.C. Presencia en la Península Ibérica de una pulga africana de murciélagos: Rhinolophopsylla unipectinata arabs (Siphonaptera: Ischnopsyllidae) y otras nuevas citas de pulgas de murciélagos para España y Marruecos. Galemys 2014, 26, 41–47. (In Spanish) [Google Scholar] [CrossRef]
- Emelyanova, A.A.; Khristenko, E.A.; Vinogradova, E.A.; Volkova, A.S. The first reliable find of the Leisler’s bat (Nyctalus leisleri) in the Tver region: Morphology, echolocation characteristics, ectoparasites. Plecotus 2022, 25, 29–43. (In Russian) [Google Scholar]
- Demidova, T.N.; Vekhnik, V.P. Bat fleas of the Samara Luka. Ecol. Probl. Prot. Areas Russia 2003, 1, 192–193. (In Russian) [Google Scholar]
- Savenko, R.F. Contribution to the flea fauna (Aphaniptera) of Georgia. Tr. Instituta Zool. GSSR 1950, 103–116. (In Russian) [Google Scholar]
- Dubovchenko, T.A. New species of fleas for the fauna of Azerbaijan, Materialy nauchnoi sessii entomologov Azerbaidzhana (Proc. Sci. Session of Entomologists of Azerbaijan). Baku Akad. Nauk Azerb. SSR 1965, 16, 83–84. [Google Scholar]
- Orlova, M.; Chistiakov, D.; Orlov, O.; Kruger, F.; Kshnyasev, I. Ectoparasite fauna of pond bat Myotis dasycneme (Boie, 1825), (Chiroptera, Vespertilionidae) in Northern Eurasia. Biol. Commun. 2014, 17, 24–38. Available online: https://biocomm.spbu.ru/article/view/1145 (accessed on 29 May 2025).
- Beaucournu, J.C.; Kowalski, K. New data on the fleas (Insecta, Siphonaptera) of Algeria. Bull. Société Pathol. Exot. Ses Fil. 1985, 78, 378–392. (In French) [Google Scholar] [PubMed]
- Ghasemi, F.; Miri, M.; Rajabloo, M. First record of Ischnopsyllus intermedius (Siphonaptera) as an ectoparasite of Myotis blythii (Chiroptera) from southwest of Iran. Small Mammal Mail. Bi-Annu. Newsl. CCINSA RISCINSA 2016, 8, 3–7. [Google Scholar]
- Tiflov, V.E. Materials on the study of the flea fauna (Aphaniptera) of the Saratov province. In Proceedings of the First All-Union Anti-Plague Conference, Saratov, Russia; 1928; pp. 268–275. [Google Scholar]
- Grigor’eva, Y.A.; Abrosimova, E.G. Ectoparasites of Myotis dasycneme (Boie, 1825) in research station of ULGPU. Priroda simbirskogo Povolzh’ya (=Nature of the Simbirsk Volga Region) 2017, 1, 91–95. (In Russian) [Google Scholar]
- Hůrka, K. Příspěvek k systematice, faunistice, bionomii a ekologii netopýřích blech v ČSR. Ceskoslov. Parasitol. 1957, 4, 143–166. (in Czech). [Google Scholar]
- Hůrka, K. On the insect bat ectoparasites of coastal Libya (Cimicidae, Nycteribiidae, Streblidae, Ischnopsyllidae). Věstník Ceskoslov. Společnosti Zool. 1982, 46, 85–91. [Google Scholar]
- Ioff, I.G. Study of vector activity of insects. Parasit. Infect. 1949, 18, 11–20. (In Russian) [Google Scholar]
- Ioff, I.G.; Mikulin, M.A.; Skalon, O.I. The Flea Keys of Central Asia and Kazakhstan; Medicina: Moscow, Russia, 1965; 371p. (In Russian) [Google Scholar]
- Hůrka, K. New data on taxonomy and distribution of Palaearctic, Oriental and Neotropical Ischnopsyllidae (Siphonaptera), Nycteribiidae and Streblidae (Diptera). Acta Soc. Zool. Bohem. 1997, 61, 23–33. [Google Scholar]
- Lewis, R.E. 1998. A catalog of primary types of bat fleas (Siphonaptera: Ischnopsyllidae) of the world. Bat Res. News 2006, 47, 43–60. [Google Scholar]
- Zahn, A.; Rupp, D. Ectoparasite load in European vespertilionid bats. J. Zool. 2004, 262, 383–391. [Google Scholar] [CrossRef]
- Strelkov, P.P.; Iin, V.Y. Bats of easternmost Europe: Distribution and faunal status. In Prague Studies in Mammalogy; Oráček, I., Vohrík, V., Eds.; Charles University Press: Praha, Czech Republic, 1992; pp. 193–205. 245p, ISBN 80-7066-556-4. [Google Scholar]
- Smirnov, D.G.; Yanyaeva, N.M.; Il’in, V.Y. On the issue of hybridization of two forms of Eptesicus serotinus. In Mammals as a Component of Arid Ecosystems (Resources, Fauna, Ecology, Medical Importance and Protection); IPEE RAN: Saratov-Moscow, Russia, 2004; pp. 140–141. [Google Scholar]
- Scheffler, I.; Jargalsaikhan, A.; Bolorchimeg, I.; Stubbe, A.; Stubbe, M.; Abraham, A.; Thiele, K. Bat Ectoparasites of Mongolia, Part 3. Erforschung biologischer Ressourcen der Mongolei. Explor. Into Biol. Resour. Mongolia 2016, 190, 395–408. [Google Scholar]
- Hsü, Y.C. Two new species of insects parasites of the bat in Soochow. Peking Nat. Hist. Bull. 1935, 9, 293–298. [Google Scholar]
- Jordan, K. On four eight-combed Chinese bat-fleas of the genus Ischnopsyllus in the collection of the British Museum (Natural History). Parasitology 1941, 33, 362–372. [Google Scholar] [CrossRef]
- Lewis, R.E. Notes on the geographical distribution and host preferences in the order Siphonaptera. Part 2. Rhopalopsyllidae, Malacopsyllidae and Vermipsyllidae. J. Med. Entomol. 1973, 10, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Litvinov, M.N.; Litvinova, E.A. Ecological and faunistic characteristics of fleas of small mammals in the south of the Far East (First report. Common species of rodent fleas). In Proceedings of the A. I. Kurentsov’s Annual Memorial Meetings, Moscow, Russia; September 2003; pp. 140–152. [Google Scholar]
- Benda, P.; Abi Said, M.R.; Bou Jaoude, I.; Karanouh, R.; Lučan, R.K.; Sadek, R.; Ševčík, M.; Uhrin, M.; Horáček, I. Bats (Mammalia: Chiroptera) of the Eastern Mediterranean and Middle East. Part 13. Review of distribution and ectoparasites of bats in Lebanon. Acta Soc. Zool. Bohemicae 2016, 80, 207–316. [Google Scholar]
- Galloway, T.; Beaucournu, J.C.; Estrada-Peña, A. Two new fleas for the Canary Islands (Siphonaptera, Ischnopsyllidae). Bull. Société Français Parasitol. 1993, 11, 159–162. [Google Scholar]
- Beaucournu, J.C.; Kock, D. Notes on the Ischnopsyllinae of the African continent, III. Additions to the distribution of species (Insecta: Siphonaptera: Ischnopsyllidae), Senckenberg. Biol. 1996, 70, 77–82. [Google Scholar]
- Peus, F. Flöne aus Anatolien und dem Iran (Insecta: Siphonaptera). IX. Beitrag der Serie “Flöne aus dem Mittelmeergebiet”. Ann. Naturhist. Mus. 1978, 81, 507–516. (In German) [Google Scholar]
- Aellen, V. Contribution a l’etude de la fauene d’Afghanistan 21. Dipterse Pupipares parasites de Chiropteres. Rev. Suisse Zool. 1959, 66, 555–567. (In French) [Google Scholar] [CrossRef]
- Smit, F.G.; Rosicky, B. Siphonaptera from eastern Afghanistan. Folia Parasitol. 1974, 21, 29–37. [Google Scholar] [PubMed]
- Farhang-Azid, A. The Flea Fauna of Iran. IV. Note on the small collection of Eat fleas. Bull. Soc. Pathol. Exot. Fil. 1969, 62, 151–152. [Google Scholar] [PubMed]
- Vshivkov, F.N.; Skalon, O.I. Fleas (Suctoria) of Crimea. Tr. Nauchno-Issledovatelskogo Protivochumnogo Instituta Kavk. Zakavk. 1961, 5, 138–155. (In Russian) [Google Scholar]
- Chirniy, V.I. Materials on the flea fauna (Siphonaptera) of the Crimean Peninsula. Probl. Dev. Crimea 2004, 193–196. (In Russian) [Google Scholar]
- Orlova, M.V.; Orlov, O.L. Contribution to the ectoparasite fauna of bats (Chiroptera: Vespertilionidae, Rhinolophidae) of Crimea. Entomol. Rev. 2018, 98, 319–323. [Google Scholar] [CrossRef]
- Orlova, M.V.; Smirnov, D.G.; Vekhnik, V.P.; Lukyanenko, A.M.; Zabashta, A.V. Ectoparasites and pathogens of Kuhl’s Pipistrelle Pipistrellus kuhli (Kuhl, 1817) (Chiroptera: Vespertilionidae): Our own and published data review. Russ. J. Biol. Invasions 2020, 11, 348–362. [Google Scholar] [CrossRef]
- Isaeva, E.V. New data on flea fauna of Azerbaijan. Izv. Azerbaijanskoy SSR 1948, 5, 86–95. (In Russian) [Google Scholar]
- Beaucournu, J.C.; Launay, H. Les Puces de France et du Bassin Méditerranéen Occidental; Faune de France, Fédération Française des Sociétés de Sciences Naturelles: Paris, France, 1990; Volume 76, ISBN 2-903052-10-7. [Google Scholar]
- Frank, R.; Kuhn, T.; Werblow, A. Parasite diversity of European Myotis species with special emphasis on Myotis myotis (Microchiroptera, Vespertilionidae) from a typical nursery roost. Parasites Vectors 2015, 8, 101. [Google Scholar] [CrossRef]
- Haas, G.E.; Walton, D.W. Fleas (Siphonaptera) infesting small mammals from the Western Oriental region. Korean, J. Parasitol. 1973, 11, 102–107. [Google Scholar] [CrossRef]
- Hůrka, K. Notes on the taxonomy and distribution of Ischnopsyllidae (Siphonaptera). Věstník Ceskoslov. Společnosti Zool. 1976, 40, 273–278. [Google Scholar]
- Lebedeva, D.I.; Belkin, V.V.; Stanyukovich, M.K.; Bespyatova, L.A.; Bugmyrin, S.V. First records of bat parasites in Karelia. Transact. Karel. Res. Cent. RAS 2020, 8, 120–125. [Google Scholar] [CrossRef]
- Burazerović, J.; Orlova, M.; Obradović, M.; Ćirović, D.; Tomanović, S. Patterns of Abundance and Host Specificity of Bat Ectoparasites in the Central Balkans. J. Med. Entomol. 2018, 55, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Haitlinger, R.; Ruprecht, A. Parasitic arthropods (Siphonaptera, Diptera, Acari) of bats from western part of the Bialowieza Primeval Forest. Nyctalus (NF) 1992, 4, 315–319. [Google Scholar]
- Haitlinger, R.; Łupicki, D. Arthropods (Acari, Siphonaptera, Heteroptera, Psocoptera) associated with Nyctalus noctula (Schreber, 1774) (Chiroptera: Vespertilionidae) in Southern Poland. Wiadomości Parazytol. 2008, 54, 123–130. [Google Scholar] [PubMed]
- Orlova, M.V. The Fauna and Ecology of the Ectoparasites of the Bats of the Urals. Ph.D. Thesis, Institute of Plants and Animals ecology Russian Academy of Sciences, Ekaterinburg, Russia, 2013; 149p. (In Russian). [Google Scholar]
- Orlova, M.V.; Zapart, A. Interaction of ectoparasites in cohabitating colonies of pond bats Myotis dasycneme (Boie, 1825) and species of genus Pipistrellus from northern Poland. Ann. Parasitol. 2012, 58, 211–215. [Google Scholar] [PubMed]
- Scheffler, I. Ektoparasiten der Fledermäuse in Winterquartieren in Brandenburg. Märkische Ent. Nachr. 2010, 12, 119–132. [Google Scholar]
- Beaucournu, J.C.; Quetglas, J. Nycteridopsylla pentactena (Kolenati, 1856), puce nouvelle pour la péninsule Ibérique (Siphonaptera, Ischnopsyllidae). Bull. Société Entomol. Fr. 2005, 110, 159–160. [Google Scholar] [CrossRef]
- Dubovchenko, T.A. Ectoparasites of Bats in Azerbaijan. Ph.D. Thesis, Institute of Zoology, Baku, Azerbaijan, 1968; 23p. [Google Scholar]
- Niewiadomska, K. Materials for flea fauna (Aphaniptera) of Poland. Fragm. Faun. Musei Zool. Pol. 1953, 6, 250–262. [Google Scholar]
- Sakalauskas, P.; Lipatova, I.; Griciuvienė, L.; Ražanskė, I.; Snegiriovaitė, J.; Paulauskas, A. Diversity of Flea Species (Siphonaptera) and Their Vector-Borne Pathogens from Bats (Chiroptera) in Lithuania. Diversity 2024, 16, 192. [Google Scholar] [CrossRef]
- Skuratowitcz, W. Pchty. In Aphaniptera; Katalog fauny Polski XXI, I—59; Museum I instytut zoologii PAN: Warszawa, Poland, 1964; 330p. (In Polish) [Google Scholar]
- Beaucournu, J.C. Provisional catalog of the Siphonaptera of the French fauna. Ann. Société Entomol. Fr. (NS) 1968, 4, 615–635. (In French) [Google Scholar] [CrossRef]
- Rosicky, B. Aphaniptera; Fauna CSR; SV: Praha, Czech, 1957; p. 446. [Google Scholar]
- Hůrka, L. Bat fleas (Aphaniptera, Ischnopsyllidae) from West Bohemia. Folia Mus. Rer. Natur. Bohem. Occid. Plzeò Zool. 1974, 4, 12–21. [Google Scholar]
- Lanza, B. I Parassiti dei Pipistrelli (Mammalia, Chiroptera) della Fauna Italiana; Museo Regionale di Scienze Naturali: Turin, Italy, 1999; ISBN 9788886041256. (In Italian) [Google Scholar]
- Krištofík, J.; Danko, Š. Arthropod ectoparasites (Acarina, Heteroptera, Diptera, Siphonaptera) of bats in Slovakia. Vespertilio 2012, 16, 167–189. [Google Scholar]
- Whiting, M.F.; Whiting, A.S.; Hastriter, M.W.; Dittmar, K. A molecular phylogeny of fleas (Insecta: Siphonaptera): Origins and host associations. Cladistics 2008, 24, 677–707. [Google Scholar] [CrossRef]
- Dick, C.W.; Gettinger, D.; Gardner, S.L. Bolivian ectoparasites: A survey of bats (Mammalia Chiroptera). Comp. Parasitol. 2007, 74, 372–377. [Google Scholar] [CrossRef]
- Sándor, A.D.; Corduneanu, A.; Hornok, S.; Mihalca, A.D.; Péter, Á. Season and host-community composition inside roosts may affect host-specificity of bat flies. Sci. Rep. 2024, 14, 41–47. [Google Scholar] [CrossRef]
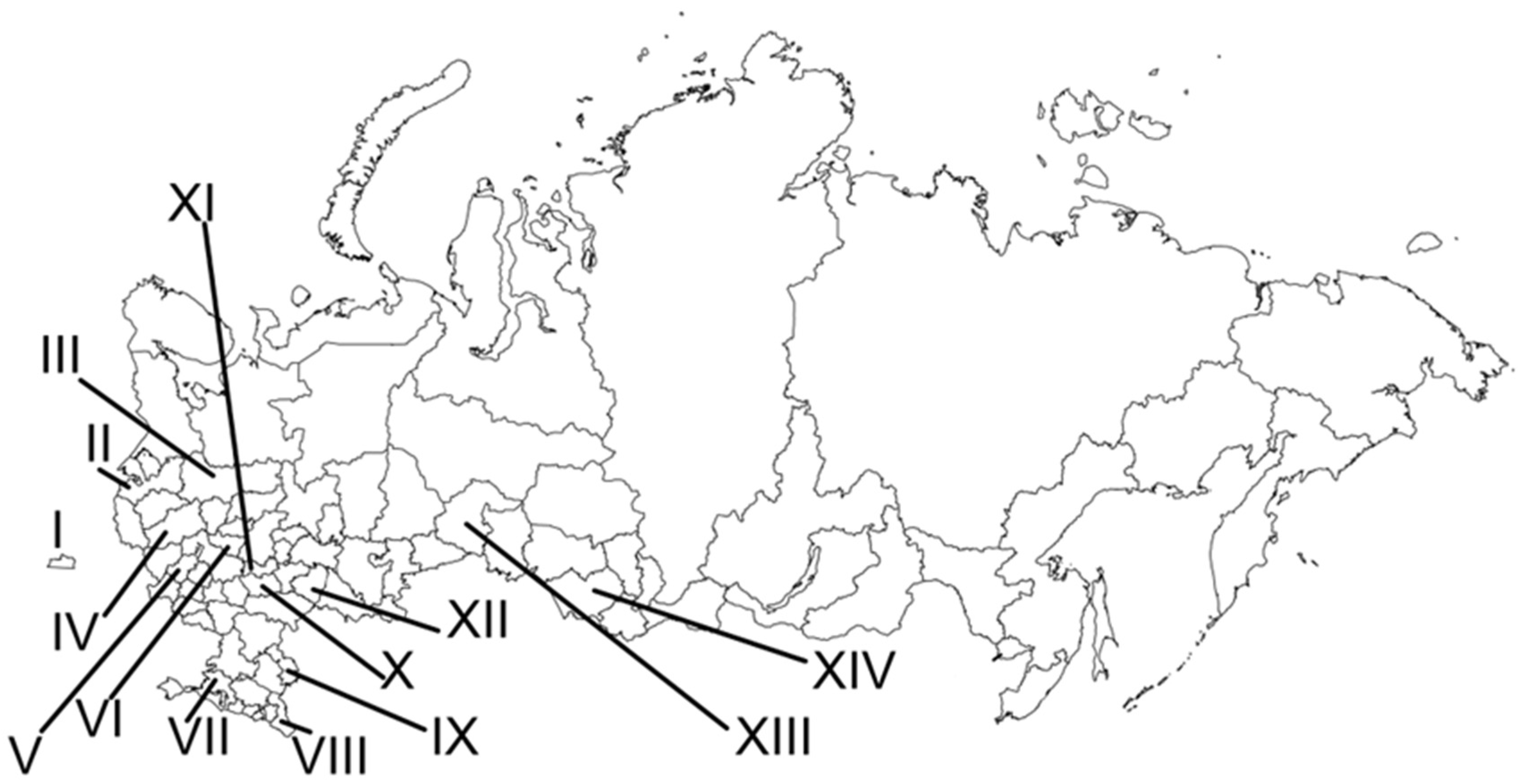
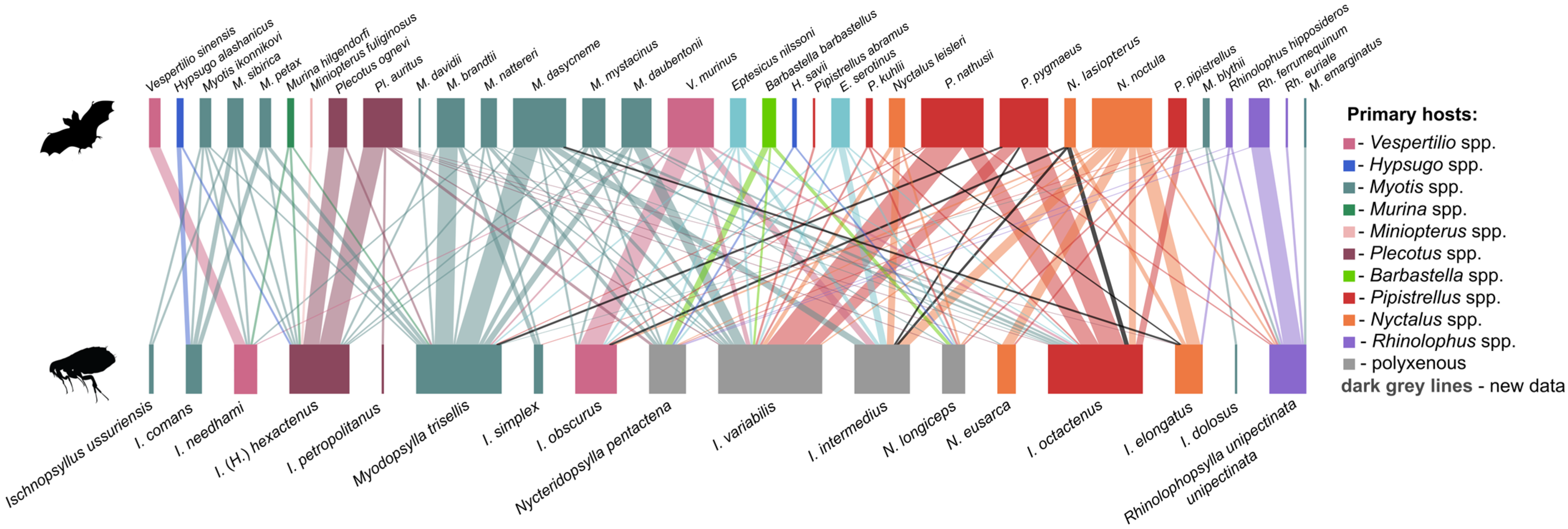
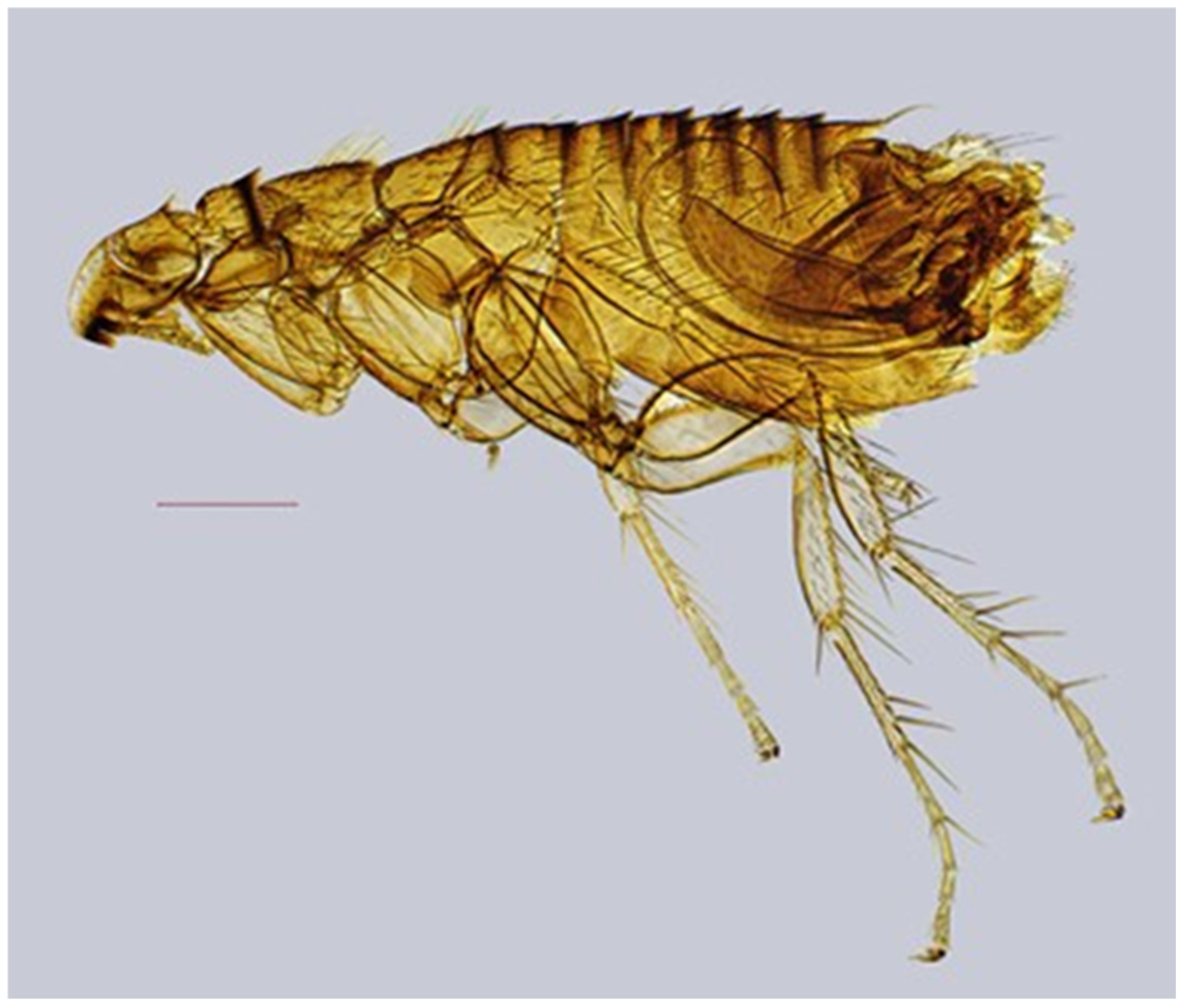
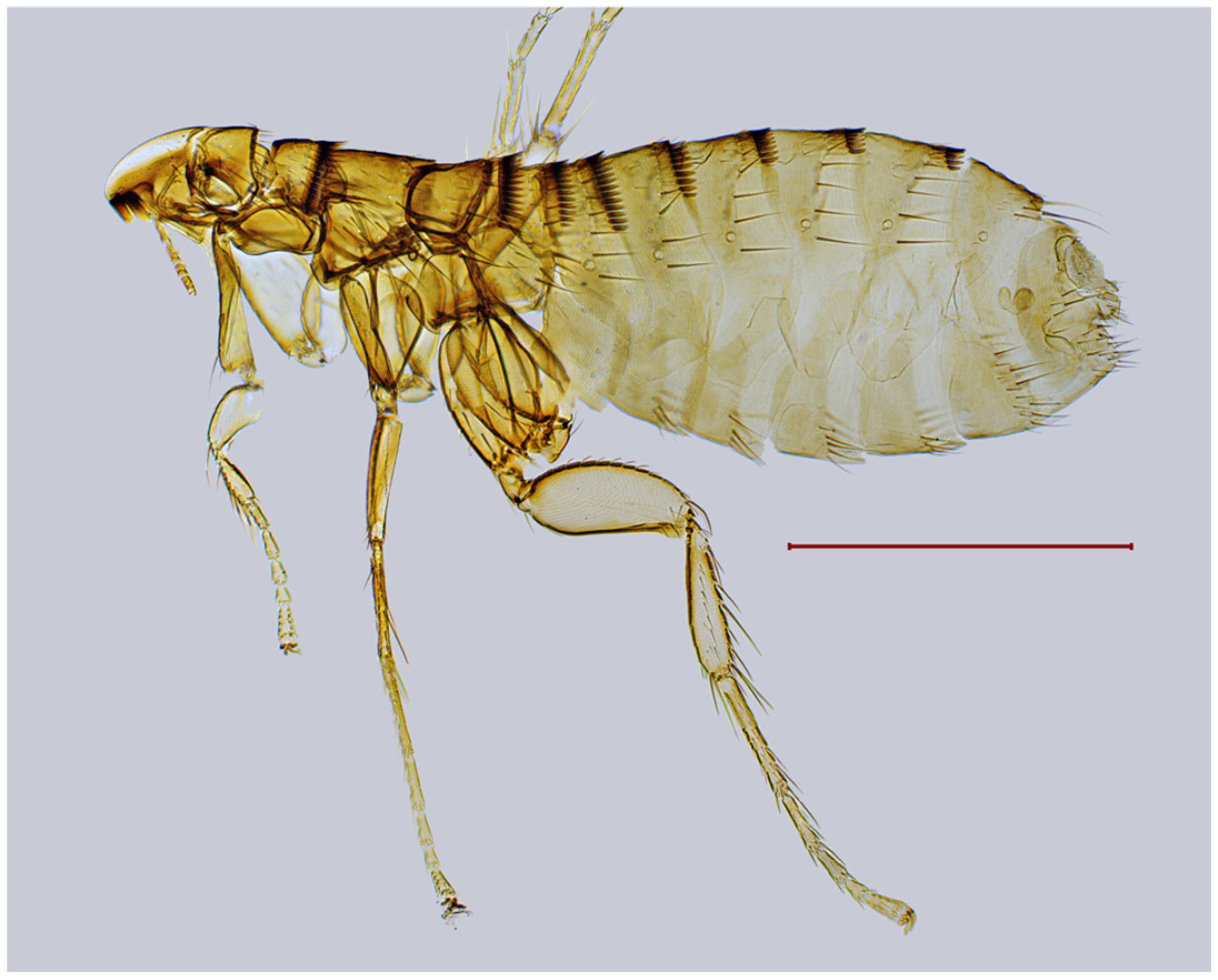
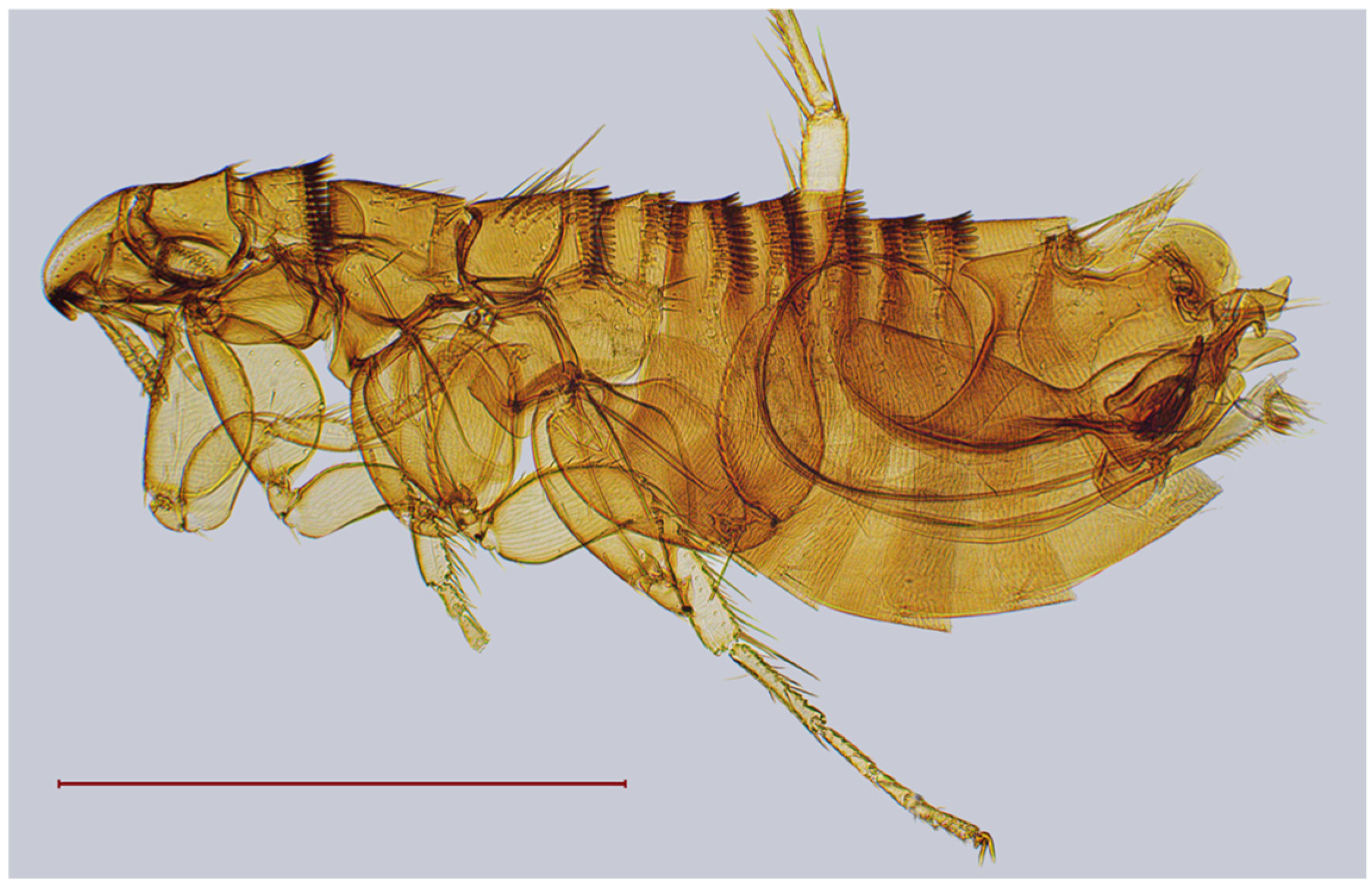
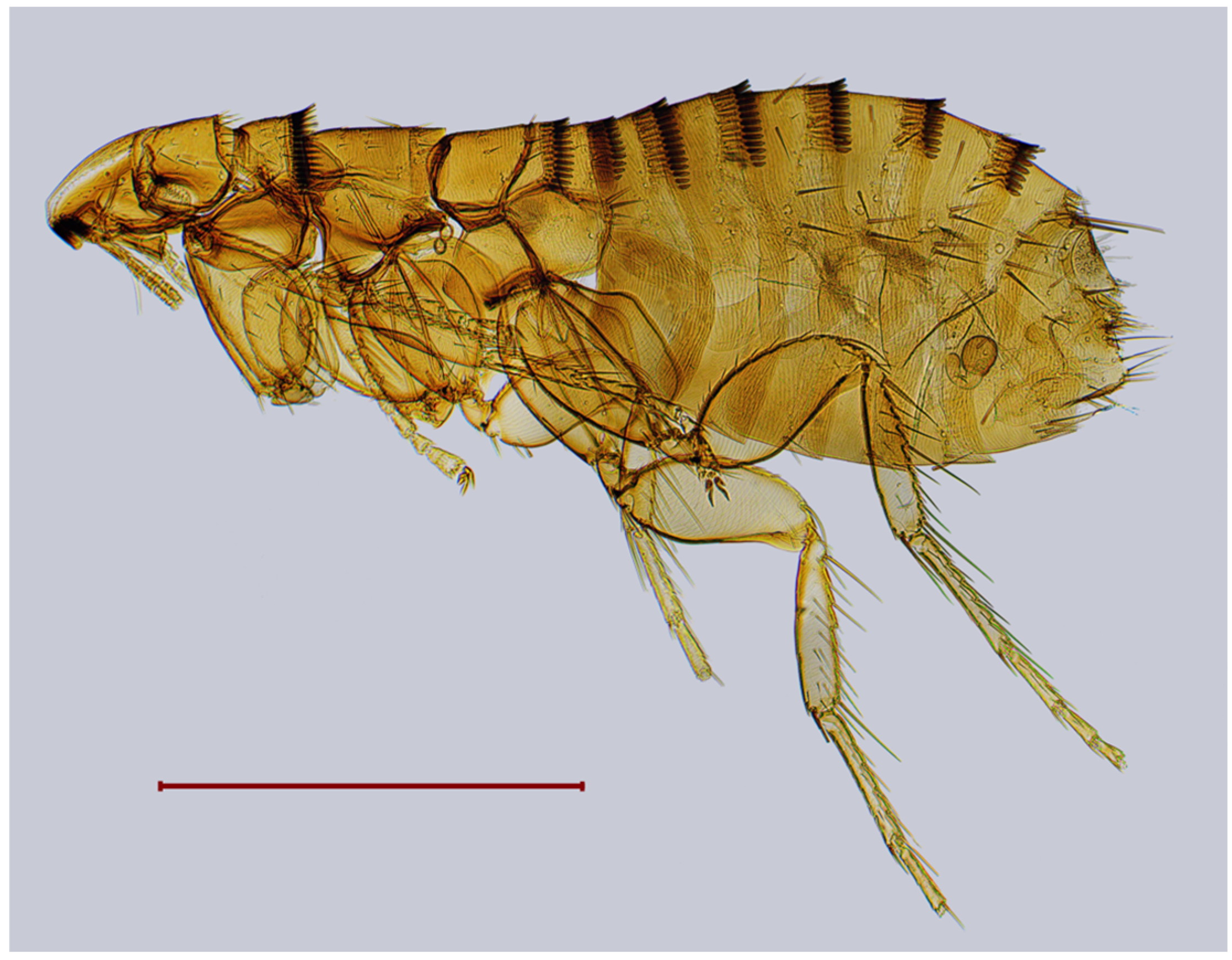

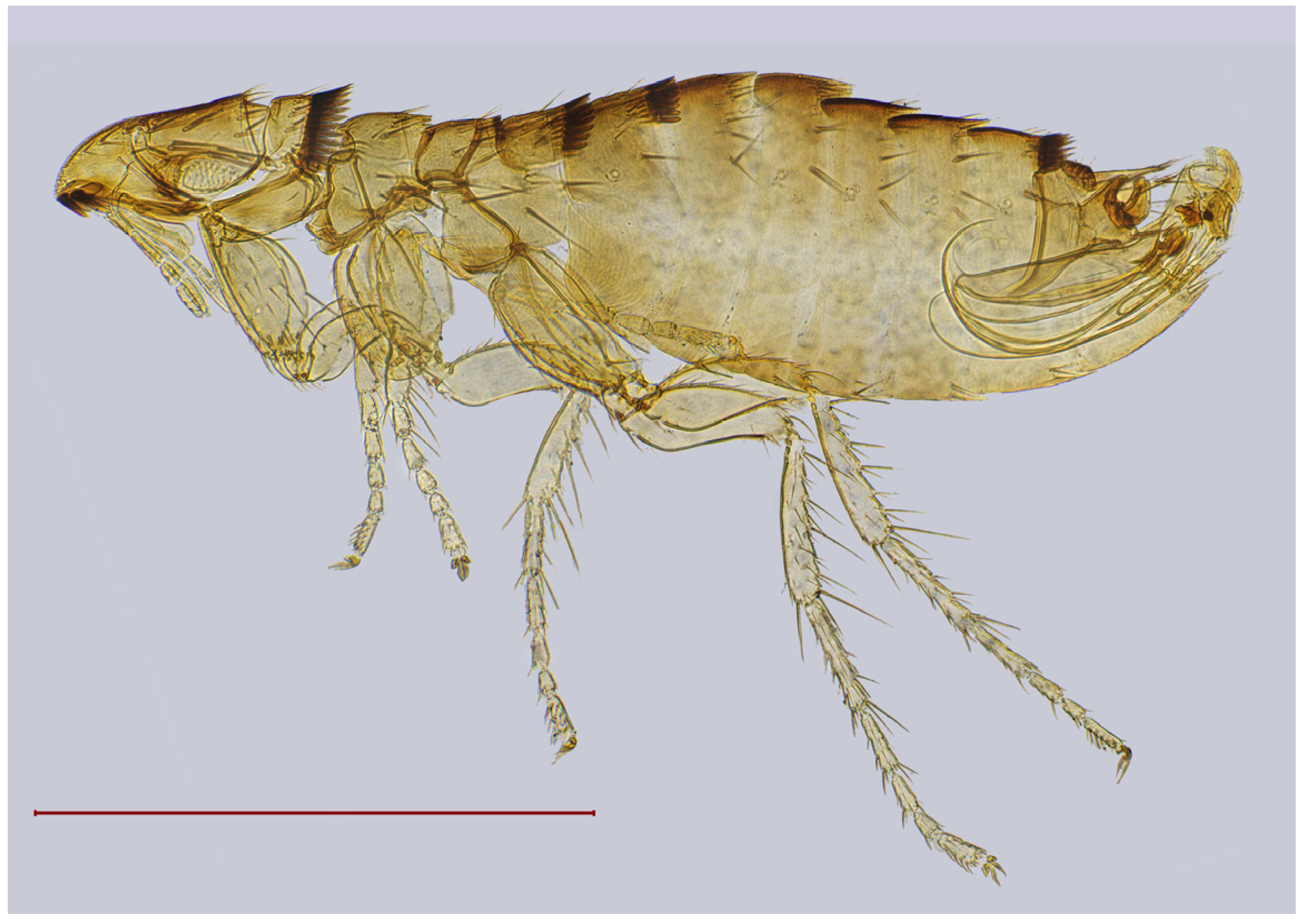
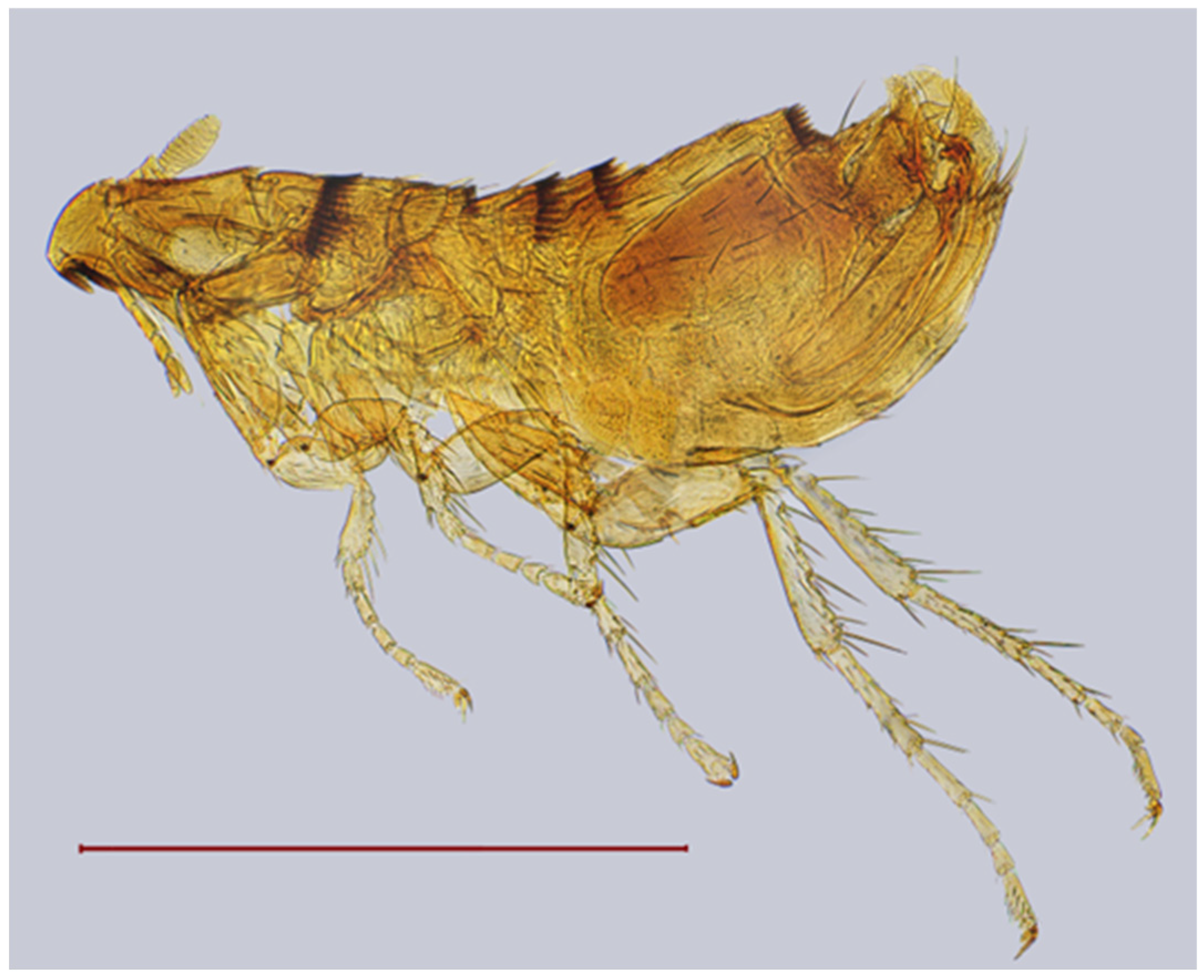
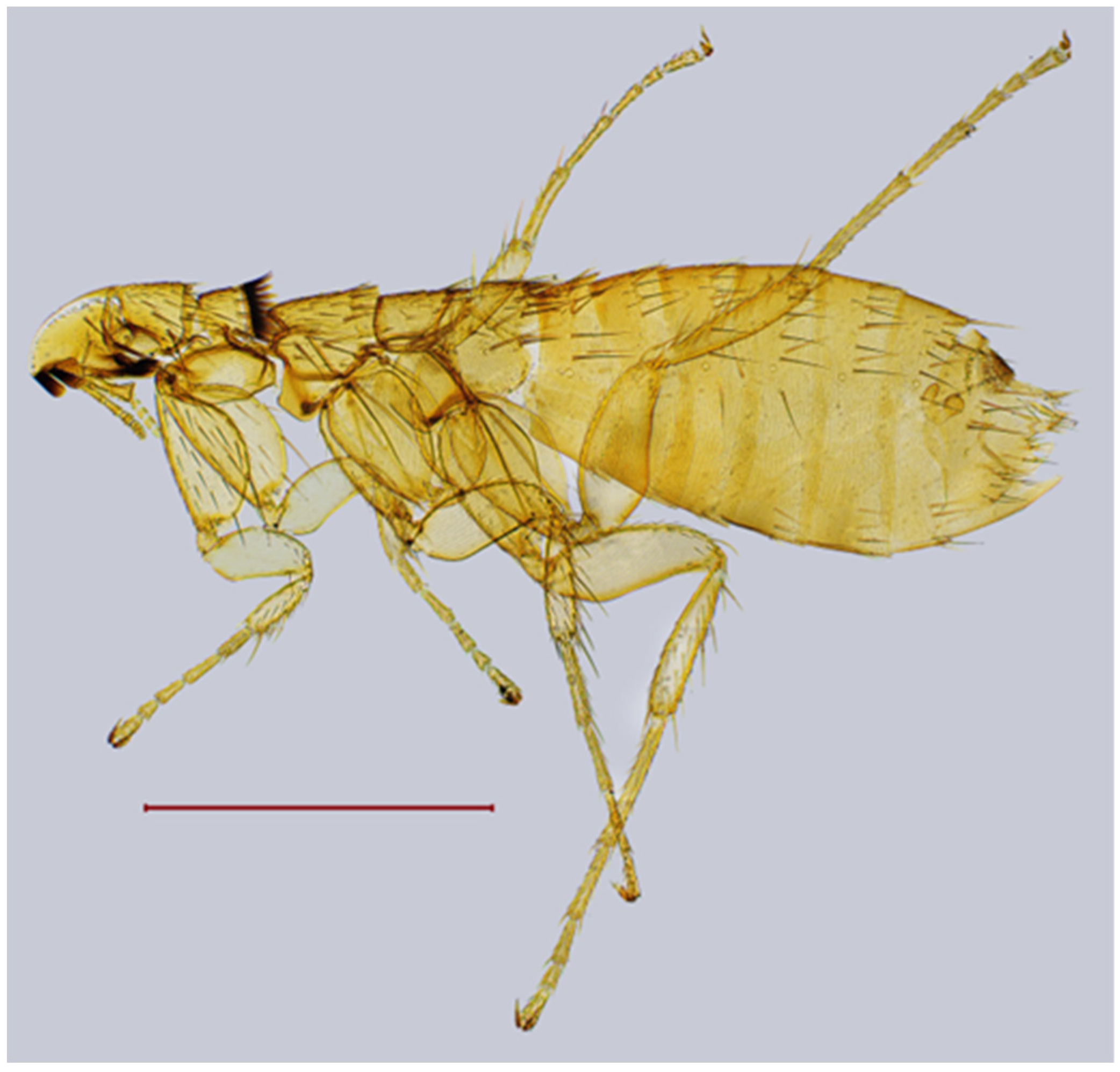
| № | Locality Name | Coordinates |
|---|---|---|
| I. Kaliningrad Region | ||
| 1. | Guryevsk municipal District, Bolshoe Isakovo Settlement, Fort № 1 ‘Steinz’ | 54°39′ N 20°31′ E |
| 2. | Central District, Fort № 5 ‘Friedrich Wilhelm III’ | 54°45′ N 20°26′ E |
| 3. | Leningradsky District, Fort № 3 ‘Friedrich III’ | 54°45′ N 20°32′ E |
| 4. | Guryevsk municipal District, Maloe Vasilkovo Settlement, Fort № 2 ‘Bronzart’ | 54°44′ N 20°36′ E |
| II. Leningrad Region | ||
| 5. | Volkhovsky District, Tanechkina Cave | 60°00′ N 32°18′ E |
| III. Vologda Region | ||
| 6. | Darwin Nature Reserve, Vauch Cordon, Losha River | 58°35′ N 37°30′ E |
| 7. | Darwin Nature Reserve, Netecha River | 58°34′ N 37°33′ E |
| 8. | Darwin Nature Reserve, Osinovik Cordon | 58°37′ N 37°46′ E |
| IV. Tver’ Region | ||
| 9. | Staritsky District, Ledyanaya Cave | 56°34′ N 34°59′ E |
| V. Kaluga Region | ||
| 12. | Kozelsky District, Optina Pustyn’ Village, pond on the Zhelezenka River ‘Valley of Love’ | 54°03′ N 35°51′ E |
| 13. | Kozelsky District, Optina Pustyn’ Village, pond at the Optina Pustyn’ Monastery near the skete and along the walls | 54°03′ N 35°50′ E |
| 14. | Kozelsky District, Nizhnie Pryski, Zhizdra River floodplain, Gorozhenoe Lake | 54°05′ N 35°53′ E |
| 15. | Kozelsky District, Berezichsky Glass Factory, near the house | 53°57′ N 35°49′ E |
| 16. | Kozelsky District, Berezichsky Glass Factory, lake shore | 53°58′ N 35°48′ E |
| 17. | Kozelsky District, Dmitrovsky, pond in broad-leaved forest near the Chechik River | 53°56′ N 35°48′ E |
| 18. | Kozelsky District, Chernysheno Village, pond | 53°48′ N 35°47′ E |
| 19. | Kozelsky District, Volosovo-Zvyagino Village, pond in spruce-broad-leaved forest | 53°54′ N 35°45′ E |
| 20. | Kozelsky District, Volosovo-Zvyagino Village, pond | 53°53′ N 35°46′ E |
| 21. | Kozelsky District, Poloshkovo Village, automobile bridge across the Serene River | 54°8′ N 35°52′ E |
| 22. | Peremyshlsky District, Ilyinskoye Settlement, floodplain of Bolshoe Lake | 54°12′ N 36°03′ E |
| 23. | Peremyshlsky District, Kaluga experimental agricultural station, ponds near the Vyssa River | 54°25′ N 36°04′ E |
| 24. | Peremyshlsky District, Vorotynsk Town, floodplain of the Vyssa River | 54°25′ N 36°02′ E |
| 25. | Dzerzhinsky District, Luzhnoe Settlement, pond on the field | 54°41′ N 36°44′ E |
| 26. | Dzerzhinsky District, Shenyano-Sloboda Village, near houses | 54°46′ N 35°54′ E |
| 27. | Dzerzhinsky District, Shenyano-Sloboda Village, pond near the highway | 54°44′ N 35°54′ E |
| 28. | Dzerzhinsky District, Rudnya, dam on the Rudnitsa River | 54°39′ N 35°53′ E |
| 29. | Dzerzhinsky District, Novaya Zhizn’ Village, dam on the road | 54°41′ N 35°49′ E |
| 30. | Yukhnovsky District, Tents, pond bank | 54°44′ N 35°22′ E |
| VI. Vladimir Region | ||
| 10. | Aleshunino Village | 55°49′ N 42°20′ E |
| 11. | Nature Reserve ‘Dyukinsky’ | 56°00′ N 41°03′ E |
| VII. Krasnodar Territory | ||
| 40. | Adler district of Sochi City, Akhshtyrskaya Cave | 43°31′ N 40°00′ E |
| VIII. Dagestan Republic | ||
| 43. | Yangiyurt State Nature Reserve, Shaitan-Kazak Settlement, Shaitan-Kazak Lake | 43°19′ N 46°56′ E |
| 44. | Suleiman-Stalsky district, Shurdere stream, surroundings of Sovetskoye Settlement | 41°45′ N 48°17′ E |
| 45. | Akushin district, Verkhnie Mulyobki Settlement | 42°19′ N 47°31′ E |
| 46. | Samurskiy National Park, Platan 800 years | 41°52′ N 48°32′ E |
| IX. Astrakhan’ Region | ||
| 41. | Astrakhan Order of the Red Banner of Labor State Nature Biosphere Reserve, Damchik Settlement | 46°18′ N 49°00′ E |
| 42. | Volodarsky District, Left Bank of Obzhorova River | 46°18′ N 48°59′ E |
| X. Penza Region | ||
| 31. | Right Bank of Surskiy Reservoir, Shemysheysky District, Research Station of Penza State University | 52°58′ N 45°21′ E |
| XI. Mordovia Republic | ||
| 32. | Ichalkovsky District, Bandasevsky Cordon tract, Mitryashka Lake | 54°44′ N 45°30′ E |
| XII. Samara Region | ||
| 33. | Nature Reserve ‘Samarskaya Luka’ | 53°18′ N; 49°49′ E |
| 34. | Zhiguli State Nature Reserve, Solnechnaya Polyana Settlement, Volga Coast, Rizhskiy beach | 53°26′ N 49°54′ E |
| 35. | Zhiguli Nature Reserve, Solnechnaya Polyana Settlement, Boarding school № 3 | 53°25′ N 49°55′ E |
| 36. | Zhiguli State Nature Reserve, Solnechnaya Polyana Settlement, Vremena Goda recreation | 53°26′ N 49°55′ E |
| 37. | Zhiguli State Nature Reserve, forestry, Bear Grotto | 53°23′ N 49°55′ E |
| 38. | Zhiguli State Nature Reserve, Temple of Nature Ecological walking route | 53°24′ N 49°55′ E |
| 39. | Zhiguli State Nature Reserve, Shiryaevo Settlement, Mount Popova | 53°25′ N 50°0′ E |
| XIII. Tyumen Region | ||
| 47. | Tyumen City | 57°09′ N 65°32′ E |
| XIV. Altai Territory | ||
| 48. | Salair National Park, Ionikha Cordon | 53°33′ N 86°07′ E |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orlova, M.V.; Viskontene, A.L.; Korzikov, V.A.; Zabashta, M.V.; Zabashta, A.V.; Kruskop, S.V.; Smirnov, D.G.; Malyavina, M.S.; Pavlov, A.V.; Orlov, O.L.; et al. A Review of Bat Fleas (Siphonaptera: Ischnopsyllidae) from Russia. Diversity 2025, 17, 419. https://doi.org/10.3390/d17060419
Orlova MV, Viskontene AL, Korzikov VA, Zabashta MV, Zabashta AV, Kruskop SV, Smirnov DG, Malyavina MS, Pavlov AV, Orlov OL, et al. A Review of Bat Fleas (Siphonaptera: Ischnopsyllidae) from Russia. Diversity. 2025; 17(6):419. https://doi.org/10.3390/d17060419
Chicago/Turabian StyleOrlova, Maria V., Alex L. Viskontene, Vyacheslav A. Korzikov, Marina V. Zabashta, Alexey V. Zabashta, Sergei V. Kruskop, Dmitriy G. Smirnov, Maria S. Malyavina, Alexandr V. Pavlov, Oleg L. Orlov, and et al. 2025. "A Review of Bat Fleas (Siphonaptera: Ischnopsyllidae) from Russia" Diversity 17, no. 6: 419. https://doi.org/10.3390/d17060419
APA StyleOrlova, M. V., Viskontene, A. L., Korzikov, V. A., Zabashta, M. V., Zabashta, A. V., Kruskop, S. V., Smirnov, D. G., Malyavina, M. S., Pavlov, A. V., Orlov, O. L., Mishchenko, V. A., Vyalykh, I. V., Boyarintsev, D. I., Kuzminov, I. V., Bryutova, K., Khizhkin, E. A., Larchanka, A. I., Shapkin, O. A., Vinogradova, E. A., ... Sakharov, S. P. (2025). A Review of Bat Fleas (Siphonaptera: Ischnopsyllidae) from Russia. Diversity, 17(6), 419. https://doi.org/10.3390/d17060419









