Abstract
Pedicularis hallaisanensis is a strictly biennial, hemiparasitic herb endemic to Republic of Korea and listed as an endangered species. Its populations are limited to high-altitude habitats, with recent surveys confirming survival only in Gayasan. This study aimed to assess the population size and ecological traits of P. hallaisanensis to inform conservation strategies. We established 23 quadrats at 1400–1410 m above sea level and collected microhabitat data (air temperature, soil moisture, electroconductivity, vegetation cover, and species richness) from 2022 to 2024. Flora composition and pollinator species were surveyed, with bumblebees (Bombus ignitus, B. hypocrita sapporoensis) identified as the most frequent pollinators. General linear mixed models and Pearson’s correlation analysis showed a strong positive relationship between species richness and population size and between vegetation cover and stem height. The study area’s average temperature was 6.3 °C below Republic of Korea’s national average, suggesting that climate change could disrupt the microclimatic conditions necessary for this species’ survival. The findings highlight the importance of maintaining plant diversity and controlling invasive woody species to sustain P. hallaisanensis populations. Targeted conservation measures, including habitat management and ex situ propagation, are recommended to safeguard this vulnerable species.
1. Introduction
Biodiversity is essential for maintaining ecosystem stability and functionality, supporting key processes, such as energy flow and nutrient cycling, through species interactions [1]. Endangered species play a vital role in these dynamics, and their loss can markedly disrupt ecological equilibrium [2,3]. Therefore, conserving endangered species is crucial for biodiversity protection. Globally, numerous plant species face extinction due to habitat destruction, climate change, overexploitation, and other anthropogenic pressures. These threats often lead to habitat fragmentation, reducing ecosystem functioning [4,5]. Consequently, many plant species are now endangered, directly affecting ecosystem services critical to human survival [6].
In this context, understanding the survival strategies and ecological roles of plant species adapted to specialized environments, such as high-altitude ecosystems, is of particular importance. Among these, species of the genus Pedicularis, which exhibit unique physiological traits and ecological interactions, play a significant role within their ecosystems.
Plants in the genus Pedicularis of the Orobanchaceae family are hemiparasitic, synthesizing nutrients through photosynthesis while extracting water and minerals from host roots, an adaptation essential for growth and survival [7].
While this physiological trait is well established, its broader ecological implications remain underexplored. Hemiparasitic plants can influence plant community composition by altering competitive interactions among species. For instance, Pedicularis canadensis has been reported to reduce host plant dominance in prairie ecosystems, thereby promoting species evenness and enhancing overall biodiversity [8]. These ecological functions suggest that hemiparasitism in Pedicularis may serve as a key adaptive strategy, warranting further investigation into its role in structuring plant communities across different environments.
Pedicularis hallaisanensis Hurus., the focus of this study, is endemic to Republic of Korea and classified as a Class II endangered wild species by Republic of Korea’s Ministry of Environment. It is also listed as Endangered in the National Red List [9,10]. This herbaceous plant inhabits isolated mountaintops at altitudes exceeding 1000 m, which historically included the Hallasan, Seoraksan, Bangtaesan, and Gayasan mountains [10]. However, recent studies have indicated a shrinking distribution of wild P. hallaisanensis populations under changing climate across mountainous habitats [11]. This rapid decline underscores the need for further population surveys, habitat assessments, and urgent conservation and restoration efforts.
Ecologically, as a hemiparasitic plant, P. hallaisanensis is likely to influence plant community dynamics by interacting with a variety of host species, potentially altering resource distribution and competitive relationships within high-altitude ecosystems. Press and Phoenix [12] highlighted that parasitic plants can function as keystone species by modulating host community composition and promoting habitat heterogeneity. In this context, P. hallaisanensis may also contribute to ecosystem complexity and stability, particularly within the specialized conditions of high-altitude environments.
The life history of P. hallaisanensis has previously been described inconsistently, with some studies identifying it as an annual [13] or perennial [4] plant. However, recent ecological and genomic evidence confirms that the species exhibits a strict biennial life cycle, by which the seedlings of this species only maintain the rosette leaves and roots during the first year and then develop flowers and seeds in successive years [14].
Beyond its ecological uniqueness, P. hallaisanensis may also hold additional value in economic, cultural, and ecological contexts. Phytochemical studies of Pedicularis species have revealed the presence of iridoid glycosides and flavonoids, which exhibit antioxidant, anti-inflammatory, and neuroprotective properties [15]. Although P. hallaisanensis itself has not yet been pharmacologically explored, related species such as P. rex and P. longiflora have shown potential for medicinal applications [16], suggesting that Korean endemic species may serve as valuable genetic resources for future bioprospecting.
Indoor seed germination experiments reveal that cold exposure is crucial for breaking dormancy [8,17]. In natural habitats, seeds likely break dormancy following winter cold exposure after autumn dispersal, germinating in the subsequent spring or summer. Consequently, winter temperatures play a key role in sustaining P. hallaisanensis populations, suggesting that climate warming could adversely affect germination. However, research on population size and optimal environmental conditions remains limited, with few studies examining the impact of habitat changes on population persistence [18].
This study aims to provide essential data for effective P. hallaisanensis conservation and restoration strategies by examining population size, primary pollinators, and microhabitat characteristics in the species’ remaining Gayasan habitats. In particular, our monitoring focuses on the environmental variables influencing the population size of P. hallaisanensis and its potential threatening factors. To address such research objective, we selected a null hypothesis that the population size of the P. hallaisanensis would be affected by the abundance and diversity of the co-occurring plants, considering the target species’ hemiparasitic nature.
2. Materials and Methods
2.1. Study Area
This research was conducted in the natural habitat of P. hallaisanensis on the southwestern slope near the Gayasan Mountain summit (1400–1410 m above sea level; coordinates: 35°49′2″ N, 128°7′10″ E; Figure 1 and Figure A1). The site shows remnants of anthropogenic disturbances, including flattened areas, gravel placement, and fence installations. The soil, classified as Entisols, consists of a thin mineral soil layer approximately 20 cm deep with poorly developed humus layers. The microtopography includes impermeable layers that causes water accumulation after rainfall. Although P. hallaisanensis historically inhabited the Hallasan, Seoraksan, and Bangtaesan mountains, no individuals have been recorded there since 2019, limiting our monitoring to Gayasan [14].

Figure 1.
Strict biennial life cycle of P. hallaisanensis with two distinct phases.
2.2. Environmental Data Collection
To analyze anthropogenic disturbances and vegetation succession, historical aerial photographs (1954–2015) were obtained from the National Geographic Information Institute (Suwon, Republic of Korea), and drone mapping was conducted in 2022–2024 for aerial imaging. Quadrats (1.5 × 1.5 m) were established at all microhabitats with P. hallaisanensis individuals (23 in total) and surveyed periodically [19]. Air temperature and relative humidity were recorded hourly using an air temperature and moisture sensor (U23-001, Onset, Bourne, MA, USA) installed at 1 m above the soil surface in June 2022. Soil moisture and electroconductivity were measured at three random points per quadrat using a portable TDR sensor (TDR 150, Spectrum Technologies Inc., Aurora, IL, USA) at 0–20 cm soil depths, which covers the entire depths of humus and mineral soil layers above the parent material of the study area.
2.3. Flora and Pollinator Survey
Plant species cooccurring with P. hallaisanensis were identified following the International Plant Names Index (IPNI). Drone-based imaging (Mavic 3 Enterprise, DJI, Shenzhen, China) captured 978 aerial photographs from an altitude of 20 m between August 2022 and 2024, which were processed using Agisoft PhotoScan (Agisoft, Saint Petersburg, Russia). Vegetation cover per quadrat was calculated using ImageJ 1.53k software. Insects visiting P. hallaisanensis flowers during blooming were photographed and identified to species level using the Integrated Taxonomic Information System (ITIS) as a reference.
To ensure temporal representativeness of pollinator observations, surveys were conducted during daylight hours, from 09:00 to 18:00, totaling 9 h per day. This approach was employed to minimize biases caused by pollinator activity variation across time and to obtain a more accurate assessment of pollinator distribution.
2.4. Population Size
The morphological traits (the rosette diameter of first-year seedlings and stem height and canopy width of second-year adults) and population size of first-year seedlings and second-year adults were recorded in April, June, August, and October from 2022 to 2024. The rosette diameter of first-year seedlings as well as the height and canopy width of second-year adults were measured during the flowering season of the target species in August.
2.5. Statistical Analysis
Quadrats containing P. hallaisanensis served as replicates (n = 23). Data normality was assessed using the Shapiro–Wilk test (α = 0.05), with log transformation applied when necessary. Data normality and homoscedasticity were assessed using Shapiro–Wilk and Levene’s tests (α = 0.05). Total population size, population size of the first-year seedlings, and population size, height, and canopy height of the second-year adults were log transformed, given the results of Shapiro–Wilk test (p < 0.05). All data complied with the assumption of homoscedasticity according to Levene’s test (p > 0.05). General linear mixed models and Pearson’s correlation analyses were performed using R version 4.3.2 with the lme4 and agricolae packages [20,21,22]. Explanatory variables included soil electroconductivity, soil moisture, vegetation cover, species richness, and their two-way interaction effects (α = 0.05). The survey year (2022–2024) was treated as a random effect within the general linear model to focus on the general, consistent effects of environmental variables across overall study periods rather than the specific patterns within each individual year [23]. Pearson’s correlation test was used to address pairwise relationships between two selected variables and multicollinearity among the explanatory variables (α = 0.05; Table A1).
3. Results
3.1. Changes Observed in Aerial Imagery
Aerial photographs from 1954 revealed extensive soil exposure and visible signs of erosion, characteristic of a colluvial slope. Major anthropogenic alterations, including a helicopter landing pad, were evident from 1982 onward. By 2015, the helicopter pad had almost vanished, and drone images from 2024 showed a substantial increase in vegetation cover, indicating ecological recovery after the site ceased functioning as a landing area in 2010 (Figure 2).
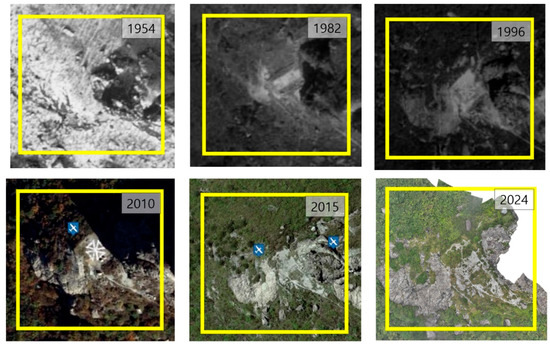
Figure 2.
Aerial photographs of the study area used to assess vegetation and topographic changes (1954–2024).
3.2. Microclimate Conditions
From June 2022 to August 2024, the mean, minimum, and maximum air temperatures in the P. hallaisanensis habitat were 8.6 °C, −23.9 °C, and 34.5 °C, respectively, reflecting cool temperate mountain conditions (Figure 3). The average relative humidity was 78.6%. Notably, the site’s average temperature was ~6.3 °C lower than the national average of 14.9 °C for Republic of Korea.
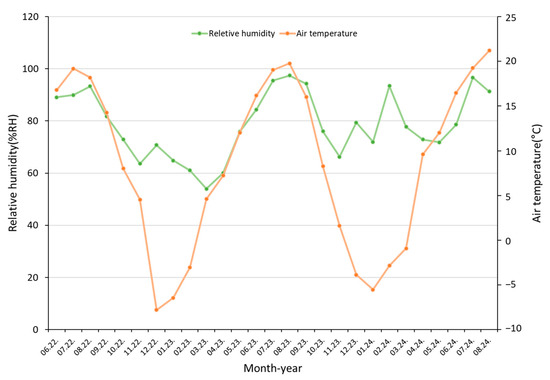
Figure 3.
Monthly air temperature and relative humidity data at the P. hallaisanensis habitat.
3.3. Flora Composition
In total, 67 vascular plant species, belonging to 24 orders and 31 families, were recorded in the study microhabitats (Table 1). Dominant species with high co-occurrence frequency with the target species comprised Thymus quinquecostatus (100%), Carex humilis var. nana (91.3%), Dendranthema zawadskii var. latilobum (87.0%), and Geranium dahuricum (78.3%) (Table 1). Cool temperate mountain species, such as Conioselinum tenuissimum, Gentiana wootchuliana, and Sorbus commixta, as well as cliff-dwelling species, including Selaginella rossii, Meterostachys sikokianus, Sedum polytrichoides, and Potentilla dickinsii, were observed. Wetland species, including Eriocaulon decemflorum and Parnassia palustris var. multiseta, were recorded owing to the presence of impermeable soil layers. The average soil electroconductivity was 0.019 ± 0.002 mS/cm, soil moisture content was 18.200% ± 0.818%, vegetation cover was 67.000% ± 3.009%, and species richness averaged 4.300 ± 0.121 per quadrat.

Table 1.
Plant species identified in the quadrat survey of P. hallaisanensis in Gayasan. Frequency data indicate the number of quadrats containing each plant species.
3.4. Pollinator Survey
During P. hallaisanensis blooming, 10 pollinator species were identified: Bombus ignitus, Bombus hypocrita sapporoensis, Apis cerana, Apis mellifera, Lasioglossum (Evylaeus) duplex, Lygaeus equestris, Carbula putoni, Eristalis tenax, Helophilus virgatus, and Argyreus hyperbius. Among these, B. ignitus and B. hypocrita sapporoensis were most frequently observed (Figure 4). These pollinators primarily visited the flowers of dominant surrounding species, including T. quinquecostatus, Sanguisorba hakusanensis, and P. hallaisanensis, for nectar collection.

Figure 4.
Pedicularis hallaisanensis pollinators in Gayasan: (A) B. ignites and (B) B. hypocrita sapporoensis.
3.5. Influential Factors for Population Size
The total population size of P. hallaisanensis individuals was 187, 340, and 334 in 2022, 2023, and 2024, respectively (Figure 5). First-year seedlings (104–305 individuals) were consistently outnumbering second-year adults (35–133 individuals) throughout the monitoring years (Figure 5). The average rosette diameter range of first-year seedlings was 2.8–4.3 cm, and second-year adults exhibited significant variation in height (mean: 12.2–16.8 cm) and canopy width (mean: 7.3–11.0 cm) (Table 2). The canopy width of second-year adults was consistently larger than the rosette diameter of first-year seedlings in all monitoring years (Table 2).
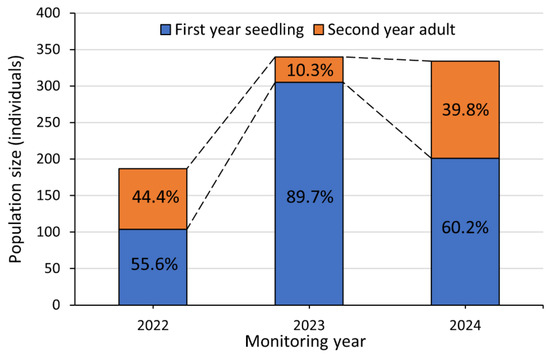
Figure 5.
Population size of P. hallaisanensis in 2022–2024. Percentage values indicate the proportion of first-year seedlings and second-year adults within the total population size in each year. Dashed lines indicate the changes in the population size of each age class of P. hallaisanensis.

Table 2.
Morphological traits (mean ± standard error) of P. hallaisanensis during each monitoring year.
A general linear mixed model (Table 3, Figure 6) showed that species richness had the significant effect on the population size of total population, first-year seedlings, and second-year adults (Table 3). Only the interaction effect of vegetation cover and species richness was significant for total population size (Table 3). Correlation analyses further revealed that the population sizes of total population (r = 0.52, p < 0.01, Figure 6), first-year seedling (r = 0.42, p < 0.01), and second-year adults (r = 0.45, p < 0.005) increased with increasing species richness. In addition, vegetation cover had a significant effect on the height of second-year adults (Table 3). The height of second-year adults increased with increasing vegetation cover (r = 0.56, p < 0.01, Figure 6). Soil electroconductivity and moisture content had no significant effect on either population size or morphological traits (p > 0.05), which may be affected by the multicollinearity between these variables (r = 0.51, p < 0.05, Table A1).

Table 3.
Results of generalized linear mixed model analysis applied to the population size and morphological traits of P. hallaisanensis with environmental variables.
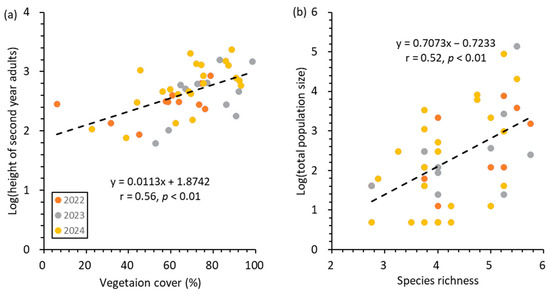
Figure 6.
Correlations between stem height of second-year adults of P. hallaisanensis and vegetation cover (a) and between total population size and species richness (b). Dashed line on each panel indicates the linear regression between explanatory and independent variables.
4. Discussion
4.1. Habitat and Population Size of P. hallaisanensis
This study analyzed flora in the P. hallaisanensis habitat at Gayasan, comparing the findings with previous surveys [13]. Results indicated an increase in plant diversity, with the taxa count rising from 25 across 16 families in 2018 to 67 across 31 families in 2024. Aerial imagery confirmed substantial growth in vegetation coverage. In 2018, only one tree species (Cornus kousa) and three shrub species (Rubus crataegifolius, Rhododendron sohayakiense var. koreanum, and Weigela subsessilis) were recorded. Conversely, in 2024, seven tree species (Quercus mongolica, Carpinus cordata, Fraxinus rhynchophylla, Pinus densiflora, Fraxinus sieboldiana, Betula costata, and Sorbus commixta) and five shrub species (R. crataegifolius, Weigela praecox, Rhododendron yedoense f. poukhanense, Lespedeza melanantha, and Rhamnus yoshinoi) were recorded. The increase in tree species, such as Q. mongolica, B. chinensis, and F. rhynchophylla, along with the shrub layer expansion, comprising species such as R. yedoense f. poukhanense and L. melanantha, indicates enhanced structural diversity in the plant community.
The P. hallaisanensis population size positively correlated with species richness within the Gayasan microhabitats, suggesting that germination and growth conditions may improve in the presence of diverse host plants. In particular, the increased abundance of species such as Artemisia indica and Dendranthema zawadskii var. latilobum which have been shown to influence germination dynamics may have contributed to the observed population persistence through facilitative effects on seedling establishment [24]. These findings fit into our null hypothesis regarding the relationship between co-occurring plant species richness and P. hallaisanensis population size.
As a hemiparasitic species, P. hallaisanensis likely depends on physiological interactions with host plants to acquire water and mineral nutrients [24]. The observed positive association between P. hallaisanensis population size and plant species richness may reflect its reliance on diverse host assemblages. Similar positive relationships between plant species richness and the population size of hemiparasitic Pedicularis species have also been documented. For example, the abundance of P. kansuensis was found to be positively associated with species richness in Tibetan alpine grasslands, suggesting that high plant diversity may provide more compatible host options and improve habitat quality for hemiparasitic plants [8]. P. canadensis in tallgrass prairie landscapes also featured a similar ecological interaction with co-occurring plant species as potential hosts [25]. Furthermore, higher plant diversity has been reported to enhance soil microbial diversity, which in turn can facilitate plant growth and seedling survival through improved nutrient cycling and mutualistic interactions [26].
Bumblebees, particularly B. ignitus and B. hypocrita sapporoensis, were the main pollinators visiting P. hallaisanensis. These bumblebees visited multiple plant species, thereby playing an essential role in sexual reproduction and biodiversity maintenance [27]. Similar ecological patterns have been observed in other regions as well. For example, Macior (1977) reported that Pedicularis species in North America primarily rely on bumblebees for pollination, and his later work in the Chinese Himalaya also confirmed bumblebees as the principal pollinators of Pedicularis species in alpine habitats [28]. These findings emphasize the critical role of bumblebees in Pedicularis reproductive success across diverse environments, although our study lacked the data on the nocturnal pollinators visiting P. hallaisanensis. flowers.
4.2. Threatening Factors
P. hallaisanensis population size is closely linked to plant species richness, meaning that a species richness decline could negatively impact its population. Therefore, management strategies must prioritize maintaining species diversity and preventing excessive expansion of certain species due to anthropogenic disturbance or invasive species. In addition, increased shrub layers and tree species in open areas can alter habitats critical to P. hallaisanensis, increasing competition and potentially threatening population stability. Prior reports from Hallasan, Jeju Island, showed that such expansion decreased mountaintop plant diversity, negatively impacting several species, including P. hallaisanensis [29]. This study found that increased vegetation cover led to taller biennial plants, likely as an adaptation to enhanced competition for light [30]. Such stem elongation is often interpreted as a shade avoidance response, which may reflect an increased competition for light availability. Increased allocation to stem growth under dense vegetation cover can reduce resource investment to reproductive organs, and hinder the fecundity and long-term population viability [31,32].
Generally, mountaintop plants are vulnerable to climate change [33,34,35]. In Republic of Korea, mountaintop species at elevations exceeding 1000 m are experiencing habitat shrinkage and population decline due to climate change [36]. Most mountaintop species are isolated, making them particularly susceptible to extinction without refuges [37,38,39]. The study area, located at 1410 m above sea level, exhibited temperatures that were about 6.3 °C lower than the national average for Republic of Korea. This substantial temperature difference reflects the high-altitude microclimate, suggesting that climate change–induced disruptions to these conditions could negatively impact P. hallaisanensis population sustainability.
As no official meteorological station is located within Gayasan National Park, long-term climate trends were assessed using temperature data from the two nearest national weather stations, Geochang and Hapcheon [40]. These stations recorded an average increase of approximately 0.9 °C in annual mean temperature over the past 30 years, indicating a gradual regional warming trend. Such shifts may significantly affect the microclimatic conditions of high-elevation habitats, which are particularly sensitive to thermal changes due to their ecological specialization and limited altitudinal buffering capacity [41]. Given that the P. hallaisanensis habitat maintains an average temperature ~6.3 °C lower than the national mean, even slight warming may disrupt key processes such as seed dormancy break and flowering synchrony. Furthermore, habitat modeling studies suggest that suitable areas for P. hallaisanensis could decline under projected warming scenarios, reinforcing the urgency of conservation efforts targeting microclimate preservation and species migration corridors [18]. Additionally, shifts in environmental conditions may promote the upward migration of non-native or invasive plant species into high-elevation habitats, potentially intensifying competition and degrading the ecological integrity of P. hallaisanensis habitats [42].
4.3. Conservation Implications and Future Research Requirements
We proposed three primary conservation measures to protect P. hallaisanensis and mitigate climate change–mediated habitat loss. First, maintaining plant species diversity within existing habitats is essential, as P. hallaisanensis population viability correlates positively with potential host plant diversity as our null hypothesis. Therefore, management efforts must extend beyond designating protected areas to actively preserving balanced plant communities.
Second, assessing and managing the impact of shrub and tree expansion is crucial, as increased vegetation cover heightens competition for light, affecting the growth and reproduction of various species. Implementing continuous monitoring and targeted control of woody plant proliferation will help maintain optimal habitat conditions. In particular, mechanical removal of encroaching woody species may serve as an effective strategy for restoring open habitat structures and alleviating competitive pressure in high-altitude habitats [43]. In parallel, conserving key pollinators such as B. ignitus and B. hypocrita sapporoensis is critical to sustaining reproductive success in P. hallaisanensis. Conservation measures should include maintaining native floral resources throughout the flowering season, protecting natural nesting habitats, and mitigating the impact of invasive species such as B. terrestris, which can cause reproductive interference and displacement of native bumblebee populations [44,45].
Finally, establishing ex situ conservation strategies and alternative habitats is essential for protecting isolated high mountain plants such as P. hallaisanensis. These efforts should be aligned with national programs like the Republic of Korea’s Endangered Wildlife Proliferation and Restoration Plan [46] and supported by further research on germination ecology and propagation methods to ensure long-term survival [17].
While this study provides important insights into the population dynamics and microhabitat characteristics of P. hallaisanensis, several future studies are still necessary to overcome the limitations of our study. First, the spatial scope of the study was limited to a single mountain region (Gayasan), where the only known population of the species currently persists. As a result, the generalizability of our findings to other historical or potential habitats may be limited. Future studies incorporating additional remnant populations, if rediscovered, or ecologically similar sites could provide broader spatial perspectives.
Second, further controlled experiments on the effects of climate change should be needed, considering the increasing patterns in the air temperature around the study area. For instance, conducting seed germination trials of P. hallaisanensis under simulated future temperature scenarios (e.g., 5–15 °C vs. 11.5–21.5 °C) could elucidate the species’ thermal thresholds and potential shifts in germination timing. Additionally, implementing reciprocal transplant experiments across different elevations may reveal how rising temperatures influence plant growth, reproductive success, and interspecific competition within its native habitat [47,48]. As climate change can accelerate the invasion of non-native plant species to the upper mountain habitats, future work should incorporate targeted monitoring of invasive species dynamics and their competitive interactions with native alpine flora, including P. hallaisanensis under climate change scenarios [49,50]
Finally, the study relied on traditional quadrat surveys and general linear mixed models for field data collection and analysis. Although these methods remain standard in ecological research, they offer limited technological innovation. Recent studies increasingly utilize unmanned aerial vehicles (UAVs) combined with machine learning algorithms to monitor vegetation patterns, population structure, and microhabitat complexity at higher spatial and temporal resolutions [51,52] While we incorporated drone-based imagery for vegetation cover analysis, such advanced automated image processing and classification techniques were not applied. Future integration of such technologies may improve monitoring efficiency, reduce observer bias, and allow for broader ecological scaling.
5. Conclusions
This study examined the population size and ecological characteristics of P. hallaisanensis, an endemic species at Gayasan. Regarding our null hypothesis, results revealed that species richness positively influences population size, highlighting the importance of preserving co-occurring plant diversity to maintain the health of the remaining P. hallaisanensis population. Furthermore, increased vegetation cover due to the introduction of tree and shrub species may led to the overly elongated stem length of the second-year adults of P. hallaisanensis, which potentially has negative effects on the fecundity and long-term population viability. Thus, controlling invasive woody species that intensify competition within habitats is vital for the long-term viability of P. hallaisanensis populations. Effective management should focus on protecting key pollinators, especially B. ignitus and B. hypocrita sapporoensis. With climate change posing potential threats, identifying alternative habitats and implementing proactive conservation strategies are essential for ensuring P. hallaisanensis’s survival.
Author Contributions
All authors contributed to the research conception and design. C.W.L. and S.K. conceptualized the study. C.W.L., H.-J.P., Y.-J.K., J.E.H., H.B.P. and S.K. collected field data. C.W.L. and S.K. wrote and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by a research grant from the National Institute of Ecology (grant number: NIE-B-2025-49) of Republic of Korea.
Data Availability Statement
The original data underlying the conclusions of this article will be provided by the authors upon request, with the first author responsible for sharing the data.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A
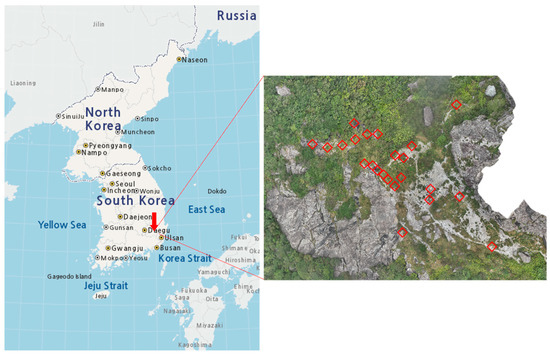
Figure A1.
Location of the study site. Red squares on the right panel indicate the 23 quadrats for P. hallaisanensis monitoring. Red arrow on the left panel indicates the location of the study site.

Table A1.
Pearson’s correlation matrix among the environmental variables.
Table A1.
Pearson’s correlation matrix among the environmental variables.
| EC | SMC | VCR | SR | |
|---|---|---|---|---|
| EC | - | r = 0.51 p < 0.001 | r = 0.25 p = 0.09 | r = 0.02 p = 0.87 |
| SMC | r = 0.51 p < 0.001 | - | r = 0.07 p = 0.64 | r = 0.21 p = 0.16 |
| VCR | r = 0.25 p = 0.09 | r = 0.07 p = 0.64 | - | r = 0.18 p = 0.23 |
| SR | r = 0.02 p = 0.87 | r = 0.21 p = 0.16 | r = 0.18 p = 0.23 | - |
EC = soil electroconductivity; SMC = soil moisture content; VCR = vegetation cover ratio; SR = species richness.
References
- Loreau, M.; Naeem, S.; Inchausti, P.; Bengtsson, J.; Grime, J.P.; Hector, A.; Wardle, D.A. Biodiversity and ecosystem functioning: Current knowledge and future challenges. Science 2001, 294, 804–808. [Google Scholar] [CrossRef] [PubMed]
- Pimm, S.L.; Russell, G.J.; Gittleman, J.L.; Brooks, T.M. The future of biodiversity. Science 1995, 269, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Soulé, M.E. What is conservation biology? A new synthetic discipline addresses the dynamics and problems of perturbed species, communities, and ecosystems. BioScience 1985, 35, 727–734. [Google Scholar] [CrossRef]
- Cho, W.B.; Choi, B.H. Taxonomic position of Pedicularis hallaisanensis Hurusawa, an endemic plant of Mt. Halla. Korean J. Pl. Taxon. 2011, 41, 130–137. [Google Scholar] [CrossRef]
- Kim, S.; Park, H.-J.; Lee, C.W.; Kim, N.Y.; Hwang, J.E.; Lee, B.-D.; Park, H.B.; An, J.; Baek, J. Endangered plant species under differing anthropogenic interventions: How to preserve Pterygopleurum neurophyllum in Wondong wetland? PeerJ 2022, 10, e14050. [Google Scholar] [CrossRef]
- IUCN. The IUCN Red List of Threatened Species. Version 2024.3. Available online: https://www.iucnredlist.org (accessed on 17 May 2025).
- Heide-Jørgensen, H.S. Parasitic Flowering Plants; Brill: Leiden, The Netherlands, 2008. [Google Scholar]
- Li, Y.; Wang, R.; Han, C.; Zhang, L. Effects of Pedicularis kansuensis on plant community structure and diversity in alpine grasslands. Plants 2022, 11, 1673. [Google Scholar] [CrossRef]
- National Institute of Ecology (NIE). Pedicularis hallaisanensis Species Information. Available online: https://www.nie.re.kr/ (accessed on 4 February 2025).
- National Institute of Biological Resources (NIBR). Pedicularis hallaisanensis Species Information. Available online: https://species.nibr.go.kr/ (accessed on 4 February 2025).
- Kim, H.-H.; Yoon, H.-J.; Kim, J.-W. Distribution characteristics and diversity of alpine and subalpine plants growing naturally in National Parks. J. Environ. Sci. Int. 2024, 33, 367–382. [Google Scholar] [CrossRef]
- Sala, O.E.; Chapin, F.S.; Armesto, J.J.; Berlow, E.; Bloomfield, J.; Dirzo, R.; Wall, D.H. Global biodiversity scenarios for the year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef]
- Kim, L.-K.; Choi, S.-D.; Choo, G.-C.; Hwang, B.-Y.; Gang, G.-H.; So, S.-K.; Park, E.-H. Environmental characteristics and floristic study of endangered Pedicularis hallaisanensis habitats. J. Agric. Life Sci. 2018, 52, 163–173. [Google Scholar] [CrossRef]
- Kim, S.; Lee, B.-D.; Lee, C.W.; Park, H.-J.; Hwang, J.E.; Park, H.B.; Kim, Y.-J.; Jeon, D.; Yoon, Y.-J. Strict biennial lifecycle and anthropogenic interventions affect temporal genetic differentiation in the endangered endemic plant, Pedicularis hallaisanensis. Front. Plant Sci. 2024, 15, 1468395. [Google Scholar] [CrossRef]
- Frezza, C.; Venditti, A.; Toniolo, C.; De Vita, D.; Serafini, M.; Nicoletti, M. Pedicularis L. Genus: Systematics, Botany, Phytochemistry, Chemotaxonomy, Ethnopharmacology, and Other. Plants 2019, 8, 306. [Google Scholar] [CrossRef] [PubMed]
- Li, M.X.; He, X.R.; Tao, R.; Cao, X. Phytochemistry and Pharmacology of the Genus Pedicularis Used in Traditional Chinese Medicine. Am. J. Chin. Med. 2014, 42, 1071–1098. [Google Scholar] [CrossRef]
- Park, H.B.; Eun-Hwang, J.; Young-Jeon, D.; Woo-Lee, C.; Joon-Park, H.; Kim, S.; Yoon, Y.J. Classification of seed dormancy in Pedicularis hallaisanensis: An endemic and endangered species native to Korea. Horticulturae 2024, 10, 1188. [Google Scholar] [CrossRef]
- Jang, R.H.; Kim, S.; Jung, J.W.; Tho, J.H.; Cheong, S.; Yoon, Y.J. Development of a habitat suitability index for the habitat restoration of Pedicularis hallaisanensis Hurusawa. J. Ecol. Environ. 2022, 46, 316–323. [Google Scholar] [CrossRef]
- Hill, D.; Fasham, M.; Tucker, G.; Shewry, M.; Shaw, P. Handbook of Biodiversity Methods: Survey, Evaluation and Monitoring; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effect models using lme4. J. Stat. Softw. 2015, 67, 48. [Google Scholar] [CrossRef]
- De Mendiburu, F.; Simon, R. Agricolae-ten years of an open source statistical tool for experiments in breeding, agriculture and biology. PeerJ Prepr. 2015, 3, e1404v1. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 1 April 2025).
- Liu, X.; Tan, N.; Zhou, G.; Zhang, D.; Zhang, Q.; Liu, S.; Chu, G.; Liu, J. Plant diversity and species turnover co-regulate soil nitrogen and phosphorus availability in Dinghushan forests, southern China. Plant Soil. 2021, 464, 257–272. [Google Scholar] [CrossRef]
- Kim, I.-K.; Park, E.-H.; Gang, G.-H.; Hwang, B.-Y.; Jung, H.-J.; Kim, M.-Y.; Park, J.-G.; Park, S.-B.; Kim, B.-G.; Choo, G.-C. Form and embryonic characteristics of Pedicularis hallaisanensis seeds as endangered wild species II-class using host plants. J. Korean Soc. For. Sci. 2019, 108, 290–295. [Google Scholar] [CrossRef]
- Hedberg, A.M.; Borowicz, V.A.; Armstrong, J.E. Interactions between a hemiparasitic plant, Pedicularis canadensis L. (Orobanchaceae), and members of a tallgrass prairie community. J. Torrey Bot. Soc. 2005, 132, 401–410. [Google Scholar] [CrossRef]
- Lange, M.; Eisenhauer, N.; Sierra, C.A.; Bessler, H.; Engels, C.; Griffiths, R.I.; Mellado-Vázquez, P.G.; Malik, A.A.; Roy, J.; Scheu, S.; et al. Plant diversity increases soil microbial activity and soil carbon storage. Nat. Commun. 2015, 6, 6707. [Google Scholar] [CrossRef]
- Macior, L.W. The Pollination Ecology of Pedicularis (Scrophulariaceae) in the Sierra Nevada of California. Bull. Torrey Bot. Club 1977, 104, 148–154. [Google Scholar] [CrossRef]
- Macior, L.W. A preliminary study of the pollination ecology of Pedicularis in the Chinese Himalaya. Plant Species Biol. 1990, 5, 215–223. [Google Scholar] [CrossRef]
- Kim, H.-C. Ecological Characteristics and Management Methods of Sasa quelpaertensis Nakai. Ph.D. Thesis, Jeju National University, Jeju, Republic of Korea, 2009. Available online: https://dcoll.jejunu.ac.kr/jsp/common/DcLoOrgPer.jsp?sItemId=000000004549 (accessed on 4 March 2025).
- Obeso, J.R. The costs of reproduction in plants. New Phytol. 2002, 155, 321–348. [Google Scholar] [CrossRef]
- Franklin, K.A. Shade avoidance. New Phytol. 2008, 179, 930–944. [Google Scholar] [CrossRef]
- Weiner, J. Allocation, plasticity and allometry in plants. Perspect. Plant Ecol. Evol. Syst. 2004, 6, 207–215. [Google Scholar] [CrossRef]
- Dullinger, S.; Gattringer, A.; Thuiller, W.; Moser, D.; Zimmermann, N.E.; Guisan, A.; Willner, W.; Plutzar, C.; Leitner, M.; Mang, T.; et al. Extinction debt of high-mountain plants under twenty-first-century climate change. Nat. Clim. Change 2012, 2, 619–622. [Google Scholar] [CrossRef]
- Scherrer, D.; Körner, C. Topographically controlled thermal-habitat differentiation buffers alpine plant diversity against climate warming. J. Biogeogr. 2011, 38, 406–416. [Google Scholar] [CrossRef]
- Salick, J.; Ghimire, S.K.; Fang, Z.; Dema, S.; Konchar, K.M.; Collaborating authors. Himalayan alpine vegetation, climate change and mitigation. J. Ethnobiol. 2014, 34, 276–293. [Google Scholar] [CrossRef]
- Kim, E.S.; Lee, J.S.; Park, G.E.; Lim, J.H. Analysis of 20-year changes in the area of subalpine coniferous forests. J. Korean Soc. For. Sci. 2019, 108, 10–20. [Google Scholar] [CrossRef]
- National Institute of Ecology (NIE). Analysis of Research Trends on Conservation Technologies for Alpine Vulnerable Ecosystems; National Institute of Ecology Report: Seocheon, Republic of Korea, 2024.
- Kidane, Y.O.; Steinbauer, M.J.; Beierkuhnlein, C. Dead end for endemic plant species? A biodiversity hotspot under pressure. Glob. Ecol. Conserv. 2019, 19, e00670. [Google Scholar] [CrossRef]
- You, J.H.; Jeon, S.K.; Seol, J.W. Flora and conservation plan of Gayasan National Park. J. Korean Soc. Environ. Restor. Technol. 2013, 16, 109–130. [Google Scholar] [CrossRef][Green Version]
- Korea Meteorological Administration (KMA). Automated Synoptic Observing System: Temperature Data from Geochang and Hapcheon Stations (1991–2020). Available online: https://data.kma.go.kr (accessed on 17 May 2025).
- Körner, C. Alpine Plant Life: Functional Plant Ecology of High Mountain Ecosystems, 3rd ed.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Petitpierre, B.; McDougall, K.; Seipel, T.; Broennimann, O.; Guisan, A.; Kueffer, C. Will climate change increase the risk of plant invasions into mountains? Ecol. Appl. 2016, 26, 530–544. [Google Scholar] [CrossRef] [PubMed]
- Devine, W.D.; Harrington, C.A.; Peter, D.H. Oak woodland restoration: Understory response to removal of encroaching conifers. Ecol. Restor. 2007, 25, 247–255. [Google Scholar] [CrossRef]
- Nagamitsu, T.; Ushirokita, F.; Konno, Y. Foraging habitats and floral resource use by colonies of long- and short-tongued bumble bee species in an agricultural landscape with kabocha squash fields. Appl. Entomol. Zool. 2005, 40, 437–446. [Google Scholar] [CrossRef]
- Kanbe, Y.; Okada, I.; Yoneda, M.; Goka, K.; Tsuchida, K. Interspecific mating of the introduced bumblebee Bombus terrestris and the native Japanese bumblebee Bombus hypocrita sapporoensis results in inviable hybrids. Sci. Rep. 2023, 13, 11506. [Google Scholar] [CrossRef]
- Ministry of Environment, Republic of Korea. The Republic of Korea’s Fifth National Biodiversity Strategy (2024–2028); Ministry of Environment: Sejong, Republic of Korea, 2023. Available online: https://www.cbd.int/doc/world/kr/kr-nbsap-v5-en.pdf (accessed on 18 May 2025).
- Dove, T. The Effect of Increasing Temperature on Germination of Native Plant Species; University of Notre Dame Environmental Research Center: Notre Dame, IN, USA, 2010; Available online: https://underc.nd.edu/assets/156376/fullsize/dove2010.pdf (accessed on 18 May 2025).
- Zhang, Q.; Lu, Z.; Guo, M.; Kang, J.; Li, J.; He, X.; Wu, J.; Liu, R.; Dang, J.; Li, Z. Responses of Three Pedicularis Species to Geological and Climatic Changes in the Qinling Mountains and Adjacent Areas in East Asia. Plants 2024, 13, 765. [Google Scholar] [CrossRef]
- Cui, H.; Töpper, J.P.; Yang, Y.; Vandvik, V.; Wang, G. Plastic Population Effects and Conservative Leaf Traits in a Reciprocal Transplant Experiment Simulating Climate Warming in the Himalayas. Front. Plant Sci. 2018, 9, 1069. [Google Scholar] [CrossRef]
- Huelsman, K. Assessing the Ability to Detect Invasive Plant Species Using Drone-Based Leaf-Scale Visible and Near-Infrared Imaging Spectroscopy; University of Virginia: Charlottesville, VA, USA, 2021; Available online: https://vsgc.odu.edu/wp-content/uploads/2021/11/Huelsman.pdf (accessed on 18 May 2025).
- Barnas, A.F.; Darby, B.J.; Vandeberg, G.S.; Rockwell, R.F.; Ellis-Felege, S.N. A comparison of drone imagery and ground-based methods for estimating the extent of habitat destruction by lesser snow geese (Anser caerulescens caerulescens) in La Pérouse Bay. PLoS ONE 2019, 14, e0217049. [Google Scholar] [CrossRef]
- Osco, L.P.; Marcato Junior, J.; Ramos, A.P.M.; Jorge, L.A.C.; Fatholahi, S.N.; Silva, J.A.; Matsubara, E.T.; Pistori, H.; Gonçalves, W.N.; Li, J. A Review on Deep Learning in UAV Remote Sensing. arXiv 2021, arXiv:2101.10861. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).