Locally Measured Functional Traits Predict Species Registrability in Herbaceous Flora
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Functional Traits Measurements
2.3. Statistical Analyses
3. Results
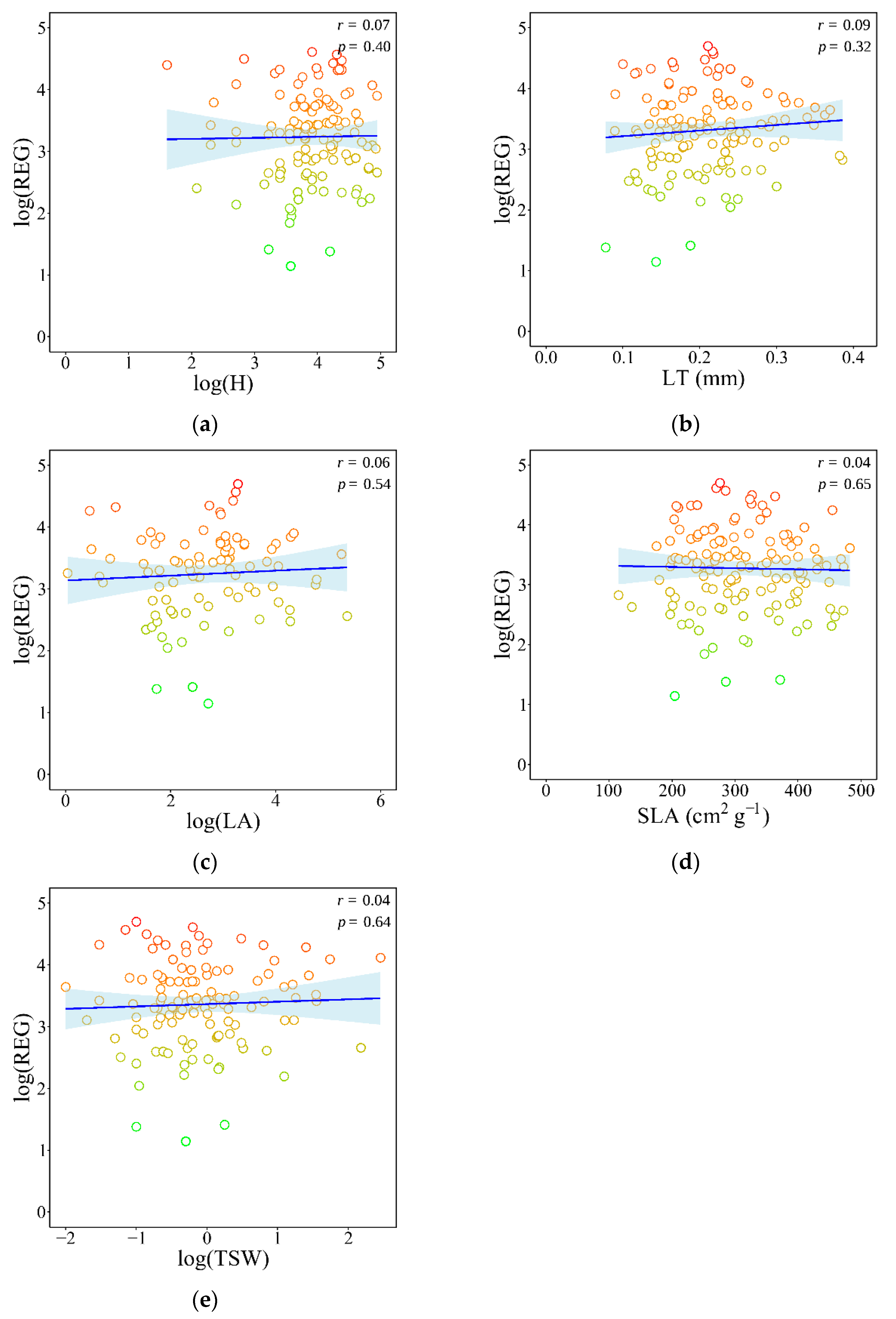
3.1. Associations Between Plant Morphological Traits and Species’ Registrability
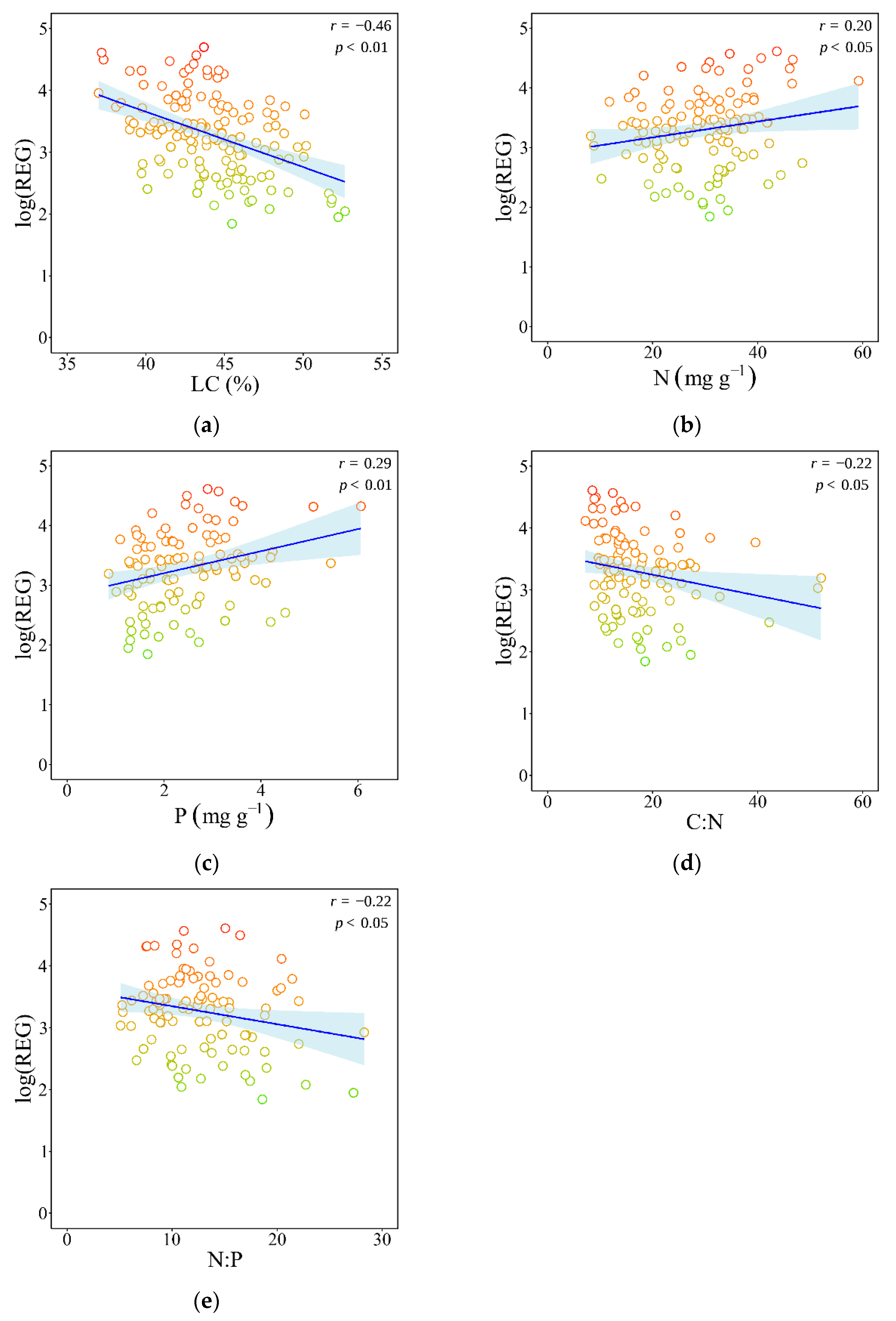
3.2. Associations Between Nutrient-Related Traits and Species’ Registrability
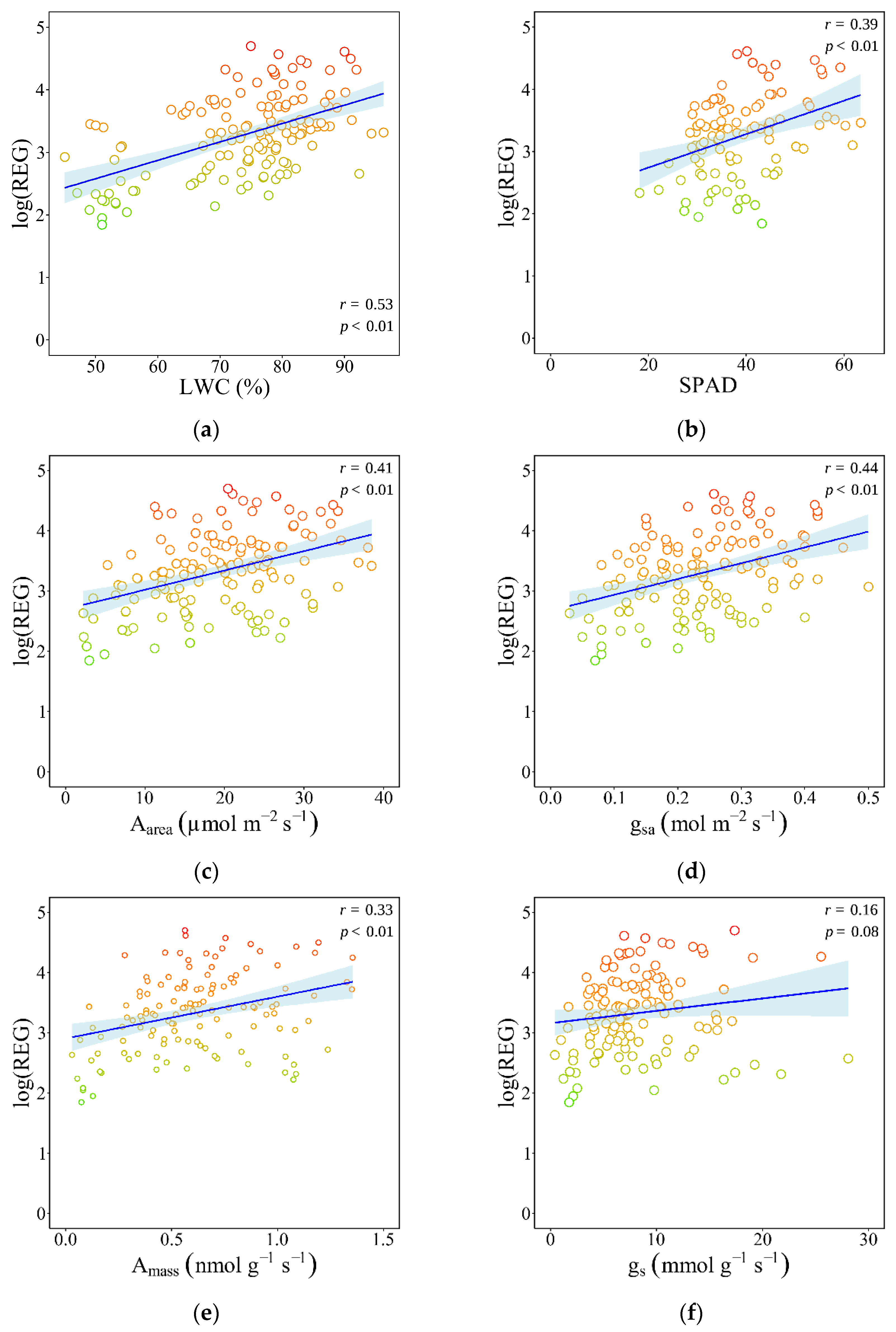
3.3. Associations Between Physiological Traits and Species’ Registrability
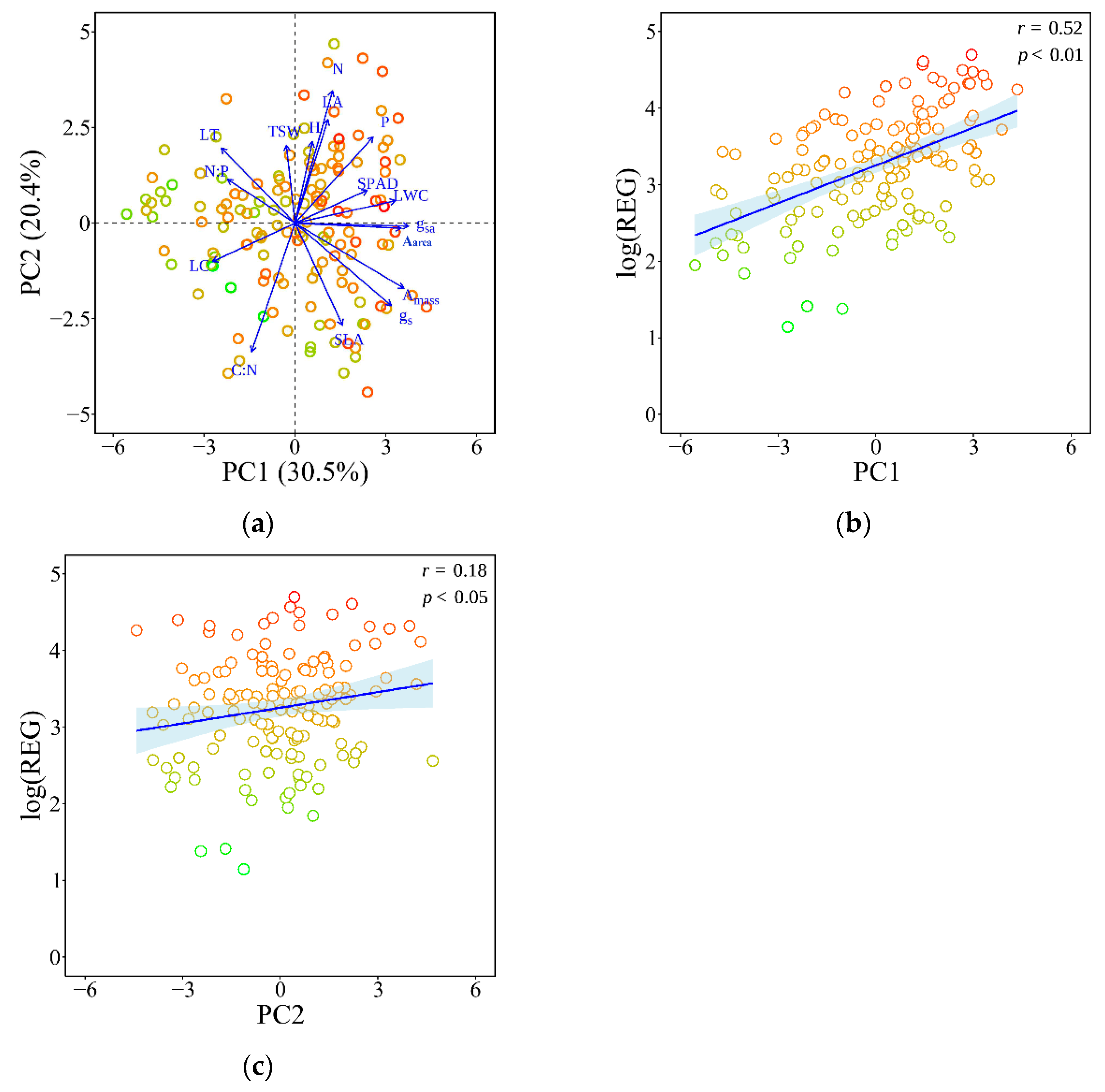
3.4. Trait Syndromes and Registrability Along Principal Component Axes
4. Discussion
4.1. Morphological Traits and Registrability
4.2. Nutrient-Related Traits Differentiate Acquisitive Versus Conservative Strategies
4.3. Physiological Traits
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shay, J.E.; Pennington, L.K.; Mandussi Montiel-Molina, J.A.; Toews, D.J.; Hendrickson, B.T.; Sexton, J.P. Rules of Plant Species Ranges: Applications for Conservation Strategies. Front. Ecol. Evol. 2021, 9, 2021. [Google Scholar] [CrossRef]
- Murray, B.R.; Lepschi, B.J. Are locally rare species abundant elsewhere in their geographical range? Austral Ecol. 2004, 29, 287–293. [Google Scholar] [CrossRef]
- Feng, X.; Peterson, A.T.; Aguirre-López, L.J.; Burger, J.R.; Chen, X.; Papeş, M. Rethinking ecological niches and geographic distributions in face of pervasive human influence in the Anthropocene. Biol. Rev. 2024, 99, 1481–1503. [Google Scholar] [CrossRef]
- Gaston, K.J.; Fuller, R.A. The sizes of species’ geographic ranges. J. Appl. Ecol. 2009, 46, 1–9. [Google Scholar] [CrossRef]
- Ellis, E.C. Ecology in an anthropogenic biosphere. Ecol. Monogr. 2015, 85, 287–331. [Google Scholar] [CrossRef]
- Koffel, T.; Umemura, K.; Litchman, E.; Klausmeier, C.A. A general framework for species-abundance distributions: Linking traits and dispersal to explain commonness and rarity. Ecol. Lett. 2022, 25, 2359–2371. [Google Scholar] [CrossRef]
- Mokany, K.; Roxburgh, S.H. The importance of spatial scale for trait–abundance relations. Oikos 2010, 119, 1504–1514. [Google Scholar] [CrossRef]
- Li, R.; Zhu, S.; Chen, H.Y.; John, R.; Zhou, G.; Zhang, D.; Zhang, Q.; Ye, Q. Are functional traits a good predictor of global change impacts on tree species abundance dynamics in a subtropical forest? Ecol. Lett. 2015, 18, 1181–1189. [Google Scholar] [CrossRef]
- Reich, P.B. The world-wide ‘fast–slow’ plant economics spectrum: A traits manifesto. J. Ecol. 2014, 102, 275–301. [Google Scholar] [CrossRef]
- Umaña, M.N.; Zhang, C.; Cao, M.; Lin, L.; Swenson, N.G. Commonness, rarity, and intraspecific variation in traits and performance in tropical tree seedlings. Ecol. Lett. 2015, 18, 1329. [Google Scholar] [CrossRef]
- Umaã±A, M.N.; Zhang, C.; Cao, M.; Lin, L.; Swenson, N.G. A core-transient framework for trait-based community ecology: An example from a tropical tree seedling community. Ecol. Lett. 2017, 20, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Díaz de León Guerrero, S.D.; González-Rebeles Guerrero, G.; Ibarra-Montes, T.M.; Rodríguez Bastarrachea, A.; Santos Cobos, R.; Bullock, S.H.; Sack, L.; Méndez-Alonzo, R. Functional traits indicate faster resource acquisition for alien herbs than native shrubs in an urban Mediterranean shrubland. Biol. Invasions 2020, 22, 2699–2712. [Google Scholar] [CrossRef]
- Dyderski, M.K.; Jagodziński, A.M. Functional traits of acquisitive invasive woody species differ from conservative invasive and native species. NeoBiota 2019, 41, 91–113. [Google Scholar] [CrossRef]
- Li, R.; Zhu, S.; Lian, J.; Zhang, H.; Liu, H.; Ye, W.; Ye, Q. Functional Traits Are Good Predictors of Tree Species Abundance Across 101 Subtropical Forest Species in China. Front. Plant Sci. 2021, 12, 541577. [Google Scholar] [CrossRef] [PubMed]
- Lisner, A.; Partel, M.; Helm, A.; Prangel, E.; Leps, J. Traits as determinants of species abundance in a grassland community. J. Veg. Sci. 2021, 32, e13041. [Google Scholar] [CrossRef]
- Maynard, D.S.; Bialic-Murphy, L.; Zohner, C.M.; Averill, C.; van den Hoogen, J.; Ma, H.; Mo, L.; Smith, G.R.; Acosta, A.T.R.; Aubin, I.; et al. Global relationships in tree functional traits. Nat. Commun. 2022, 13, 3185. [Google Scholar] [CrossRef]
- Liao, H.X.; Li, D.J.; Zhou, T.; Huang, B.; Zhang, H.J.; Chen, B.M.; Peng, S.L. The role of functional strategies in global plant distribution. Ecography 2021, 44, 493–503. [Google Scholar] [CrossRef]
- Guo, K.; Pysek, P.; van Kleunen, M.; Kinlock, N.L.; Lucanova, M.; Leitch, I.J.; Pierce, S.; Dawson, W.; Essl, F.; Kreft, H.; et al. Plant invasion and naturalization are influenced by genome size, ecology and economic use globally. Nat. Commun. 2024, 15, 1330. [Google Scholar] [CrossRef]
- Segrestin, J.; Sartori, K.; Navas, M.L.; Kattge, J.; Diaz, S.; Garnier, E. PhenoSpace: A Shiny application to visualize trait data in the phenotypic space of the global spectrum of plant form and function. Ecol. Evol. 2021, 11, 1526–1534. [Google Scholar] [CrossRef]
- Sih, A.; Ferrari, M.C.; Harris, D.J. Evolution and behavioural responses to human-induced rapid environmental change. Evol. Appl. 2011, 4, 367–387. [Google Scholar] [CrossRef]
- Zenni, R.D.; Bailey, J.K.; Simberloff, D. Rapid evolution and range expansion of an invasive plant are driven by provenance-environment interactions. Ecol. Lett. 2014, 17, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Rubio, V.E. Chapter 4—Traits, occurrence, and abundance of plants. In Plant Functional Traits; Kumar, M., Bussmann, R.W., Swenson, N.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2025; pp. 43–55. [Google Scholar]
- Loranger, J.; Munoz, F.; Shipley, B.; Violle, C. What makes trait-abundance relationships when both environmental filtering and stochastic neutral dynamics are at play? Oikos 2018, 127, 1735–1745. [Google Scholar] [CrossRef]
- Spaak, J.; Schreiber, S. Building modern coexistence theory from the ground up: The role of community assembly. Ecol. Lett. 2023, 26, 1840–1861. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, Y.; Wei, C.; Liu, S.; Liang, X.; Zhang, J.; Li, R. Invasive Plant Species Demonstrate Enhanced Resource Acquisition Traits Relative to Native Non-Dominant Species but not Compared with Native Dominant Species. Diversity 2024, 16, 317. [Google Scholar] [CrossRef]
- Sporbert, M.; Welk, E.; Seidler, G.; Jandt, U.; Aćić, S.; Biurrun, I.; Campos, J.A.; Čarni, A.; Cerabolini, B.E.L.; Chytrý, M.; et al. Different sets of traits explain abundance and distribution patterns of European plants at different spatial scales. J. Veg. Sci. 2021, 32, e13016. [Google Scholar] [CrossRef]
- Sonkoly, J.; Deak, B.; Valko, O.; Molnar, V.A.; Tothmeresz, B.; Torok, P. Do large-seeded herbs have a small range size? The seed mass-distribution range trade-off hypothesis. Ecol. Evol. 2017, 7, 11204–11212. [Google Scholar] [CrossRef] [PubMed]
- Westerband, A.C.; Funk, J.L.; Barton, K.E. Intraspecific trait variation in plants: A renewed focus on its role in ecological processes. Ann. Bot. 2021, 127, 397–410. [Google Scholar] [CrossRef]
- Barabás, G.; Parent, C.; Kraemer, A.; Van de Perre, F.; De Laender, F. The evolution of trait variance creates a tension between species diversity and functional diversity. Nat. Commun. 2022, 13, 2521. [Google Scholar] [CrossRef]
- Yang, J.; Cao, M.; Swenson, N.G. Why Functional Traits Do Not Predict Tree Demographic Rates. Trends Ecol. Evol. 2018, 33, 326–336. [Google Scholar] [CrossRef]
- Wright, I.J.; Dong, N.; Maire, V.; Prentice, I.C.; Westoby, M.; Diaz, S.; Gallagher, R.V.; Jacobs, B.F.; Kooyman, R.; Law, E.A.; et al. Global climatic drivers of leaf size. Science 2017, 357, 917–921. [Google Scholar] [CrossRef]
- Martin, A.R.; Rapidel, B.; Roupsard, O.; Van den Meersche, K.; de Melo Virginio Filho, E.; Barrios, M.; Isaac, M.E. Intraspecific trait variation across multiple scales: The leaf economics spectrum in coffee. Funct. Ecol. 2017, 31, 604–612. [Google Scholar] [CrossRef]
- Craine, J.M.; Dybzinski, R. Mechanisms of plant competition for nutrients, water and light. Funct. Ecol. 2013, 27, 833–840. [Google Scholar] [CrossRef]
- Tilman, D. The resource-ratio hypothesis of plant succession. Am. Nat. 1985, 125, 827–852. [Google Scholar] [CrossRef]
- Zhu, S.-D.; Li, R.-H.; Song, J.; He, P.-C.; Liu, H.; Berninger, F.; Ye, Q. Different leaf cost–benefit strategies of ferns distributed in contrasting light habitats of sub-tropical forests. Ann. Bot. 2016, 117, 497–506. [Google Scholar] [CrossRef]
- Osnas, J.L.D.; Lichstein, J.W.; Reich, P.B.; Pacala, S.W. Global leaf trait relationships: Mass, area, and the leaf economics spectrum. Science 2013, 340, 741–744. [Google Scholar] [CrossRef] [PubMed]
- Onoda, Y.; Westoby, M.; Adler, P.B.; Choong, A.M.; Clissold, F.J.; Cornelissen, J.H.; Diaz, S.; Dominy, N.J.; Elgart, A.; Enrico, L.; et al. Global patterns of leaf mechanical properties. Ecol. Lett. 2011, 14, 301–312. [Google Scholar] [CrossRef]
- Medeiros, C.D.; Henry, C.; Trueba, S.; Anghel, I.; Guerrero, S.D.D.d.L.; Pivovaroff, A.; Fletcher, L.R.; John, G.P.; Lutz, J.A.; Méndez Alonzo, R.; et al. Predicting plant species climate niches on the basis of mechanistic traits. Funct. Ecol. 2023, 37, 2786–2808. [Google Scholar] [CrossRef]
- Leishman, M.R.; Haslehurst, T.; Ares, A.; Baruch, Z. Leaf trait relationships of native and invasive plants: Community- and global-scale comparisons. New Phytol. 2007, 176, 635–643. [Google Scholar] [CrossRef]
- Croft, H.; Chen, J.M.; Wang, R.; Mo, G.; Luo, S.; Luo, X.; He, L.; Gonsamo, A.; Arabian, J.; Zhang, Y.; et al. The global distribution of leaf chlorophyll content. Remote Sens. Environ. 2020, 236, 111479. [Google Scholar] [CrossRef]
- Zhu, S.-D.; Song, J.-J.; Li, R.-H.; Ye, Q. Plant hydraulics and photosynthesis of 34 woody species from different successional stages of subtropical forests. Plant Cell Environ. 2013, 36, 879–891. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, H.; Wang, H.; Peñuelas, J.; Sardans, J.; Niinemets, Ü.; Niklas, K.J.; Li, Y.; Xie, J.; Wright, I.J. Leaf water content contributes to global leaf trait relationships. Nat. Commun. 2022, 13, 5525. [Google Scholar] [CrossRef] [PubMed]
- Kursar, T.A.; Engelbrecht, B.M.; Burke, A.; Tyree, M.T.; EI Omari, B.; Giraldo, J.P. Tolerance to low leaf water status of tropical tree seedlings is related to drought performance and distribution. Funct. Ecol. 2009, 23, 93–102. [Google Scholar] [CrossRef]
- Smart, S.M.; Glanville, H.C.; Blanes, M.d.C.; Mercado, L.M.; Emmett, B.A.; Jones, D.L.; Cosby, B.J.; Marrs, R.H.; Butler, A.; Marshall, M.R. Leaf dry matter content is better at predicting above-ground net primary production than specific leaf area. Funct. Ecol. 2017, 31, 1336–1344. [Google Scholar] [CrossRef]
- Violle, C.; Reich, P.B.; Pacala, S.W.; Enquist, B.J.; Kattge, J. The emergence and promise of functional biogeography. Proc. Natl. Acad. Sci. USA 2014, 111, 13690–13696. [Google Scholar] [CrossRef]
- Beck, J.; Böller, M.; Erhardt, A.; Schwanghart, W. Spatial bias in the GBIF database and its effect on modeling species’ geographic distributions. Ecol. Inform. 2014, 19, 10–15. [Google Scholar] [CrossRef]
- Hughes, A.C.; Orr, M.C.; Ma, K.; Costello, M.J.; Waller, J.; Provoost, P.; Yang, Q.; Zhu, C.; Qiao, H. Sampling biases shape our view of the natural world. Ecography 2021, 44, 1259–1269. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, C.; Liu, S.; Liang, X.; Chen, Y.; Zhang, J.; Li, R. Locally Measured Functional Traits Predict Species Registrability in Herbaceous Flora. Diversity 2025, 17, 408. https://doi.org/10.3390/d17060408
Wei C, Liu S, Liang X, Chen Y, Zhang J, Li R. Locally Measured Functional Traits Predict Species Registrability in Herbaceous Flora. Diversity. 2025; 17(6):408. https://doi.org/10.3390/d17060408
Chicago/Turabian StyleWei, Caihong, Si Liu, Xiaoyue Liang, Yingcan Chen, Jiaen Zhang, and Ronghua Li. 2025. "Locally Measured Functional Traits Predict Species Registrability in Herbaceous Flora" Diversity 17, no. 6: 408. https://doi.org/10.3390/d17060408
APA StyleWei, C., Liu, S., Liang, X., Chen, Y., Zhang, J., & Li, R. (2025). Locally Measured Functional Traits Predict Species Registrability in Herbaceous Flora. Diversity, 17(6), 408. https://doi.org/10.3390/d17060408






