Abstract
Global warming is influencing marine dynamics, with marine heat waves (MHWs) threatening the survival of several species. After observing mussels’ massive mortality for three consecutive years (2022–2024) along the Italian Mid-Adriatic Coast, the present study aimed to evaluate, from an ecological and molecular perspective, the evolution of the health state of the Mediterranean mussel (Mytilus galloprovincialis) population facing the effects of summer heatwave in 2024, in situ. Three MHWs were recorded over the summer, with the second being 41 days long and having temperatures higher than 30 °C. In both sites considered (at the Tronto River mouth and the Frana San Nicola) inside the Piceno coast, the mussel beds experienced a clear decrease in individual density from April (the reference month) to August, with the total mortality recorded in September. The transcriptional levels of the molecular biomarkers analyzed during this time span revealed a state of heat stress with HSP70 (heat shock protein 70) and HSP90 (heat shock protein 90) upregulated in July and August. The apoptotic signal measured through the branchial transcript quantification of p53 and caspase 3 is less clear. The occurrence of MHWs is reshaping the local macrozoobenthonic community structure: the permanent mussel beds that characterized the intertidal and shallow submerged reef along the Mid-Adriatic coast are shifting to a temporary population that renovates yearly.
1. Introduction
As a component of the climate crisis, ocean warming alters global evaporation rates, precipitation, and local factors like salinity [1]. One of the ways ocean warming exerts its effects is through Marine Heat Waves (MHW), anomalously warm water events [2]. Temperature anomalies are considered MHWs when they exceed a threshold of the 90th percentile for at least 5 days during a climatological period of 30 years [3]. The duration, magnitude, and frequency of extreme events like MHWs are exacerbated by the surge of the climate crisis [4,5].
In recent decades, local marine ecosystems have been particularly affected by MHWs worldwide, influencing local ecological dynamics [3]. Marine species respond differently to the impact of the climate crisis, which is often not uniform over time and space, as they have different levels of vulnerability, exposure, sensitivity, and adaptation [6]. The synergic action of extreme events like MHWs and other abiotic or biotic factors like ocean acidification or infection/parasitism challenge the resilience of marine habitats and the ecosystem services they provide [4,5,7,8].
Coastal environments are subjected to the most significant ecological impact by MHWs, like changes in local species composition and abundance [9] or sessile species mass mortality events (MME), like sponges, gorgonians, or mussels [10,11,12]. Indeed, mobile species can migrate toward waters with more favorable conditions when MHWs hit their habitat and, being active, may have a higher thermal tolerance. On the contrary, sessile species are unable to escape [5] and are consequently subjected to stress conditions [10,11,12].
A semi-closed basin like the Mediterranean Sea is particularly susceptible to the climate crisis. Indeed, the warming rate, caused by extreme temperatures and the numerous, more frequent, and higher magnitude MHWs of this area are more intense in a global scenario [10,13,14,15]. Within this geographical context, the effects of the climatic anomalies are observed more quickly in the Adriatic basin due to its shallow depths (in the northern and central parts) and its semi-closed conformation that allows a slow water turnover with the rest of the Mediterranean through the 70 km-large Otranto channel [13,16]. Numerous Italian rivers discharge their flow along the Adriatic western side, with the Po river being the main contributor of the high productivity of Adriatic waters. However, the increase of drought occurrence in the Mediterranean scenario pushes organisms’ lives to their limits of tolerance and affects the Adriatic marine food web [16,17].
While MHWs occurred in the Adriatic area [18], the first MMEs of the Mediterranean mussel Mytilus galloprovincialis were reported only recently [10,14,15]. A massive die-off of M. galloprovincialis along the Central Adriatic coast characterized the coastal reefs during the late summer of 2022 [10] and once again in the late summer of 2023 (unpublished observation by the same authors). Analyzing the climatic data (temperature, precipitation, river flow) that influenced that region, these authors suggested that the mussel MME may be due to prolonged anomalously warm water combined with reduced nutrient supply. Indeed, this benthic species, which often dominates intertidal and subtidal zones, is exposed to a variety of harsh marine and terrestrial conditions, like MHWs, that could affect their survival [19]. The Mediterranean mussel is a eurytherm species with wide optimum thermal limits between 9 °C and 25 °C for optimal physiological processes [20,21,22]. In fact, Ref. [20] suggests that natural populations of Mediterranean mussels already live in conditions close to their thermal acclimation limits during the summer season.
In addition to behavioral changes like valve opening, mussel metabolism and transcriptome are factors negatively affected by MHWs [23]. Transcriptome modulation is indeed an early indicator of the physiological response to stress. Molecular investigations on bivalves exposed to artificially induced but realistic MHWs in a controlled setting demonstrate the activation of the cellular stress response, with the regulation of molecular biomarkers such as chaperones and molecular protector heat-shock proteins (HSPs) or genes associated with apoptotic processes like caspase or p53 [23,24,25,26].
The present study aimed to assess in situ the response of the Mediterranean mussel Mytilus galloprovincialis to real MHWs and detect any transcription alteration of molecular biomarkers of exposure in this species from natural populations inhabiting coastal habitats of the Italian Central Adriatic Sea from Spring 2024 to the end of Summer 2024. The chaperones (HSPs) and the regulators of the cell cycle and apoptosis (p53 protein and caspase 3) are the biomarkers selected due to their wide use in MHW studies [23,24,26]. Molecular profiles are supported by ecological and climatic data.
2. Materials and Methods
2.1. Study Area
The study was conducted along the Piceno Coast (Central Adriatic Sea, Italy) from February to September 2024. Mussel bed coverage and individuals were collected in situ from natural populations inhabiting submerged reefs. Figure 1 shows the two sampling stations, identified as “Frana San Nicola a mare” (FSN) in Grottammare (AP) Municipality (43°00′01″ N, 13°52′21″ E—33T), and “Tronto” (TRN) at the mouth of the Tronto river, between San Benedetto del Tronto (AP) and Martinsicuro (TE) Municipalities (42°53′36″ N, 13°55′11″ E—33T). The area is characterized by shallow sandy bottoms where the sea temperatures are almost constant throughout the shallow water column. The FSN site consists of a natural reef, while in TRN the mussel beds insist on artificial anti-erosion barriers. In the study area, the Mediterranean mussel forms vast and dense monospecific beds at a level ranging from the surface to around −3 m depth.
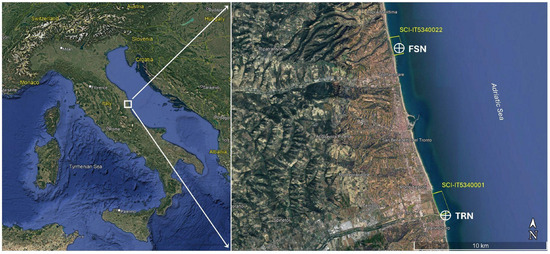
Figure 1.
Location of studied sites; the right figure represents the zoom of the area delineated in the left picture. FSN stands for Frana San Nicola and TRN for Tronto River, SCI stands for Site Community Importance.
Both sites do not exceed 4.5 m in depth. With respect to the previous study [10], these two sites were selected on the basis of their differences: one (TRN) is an artificial reef at the river mouth (potential temperature mitigation and good food availability actions by riverine freshwater), and the other one is a natural reef not influenced by riverine inputs. Both sites deal with marine Natura 2000 network sites; FSN is included in IT5340022, and TRN is close to the southern limit of IT5340001.
2.2. Environmental Data and Analyses
MHWs are defined as periods during which temperatures exceed the threshold of the 90th percentile relative to long-term local climatology for at least five days [3]. The analysis of the last summer (2024) of our study area was based on data exported by three official sources: 1. The Italian Institute for Environmental Protection and Research (ISPRA) San Benedetto del Tronto meteo-marine station (42°57′18″ N, 13°53′22″ E—33T); 2. The meteoclimatic station provided by regional civil Protection, close to URDIS campus, (42°55′58″ N, 13°53′25″ E—33T); 3. Copernicus Marine Environment Monitoring Service.
Weather data from ISPRA had a variable time step between 10 and 60 min, which is in line with recommendations from the 2008 World Meteorological Organization guide [27,28]. We focused on data from 1 January 2012 to 31 December 2024, with the only exception of a very short recording gap; the monitoring climate signal was really considered homogeneous because the data are derived from the monitoring of a meteo-marine station that has not moved at all. At the same time, the signal is continuous because it has no temporally extended recording gaps of more than 10 percent of the total data.
The 90th percentile of the sea surface temperature (SST) for each day from 15 April to 15 September was extracted from San Benedetto del Tronto ISPRA meteo-marine station while for the same period, daily data are available for the Copernicus dataset, considering the 30-year period from 1994 to 2023 (CMEMS—Reprocessed (REP) Mediterranean (MED) dataset—see SST_MED_SST_L4_REP_OBSERVATIONS_010_021 product). After conducting a nesting operation, we cross-referenced these data with the hourly data noted above [29].
We referred to the University of Urbino Bulletin of coastal waters [30] to estimate the seawater trophic situation of the study area.
2.3. Mussel Bed Coverage
Visual methods were used to obtain an accurate representation of the percentage cover of the mussels in colonizing a specific reef block (1.5–2.5 m depth) in each of the two sampling stations [31]. A 50 × 50 cm quadrat was placed randomly on 10 different points of the reference rocks. Each 10 × 10 cm square of the quadrat completely covered in mussels counted as 4% coverage, while if mussels occupied ¾ of the square, it was considered a 3% coverage, etc. [31]. Data from each of the 10 quadrat replicas were used to estimate the percentage of the total mussel bed coverage in each station. The presence of seaweed, like Ulva lactuca, that could cover the presence of the bivalves, made it difficult to use digital analyses of the pictures; for this reason, the data were acquired visually by the two underwater operators.
2.4. Mussel Collection and Processing
To evaluate the evolution of M. galloprovincialis populations, individuals of this species were collected at a depth of 1.5–2.5 m from each sampling station from a reef block adjacent to the area taken as a reference for the visual assessment of the bed coverage. After gently cutting the byssus thread, the bivalves were kept at a stable temperature during the immediate transfer to the laboratory. The valves of 90–100 mussel individuals from each station were measured over the months in order to observe the morphological dimension dynamics of the considered populations. A batch of 10 individuals was used for molecular analyses. After cutting the posterior adductor muscle, the gills were dissected using sterilized scissors and forceps and stored in Trizol Reagent (Thermo Scientific, Waltham, MA, USA) at −80 °C until later RNA isolation.
2.5. Molecular Analyses
Total RNA was extracted from 50 mg of gill tissue using the Trizol Reagent (Thermo Scientific) according to the manufacturer’s protocol (Invitrogen, Waltham, MA, USA). The concentration and quality of RNA were achieved using Qubit™ 4 Fluorometer (Thermo Fisher Scientific) with the Qubit RNA BR Assay and the Qubit™ RNA IQ Assay Kits (Thermo Fisher Scientific). Purified RNA was reverse transcribed into cDNA using the All-In-One 5X RT Master Mix according to the manufacturer’s instructions (Applied Biological Material, Richmond, BC, Canada). The mixture was incubated at 37 °C for 15 min for genomic DNA removal, followed by 60 °C for 10 min for cDNA synthesis. The reaction was stopped by heating at 95 °C for 2 min.
SYBR green-based real-time PCR with specific primer pairs (Table 1) was used for assessing the transcription profile of the chaperones heat shock protein 70 (HSP70) and HSP90, selected for their role in maintaining the protein conformation stability [32], the pro-apoptotic factor p53 [33] and the apoptotic executor caspase 3 (Cas3) [34] target genes, and 18S ribosomal RNA as a housekeeping gene.

Table 1.
List of RNA primers used in this study.
The PCR reaction included 5 µL yourSIAL® Green Mix 2X, 0.5 µL of each forward and reverse primer (10 µM), 2 µL template cDNA, and 2 µL DEPC-H2O. Thermal cycling temperatures were 95 °C for 2 min, followed by 40 cycles at 95 °C for 5 s and 60 °C for 30 s. Dissociation curve analysis indicated the production of a single amplification product. Results were normalized to 18s rRNA and expressed as fold changes with respect to the April group.
The selection of April (surface seawater T = 19 °C) as the reference month for normalizing raw PCR data was based on the fact that mussels exhibit their optimal physiological performance at approximately 18–20 °C [8,23].
Data were assessed with GraphPad prism v 8.0.2 software (GraphPad Software Inc., La Jolla, CA, USA). Statistical analysis was performed using one-way ANOVA followed by Dunnet’s test for comparisons with respect to the control group (i.e, April). Differences with p < 0.05 were considered statistically significant.
3. Results
3.1. Climatic Analysis
The SST historical series, related to more than 1.41 mln of numerical data (period 15 March–15 September 2024), was analyzed in detail. For the time series under study, the maximum sea temperature was 31 °C and was registered on 20 July. As shown in Figure 2, four MHWs occurred during this period, and only one of them (in June) had temperatures below 30 °C. The other three MHWs were particularly intense both in duration and intensity; in fact, they had a total duration of 52 days, in which the temperature always exceeded 30 °C. Note that while the MHW of July and the first MHW of August appear separated in Figure 2, those are actually the same event, characterized by a 41-day time span. Only two days, during which the temperatures recorded were slightly lower (29.6 and 29.7 °C), separate the last MHW from the previous one.
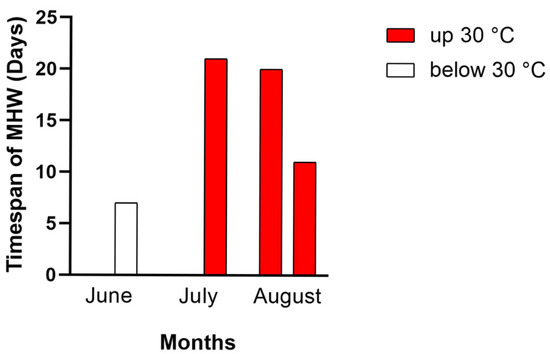
Figure 2.
Marine heat waves (MHWs) occurred in the study area in the 2024 year; in red the MHWs in which the temperature was higher than 30 °C; MHWs below 30 °C were presented in white. The second heat wave of August also incorporates the first two days of September.
Figure 3 shows the flow of the Po river, the most important Italian river basin, registered the second-highest average runoff in the past 50 years. Based on a dominant sea current that runs southward and transports the principal Italian rivers’ runoff along the Italian Adriatic coast, the trophic status of the sea waters in the studied area depends mainly on what happens in the upper Adriatic basin [10,39,40].
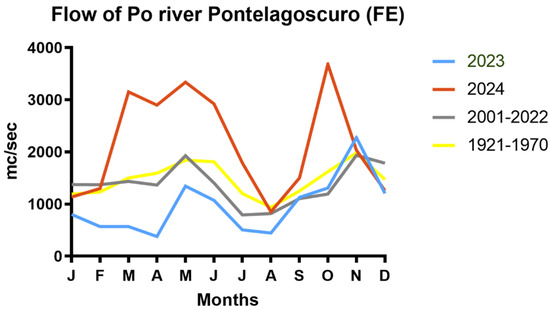
Figure 3.
Flow river comparison between the years 2023, 2024 and two previous periods, at Pontelagoscuro (FE) station (data source [https://simc.arpae.it/dext3r/] accessed on 7 February 2025).
3.2. Mussel Coverage and Growing
Table 2 enlists the mussels’ percentages of coverage observed in the two sites during the period February–September 2024. For the TRN site, there was a strong coverage percentage of the mussel bed starting from the first sampling month.

Table 2.
Mussel coverage percentages in the two considered sites from February to September 2024 in the Tronto river mouth (TRN) and Frana San Nicola (FSN) sites.
Interestingly, the coverage percentage value in February in the FSN site was almost half compared to the other site; furthermore, in FSN, the decline of mussel coverage begins earlier and with greater intensity.
Figure 4 shows the frequencies of individuals for six size classes. In the TRN site, there were adult individuals during the first sampling in February, who were then joined by juveniles in June (two modes in histograms: 10–20 mm and 30–40 mm). In the FSN site, only specimens smaller than 5 mm were observed on the rocky substrate in February and March. The number of individuals decreased significantly in August (also confirmed by the percentage coverage), dropping to zero in September (see also Figure S1 and Video S1 in Supplementary Materials).
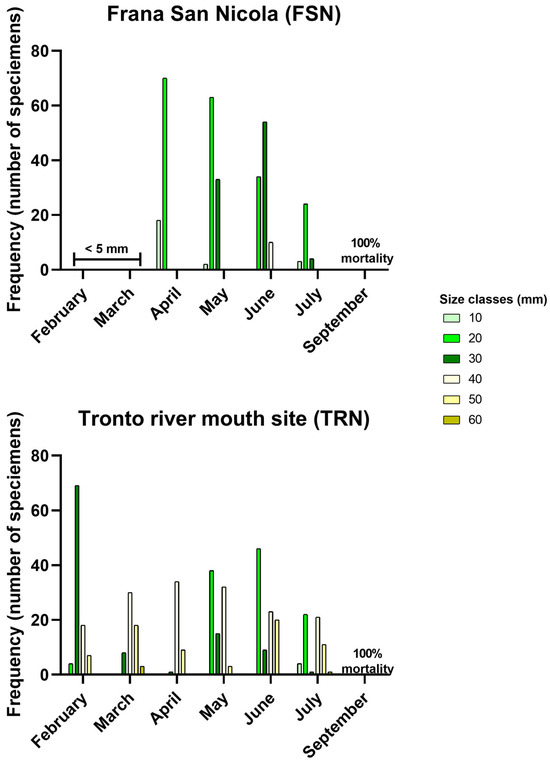
Figure 4.
The dimensional evolution of mussel populations in the two considered sites from February to September 2024, studied considering six size classes. The February and March graphs for the FSN site do not report any data, as small specimens (<5 mm long) were not collected, and September for both sites, do not report any data as a complete mortality was recorded.
3.3. Transcription Modulation over the Months
HSP70 transcripts in the gills isolated from the Mediterranean mussels inhabiting the TRN site revealed significantly higher values in July and August (2.44 and 2.94-fold respectively) than those collected in April, the reference month, and June (Figure 5a). Similarly, individuals from the FSN site expressed 1.5, 2.04, and 2.38-fold-higher levels of this gene in June, July, and August, respectively, with respect to April (Figure 5b).
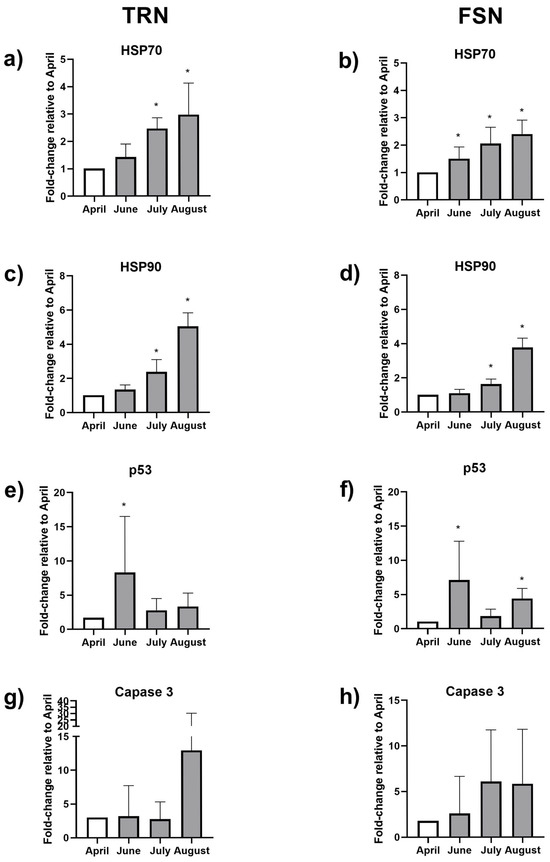
Figure 5.
Relative gene expression of heat shock proteins 70 (HSP70) (a,b), HSP90 (c,d), p53 (e,f) and caspase 3 (g,h) in the gills of the Mediterranean mussel Mytilus galloprovincialis individuals from the Tronto River mouth (TRN) and Frana San Nicola (FSN) sites. April was considered the reference month. Asterisks indicate significant differences respect to April (p < 0.05; n = 8–10).
The gene expression levels of the chaperone HSP90 showed a gradual and significant upregulation in mussels from the Spring months (April/June) to the summer months (July/August), with a similar trend in both stations, with August transcription levels being 4.99 and 3.73 times higher than April levels, in TRN and FSN, respectively. No significant differences were observed between June and the reference month (Figure 5c,d).
The pro-apoptotic factor p53 transcription levels increased 6.40 times in the gills of bivalves collected in June in TRN site, with respect to the control, and were found to be not significantly modulated during all the other months (Figure 5e). Mussels from FSN site collected in June and August had a 6.78 and 4.18-time higher mRNA content of p53 with respect to the gills of mussels from the reference month, a difference not observed in individuals collected in July (Figure 5f).
On the contrary, the caspase did not appear differently modulated by MHWs, neither in mussels from TRN nor in those from FSN (Figure 5g,h).
4. Discussion
This study examined the monthly gene expression profile in wild Mediterranean mussel populations inhabiting submerged reefs of the Piceno Coast (Central Adriatic Sea) and aimed to search for a significant relation with environmental conditions related to the MHWs. We observed a clear upregulation of the HSPs in mussels collected in the summer with respect to individuals collected in April, the reference month. The mussel coverage gradually reduced while approaching the end of summer 2024, when the total mortality was recorded in early September.
4.1. Ecological Observation at the Population Level
MHWs can impact the biological compartments of marine environments at different levels and with different magnitudes [2]. Seawater temperature seems not to affect the ability of Mytilus ssp. to filter food particles [41], and M. galloprovincialis may potentially maintain a positive energy balance at SST below 29–30 °C [22]. However, M. galloprovincialis is an eurytherm species with wide optimum thermal limits between 9 °C and 25 °C for optimal physiological processes [20,22]. Indeed, extreme SST higher than 29 °C may define the Mediterranean mussel (M. galloprovincialis)’s geographic distribution of this species [22].
Mussel mortality can be observed at an environmental temperature of 26 °C: Ref. [20] recorded a 5% death rate at this temperature within 5 days from the exposure start and a 20% death rate after 30 days. The same authors [20] also observed that 20% of the mussels reared at 28 °C died after 5 days from the experiment start, and in groups exposed to 30 °C, the mortality reached 80% after 15 days. In our case, that is an in situ study, the mussel bed coverage percentage in the two sites shifted from 95% in April (monthly temperature from 15.6 to 18.7 °C) to 5% (FSN) and 20% (TRN) in August (monthly temperature from 29.3 to 30.9 °C), reaching 100% mortality in early September. As described in Bracchetti et al. [10] we exclude the intraspecific competition. In the study area, in fact, the predatory pressure is not strong enough to justify a quick, intense and massive population decline that leads to a complete mortality in such a vast area. On the basis of what is described above in terms of high temperature effects on the mussels’ mortality, the occurrence of severe and prolonged MHWs challenges their survival. In our observation, during August–July 2024 the seawater temperature was above 30 °C for over 50 days, of which 41 were consecutive, pushing mussels’ thermal stress to the highest levels.
The continuous exposure to extremely high summer temperatures that occurred yearly, starting in 2022 [10], has determined a change in the benthic community structure, characterized by a shift from a permanent mussel bed [42] to a transitory bed in the FSN site. These bivalves colonize the substrate the first months of the year (i.e., January/February), grow until the summer months, and then die in late summer ([10] and direct observation from the authors in 2023). This phenomenon is clearly visible in Figure 4, in which the frequencies of the size classes of the samples collected in situ are reported. No individuals were collected in February and March for molecular analyses due to the small dimensions (<5 mm).
On the contrary, the presence of adult individuals was observed in the TRN site since the beginning of sampling, together with juveniles that settled on the substrate during the winter months (Figure 4); this means that the mortality rate in this site was not total in 2023, as observed in the FSN site the same year.
Indeed, while the temperature peaked at 30.7 °C in the summer of 2023 (22 July), only two very short MHW occurred (the first in June with T < 30 °C and the second in July with T > 30 °C) that never exceeded 6 days in time span [28]. These conditions have been critical for the FSN mussel population but not for the TRN population, where the riverine freshwater may have locally reduced the harsh effects of the sea warming. This possible mitigation effect was probably not enough in 2024, during which the seawater was highly warm for a long period of time (Figure 2). In 2022 we hypothesized that a combination of environmental factors such as increased heat and low trophic load would have been the causes of the mussel mortality [10]. However, the chlorophyl level was within a normal range in 2024 and so the mussel mass mortality event recorded in 2024 seemed to be caused exclusively by the severe and prolonged (more than 50 days) MHW conditions [10].
4.2. Modulation of Molecular Biomarkers
Biochemical mechanisms contribute to defining organisms’ optimal level of performance within a window of thermal tolerance, which is reflected in behavioral and ecological traits. For the Mediterranean mussels, the upper thermal limit has been identified at 25 °C [20,21]. Indeed, thermal stress is one of the main factors promoting protein denaturation [21]. Among the molecular biomarkers of exposure considered valuable tools to determine the heat-stress response in bivalves, the HSPs are the most used [20,23,24,43]. HSPs are chaperones with a protective mechanism for protein conformation stability and a three-dimensional structure remodeling activity of denatured proteins [32]. HSPs are induced by several kinds of stress conditions like hypoxia, toxicant exposure, and high temperatures, with HSP70 and HSP90 being the HSP families most considered in MHW studies [20,21,23,24,43]. Ref. [21] identified 25 °C as the temperature threshold for inducing HSP70 synthesis in M. galloprovincialis. Our results show that mussels from both TRN and FSN sites increased their branchial HSP70 and HSP90 transcription activity over time, with a significant difference between April (T = 19 °C) and July/August (T = 29–31 °C) 2024. Indeed, the 41-day MHW occurred in July and lasted until August. Similarly, Ref. [14], during an in situ study that aimed to analyze the molecular response of farmed M. galloprovincialis in a Mediterranean scenario, showed how HSP70 mRNA levels significantly increased in individuals collected in the summer (July/August; T = 26.5–29.5 °C) compared to specimens collected in late Spring (May; T = 18 °C).
Our results are consistent with other studies showing that the Pacific oyster exposed to a 28 °C temperature activated HSP transcription in the gills just after three days from the beginning of exposure and it remained high for the following days [24]. Interestingly, these authors found that both HSP70 and HSP90 can be activated in a temporally different manner in bivalves, with HSP70 immediately transcribed and HSP90 activated later (even after 10 days from exposure to the high temperatures). In this regard, it has been suggested that the requirement for the high cellular concentration of HSP70 family proteins may inhibit the production of HSP90 [24].
HSPs have an important role in the activation of pro-apoptotic factors such as p53. Indeed, the chaperone family of HSP70 can selectively recognize and bind p53 proteins [33]. As a consequence, HSP expression may follow the same trend as p53 in bivalves [24], and, in Mediterranean mussels specifically, we observed a different tendency of the two biomarkers, whose gene expression with respect to April was upregulated in June (the first MHW) and August (in FSN site) but not in July. Mussels from TRN site on the other hand only showed a significant modulation of the p53 branchial gene in individuals collected in June, when the first MHW was recorded. Ref. [24] showed a time pattern modulation of the p53 gene, that was overexpressed after 10 days from the start of the heat stress (28 °C) in their controlled setting, and later, at a 30 day timepoint, reduced its transcripts. Interestingly, the first MHW recorded in June 2024 started exactly 9 days before the mussels were collected from the field, and similarly to [24], the p53 gene was upregulated only during this month and not in July, thus indicating a rapid return to the basal level after the end of the first MHW. Data also suggest that starting from the second MHW, p53 expression increased again, reaching a significant level in August compared to April (FSN site). The role of the increased heat as a stressing factor affecting the cellular p53 transcript levels is also confirmed by [44], who observed an upregulation in mussels during the summer.
p53 can be activated in case of DNA damage: by binding the DNA it promotes the transcription of a number of genes involved in DNA repair, cell cycle arrest, or apoptosis [45]. For this cellular function, p53 expression is used as a molecular biomarker to assess cell stress [46].
Extensive cellular damage that cannot be properly repaired leads to programmed cell destruction aimed at maintaining tissue homeostasis, with caspases playing a key role in this apoptotic pathway and contributing to the disassembling of important architectural components of the cell [34,47]. In situ assessments by [14] of the molecular response of the Mediterranean mussel showed an upregulation of cleaved caspases in individuals collected in the summer (July/August; T = 26.5–29.5 °C) with respect to those analyzed in May (T = 18 °C), suggesting the activation of the apoptotic apparatus that leads to extensive mortality of this population. The transcriptomic results presented in the present work did not show a clear modulation of caspase 3, the main executor of the apoptotic cascade [34], used as a biomarker of cell death to assess the response to the MHWs. On the contrary, Ref. [23] observed a significant gills downregulation of this gene during the artificial MHWs (T = 25 °C) in Mytilus edulis and also recorded a restored transcription activity at the end of their long-term experiment during the recovery phase. Another congener, Mytilus coruscus, showed an upregulation of caspase 3 protein at 26 °C and a downregulation of the same gene at 33 °C [48]. Overall, the available data indicate a controversial role of caspase 3 as a molecular biomarker of early response to heat stress in mussels.
5. Conclusions
After three years of observation of the study area in the same sites ([10] and direct observation of the authors), we conclude that the mussel mass mortality event is mainly caused by the magnitude and duration of the MHWs that pushed the Mediterranean mussel to its physiological limits, as also observed by the gene expression analyses. This phenomenon has ecological consequences on the Piceno coast by reshaping the benthic community structure and by moving from a permanent to a transient mussel bed.
Most of the investigations aiming to assess the biological implications of MHWs on marine ecosystems are conducted in controlled conditions, where only one or few parameters are taken into consideration [23,24,25,49]. However, in situ studies are needed in order to assess the real physiological and molecular state of an organism.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d17060385/s1, Figure S1: Quadrat underwater photos capturing the change of mussel bed coverage in the Frana San Nicola a mare site (FSN) from Apil to September 2024; Video S1: Underwater video showing the change in mussel bed coverage in the Frana San Nicola a mare site (FSN) from April to September 2024.
Author Contributions
Conceptualization, M.C., L.B., and F.A.P.; methodology, M.C., L.B., P.C., and F.A.P.; formal analysis, L.B., M.F., F.C., and F.A.P.; investigation, M.C., L.B., and V.M.; visualization L.B., M.F., and F.A.P.; writing—original draft preparation, M.C., L.B., and M.F.; writing—review and editing, M.C., L.B., P.C., M.F., F.C., and F.A.P.; supervision, L.B., and F.A.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available as they are partly owned by local governments.
Acknowledgments
We would like to thank the “Autorità di bacino distrettuale fiume Po” for the historical flows of the Po River data provided.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Cheng, L.; von Schuckmann, K.; Abraham, J.P.; Trenberth, K.E.; Mann, M.E.; Zanna, L.; England, M.H.; Zika, J.D.; Fasullo, J.T.; Yu, Y.; et al. Past and future ocean warming. Nat. Rev. Earth Environ. 2022, 3, 776–794. [Google Scholar] [CrossRef]
- Hobday, A.J.; Oliver, E.C.; Gupta, A.S.; Benthuysen, J.A.; Burrows, M.T.; Donat, M.G.; Holbrook, N.J.; Moore, P.J.; Thomsen, M.S.; Wernberg, T.; et al. Categorizing and naming marine heatwaves. Oceanography 2018, 31, 162–173. [Google Scholar] [CrossRef]
- Hobday, A.J.; Alexander, L.V.; Perkins, S.E.; Smale, D.A.; Straub, S.C.; Oliver, E.C.J.; Benthuysen, J.A.; Burrows, M.T.; Donat, M.G.; Feng, M.; et al. A hierarchical approach to defining marine heatwaves. Prog. Oceanogr. 2016, 141, 227–238. [Google Scholar] [CrossRef]
- Malhi, Y.; Franklin, J.; Seddon, N.; Solan, M.; Turner, M.G.; Field, C.B.; Knowlton, N. Climate change and ecosystems: Threats, opportunities and solutions. Philos. Trans. R. Soc. A 2020, 375, 20190104. [Google Scholar] [CrossRef]
- Smale, D.A.; Wernberg, T.; Oliver, E.C.J.; Thomsen, M.; Harvey, B.P.; Straub, S.C.; Burrows, M.T.; Alexander, L.V.; Benthuysen, J.A.; Donat, M.G.; et al. Marine heatwaves threaten global biodiversity and the provision of ecosystem services. Nat. Clim. Change 2019, 9, 306–312. [Google Scholar] [CrossRef]
- Beever, E.A.; O’Leary, J.; Mengelt, C.; West, J.M.; Julius, S.; Green, N.; Managness, D.; Petes, L.E.; Stein, B.A.; Nicotra, A.B.; et al. Improving conservation outcomes with a new paradigm for understanding species’ fundamental and realized adaptive capacity. Conserv. Lett. 2016, 9, 131–137. [Google Scholar] [CrossRef]
- Greenhough, H.; Vignier, J.; Smith, K.F.; Brown, C.M.; Kenny, N.J.; Rolton, A. Multi-stressor dynamics: Effects of marine heatwave stress and harmful algal blooms on juvenile mussel (Perna canaliculus) survival and physiology. Sci. Total Environ. 2025, 964, 178590. [Google Scholar] [CrossRef]
- Lattos, A.; Papadopoulos, D.K.; Feidantsis, K.; Giantsis, I.A.; Georgoulis, I.; Karagiannis, D.; Michaelidis, B. Antioxidant Defense of Mytilus galloprovincialis Mussels Induced by Marine Heatwaves in Correlation with Marteilia Pathogen Presence. Fishes 2023, 8, 408. [Google Scholar] [CrossRef]
- Joyce, P.W.; Tong, C.B.; Yip, Y.L.; Falkenberg, L.J. Marine heatwaves as drivers of biological and ecological change: Implications of current research patterns and future opportunities. Mar. Biol. 2024, 171, 20. [Google Scholar] [CrossRef]
- Bracchetti, L.; Capriotti, M.; Fazzini, M.; Cocci, P.; Palermo, F.A. Mass Mortality Event of Mediterranean Mussels (Mytilus galloprovincialis) in the Middle Adriatic: Potential Implications of the Climate Crisis for Marine Ecosystems. Diversity 2024, 16, 130. [Google Scholar] [CrossRef]
- Galil, B.S.; Mienis, H.K.; Mendelson, M.; Gayer, K.; Goren, M. Here today, gone tomorrow-the Levantine population of the Brown mussel Perna perna obliterated by unprecedented heatwave. Aquat. Invasions 2022, 17, 174–185. [Google Scholar] [CrossRef]
- Garrabou, J.; Coma, R.; Bensossan, N.; Bally, M.; Chevaldonné, P.; Cigliano, M.; Diaz, D.; Harmelin, J.G.; Gambi, M.C.; Kersting, D.K.; et al. Mass mortality in Northwestern Mediterranean rocky benthic communities: Effects of the 2003 heat wave. Glob. Change Biol. 2009, 15, 1090–1103. [Google Scholar] [CrossRef]
- Bonacci, O.; Bonacci, D.; Patekar, M.; Pola, M. Increasing Trends in Air and Sea Surface Temperature in the Central Adriatic Sea (Croatia). J. Mar. Sci. Eng. 2021, 9, 358. [Google Scholar] [CrossRef]
- Lattos, A.; Papadopoulos, D.K.; Feidantsis, K.; Karagiannis, D.; Giantsis, I.A.; Michaelidis, B. Are marine heatwaves responsible for mortalities of farmed Mytilus galloprovincialis? A pathophysiological analysis of Marteilia infected mussels from Thermaikos Gulf, Greece. Animals 2022, 12, 2805. [Google Scholar] [CrossRef] [PubMed]
- Mandić, M.; Nikolić, S.; Kokić, I.; Jokanović, S. Mass mortality of farmed mussels—A phenomenon without explanation? Studia Marina 2024, 37, 5–21. [Google Scholar]
- Marini, M.; Grilli, F. The Role of Nitrogen and Phosphorus in Eutrophication of the Northern Adriatic Sea: History and Future Scenarios. Appl. Sci. 2023, 13, 9267. [Google Scholar] [CrossRef]
- Cozzi, S.; Giani, M. River water and nutrient discharges in the Northern Adriatic Sea: Current importance and long term changes. Cont. Shelf Res. 2011, 31, 1881–1893. [Google Scholar] [CrossRef]
- Juza, M.; Fernández-Mora, À.; Tintoré, J. Sub-Regional Marine Heat Waves in the Mediterranean Sea from Observations: Long-Term Surface Changes, Sub-Surface and Coastal Responses. Front. Mar. Sci. 2022, 9, 785771. [Google Scholar] [CrossRef]
- Harley, C.D. Tidal dynamics, topographic orientation, and temperature-mediated mass mortalities on rocky shores. Mar. Ecol. Prog. Ser. 2008, 371, 37–46. [Google Scholar] [CrossRef]
- Anestis, A.; Lazou, A.; Portner, H.O.; Michaelidis, B. Behavioral, metabolic, and molecular stress responses of marine bivalve Mytilus galloprovincialis during long-term acclimation at increasing ambient temperature. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R911–R921. [Google Scholar] [CrossRef]
- Hofmann, G.E.; Somero, G.N. Interspecific variation in thermal denaturation of proteins in the congeneric mussels Mytilus trossulus and M. galloprovincialis: Evidence from the heat-shock response and protein ubiquitination. Mar. Biol. 1996, 126, 65–75. [Google Scholar] [CrossRef]
- Fly, E.K.; Hilbish, T.J.; Wethey, D.S.; Rognstad, R.L. Physiology and Biogeography: The Response of European Mussels (Mytilus spp.) to Climate Change. Am. Malacol. Bull. 2015, 33, 136–149. [Google Scholar] [CrossRef]
- Grimmelpont, M.; Payton, L.; Lefrançois, C.; Tran, D. Molecular and behavioural responses of the mussel Mytilus edulis exposed to a marine heatwave. Mar. Environ. Res. 2024, 196, 106418. [Google Scholar] [CrossRef]
- De Marco, A.; Baldassarro, V.A.; Calzà, L.; Giardino, L.; Dondi, F.; Ferrari, M.G.; Bignami, G.; Parma, L.; Bonaldo, A. Prolonged heat waves reduce the condition index and alter the molecular parameters in the pacific oyster Crassostrea gigas. Fish Shellfish Immunol. 2023, 133, 108518. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, Z.; Zhang, Y.; Liang, J.; He, G.; Liu, X.; Zhao, L. Transcriptome analysis reveals acclimation responses of pearl oysters to marine heatwaves. Sci. Total Environ. 2022, 810, 151189. [Google Scholar] [CrossRef]
- Fabbri, E.; Valbonesi, P.; Franzellitti, S. HSPexpression in bivalves. Invertebr. Surviv. J. 2008, 5, 135–161. [Google Scholar]
- WMO (World Meteorological Organization). Guide to Meteorological Instruments and Methods of Observation. 2008. Available online: https://www.weather.gov/media/epz/mesonet/CWOP-WMO8.pdf (accessed on 12 February 2025).
- SNPA. Il Clima in Italia Nel 2023, Report Ambientali SNPA, n. 42/2024. Available online: https://www.puntosicuro.it/_resources/files/Rapporto-SNPA-clima-2023.pdf (accessed on 12 February 2025).
- Pastor, F.; Valiente, J.A.; Khodayar, S. A Warming Mediterranean: 38 Years of Increasing Sea Surface Temperature. Remote Sens. 2020, 12, 2687. [Google Scholar] [CrossRef]
- Qualità delle Acque della Costa. Available online: https://www.uniurb.it/ricerca/organizzazione-della-ricerca/strutture-della-ricerca/qualita-delle-acque-della-costa (accessed on 6 December 2024).
- Murray, S.N.; Ambrose, R.F.; Dethier, M.N. Methods for Performing Monitoring, Impact, and Ecological Studies on Rocky Shores; MMS U.S. Department of the Interior Minerals Management Service: Pacific OCS Region, USA, 2002; pp. 116–147.
- Parsell, D.A.; Lindquist, S. The function of heat-shock proteins in stress tolerance: Degradation and reactivation of damaged proteins. Annu. Rev. Genet. 1993, 27, 437–497. [Google Scholar] [CrossRef]
- Fourie, A.M.; Hupp, T.R.; Lane, D.P.; Sang, B.C.; Barbosa, M.S.; Sambrook, J.F.; Gething, M.J.H. HSP70 binding sites in the tumor suppressor protein p53. J. Biol. Chem. 1997, 272, 19471–19479. [Google Scholar] [CrossRef]
- Earnshaw, W.C.; Martins, L.M.; Kaufmann, S.H. Mammalian caspases: Structure, activation, substrates, and functions during apoptosis. Annu. Rev. Biochem. 1999, 68, 383–424. [Google Scholar] [CrossRef]
- Meng, X.; Li, F.; Wang, X.; Liu, J.; Ji, C.; Wu, H. Toxicological effects of graphene on mussel Mytilus galloprovincialis hemocytes after individual and combined exposure with triphenyl phosphate. Mar. Pollut. Bull. 2020, 151, 110838. [Google Scholar] [CrossRef] [PubMed]
- Canesi, L.; Borghi, C.; Fabbri, R.; Ciacci, C.; Lorusso, L.C.; Gallo, G.; Vergani, L. Effects of 17β-estradiol on mussel digestive gland. Gen. Comp. Endocrinol. 2007, 153, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Banni, M.; Sforzini, S.; Franzellitti, S.; Oliveri, C.; Viarengo, A.; Fabbri, E. Molecular and cellular effects induced in Mytilus galloprovincialis treated with oxytetracycline at different temperatures. PLoS ONE 2015, 10, e0128468. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Ji, C.; Wei, L.; Zhao, J.; Lu, H. Proteomic and metabolomic responses in hepatopancreas of Mytilus galloprovincialis challenged by Micrococcus luteus and Vibrio anguillarum. J. Proteom. 2013, 94, 54–67. [Google Scholar] [CrossRef]
- Laurent, L.; Buoncristiani, J.-F.; Pohl, B.; Zekollari, H.; Farinotti, D.; Huss, M.; Mugnier, J.-L.; Pergaud, J. The impact of climate change and glacier mass loss on the hydrology in the Mont-Blanc massif. Sci. Rep. 2020, 10, 10420. [Google Scholar] [CrossRef]
- Fazzini, M.; Baione, L.; Raspanti, A.; Capizzi, P.; Casagli, N. Climate crisis and influence on snowfall in the Italian physical territory in the last thirty years—CLINO 1991–2020. In Proceedings of the 6th World Landslide Forum. Landslide Science for Sustainable Development, Florence, Italy, 14–17 November 2023; ISBN 9791221048063. [Google Scholar]
- Rosa, M.; Capriotti, M.; Austin, K.; Shumway, S.E.; Ward, J.E. Effect of seasonal changes in temperature on capture efficiency in the blue mussel, Mytilus edulis, fed seston and microplastics. Invertebr. Biol. 2024, 143, e12446. [Google Scholar] [CrossRef]
- Bracchetti, L.; Capriotti, M. Le formazioni a Reef della Costa Picena. Studi Costieri. I Litorali Marchig. E Il Nuovo Piano di Gestione Integr. Delle Zone Costiere 2021, 29, 83. [Google Scholar]
- Masanja, F.; Xu, Y.; He, G.; Liang, F.; Liu, X.; Yang, K.; Zhao, L. Exploring HSP90 as a biomarker for marine heatwaves in pinctada maxima. Front. Mar. Sci. 2022, 9, 913920. [Google Scholar] [CrossRef]
- Carella, F.; Aceto, S.; Mangoni, O.; Mollica, M.P.; Cavaliere, G.; Trinchese, G.; Aniello, F.; De Vico, G. Assessment of the health status of mussels Mytilus galloprovincialis along the Campania coastal areas: A multidisciplinary approach. Front. Physiol. 2018, 9, 683. [Google Scholar] [CrossRef]
- Lakin, N.D.; Jackson, S.P. Regulation of p53 in response to DNA damage. Oncogene 1999, 18, 7644–7655. [Google Scholar] [CrossRef]
- Cocci, P.; Capriotti, M.; Mosconi, G.; Palermo, F.A. Transcriptional variations in biomarkers of Mytilus galloprovincialis sampled from Central Adriatic coastal waters (Marche region, Italy). Biomarkers 2017, 22, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Xu, L.; Xiong, X.; Liu, X. Expansion and diversity of caspases in Mytilus coruscus contribute to larval metamorphosis and environmental adaptation. BMC Genom. 2024, 25, 314. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, L.; Wang, Y.; Lin, S.; Li, H.; Qi, P.; Buttino, I.; Wang, W.; Guo, B. Insights into the Response in Digestive Gland of Mytilus coruscus under Heat Stress Using TMT-Based Proteomics. Animals 2023, 13, 2248. [Google Scholar] [CrossRef] [PubMed]
- Peruzza, L.; Tucci, C.F.; Frizzo, R.; Riello, T.; Quagliariello, A.; Martino, M.E.; Manuzzi, A.; Rovere, G.D.; Bonsembiante, F.; Gelain, M.E.; et al. Impaired reproduction, energy reserves and dysbiosis: The overlooked consequences of heatwaves in a bivalve mollusc. Mar. Pollut. Bull. 2023, 193, 115192. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).