Abstract
Giant clams are ecologically important coral reef animals, with many species facing imminent local extinction. While many regions have undertaken recent assessments of their biodiversity assets, persistent gaps remain even in otherwise well-surveyed areas. This study sought to understand the geographic distribution of smaller-bodied and morphologically similar giant clams, specifically Tridacna maxima and T. noae, in the eastern Indian Ocean. Due to the difficulties in reliably identifying these species using morphological characters, we confirmed species identity and investigated intraspecific variation using sequence data from the mitochondrial cytochrome C oxidase subunit I gene (COI). Seventy whole animal vouchers were newly sampled from a 1500 km span of remote northwestern Australian coastline over a decade, as part of an ongoing coral reef survey expedition of the Western Australian Museum and partners. Tridacna maxima had a limited distribution and was only genotyped from offshore oceanic reefs in the Rowley Shoals and Cocos Keeling Islands. In contrast, T. noae was well established beyond Ningaloo Reef, and was abundant at inshore sites throughout the Pilbara and Kimberley, and even offshore to Ashmore Reef. Phylogeographically, T. maxima did not group with conspecifics from the Western Pacific Ocean, including the east coast of Australia, but instead clustered with individuals from Malaysia, China, Taiwan, and Indonesia; T. noae exhibited a similar pattern. The affinity of Western Australian individuals with representatives from the Indo-Malay region and not eastern Australia will be an important consideration for these commercially important species. Novel haplotypes in both tested species occur in Western Australia. Continued sampling of eastern and central Indian Ocean giant clams, especially to continue to document the range of T. noae, is encouraged to understand connectivity in this basin. Together, these findings contribute to an improved baseline for conservation initiatives of these iconic coral reef animals in Western Australia.
1. Introduction
Giant clams in the genera Tridacna and Hippopus are the most captivating and well-studied coral-reef-inhabiting bivalves [1,2,3,4]. Their large size and striking mantle coloration makes some giant clams conspicuous and attractive, while others can be camouflaged. All species are photosymbiotic and most common in shallow-to-subtidal coral reef habitats across the Indo-West Pacific (IWP) (although the maximum recorded depth is 42 m for T. squamosa [5,6]). All giant clams retain the ability to filter feed, but they rely substantially on autotrophy, with some being exclusively phototrophic [7]. Giant clams can be locally abundant, carpeting inshore coral reef habitats where they occur, such as on French Polynesian coral reefs, where their average density can be 1.6–2.7 individuals m−2 [8] and a maximum of 544 individuals per m2 has been reported [9]. However, ongoing human harvesting for consumption, decoration, and the aquarium trade has taken its toll, resulting in serious declines, especially for larger-bodied species, with local extinction documented for six species [3]. Demographic analyses based on the recently sequenced T. maxima genome indicate a population expansion from ~3 mya, followed by a decline after ~1.3 mya at the onset of the Mid-Pleistocene Transition and a second steep decline that coincides with the Last Glacial Period (LGP), from ~125 kya to ~10 kya [4]. All giant clams (Hippopus and Tridacna species) are listed in Appendix A of the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES). Furthermore, Lee et al. [10] recently called for heightened conservation status of many larger-bodied species, and this is reflected in the International Union for Conservation of Nature (IUCN)’s updated listings, which have now assessed Tridacna gigas as critically endangered (CR; previously vulnerable, VU) as well as T. derasa, T. squamosina, and T. mbalavuana as endangered (EN). Hippopus hippopus has moved from least concern (LC) to VU. Many of the small-bodied species are assessed as LC but are worryingly data-deficient in metrics like population trends.
Analysis of genetic sequence data has improved our understanding of marine biodiversity, species boundaries, and distributions for many well-known marine molluscs for decades [11]. These data have revealed numerous cryptic species of tropical IWP molluscs, including cowries [12], the turbinid Astralium [13], Sepioteuthis cf. lessoniana, the big fin reef squid [14], and Pinctada pearl oysters [15]. The discovery of cryptic diversity is critical to address, especially for commercially important taxa that can be overexploited if ‘hidden’ (e.g., [6,16,17]). Such vulnerability has been reinforced over the last decade for giant clams, with the rediscovery of cryptic lineages, e.g., T. noae in the IWP [18,19], T. squamosina from the Red Sea [20], and T. elongatissima from Madagascar, La Reunion, Mauritius, Mozambique [21], and Tanzania [22]. These species have all previously been confused with T. maxima and, as a result, all species, including even the widespread T. maxima, have been historically overestimated.
Genetic sequence data from rapidly evolving gene regions offer a robust identification tool and can also inform us about phylogeography and population genetics. Molecular markers (e.g., COI) are a reliable method to differentiate among closely similar species of all giant clam species, including juveniles. Ma et al. [23] recently provided a consensus Tridacna phylogeny based on 13 protein-coding genes (mitochondrial DNA; mtDNA) and 18s rRNA that reinforced the relationships captured by smaller datasets utilizing mtDNA. Ma et al. [23] dated the split between T. maxima and a clade containing T. noae, T. crocea, and T. squamosa at approximately 10 Ma (late Miocene), reinforcing the finding that while T. maxima and T. noae are most often confused for one another, T. noae actually falls in a separate clade from T. maxima and is most closely related to T. squamosa and T. crocea—an often overlooked detail potentially suggestive of morphological convergence in shell characters, such as ribbing.
Tridacna maxima is the most well-studied species and has been the subject of phylogeographic analyses across much of its vast distribution from Africa to the Red Sea, north to Japan, and west to French Polynesia (e.g., [18,21,24,25,26,27]). This species exhibits pronounced genetic differentiation in peripheral populations (summarized in [27]) of the West Pacific, such as French Polynesia. Within the Indo-Malay focal region, numerous studies have documented novel haplotypes and genetic differentiation, as well as panmixia, suggesting that many factors structure populations (e.g., [19,27,28,29,30,31,32]) around large landmasses with complex connectivity. In contrast, there are fewer data available to evaluate the phylogeography and population genetics of T. noae at this stage.
Focusing on the Indian Ocean (IO), there is genetic evidence that T. maxima occurs along the coast of Kenya [22,29] and the Red Sea [33] in the western Indian Ocean (WIO), aside from records of this species from the eastern Indian Ocean (EIO) around the Indo-Malay triangle (e.g., [29]). Fauvelot et al. [21] found little evidence of contemporary gene flow between T. maxima in the Red Sea and the WIO, and this may characterize IO populations more broadly, given the patchy reefal habitat in the IO compared with the more contiguous reefal habitat found in the western Pacific [34]. Many sampling gaps still remain in the IO relative to work in the tropical Pacific for the widespread T. maxima [35], and currently, T. noae is either absent or poorly known in the IO (see [36] for a visual record of T. noae at Christmas Island).
In Western Australia, the world-heritage-listed Ningaloo Reef is a regional beacon of coral reef studies, with a history of giant clam research there [37,38,39]. Early studies of giant clam population demographics were thought to represent T. maxima, based on morphological identification [37], until a later genetic study found no evidence of T. maxima at Ningaloo [39]. Species are similar in size and shell characters, with mantle differences often cited as the best means of differentiating these two species in the field. Tridacna maxima exhibits concentrated hyaline organs along the mantle periphery and the absence of teardrop features on the mantle edge, while T. noae does not exhibit concentrated hyaline organs along the mantle periphery but is often identified through the presence of often light or white-colored teardrop markings on the mantle edge [37]. The absence of T. maxima at this site then prompted Johnson et al. [39] to query the occurrence of T. maxima in Western Australia more broadly. While photographic records [40] and museum vouchers (often dead shells) form the basis of speculations that T. maxima exists in Western Australia, genetic-based validation is absent. Moreover, T. noae has not been genetically validated outside of Ningaloo Reef in the state [19,39].
In summary, it is thought that tropical Australia is home to T. maxima in the east (southwestern Pacific Ocean at the Great Barrier Reef) and T. noae in the west (the EIO at Ningaloo Reef). Given the conservation importance of Tridacna species more broadly, a modern survey to establish the phylogeography of these two species, especially considering their harvest for the aquarium trade in Western Australia, is critical. For example, overestimating the population size and number of T. maxima, which has surpassed T. crocea as most the popular aquarium species harvested from the wild, is detrimental to protecting this species [41].
Our goal with this study was threefold: (1) identify species of small-bodied giant clams, specifically T. noae and T. maxima, in the EIO through genetic survey; (2) place Western Australian populations of these species in a larger regional context to assess phylogeographic affinities; and (3) quantify genetic differentiation within species to flag deeply divergent populations for conservation consideration.
2. Materials and Methods
2.1. Specimen Collection
Seventy-nine samples (tissue subsamples from retained whole vouchers plus tissue subsamples only) were collected specifically for this study between 2010 and 2017, during ten expeditions (Table A2). Collection permits were issued under the authority of the Department of Primary Industries and Regional Development (DPIRD) and the Department of Biodiversity, Conservation and Attractions (DBCA). Sampling in Western Australia was carried out by the first author or the Western Australian Museum’s Aquatic Zoology staff. Cocos Keeling Islands tissue subsamples and photos of T. maxima were generously provided by Dr. Scott Evans (DPIRD). Specimens were photographed in situ prior to collection, or on the boat following collection and tissue sampling. Tissue samples were immediately placed in pure ethanol. Voucher and tissue samples were housed at the Collections and Research Centre in the WA Museum’s Welshpool location. All specimen collection was conducted in compliance with relevant local permits and international regulations.
2.2. DNA Extraction and Amplifications
Whole genomic DNA was extracted from tissue samples using DNeasy blood and tissue kits (Qiagen, Clayton, Australia), following the manufacturer’s instructions for animal tissue, with the following modifications: 20 h incubation prior to extraction, the AW2 wash step was repeated twice, and DNA was eluted into 100–120 µL of AE buffer.
Amplifications were carried out in 25 µL reactions, which included 1–2 µL of DNA template (neat or dilute to 1:75), 1x Meridian Bioscience MyTaq or MiFi reaction buffer, 1 unit of MyTaq HS or 2 units of MiFi DNA polymerase, and 0.2–0.3 µM of each primer.
2.3. DNA Sequencing and Data Collection
We amplified and obtained partial sequence data for three mitochondrial DNA genes (cytochrome c oxidase subunit I [COI] and 28S; Table A1) for 79 specimens (Table A2). 25 µL PCR reactions comprised 0.5 µL of MyTaq DNA polymerase, 5 µL of reaction buffer (5x; Bioline), 0.5 µL of forward primer (10 µM), 0.5 µL of reverse primer (10 µM), 17.5 µL of ddH2O, and 1 µL of DNA (diluted to 1–5 ng/µL). PCR conditions were as follows: initial denature at 94 °C for 2 min, 35 amplification cycles (denature: 94 °C for 40 s; anneal: 50 °C for 40 s; extension: 72 °C for 90 s), and a final extension step of 72 °C for 10 min. PCR products were checked via gel electrophoresis using a HyperLadder 100 bp (Bioline) for reference. Unpurified PCR products were sent to the Australian Genome Research Facility (AGRF) in Perth, Western Australia for purification and single-direction sequencing. Electropherograms were aligned, visually inspected and, when necessary, manually corrected and trimmed using Geneious Prime 2021.1 [42].
For COI, 2759 additional sequences were obtained from GenBank (Hippopus hippopus, n = 2; T. crocea, n = 1147; T. derasa, n = 6; T. elongatissima, n = 107; T. gigas, n = 3; T. maxima, n = 927; T. mbalavuana, n = 1; T. noae, n = 266; T. rosewateri, n = 5; T. squamosa, n = 263; T. squamosina, n = 2; unidentified Tridacna sp., n = 30).
The 28S sequences were conserved and invariable, so they were not explored any further for the purposes of this paper; however, this dataset is available upon request to the first author.
Using COI data, we constructed a Maximum Likelihood (ML) topology using RAxML v8.0.19 [43]. To do so, we imported our COI alignment and identified 271 unique COI sequences. These haplotypes were used for downstream model testing and phylogenetic construction. We used jModelTest v0.1.1 [44] to carry out statistical selection of best-fit models of nucleotide substitution and selected the most appropriate model (GTR + Γ + I) based on ‘goodness of fit’ via the Akaike information criterion (AIC; [45]). The strength of support for internal nodes of ML construction was measured using 1000 rapid bootstrap (BS) replicates.
We generated median-joining haplotype networks [46] for T. maxima (n = 932) and T. noae (n = 269) using PopART v1.7 [47]. For T. maxima, we removed 365 individuals with an excess of missing bases (n = 567). We then trimmed both species’ input sequence alignments at both ends to reduce missing data to ≤5%. After visualization of our networks, we grouped T. maxima individuals into four putative populations and estimated genetic differentiation among these groups via DNAsp v6.12 [48].
3. Results
3.1. Distribution and Phylogeographic Affinities of T. noae and T. maxima
3.1.1. Tridacna maxima in the Eastern Indian Ocean
Tridacna maxima occurs offshore of continental Western Australia (Figure 1). It is currently genetically confirmed only from the offshore islands of the Rowley Shoals Marine Park, at Imperieuse Reef and at Mermaid Reef Marine Park. It has also been genotyped from the Cocos Keeling Islands in the Indian Ocean Territories, including at West Island. The depth range of sampled individuals was 0–20 m (at Imperieuse Reef and Mermaid Reef) and 0–1 m (Cocos Keeling). Tridacna maxima was not genotyped at any sites where T. noae was genetically confirmed during this study. This included an extensive survey along 1500 km of the Western Australian coastline.
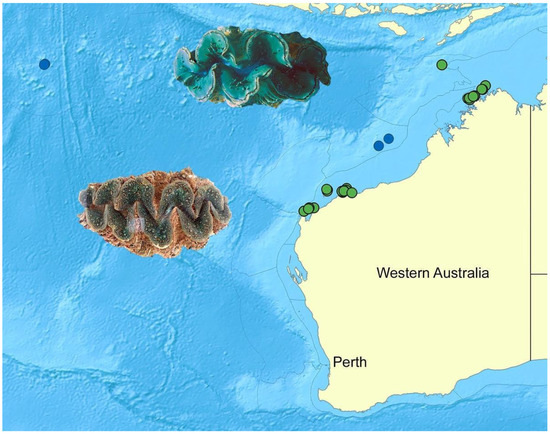
Figure 1.
Distribution of Tridacna maxima upper image WAMS68248; blue dots including the Rowley Shoals (right) and Cocos Keeling Islands (left) and T. noae lower image WAMS78647; green dots along the Western Australia coast from Ningaloo, to the Pilbara and northern Kimberley coastlines, and out to Ashmore Reef (top) in the eastern Indian Ocean.
3.1.2. Tridacna noae in the Eastern Indian Ocean
Tridacna noae is widespread at inshore sites in the eastern IO and occurs well beyond its currently documented range of Ningaloo [39] in Western Australia. It is now known from sites north of Ningaloo (Figure 1), based on 63 samples of whole vouchers, as follows: from Muirons (North and South Islands), Montebellos (Trimouille, Epsilon, Chong Islands), Great Sandy Islands, Dampier Archipelago (seven sites), Point Sampson, Montalivet Islands, Bonaparte Archipelago (six sites), East Holothuria Reef, Long Reef, and Ashmore Reef (Table A2). Where found, specimens occurred from 0 to 15 m, but they were most commonly collected intertidally. As mentioned above, T. noae was not genetically confirmed at any sites where T. maxima was genotyped, and it was not encountered in the Rowley Shoals Marine Park, Mermaid Marine Park, or Cocos Keeling Islands during our surveys.
3.1.3. Phylogeographic Affinities of Tridacna maxima in the Broader Indo-West Pacific
Our T. maxima haplotype network revealed that between three and four discrete haplotype groups were present among our sampled individuals (Figure 2). Individuals from the Western Pacific (bottom of Figure 1) were separated from the Indian Ocean and Red Sea (central) group by ≥26 mutations. Similarly, ≥26 mutations also separated individuals from French Polynesia (upper right of Figure 1) and those from the Indian Ocean and Red Sea. Interestingly, double the mutations (>52) separated French Polynesia from the Western Pacific group. Within the central group, Red Sea individuals were separated from Indian Ocean individuals by ≥10 mutations.
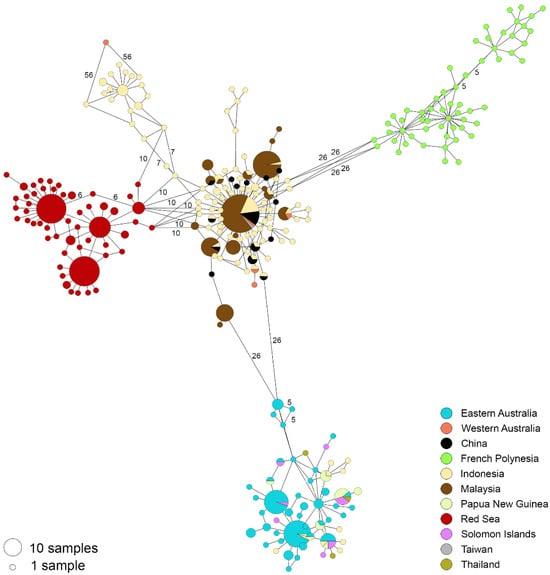
Figure 2.
Phylogeographic affinities of Tridacna maxima in the Indo-West Pacific, illustrated in a minimum-spanning haplotype network of 567 individuals. Analysis is based on 445 bp of the mitochondrial COI gene. The number of mutations separating haplotypes is indicated by text (≥5).
Tridacna maxima from Western Australia formed a haplogroup with Malaysia, China, Taiwan, and, in Indonesia, Sulawesi and Timor, as well as Pulau Seribu, Padang, and Java. East Australian T. maxima formed a discrete haplogroup separate from the above one.
Generally, geographic distance was correlated with genetic differentiation among our four inferred haplotype groups (Table 1). Interestingly, however, the nucleotide-based differences were greatest between the Western Pacific and French Polynesia. The Red Sea and IO/South and East China Sea groups were the most similar—less than 1/2 the average nucleotide differences (Kxy = 15.5; mean Kxy = 31.3).

Table 1.
Pairwise genetic diversity metrics among four Tridacna maxima haplotype groups. Analyses are based on 445 bp of the mitochondrial COI gene.
The Red Sea group also had the lowest haplotype and nucleotide diversity (Table 2).

Table 2.
Haplotype (Hd) and nucleotide (Pi) diversity estimates among four Tridacna maxima haplotype groups. Analyses are based on 445 bp of the mitochondrial COI gene.
3.1.4. Phylogeographic Affinities of T. noae in the Indo-Pacific
Visualization of our T. noae haplotype network revealed less population structure, relative to T. maxima—that is, only 1–13 mutations separated the five large haplotypes (Figure 3). However, fewer bases were analyzed, due to a higher proportion of missing data and non-overlapping sequences (T. maxima = 445 bp; T. noae = 378 bp). Tridacna noae from Western Australia fell in a haplogroup with the Philippines and, like T. maxima, Taiwan, China, and Indonesia, as well as Micronesia. East Australian T. noae was not available so affinities there could not be tested.
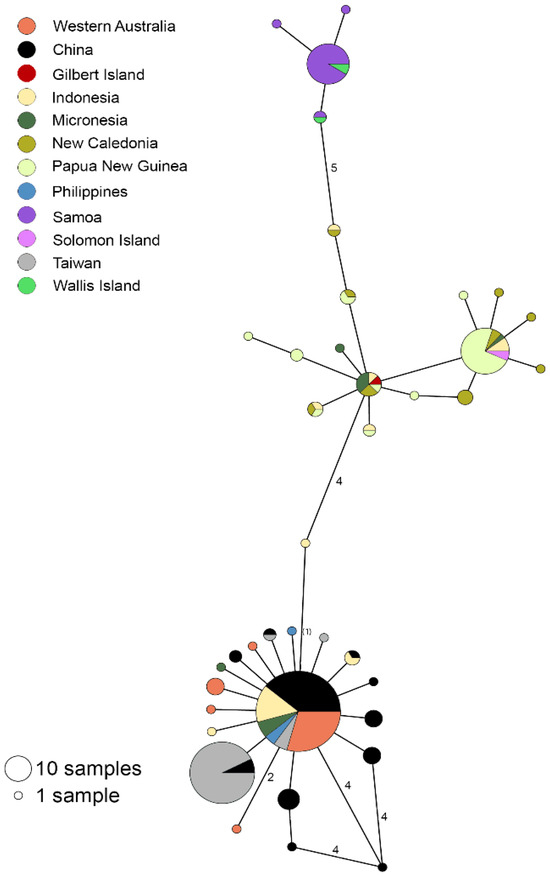
Figure 3.
Phylogeographic affinities of Tridacna noae in the Indo-West Pacific, illustrated in a minimum-spanning haplotype network of 269 T. noae individuals based on 378 bp of the mitochondrial COI gene. The number of mutations separating haplotypes is indicated by text (≥2).
4. Discussion
Distributed from the Red Sea to French Polynesia, T. maxima and T. squamosa exhibit the widest distributions of all giant clams [27]. However, many areas are unsampled or undersampled within this enormous range, and given the uncertainty of morphological identification, they require genotyping to confirm identity. Additionally, a significant structure characterizes verified T. maxima populations across this vast range, and deep divisions may indicate incipient species formation ([49], Figure 4). While T. maxima populations have been well studied in the western IO, Indo-Malay region, and Western Pacific, they have been little surveyed along and off the coastline of Western Australia in the eastern IO until now [35] (Figure 5). We feel that T. maxima is restricted to select offshore island groups in the eastern IO, based on the absence of this species during extensive surveys at more than 20 inshore sampling sites (Table A2). While it was not collected and sequenced at Ashmore Reef, where T. noae was collected and sequenced, specimens have been provisionally identified based on photographs (L. Kirkendale, unpublished data). As for all in situ photographic or shell records of closely similar species, the presence of T. maxima needs to be confirmed by genetic sequencing of vouchered material.
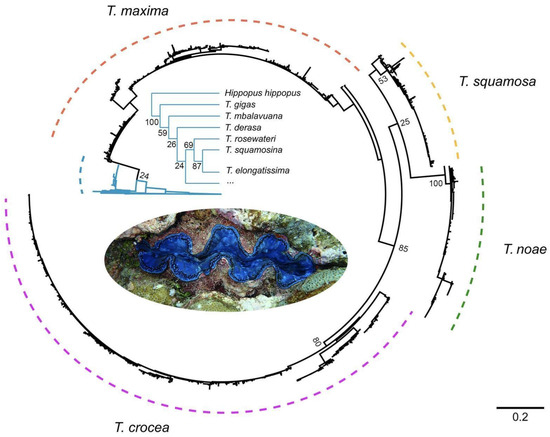
Figure 4.
Schematic COI phylogram for Tridacna maxima, T. noae, T. squamosa, and T. crocea to illustrate divergences within species.
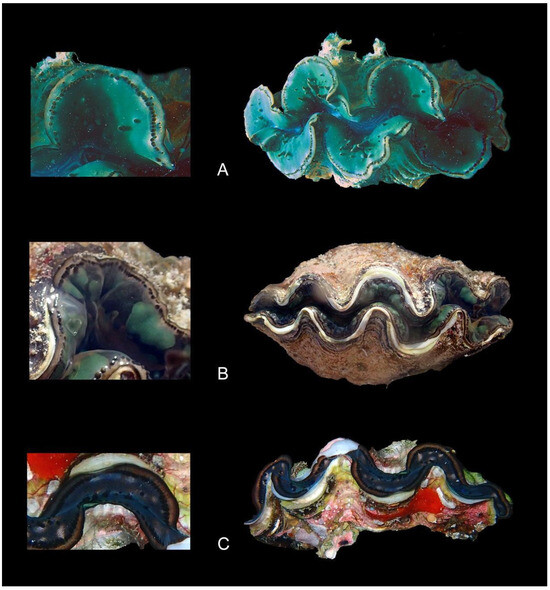
Figure 5.
Color forms and mantle features of Tridacna maxima from Western Australia: (A) WAM S68248 and (B) WAM S 68249, both Imperieuse Reef; (C) WAM S68179, Rowley Shoals.
Tridacna maxima exhibits strong population structure with four clear haplogroups, most divergent in peripheral locations, consistent with earlier work [27]. Novel haplotypes were evident within Western Australian samples of T. maxima that fell within a larger haplogroup with Indo-Malay representatives (Malaysia, China, Taiwan, and, in Indonesia, Sulawesi and Timor, as well as Pulau Seribu, Padang, and Java) (Figure 2). Together with the nucleotide diversity data (Table 1), these results indicate that T. maxima exhibits greater similarity and inferred connectivity with the China Seas and with the Red Sea than with the Western Pacific and French Polynesia (mid-Pacific). Thus, T. maxima populations are not closely similar within Australia or other southwestern Pacific localities, including neighboring Papua New Guinea, although they are connected by coastline, or they were in the recent past during lowered sea stands [50]. That West Australian T. maxima forms a discrete haplogroup distinct from east-coast T. maxima is an important finding with relevance to conservation management. This distribution is presently both inconsistent and consistent with the ‘Leeuwin Effect’ [35], a phenomenon where the southward flow of the Leeuwin Current is responsible for transporting larval propagules from the Coral Triangle region down the coast of Western Australia, resulting in broader Indo-West Pacific affinities. It is inconsistent with the Leeuwin effect given that connectivity across the top end of northern Australia appears compromised for populations of T. maxima, but is consistent with the Leeuwin Effect given genetic similarity between T. maxima populations exist between Western Australia and the China Seas. Herwerden et al. [51] reported that while panmixia typified trends in commercially important Lethrinus miniatus between the east and west coasts of Australia, in contrast, L. sebae was divergent enough on each coast that it was recommended that each population be managed as discrete stocks. While [52] reported that spatial subdivision and high genetic diversity characterized populations of Nautilus pompilius from east and west Australia, it has more recently been found that the west and east coasts may harbor different species (N. repertus from Scott Reef and N. stenomphalus from the GBR) (summarized in [53], but see the taxonomic controversy outlined in [54]). Together, this indicates a more complicated connectivity, likely driven by the highly complex insular setting and oceanography that together determine the flow of water and propagules through the Indo-Malay region [55].
Tridacna noae was first documented in Ningaloo in Western Australia, one of the earliest records of this cryptic species [19]. However, we now know that it occurs far beyond Ningaloo, having been recorded from more than 20 sites from the mid-Australian coast at Ningaloo to northwestern Australia and offshore to Ashmore Reef. This work has revealed that it is the most commonly encountered giant clam in inshore tropical waters along the Western Australian coastline, often found in the tide pools alongside T. squamosa. Surprisingly, it is also found at Ashmore Reef, which is situated 830 km west of Darwin and 630 km north of Broome [56], a site where T. maxima would be expected given its known distribution, which encompasses remote atolls (see [27]). Intriguingly, T. maxima was not sequenced or sampled at either Ashmore Reef or Hibernia Reef during the survey work there, even though a review of legacy photographic records housed at the Western Australian Museum revealed characters consistent with T. maxima (e.g., concentrated hyaline organs along the mantle periphery, and the absence of teardrop features on the mantle edge) (Figure 5), like [40] from Ashmore Reef—although these characters are not always taxonomically informative for separating T. maxima and T. noae [57,58]. Further sampling at the outermost islands of the Kimberley would help to further refine our understanding of the distributional boundaries of Tridacna maxima in relation to T. noae in the eastern IO.
Distributed from Japan, south to New Caledonia, southwest to Western Australia, and to the far-flung eastern Line Islands, T. noae currently exhibits a patchier and expanded Indo-Malay distribution compared to T. maxima [2]. As in T. maxima, substantial genetic differences characterize T. noae populations from peripheral locations [19]. Three haplogroups (previously recovered by [2]) were resolved with samples of Western Australian T. noae falling in a larger haplogroup with Indo-Malay representatives (China, Taiwan, and Indonesia, as well as the Philippines and Micronesia), to the exclusion of the southwest Pacific and Papua New Guinea, reinforcing trends observed for T. maxima (Figure 2). Additional genetic novelty was evident, with the increased sampling in Western Australia indicating new haplotypes there (Figure 3). Samples of T. noae from eastern Australia are lacking, and affinities with WA populations of this species could not be tested. However, given the inclusion of Papua New Guinea in the sample set and the lack of a close relationship there, it is possible that, as for T. maxima, the east and west Australian coasts may show limited similarity. In addition to the well-documented occurrence of T. noae from Ningaloo Reef [38,39], these new records of T. noae represent only the second genetic validation of T. noae from the eastern IO. The limited sampling of T. noae in the IO, if it is found beyond the coast of Western Australia at all, precludes tests of phylogeographic affinity within the basin. Increased sampling of eastern and central IO giant clams, including the intriguing record of T. noae at Christmas Island [36], would allow for a better test of this hypothesis and provide data to compare with T. maxima.
Pronounced regional variation in mantle coloration was observed in T. noae within Western Australia over the course of this study (compare Figure 6 and Figure 7). These differences represent the most striking range of intraspecific mantle color variation reported for a giant clam species to date. At sites from Ningaloo Reef and South Muiron Island, up to Dampier Archipelago, across depth ranges from 0 to 7 m, mantles were “typical” T. noae [57] (Figure 6). In contrast, populations of T. noae from inshore locations in the Kimberley across depth ranges from 0 to 15 m displayed “atypical” mantles. These individuals all exhibited mantles with dark, chocolate-brown coloration [59] (Figure 7). Until now, teardrop or oval patterns on the mantle edge and less pronounced and less orderly hyaline organs or eyes have been considered to be quite reliable evidence for identifying T. noae, as they were less commonly observed in other species [26] (although some [39] have cautioned against this, stating that ‘previously reliable mantle patterning and shell shape trends were unreliable indicators of species’). Our study indicates that there is no mantle magic bullet for reliable species identification, especially in Western Australia, where traditional mantle characteristics that have been useful in distinguishing between T. maxima and T. noae are not apparent and, thus, not useful. Reliable new characters are needed, and we are working to address this limitation. Testing of traditional (e.g., shell length, height, and width, as well as rib number and rib ornamentation) and novel shell characters for genotyped vouchers across ontogenetic series to identify reliable shell and mantle features for field identification is ongoing at the WA Museum.
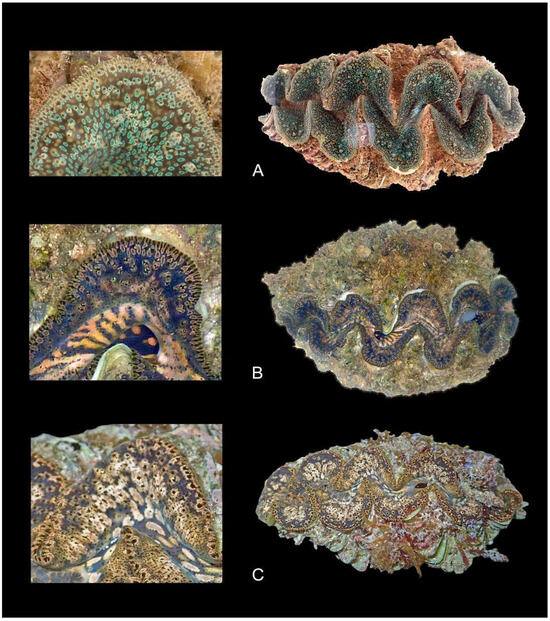
Figure 6.
Typical color forms of Tridacna noae from Western Australia: (A) WAM S78647 from Dampier Archipelago sampled at 3 m depth in 2017; (B) WAM S96406 from the Montebello Islands sampled at 0–1 m depth in 2015; (C) WAM S92107 sampled from a Ningaloo Reef flat at 0–1 m depth in 2016.
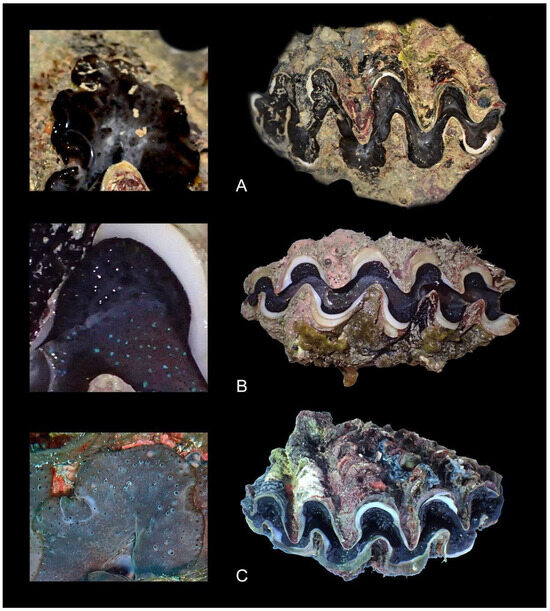
Figure 7.
Atypical color forms of Tridacna noae from Western Australia: (A) WAM S98329 from the Eclipse Archipelago, sampled at 1 m; (B) WAM S98805 from the Bonaparte Archipelago (East Montalivet Island), sampled at 0–1 m depth; (C) WAM S98701 from the Bonaparte Archipelago (Robroy Reefs) at 15 m depth. All were sampled in 2016.
Important unsampled sites exist that have faced and continue to face significant marine heatwaves, coral bleaching, and coral death in Western Australia. Scott Reef is one such area, located far offshore from many human pressures and impacts, under threat from elevated sea surface temperatures [60]. Documenting the giant clam species from this vulnerable location will be important to obtain baseline data as marine heatwaves continue. Another key unsampled location is the Houtman Abrolhos Islands, which represent some of the most southerly coral reefs in Australia, with large branching Acropora stands forming complex reef matrices. These islands harbor the most southerly populations of giant clams in the world. A marine heatwave impacted these islands in 2011, followed by coral bleaching in 2012 [61]. A provisional review of giant clam mantle images indicated that T. noae is present at this site [40]; however, as for Scott Reef individuals, genetic testing is critical to confirm this preliminary finding. Records of T. noae from Christmas Island are intriguing [36] and currently represent the most westerly populations of T. noae in the IO. Genotyping is necessary to confirm these observations, but the mantle does show features consistent with T. noae (teardrop or oval patterns on the mantle edge, and less pronounced and less orderly hyaline organs or eyes).
Studies of photosymbiont genetic diversity are underway to better understand whether the two different mantle coloration patterns observed in Western Australian T. noae are due to differences in photosymbiont assemblages (Meggan Miller, unpublished study), a result of iridophore variability, or both [62,63,64]. Perhaps brown mantle coloration of inshore Kimberley T. noae could be photoprotective and functionally adaptive for massive tidal regimes that leave intertidal species exposed to very high temperatures at low tides. No bleaching was observed in these or any other specimens of giant clam during the course of the study [65].
5. Conclusions
Continued sampling of giant clams from new coral reef habitats is needed to better understand population connectivity among species, reveal novel haplogroups of priority for conservation consideration, and assess the health of clams through bleaching assessments and symbiont genetic screening. This is especially critical for peripheral populations, such as in Western Australia. Intriguingly, artificial hybridization has been successful between T. noae and T. maxima, and it will be important now to validate this in wild populations, given the implications for species boundaries [66]). Future work is planned to review morphological features and mantle patterns to find new synapomorphies that are diagnostic for T. noae, given the extensive photographic, soft tissue, and shell vouchers accumulated over the course of this study from the eastern IO. This will help to delineate key characters that will be useful in the field.
Author Contributions
L.K.: Conceptualization, methodology, validation, investigation, negotiating resources, data curation, writing—original draft preparation, project management; M.A.: methodology, software, validation, formal analysis, data curation; writing—review and editing; P.M.: writing—review and editing, mapping, plate preparation. All authors have read and agreed to the published version of the manuscript.
Funding
The molecular sequence data were obtained via a Net Conservation Benefits (NCB) grant administered by the Western Australian Department of Biodiversity, Conservation and Attractions (DBCA).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Sequence data will be available at GenBank, including PV684038-PV684113 for COI. Alignments and accessions for external GenBank data are available at Mendelay Data https://doi.org/10.17632/gx3d7kxvsw.1. Please contact the first author for any additional data enquiries.
Acknowledgments
Tridacna samples from Cocos Keeling Islands, Indian Ocean Territories, were generously provided by the Ecological Monitoring and Assessments Aquatic Science team of the Western Australian Department of Primary Industries and Regional Development (DPIRD), under a service delivery arrangement with the Commonwealth Department of Infrastructure, Transport, Regional Development, Communications, and the Arts. The Western Australian Museum’s Molecular Systematic Unit (now Genomic Resources, GR) conducted subsampling through to sequence editing, alignment, and management. Sequencing was outsourced to AGRF. We are grateful to the Aquatic Zoology NCB team through the years, namely, Kim Lema, Michelle Condy, Diana Prada, Priya Krishnamurthy, and especially Nerida Wilson, for sequence data management and early discussions about the project. We also thank Melissa Danks for her assistance with our queries related to molecular data, and Arianna Urso for uploading sequence data to GenBank. Fieldwork support was provided by the Western Australian Museum’s Aquatic Zoology staff, and we are especially grateful to Corey Whisson for database management and tracking of voucher specimens and tissue subsamples. We would like to acknowledge work on sea country of Wunambal Gaambera custodians, as well as the participation of Uunguu Rangers in the 2016 coral reef expedition to the Bonaparte Archipelago.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Appendix A

Table A1.
Mitochondrial genes partially sequenced to investigate the phylogeographic relationships of Tridacna.
Table A1.
Mitochondrial genes partially sequenced to investigate the phylogeographic relationships of Tridacna.
| Gene | Forward Primer | Reverse Primer | Reference |
|---|---|---|---|
| Cytochrome c oxidase subunit I | LCO1490 5′GGTCAACAAATCATAAAGATATTGG 3′ | HC02198 5′TAAACTTCAGGGTGACCAAAAAATCA3′ | Folmer et al. [67] |
| 28S ribosomal RNA | 5′TCAGTAAGCGGAGGAA3′ | 5′CCAGCTATCCTGAGGGAAACTTC3′ | Park and O’Foighil [68] |

Table A2.
WAM registration numbers, collection localities, and depths for giant clams in the genus Tridacna that were newly sampled in this study (full information available upon request from L.K.).
Table A2.
WAM registration numbers, collection localities, and depths for giant clams in the genus Tridacna that were newly sampled in this study (full information available upon request from L.K.).
| WAM Registration | Species | Site | LatDec | LongDec | Depth (m) |
|---|---|---|---|---|---|
| WAMS68041 | maxima | Rowley Shoals; Mermaid Reef | −17.0726 | 119.6271 | 22 |
| WAMS68042 | maxima | Rowley Shoals; Mermaid Reef | −17.0726 | 119.6271 | 14 |
| WAMS68043 | maxima | Rowley Shoals; Mermaid Reef | −17.0726 | 119.6271 | 14 |
| WAMS68179 | maxima | Rowley Shoals; Imperieuse Reef | −17.5359 | 118.9733 | 20 |
| WAMS68248 | maxima | Rowley Shoals; Imperieuse Reef | −17.555 | 118.9651 | 14 |
| WAMS68249 | maxima | Rowley Shoals; Imperieuse Reef | −17.5361 | 118.9709 | 0 |
| WAMS95499 | maxima | Rowley Shoals; Imperieuse Reef | −17.5479 | 118.9738 | 18 |
| WAMS67959 | maxima | Cocos Keeling Islands | −12.1515 | 96.9179 | 1 |
| WAMS67960 | maxima | Cocos Keeling Islands | −12.1515 | 96.9179 | 1 |
| WAMS67961 | maxima | Cocos Keeling Islands | −12.1515 | 96.9179 | 1 |
| WAMS67962 | maxima | Cocos Keeling Islands | −12.1515 | 96.9179 | 1 |
| WAMS67964 | maxima | Cocos Keeling Islands | −12.1515 | 96.9179 | 1 |
| WAMS67965 | maxima | Cocos Keeling Islands | −12.2037 | 96.8617 | 1 |
| WAMS67966 | maxima | Cocos Keeling Islands | −12.2037 | 96.8617 | 1 |
| WAMS67967 | maxima | Cocos Keeling Islands | −12.2037 | 96.8617 | 1 |
| WAMS67968 | maxima | Cocos Keeling Islands | −12.2037 | 96.8617 | 1 |
| WAMS71000 | noae | Montalivet Islands, West Montalivet Island | −14.2861 | 125.2229 | 1 |
| WAMS98324 | noae | Eclipse Archipelago, East Holothuria Reef | −13.5825 | 126.0189 | 1 |
| WAMS98325 | noae | Eclipse Archipelago, East Holothuria Reef | −13.5825 | 126.0189 | 1 |
| WAMS98326 | noae | Eclipse Archipelago, East Holothuria Reef | −13.5825 | 126.0189 | 1 |
| WAMS98328 | noae | Eclipse Archipelago, Long Reef | −13.8301 | 125.8328 | 1 |
| WAMS98329 | noae | Eclipse Archipelago, Long Reef | −13.8301 | 125.8328 | 1 |
| WAMS97233 | noae | Great Sandy Islands, Serrurier island | −21.6262 | 114.6873 | 1 |
| WAMS91596 | noae | Kimberley; Ashmore Reef | −12.2077 | 123.1456 | 15 |
| WAMS110144 | noae | Dampier Islands, West Lewis Island | −20.6047 | 116.5961 | 0 |
| WAMS110145 | noae | Dampier Islands, West Lewis Island | −20.6047 | 116.5961 | 0 |
| WAMS110146 | noae | Dampier Islands, West Lewis Island | −20.6047 | 116.5961 | 0 |
| WAMS58832 | noae | Kimberley, Long Reef | −13.8197 | 125.78 | 0 |
| WAMS75437 | noae | Kimberley; Ashmore Reef | −12.2102 | 123.1441 | 1 |
| WAMS78500 | noae | Dampier Islands, Legendre Island | −20.352 | 116.85 | 16 |
| WAMS78519 | noae | Dampier Islands, Legendre Island | −20.352 | 116.85 | 6 |
| WAMS78647 | noae | Dampier Archipelago; Flying Foam Passage near Angel Island | −20.4674 | 116.8278 | 3 |
| WAMS78665 | noae | Dampier Archipelago; Flying Foam Passage near Angel Island | −20.4674 | 116.8278 | 2 |
| WAMS78737 | noae | Dampier Archipelago; Malus Island | −20.5024 | 116.6808 | 3 |
| WAMS78738 | noae | Dampier Archipelago; Malus Island | −20.5024 | 116.6808 | 3 |
| WAMS78739 | noae | Dampier Archipelago; Malus Island | −20.5024 | 116.6808 | 3 |
| WAMS78910 | noae | Dampier Archipelago; off Goodwin Island | −20.5367 | 116.5441 | 0 |
| WAMS78911 | noae | Dampier Archipelago; off Goodwin Island | −20.5367 | 116.5441 | 0 |
| WAMS78912 | noae | Dampier Archipelago; off Goodwin Island | −20.5367 | 116.5441 | 0 |
| WAMS78917 | noae | Dampier Archipelago; off Goodwin Island | −20.5367 | 116.5441 | 0 |
| WAMS78979 | noae | Dampier Archipelago; between Malus Islands and Rosemary Island | −20.52 | 116.672 | 3 |
| WAMS92259 | noae | Muiron Islands, Peak Island | −21.6277 | 114.3737 | 11 |
| WAMS98701 | noae | Bonaparte Archipelago; Robroy Reefs | −14.4295 | 124.8614 | 15 |
| WAMS98786 | noae | Bonaparte Archipelago; North Maret Island | −14.3977 | 124.9591 | 0 |
| WAMS98787 | noae | Bonaparte Archipelago; North Maret Island | −14.3977 | 124.9591 | 0 |
| WAMS98790 | noae | Bonaparte Archipelago; North Maret Island | −14.3977 | 124.9591 | 0 |
| WAMS98803 | noae | Bonaparte Archipelago; East Montalivet Island | −14.2666 | 125.297 | 0 |
| WAMS98805 | noae | Bonaparte Archipelago; East Montalivet Island | −14.2666 | 125.297 | 0 |
| WAMS98809 | noae | Bonaparte Archipelago; Patricia Island; Northwest Reef | −14.2528 | 125.3068 | 0 |
| WAMS98814 | noae | Bonaparte Archipelago; Patricia Island; Northwest Reef | −14.2528 | 125.3068 | 0 |
| WAMS98816 | noae | Bonaparte Archipelago; Patricia Island; Northwest Reef | −14.2528 | 125.3068 | 0 |
| WAMS98857 | noae | Bonaparte Archipelago; West Montalivet Island | −14.2822 | 125.2219 | 0 |
| WAMS98871 | noae | Bonaparte Archipelago; West Montalivet Island | −14.2822 | 125.2219 | 0 |
| WAMS98876 | noae | Bonaparte Archipelago; West Montalivet Island | −14.2822 | 125.2219 | 0 |
| WAMS98880 | noae | Bonaparte Archipelago; West Montalivet Island | −14.2822 | 125.2219 | 0 |
| WAMS98885 | noae | Bonaparte Archipelago; West Montalivet Island | −14.2822 | 125.2219 | 0 |
| WAMS98941 | noae | Bonaparte Archipelago; Berthier Island | −14.504 | 124.9821 | 0 |
| WAMS98942 | noae | Bonaparte Archipelago; Berthier Island | −14.504 | 124.9821 | 0 |
| WAMS98943 | noae | Bonaparte Archipelago; Berthier Island | −14.504 | 124.9821 | 0 |
| WAMS98952 | noae | Bonaparte Archipelago; Berthier Island | −14.504 | 124.9821 | 0 |
| WAMS98804 | noae | Bonaparte Archipelago; East Montalivet Island | −14.2666 | 125.297 | 0 |
| WAMS69292 | noae | Bonaparte Archipelago; West Montalivet Island | −20.6331 | 117.1987 | 0 |
| WAMS92107 | noae | Ningaloo, Jurabi Marine Park | −21.8483 | 114.0314 | 0 |
| WAMS92109 | noae | Ningaloo, Jurabi Marine Park | −21.8483 | 114.0314 | 0 |
| WAMS92110 | noae | Ningaloo, Jurabi Marine Park | −21.8483 | 114.0314 | 0 |
| WAMS92111 | noae | Ningaloo, Jurabi Marine Park | −21.8483 | 114.0314 | 0 |
| WAMS92112 | noae | Ningaloo, Jurabi Marine Park | −21.8483 | 114.0314 | 0 |
| WAMS92113 | noae | Ningaloo, Jurabi Marine Park | −21.8483 | 114.0314 | 0 |
| WAMS92114 | noae | Ningaloo, Jurabi Marine Park | −21.8483 | 114.0314 | 0 |
| WAMS96093 | noae | Montebello Islands, Quandong Islands | −20.4513 | 115.5827 | 7 |
| WAMS96173 | noae | Montebello Islands, Ah Chong Islands | −20.5192 | 115.5677 | 11 |
| WAMS98789 | noae | Bonaparte Archipelago; North Maret Island | −14.3977 | 124.9591 | 0 |
| WAMS92180 | noae | Muiron Islands, South Muiron Island | −21.6936 | 114.3289 | 7 |
| WAMS92181 | noae | Muiron Islands, South Muiron Island | −21.6936 | 114.3289 | 7 |
| WAMS92182 | noae | Muiron Islands, South Muiron Island | −21.6936 | 114.3289 | 7 |
| WAMS96406 | noae | Montebello Islands; Trimouille Island | −20.4055 | 115.5307 | 0 |
| WAMS98933 | noae | Bonaparte Archipelago; West Montalivet Island | −14.2877 | 125.2127 | 12 |
| WAMS92179 | noae | Muiron Islands, South Muiron Island | −21.6936 | 114.3289 | 7 |
| WAMS98923 | noae | Bonaparte Archipelago; West Montalivet Island | −14.2877 | 125.2127 | 12 |
References
- Kirkendale, L.; Paulay, G. Treatise Online no. 89: Part N, Revised, Volume 1, Chapter 9: Photosymbiosis in Bivalvia. Treatise Online 2017, 1, 89. [Google Scholar] [CrossRef]
- Fauvelot, C.; Andréfouët, S.; Grulois, D.; Tiavouane, J.; Wabnitz, C.C.C.; Magalon, H.; Borsa, P. Phylogeography of Noah’s giant clam. Mar. Biodivers. 2019, 49, 521–526. [Google Scholar] [CrossRef]
- ter Poorten, J.J. A Taxonomic Iconography of Living Cardiidae; ConchBooks: Harxheim, Germany, 2024; 600p. [Google Scholar]
- Li, R.; Leiva, C.; Lemer, S.; Kirkendale, L.; Li, J. Photosymbiosis shaped animal genome architecture and gene evolution as revealed in giant clams. Commun. Biol. 2025, 8, 7. [Google Scholar] [CrossRef]
- Jantzen, C.; Wild, C.; El-Zibdah, M.; Roa-Quiaoit, H.A.; Haacke, C.; Richter, C. Photosynthetic performance of giant clams, Tridacna maxima and T. squamosa, Red Sea. Mar. Biol. 2008, 155, 211–221. [Google Scholar] [CrossRef]
- Neo, M.L.; Wabnitz, C.C.C.; Braley, R.D.; Heslinga, G.A.; Fauvelot, C.; van Wynsberge, S.; Andrefouet, S.; Waters, C.; Shau-Hwai, T.A.; Gomez, E.D.; et al. Giant clams (Bivalvia: Cardiidae: Tridacninae): A comprehensive update of species and their distribution, current threats and conservation status. Oceanogr. Mar. Biol. Annu. Rev. 2017, 55, 2–303. [Google Scholar]
- Fitt, W.K.; Trench, R.K. Spawning, development and acquisition of zooxanthellae by Tridacna squamosa (Mollusca: Bivalvia). Biol. Bull. 1981, 161, 213–235. [Google Scholar] [CrossRef]
- Andréfouët, S.; Friedman, K.; Gilbert, A.; Remoissenet, G. A comparison of two surveys of invertebrates at Pacific Ocean islands: The giant clam at Raivavae Island, Australes Archipelago, French Polynesia. ICES J. Mar. Sci. 2009, 66, 1825–1836. [Google Scholar] [CrossRef]
- Gilbert, A.; Yan, L.; Remoissenet, G.; Andréfouët, S.; Payri, C.; Chancerelle, Y. Extraordinarily high giant clam density under protection in Tatakoto Atoll (eastern Tuamotu Archipelago, French Polynesia). Coral Reefs 2005, 24, 495. [Google Scholar] [CrossRef]
- Lee, L.K.; Neo, M.L.; Hii, K.S.; Gu, H.; Chen, C.A.; Lim, P.T.; Leaw, C.P. Vanishing giants: An assessment on the population status of giant clams across Malaysia. Reg. Stud. Mar. Sci. 2024, 74, 103546. [Google Scholar] [CrossRef]
- Appeltans, W.; Ahyong, S.T.; Anderson, G.; Angel, M.V.; Artois, T.; Bailly, N.; Bamber, R.; Barber, A.; Bartsch, I.; Berta, A.; et al. The magnitude of global marine species diversity. Curr. Biol. 2012, 22, 2189–2202. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.P. Molecular systematics of cowries (Gastropoda: Cypraeidae) and diversification patterns in the tropics. Biol. J. Linn. Soc. 2003, 79, 401–459. [Google Scholar] [CrossRef]
- Meyer, C.P.; Geller, J.B.; Paulay, G. Fine scale endemism on coral reefs: Archipelagic differentiation in turbinid gastropods. Evolution 2005, 59, 113–125. [Google Scholar]
- Cheng, S.; Anderson, F.E.; Bergman, A.; Mahardika, G.; Muchlisin, Z.; Dang, B.; Calumpong, H.P.; Mohamed, K.S.; Sasikumar, G.; Venkatesan, V.; et al. Molecular evidence for co-occurring cryptic lineages within the Sepioteuthis cf. lessoniana species complex in the Indian and Indo-West Pacific Oceans. Hydrobiologia 2014, 725, 165–188. [Google Scholar] [CrossRef]
- Lemer, S.; Planes, S. Effects of habitat fragmentation on the genetic structure and connectivity of the black-lipped pearl oyster Pinctada margaritifera populations in French Polynesia. Mar. Biol. 2014, 161, 2035–2049. [Google Scholar] [CrossRef]
- Amor, M.D.; Hart, A.M. Octopus djinda (Cephalopoda: Octopodidae): A new member of the Octopus vulgaris group from southwest Australia. Zootaxa 2021, 5061, 145–156. [Google Scholar] [CrossRef]
- Neo, M.L.; Eckman, W.; Vicentuan-Cabaitan, K.; Teo, S.L.-M.; Todd, P.A. The ecological significance of giant clams in coral reef ecosystems. Biol. Conserv. 2015, 181, 111–123. [Google Scholar] [CrossRef]
- Tang, Y. The Systematic Status of Tridacna maxima (Bivalvia: Tridacnidae) Based on Morphological and Molecular Evidence. Master’s Thesis, National Taiwan Ocean University, Keelung, Taiwan, 2005. [Google Scholar]
- Huelsken, T.; Keyse, J.; Liggins, L.; Penny, S.; Treml, E.A.; Riginos, C. A novel widespread cryptic species and phylogeographic patterns within several giant clam species (Cardiidae: Tridacna) from the Indo-Pacific Ocean. PLoS ONE 2013, 8, e80858. [Google Scholar] [CrossRef]
- Richter, C.; Roa-Quiaoit, H.; Jantzen, C.; Al-Zibdah, M.; Kochzius, M. Collapse of a new living species of giant clam in the Red Sea. Curr. Biol. 2008, 18, 1349–1354. [Google Scholar] [CrossRef]
- Fauvelot, C.; Zuccon, D.; Borsa, P.; Grulois, D.; Magalon, H.; Riquet, F.; Andréfouët, S.; Berumen, M.L.; Sinclair-Taylor, T.H.; Gélin, P.; et al. Phylogeographical patterns and a cryptic species provide new insights into western Indian Ocean giant clams phylogenetic relationships and colonization history. J. Biogeogr. 2020, 47, 1086–1105. [Google Scholar] [CrossRef]
- Velkeneers, X.; Dissanayake, P.A.K.N.; Huyghe, F.; Nehemia, A.; Ratsimbazafy, H.A.; Kochzius, M. DNA barcoding validates new sightings of Tridacna elongatissima in Tanzania and Mozambique (Western Indian Ocean). Coral Reefs 2022, 41, 837–842. [Google Scholar] [CrossRef]
- Ma, H.; Yu, D.; Li, J.; Qin, Y.; Zhang, Y.; Xiang, Z.; Zhang, Y.; Yu, Z. Molecular phylogeny and divergence time estimates for native giant clams (Cardiidae: Tridacninae) in the Asia-Pacific: Evidence from mitochondrial genomes and nuclear 18S rRNA genes. Front. Mar. Sci. 2022, 9, 964202. [Google Scholar] [CrossRef]
- Benzie, J.A.H.; Williams, S.T. Genetic structure of giant clam (Tridacna maxima) populations in the West Pacific is not consistent with dispersal by present-day ocean currents. Evolution 1997, 51, 768–783. [Google Scholar] [CrossRef]
- Gardner, J.P.A.; Boesche, C.; Meyer, J.M.; Wood, A.R. Analyses of DNA obtained from shells and brine-preserved meat of the giant clam Tridacna maxima From the central Pacific Ocean. Mar. Ecol. Prog. Ser. 2012, 453, 297–301. [Google Scholar] [CrossRef]
- Su, Y.; Hung, J.-H.; Kubo, H.; Liu, L.-L. Tridacna noae (Röding, 1798)—A valid giant clam species separated from T. maxima (Röding, 1798) by morphological and genetic data. Raffles Bull. Zool. 2014, 62, 124–135. [Google Scholar]
- Riquet, F.; Horaud, M.; Dubousquet, V.; Tiavouane, J.; Lopes, C.; Raharivelomanana, P.; Berteaux-Lecellier, V.; Planes, S.; Grulois, D.; Andréfouët, S.; et al. Insights into the genetic makeup of French Polynesian peripheral populations of the small giant clam Tridacna maxima. Aquat. Conserv. Mar. Freshw. Ecosyst. 2023, 33, 1514–1534. [Google Scholar] [CrossRef]
- Nuryanto, A.; Kochzius, M. Highly restricted gene flow and deep evolutionary lineages in the giant clam Tridacna maxima. Coral Reefs 2009, 28, 607–619. [Google Scholar] [CrossRef]
- Hui, M.; Kraemer, W.E.; Seidel, C.; Nuryanto, A.; Joshi, A.; Kochzius, M. Comparative genetic population structure of three endangered giant clams (Cardiidae: Tridacna species) throughout the Indo-West Pacific: Implications for divergence, connectivity and conservation. J. Molluscan Stud. 2016, 82, 403–414. [Google Scholar] [CrossRef]
- Van Wynsberge, S.; Andréfouët, S.; Gaertner-Mazouni, N.; Tiavouane, J.; Grulois, D.; Lefèvre, J.; Pinsky, M.L.; Fauvelot, C. Considering reefscape configuration and composition in biophysical models advance seascape genetics. PLoS ONE 2017, 12, e0178239. [Google Scholar] [CrossRef]
- Keyse, J.; Treml, E.A.; Huelsken, T.; Barber, P.H.; DeBoer, T.; Kochzius, M.; Nuryanto, A.; Gardner, J.P.A.; Liu, L.-L.; Penny, S.; et al. Historical divergences associated with intermittent land bridges overshadow isolation by larval dispersal in co-distributed species of Tridacna giant clams. J. Biogeogr. 2018, 45, 848–858. [Google Scholar] [CrossRef]
- Liggins, L.; Carvajal, J.I. Genomic Diversity and Connectivity of Small Giant Clam (Tridacna maxima) Populations Across the Cook Islands. Report for the Ministry of Marine Resources, Government of the Cook Islands. 2021; p. 18. Available online: https://environment.gov.ck/wp-content/uploads/2022/06/21.-MMR-Paua-Research-Report-2021.pdf (accessed on 15 February 2025).
- Pappas, M.K.; He, S.; Hardenstine, R.S.; Kanee, H.; Berumen, M.L. Genetic diversity of giant clams (Tridacna spp.) and their associated Symbiodinium in the central Red Sea. Mar. Biodivers. 2017, 47, 1209–1222. [Google Scholar] [CrossRef]
- Burke, L.; Reytar, K.; Spalding, M.; Perry, A. Reefs at Risk Revisited; World Resources Institute: Washington, DC, USA, 2011. [Google Scholar]
- Wilson, N.G.; Kirkendale, L.A. Putting the ‘Indo’ back into the Indo-Pacific: Resolving marine phylogeographic gaps. Invertebr. Syst. 2016, 30, 86–94. [Google Scholar] [CrossRef]
- Neo, M.L.; Low, J.K.Y. First observations of Tridacna noae (Roding, 1798) (Bivalvia: Heterodonta: Cardiidae) in Christmas Island (Indian Ocean). Mar. Biodivers. 2017, 48, 2183–2185. [Google Scholar] [CrossRef]
- Black, R.; Johnson, M.; Prince, J.; Brearley, A.; Bond, T. Evidence of large, local variations inrecruitment and mortality in the small giant clam, Tridacna maxima, at Ningaloo Marine Park, Western Australia. Mar. Freshw. Res. 2011, 62, 1318–1326. [Google Scholar] [CrossRef]
- Penny, S.S.; Willan, R.C. Description of a new species of giant clam (Bivalvia: Tridacnidae) from Ningaloo Reef, Western Australia. Molluscan Res. 2014, 34, 201–211. [Google Scholar] [CrossRef]
- Johnson, M.S.; Prince, J.; Brearley, A.; Rosser, N.L.; Black, R. Is Tridacna maxima (Bivalvia: Tridacnidae) at Ningaloo Reef, Western Australia? Molluscan Res. 2016, 36, 264–270. [Google Scholar] [CrossRef]
- iNaturalist Australia ALA. Available online: https://inaturalist.ala.org.au/ (accessed on 16 March 2025).
- Vogel, M.L.; Hoeksema, B.W. The role of aquaculture in the International trade of giant clams (Tridacninae) for the aquarium industry (2001–2019). Aquaculture 2024, 583, 740563. [Google Scholar] [CrossRef]
- Available online: https://www.Geneious.com (accessed on 30 October 2024).
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Posada, D. jModelTest: Phylogenetic model averaging. Mol. Biol. Evol. 2008, 25, 1253–1256. [Google Scholar] [CrossRef]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control. 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Bandelt, H.J.; Forster, P.; Röhl, A. Median-joining networks for inferring intraspecific phylogenies. Mol. Biol. Evol. 1999, 16, 37–48. [Google Scholar] [CrossRef]
- Leigh, J.W.; Bryant, D. Popart: Full-feature software for haplotype network construction. Methods Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef] [PubMed]
- Nevatte, R.J.; Gillings, M.R.; Morejohn, K.; Ainley, L.; Liggins, L.; Pratchett, M.S.; Hoey, A.S.; Doll, P.C.; Pasisi, B.; Williamson, J.E. Of clams and clades: Genetic diversity and connectivity of small giant clams (Tridacna maxima) in the southern Pacific Ocean. Ecol Evol. 2024, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Morrison, P.; O’Leary, M.; McDonald, J. The evolution of Australian island geographies and the emergence and persistence of Indigenous maritime cultures. Quat. Sci. Rev. 2023, 308, 108071. [Google Scholar] [CrossRef]
- van Herwerden, L.; Aspden, W.J.; Newman, S.J.; Pegg, G.G.; Briskey, L.; Sinclair, W. A comparison of the population genetics of Lethrinus miniatus and Lutjanus sebae from the east and west coasts of Australia: Evidence for panmixia and isolation. Fish. Res. 2009, 100, 148–155. [Google Scholar] [CrossRef]
- Sinclair, W.; Newman, S.J.; Vianna, G.M.S.; Williams, S.; Aspden, W.J. Spatial subdivision and genetic diversity in populations on the east and west coasts of Australia: The multi-faceted case of Nautilus pompilius (Mollusca, Cephalopoda). Rev. Fish. Sci. Aquacult. 2010, 19, 52–61. [Google Scholar] [CrossRef]
- Barord, G.J.; Combosch, D.J.; Giribet, G.; Landman, N.; Lemer, S.; Veloso, J.; Ward, P.D. Three new species of Nautilus Linnaeus, 1758 (Mollusca, Cephalopoda) from the Coral Sea and South Pacific. ZooKeys 2023, 1143, 51–69. [Google Scholar] [CrossRef]
- WoRMS. Available online: https://www.marinespecies.org/aphia.php?p=taxdetails&id=342238 (accessed on 24 March 2025).
- Feng, M.; Zhang, N.; Liu, Q.; Wijffels, S. The Indonesian throughflow, its variability and centennial change. Geosci. Lett. 2018, 5, 3. [Google Scholar] [CrossRef]
- Commonwealth of Australia. Ashmore Reef National Nature Reserve and Cartier Island Marine Reserve (Commonwealth Waters) Management Plans; Environment Australia: Canberra, Australia, 2002. [Google Scholar]
- Marra-Biggs, P.; Fatherree, J.; Green, A.; Toonen, R.J. Range expansion and first observation of Tridacna noae (Cardiidae: Tridacninae) in American Sāmoa. Ecol. Evol. 2022, 12, e9635. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Neo, M.L.; Liu, L.L.; Huang, D.; Soong, K. Thriving populations with low genetic diversity in giant clam species, Tridacna maxima and Tridacna noae, at Dongsha Atoll, South China Sea. Reg. Stud. Mar. Sci. 2018, 24, 278–287. [Google Scholar] [CrossRef]
- Militz, T.A.; Kinch, J.; Southgate, P.C. Population demographics of Tridacna noae (Röding, 1798) in New Ireland, Papua New Guinea. J. Shellfish Res. 2015, 34, 329–335. [Google Scholar] [CrossRef]
- Liyanaarachchige, P.T.A.W.; Fisher, R.; Thompson, H.; Menendez, P.; Gilmour, J.; McGree, J.M. Adaptive monitoring of coral health at Scott Reef where data exhibit nonlinear and disturbed trends over time. Ecol. Evol. 2022, 12, e9233. [Google Scholar] [CrossRef]
- Abdo, D.A.; Bellchambers, L.M.; Evans, S.N. Turning up the heat: Increasing temperature and coral bleaching at the high latitude coral reefs of the Houtman Abrolhos Islands. PLoS ONE 2012, 7, e43878. [Google Scholar] [CrossRef]
- Kawaguti, S. Electron microscopy on the mantle of the giant clam with special references to zooxanthelar and iridophores. Biol. J. Okayama Univ. 1966, 12, 81–92. [Google Scholar]
- Kamishima, Y. Organization and development of reflecting platelets in iridophores of the giant clam, Tridacna crocea Lamarck. Zool. Sci. 1990, 1, 63–72. [Google Scholar]
- Li, J.; Lemer, S.; Kirkendale, L.; Bieler, R.; Cavanaugh, C.; Giribet, G. Shedding light: A phylotranscriptomic perspective illuminates the origin of photosymbiosis in marine bivalves. BMC Evol. Biol. 2020, 20, 50. [Google Scholar] [CrossRef]
- Richards, Z.T.; Garcia, R.; Moore, G.; Fromont, J.; Kirkendale, L.; Bryce, M.; Bryce, C.; Hara, A.; Ritchie, J.; Gomez, O.; et al. A tropical Australian refuge for photosymbiotic benthic fauna. Coral Reefs 2019, 38, 669–676. [Google Scholar] [CrossRef]
- Militz, T.A.; Braley, R.D.; Southgate, P.C. Captive hybridization of the giant clams Tridacna maxima (Röding, 1798) and Tridacna noae (Röding, 1798). J. Shellfish. Res. 2017, 36, 585–591. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar] [PubMed]
- Park, J.K.; O’Foighil, D. Sphaeriid and corbiculid clams represent separate heterodont bivalve radiations into freshwater environments. Mol. Phylogenet. Evol. 2000, 14, 75–88. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).