Description of a New Genus and Species of Semi-Aquatic Rodent (Cricetidae, Sigmodontinae, Ichthyomyini) from the Southern Peruvian Andes †
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Specimen Collection
2.2. Morphological Characters
2.3. Morphometric Analyses
2.4. Phylogenetic Analysis
3. Results
3.1. Multivariate Statistics
3.2. Morphology
3.3. Morphologic Phylogeny
3.4. Molecular Phylogenies
3.5. Genus and Species Description
- Anotomys Gardner, 1971:1088. Not Anotomys leander Thomas, 1906.
- Chibchanomys Voss, 1988:321. Not Ichthyomys trichotis Thomas, 1897. Name combination
- Chibchanomys Jenkins & Barnett, 1997:126. Not Chibchanomys orcesi Jenkins & Barnett, 1997. Name combination
- “Chibchanomys” sensu Salazar-Bravo et al. 2023. Not Chibchanomys Voss, 1988.
- Anotomys leander Gardner, 1971:1088. Not Anotomys leander Thomas, 1906
- Chibchanomys trichotis Voss, 1988:321. Not Ichthyomys trichotis Thomas, 1897. Name combination
- Chibchanomys orcesi Jenkins & Barnett, 1997:126. Not Chibchanomys orcesi Jenkins & Barnett, 1997. Misidentification
- Chibchanomys orcesi Voss, 2015:282–283. Not Chibchanomys orcesi Jenkins & Barnett, 1997. Name combination
- Holotype. the holotype is an adult male collected by A. Pari and H. Zeballos (24 October 2012; field number APC 1755). It consists of a well-preserved skin and skull, with tissues preserved in ethanol. TWC 4, with basioccipital/basiesphenoid suture close and adult pelage, deposited in Museo de Historia Natural de la Universidad Nacional de San Agustín, Arequipa, Peru (MUSA 13864).
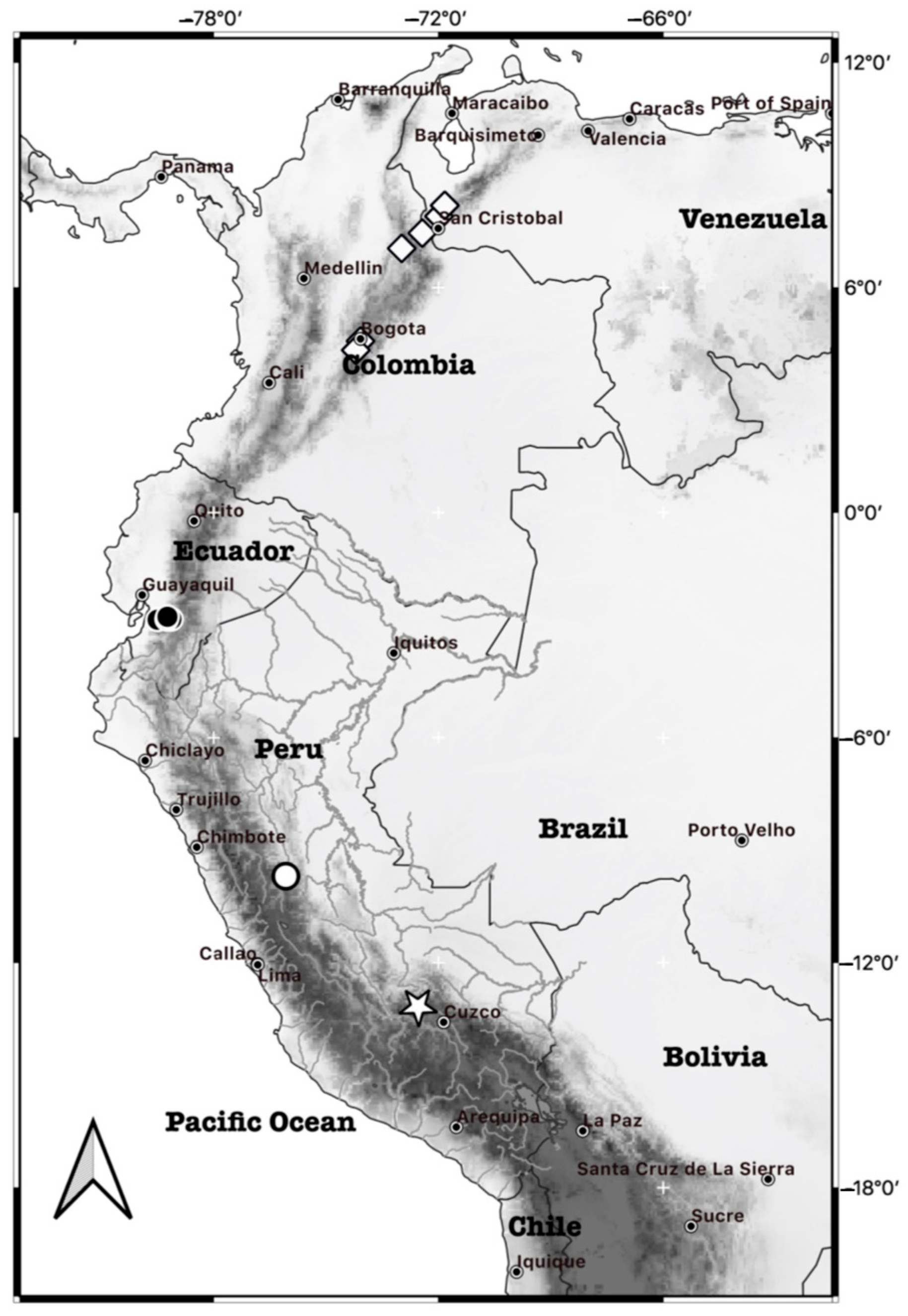
- Type locality. Peru, Department of Cusco, Province of Urubamba, Wiñaywayna, located 4 km southwest of the Aguas Calientes town (also known as Machupicchu Pueblo). The geographical coordinates are approximately 13°11′09′′ S and 72°32′34.5′′ W, at an elevation of 2825 m. The locality is within the Santuario Histórico de Machupicchu on the eastern slope of the Peruvian Andes (Figure 1).
- Paratypes. Two specimens collected by Sandra Arias (field number SAP 01, 02) from Wiñaywayna. The first is an immature female (MUSA 18964), which is a well-preserved specimen with the skin and skull in good condition, with TWC 2 and including basioccipital open suture and adult skin. The second is a male specimen (MUSA 18965) preserved in alcohol, with the skull removed, TWC 3, including basioccipital close suture and adult pelage.
- Etymology. The specific name mayopuma derives from Quechua, combining mayu (river) and puma (mountain lion). This name reflects the species’ semi-aquatic habits and carnivorous nature, akin to the neotropical otter. It emphasizes the animal’s adaptations to aquatic environments, predatory behavior, and ecological niche.
- Diagnosis. As for genus (see above)
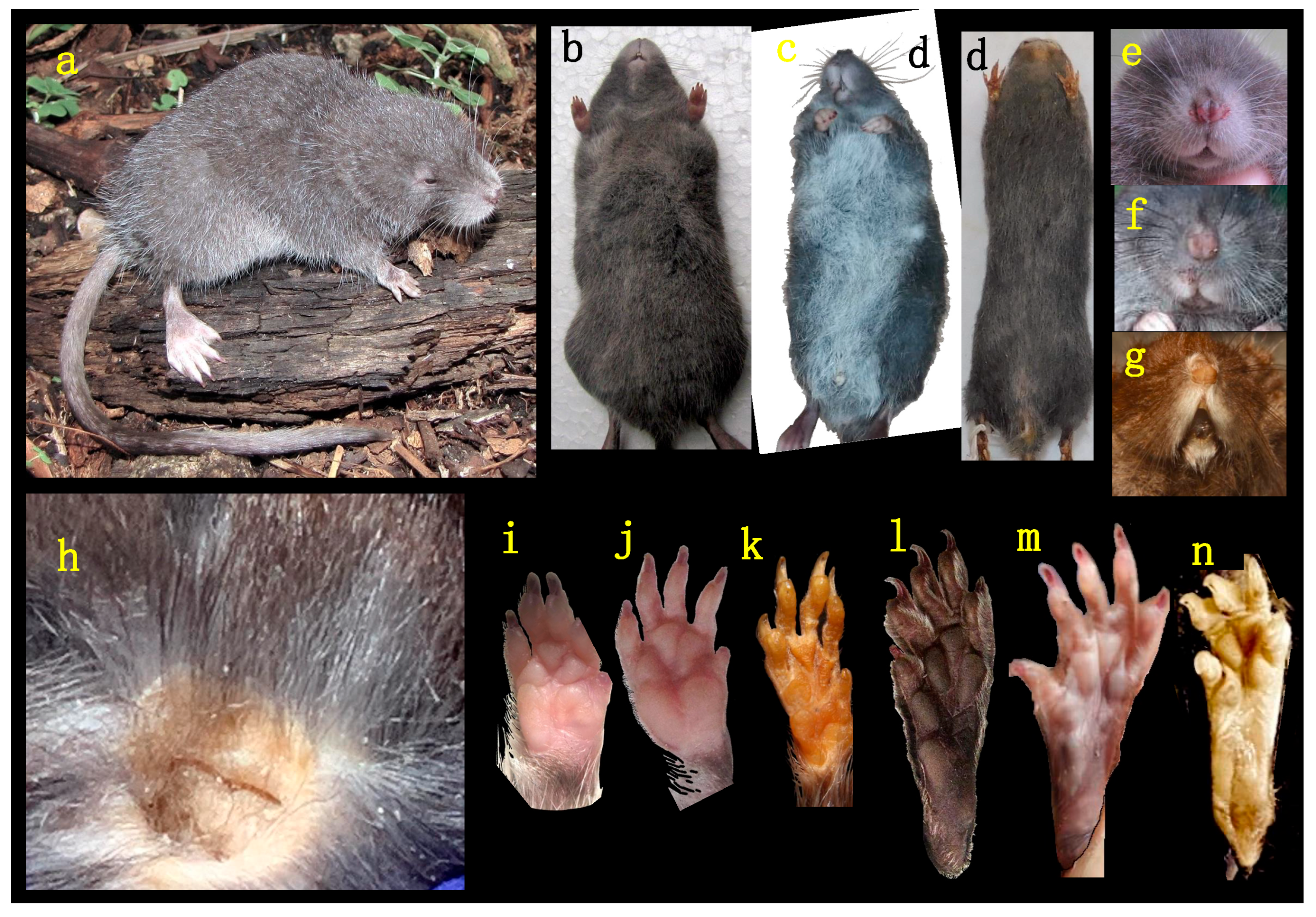
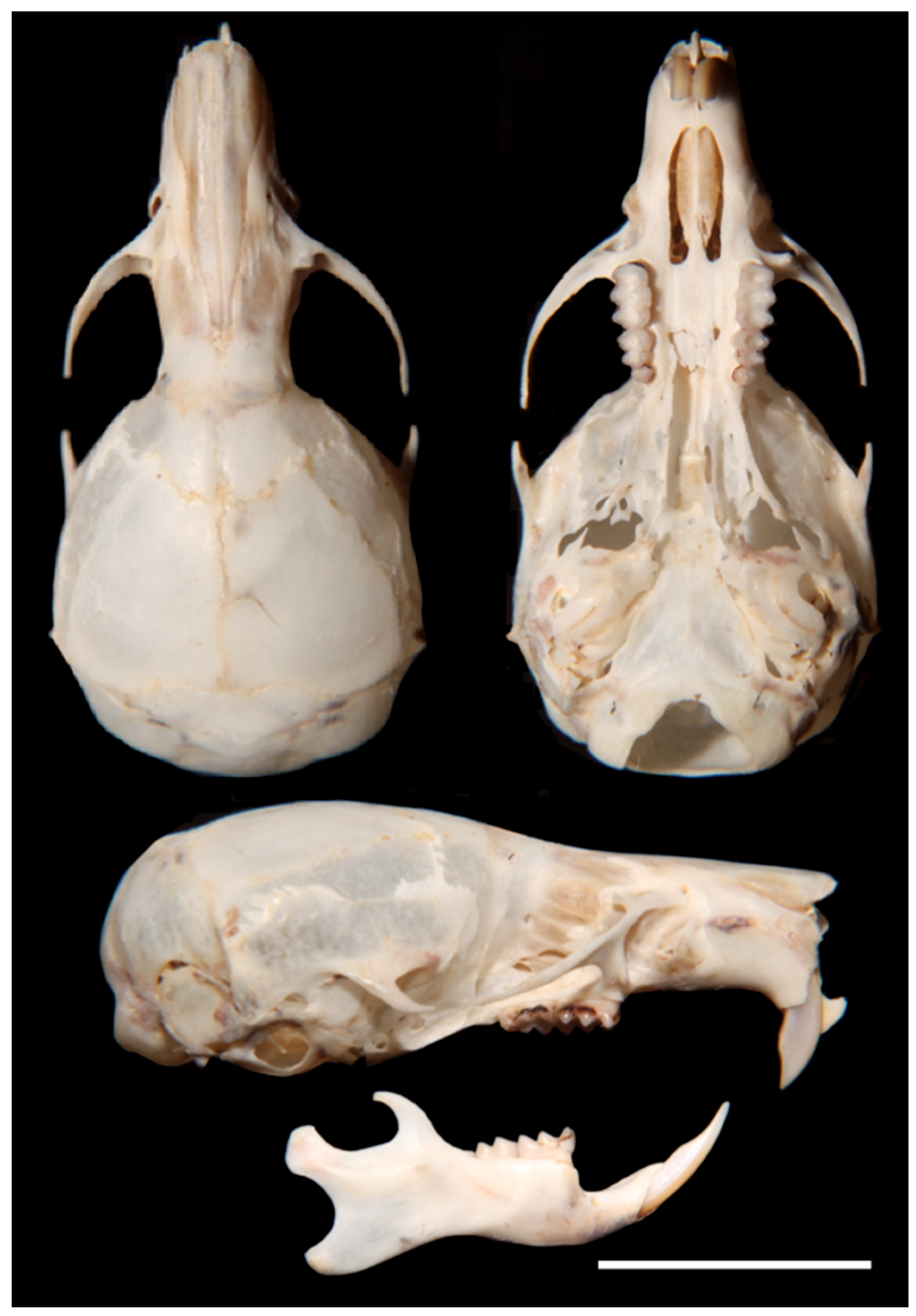
- Description. A medium-size Ichthyomyini (Table 2). The dorsal skin is soft and dull. The coloration [38,39] is deep grayish olive to hair brown (Y90 M80 C80 to Y99 M70 C90). The dorsal guard hairs are deep grayish olive (Y90 M80 C80), while the bicolor hairs have a proximal part that is pale olive gray or pearl gray (N20 Y00 M00). The underparts are slightly paler than the back, lacking contrast (Figure 3b) and have bicolor hairs that are pale olive gray distally and olive gray or deep olive gray proximally. The muzzle is pale olive-buff (N10 Y40 M20; Figure 3a,e). The dorsal hairs measure 7.9 mm ± 0.29 and consists of long dorsal guard hairs that are deep grayish olive (Y90 M80 C80) and are more abundant on the sides. The color of the dorsal guard hairs is pale olive gray (N20 Y00 M00). The ventral fur is short (6.72 mm ± 0.62), while the underfur is long (7.90 ± 0.29) and fine. The large guard hairs measure 12.38 ± 0.04. The mystacial bristles are abundant and variable in size, and the supraorbital vibrissae are absent (Figure 3a,e). The tail is longer than combined length of head and body, measuring 121.30 mm ± 5.28. It is compressed laterally in the middle and distal part and has a terminal tuft of hairs measuring 6.3 mm. The tail is well-haired (Figure 3a), lacking visible epidermal scales. The dorsal hairs on the tail measure 3.03 mm ± 0.54, while the ventral hairs measure 6.83 mm ± 0.52. The color of the tail is unicolored Olive Brown (N90 Y80 M50, Figure 3a). The pinnae are significantly reduced (Figure 3a, h), measuring 1.83–3.11 mm from the border of the auditory meatus and are hidden among the fur. The philtrum absent or indistinct (Figure 3e). The manus has grayish upper parts and pale pinkish palms with whitish fingers. There are five pads on the manus (Figure 3i): three separate interdigital pads and two separate carpal pads. The metatarsal pattern is IV > III > II = V > I. The hind feet are very wide and large (Figure 3a,l); 30.41 mm ± 1.01, with brown soles that are naked (Figure 3l). The hypothenar pad of the pes is absent, and the thenar pad is developed. The hallux has a bearing nail, and the other four fingers have claws. The pes has a fringe of stiff hairs along the plantar margins and digits (Figure 3l), measuring 1.86 mm ± 0.07 on the internal margin and 2.23 mm ± 0.10 on the external margin. The dorsal parts of the pes have brown hairs, while the fingers are paler. The rostrum is narrow, and the nasolacrimal capsules are visible on dorsal view. The nasals are long (LN/CIL = 0.40–0.42; Figure 4A) and extend anteriorly over the incisors and nasal orifice, penetrating deeply into the skull to the middle part of the interorbital constriction. The zygomatic arch is complete (Figure 4H). The posterior border of the inferior zygomatic root is over the M1 anterocone. The interorbital is narrow, with a LIB/BB ratio of 0.34–0.36 in TWC 1–4 (Figure 4I and Figure 8). The interorbital is smooth without a postorbital ridge, and the supraorbital foramina are located on the border of the interorbital constriction. The braincase is flattened and globular, giving it a young appearance compared to other genera. The braincase (Figure 4H) is flattened and globular, giving it a young appearance compared to other genera. The incisive foramen broad anteriorly (Figure 4D). The palatal process of the premaxillary bone reaches the posterior edge of the incisive foramen. The occipital condyles are not visible dorsally (Figure 4H). The posterior edge of the incisive foramen is located at the level with the anterior border of M1, and it is shorter than or does not reach the posterior border of M3 (Figure 4D). The anterior palatal pits are conspicuous (Figure 4D), and the bullae are smaller and not inflated (Figure 8). The orbicular apophysis of the malleus is absent (Figure 4O,P). The carotid circulation pattern is type 1 (sensu Voss [14]). The dental formulae: I 1/1, C 0/0 PM 0/0 M 2–3/3 = 15–16 (MUSA 18965 with dM3 absent, Figure 7). The principal cusps of the molars are tall and sharp. M1 lacks an anteromedian flexus (Figure 7A), while M2 has a delicate anteromedian style. M1 has a small mesoloph, visible in young specimens (Figure 7A), while M2 lacks mesolophs. There are small posterolophs in M1 and M2. M3 lacks a conule behind the protocone/paracone. There is a small anteroloph in M2 and M3 (Figure 7A). M3 is small without a conulid behind the protoconid/metaconid. Both m1 and m2 have an anteromedian flexid (Figure 7B) and lack mesolophids (Figure 7B). They have small entolophulids, visible in young specimens. There are also small posterolophids in m1 and m2. The upper incisors are slightly opisthodonts, the angle at which incisors protrude from the jaw was 105 (Figure 4L and Figure 8) and have a very pale-yellow anterior face. The lower incisors are slender. An omohyoid muscle is present and a unilocular–hemiglandular stomach with glandular epithelium between the esophagus and pyloric sphincter, covering the antrum to the pyloric region and bordering fold insert on the small curvature between the esophagus and sphincter. It does not have contact with the incisura angularis, and it has a gall bladder. Karyotype 2N: 92, FN: 102 [21,22].
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| UCSM | Universidad Católica de Santa María |
| SERNANP | Servicio Naciona de Áreas Naturales Protegidas |
References
- Catzeflis, F.M.; Aguilar, J.P.; Jaeger, J.J. Muroid rodents: Phylogeny and evolution. Trends Ecol. Evol. 1992, 7, 122–126. [Google Scholar] [CrossRef]
- Hershkovitz, P. Evolution of Neotropical cricetine rodents (Muridae), with special reference to the phyllotine group. Fieldiana Zool. 1962, 46, 1–524. [Google Scholar]
- Musser, G.G.; Carleton, M.D. Superfamily Muroidea. In Mammal Species of the World, A Taxonomic and Geographic Reference; Wilson, D.E., Reeder, D.A., Eds.; Johns Hopkins University Press: Baltimore, MD, USA, 2005; Volume 2, pp. 894–1531. [Google Scholar]
- Pardiñas, U.F.J.; Shenbrot, G.; Patton, J.L.; Myers, P.; León-Paniagua, L.; Ordóñez Garza, N.; Cook, J.A.; Kryštufek, B.; Haslauer, R.; Bradley, R. Family Cricetidae (True Hamsters, Voles, Lemmings and New World Rats and Mice). In Handbook of the Mammals of the World, Rodents II; Wilson, D.E., Lacher, T.E., Jr., Mittermeier, R.A., Eds.; Lynx Edicions: Barcelona, Spain, 2017; Volume 7, pp. 204–279. [Google Scholar]
- Pardiñas, U.F.J.; Ruelas, D.; Brito, J.; Bradley, L.C.; Bradley, R.D.; Ordóñez Garza, N.; Krystufek, B.; Cook, J.A.; Soto, E.C.; Salazar-Bravo, J.; et al. Species Accounts of Cricetidae. In Handbook of the Mammals of the World, Rodents II; Wilson, D.E., Lacher, T.E., Jr., Mittermeier, R.A., Eds.; Lynx Edicions: Barcelona, Spain, 2017; Volume 7, pp. 280–535. [Google Scholar]
- Patton, J.; Pardiñas, U.F.J.; D’elía, G. (Eds.) Mammals of South America, Rodents; University of Chicago Press: Chicago, IL, USA, 2015; Volume 2, pp. 1–1336. [Google Scholar]
- Reig, O.A. Diversity pattern and differentiation of High Andean rodents. In High Altitude Tropical Biogeography; Vuillemier, F., Monasterio, M., Eds.; Oxford University Press: New York, NY, USA, 1986; pp. 404–439. [Google Scholar]
- Smith, M.F.; Patton, J.L. The diversification of South American murid rodents: Evidence from mitochondrial DNA sequence data for the Akodontine tribe. Biol. J. Linn. Soc. 1993, 50, 149–177. [Google Scholar] [CrossRef]
- Smith, M.F.; Patton, J.L. Phylogenetic relationships and the radiation of Sigmodontine rodents in South America: Evidence from Cytochrome b. J. Mamm. Evol. 1999, 6, 89–128. [Google Scholar] [CrossRef]
- Wilson, D.E.; Reader, D.A. (Eds.) Mammal Species of the World: A Taxonomic and Geographic Reference, 3rd ed.; Johns Hopkins University Press: Baltimore, MD, USA, 2005; pp. i–xvii+743–2142. [Google Scholar]
- Anthony, H.E.; Tate, G.H.H. Two new genera of rodents from South America. Am. Mus. Novit. 1929, 383, 6. Available online: http://hdl.handle.net/2246/4089 (accessed on 15 January 2023).
- Barnett, A.A. The ecology and natural history of a fishing mouse Chibchanomys spec. nov. (Ichthyomyini: Muridae) from the Andes of southern Ecuador. Mamm. Biol. 1997, 62, 43–52. [Google Scholar]
- Salazar-Bravo, J.; Tinoco, N.; Zeballos, H.; Brito, J.; Arenas-Viveros, D.; Marín-C, D.; Ramírez-Fernández, J.D.; Percequillo, A.R.; Lee, T.E., Jr.; Solari, S.; et al. Systematics and diversification of the Ichthyomyini (Cricetidae, Sigmodontinae) revisited: Evidence from molecular, morphological, and combined approaches. PeerJ 2023, 11, e14319. [Google Scholar] [CrossRef] [PubMed]
- Voss, R.S. Systematics and ecology of ichthyomyine rodents (Muroidea): Patterns of morphological evolution in a small adaptive radiation. Bull. Am. Mus. Nat. Hist. 1988, 188, 259–493. Available online: http://hdl.handle.net/2246/927 (accessed on 5 February 2006).
- Voss, R.S. Tribe Ichthyomyini Vorontsov, 1959. In Mammals of South America; Patton, J.L., Pardiñas, U.F.J., D’Elía, G., Eds.; University of Chicago Press: Chicago, IL, USA, 2015; pp. 279–291. [Google Scholar]
- Zeballos, H.; Díaz, R.; Rodriguez, N.; Delgado, W.; Tuwits-Wajai, O.; Villoslaba, D.; Cahuaza, F.J.; Arce, A. Pequeños mamíferos no voladores del paisaje Alto Mayo, San Martín, Peru. In Capítulo 8, Evaluación Biológica Rápida del Paisaje Alto Mayo, San Martín Perú; Boletín RAP de Evaluación Biológica 73; Larsen, T.H., Palomino, W., Zeballos, H., Carrillo, P., Eds.; Conservation International: Arlington, VA, USA, 2024; pp. 307–326. [Google Scholar]
- Musser, G.G.; Gardner, A.L. A new species of the ichthyomyine Daptomys from Peru. Am. Mus. Novit. 1974, 2537, 1–23. Available online: http://hdl.handle.net/2246/2740 (accessed on 23 November 2006).
- SERNANP [Servicio Nacional de Áreas Naturales Protegidas]. Parque Nacional Tingo María 50 Años; SERNANP y Empresa de Generación Huallaga, S.A-Odebrecht: Lima, Peru, 2014; pp. 1–160. [Google Scholar]
- Pacheco, V.; Ugarte-Nuñez, J. New records of Stolzmann’s fish-eating rat Ichthyomys stolzmanni (Cricetidae. Sigmodontinae) in Peru: A rare species becoming a nuisance. Mamm. Biol. 2011, 76, 657–661. [Google Scholar] [CrossRef]
- Thomas, O. New mammals from Peru and Bolivia, with list of those recorded from Inambari River, Upper Madre de Dios. Ann. Mag. Nat. Hist. 1901, 7, 178–190. [Google Scholar] [CrossRef]
- Gardner, A.L. Karyotypes of two rodents from Peru, with a description of the highest diploid number recorded from a mammal. Experientia 1971, 27, 1088–1089. [Google Scholar] [CrossRef]
- Gardner, A.L.; Patton, J.L. Karyotypic variation in oryzomyine rodents (Cricetidae) with comments on Chromosomal evolution in the Neotropical Cricetine Complex. Occas. Pap. Mus. Nat. Sci. La. State Univ. 1976, 49, 1–48. [Google Scholar]
- Jenkins, P.D.; Barnett, A.A. A new species of water mouse of the genus Chibchanomys (Rodentia: Muridae: Simodontinae) from Ecuador. Bull. Nat. Hist. Mus. Lond. (Zool.) 1997, 63, 123–128. [Google Scholar]
- Sikes, R.S.; Gannon, W.L.; Animal Care and Use Committee of the ASM. 2016 Guidelines of the American Society of Mammalogists for the use of wild mammals in research and education. J. Mamm. 2016, 97, 663–688. [Google Scholar] [CrossRef]
- Anthony, H.E. Preliminary report on Ecuadorean mammals, No. 1. Am. Mus. Novitates 1921, 20, 1–6. Available online: http://hdl.handle.net/2246/4636 (accessed on 1 April 2014).
- Anthony, H.E. 1923. Preliminary report on Ecuadorean mammals, No. 3. Am. Mus. Novitates 1923, 55, 1–13. Available online: http://hdl.handle.net/2246/4635 (accessed on 13 September 2013).
- Ellerman, J.R. The families and Genera of Living Rodents. In Family Muridae; Trustees British Mus (Nat Hist): London, UK, 1941; Volume 2, pp. xii+690. [Google Scholar]
- Hanson, J.D.; D’Elía, G.; Ayers, S.B.; Cox, S.B.; Burneo, S.F.; Lee, T.E. A new species of fish-eating rat, genus Neusticomys (Sigmodontinae), from Ecuador. Zool. Stud. 2015, 54, 49. [Google Scholar] [CrossRef]
- Ochoa, J.; Soriano, P. A new species of water rat, genus Neusticomys Anthony, from the Andes of Venezuela. J. Mamm. 1991, 72, 97–103. [Google Scholar] [CrossRef]
- Percequillo, A.R.; Carmignotto, A.P.; de Silva, M.J. A new species of Neusticomys (Ichthyomyini. Sigmodontinae) from central Brazilian Amazonia. J. Mamm. 2005, 86, 873–880. [Google Scholar] [CrossRef]
- Percequillo, A.R.; Dalapicolla, J.; Abreu-Júnior, E.F.; Roth, P.R.O.; Ferraz, K.M.P.M.B.; Chiquito, E.A. How many species of mammals are there in Brazil? New records of rare rodents (Rodentia: Cricetidae: Sigmodontinae) from Amazonia raise the current known diversity. PeerJ 2017, 5, e4071. [Google Scholar] [CrossRef] [PubMed]
- Thomas, O. On some mammals from Central Peru. Proc. Zool. Soc. Lond. 1893, XXIII, 333–340. [Google Scholar]
- Thomas, O. Descriptions of four new South American mammals. Ann. Mag. Nat. Hist. 1897, 20, 218–221. [Google Scholar] [CrossRef][Green Version]
- Thomas, O. A new aquatic genus of Muridae discovered by Consul, L. Soderström in Ecuador. Ann. Mag. Nat. Hist. 1906, 17, 86–88. [Google Scholar] [CrossRef]
- Thomas, O. A third genus of the Ichthyomys group. Ann. Mag. Nat. Hist. 1906, 17, 421–423. [Google Scholar] [CrossRef][Green Version]
- Voss, R.S.; Lunde, D.R.; Simmons, N.B. The Mammals of Paracou, French Guiana: A Neotropical Lowland Rainforest Fauna Part 2. Nonvolant Species. Bull. Am. Mus. Nat. Hist. 2001, 263, 1–236. Available online: http://hdl.handle.net/2246/386 (accessed on 18 August 2001). [CrossRef]
- Carleton, M.D. A Survey of Gross Stomach Morphology in New World Cricetinae (Rodentia, Muroidea), with Comments on Functional Interpretations; Miscellaneous Publications of the Museum of Zoology; University of Michigan Museum of Zoology: Ann Arbor, MI, USA, 1973; Volume 146, pp. 1–43. Available online: http://hdl.handle.net/2027.42/56390 (accessed on 13 May 2025).
- Reig, O.A. A proposed unified nomenclature for the enameled components of the molar teeth of Cricetidae (Rodentia). J. Zool. 1977, 181, 227–241. [Google Scholar] [CrossRef]
- Ridgway, R. Color Standards and Color Nomenclature; reprint by Elibron Clasics; Elibron Clasics: Washington, DC, USA, 1912; pp. i–v+1–43+LIII plates. [Google Scholar]
- Küppers, H. Atlas de Los Colores, 1st ed.; Blume Barcelona: Barcelona, España, 2002; pp. 1–165. [Google Scholar]
- RStudio Team. Integrated Development for R; RStudio PBC: Boston, MA, USA, 2020; Available online: http://www.rstudio.com/ (accessed on 29 January 2024).
- Hooper, E.; Musser, G.G. The glans penis in Neotropical cricetines (family Muridae) with comments on classification of muroid rodents. Mis. Pub. Mus. Zool. Univ. Mich. 1964, 123, 1–57. [Google Scholar]
- Starrett, A.; Fisler, G.F. Aquatic adaptations of the water mouse, Rheomys underwoodi. Contrib. Sci. 1970, 182, 1–14. [Google Scholar] [CrossRef]
- Vorontzov, N.N. Evolution of the Alimentary System in Myomorph Rodents; Smithsonian Institution: Washington, DC, USA, 1979; pp. 1–346. [Google Scholar]
- Voss, R.S.; Linzey, A.V. Comparative gross morphology of male accessory glands among Neotropical Muridae (Mammalia: Rodentia) with comments on systematic implications. Misc. Pub Mus. Zool. Univ. Mich. 1981, 159, 1–41. [Google Scholar]
- Goloboff, P.; Catalano, S.A. TNT version 1.5, including a full implementation of phylogenetic morphometrics. Cladistics 2016, 32, 221–238. [Google Scholar] [CrossRef] [PubMed]
- Goloboff, P.; Farris, J.S.; Nixon, K.C. TNT, a free program for phylogenetic analysis. Cladistics 2008, 24, 774–786. [Google Scholar] [CrossRef]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Fluori, T.; Zozlov, A.; Morel, B.; Posada, D.; Stamatakis, A. Modeltest NG v0.1.7. Exilixis Lab. 2021. Available online: https://github.com/ddarriba/modeltest (accessed on 2 March 2025).
- Stamatakis, A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 2006, 22, 2688–2690. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Edler, D.; Klein, J.; Antonelli, A.; Silvestro, D. raxmlGUI 2.0: A graphical interface and toolkit for phylogenetic analyses using RAxML. Methods Ecol. Evol. 2020, 12, 373–377. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Pavan, S.E.; Abreu, E.F.; Sánchez-Vendizú, P.Y.; Batista, R.; Murta-Fonseca, R.A.; Pradel, R.; Rengifo, E.M.; Leo, M.; Pacheco, V.; Aleixo, A.; et al. A hint on the unknown diversity of eastern Andes: High endemicity and new species of mammals revealed through DNA barcoding. Syst. Biodivers. 2024, 21, 2302196. [Google Scholar] [CrossRef]
- Anderson, S. Mammals of Bolivia, taxonomy and distribution. Bull. Am. Mus. Nat. Hist. 1997, 231, 1–652. Available online: http://hdl.handle.net/2246/1620 (accessed on 23 January 2006).
- Hooper, E.T. Habitats and food of amphibious mice of the genus Rheomys. J. Mamm. 1968, 49, 550–553. [Google Scholar] [CrossRef]
- Fish, F.E. Transitions from drag-based to lift-based propulsion in mammalian swimming. Am. Zool. 1996, 36, 628–641. Available online: https://www.jstor.org/stable/3884002 (accessed on 6 August 2018). [CrossRef]
- Fish, F.E. Secondary evolution of aquatic propulsion in higher vertebrates: Validation and prospect. Integr. Comp. Biol. 2016, 56, 1285–1297. [Google Scholar] [CrossRef]
- Kükenthal, W. XIX. On the adaptation of mammals to aquatic life. Ann. Mag. Nat. Hist. 1891, 38, 153–179. [Google Scholar] [CrossRef]
- Dubost, G.; Petter, F. Une espèce nouvelle de “rat-pechêur” de Guyane Francaise: Daptomys oyapocki sp. nov. (Rongeurs, Cricetidae). Mammalia 1978, 42, 435–439. [Google Scholar] [CrossRef]
- Handley, C.O.; Mondolfi, E. A new species of fish-eating rat, Ichthyomys, From Venezuela (Rodentia Cricetidae). Acta Biol. Venez. 1963, 3, 417–419. [Google Scholar]
- Fernández de Córdova, J.; Nivelo-Villavicencio, C.; Reyes-Puig, C.; Pardiñas, U.F.J.; Brito, J. A new species of crab-eating rat of the genus Ichthyomys, from Ecuador (Rodentia, Cricetidae, Sigmodontinae). Mammalia 2019, 84, 377–391. [Google Scholar] [CrossRef]
- Gonzales, F.N.; Arce-Merma, A.; Zeballos, H. Range extension of the Peruvian fish-eating rat Neusticomys peruviensis (Rodentia: Cricetidae) in Peru. Rev. Peru. Biol. 2017, 24, 413–416. [Google Scholar] [CrossRef]
- Leite, R.N.; Silva, M.N.F.; Gardner, T.A. New records of Neusticomys oyapocki (Rodentia, Sigmodontinae) from a human-dominated forest landscape in northeastern brazilian Amazonia. Mastozool. Neotrop. 2007, 14, 257–261. [Google Scholar]
- Medina, C.E.; Lopez, E.; Pino, K.; Pari, A.; Zeballos, H. Biodiversidad de la zona reservada Sierra del Divisor (Perú): Una visión desde los mamíferos pequeños. Rev. Peru. Biol. 2015, 22, 199–212. [Google Scholar] [CrossRef][Green Version]
- Miranda, C.L.; Rossi, V.; Semedo, T.B.F.; Flores, T.A. New records and geographic distribution extension of Neusticomys ferreirai and N. oyapocki (Rodentia, Sigmodontinae). Mammalia 2012, 76, 335–339. [Google Scholar] [CrossRef]
- Pacheco, V.; Sánchez-Vendizú, P.; Loaiza-Salazar, C.R.; Pino, K.; Medina, C.; Vivas-Ruiz, D. A revision of Neusticomys peruviensis (Rodentia: Cricetidae) with the description of a new subspecies. J. Mamm. 2020, 101, 858–871. [Google Scholar] [CrossRef]
- Rodríguez-Posada, M.E. Primer registro del ratón de agua del Táchira, Neusticomys mussoi (Rodentia, Cricetidae) en Colombia. Mastozool. Neotrop. 2014, 21, 367–372. [Google Scholar]
- Velandia-Perilla, J.H.; Saavedra-Rodríguez, C.A. Mammalia, Rodentia, Cricetidae, Neusticomys monticolus (Anthony, 1921): Noteworthy records of the Montane fish-eating rat in Colombia. Check List 2013, 9, 686–688. [Google Scholar] [CrossRef]





| Genera | Between Genera | Within Genus | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Anotomys | New Species | Neusticomys | Ichthyomys | Daptomys | Rheomys | Chibchanomys | Distance | S. E. | |
| Anotomys | 0.016 | 0.016 | 0.014 | 0.017 | 0.014 | 0.015 | n/c | n/c | |
| New species | 0.224 | 0.013 | 0.011 | 0.012 | 0.012 | 0.012 | 0.0691 | 0.007 | |
| Neusticomys | 0.224 | 0.1617 | 0.012 | 0.011 | 0.011 | 0.012 | 0.0699 | 0.007 | |
| Ichthyomys | 0.2215 | 0.17 | 0.1723 | 0.012 | 0.009 | 0.012 | 0.0887 | 0.007 | |
| Daptomys | 0.2301 | 0.1499 | 0.1456 | 0.1705 | 0.011 | 0.012 | 0.0373 | 0.005 | |
| Rheomys | 0.2354 | 0.1774 | 0.1754 | 0.1408 | 0.1774 | 0.011 | 0.1276 | 0.01 | |
| Chibchanomys | 0.2048 | 0.1578 | 0.1477 | 0.1631 | 0.1382 | 0.1739 | n/c | n/c | |
| Anotomys | Chibchanomys | Neusticomys | Daptomys | Ichthyomys | Rheomys | Incanomys | |
|---|---|---|---|---|---|---|---|
| Adult body size (HBL), weight (W) | Medium, 110–128 mm, 40–62 g | Medium, 105–130 mm, 43–57 g | Small, 87–116 mm, 18–35 g | Medium, 95–133 mm, 32–66 g | Large, 100–205 mm, 70–155 g | Small to large 85–148 mm, 18–88 g | Medium, 103–118 mm, 35–47 g |
| Hindfoot (range mm) | Long (32–37), broad | Long (28–33), broad | Short (23–27), narrow | Short (23–30), narrow | Long (30–41), broad | Short or long (25–41), narrow or broad | Long (29–31), broad |
| Tail as % of head and body, X ± SD (range) | Long, 124 ± 0.07 (114–124) | Equal, 110.3 ± 0.03 (105–114) | Equal, 95.85 ± 9.23 (70–110) | Short, 78.52 ± 5.92 (65–86) | Equal, 99.83 ± 11.20 (80–122) | Equal to long, 107.74 ± 14.88 (82–133) | Long, 121.3 ± 0.05 (114–127%) |
| Ventral pelage and contrast with the back | Whitish, without contrast | Whitish, with contrast | Smoke gray, without contrast | Slate gray or cream without contrast | White or cream, with contrast | White or cream, with contrast | Smoke gray, without contrast |
| Naso-labial sulcus (philtrum) | Absent | Absent | Present | Present | Present | Present or absent | Incomplete or partial |
| Pinna | Absent or vestigial | Vestigial as a small fold | Long, visible above the fur | Long, visible above the fur | Small, visible above the fur | Vestigial or small | Vestigial as a small fold |
| Pads on manus | 4 | 5 | 5 | 5 | 5 | 3 or 4 | 5 |
| Metatarsal pattern | IV > III > V > II > I | IV > III > II = V > I | III ≥ IV > II > >V > I | III ≥ IV > II > >V > I | IV > III > II > V > I | IV > III > II ≥ V > I | IV > III > II = V > I |
| Hairy fringe in plantar margins | Well-developed stiff hairs | Well-developed stiff hairs | Weakly developed stiff hairs | Weakly developed stiff hairs | Well-developed stiff hairs | Weakly or well-developed stiff hairs | Well-developed stiff hairs |
| Orbicular apophysis of malleus | Absent | Absent | Present | Absent | Present | Present | Absent |
| Carotid circulation | Type 2 | Type 1 | Type 1 | Type 1 | Type 3 | Type 3 | Type 1 |
| Posteroloph (juveniles) | M1 present or absent in M2 | M1 and M2 | Absent | Small M1 and M2 | Tiny in M1 and M2 | Small M1 and M2 | M1 and M2 |
| Neusticomys orcesi | Chibchanomys trichotis | Incanomys mayopuma | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Ecuador | Venezuela and Colombia | Peru | |||||||
| Range (n) | X | SE | Range | x | SE | Range (n) | X | SE | |
| TL | 211–217 (3) | 213.33 | 1.86 | 215–256 (8) | 244.25 | 6.82 | 225–253 (4) | 236.00 ^ | 6.10 |
| LT | 108–112 (3) | 110.67 | 1.33 | 87–133 (8) | 124.50 | 6.08 | 123–135 (4) | 129.25 ^ | 2.66 |
| HBL | 100–105 (3) | 102.67 | 1.45 | 105–128 (8) | 119.75 | 2.93 | 102–118 (4) | 106.75 * | 3.77 |
| HF | 22–24 (3) | 23.17 | 0.60 | 30–33 (8) | 30.50 | 0.76 | 28.9–31.1 (4) | 30.41 ^ | 0.50 |
| EL | 10–14 (3) | 11.17 | 1.42 | 6–10 (8) | 7.21 | 0.70 | 5.9–6.7 (4) | 6.21 ^ | 0.18 |
| W | 35–41 (2) | 38.00 | 3.00 | 49.10 (2) | 49.10 | 0.10 | 37–47 (3) | 41.00 | 3.06 |
| CIL | 23.0–26.1 (4) | 24.59 | 0.64 | 22.5–26.9 (7) | 25.31 | 0.56 | 23.8–25.5 (4) | 24.77 | 0.41 |
| LN | 8.8–9.9 (4) | 9.30 | 0.26 | 7.7–9.5 (6) | 8.86 | 0.14 | 10.1–10.2 (3) | 10.21 * ^ | 0.02 |
| BN | 2.9–3.7 (4) | 3.24 | 0.15 | 2.2–3.0 (7) | 2.80 | 0.10 | 2.8–3.4 (4) | 3.19 | 0.13 |
| LIB | 4.5–4.8 (4) | 4.61 | 0.07 | 4.4–4.9 (7) | 4.70 | 0.08 | 4.3–4.5 (4) | 4.41 * ^ | 0.04 |
| BB | 11.5–12.4 (4) | 11.93 | 0.19 | 12.7–13.8 (7) | 13.33 | 0.15 | 12.4–12.8 (4) | 12.46 * | 0.12 |
| DI | 1.2–1.3 (4) | 1.28 | 0.03 | 1.1–1.5 (7) | 1.27 | 0.05 | 1.0–1.5 (4) | 1.37 ^ | 0.12 |
| HI | 3.6–4.5 (4) | 4.07 | 0.20 | 4.1–5.3 (7) | 4.64 | 0.14 | 3.9–4.6 (4) | 4.11 | 0.18 |
| BIT | 1.4–1.5 (4) | 1.46 | 0.02 | 1.0–1.3 (8) | 1.21 | 0.04 | 1.0–1.5 (4) | 1.31 | 0.11 |
| LIF | 4.2–4.7 (4) | 4.49 | 0.11 | 3.9–5.3 (7) | 4.83 | 0.18 | 4.4–4.6 (4) | 4.51 | 0.06 |
| BIF | 1.9–2.2 (4) | 2.06 | 0.08 | 1.7–2.4 (7) | 1.94 | 0.09 | 1.7–1.9 (4) | 1.82 ^ | 0.05 |
| LD | 5.0–6.1 (4) | 5.68 | 0.24 | 5.3–7.1 (6) | 6.34 | 0.31 | 5.7–6.3 (4) | 5.99 | 0.14 |
| LM | 4.1–4.3 (4) | 4.21 | 0.05 | 3.5–4.9 (8) | 4.42 | 0.15 | 4.2–4.4 (4) | 4.30 | 0.05 |
| BM1 | 1.3–1.5 (4) | 1.39 | 0.06 | 1.4–1.7 (8) | 1.53 | 0.03 | 1.4–1.5 (4) | 1.42 | 0.01 |
| BPB | 2.1–3.2 (4) | 2.55 | 0.24 | 2.7–3.4 (6) | 3.11 | 0.16 | 2.6–3.0 (4) | 2.83 | 0.10 |
| ZB | 12.4–13.5 (4) | 12.86 | 0.23 | 11.9–14.1 (7) | 13.25 | 0.31 | 11.7–12.9 (4) | 12.19 | 0.28 |
| BZP | 1.1–1.3 (4) | 1.16 | 0.04 | 0.9–1.2 (8) | 1.08 | 0.04 | 0.9–1.0 (4) | 0.96 ^ | 0.02 |
| BOC | 6.6–7.5 (4) | 6.97 | 0.21 | 7.03–7.6 (7) | 7.38 | 0.09 | 7.7–7.9 (4) | 7.80 * ^ | 0.06 |
| MAL | 16.1–17.3 (4) | 16.42 | 0.30 | 15.3–17.6 (4) | 16.61 | 0.52 | 15.6–16.3 (4) | 16.03 | 0.23 |
| LMM | 4.3–4.4 (4) | 4.31 | 0.04 | 4.61–4.6 (4) | 4.34 | 0.20 | 4.1–5.0 (4) | 4.49 | 0.26 |
| BUL | 4.5–4.7 (4) | 4.60 | 0.03 | 4.01–6.0 (4) | 4.97 | 0.17 | 3.8–4.3 (4) | 4.13 * ^ | 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeballos, H.; Pari, A.; Medina, C.E.; Pino, K.; Arias, S.; Arce, A.L.; Gonzales, F.N. Description of a New Genus and Species of Semi-Aquatic Rodent (Cricetidae, Sigmodontinae, Ichthyomyini) from the Southern Peruvian Andes. Diversity 2025, 17, 406. https://doi.org/10.3390/d17060406
Zeballos H, Pari A, Medina CE, Pino K, Arias S, Arce AL, Gonzales FN. Description of a New Genus and Species of Semi-Aquatic Rodent (Cricetidae, Sigmodontinae, Ichthyomyini) from the Southern Peruvian Andes. Diversity. 2025; 17(6):406. https://doi.org/10.3390/d17060406
Chicago/Turabian StyleZeballos, Horacio, Alexander Pari, César E. Medina, Kateryn Pino, Sandra Arias, Alayda L. Arce, and Fiorella N. Gonzales. 2025. "Description of a New Genus and Species of Semi-Aquatic Rodent (Cricetidae, Sigmodontinae, Ichthyomyini) from the Southern Peruvian Andes" Diversity 17, no. 6: 406. https://doi.org/10.3390/d17060406
APA StyleZeballos, H., Pari, A., Medina, C. E., Pino, K., Arias, S., Arce, A. L., & Gonzales, F. N. (2025). Description of a New Genus and Species of Semi-Aquatic Rodent (Cricetidae, Sigmodontinae, Ichthyomyini) from the Southern Peruvian Andes. Diversity, 17(6), 406. https://doi.org/10.3390/d17060406







